High-stability ibuprofen injection and preparation method thereof
A high stability, injection technology, applied in pharmaceutical formulations, organic active ingredients, non-central analgesics, etc., can solve the problem of lack of nitrogen protection, low solution stability, alkaline stabilizer and buffer salt safety Risks and other issues, to achieve the effects of high compatibility stability, prevention of oxidative deterioration, and stable quality indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
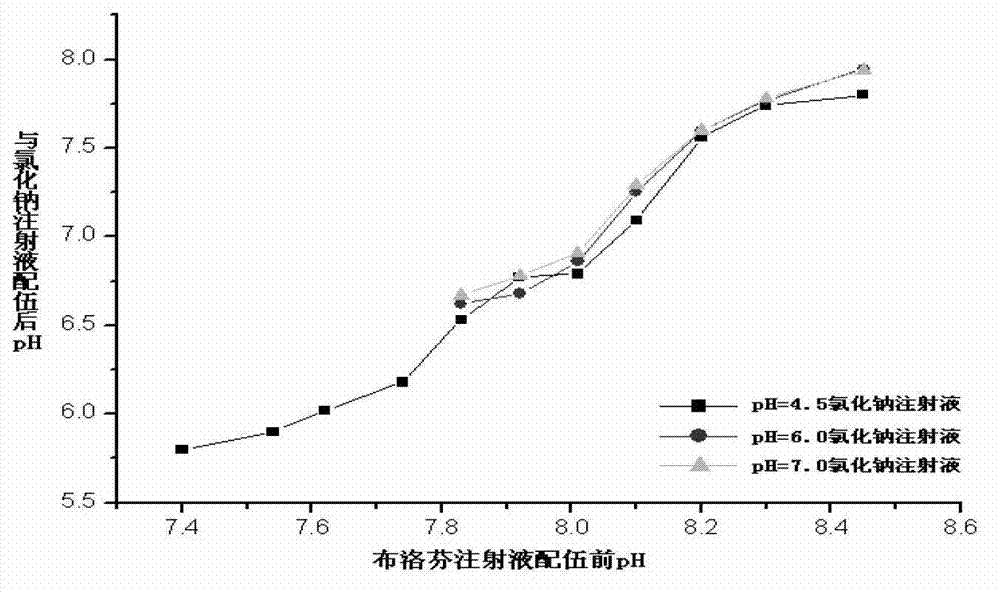
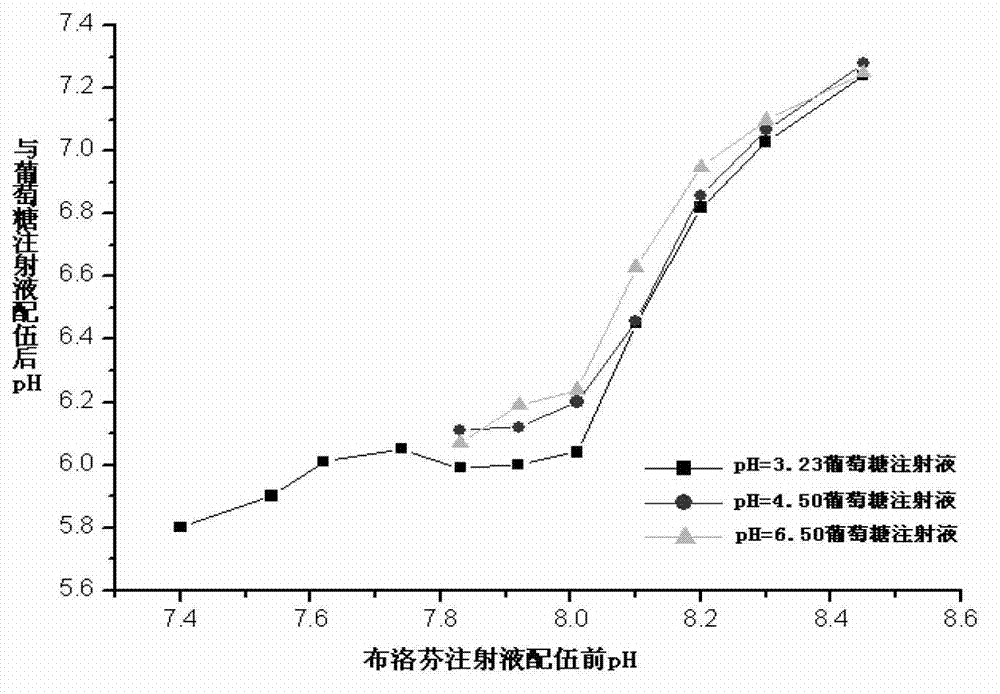
Image
Examples
Embodiment 1
[0029] prescription:
[0030]
[0031] Weigh arginine according to the prescription amount, take 80% of the total amount of water for injection, pass nitrogen to deoxygenate for 20 minutes, add arginine to dissolve, add ibuprofen raw material under stirring, and stir until completely dissolved while adding; add injection Water to the total amount, adjust the pH of the solution with 4% sodium hydroxide solution; add 0.1% of the total volume of activated carbon, heat and stir for 15 minutes, decolorize, filter through a 0.22 μm membrane; fill with nitrogen, fill in a 5ml ampoule, Sterilize at 121°C for 15 minutes; shorten the cooling time after sterilization as much as possible, and the measured pH after sterilization is 7.40.
Embodiment 2
[0033] The specific process is the same as that in Example 1, except that nitrogen flow and nitrogen protection are not carried out during the whole preparation process.
Embodiment 3
[0035] prescription:
[0036]
[0037] The specific process is the same as in Example 1, except that the prescription amount of cosolvent arginine is different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com