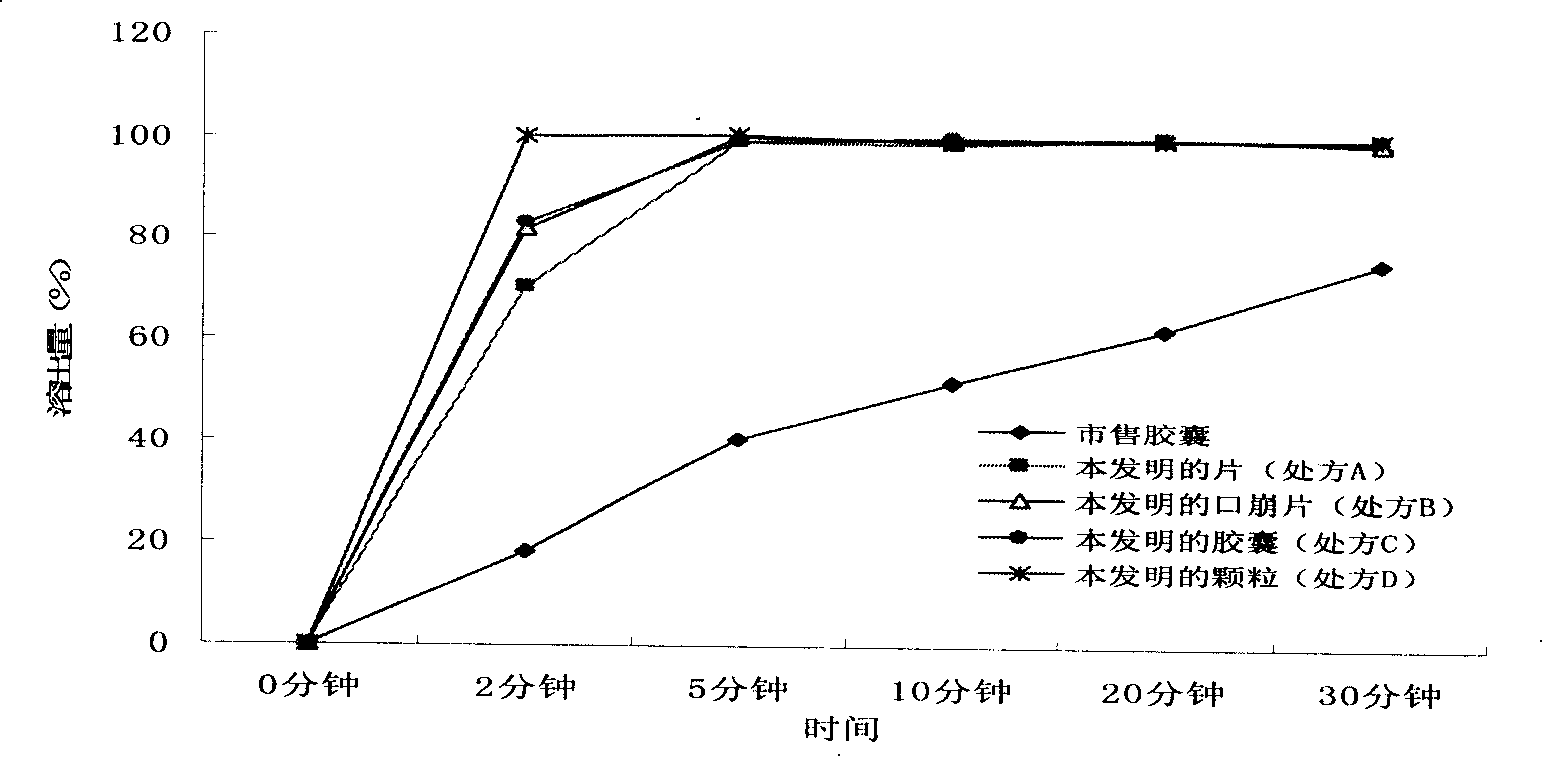
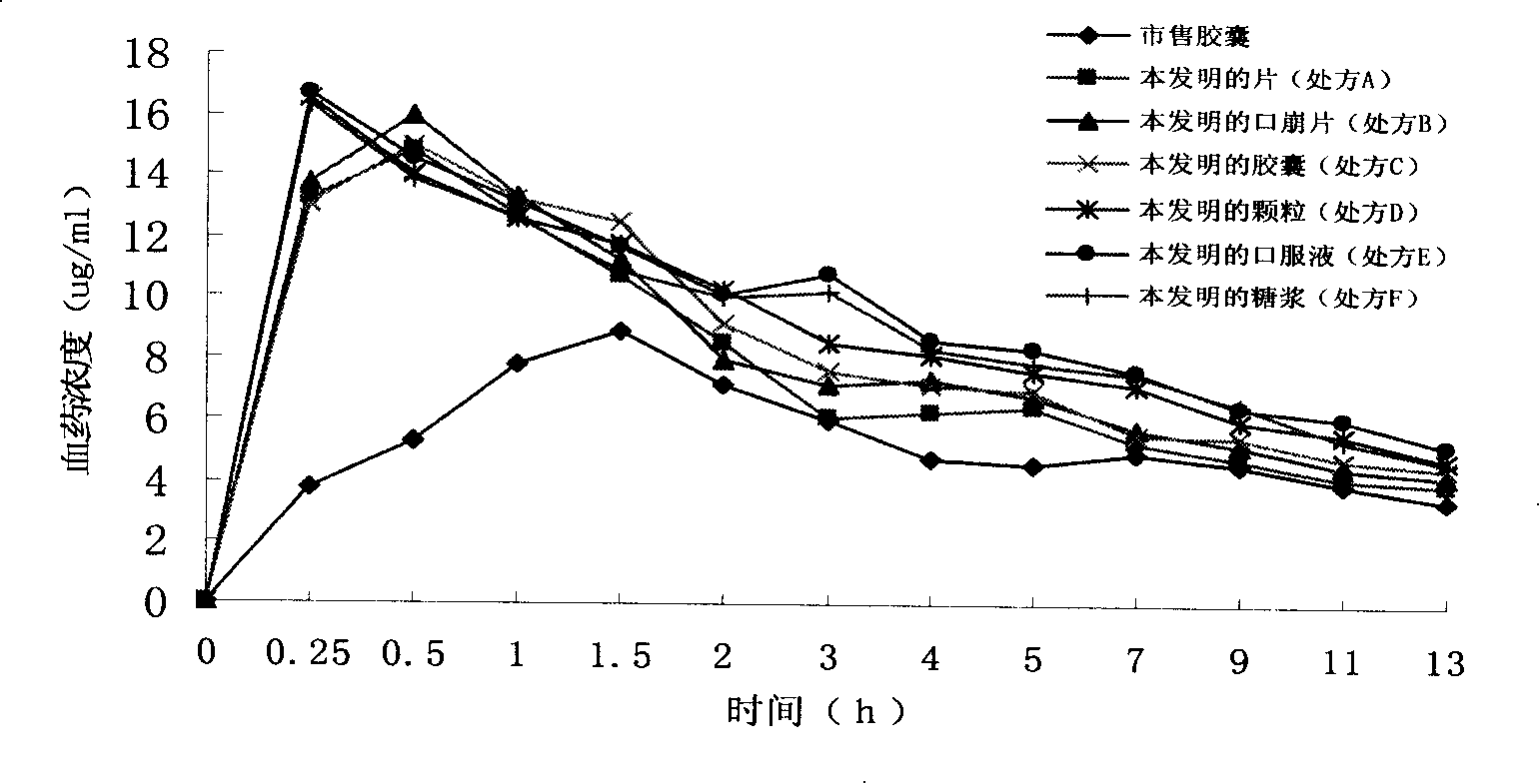
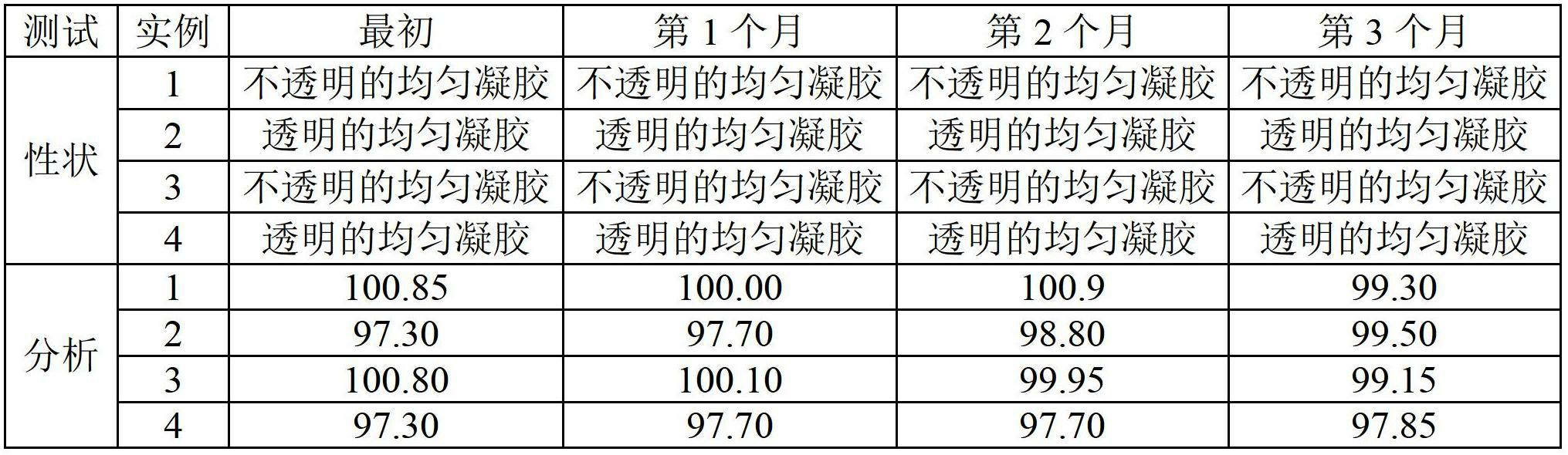
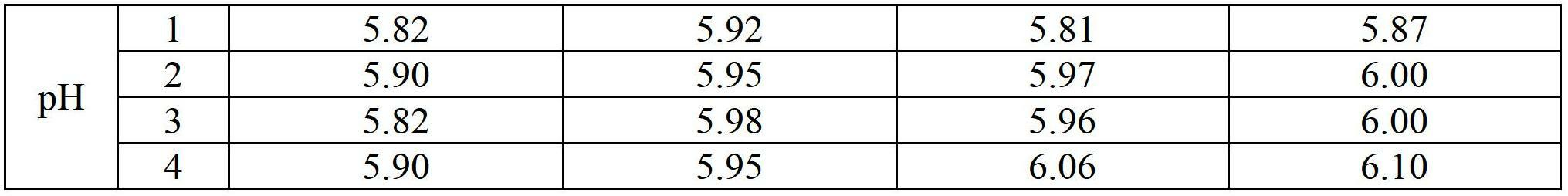
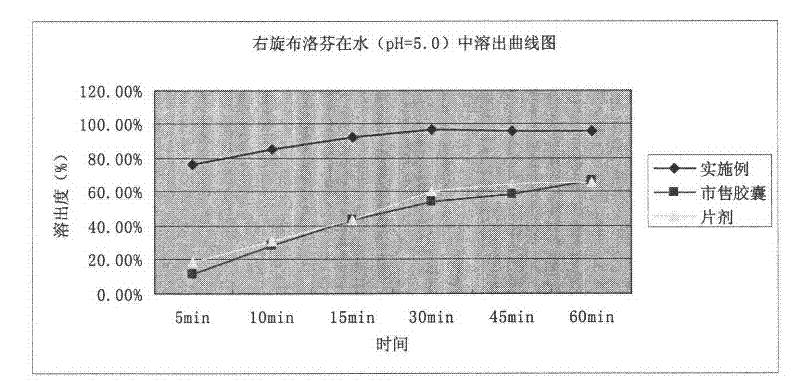
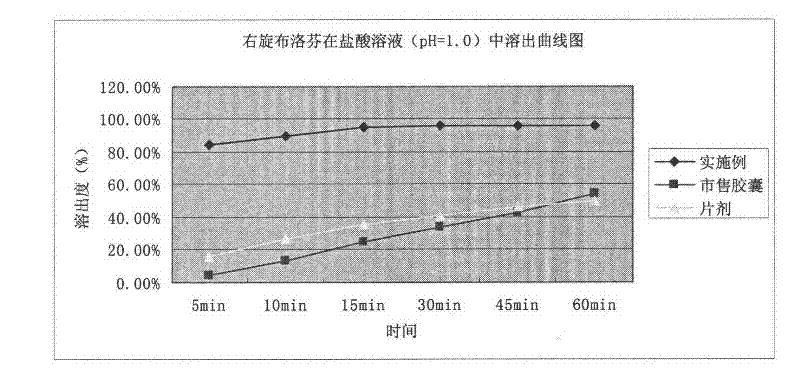
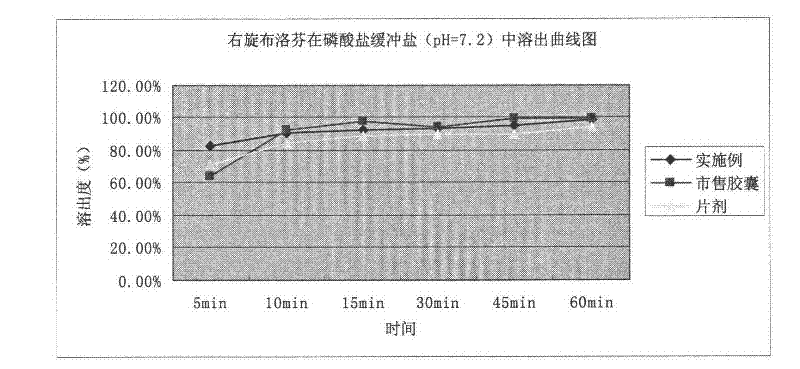
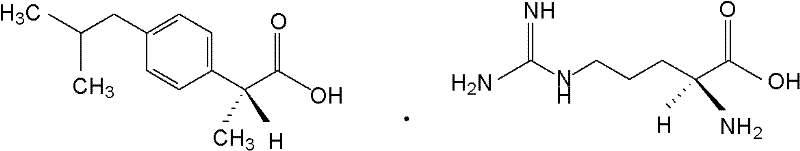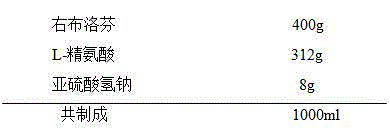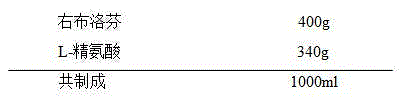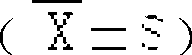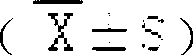Patents
Literature
40 results about "Dexibuprofen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
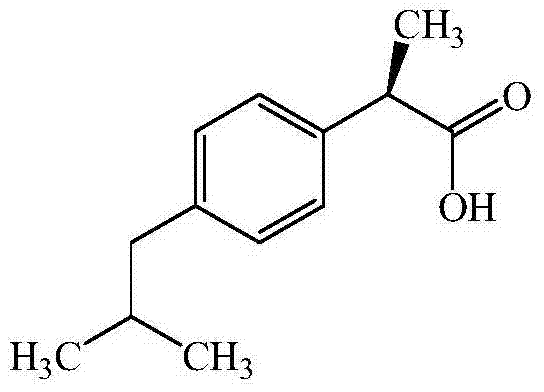
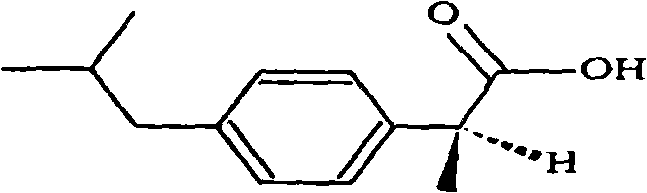
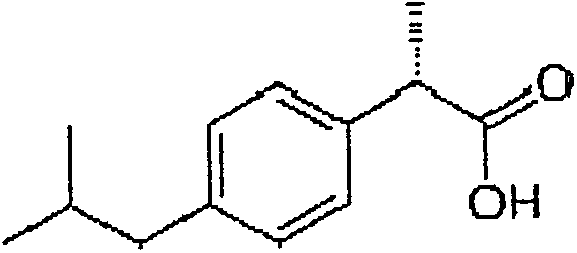
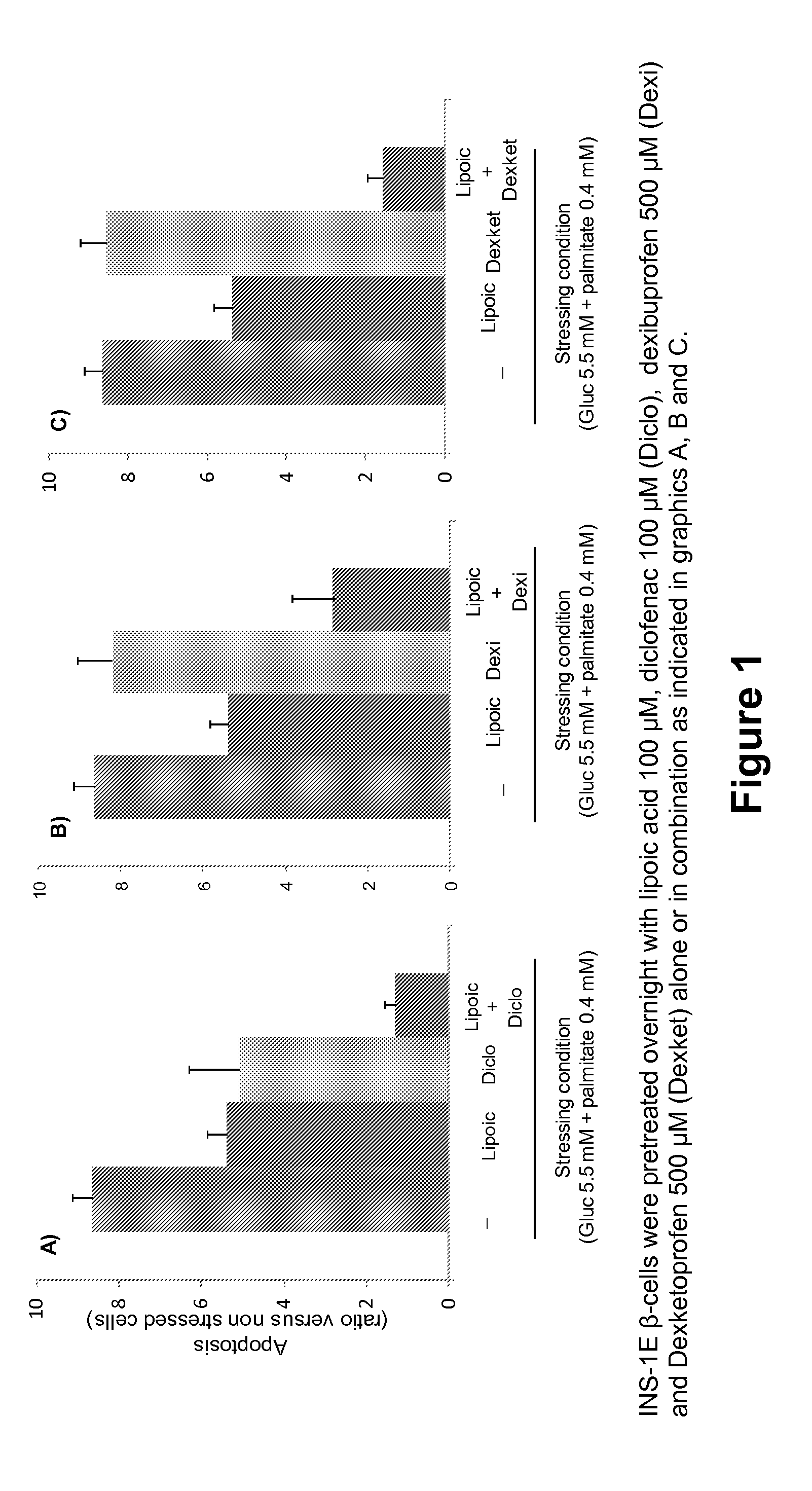
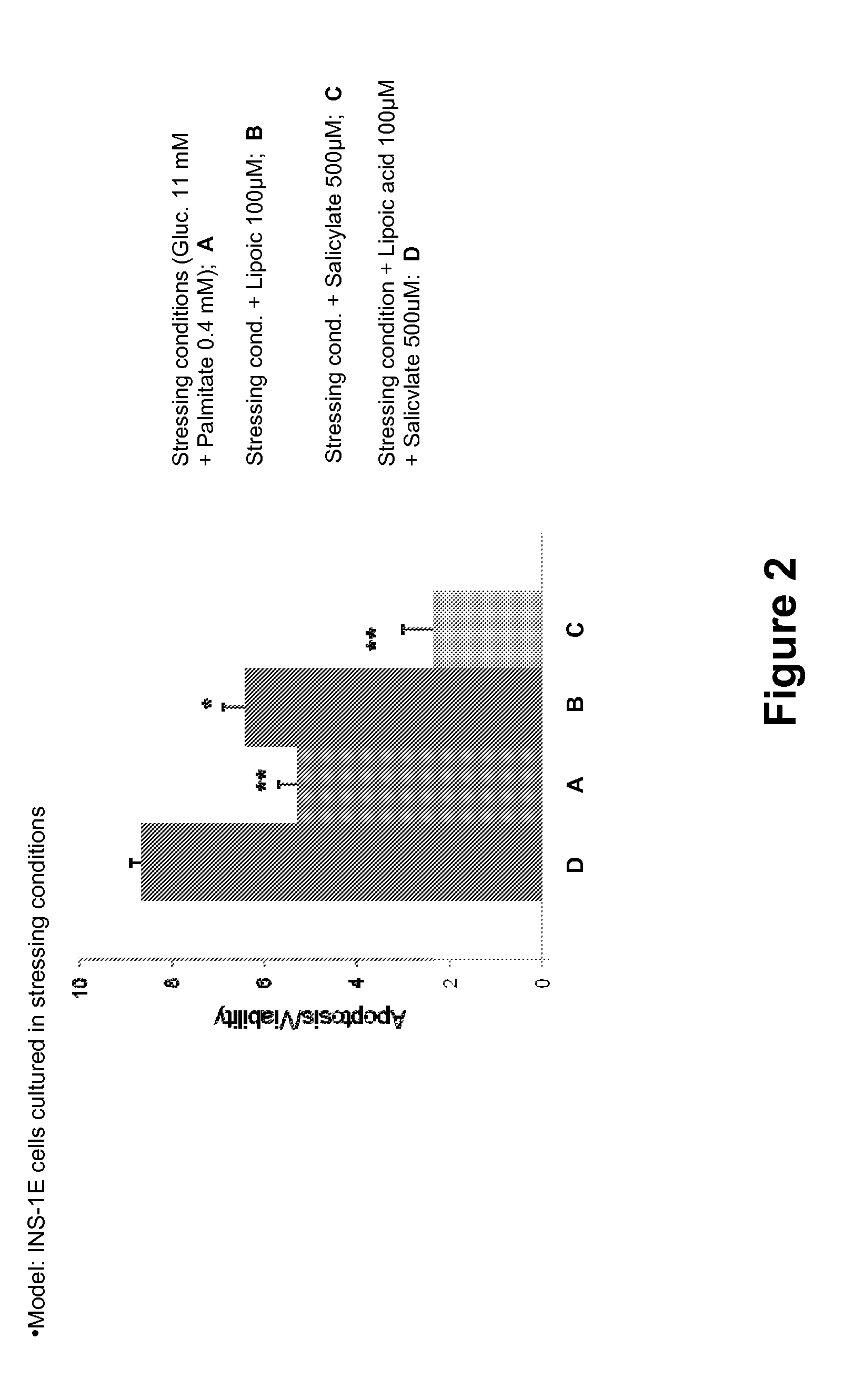
Dexibuprofen is a nonsteroidal anti-inflammatory drug (NSAID). It is the active dextrorotatory enantiomer of ibuprofen. Most ibuprofen formulations contain a racemic mixture of both isomers.
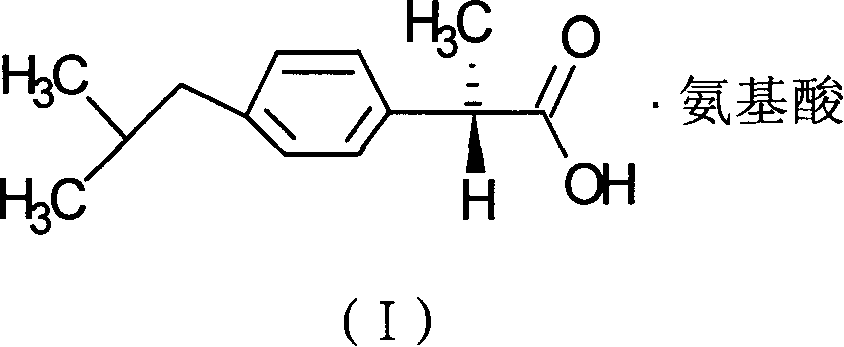
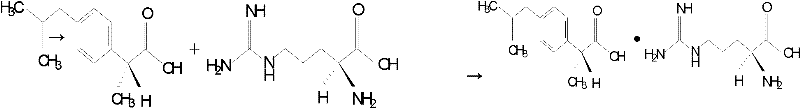
Preparation method of dexibuprofen amino acid salt and application
InactiveCN1923798ABioavailability unchangedLess irritatingOrganic active ingredientsAntipyreticDexibuprofenAmino acid
The invention discloses a nalorphine amino compound and preparing method with structural formula as (I), wherein the amino can be Arg, Lys or His; the molecular rate of nalorphine and amino is 1:1-1: 5 or 5:1-1:1.
Owner:陈亦林
Dexibuprofen slow-release capsule and production method thereof
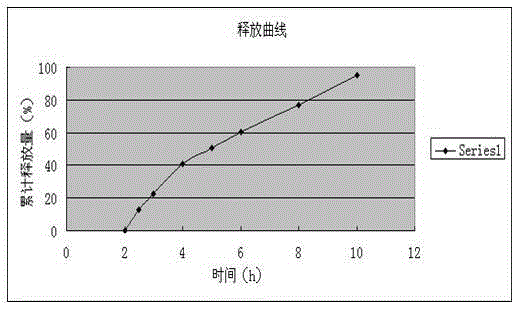
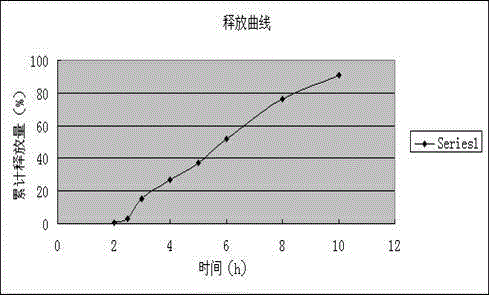
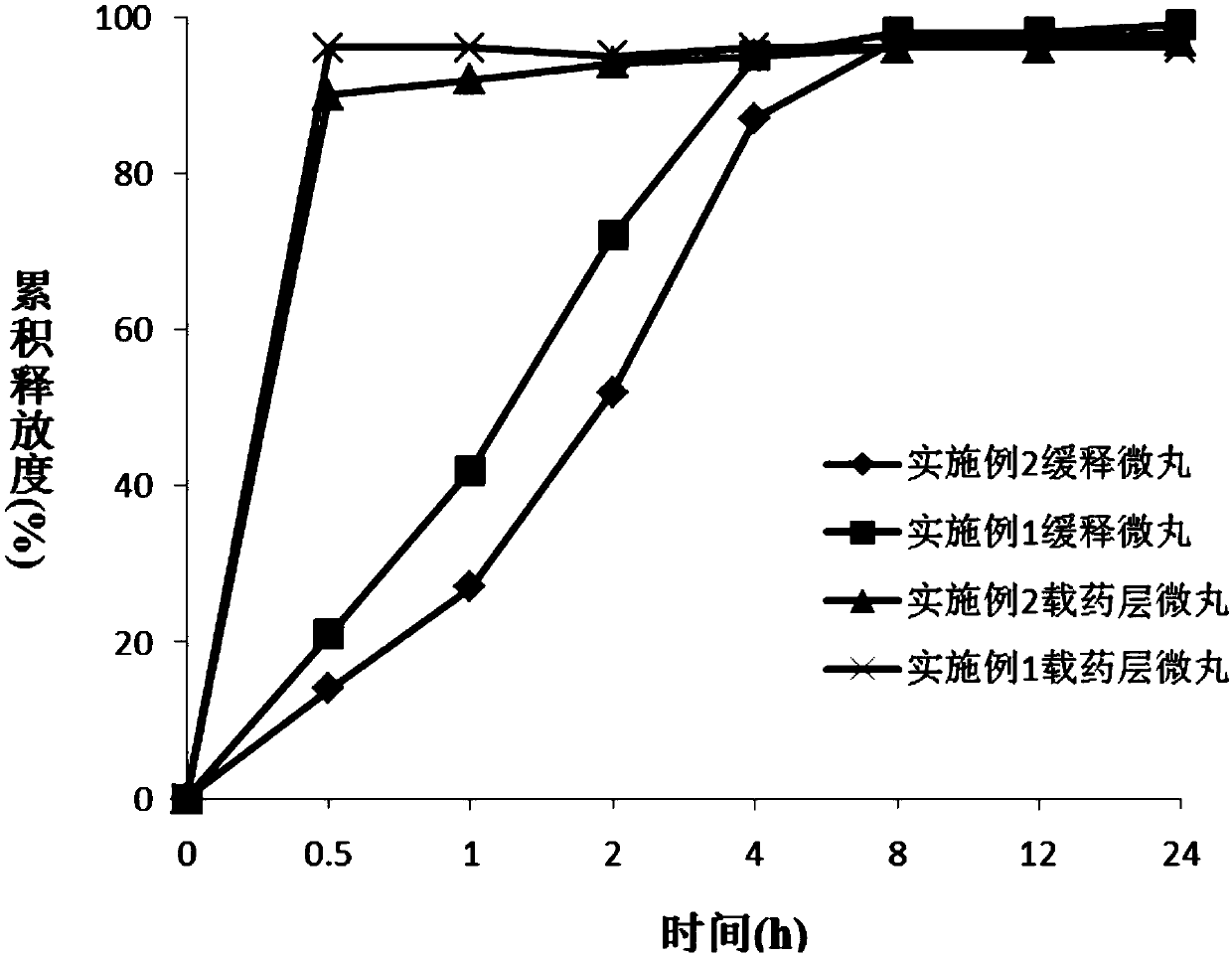
The invention discloses a dexibuprofen slow-release capsule. Pellets are filled in the capsule, each pellet consists of a pellet core and four layers of materials wrapped outside the pellet core, the four layers of materials are sequentially an inner isolation layer, a first coating layer, a second coating layer and a third coating layer from inside to outside, and the pellet core, the inner isolation layer, the first coating layer, the second coating layer and the third coating layer respectively have the following compositions: the pellet core is prepared by medicine auxiliary materials, the inner isolation layer is stearic acid, the first coating layer is a coating mixture, the second coating layer comprises the coating mixture and the stearic acid, the third coating layer is the coating mixture, and the coating mixture consists of dexibuprofen and polyvidone K30. The invention also provides a production method of the dexibuprofen slow-release capsule. Compared with an ordinary capsule, the dexibuprofen slow-release capsule disclosed by the invention has the same absorption degree, but the dexibuprofen blood maximum concentration (Cmax) of the dexibuprofen slow-release capsule disclosed by the invention in human bodies is lower, the maximum time (Tmax) from the administration to the blood Cmax reaching is longer, and a good slow release effect is realized.
Owner:武汉长联来福制药股份有限公司
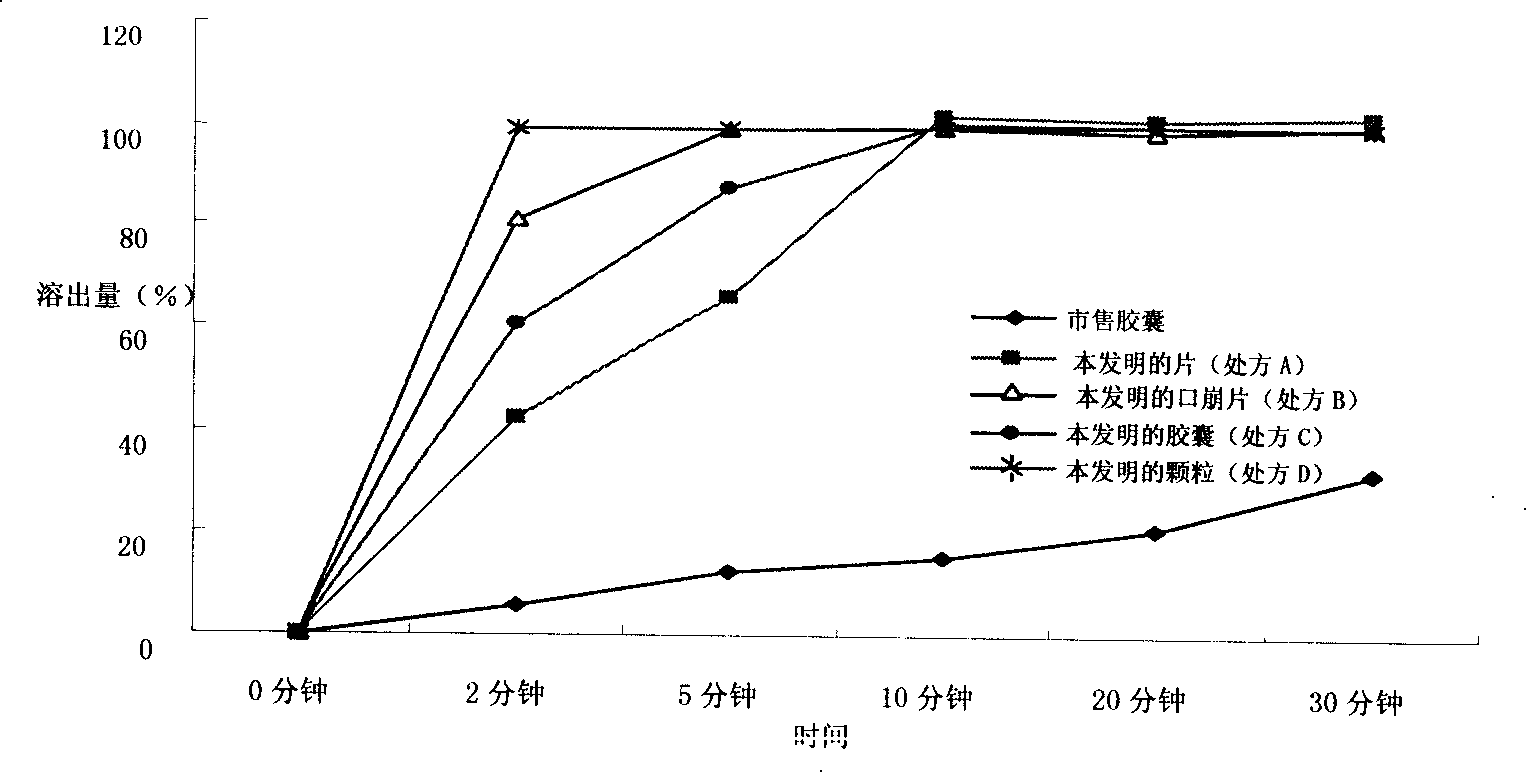
Dexibuprofen pharmaceutical composition with improved dissolving out capability and method for preparing the same
InactiveCN101219128AImprove bioavailabilityReduce adverse reactionsOrganic active ingredientsAntipyreticDiseaseSodium bicarbonate
The invention provides a dexibuprofen medical composition that can improve dissolution and a preparation method thereof, relating to the method technology field that improves the dissolution rate of the dexibuprofen medical compositions which comprise amino acid, sodium carbonate and sodium bicarbonate, being added with proper amount of other pharmaceutic adjuvant to form a new dexibuprofen medical composition. The dexibuprofen medical composition added with solubilization composition can be dissolved quickly in vivo and in vitro to shorten the absorption time and to improve the bioavailability of the blood plasma, thus resisting disease more quickly and effectively.
Owner:海南高升医药科技开发股份有限公司
Composition of dexibuprofen transdermal hydrogel
Stable non-alcoholic transdermal hydrogel of dexibuprofen was prepared by using a simple manufacturing process, and the experimental trials showed that the pH modifying agent, antioxidant and water miscible solvent are the essential excipients to obtain stable non-alcoholic transdermal hydrogel of dexibuprofen. The dexibuprofen hydrogel prepared using carbopol as a gelling polymer produced an opaque gel, whereas hydrogel prepared using hyroxypropyl methylcellulose (HPMC) as a gelling polymer produced a transparent gel. There was no significant changes observed with respect to physical description, pH, assay and particularly to the related substance values when the hydrogels were subjected to the stability study at accelerated condition (40 DEG C / 75% RH) for 3 months in laminated tubes.
Owner:SHASUN CHEM & DRUGS LTD
Dexibuprofen amino acid salt injection and preparation method thereof
InactiveCN101569604ATake a small doseReduce adverse reactionsPowder deliveryOrganic active ingredientsSodium bicarbonateAdjuvant
Owner:航天中心医院 +1
Preparation method of dexibuprofen amino acid salt
InactiveCN101570480ASimple preparation processShorten the production cycleOrganic active ingredientsAntipyreticRoom temperatureAbsorption rate
The invention discloses a preparation method of dexibuprofen amino acid salt. The method is characterized by adding amino acid to dexibuprofen ethanol solution while stirring at room temperature, and completing salt forming and crystallization in turn in a container, and the method has the advantages of simple and convenient preparation process, short production period, low impurity level, high yield and good reproducibility. The dexibuprofen amino acid salt can be used for preparing oral solution preparation, also can be used for preparing injection preparation, accelerates absorption rate, enhances bioavailability and enlarges application range.
Owner:航天中心医院 +1
Emulsion injection containing dexibuprofen and preparation method of emulsion injection
The invention belongs to the field of medical technology, and discloses emulsion injection taking dexibuprofen as a raw material and a preparation method of the emulsion injection. The emulsion injection is characterized in that each 1 L of the emulsion injection comprises the following components by mass: 30 to 100 g of dexibuprofen, 100 to 250 g of soybean oil, 9 to 24 g of phospholipid, 2 to 6 g of oleinic acid, 1 to 16 g of poloxamer, 20 to 25 g of glycerol, pH adjusting ingredients, and the balance of water for injection. The emulsion injection adopts the emulsification technology, and takes an oil-water surface as a film to carry medicine, so as to solve the problem that as dexibuprofen is difficult to dissolve in water, and improve the drug loading capacity; compared with other preparations, the emulsion injection is lower in adverse reaction and toxic side effects, higher in bioavailability, lower in preparation cost, and more convenient to use by patients.
Owner:BIOCHEM ENG COLLEGE OF BEIJING UNION UNIV
Dexibuprofen injection composition
InactiveCN103720647AThe preparation process is stable and feasibleGood and stable contentOrganic active ingredientsAntipyreticUse medicationIbuprofen Injection
The invention provides a dexibuprofen injection composition which comprises dexibuprofen, arginine and water for injection, wherein other regulating agents and organic solvents do not need to be added; the dexibuprofen can be fully dissolved to form a stable medicament composition by regulating the ratio of the arginine to the dexibuprofen. The dexibuprofen injection composition provided by the invention is higher in stability; the pH of the injection is in a blood tolerance range of a human body, so that a requirement on clinical medication can be met.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD
Preparation method of arginine dexibuprofen
ActiveCN102344360AHigh yieldHigh purityOrganic compound preparationMetabolism disorderArginineFiltration
The invention discloses a preparation method of an arginine dexibuprofen raw medicine. The method comprises the steps of: dissolving dexibuprofen in ethanol, heating the mixture to a temperature of 50DEG C-60DEG C, adding L-arginine in a dropwise manner slowly and continuously under stirring within 10min-20min, continuing stirring for reaction for 2h-3.5h with the temperature maintained at 50DEG C-60DEG C, leaving the mixture to stand for 25min-35min and cool to a temperature of 18DEG C-30DEG C, conducting pumping filtration so as to obtain crystals, then washing and drying the crystals, thus obtaining white crystals, i.e. arginine dexibuprofen. The preparation method of the invention has simple process, high yield, no refining step, shortened production period, and no need for any special equipment, thus being suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Use of Dexibuprofen and Levocetirizine Sustained-release Double-Layer Tablets in the Treatment of Airway Inflammation
InactiveCN102258519ARelieve inflammatory swellingRelieve nasal inflammationOrganic active ingredientsAntipyreticSustained Release TabletMechanism of action
The present invention relates to a use of a dexibuprofen levocetirizine double-layer sustained release tablet in treatment of airway inflammation. The tablet is composed of two layers, wherein one layer is the rapid-release part, composed of dexibuprofen, levocetirizine dihydrochloride and a pharmaceutically acceptable rapid-release additive; the other layer is the sustained-release part, composed of dexibuprofen and a pharmaceutically acceptable sustained-release additive.
Owner:XIAN LIJUN PHARMA CO LTD
Method for determining dexibuprofen related matter
InactiveCN106645542AHigh precisionIncreased durabilityComponent separationRelated impuritiesConventional analysis
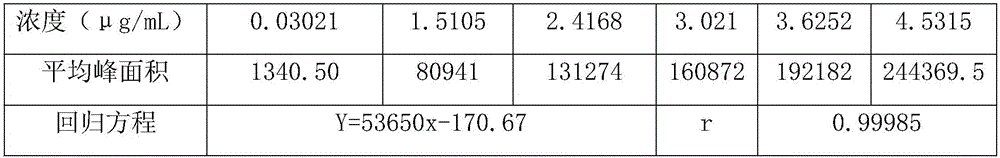
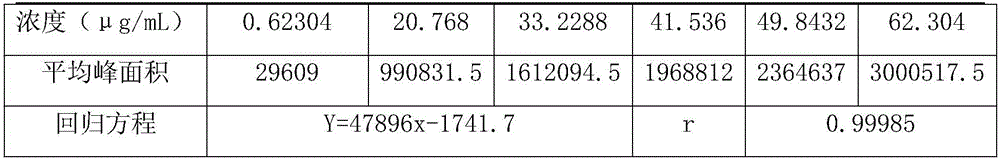
The invention discloses a method for determining a dexibuprofen related matter. According to the method, a high performance liquid chromatography method is adopted for simultaneously determining and checking 17 related impurities, such as, impurity A, impurity B, impurity C, and the like, possible to appear in dexibuprofen bulk drug. The method is time-saving, labor-saving and high in precision. Through verification, the method can be used for conventional analysis and quality control for the dexibuprofen raw material and the preparation sample.
Owner:NANJING CORE TECH CO LTD
Dexibuprofen purifying method
InactiveCN104744236AHigh purityHigh yieldOrganic compound preparationOrganic chemistry methodsOrganic solventDistillation
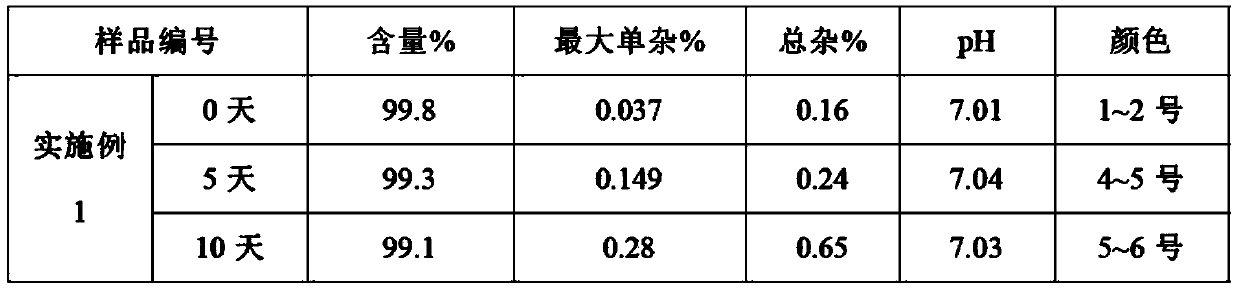
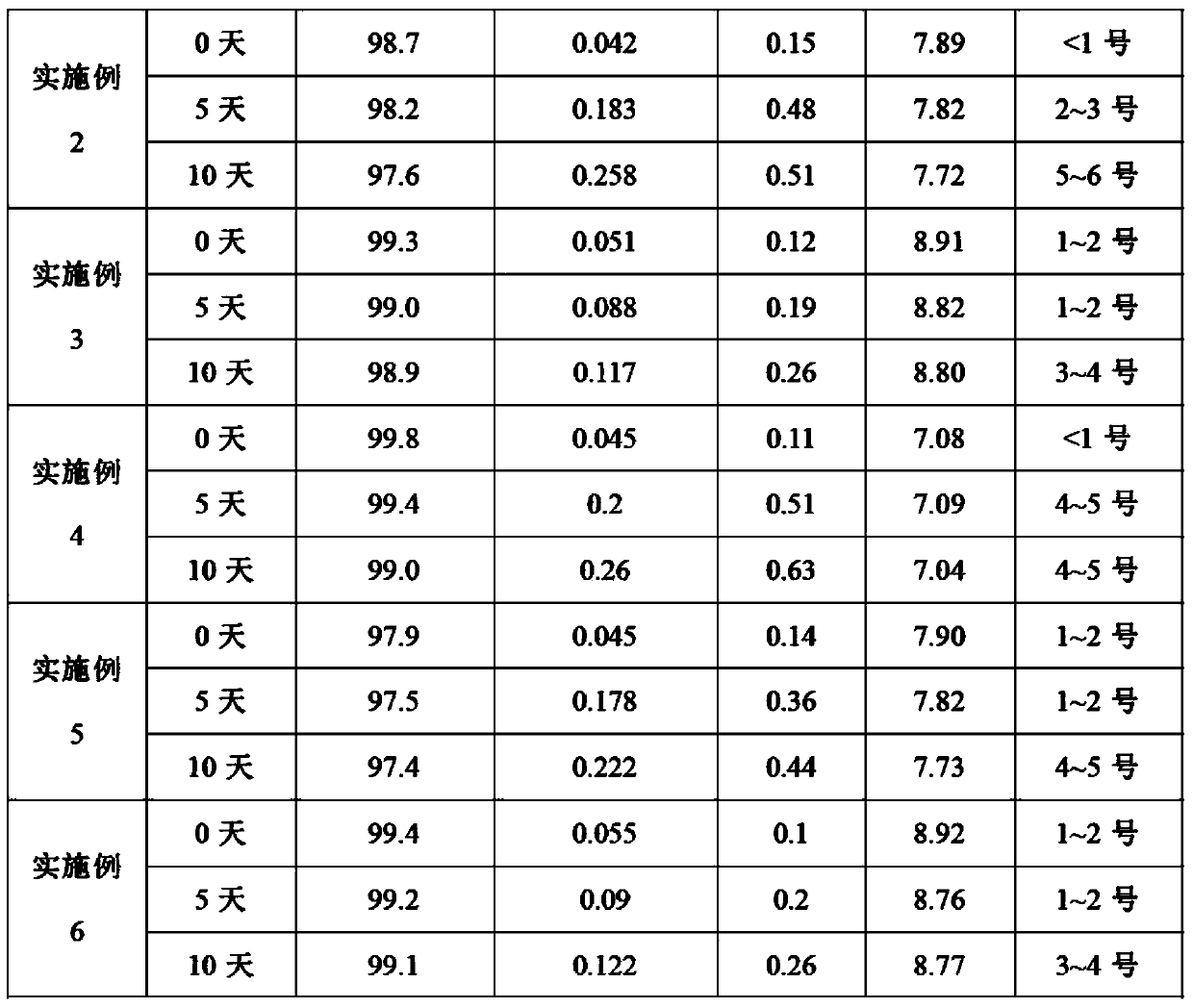
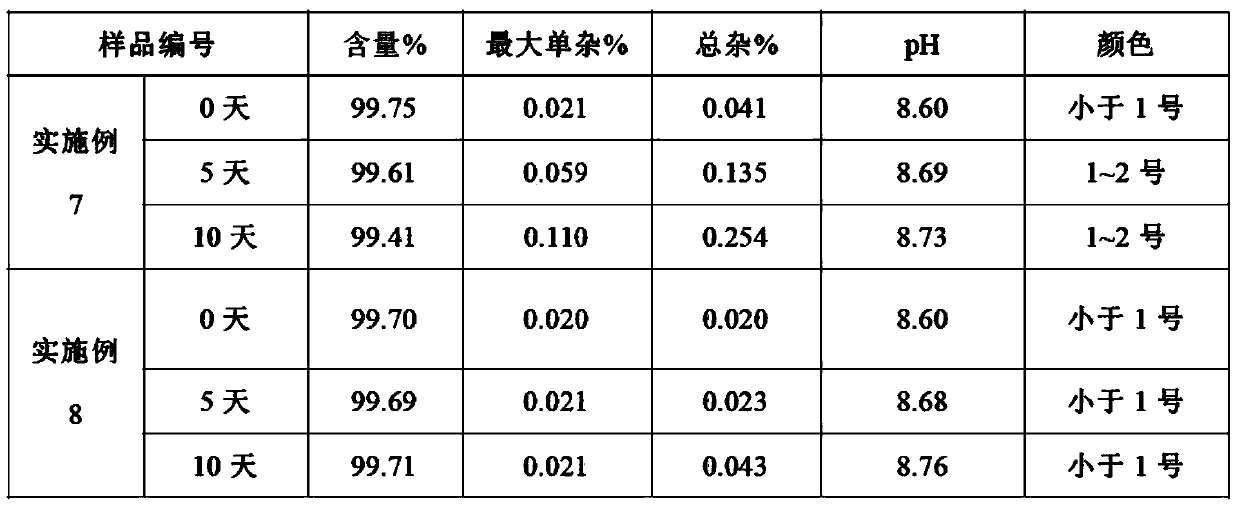
The invention relates to a dexibuprofen purifying method. The method comprises the following steps: dissolving crude dexibuprofen in an organic solvent 1, adding a sodium hydroxide solution in a dropwise manner to adjust the pH value to 9-10, carrying out cooling crystallization to obtain dexibuprofen sodium; dissolving dexibuprofen sodium in purified water, adding an organic solvent 2, and adding hydrochloric acid in a dropwise manner under stirring in order to adjust the pH value to 1-2; and carrying out liquid separation, taking the obtained organic phase, and carrying out reduced pressure distillation on the organic phase to obtain a solid which is purified dexibuprofen. The product obtained through the method has high purity of above 99.7% and high yield of above 75%; the method can completely eliminate ibuprofen ester impurities, and allows the maximal content of single impurities to be smaller than 0.1% and the total content of the impurities to be smaller than 0.3%; and the method has the advantages of low cost, simplicity and easy control of process steps, and suitableness for large scale production.
Owner:LUNAN BETTER PHARMA
Dexibuprofen sustained-release dropping pill and preparation method thereof
The invention discloses a pharmaceutical preparation used for resisting inflammation, alleviating pain and eliminating fever, and particularly relates to a prepared sustained-release oral formulation adopting dexibuprofen as the ingredient. The pharmaceutical preparation aims to supplement the deficiency of the prior art and provide a sustained-release dexibuprofen dropping pill. The sustained-release dexibuprofen dropping pill overcomes the defects in the prior art effectively, guarantees no occurrence of an obvious quality change for the drug during the effective storage period and has the advantages of controllable release time, full release and high bioavailability simultaneously.
Owner:北京博智绿洲医药科技有限公司
Dexibuprofen particle and preparation method thereof
The invention discloses a dexibuprofen particle which is mainly prepared from the following raw materials in parts by weight: 1-10 parts of dexibuprofen, 1-6 parts of sodium carbonate, 1-35 parts of sucrose, 0.1-0.8 part of aspartame and 3-12 parts of ethanol. The invention also discloses a preparation method of the dexibuprofen particle, which comprises the following steps of: sieving each raw material, and then, fully mixing the raw materials uniformly; granulating; and drying at high temperature, and then, refining to obtain the dexibuprofen particle. The dexibuprofen particle of the invention can endure high-temperature drying, has qualified indexes of microbial limit, solubility and the like and has stable and controllable quality; in addition, the preparation method is simple to operate has short production cycle and low cost, and is suitable for industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Dexibuprofen oral liquid preparation and preparation method thereof
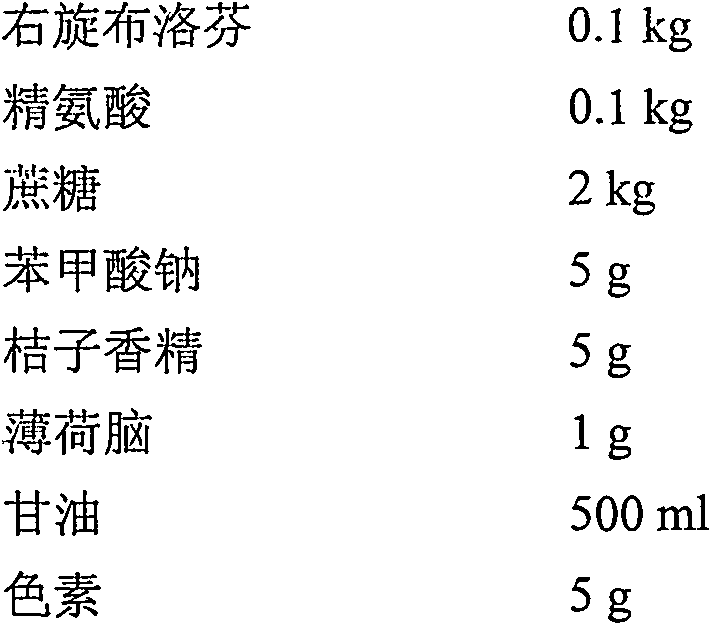
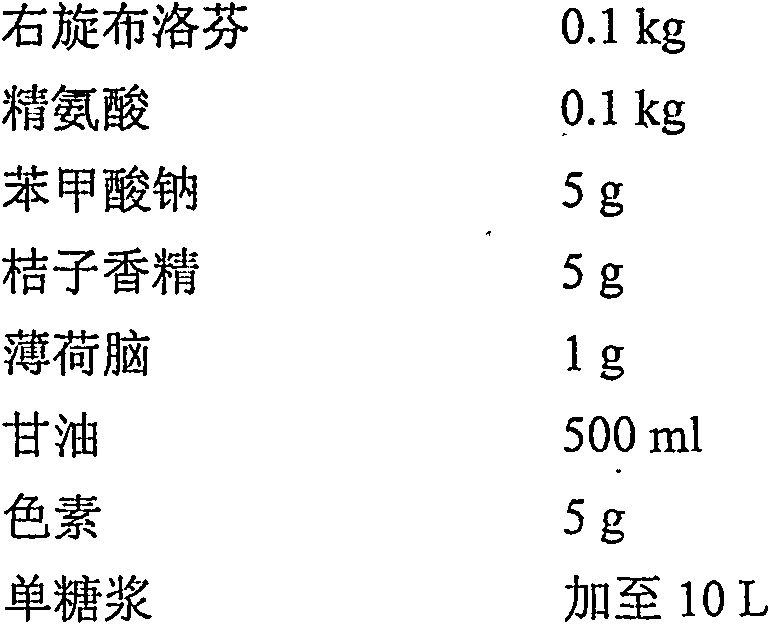
Belonging to the field of pharmaceutical preparations, the invention relates to a dexibuprofen oral liquid preparation. The oral liquid preparation adopts dexibuprofen as the effective component, and is added with proper auxiliary materials like a pH regulator, a solubilizer, a flavoring agent, an antiseptic agent and a coloring agent. A solution preparation process or syrup preparation process is employed to prepare the clear and transparent liquid preparation. The dexibuprofen liquid preparation provided by the invention is dispersed in the medium in a molecular state, has the characteristics of quick absorption, rapid effect, convenient oral administration and easy fractionation dose, and can simultaneously satisfy clinical medication, patient compliance, industrial scale production and other requirements.
Owner:SHANGHAI SCIENPHARM CO LTD
Dexibuprofen slow-release capsule and production method thereof
The invention discloses a dexibuprofen slow-release capsule. Pellets are filled in the capsule, each pellet consists of a pellet core and four layers of materials wrapped outside the pellet core, the four layers of materials are sequentially an inner isolation layer, a first coating layer, a second coating layer and a third coating layer from inside to outside, and the pellet core, the inner isolation layer, the first coating layer, the second coating layer and the third coating layer respectively have the following compositions: the pellet core is prepared by medicine auxiliary materials, the inner isolation layer is stearic acid, the first coating layer is a coating mixture, the second coating layer comprises the coating mixture and the stearic acid, the third coating layer is the coating mixture, and the coating mixture consists of dexibuprofen and polyvidone K30. The invention also provides a production method of the dexibuprofen slow-release capsule. Compared with an ordinary capsule, the dexibuprofen slow-release capsule disclosed by the invention has the same absorption degree, but the dexibuprofen blood maximum concentration (Cmax) of the dexibuprofen slow-release capsule disclosed by the invention in human bodies is lower, the maximum time (Tmax) from the administration to the blood Cmax reaching is longer, and a good slow release effect is realized.
Owner:武汉长联来福制药股份有限公司
Preparation method of dexibuprofen injection
InactiveCN105267140AImprove solubilityAvoiding the Problem of DegradationOrganic active ingredientsInorganic non-active ingredientsEnteral administrationOral medication
The invention provides a preparation method of a dexibuprofen injection. The method prepares dexibuprofen and appropriate auxiliary materials into a dexibuprofen injection with stable and controllable quality. The dexibuprofen injection relieves pain and fever of inpatients unable to employ oral administration, also has the advantages of avoidance of first pass effect and gastrointestinal reaction of drugs, quick effect, and good stability, etc.
Owner:NANJING KEFEI PINGSHENGHUI PHARMA CO LTD +2
Dexibuprofen microemulsion gel and preparation method thereof
The invention relates to dexibuprofen microemulsion gel and a preparation method thereof. The dexibuprofen microemulsion gel comprises components including dexibuprofen, a surfactant, a cosurfactant, an oil phase, carbomer and water. The dexibuprofen microemulsion gel is subjected to colon-drip delivery, the drug takes a microemulsion as a supporter, the drug solubility is increased, besides, the dexibuprofen microemulsion gel has proper flexibility and biological adhesion, a patient has a week foreign body sensation, the gel cannot flow out of the rectum easily after delivery, and absorption of the drug is facilitated, so that the better efficacy is guaranteed.
Owner:山东仁瑞生物科技有限公司
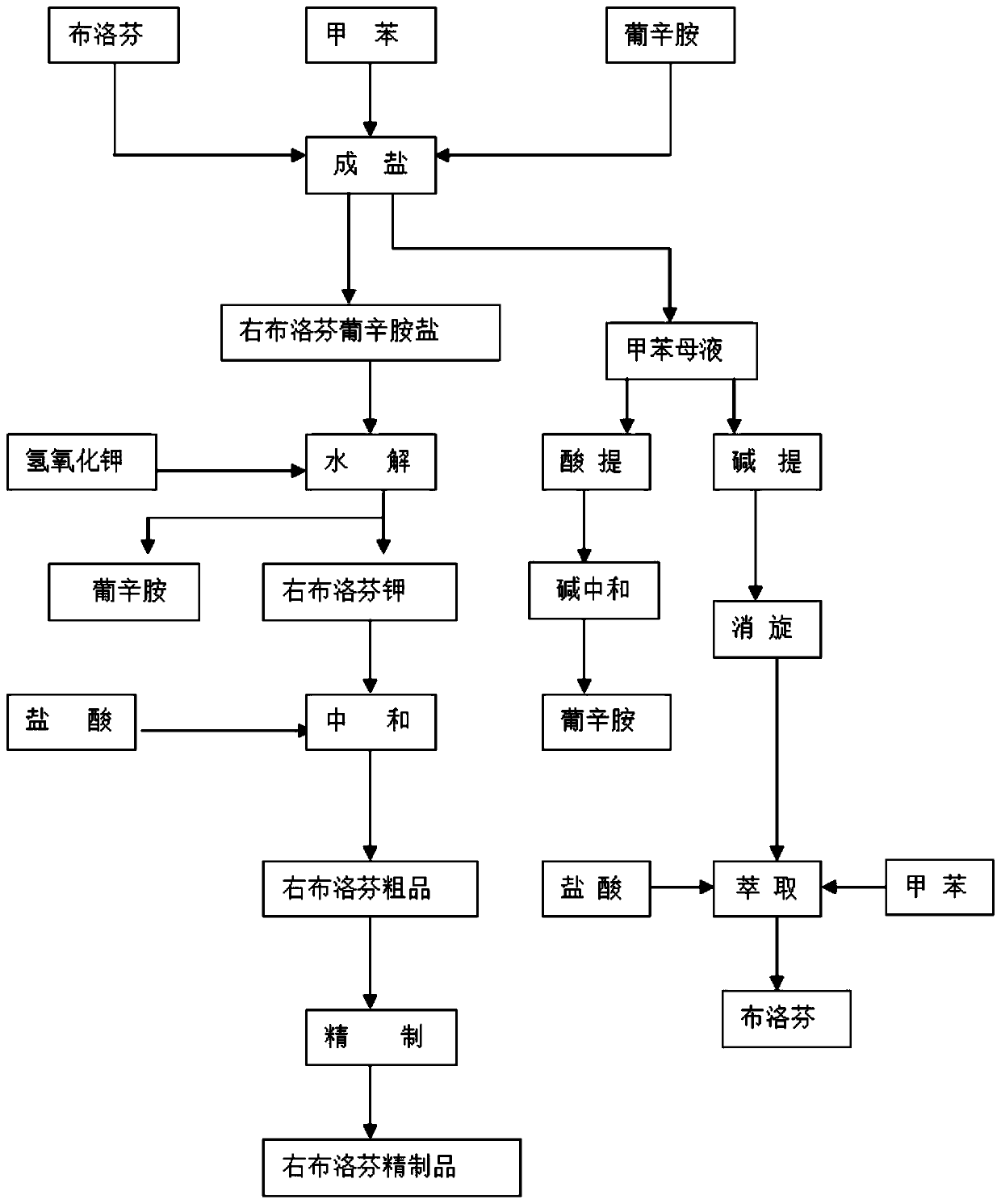
Preparation method of dexibuprofen
InactiveCN110615735AHigh molar yieldLower yield yieldPreparation from carboxylic acid saltsOrganic compound preparationTolueneHydrolysis
The invention discloses a preparation method of dexibuprofen. The method comprises the following steps: (1) adding toluene into a reaction kettle, adding ibuprofen and N-n-octyl-D-glucamine, heating to 76-80 DEG C, keeping the temperature to react for more than 0.5 hour, transferring the material into an amine salt crystallization kettle, adding purified water, cooling to crystallize, cooling to 18-22 DEG C, performing centrifugal separation, washing a product and drying to obtain a dexibuprofen salt of the N-n-octyl-D-glucamine, with the feeding ratio of the ibuprofen, the N-n-octyl-D-glucamine, the toluene and the purified water is 1:(0.65-0.75):(7.5-8):(0.01-0.02); (2) carrying out a hydrolysis reaction to separate out the N-n-octyl-D-glucamine; and (3) carrying out an acidification reaction to separate out the dexibuprofen. The applicant finally determines that the ratio of the volume of the added purified water to the weight of the ibuprofen is (0.01-0.02) L: 1kg when the amine salt is prepared, so that the melting point of the dexibuprofen reaches the qualified standard of 135-140 DEG C while the molar yield of the dexibuprofen is high.
Owner:HUBEI BIOCAUSE HEILEN PHARM CO LTD +1
Dexibuprofen suspension composition and preparation method thereof
InactiveCN106176596ASame sizeGood dispersionOrganic active ingredientsAntipyreticGellan gumCarrageenan
The invention discloses dexibuprofen suspension and a preparation method thereof. The preparation comprises dexibuprofen, low acyl gellan gum, carrageenan, pectin and a colloidality enhancer. The dexibuprofen suspension prepared by adopting the method is a thixotropic liquid, has the characteristics of low viscosity, high suspending property and no layering forever and has the beneficial effect that the particle size of dexibuprofen can remain stable in long-term storage.
Owner:SHANGHAI YANAN PHARM
Dexibuprofen enteric-coated and sustained-release tablet and preparation method thereof
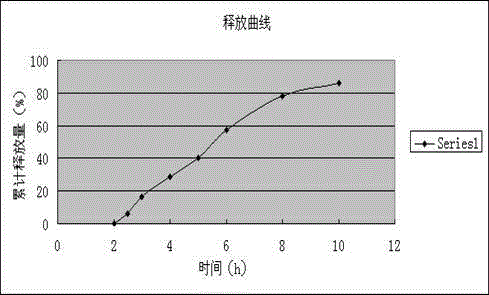
ActiveCN105250233AAvoid irritationGood slow releaseOrganic active ingredientsAntipyreticSustained Release TabletMicrobiology
The invention relates to a dexibuprofen enteric-coated and sustained-release tablet. The dexibuprofen enteric-coated and sustained-release tablet comprises a tablet core with a sustained release effect and a coating for coating the table core, wherein the tablet core is prepared from the following components: 70-90 parts of dexibuprofen, 10-30 parts of sustained-release skeleton stabilizer, 0-10 parts of filler, 0.2-1.5 parts of lubricating agent and 0.2-1.5 parts of flow aid; the effective quantity of active ingredient dexibuprofen takes more than 70% of the weight of table core. The invention has the advantages of excellent formula, reliable preparation process and easiness in industrial production; the prepared enteric-coated and sustained-release tablet in-vitro dissolution experiment shows: the releasing rate of gastric juice in 2 hours is smaller than 10%, which meets the requirements; the releasing rate of intestinal juice in 2 hours is 25-55%, the accumulative releasing rate in 4 hours is 50-80%, the accumulative releasing rate in 8 hours is more than 80%, and therefore, the releasing effect is excellent. The dexibuprofen enteric-coated and sustained-release tablet has effects of undissolving in the stomach and slowly releasing in the intestines so as to prevent irritation to the stomach and prolong the in-vivo releasing time of medicines simultaneously; the dexibuprofen enteric-coated and sustained-release tablet facilitates absorption to human bodies and has lasting effects.
Owner:湖北省医药工业研究院有限公司
Dexibuprofen lipid emulsion injection and preparation method thereof
PendingCN110339165AThe quality of clinical compatibility is stableComply with USP41 requirementsOrganic active ingredientsAntipyreticFiltrationHydroxystearic Acid
The invention relates to a preparation method of a dexibuprofen lipid emulsion injection. The preparation method comprises the following steps that long-chain fatty acid, medium-chain fatty acid and KolliphorHS 15 are heated and stirred to form a homogeneous oil-phase solution; an emulsifier, an osmotic pressure regulating agent and water are evenly mixed to form a water-phase solution; the oil phase and the water phase are mixed, stirred at a certain temperature, sheared and emulsified, and after high-pressure homogenization, pH regulating, filtration, sterilization and the like, the dexibuprofen lipid emulsion injection is obtained. The novel solubilizer KolliphorHS 15 for injection is adopted, the problems about the compatible stability and the drug loading capacity of a dexibuprofen injection during clinical use are effectively solved, and the safety of a preparation is expected to be improved.
Owner:SUZHOU UNIV
Dexibuprofen particles and preparation method thereof
ActiveCN102389401AEffective absorptionImprove bioavailabilityOrganic active ingredientsAntipyreticEmulsionHigh absorption
The invention discloses dexibuprofen particles and a preparation method thereof. According to the invention, appropriate auxiliary materials are combined with dexibuprofen, such that dexibuprofen is subject to self-emulsification in gastrointestinal tracts, and is maintained an emulsion. Compared with common dexibuprofen preparations, the dexibuprofen particles provided by the invention has advantages of good dissolution rate, high absorption speed, good controllability of preparation technological quality, good stability, and the like. The invention provides a dexibuprofen preparation with higher security, better curative effect, simpler preparation technology and good stability.
Owner:NANJING ZENKOM PHARMA
New medical salt of dexibuprofen
The invention discloses new medical salt of dexibuprofen. According to the salt, x can be 0, 0.5, 1, 1.5, 2, 2.5 and 3, n can be 1, 2, and 3, and M can be sodium, potassium, magnesium, zinc, arginine and lysine. The invention provides a preparation method of the salt, and also a medicine composite containing the salt. The composite contains a carrier which can be accepted by medicines; and the medicine composite can be produced in an appropriate and medical dosage form during the producing of medicaments.
Owner:FUKANGREN BIO PHARMA
Dexibuprofen liposome as well as preparation method and application thereof
InactiveCN108186570APromote transdermalThrough highOrganic active ingredientsAntipyreticHigh concentrationCholesterol
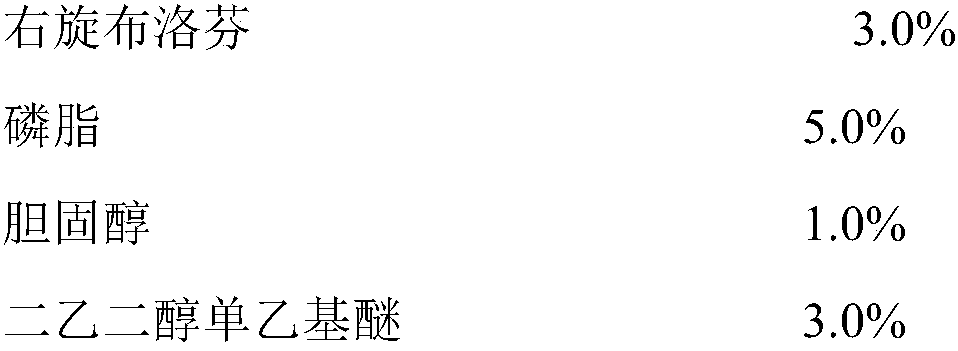
The invention relates to a dexibuprofen liposome as well as a reparation method and application thereof. The dexibuprofen liposome is prepared from the following components in percentage by mass: 1.0to 10.0 percent of dexibuprofen, 3.0 to 8.0 percent of phospholipid, 0.5 to 5.0 percent of cholesterol, 1.0 to 10.0 percent of diethylene glycol monoethyl ether, 0.5 to 5.0 percent of vitamin E polyethylene glycol succinate, 10.0 to 30.0 percent of polylol, 0.1 to 0.2 percent of citric acid and the balance of purified water. An oil phase prepared from the dexibuprofen, the phospholipid, the cholesterol and the diethylene glycol monoethyl ether is dropwise added into a water phase prepared from the vitamin E polyethylene glycol succinate, the polylol, the citric acid and the purified water, andthen an obtained mixture is treated through a high-speed shearing emulsification, high-pressure homogenization or high-pressure micro jet device, and the dexibuprofen liposome prepared by the preparation method is high in drug loading capacity, can be used for promoting the dexibuprofen to penetrate through a skin and retain for a long time in a high-concentration manner, and meanwhile, has favorable stability and safety.
Owner:张恩景
Preparation method of dexibuprofen sustained-release agent
ActiveCN106377506ANo pollutionAdvancedOrganic active ingredientsPowder deliveryFreeze-dryingUltrasonic dispersion
The invention relates to a preparation method of a dexibuprofen sustained-release agent, and aims to overcome the medical treatment and preparation defects of dexibuprofen medicine. The preparation method comprises the following steps: taking montmorillonite, hydrochloric acid, absolute ethanol and deionized water as raw materials; modifying the montmorillonite; preparing an aqueous hydrochloric acid solution and montmorillonite mixed liquor; performing ultrasonic dispersion, centrifugal separation, vacuum freeze drying, grinding and sieving to prepare dexibuprofen sustained-release agent powder particles, wherein the particle diameters of the particles are smaller than or equal to 200nm, and the product purity is up to 99.6 percent. The preparation method has the advantages of advanced process, accurate and complete data, short process flow and freeness from environmental pollution, and is an advanced method for preparing the dexibuprofen sustained-release agent; the dexibuprofen sustained-release agent can be applied to clinical medication in the department of pediatrics.
Owner:SHANXI MEDICAL UNIV
Pharmaceutical composition of dexibuprofen and medicinal use thereof
InactiveCN106008550AHas therapeutic effectOrganic active ingredientsOrganic chemistryNatural productGN - Glomerulonephritis
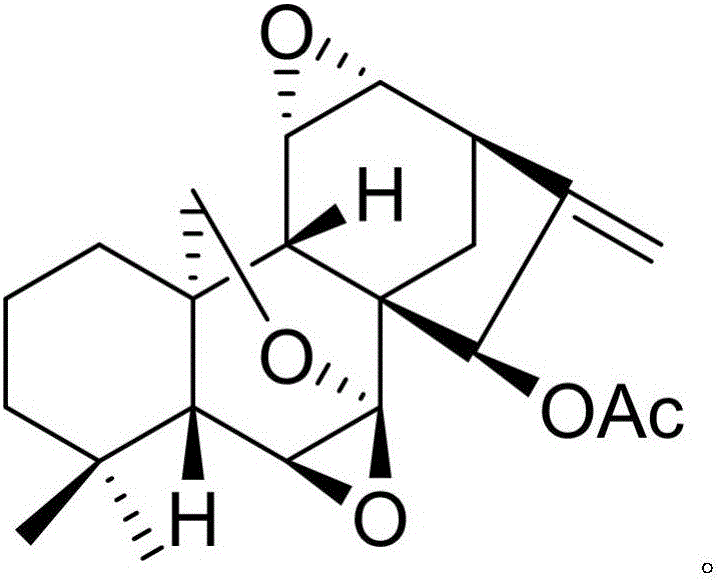
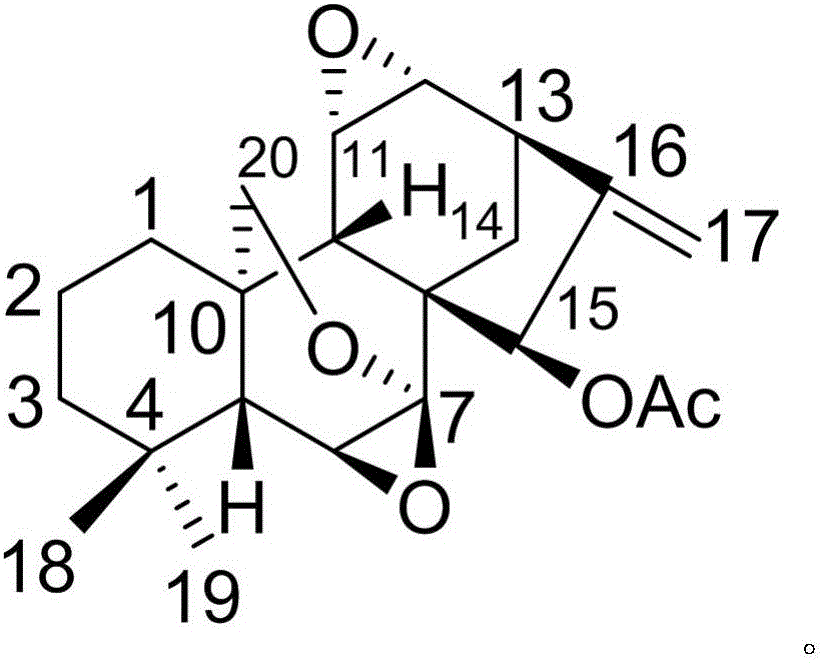
The invention discloses a pharmaceutical composition of dexibuprofen and its medical use. The pharmaceutical composition of dexibuprofen provided by the invention contains dexibuprofen and a natural product compound (I) with a novel structure. When dexibuprofen and compound (I) act alone, they have a therapeutic effect on glomerulonephritis; when dexibuprofen and compound (I) act in combination, the therapeutic effect is further improved, and can be developed into a drug for treating glomerulonephritis , compared with the prior art, has outstanding substantive features and remarkable progress.
Owner:王昌荣
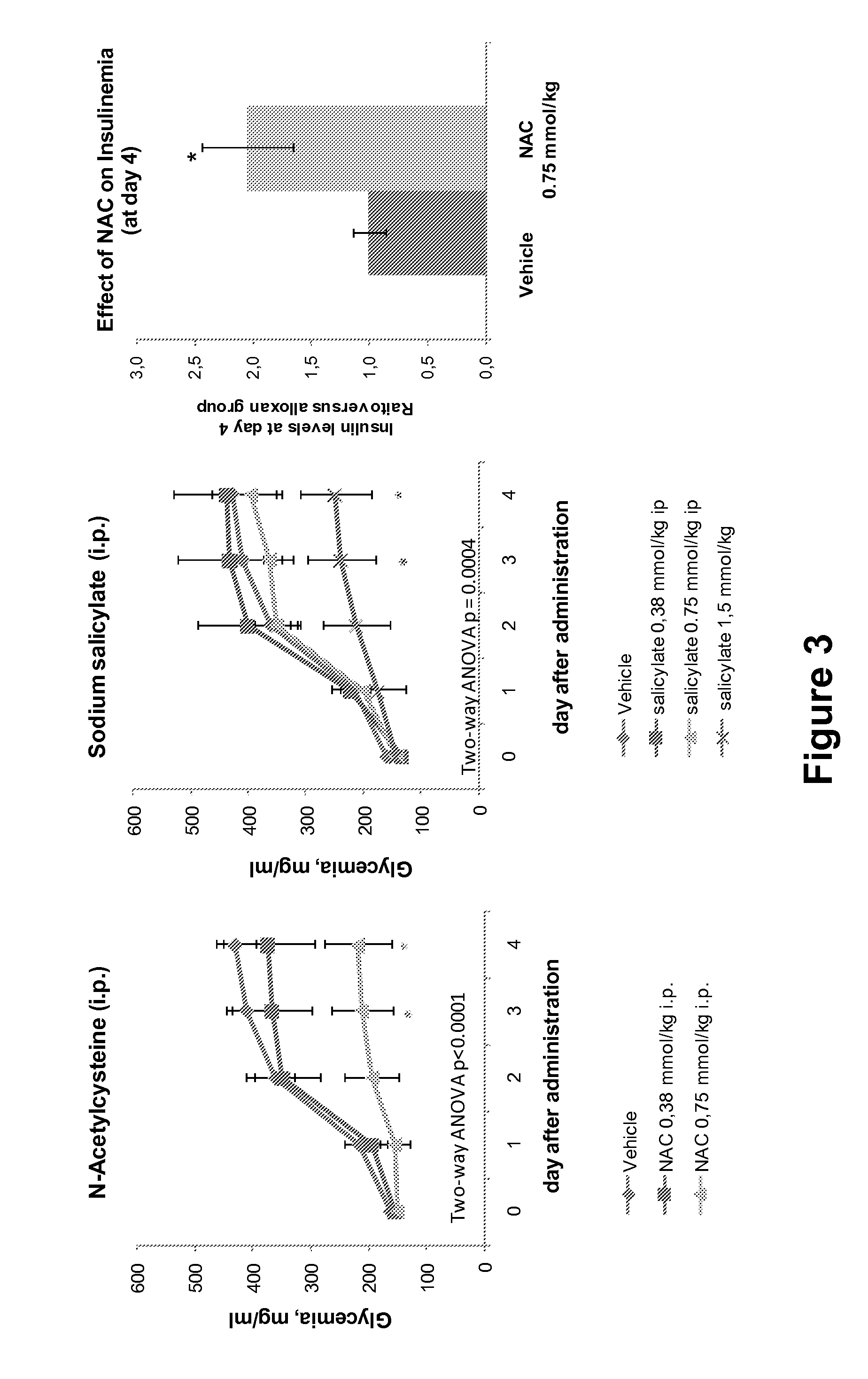
Combination Therapies For Treating Metabolic Disorders
InactiveUS20140357602A1Improve the level ofAvoid complicationsBiocideSalicyclic acid active ingredientsCombined Modality TherapyInsulin resistance
Owner:GENMEDICA THERAPEUTICS SL
Slow-release dry dexibuprofen suspension and preparation method thereof
InactiveCN109528647ASpeed up disintegrationImprove solubilityOrganic active ingredientsAntipyreticFiller ExcipientPreservative
The invention relates to a slow-release dry dexibuprofen suspension. The slow-release dry dexibuprofen suspension comprises slow-release dexibuprofen pellets, dilute particles and auxiliary materials,wherein each slow-release dexibuprofen pellet comprises a blank pellet core, a medicine-carrying layer and a slow-release layer; each dilute particle comprises a surfactant, a first filler, a flavoring agent and a preservative; the auxiliary materials include a second filler, a suspending aid, a first anti-adhesive agent, a flow aid and an essence; based on the total weight of the slow-release dry dexibuprofen suspension, the slow-release dry dexibuprofen suspension comprises the following components in percent by weight: 10-15% of the slow-release dexibuprofen pellets, 0.2-0.5% of the surfactant, 65-85% of the first filler and the second filler together, 2-7% of the flavoring agent, 0.5-3% of the suspending aid, 0.5-3% of the preservative, 0.5-3% of the first anti-adhesive agent, 0.5-3%of the flow aid and 0.1-2% of the essence. The slow-release dry dexibuprofen suspension provided by the invention has a relatively good slow-release effect and relatively good preparation stability, so that the number of medications is reduced and the medication compliance of a patient is improved.
Owner:SUZHOU UNIV
A grinding method of Dexibuprofen and the preparation method of its suspension
The invention relates to a grinding method of dexibuprofen, a preparation method of dexibuprofen suspension, and the dexibuprofen suspension obtained through the preparation method. The grinding method comprises the following steps: S1, adding dexibuprofen into a mixed solvent, and carrying out sufficient stirring, thus obtaining the dexibuprofen mixture; S2, preparing an acid sol; and S3, mixing the dexibuprofen mixture obtained in S1 with the acid sol obtained in S2, and carrying out grinding, thus the dexibuprofen grinded matter is obtained, and the grinding treatment is completed. According to the grinding method, through the comprehensive selection and synergy of a plurality of specific technical features, the dexibuprofen suspension with excellent performance is finally obtained. When the technical features are changed, the performance of the finally obtained suspension can be reduced, which means that the most stable dexibuprofen suspension can be obtained by adopting the grinding method, and therefore, grinding method and the preparation method have good application prospect and industrial production potential in the field of medicines.
Owner:湖北唯森制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com