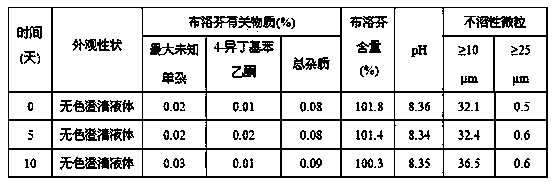
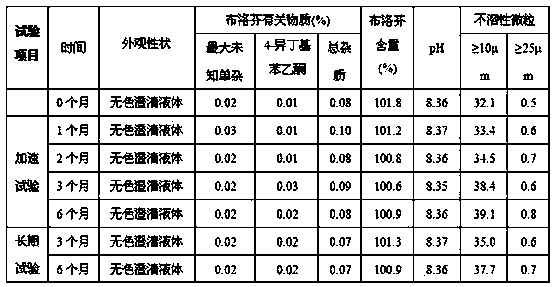
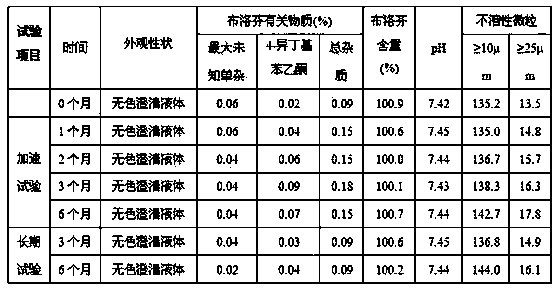
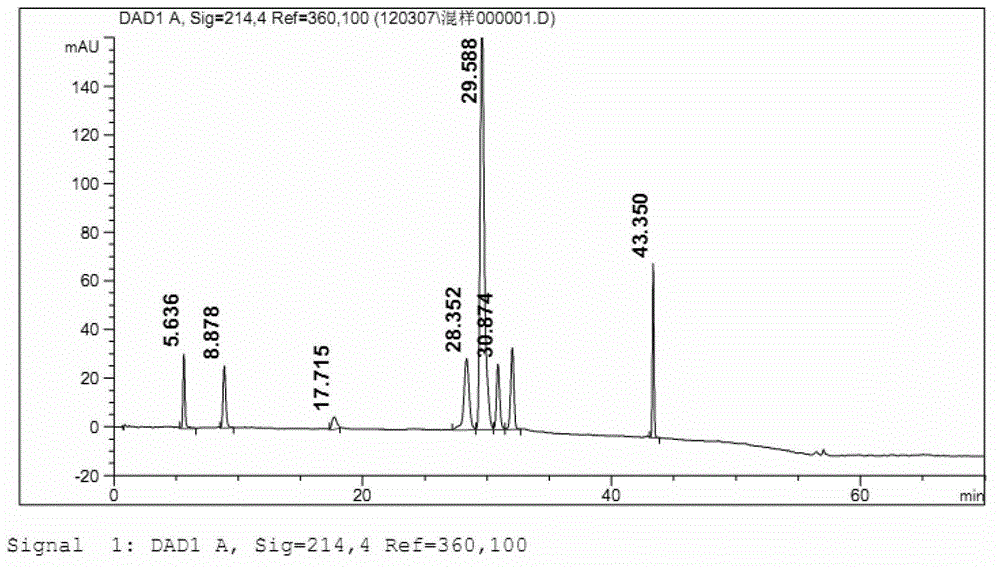
Patents
Literature
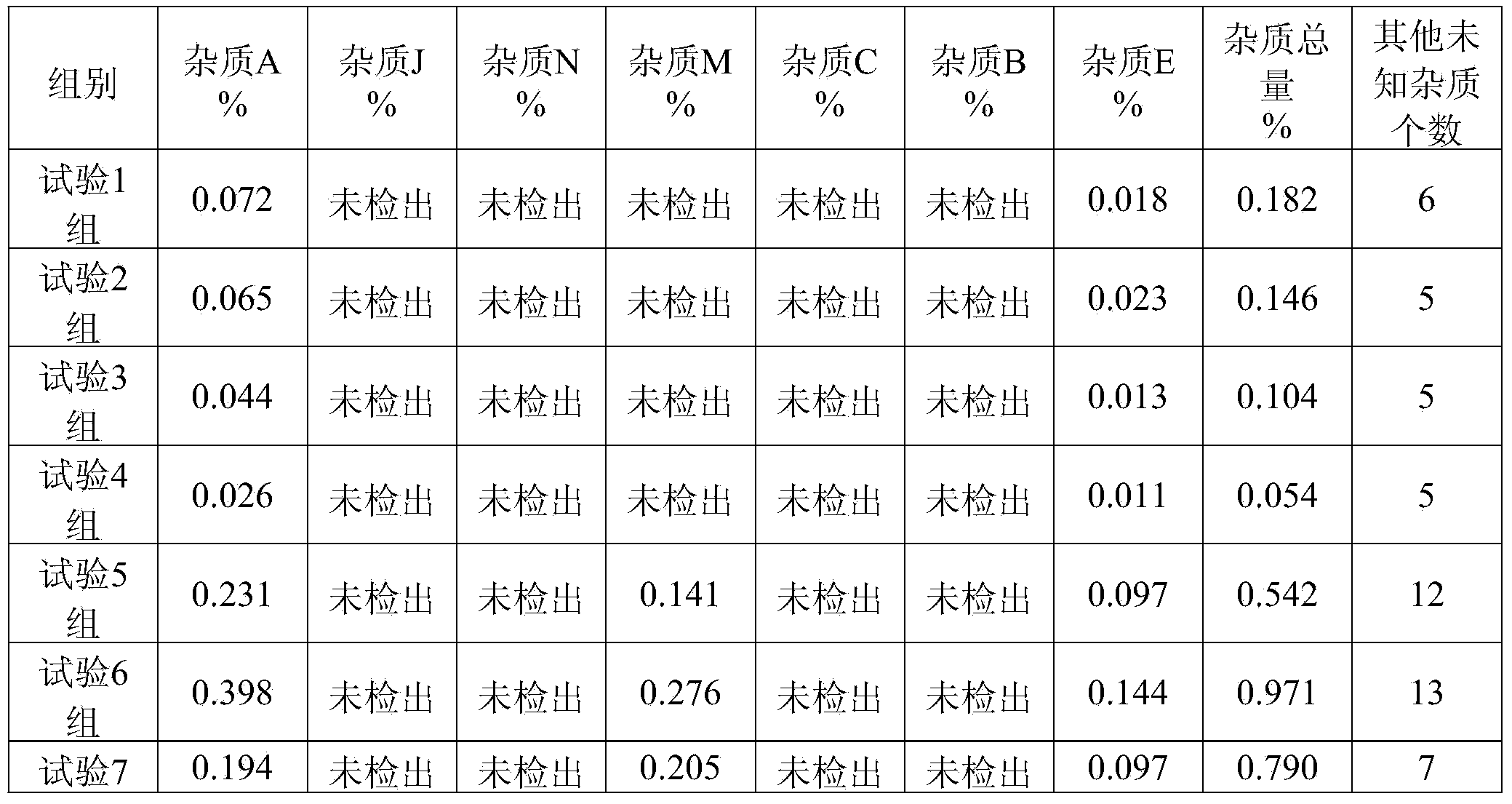
48 results about "Ibuprofen Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
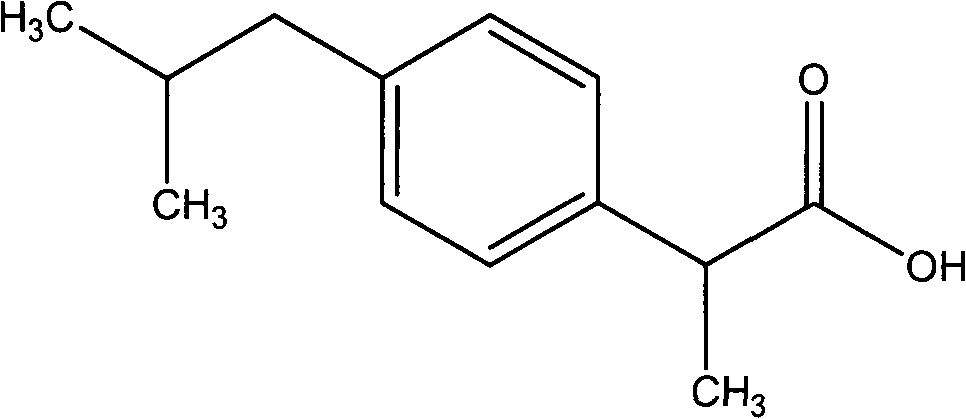
A formulation for intravenous injection containing ibuprofen, a propionic acid derivate and nonsteroidal anti-inflammatory drug (NSAID), with anti-inflammatory, analgesic, and antipyretic activities. Upon intravenous injection, ibuprofen inhibits the activity of the enzymes cyclooxygenase I (COX-1) and II (COX-2), resulting in a decreased formation of precursors of prostaglandins and thromboxanes. Inhibition of COX-2 specifically blocks the conversion of arachidonic acid (AA) to prostaglandins, which mediate inflammation, fever and pain.
Injection containing burufen
InactiveCN101069681AReduce solubilitySlow absorptionOrganic active ingredientsAntipyreticSolubilityIbuprofen Injection
The present invention provides an injection preparation containing ibuprofen and its preparation method. Said invention provides the concrete steps of its preparation method. Said ibuprofen injection preparation has high solubility and bioavailability, and its product quality is stable.
Owner:汪洪湖
Ibuprofen injection and preparation method thereof
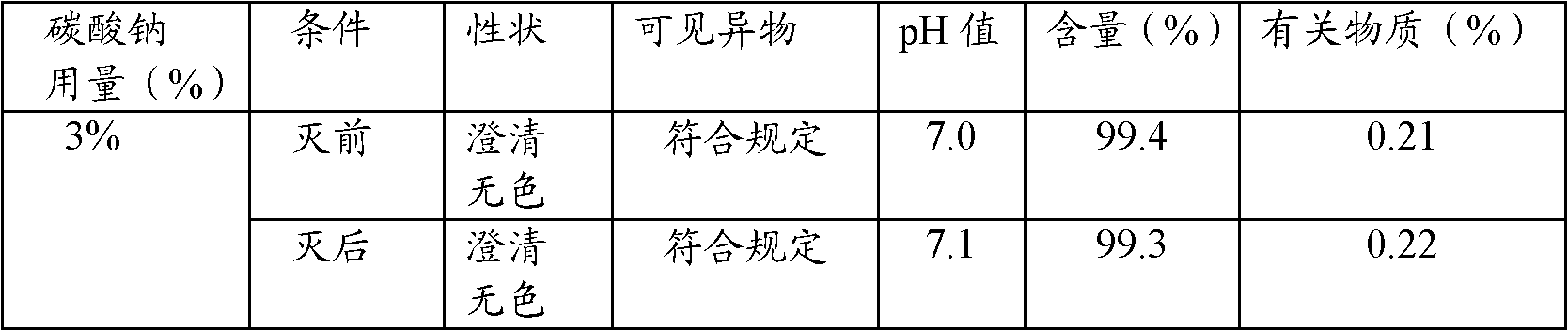
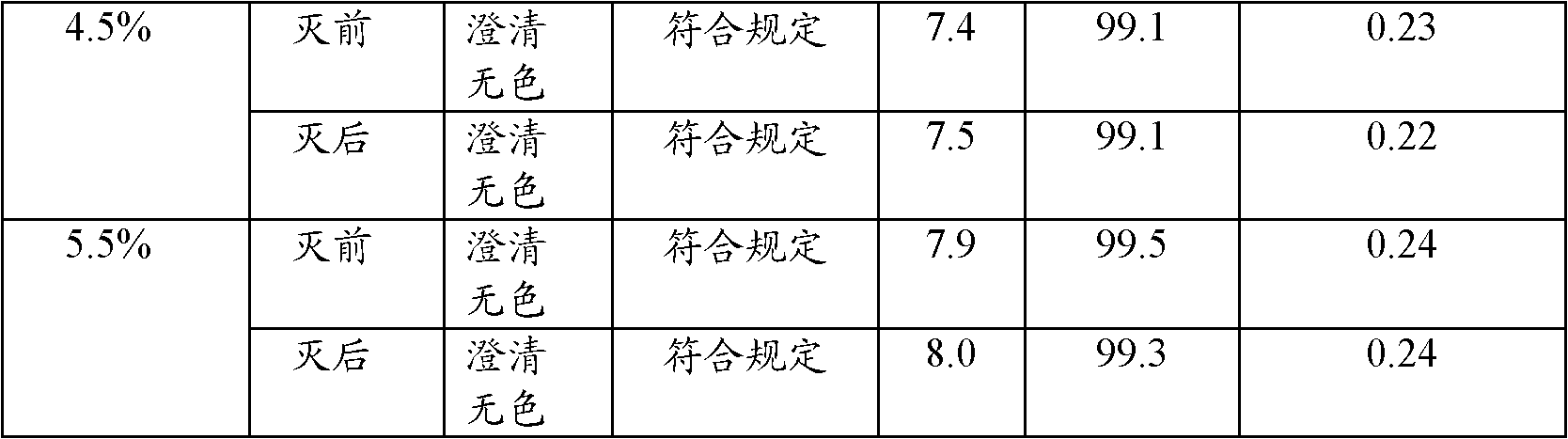
InactiveCN102085179ALow priceImprove securityOrganic active ingredientsAntipyreticSodium bicarbonateSodium acetate
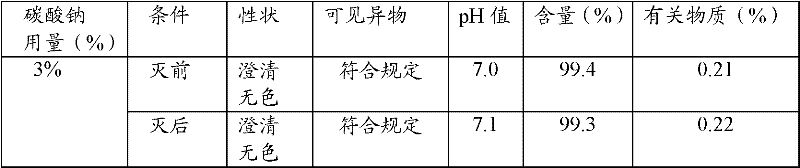
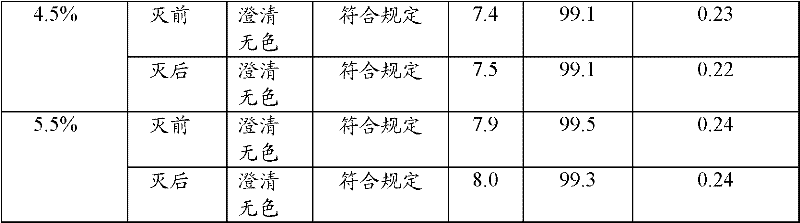
The invention relates to an ibuprofen injection, which comprises ibuprofen and one or a plurality of alkaline cosolvents pharmaceutically acceptable selected from anhydrides or hydrates of sodium carbonate, sodium bicarbonate,sodium citrate, sodium citrate, sodium phosphate, disodium dihydrogen pyrophosphate, sodium tartrate and sodium acetate. The invention also relates to a preparation method of the ibuprofen injection.
Owner:罗军
Method for preparing ibuprofen injection
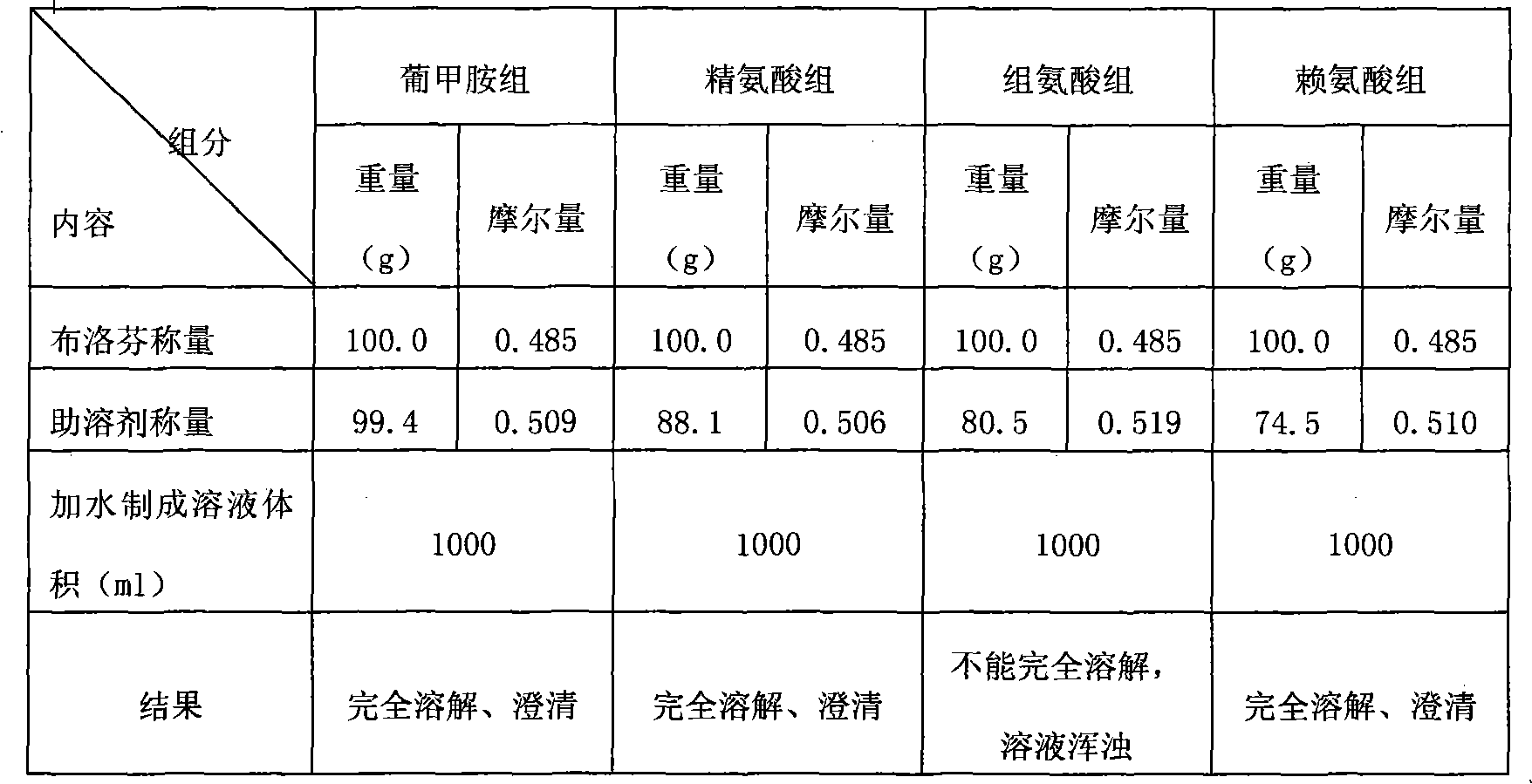
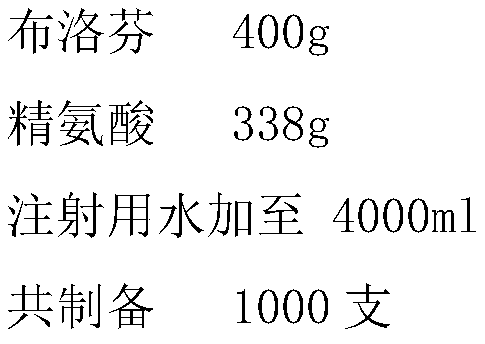
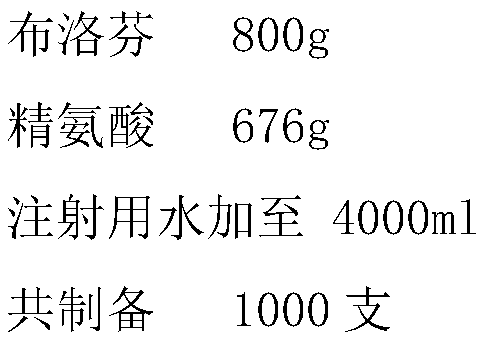
The invention discloses a method for preparing an ibuprofen injection, which is characterized in that the injection is a more stable injection prepared from ibuprofen and an alkaline cosolvent, wherein the cosolvent is one or more of arginine, lysine, histidine, anhydrous ethylenediamine, diethanol amine, and the like. The process has the advantages of simple operation and easy application and is easy for industrialized scale production.
Owner:南京威尔曼药物研究所有限公司
Ibuprofen injection composite and preparation method thereof
ActiveCN101966147AImprove stabilityMeet the needs of clinical medicineOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
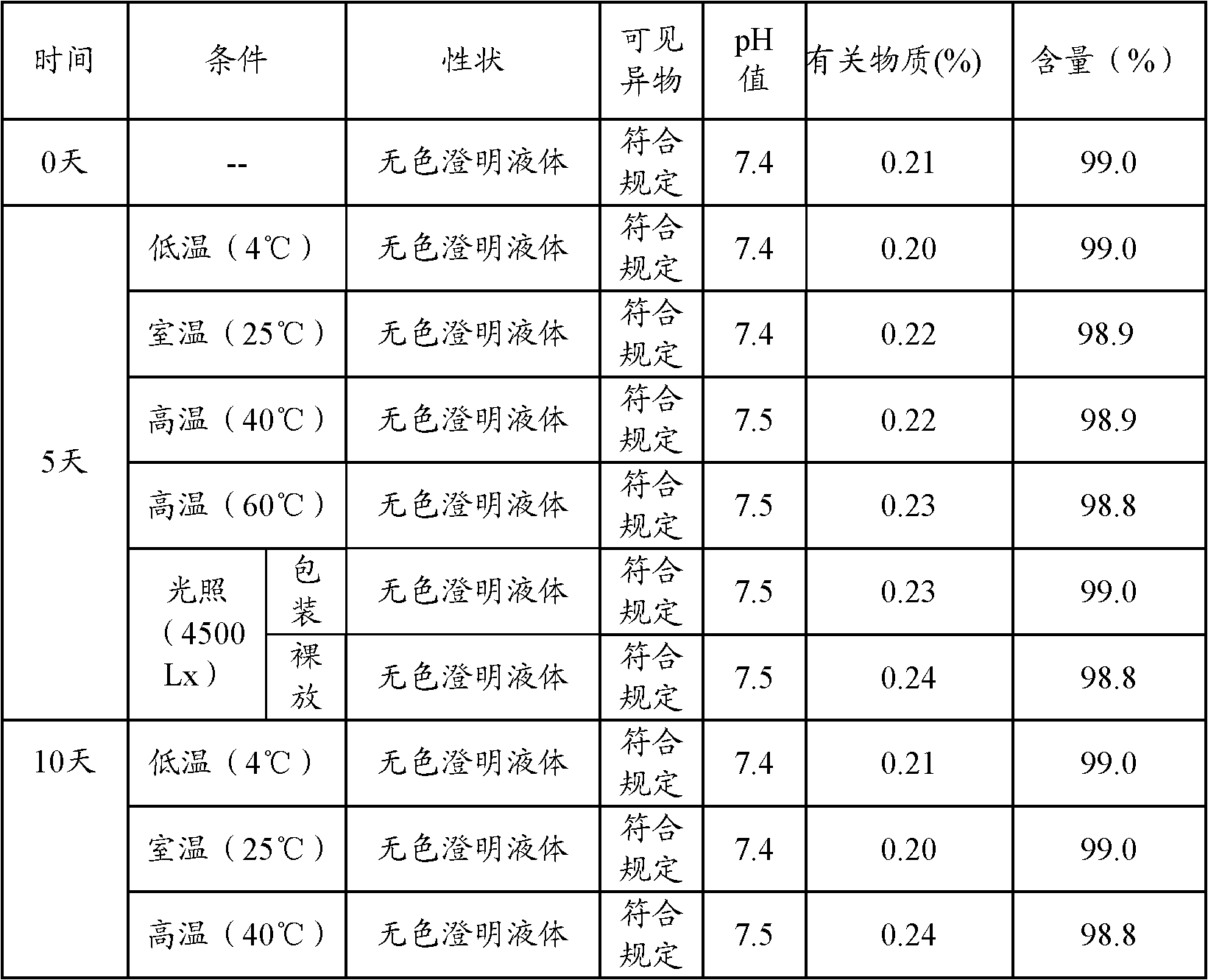
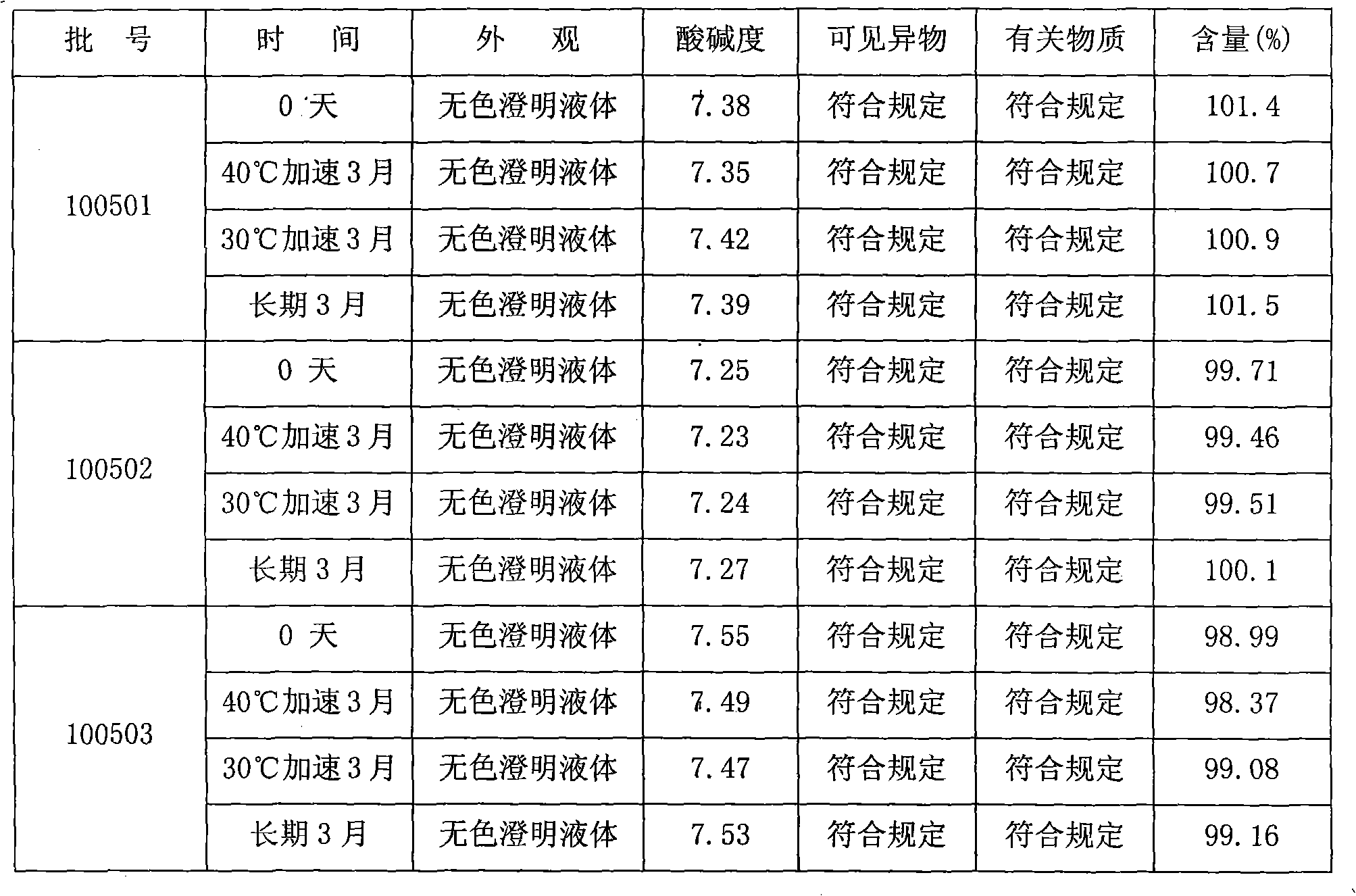
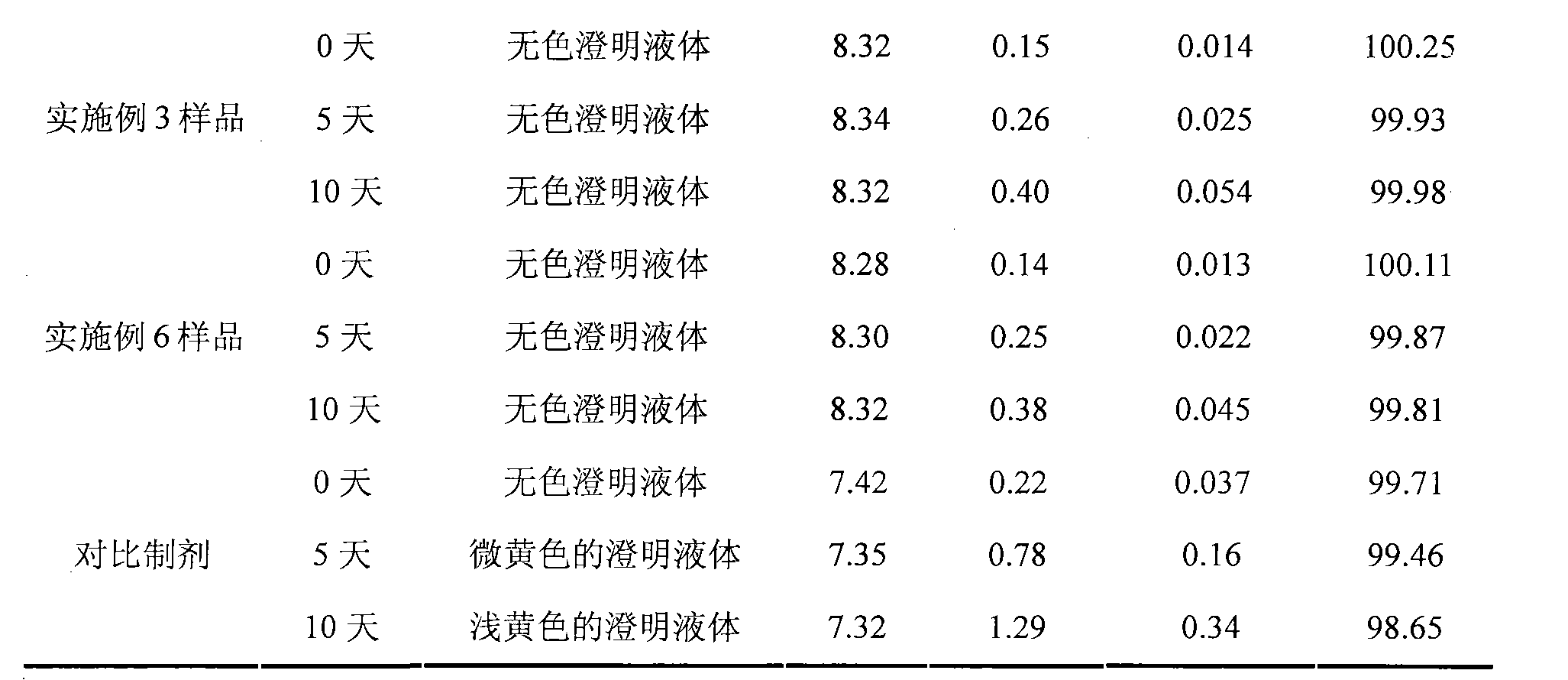
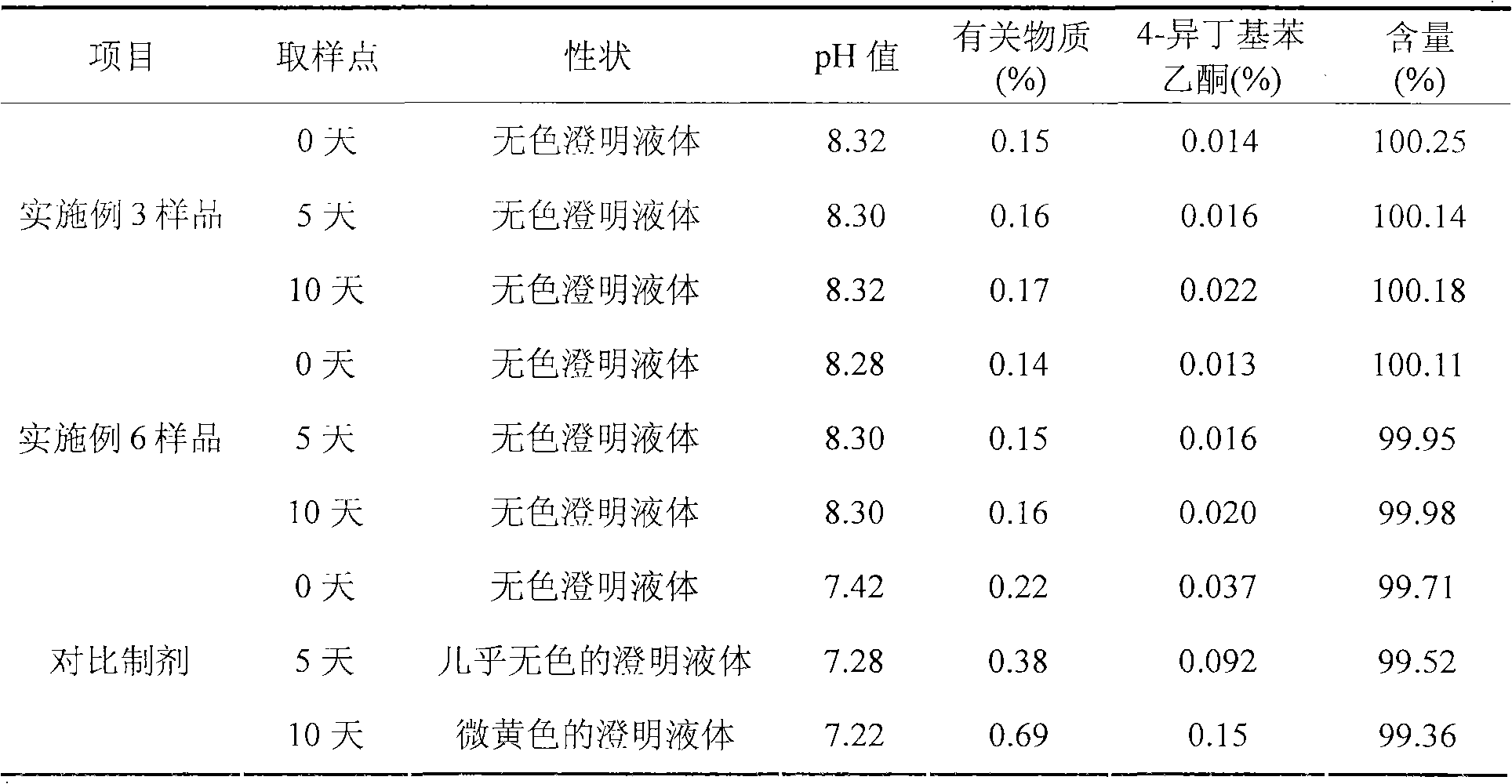
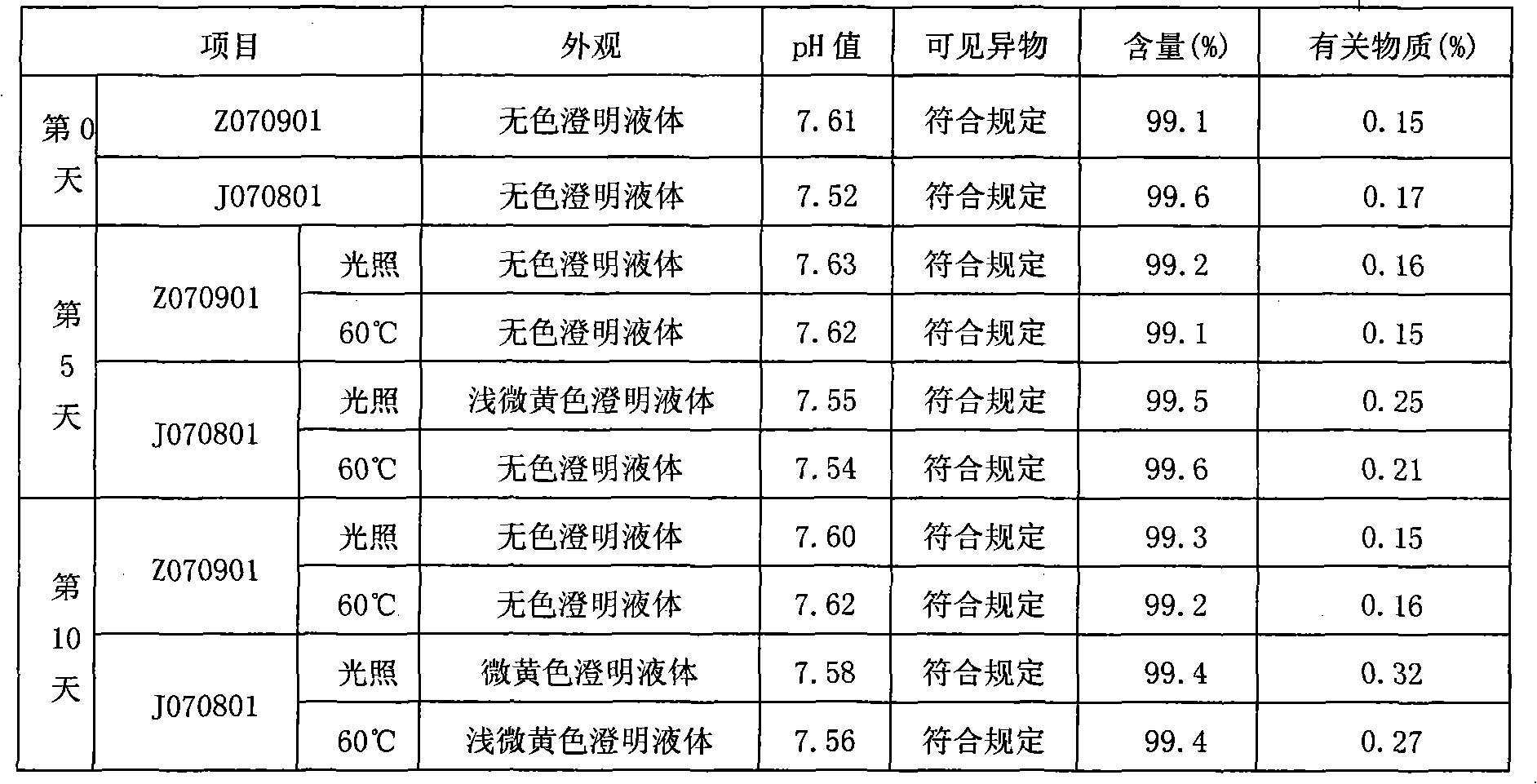
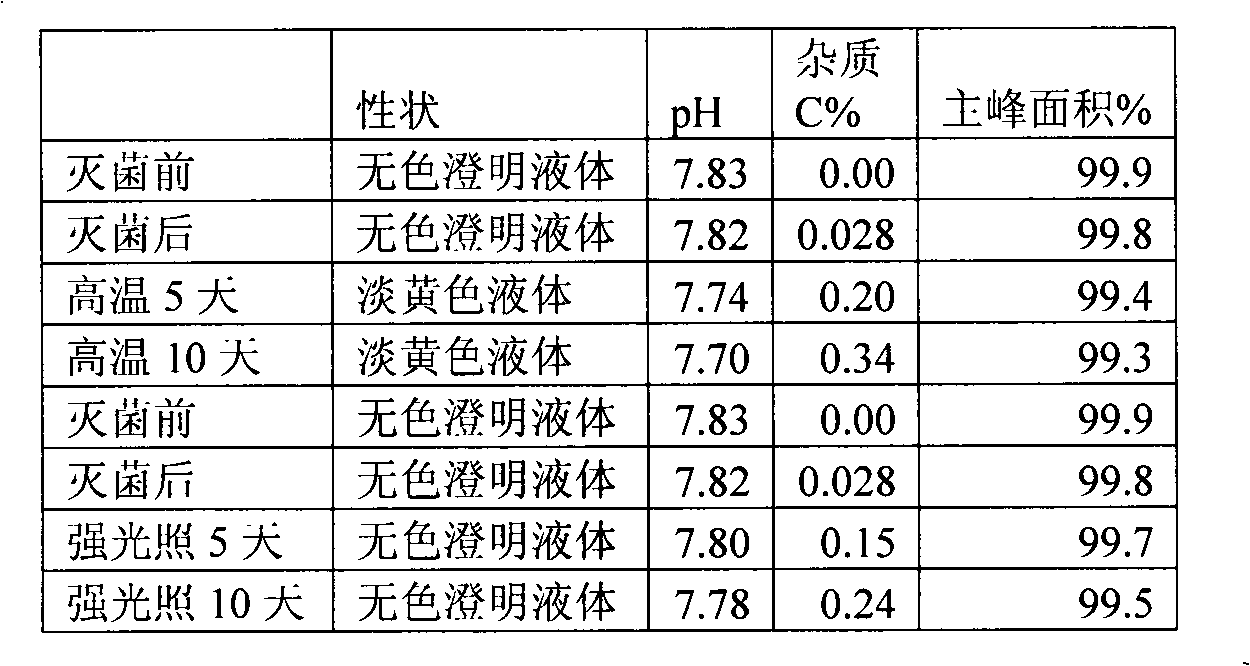
The invention discloses an ibuprofen injection composite which can obviously improve the quality stability of products and meet the clinical compatibility demand and a preparation method thereof to overcome the defect of the traditional ibuprofen injection. The injection is prepared by mixing ibuprofen and cosolvent according to a molar ratio of 1: 1.001-2, and the pH value of the injection is 7.5-9.0. The ibuprofen injection prepared through the method has obviously improved tolerance capability to high temperature and strong light and better stability and no irritant to vessels, and can effectively meet the requirement of the clinical intravenous drip. Moreover, the preparation method of the invention has the advantages of simple process and good stability of the prepared product.
Owner:四川阳光润禾药业有限公司
Ibuprofen injection and preparation method thereof
The invention relates to an ibuprofen injection and a preparation method thereof. The ibuprofen injection is characterized in that the concentration of ibuprofen in the injection is 100mg / ml, arginine is used as a cosolvent, and the molar ratio of the arginine to the ibuprofen is greater than or equal to 1:1. The invention also provides a method for preparing the injection. The prepared injection which has good dissolvability is added into a 0.9% sodium chloride injection or a 5% glucose injection, the concentration of the ibuprofen is 4mg / ml or less than 4mg / ml, and the solution is clear and is stabilized for a long time. The ibuprofen injection is suitable for intravenous drip for patients for whom the oral administration is not suitable.
Owner:付正香
Ibuprofen injection and preparation method thereof
InactiveCN101991540ALittle side effectsSize of side effectsPowder deliveryOrganic active ingredientsMedication injectionIbuprofen Injection
The invention relates to ibuprofen injection and a preparation method thereof. The ibuprofen injection comprises 0.5 to 20 weight percent of ibuprofen, 10 to 60 weight percent of cyclodextrin or cyclodextrin derivatives, 1 to 40 weight percent of water soluble excipient and the balance of water, wherein ibuprofen serves as a main medicament; and the cyclodextrin or cyclodextrin derivatives and the water soluble excipient serve as medicinal auxiliary materials. In the invention, the cyclodextrin or cyclodextrin derivatives represented by hydroxypropyl-beta-cyclodextrin is used to make the ibuprofen, which used to be water insoluble, very water soluble. The stability of the medicament is improved. The clathrate compounds can be made into injection using water as a solvent or power for injection. Thus, it is very convenient to administrate the medicament in clinic and it is easy to control injection volume. Meanwhile, the drawback of the universal adoption of auxiliary materials and solvents for injection, which are very dangerous, such as organic solvents and cosolvents, of the conventional insoluble and soluble medicinal injection is overcome.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
A low irritation ibuprofen injection
The invention discloses a low-irritation ibuprofen injection and a preparation method thereof. In the ibuprofen injection, the drug active ingredient is ibuprofen, and the auxiliary materials are amino acid and water for injection. The molar ratio of ibuprofen to amino acid ranges from 1:1.0 to 1:1.4. The ibuprofen injection of the present invention mainly adopts amino acid as a solubilizer and a pH regulator, which reduces the types of auxiliary materials used in the preparation process of the injection, thereby preparing a low-irritation ibuprofen injection beneficial to clinical application. The injection described in the invention is mainly used for treating pain, inflammation, fever and / or other diseases relieved by ibuprofen.
Owner:沈阳双鼎制药有限公司
Ibuprofen injection and preparation method thereof
The invention relates to ibuprofen injection and a preparation method thereof. The ibuprofen injection is characterized in that: amino acid in a certain ratio is adopted as a cosolvent to regulate the pH range of the ibuprofen injection, and the prepared injection has the advantages of high dissolubility, high stability and quick response and is suitable for patients who are not suitable for oral administration.
Owner:付正香
Ibuprofen injection preparation and preparation method thereof
ActiveCN102370615AFast playFully playOrganic active ingredientsPowder deliveryIbuprofen InjectionFreeze-drying
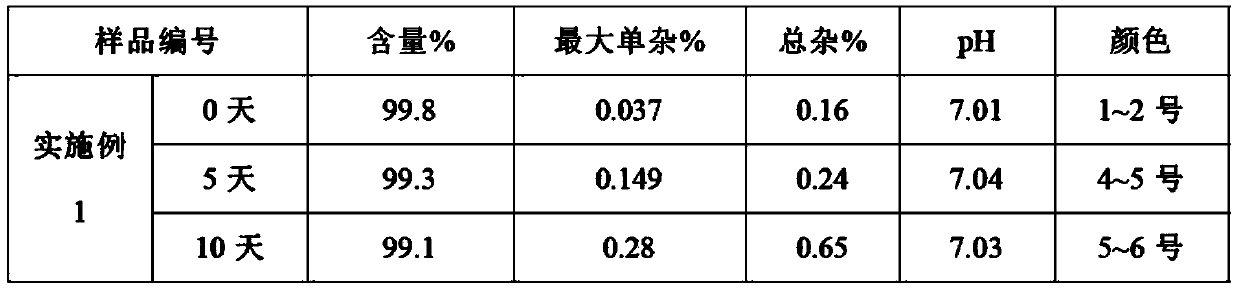
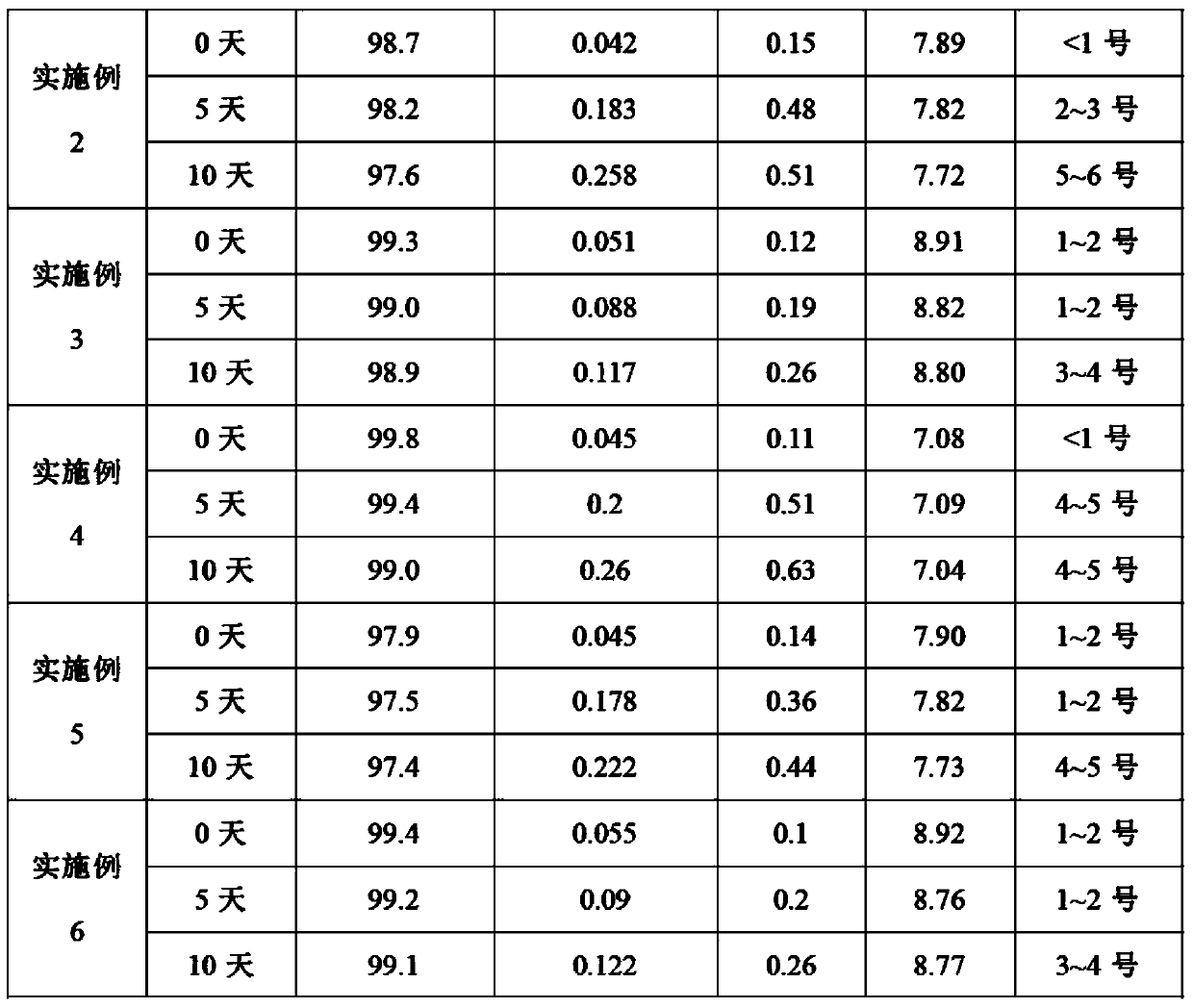
The invention relates to an ibuprofen injection preparation and a preparation method thereof, and especially relates to an ibuprofen injection preparation with meglumine as a cosolvent and a preparation method thereof, wherein the molar ratio of meglumine and ibuprofen is 0.1-2.0:1. The invention provides a new effective method which allows ibuprofen to be dissolved, the drug effect of ibuprofen to be rapidly and fully performed, and the solution formed by ibuprofen to be used for preparing a solution for injection or freeze-dried powder for injection. By carrying out pharmic stability tests,animal safety tests, pharmacokinetics and the like to realize the multitime investigation of the stability, the safety and the effectiveness of the ibuprofen injection preparation, results show that the ibuprofen injection preparation has a strong stability. So compared with the prior part, the preparation method of the invention allows the safety and the effectiveness of drugs to be guaranteed.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
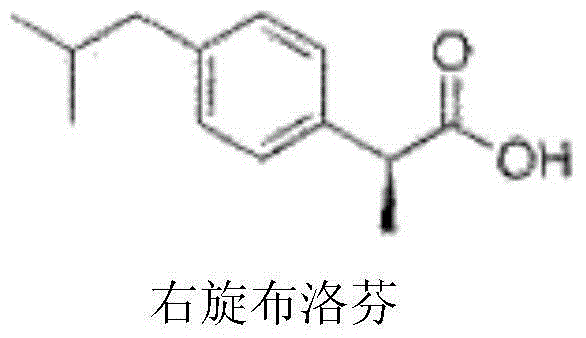
Dexibuprofen injection composition
InactiveCN103720647AThe preparation process is stable and feasibleGood and stable contentOrganic active ingredientsAntipyreticUse medicationIbuprofen Injection
The invention provides a dexibuprofen injection composition which comprises dexibuprofen, arginine and water for injection, wherein other regulating agents and organic solvents do not need to be added; the dexibuprofen can be fully dissolved to form a stable medicament composition by regulating the ratio of the arginine to the dexibuprofen. The dexibuprofen injection composition provided by the invention is higher in stability; the pH of the injection is in a blood tolerance range of a human body, so that a requirement on clinical medication can be met.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD
Ibuprofen injection
The invention provides an ibuprofen injection which includes ibuprofen, solubilizer and injection water.Solubilizer is selected from disodium hydrogen phosphate, dipotassium phosphate, sodium dihydrogen phosphate, monopotassium phosphate or any combination of disodium hydrogen phosphate, dipotassium phosphate, sodium dihydrogen phosphate and monopotassium phosphate and can also be selected from sodium phosphate, potassium phosphate or combination of sodium phosphate and potassium phosphate.Ibuprofen has concentration of 1-6 mg / ml and preferentially 2-4 mg / ml.The ibuprofen injection is high in stability in the transportation process, and the quality of a compatible product is not influenced in the clinical compatibility use process.
Owner:BEIJING LANDAN PHARMA TECH
Dexibuprofen injection drug composition and preparation method thereof
ActiveCN104546697AImprove complianceGood analgesic effectOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a dexibuprofen injection and a preparation method thereof. The dexibuprofen injection per ml comprises 10-90 mg of dexibuprofen. The high temperature resistance and hard light tolerance of a dexibuprofen injection prepared according to the invention are obviously superior to those in the prior art, the product stability is better, the quality is better, the requirements of clinical intravenous drip can be satisfied, and the clinical compliance is better.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
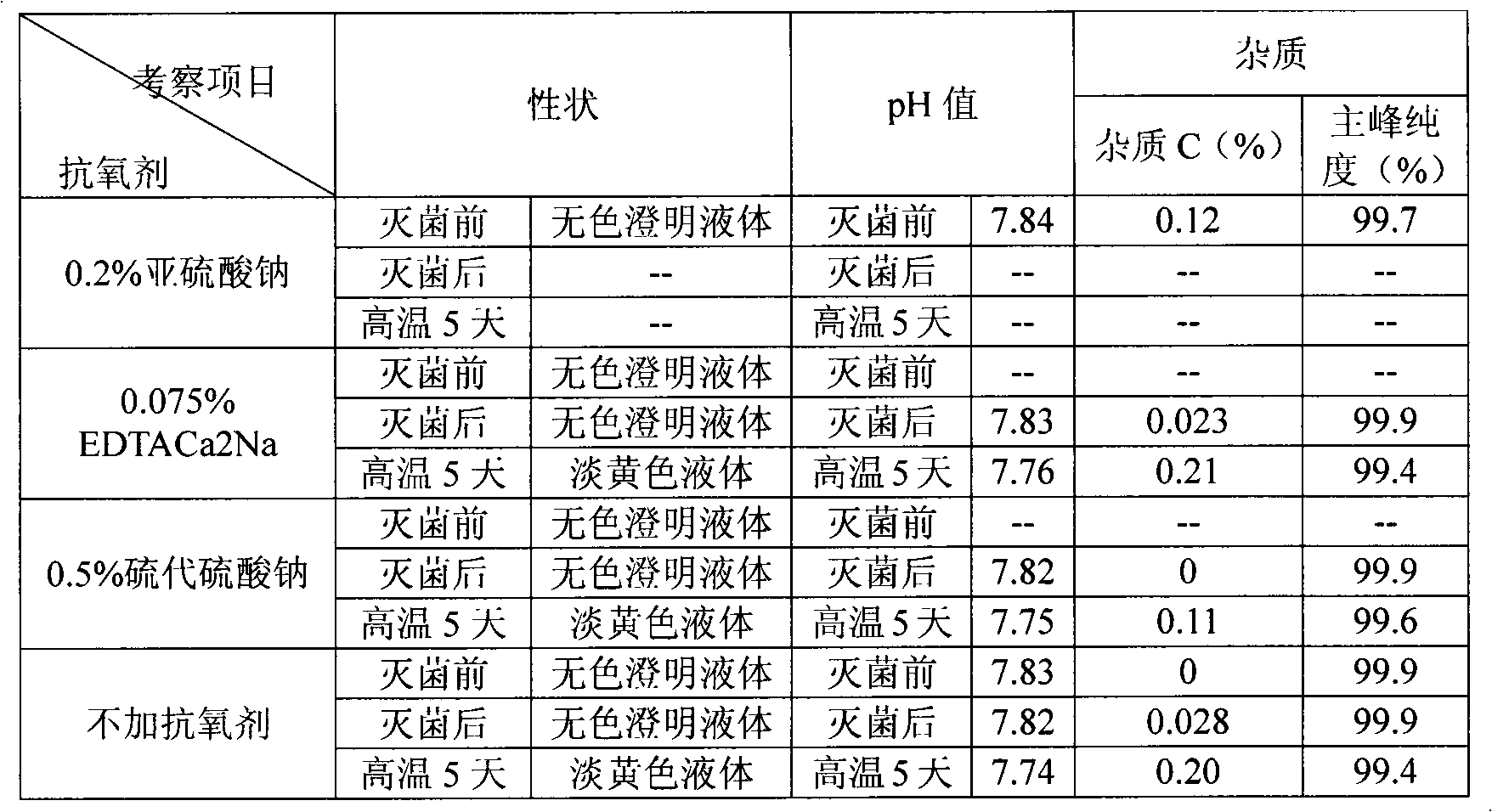
High-stability ibuprofen injection and preparation method thereof
InactiveCN103040734AReduce oxygen contentPrevent oxidative deteriorationOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
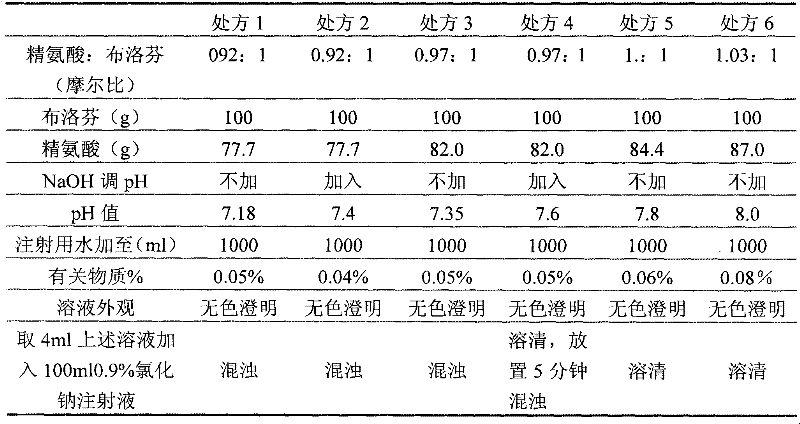
The invention relates to high-stability ibuprofen injection and a preparation method thereof. The injection comprises the following components of arginine, ibuprofen and water for injection, wherein the mol ratio of the arginine to the ibuprofen is (0.85-0.95): 1; the weight / volume ratio of the ibuprofen in the injection is 10 percent; the weight / volume ratio of the arginine in the injection is 7.2-8.0 percent; and the pH value of the injection is 7.5-8.5. The method creatively adopts the fewest and the smallest accessories to solve the stability difficulty of the ibuprofen injection, and successfully prepares the ibuprofen injection with safety in clinical use and good stability.
Owner:蚌埠丰原涂山制药有限公司
Ibuprofenlyophilized powder composition and preparation method
The invention provides anibuprofenlyophilized powder composition and a preparation method. The ibuprofenlyophilized powder composition comprises ibuprofen,a solubilizer arginine and sodium chloride of a stabilizer. The problems of instability of an existing ibuprofeninjection and related substance increase are solved; and the compliance of a patient is improved.
Owner:JINAN ORIENT KAIYUAN PHARMA TECH
Ibuprofen liquid injection composition and preparation method thereof
ActiveCN102697718AImprove the inspection items of visible foreign matterImprove complianceOrganic active ingredientsAntipyreticIbuprofen InjectionGLUCOSE LIQUID
The invention relates to an ibuprofen liquid injection which is characterized by comprising active ingredient ibuprofen and cosolvent arginine at molar ratio of 1:1-1:1.2, and adopting ammonia water and hydrochloric acid as pH regulator. Ammonia water is added into solution of ibuprofen and arginine to regulate pH value to 7.8-9.0, to obviously reduce insoluble particles, and solve compatibility problem between the liquid injection and sodium chloride and glucose liquid injection; then hydrochloric acid is adopted to regulate pH value back to 7.0-8.0, to enable solution pH value to be same asor approximate to human body blood pH value, and improve administration compliance and comfort of patient.
Owner:WUHAN WUYAO SCI & TECH
Method for simultaneous determination of content of ibuprofen and arginine in ibuprofen injection
ActiveCN102854263AEasy to operateStrong specificityComponent separationIbuprofen InjectionRepeatability
The invention provides a method for simultaneous determination of content of ibuprofen and arginine in ibuprofen injection. In the method, a cyano column and high performance liquid chromatography are used for simultaneous determination of content of ibuprofen and arginine. According to the invention, tedious procedures in respective detection of ibuprofen and arginine are omitted, a cumbersome process for derivatization of arginine is avoided, and the method is fast and accurate and has good repeatability.
Owner:JILIN SIHUAN PHARM CO LTD +1
Detection method of relevant substances of ibuprofen injection
InactiveCN109521117AIncrease concentrationHigh detection sensitivityComponent separationIbuprofen InjectionSilica gel
The invention discloses a detection method of relevant substances of ibuprofen injection. The relevant substances comprise arginine. A high performance liquid chromatography method is used for detection; the relevant substances are subjected to qualitative or quantitative detection. The detection conditions comprise a detector being an ELSD detector, a chromatographic column being an alkyl silanebonded silica gel column or an amino column, flowing phases including an organic phase and a water phase at the volume ratio of (50 to 70):(50 to 30) (preferably 65:35). By using the method provided by the invention, the relevant substance of the arginine can be accurately monitored; the guarantee is provided for the quality and safety of the ibuprofen injection.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
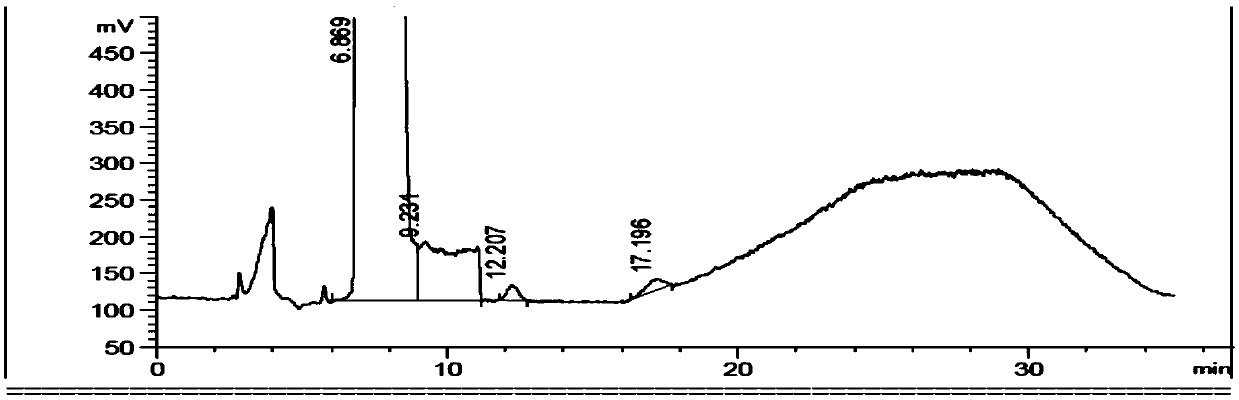
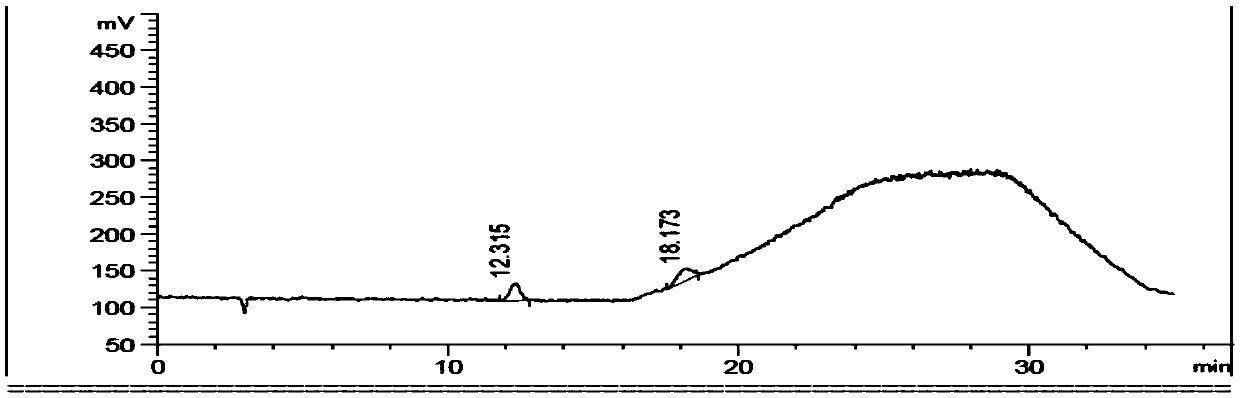
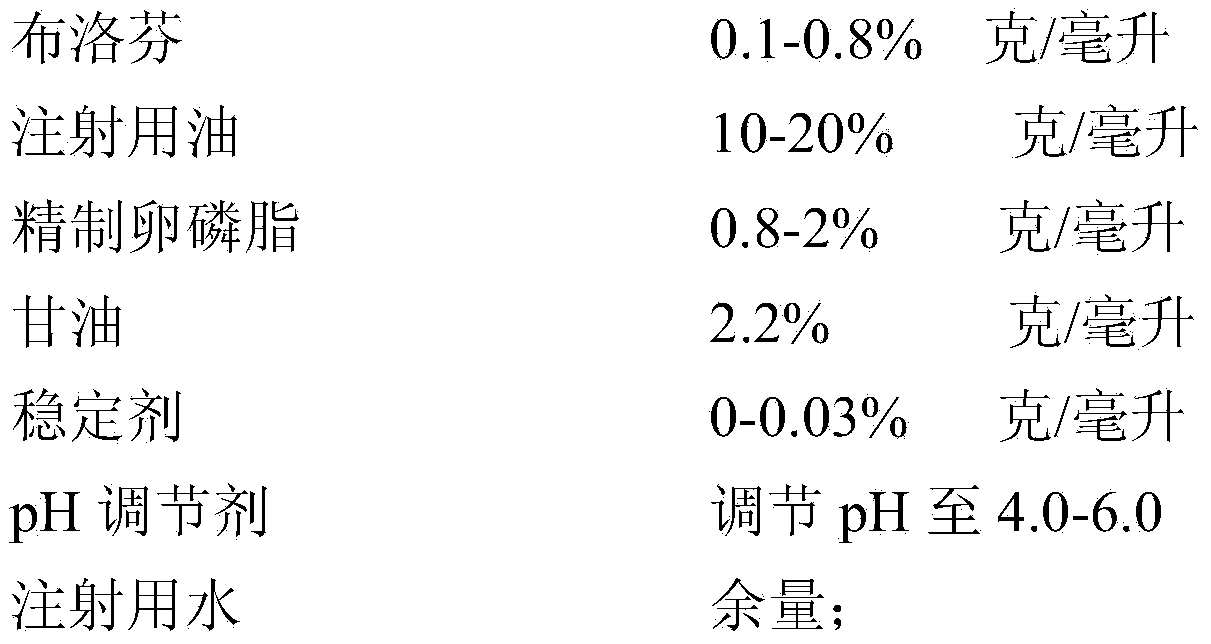
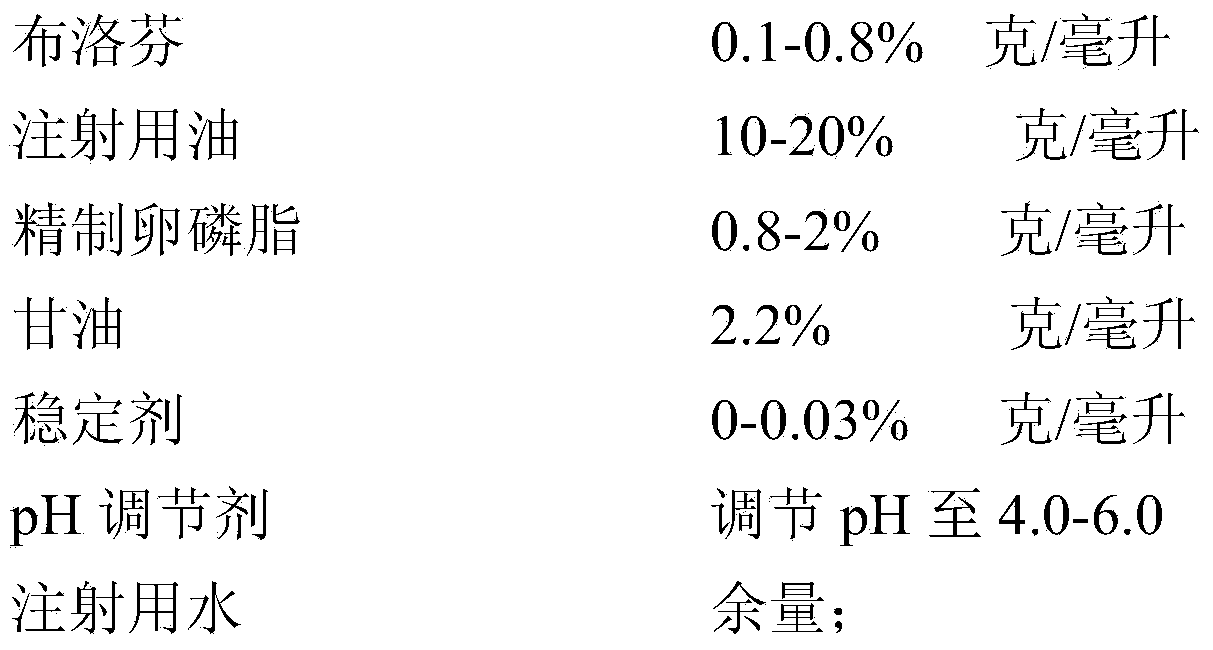
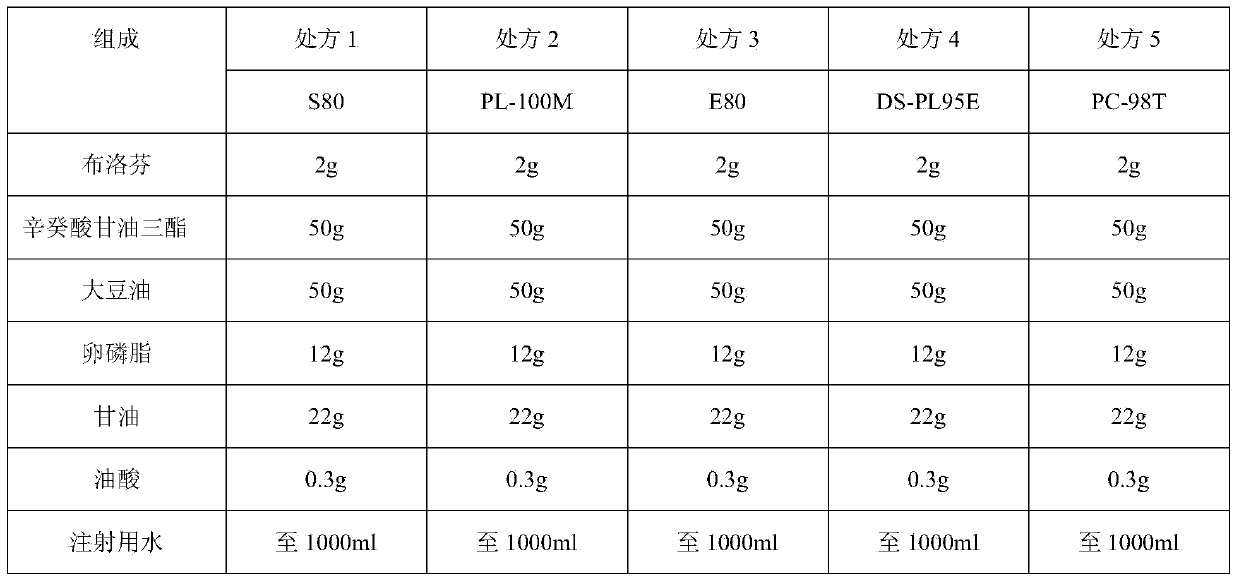
Ibuprofen fat emulsion injection and preparation method thereof
InactiveCN103622907AAvoid the risk of cross-contaminationGood for clinical useOrganic active ingredientsAntipyreticIbuprofen InjectionFat emulsion
The invention relates to the technology of pharmaceutical preparation. The invention provides an ibuprofen fat emulsion injection which comprises ibuprofen, injection oil, refined lecithin, glycerol, a stabilizing agent, a pH regulator and injection water. Through animal experiments, the pain easing effect of the ibuprofen fat emulsion injection is 1.59-4.31 times that of the commercially available ibuprofen injection, the antipyretic and anti-inflammatory effects can also be obviously improved, and the ibuprofen fat emulsion injection has certain long-acting effect. Proved by long-time stability experiments, the quality of the ibuprofen fat emulsion injection at normal temperature has no obvious change within two years. The invention provides the ibuprofen fat emulsion injection which can be intravenous, stable, safe, and high-efficiency.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Ibuprofen injection and its preparation method
The invention provides a method for stabilizing ibuprofen injections, as well as an ibuprofen injection prepared by the method. The main points of the method lie in that nitrogen gas is introduced one or repeatedly during preparation for expelling the oxygen in an enclosed packing container, and finally the oxygen content inside the enclosed container is controlled at 0-3%, thus reaching the purpose of avoiding oxidizing ibuprofen.
Owner:通辽市华邦药业有限公司
Ibuprofen injection composition and preparation method thereof
The invention relates to an ibuprofen injection. The injection consists of an active component ibuprofen and a cosolvent arginine, and is characterized in that the molar ratio of ibuprofen to arginine is 1:1.08-1.12, pH conditioning agents are not used, the amount and kind of accessories during injection preparation are reduced, irritation is low and security is higher. The prepared ibuprofen injection is substantially improved in resistance of high temperature and hard light; not only insoluble particles are obviously minimized, but also the compatibility problems of the injection with common salt and glucose injections are solved; and the product quality stability is substantially improved, and clinic compatibility demands are satisfied. Also, the invention provides a preparation method which is simple in technology and suitable for industrialized production.
Owner:WUHAN WUYAO SCI & TECH
Injection liquid containing ibuprofen and preparation process of injection liquid
InactiveCN102716069AAvoid adverse reactionsImprove efficiencyOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
The invention relates to a proportioning method of ibuprofen injection liquid, a preparation process and relevant substance limitation and belongs to the technical field of medicine. The medical auxiliary materials of the injection liquid are arginine, the injection liquid is prepared by mixing the ibuprofen and arginine according to a mol ratio, the total quantity of relevant substances in the injection liquid is lower than 0.5 percent, and in addition, the content of each kind of impurities is respectively lower than 0.1 percent. The pH value of the injection liquid is controlled in a range that the ibuprofen can be completely dissolved, and the crystal separation phenomenon cannot occur. The content of relevant substances of the injection liquid is strictly controlled in a certain range so that the adverse effect generated on human bodied is avoided to the furthest degree during the intravenous drip on patients, and the safety is higher. The ibuprofen injection liquid provided by the invention has the advantages that the preparation process is simple, the operation is easy, the efficiency is high, and products with stable quality can be prepared.
Owner:无锡康福特药物科技有限公司
Medicinal composition and preparation thereof
InactiveCN103845286AQuality improvementOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
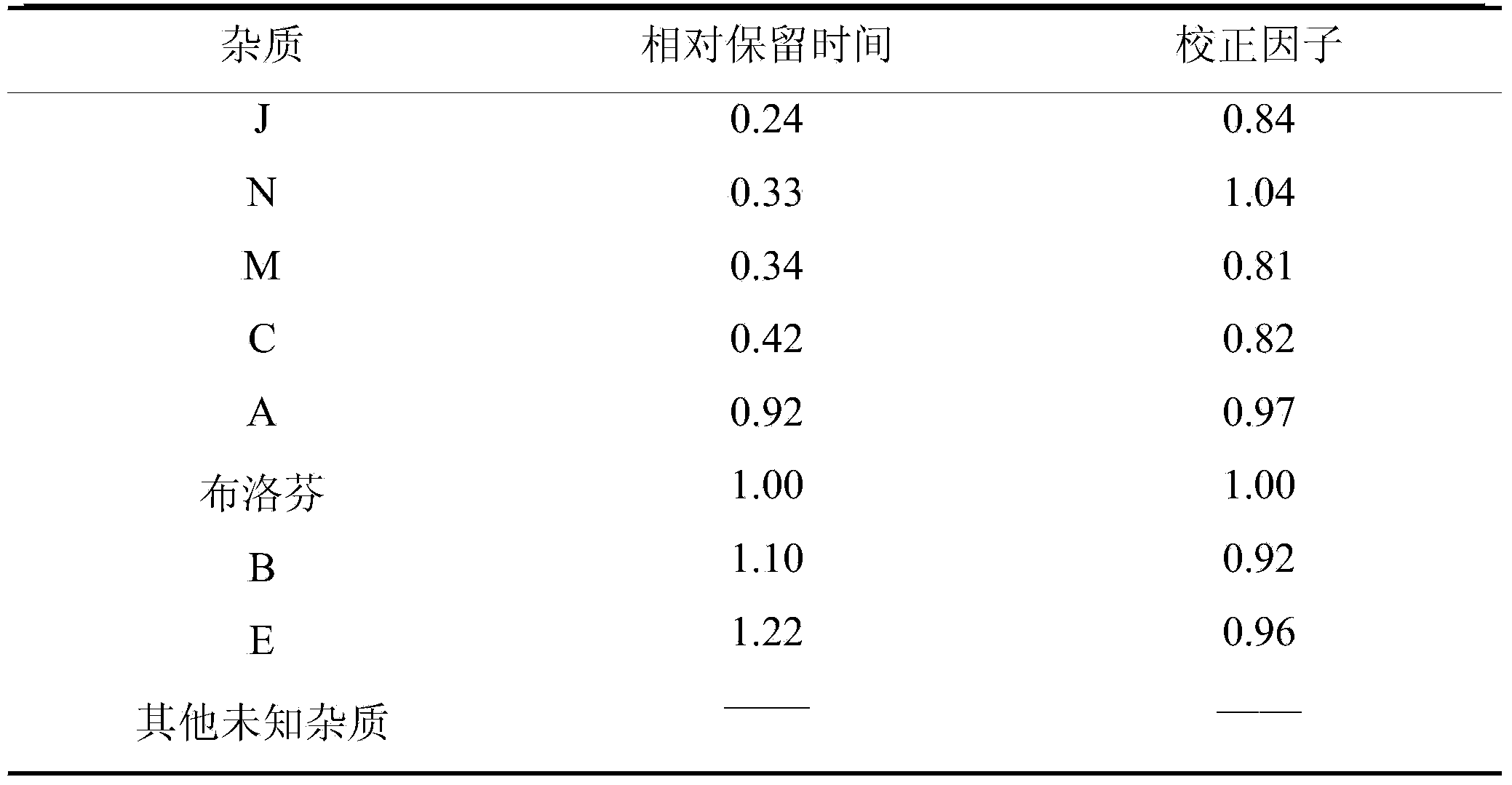
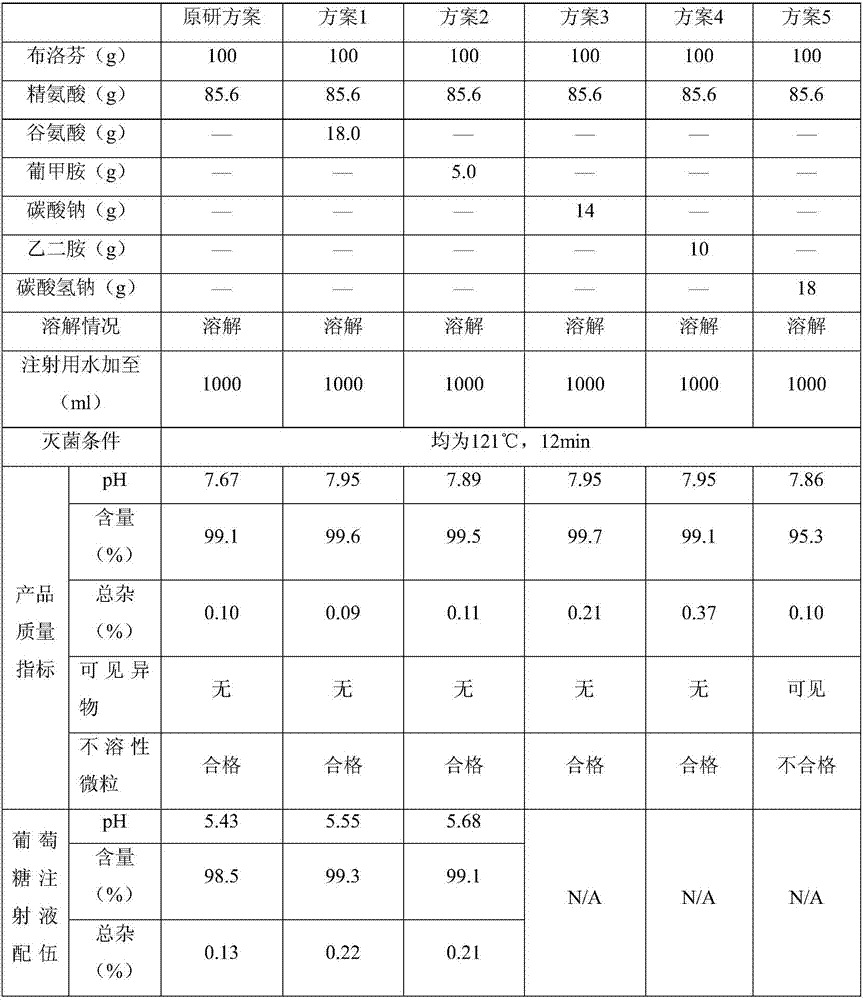
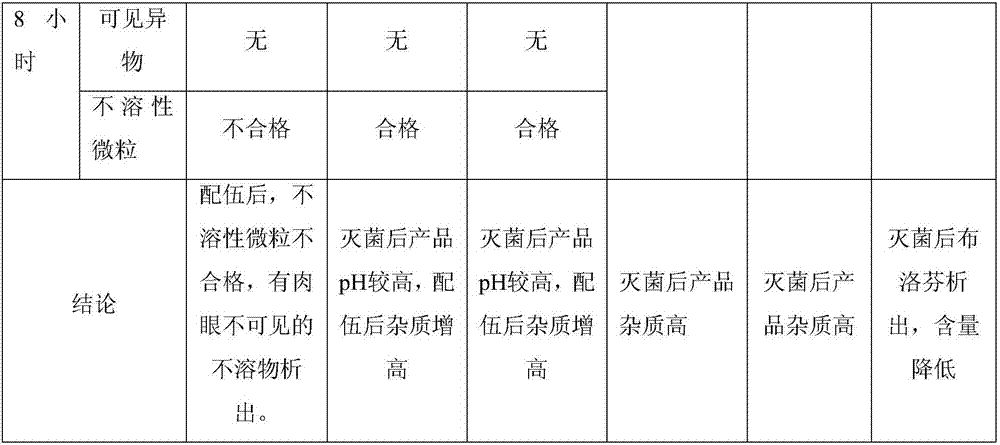
The invention belongs to the technical field of medicines, and discloses a medicinal composition and a preparation thereof. The medicinal composition is prepared into a water injection which consists of ibuprofen, arginine, a pH regulating agent and water for injection, wherein the pH regulating agent comprises arginine. As proved by experimental study, the number of increased impurities in an ibuprofen injection which meets the requirement is small, and a new impurity M is not produced. The preparation prepared by using the medicinal composition is more excellent in quality.
Owner:北京美迪康信医药科技有限公司 +1
Ibuprofen injection for intravenous administration
ActiveCN107198676AImprove solubilityImprove sterilityOrganic active ingredientsAntipyreticIbuprofen InjectionPoloxamer
The invention discloses an ibuprofen injection for intravenous administration and a preparation method thereof. The ibuprofen injection for intravenous administration is prepared from ibuprofen, arginine, a cosolvent and water for injection, wherein the cosolvent is poloxamer 188. The ibuprofen injection disclosed by the invention has the advantages of stable quality, high sterile level, moderate pH and good compatibility of clinical application. The preparation method of the ibuprofen injection disclosed by the invention has the advantages that the technology is simple and efficient, the quality is controllable, and industrial mass production is facilitated.
Owner:燃点(南京)生物医药科技有限公司
Ibuprofen injection and preparation method thereof
InactiveCN107233307AEnsure safetyImprove stabilityOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
The invention relates ibuprofen injection. The ibuprofen injection is prepared from ibuprofen, arginine, hydrochloric acid, a buffer pair and injection water. The invention further provides a preparation method of the ibuprofen injection. The ibuprofen injection prepared with the method is good in stability, a preparationmethod is simple, and popularization is facilitated.
Owner:JINAN LIMIN PHARMA
Ibuprofen injection composite and preparation method thereof
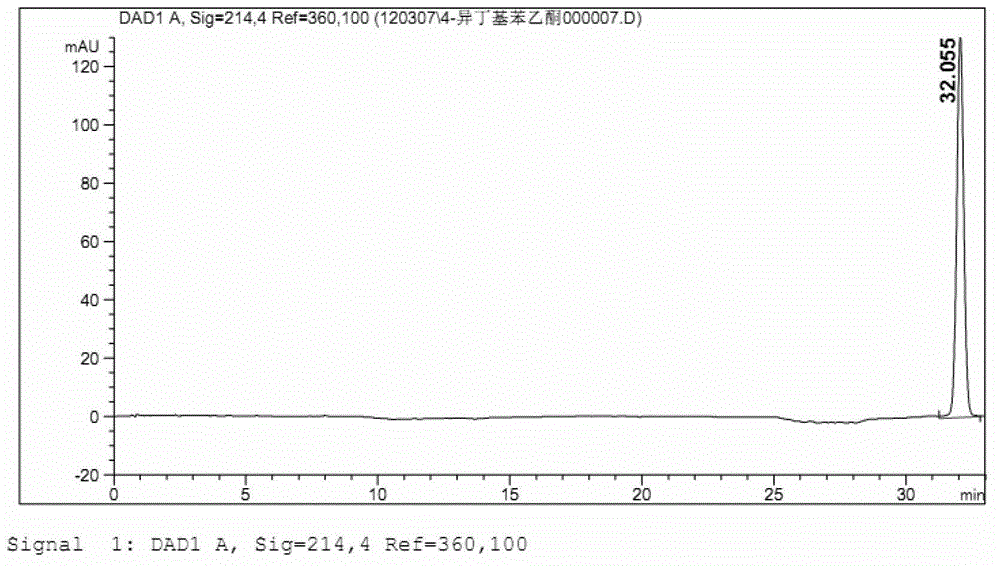
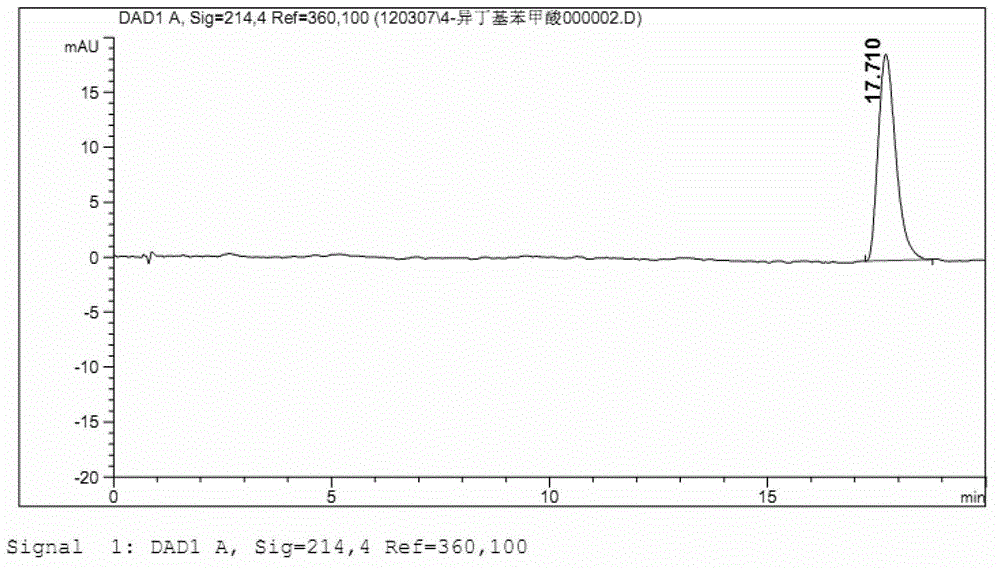
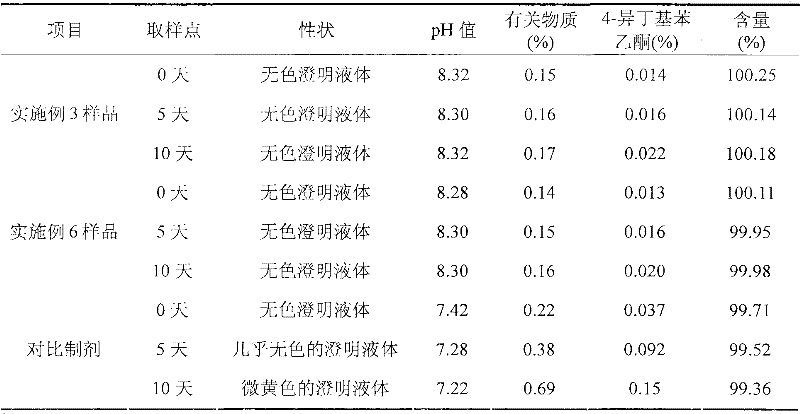
ActiveCN101966147BImprove stabilityLow in 4-isobutylacetophenoneOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
The invention discloses an ibuprofen injection composite which can obviously improve the quality stability of products and meet the clinical compatibility demand and a preparation method thereof to overcome the defect of the traditional ibuprofen injection. The injection is prepared by mixing ibuprofen and cosolvent according to a molar ratio of 1: 1.001-2, and the pH value of the injection is 7.5-9.0. The ibuprofen injection prepared through the method has obviously improved tolerance capability to high temperature and strong light and better stability and no irritant to vessels, and can effectively meet the requirement of the clinical intravenous drip. Moreover, the preparation method of the invention has the advantages of simple process and good stability of the prepared product.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD +1
Dexibuprofen lipid emulsion injection and preparation method thereof
PendingCN110339165AThe quality of clinical compatibility is stableComply with USP41 requirementsOrganic active ingredientsAntipyreticFiltrationHydroxystearic Acid
The invention relates to a preparation method of a dexibuprofen lipid emulsion injection. The preparation method comprises the following steps that long-chain fatty acid, medium-chain fatty acid and KolliphorHS 15 are heated and stirred to form a homogeneous oil-phase solution; an emulsifier, an osmotic pressure regulating agent and water are evenly mixed to form a water-phase solution; the oil phase and the water phase are mixed, stirred at a certain temperature, sheared and emulsified, and after high-pressure homogenization, pH regulating, filtration, sterilization and the like, the dexibuprofen lipid emulsion injection is obtained. The novel solubilizer KolliphorHS 15 for injection is adopted, the problems about the compatible stability and the drug loading capacity of a dexibuprofen injection during clinical use are effectively solved, and the safety of a preparation is expected to be improved.
Owner:SUZHOU UNIV
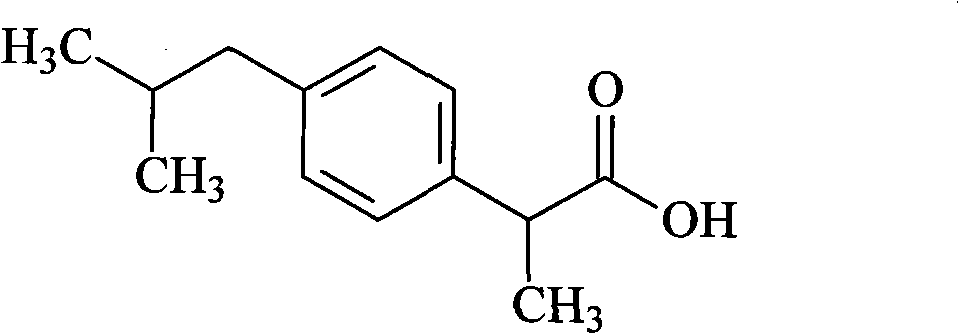
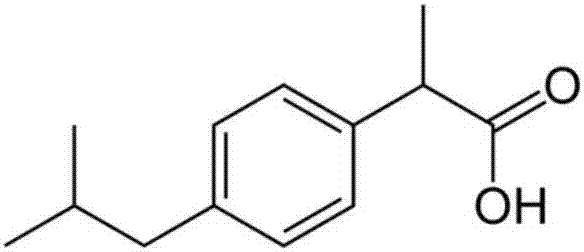
Ibuprofen compound and pharmaceutical composition thereof
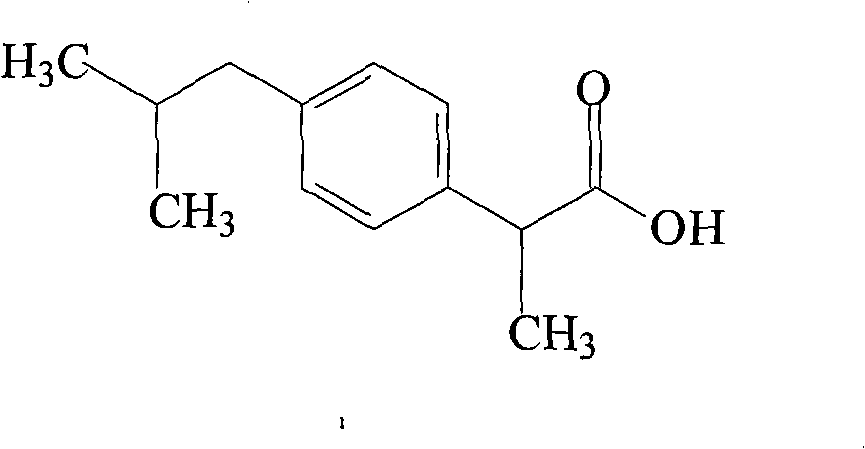
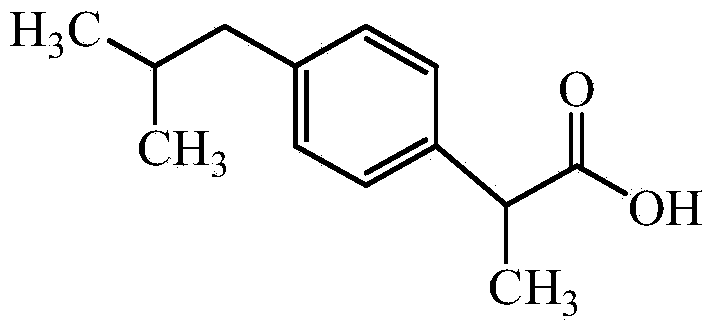
InactiveCN103204774ALess impurity content in storageGood storage stabilityOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
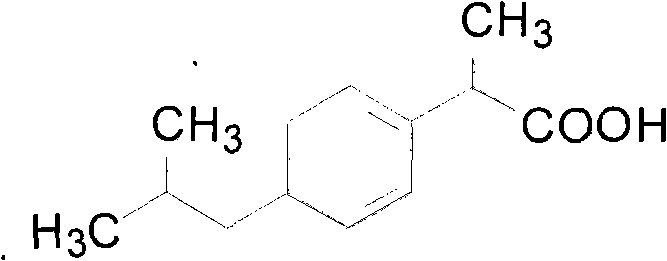
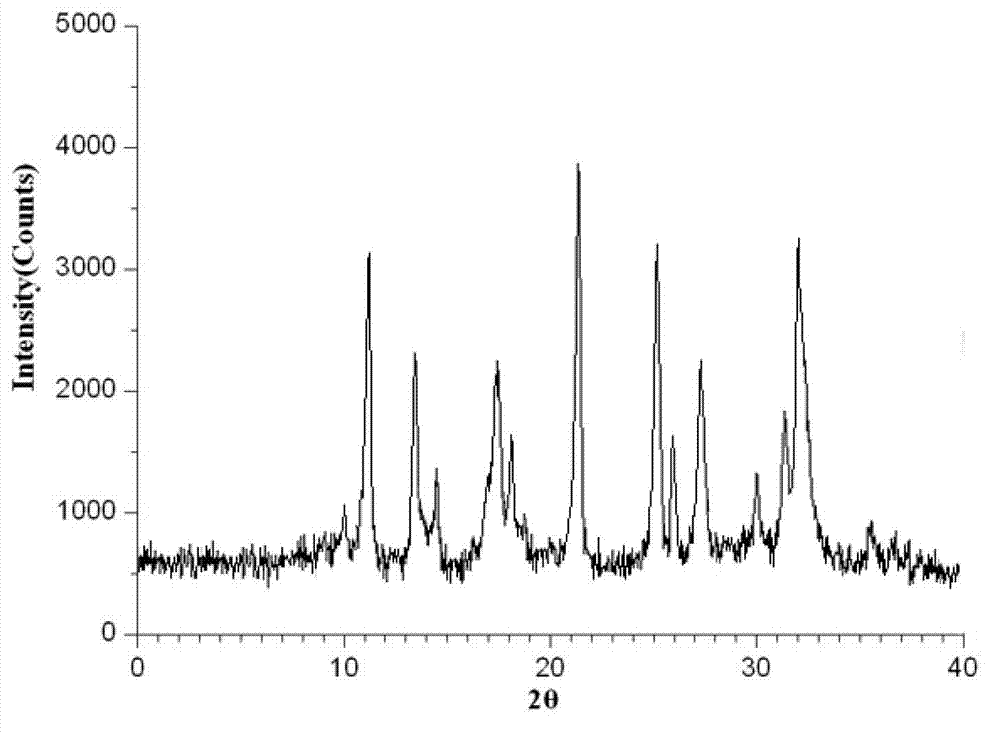
The invention belongs to the technical field of medicines, and particularly relates to an ibuprofen compound. The structure formula of the ibuprofen compound is shown in the specification. An X-ray powder diffraction spectrum of the Ibuprofen compound measured by a Cu-K alpha ray is shown in figure 1. A preparation method of the ibuprofen compound and a pharmaceutical composition containing the ibuprofen compound are also provided by the invention. The ibuprofen pharmaceutical composition is ibuprofen injection and Ibuprofen sterile powder injection. Compared with the prior art, the ibuprofen compound and the pharmaceutical composition thereof provided by the invention have better storage stability; and the medication safety of a sufferer is greatly improved.
Owner:北京康瑞达彤科技有限公司
Ibuprofen injection and preparation method
InactiveCN110693822ASolve the technical problems of crystallizationImprove securityOrganic active ingredientsAntipyreticIbuprofen InjectionMedicinal chemistry
The invention relates to an ibuprofen injection. A prescription of the injection comprises a main drug ibuprofen and a cosolvent arginine, the dose of arginine in the prescription is 83.75 mg / ml-95 mg / ml, the molar ratio of the arginine to the ibuprofen is 0.99:1-1.18:1, and the pH value range of the injection is 7.8-8.8. The prepared product is superior to the prior art in quality and has good stability and safety.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Application of ibuprofen to preparation of antitumor drugs
InactiveCN108186620AHas antitumor activitySignificant effectOrganic active ingredientsPharmaceutical delivery mechanismIbuprofen InjectionCurative effect
The invention discloses application of ibuprofen to preparation of antitumor drugs, especially application of an ibuprofen injection with a specific concentration (10% to 19%) to treatment of pancreatic cancer, etc. Conventionally, ibuprofen is only used as a drug for relieving fever, easing pain and the like; the results of scientific experiments in the invention prove that ibuprofen has antineoplastic activity; research results have been applied to treatment of patients with tumors, and obvious curative effect is obtained; so theoretical bases and actual cases are provided for application and clinical promotion of ibuprofen in preparation of antitumor drugs.
Owner:杨韶彰
Ibuprofen injection and preparation method thereof
InactiveCN102085179BLow priceImprove securityOrganic active ingredientsAntipyreticSodium bicarbonateSodium acetate
The invention relates to an ibuprofen injection, which comprises ibuprofen and one or a plurality of alkaline cosolvents pharmaceutically acceptable selected from anhydrides or hydrates of sodium carbonate, sodium bicarbonate,sodium citrate, sodium citrate, sodium phosphate, disodium dihydrogen pyrophosphate, sodium tartrate and sodium acetate. The invention also relates to a preparation method of the ibuprofen injection.
Owner:罗军
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com