Dexibuprofen injection composition
A composition and injection technology, applied in the field of medicine, can solve the problems of low bioavailability, low solubility, inconvenience to patients, etc., and achieve the effects of good stability, easy production control, and stable and safe preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
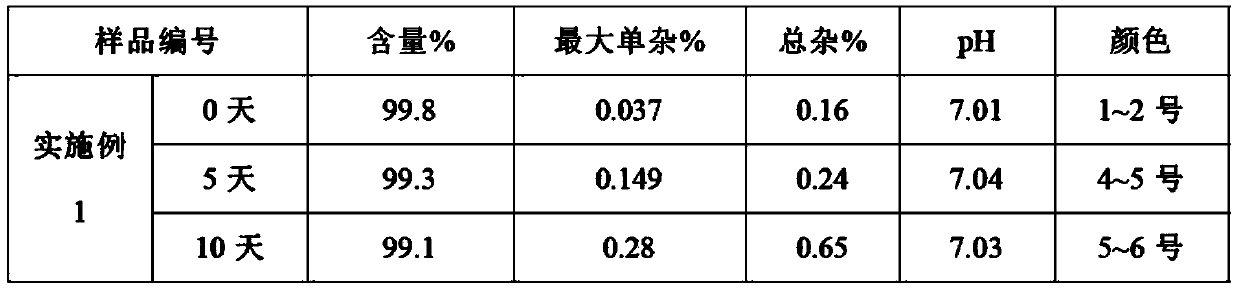
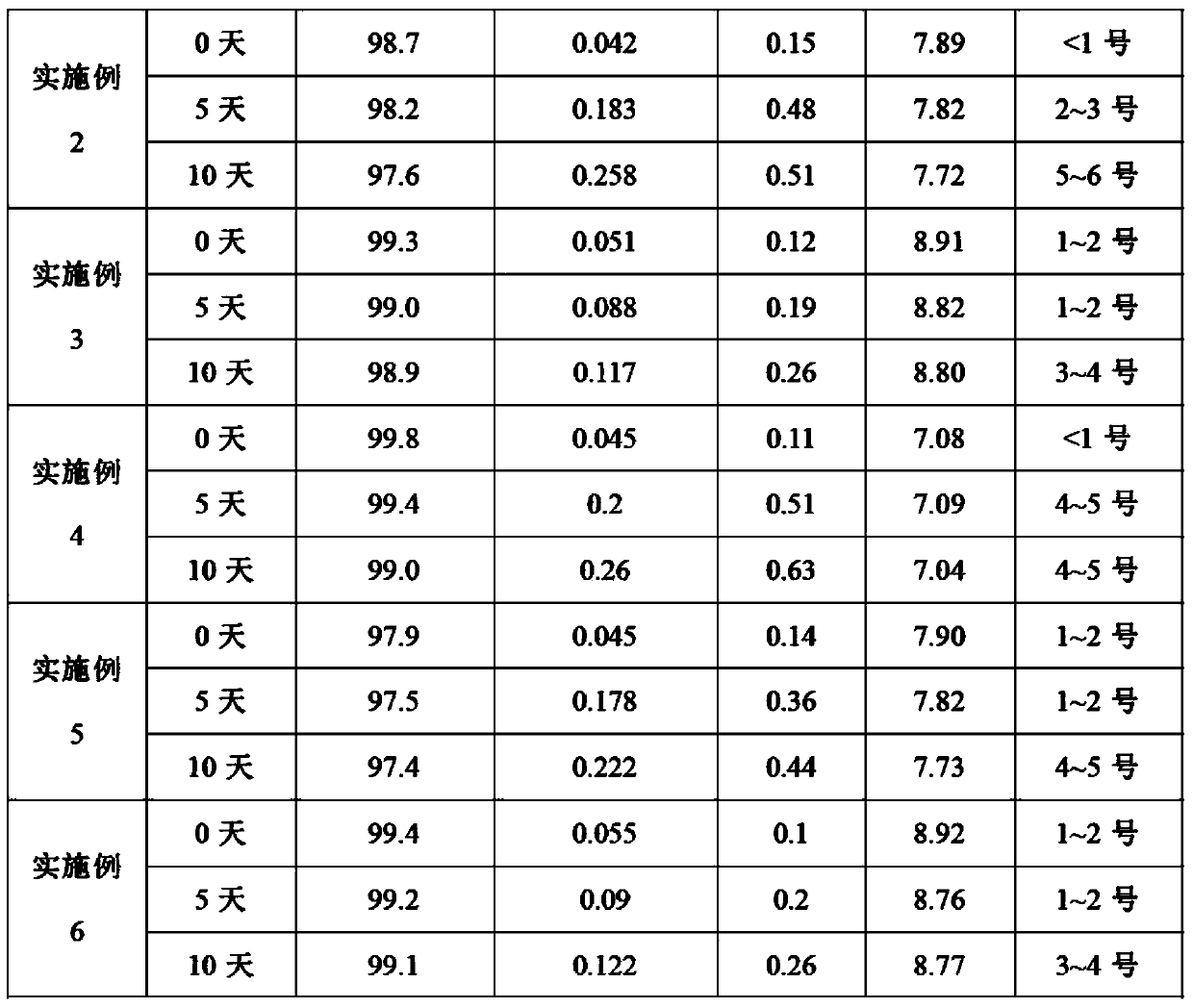
[0027] According to the formulation and preparation conditions in Table 1, different proportions of the dextroibuprofen compositions of Examples 1-6 (the content of dextrobuprofen in the formula is 100 mg / ml) were prepared by the following preparation methods.
[0028] Formulation and preparation conditions of table 1 embodiment 1-6 Dexibuprofen composition
[0029] test
Raw materials
The molar ratio of
concentration
Sterilization conditions
Example 1
Dexibuprofen: Arginine
1:0.82
100mg / ml
121℃ damp heat
Example 2
Dexibuprofen: Arginine
1:1.03
100mg / ml
121℃ damp heat
Example 3
Dexibuprofen: Arginine
1:1.30
100mg / ml
121℃ damp heat
Example 4
Dexibuprofen: Arginine
1:0.82
100mg / ml
121℃ damp heat
Example 5
Dexibuprofen: Arginine
1:1.03
l00mg / ml
121℃ damp heat
Example 6
Dexibuprofen: Arginine
1:1.30
100mg / ml
121℃ damp hea...
Embodiment 7-8
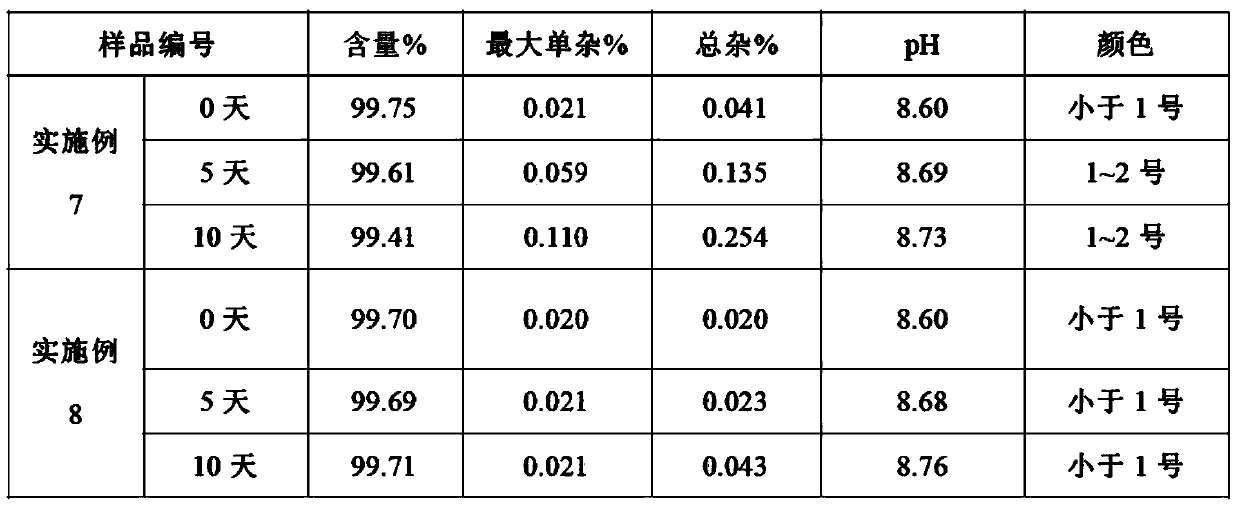
[0045] Formulation and preparation conditions of table 3 embodiment 7-8 Dexibuprofen composition
[0046] test
Raw materials
The molar ratio of
concentration
Sterilization conditions
Whether to fill nitrogen
Example 7
Dexibuprofen: Arginine
1:1.30
100mg / ml
121℃ damp heat
N
[0047] Example 8
Dexibuprofen: Arginine
1:1.30
100mg / ml
121℃ damp heat
Y
[0048] 1. Weigh the prescribed amount of arginine and ibuprofen;
[0049] 2. Dissolve arginine with an appropriate amount of water for injection (about 80ml) until it is clear and transparent, add the prescribed amount of Dexibuprofen, and stir until it is completely dissolved;
[0050] 3. Add an appropriate amount of activated carbon, stir for 15 minutes, and decarbonize by filtering with a 0.45 μm filter membrane.
[0051] 4. Dilute to full volume, filter with 0.22μm filter membrane, fill, 4ml / bottle, and seal.
[0052] 5. Steri...
Embodiment 8-10
[0059] Formulation and preparation conditions of table 5 embodiment 8-10 Dexibuprofen composition
[0060] test
Raw materials
The molar ratio of
Sterilization conditions
Whether to fill nitrogen
Example 8
Dexibuprofen: Arginine
1:1.30
121℃ damp heat for 15min
Y
Example 9
Dexibuprofen: Arginine
1:1.30
121°C damp heat for 8 minutes
Y
[0061] Example 10
Dexibuprofen: Arginine
1:1.30
115°C damp heat for 30min
Y
[0062] 1. Weigh the prescribed amount of arginine and ibuprofen;
[0063] 2. Dissolve arginine with an appropriate amount of water for injection (about 80ml) until it is clear and transparent, add the prescribed amount of Dexibuprofen, and stir until it is completely dissolved;
[0064] 3. Add 0.5% activated carbon, stir for 15 minutes, and remove carbon by filtering with a 0.45 μm filter membrane.
[0065] 4. Dilute to full volume, filter with 0.22μm filter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com