Ibuprofen injection and preparation method
A technology for injection and water for injection, applied in the field of medicine, can solve the problems of unstable raw materials of ibuprofen and prone to crystallization, and achieve the effect of excellent product quality and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] prescription:
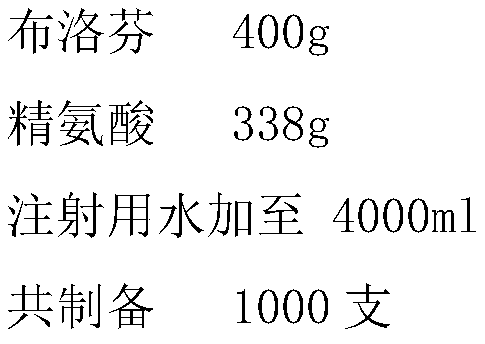
[0021] Ibuprofen 8.0g
[0022] Arginine 6.2g
[0023] Add water for injection to 80ml
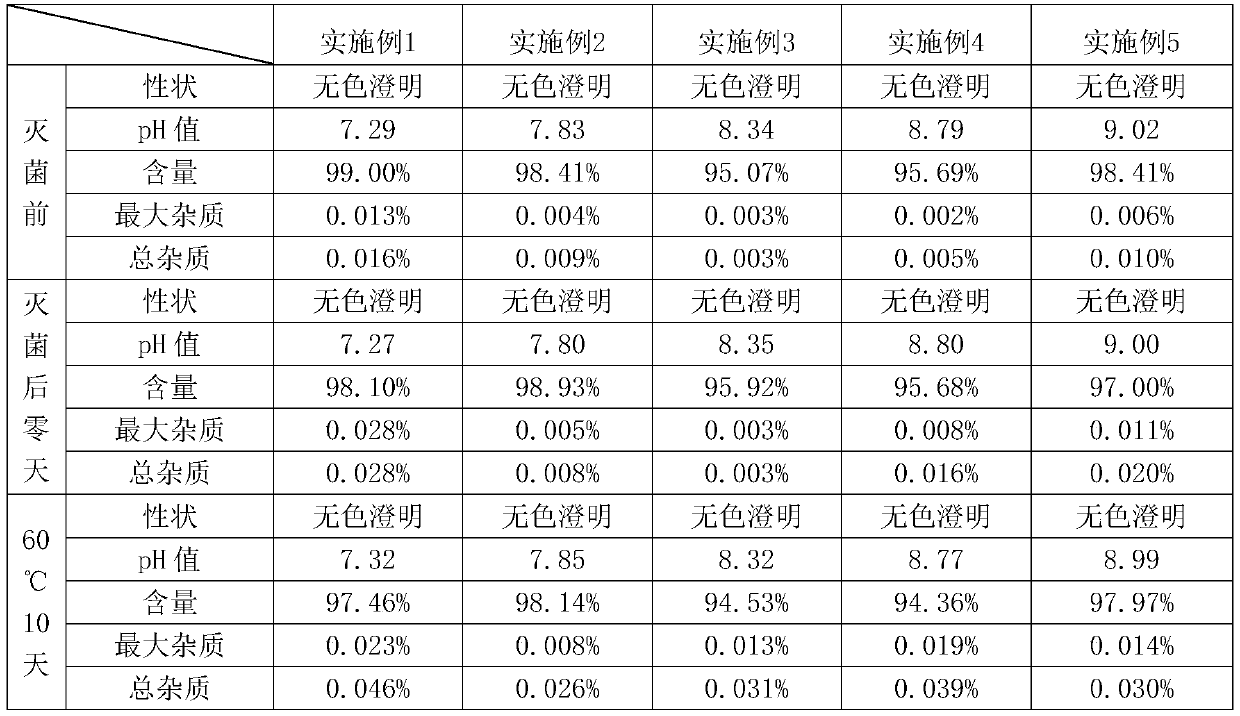
[0024] Preparation method: take an appropriate amount of water for injection and pass it with nitrogen gas for 30 minutes, seal it for later use; take the above water for injection with a liquid volume of 80%, add ibuprofen and stir at 70-80°C, then add the prescribed amount of arginine and stir to dissolve, and constant volume , control the pH in the range of 7.8-8.8, replenish the water to the full amount; add activated carbon for needles with a liquid volume of 0.1% (0.1g / 100ml), stir and adsorb at room temperature for 20min, and decarburize by filtering through a 0.45μm microporous membrane; The 0.45 μm microporous membrane is filtered twice, and nitrogen is protected; the ampoule is filled with nitrogen, and then filled with liquid medicine, nitrogen, and sealed; sterilized by autoclaving at 121 ° C for 15 minutes.
Embodiment 2
[0026] prescription:
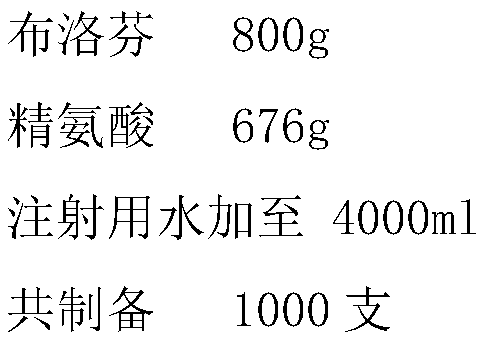
[0027] Ibuprofen 8.0g
[0028] Arginine 6.7g
[0029] Add water for injection to 80ml
[0030] Preparation method: take an appropriate amount of water for injection, pass nitrogen gas for 30 minutes, and seal it for later use; take the above-mentioned water for injection with a dosing volume of 80%, add ibuprofen and stir at 70-80°C, then add the prescribed amount of arginine, stir to dissolve, and set to control the pH in the range of 7.8-8.8; add activated carbon for needles with a dosing volume of 0.1% (0.1g / 100ml), stir and adsorb at room temperature for 20min, and decarburize by filtration through a 0.45μm microporous membrane; the liquid is passed through a 0.45μm The microporous filter membrane is filtered twice, and nitrogen protection is passed; the ampoule is filled with nitrogen gas, then filled with liquid medicine, nitrogen gas is passed, and the seal is sealed; sterilized by autoclaving at 121°C for 15 minutes.
Embodiment 3
[0032] prescription:
[0033] Ibuprofen 8.0g
[0034] Arginine 7.0g
[0035] Add water for injection to 80ml
[0036] Preparation method: take an appropriate amount of water for injection, pass nitrogen gas for 30 minutes, and seal it for later use; take the above-mentioned water for injection with a dosing volume of 80%, add ibuprofen and stir at 70-80°C, then add the prescribed amount of arginine, stir to dissolve, and set to control the pH in the range of 7.8-8.8; add activated carbon for needles with a dosing volume of 0.1% (0.1g / 100ml), stir and adsorb at room temperature for 20min, and decarburize by filtration through a 0.45μm microporous membrane; the liquid is passed through a 0.45μm The microporous filter membrane is filtered twice, and nitrogen protection is passed; the ampoule is filled with nitrogen gas, then filled with liquid medicine, nitrogen gas is passed, and the seal is sealed; sterilized by autoclaving at 121°C for 15 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com