Ibuprofenlyophilized powder composition and preparation method
A technology of freeze-dried powder injection and freeze-dried powder injection, applied in the field of freeze-dried composition for injection, which can solve the problems affecting the stability of the appearance and properties of powder injection for injection, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
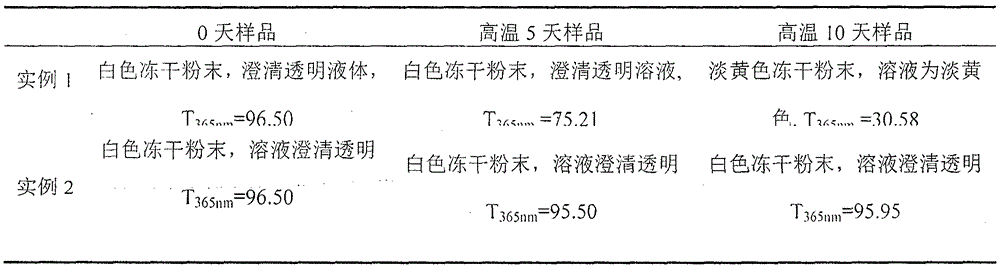
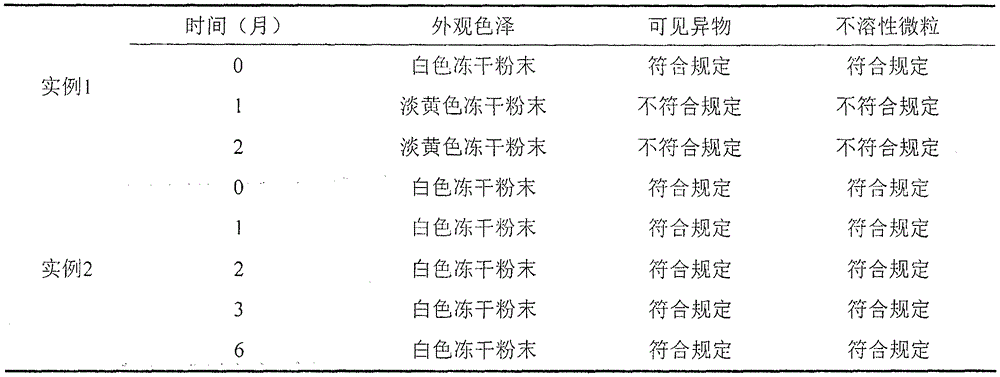
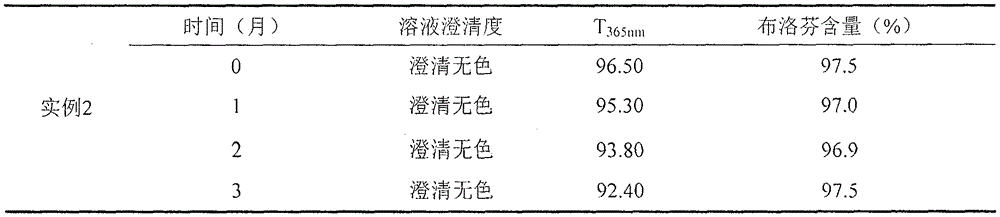
Examples
example 2
[0022] prescription:
[0023] Ibuprofen 400g
[0025] L-Arginine 300~400g
[0026] 1) Mix the ingredients of arginine, sodium chloride and ibuprofen into water for injection, stir until fully dissolved, and the mass ratio of water for injection to ibuprofen is 10:1;
[0027] 2) ultrafiltration: the solution is subjected to ultrafiltration in a sterile environment, then aseptically subpackaged, and the packed solution is divided;
[0028] 3) Freeze-dry the filled semi-finished product, process parameters of freeze-drying curve: cool down to -40°C at full speed, keep warm for 2-5 hours; turn on the condenser, and turn on the vacuum pump when the temperature of the condenser drops to -80°C; When it drops below 10Pa, turn on the heating, raise the temperature to -15°C, and keep it warm for 6-10 hours; at 0°C, keep it warm for 1-5 hours, then raise the temperature to 25°C, and keep it warm for 8-20h; Seal the aluminum cover.
Embodiment 3
[0029] Implementation 3: The difference between this embodiment and Example 2 is that first arginine and sodium chloride will be dissolved in water for injection at 20-30° C. in step 1), and the others are the same as Example 2.
example 4
[0031] prescription:
[0032] Ibuprofen 400g
[0033] Sodium chloride 4~7g
[0034] L-Arginine 300~400g
[0035] 1) Mix the ingredients of arginine and sodium chloride into water for injection, slowly add ibuprofen under stirring and stir until completely dissolved, the mass ratio of water for injection and ibuprofen is 10:1;
[0036] 2) ultrafiltration: the solution is subjected to ultrafiltration in a sterile environment, then aseptically subpackaged, and the packed solution is divided;
[0037] 3) Freeze-dry the filled semi-finished product, freeze-drying curve process parameters: cool down to -40°C at full speed, keep warm for 2 to 5 hours; turn on the condenser, and turn on the vacuum pump when the temperature of the condenser drops to -80°C; When the temperature is below 10Pa, turn on the heating, raise the temperature to -15°C, and keep it warm for 6-10 hours; at 0°C, keep it warm for 1-5 hours, then raise the temperature to 25°C, and keep it warm for 8-20 hours; Al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com