Detection method of relevant substances of ibuprofen injection
A technology of related substances and detection methods, applied in the field of drug-related substances, can solve the problems of injection quality and safety performance, inability to detect, etc., and achieves good chromatographic peak symmetry, quality and safety assurance, and high number of theoretical plates. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
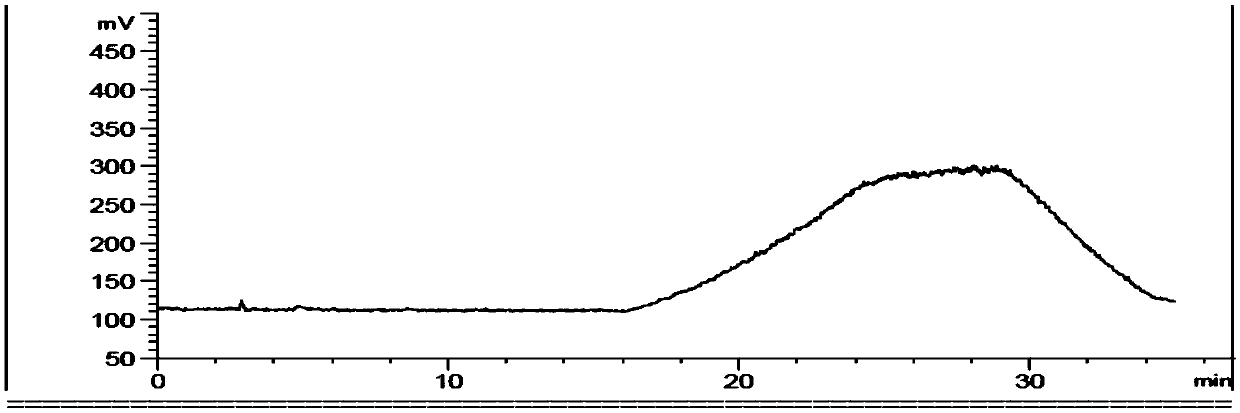
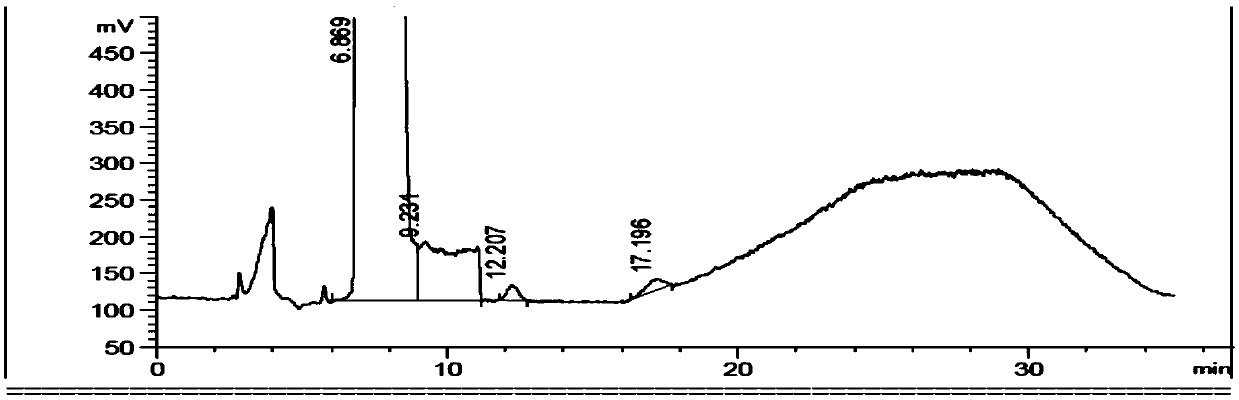
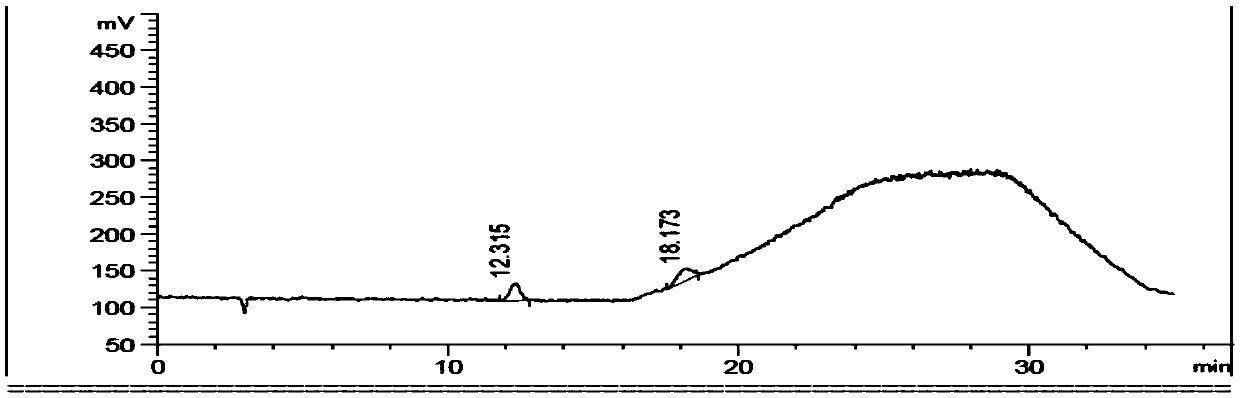
Image
Examples
Embodiment 1
[0042] Inspection method: ChP2015 Four General Rules 0512, determination by high performance liquid chromatography.
[0043] Instrument: Agilent 1260 with ELSD detector
[0044] Chromatographic column: Kromasil 100-5-NH2, 4.6mm×250mm, 5μm or equivalent chromatographic column;
[0045] Mobile phase: acetonitrile-5mmol / L ammonium bicarbonate solution=65:35;
[0046] Injection volume: 50μl; Column temperature: 30°C; Drift tube temperature: 110°C; Carrier gas flow rate (air): 2.0L / min;
[0047] Mode: Split.
[0048]Diluent (blank solution): 65% acetonitrile solution.
[0049] Citrulline reference substance stock solution (0.2mg / ml): Accurately weigh an appropriate amount of citrulline reference substance, dissolve it in water and quantitatively dilute it with acetonitrile to make a 0.2mg / ml solution, shake well, and obtain.
[0050] Ornithine reference substance stock solution (0.2mg / ml): Accurately weigh an appropriate amount of ornithine reference substance, dissolve it in w...
Embodiment 2
[0057] Inspection method: ChP2015 Four General Rules 0512, determination by high performance liquid chromatography.
[0058] Instrument: Agilent 1260 with DAD detector.
[0059] Chromatographic column: Kromasil 100-5-NH2, 4.6mm×250mm, 5μm or equivalent chromatographic column.
[0060] Mobile phase: acetonitrile-5mmol / L ammonium bicarbonate solution=60:40.
[0061] Injection volume: 20μl; Column temperature: 30°C; Wavelength: 205nm; Flow rate: 1.3m / min.
[0062] Diluent (blank solution): 60% acetonitrile solution.
[0063] Citrulline reference substance stock solution (0.6mg / ml): Accurately weigh an appropriate amount of citrulline reference substance, dissolve it with a diluent and quantitatively dilute it to make a 0.6mg / ml solution, shake well, and obtain.
[0064] Ornithine reference substance stock solution (1mg / ml): Accurately weigh an appropriate amount of ornithine reference substance, dissolve it with a diluent and quantitatively dilute it to make a 1mg / ml solution,...
Embodiment 3
[0069] Inspection method: ChP2015 Four General Rules 0512, determination by high performance liquid chromatography.
[0070] Instrument: Agilent 1260 with DAD detector.
[0071] Chromatographic column: Kromasil 100-5-NH2, 4.6mm×250mm, 5μm or equivalent chromatographic column.
[0072] Mobile phase: acetonitrile-30mmol / L potassium dihydrogen phosphate solution (pH5.4)=60:40.
[0073] Injection volume: 20μl; Column temperature: 30°C; Wavelength: 205nm; Flow rate: 1.3m / min.
[0074] Diluent (blank solution): 60% acetonitrile solution. Citrulline reference substance stock solution (0.6mg / ml): Accurately weigh an appropriate amount of citrulline reference substance, dissolve it with a diluent and quantitatively dilute it to make a 0.6mg / ml solution, shake well, and obtain.
[0075] Ornithine reference substance stock solution (1mg / ml): Accurately weigh an appropriate amount of ornithine reference substance, dissolve it with a diluent and quantitatively dilute it to make a 1mg / ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com