Oral solid pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the field of medicine, to achieve stable vasodilation, increase deformability, and prevent structural and functional disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] The preparation of embodiment 1 hydrochlorothiazide crystal
[0093] (1) 1kg hydrochlorothiazide is dissolved in acetone to obtain acetone solution whose concentration is 0.1g / ml hydrochlorothiazide;
[0094] (2) Add distilled water dropwise to the acetone solution under stirring at 160r / min until the solution becomes turbid;
[0095] (3) under the ultrasonic field that power is 0.5KW, flow the organic mixed solution of ethanol and ether in the solution gained in step 2, continue the stirring of 25r / min; Wherein the volume ratio of ethanol and ether in the organic mixed solution is 5: 6, The volume ratio of described mixed solution and acetone is 1: 1;
[0096] (4) Continue ultrasonication for 2 minutes, let stand, grow crystals at 16° C. for 2 hours, filter, wash the filter cake with ether, and vacuum-dry to obtain hydrochlorothiazide crystals.
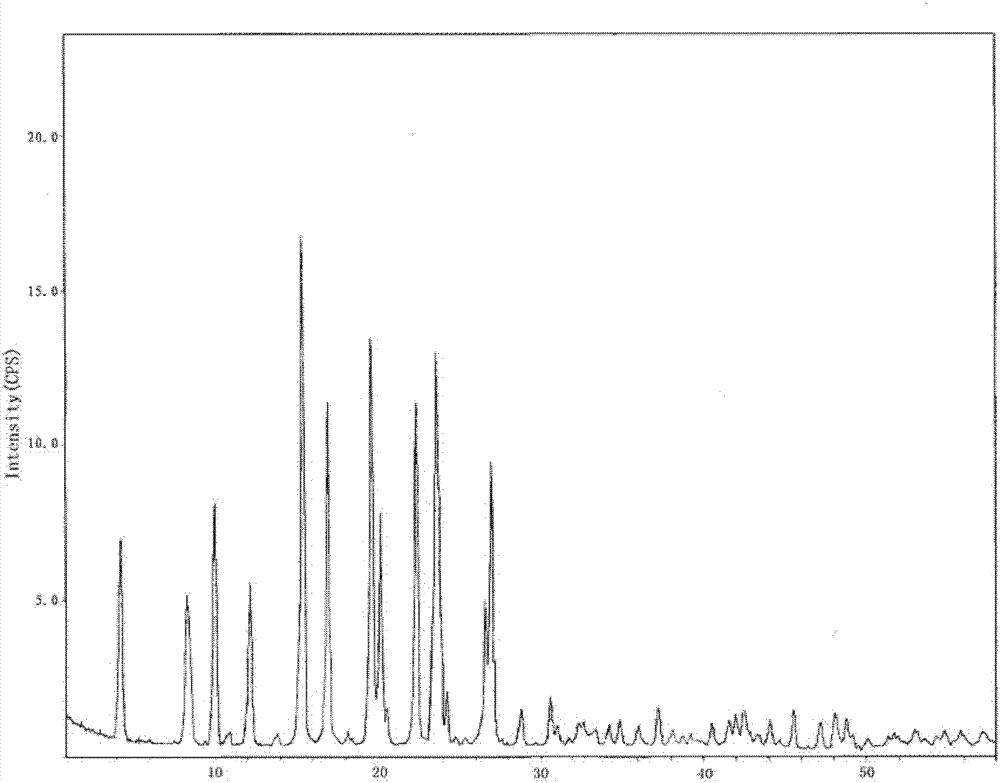
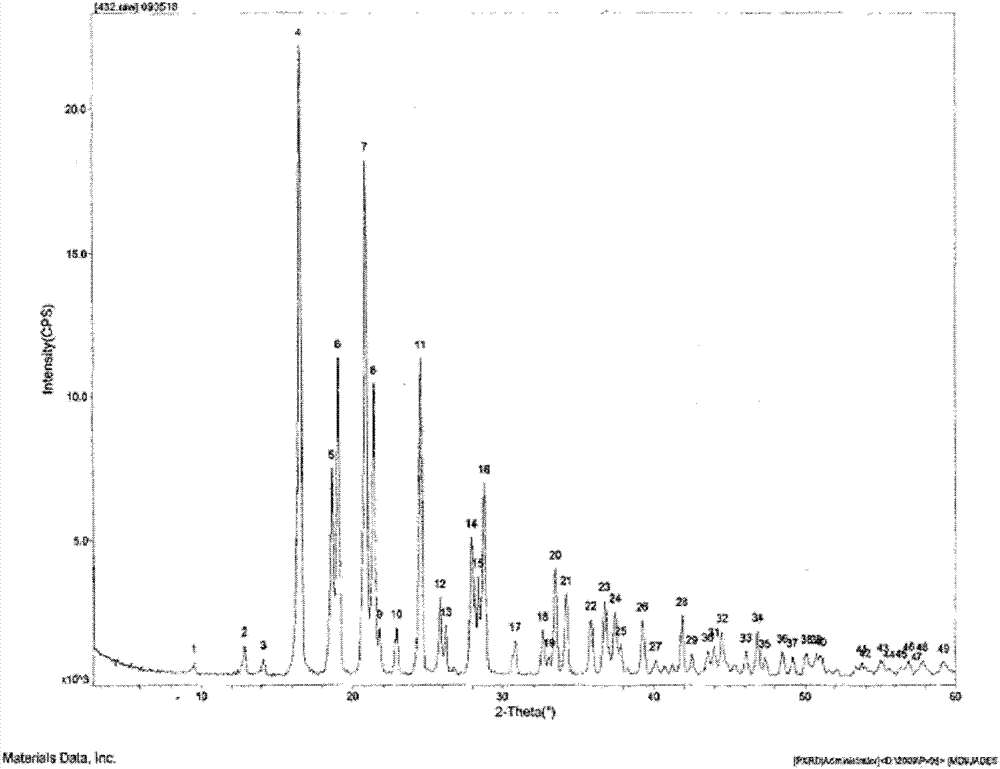
[0097] Such as figure 1 As shown, the characteristic peaks in the X-ray powder diffraction pattern obtained by measuring...
Embodiment 2
[0098] The preparation of embodiment 2 hydrochlorothiazide crystals
[0099] (1) 1kg hydrochlorothiazide is dissolved in acetone to obtain acetone solution whose concentration is 0.2g / ml hydrochlorothiazide;
[0100] (2) Add distilled water dropwise to the acetone solution under stirring at 120r / min until the solution becomes turbid;
[0101] (3) under the ultrasonic field that power is 0.4KW, flow the organic mixed solution of ethanol and ether in the solution gained in step 2, continue the stirring of 20r / min; Wherein the volume ratio of ethanol and ether in the organic mixed solution is 2: 3, The volume ratio of described mixed solution and acetone is 4: 5;
[0102] (4) Continue ultrasonication for 2 minutes, let stand, grow crystals at 12° C. for 1.5 hours, filter, wash the filter cake with ether, and vacuum-dry to obtain hydrochlorothiazide crystals.
[0103] Such as figure 1 As shown, the characteristic peaks in the X-ray powder diffraction pattern obtained by measurin...
Embodiment 3
[0104] The preparation of embodiment 3 hydrochlorothiazide crystals
[0105] (1) 1kg hydrochlorothiazide is dissolved in acetone to obtain a solution of acetone with a concentration of 0.08g / ml hydrochlorothiazide;
[0106] (2) Add distilled water dropwise to the acetone solution under stirring at 180r / min until the solution becomes turbid;
[0107] (3) under the ultrasonic field that power is 0.6KW, flow the organic mixed solution of ethanol and ether in the solution gained in step 2, continue the stirring of 30r / min; Wherein the volume ratio of ethanol and ether in the organic mixed solution is 7: 6, The volume ratio of described mixed solution and acetone is 8:5;
[0108] (4) Continue ultrasonication for 3 minutes, let stand, grow crystals at 18° C. for 2.5 hours, filter, wash the filter cake with ether, and vacuum-dry to obtain hydrochlorothiazide crystals.
[0109] Such as figure 1 As shown, the characteristic peaks in the X-ray powder diffraction pattern obtained by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com