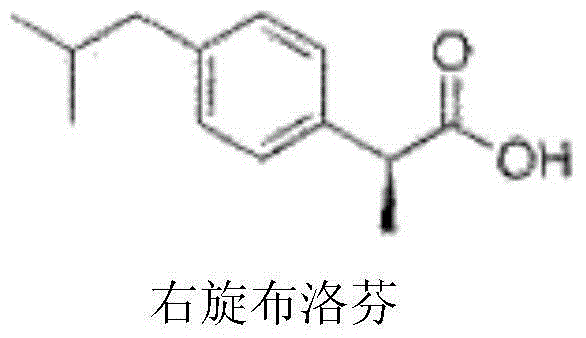
Dexibuprofen injection drug composition and preparation method thereof
A technology of dextro-ibuprofen and dex-profen, which is applied in the directions of drug combination, pharmaceutical formula, drug delivery, etc., can solve problems such as unreasonable dosage, undisclosed product quality research data, and difficulty in confirming product quality and stability. , to achieve the effect of reducing production costs and steps, significant antipyretic cooling effect, and good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Each 1000 injections preparation contains the following ingredients (75mg / 2ml):
[0030] Dextroibuprofen
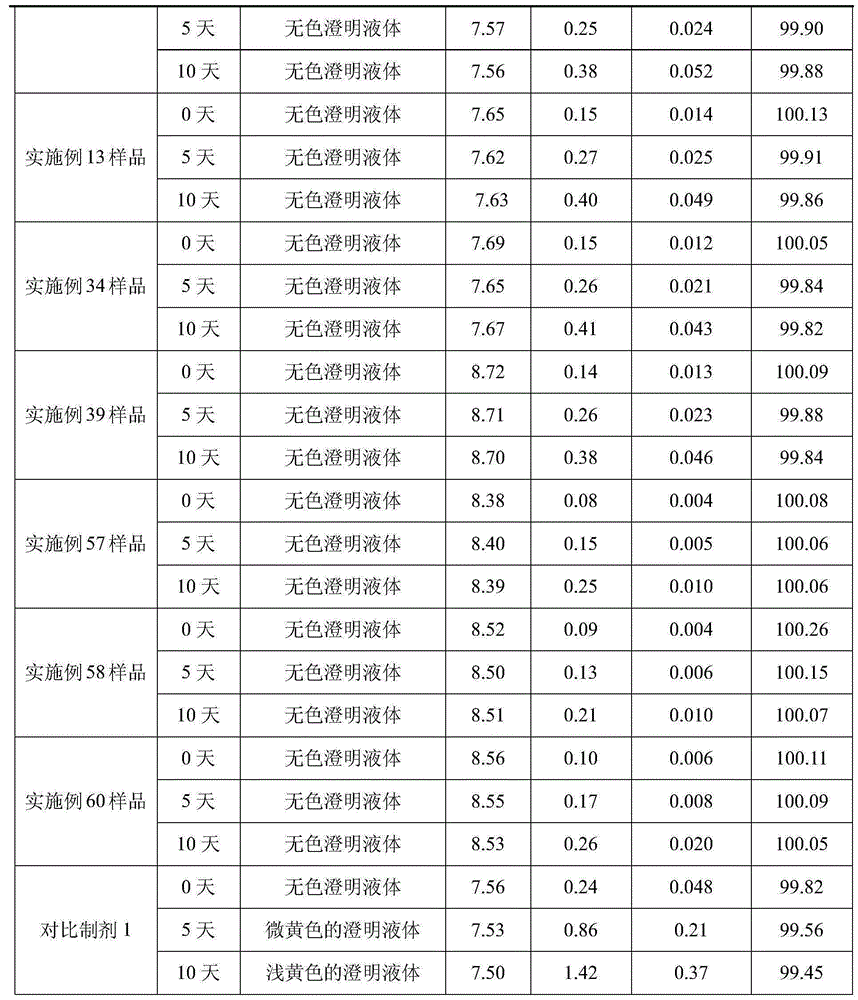
[0031] Weigh arginine according to the prescription amount, add 800-1000ml water for injection, stir to dissolve it, then add the prescription amount of dextroibuprofen, keep stirring to dissolve it, then add water for injection to 2000ml, stir and mix well; add Prepare a total amount of 0.1% (W / V) activated carbon for needles, keep at 40~60℃ for 20 minutes, stir and absorb, filter and decarburize for later use; filter the above solution with 0.22μm and 0.45μm microporous membranes until the filtrate is clear. After the semi-finished product passes the inspection, it is packed and filled into 1000 2ml ampoules, so that the content of the main drug in each ampoule is 75mg / 2ml. Sterilize at 115℃ for 30 minutes with hot and humid steam, leak detection, and light inspection to obtain the finished product. .
Embodiment 2
[0033] Each 1000 injections preparation contains the following ingredients (75mg / 2ml):
[0034] Dextroibuprofen
[0035] Weigh arginine according to the prescription amount, add 800-1000ml water for injection, stir to dissolve it, then add the prescription amount of dextroibuprofen, keep stirring to dissolve it, then add water for injection to 2000ml, stir and mix well; add Prepare a total amount of 0.1% (W / V) activated carbon for needles, keep at 40~60℃ for 20 minutes, stir and absorb, filter and decarburize for later use; filter the above solution with 0.22μm and 0.45μm microporous membranes until the filtrate is clear. After the semi-finished product passes the inspection, it is packed and filled into 1000 2ml ampoules, so that the content of the main drug in each ampoule is 75mg / 2ml. Sterilize at 115℃ for 30 minutes with hot and humid steam, leak detection, and light inspection to obtain the finished product. .
Embodiment 3
[0037] Each 1000 injections preparation contains the following ingredients (75mg / 2ml):
[0038] Dextroibuprofen
[0039] Weigh arginine according to the prescription amount, add 800-1000ml water for injection, stir to dissolve it, then add the prescription amount of dextroibuprofen, keep stirring to dissolve it, then add water for injection to 2000ml, stir and mix well; add Prepare a total amount of 0.1% (W / V) activated carbon for needles, keep at 40~60℃ for 20 minutes, stir and absorb, filter and decarburize for later use; filter the above solution with 0.22μm and 0.45μm microporous membranes until the filtrate is clear. After the semi-finished product passes the inspection, it is packed and filled into 1000 2ml ampoules, so that the content of the main drug in each ampoule is 75mg / 2ml. Sterilize at 115℃ for 30 minutes with hot and humid steam, leak detection, and light inspection to obtain the finished product. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Division value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com