Zeaxanthine dipalmitate liposome eye drops and preparation method thereof
A technology of palmitate and zeaxanthin, which is applied in the direction of liposome delivery, pharmaceutical formulations, active ingredients of esters, etc., can solve the problems of no safety testing and efficacy verification, differences, and unclear use effects, etc., to improve biological Utilization, various action effects, macular degeneration prevention effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
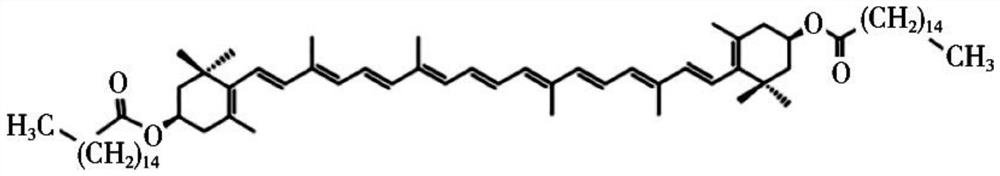
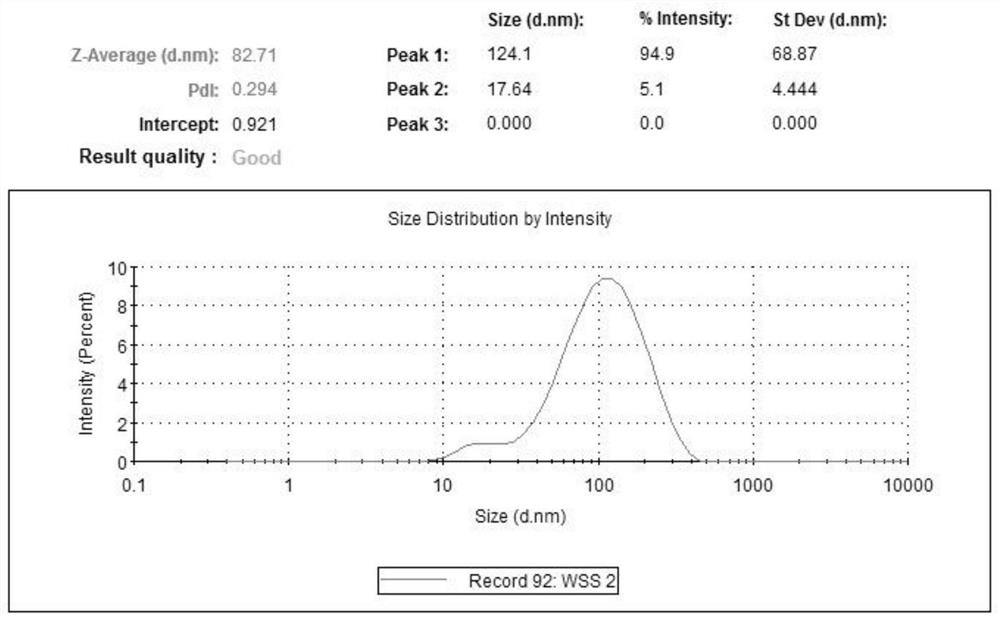
[0048] Zeaxanthin dipalmitate was dissolved with a small amount of absolute ethanol (see Annex formula figure 1 ) 20mg, Compound lecithin and Tween 800.5mg 400mg, rotary evaporated under reduced pressure to the film formation; the amount of 10mL water for injection preheated at 50 ℃; post under the same conditions, hydration water for injection, the solution was completely dissolved to an ultrasonic homogenous, clear, filter sterilized 0.22 m, to obtain 2mg / mL zeaxanthin dipalmitate liposomes (with figure 2 ), Particle size distribution mitsuke image 3 . Then diluted with MEM medium containing serum and double antibody zeaxanthin dipalmitate into liposomes 1.6mg / mL, 1.2mg / mL, 0.8mg / mL, 0.4mg / mL.
[0049] A compound described above in the embodiment lecithin embodiment comprises egg yolk lecithin, anhydrous butter, cholesterol, which is a mass ratio of 3: 2: 1.
Embodiment 2
[0051] Percentage by mass weighed raw glycerol and 0.05% Phenoxyethanol 0.20% mixture is placed on a magnetic stirrer set speed of 800rpm and 35 ℃, dissolved stirred until homogeneous; mass percentage of the water for injection feed weighed 93.95%, transparent sodium hyaluronate 0.10%, 0.10% panthenol, vitamin B120.05%, vitamin B60.05% sodium chloride and 0.5%, the raw material added in small amounts several times, dissolved stirred until homogeneous; mass percentage of the raw materials weighed zeaxanthin dipalmitate esters 5.00% was added to the liposome raw material, stirred until homogeneous, to give a clear pale yellow transparent liquid, liquid with boric acid to adjust the pH to 6.5 to 5.1, filter sterilized, bottling, that is, the zeaxanthin dipalmitate ester liposome eye drops.
Embodiment 3
[0053] Percentage by mass weighed raw glycerol and 0.05% Phenoxyethanol 0.20% mixture is placed on a magnetic stirrer set speed of 800rpm and 35 ℃, dissolved stirred until homogeneous; mass percentage of the water for injection feed weighed 88.95%, transparent sodium hyaluronate 0.10%, 0.10% panthenol, vitamin B120.05%, vitamin B60.05% sodium chloride and 0.5%, the raw material added in small amounts several times, dissolved stirred until homogeneous; mass percentage of the raw materials weighed zeaxanthin dipalmitate 10.00% acetate was added to the liposome raw material, stirred until homogeneous, to give a clear pale yellow transparent liquid, liquid with boric acid to adjust the pH to 6.5 to 5.1, filter sterilized, bottling, that is, the zeaxanthin dipalmitate ester liposome eye drops.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com