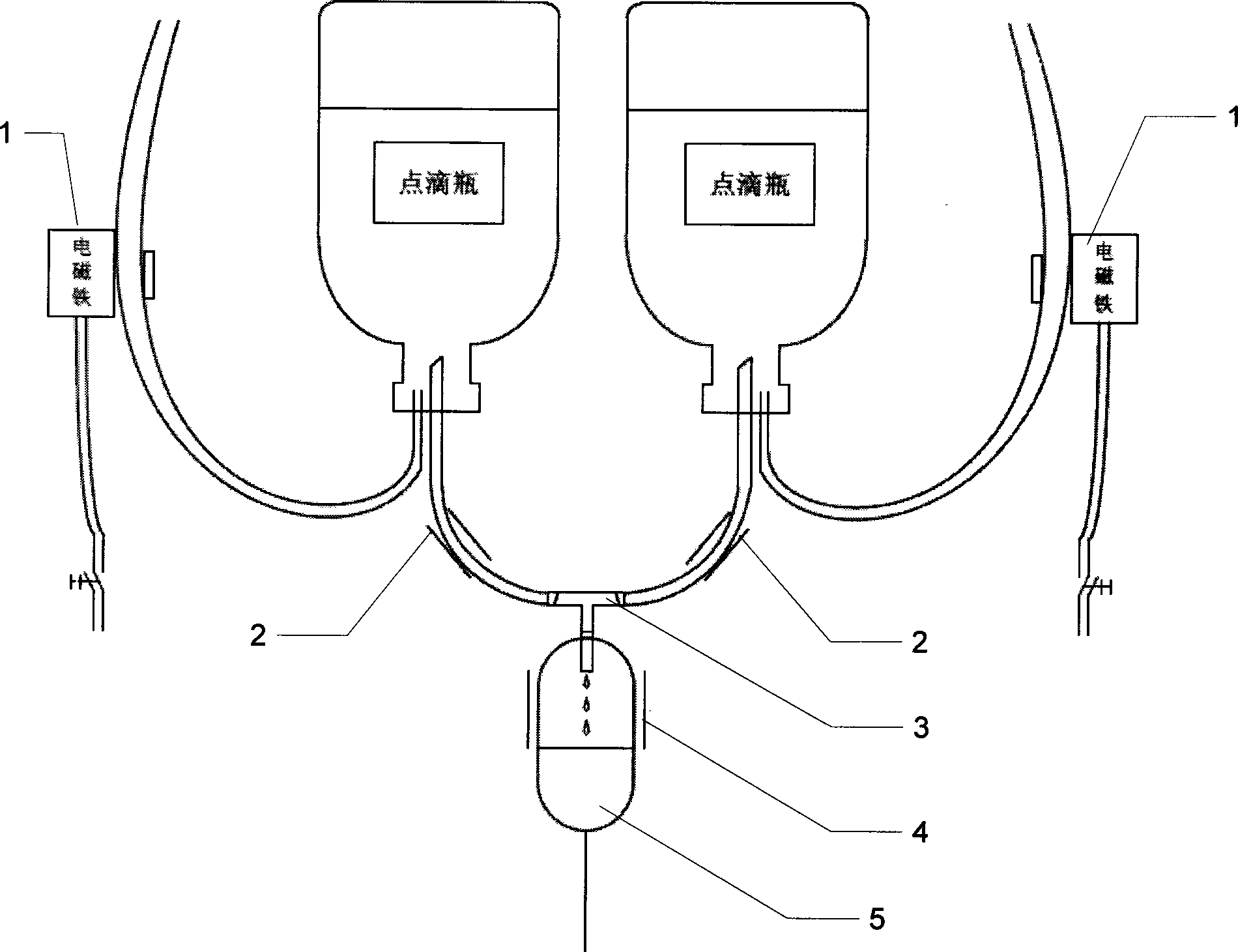
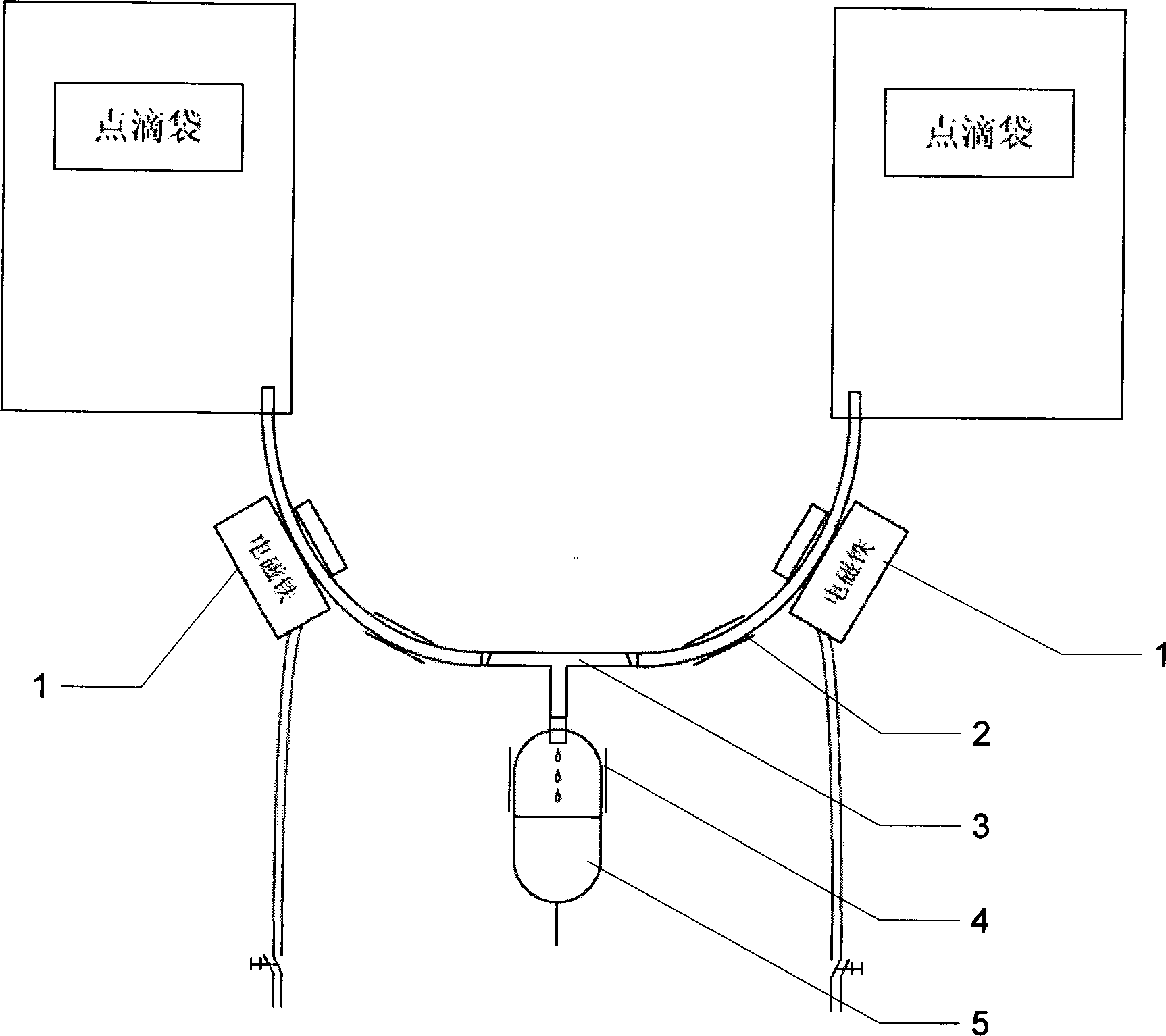
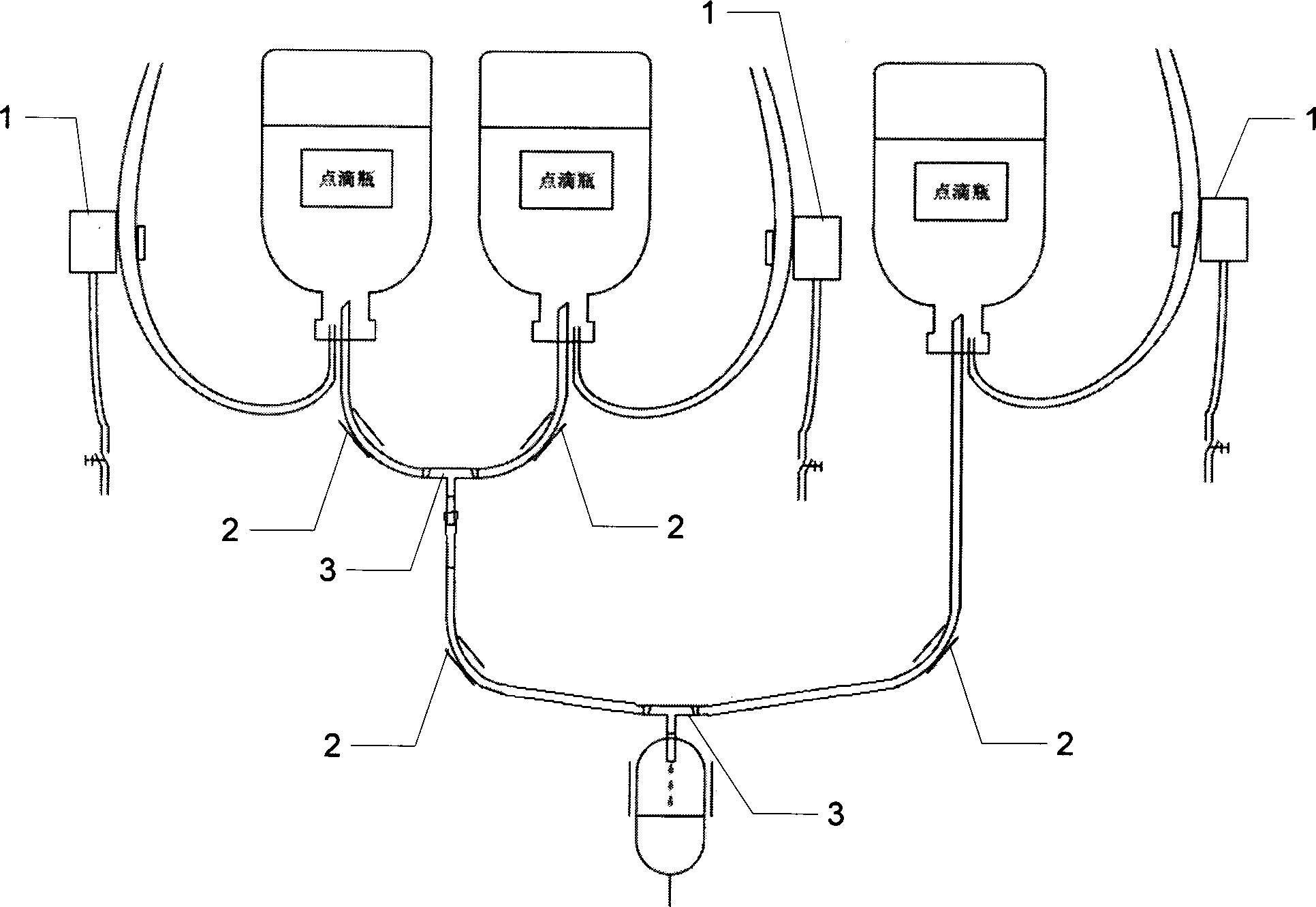
Patents
Literature
373 results about "Intravenous use" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intravenous therapy (IV) is a therapy that delivers liquid substances directly into a vein (intra- + ven- + -ous). The intravenous route of administration can be used for injections (with a syringe at higher pressures) or infusions (typically using only the pressure supplied by gravity).
Micro vein enhancer
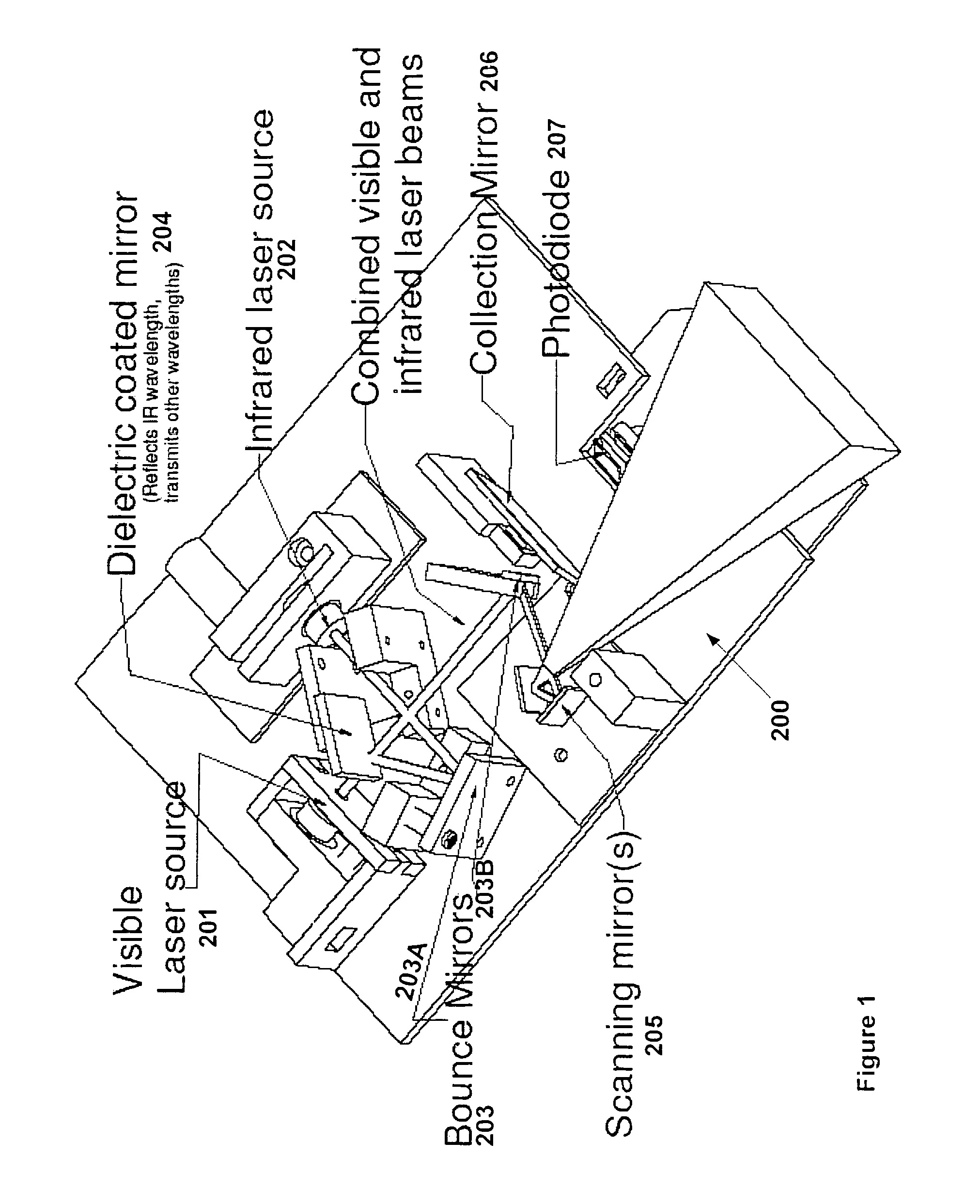
ActiveUS7904138B2Reduce impactExpand the scope of workImage analysisDiagnostics using lightVeinBlood test
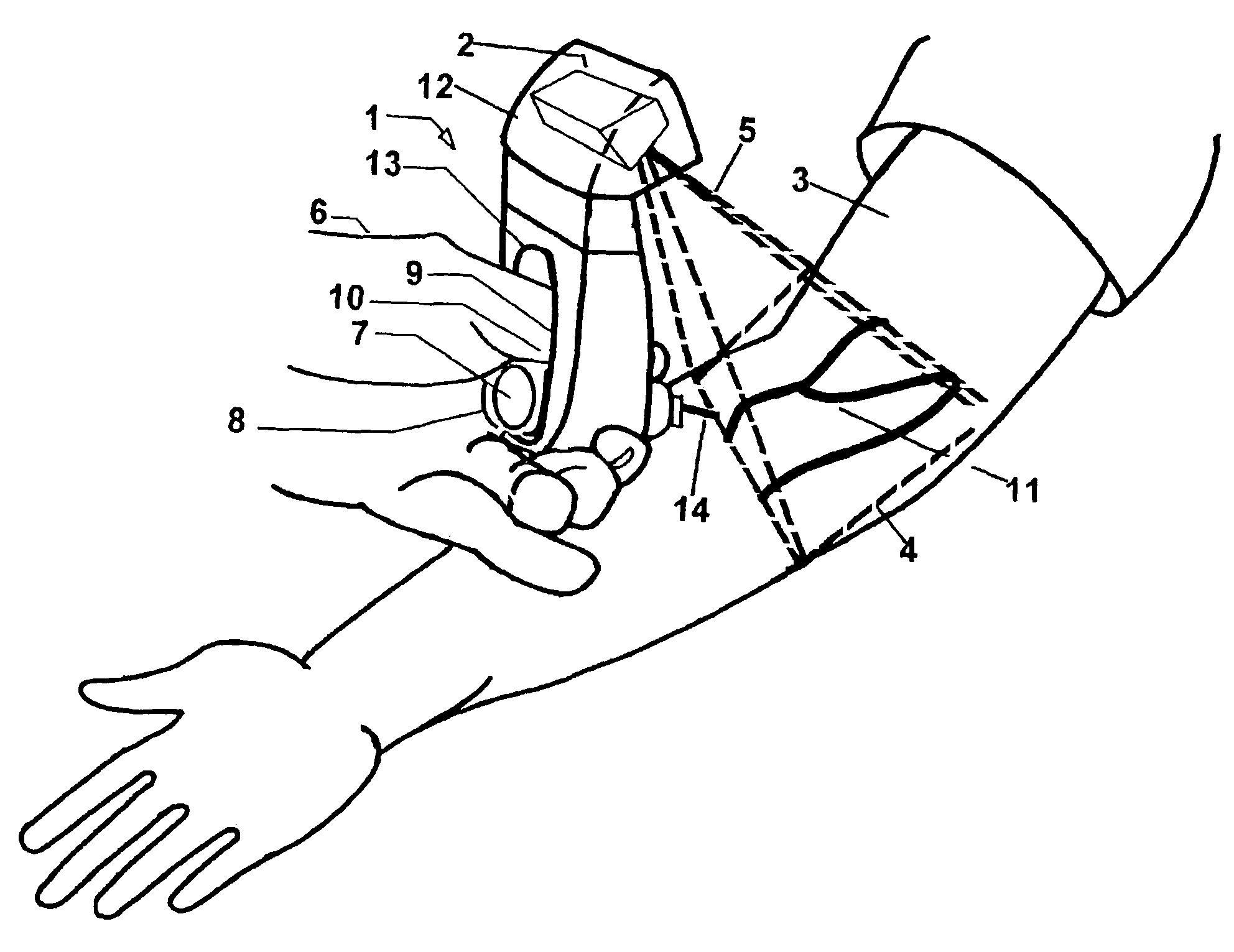
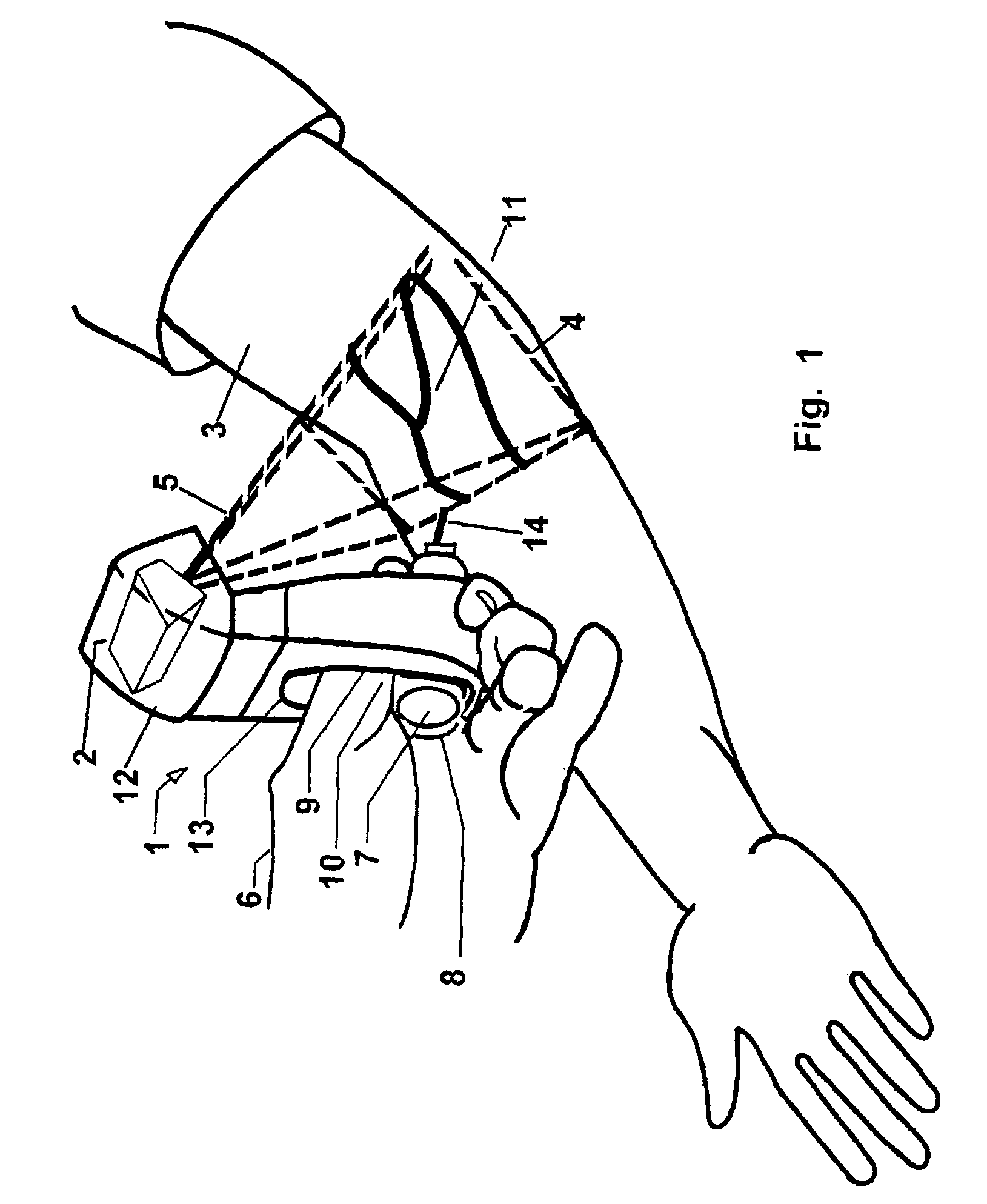
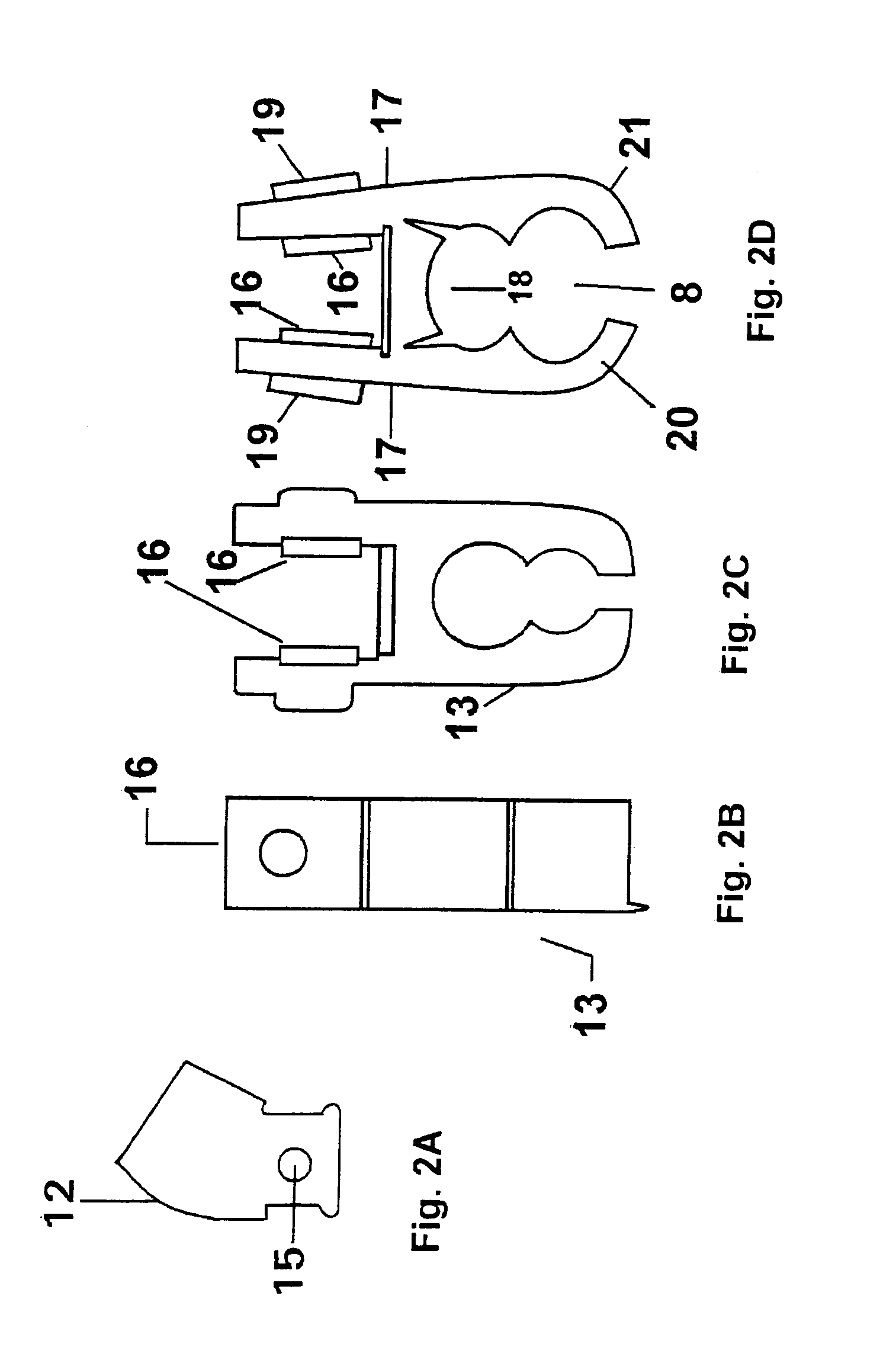
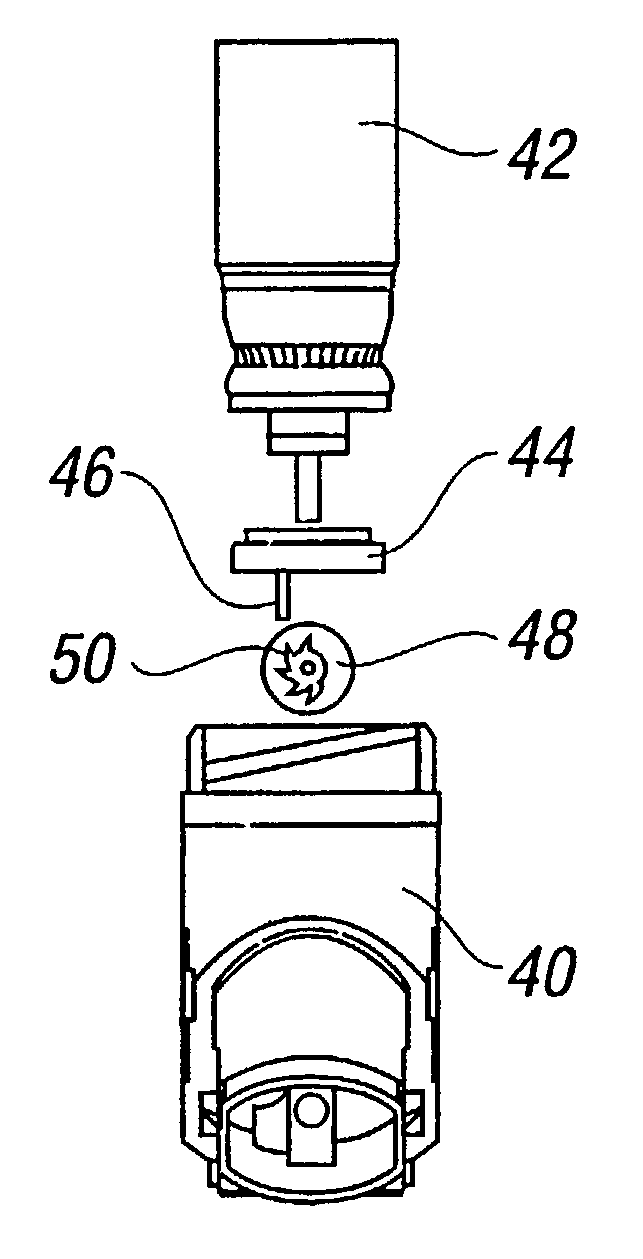
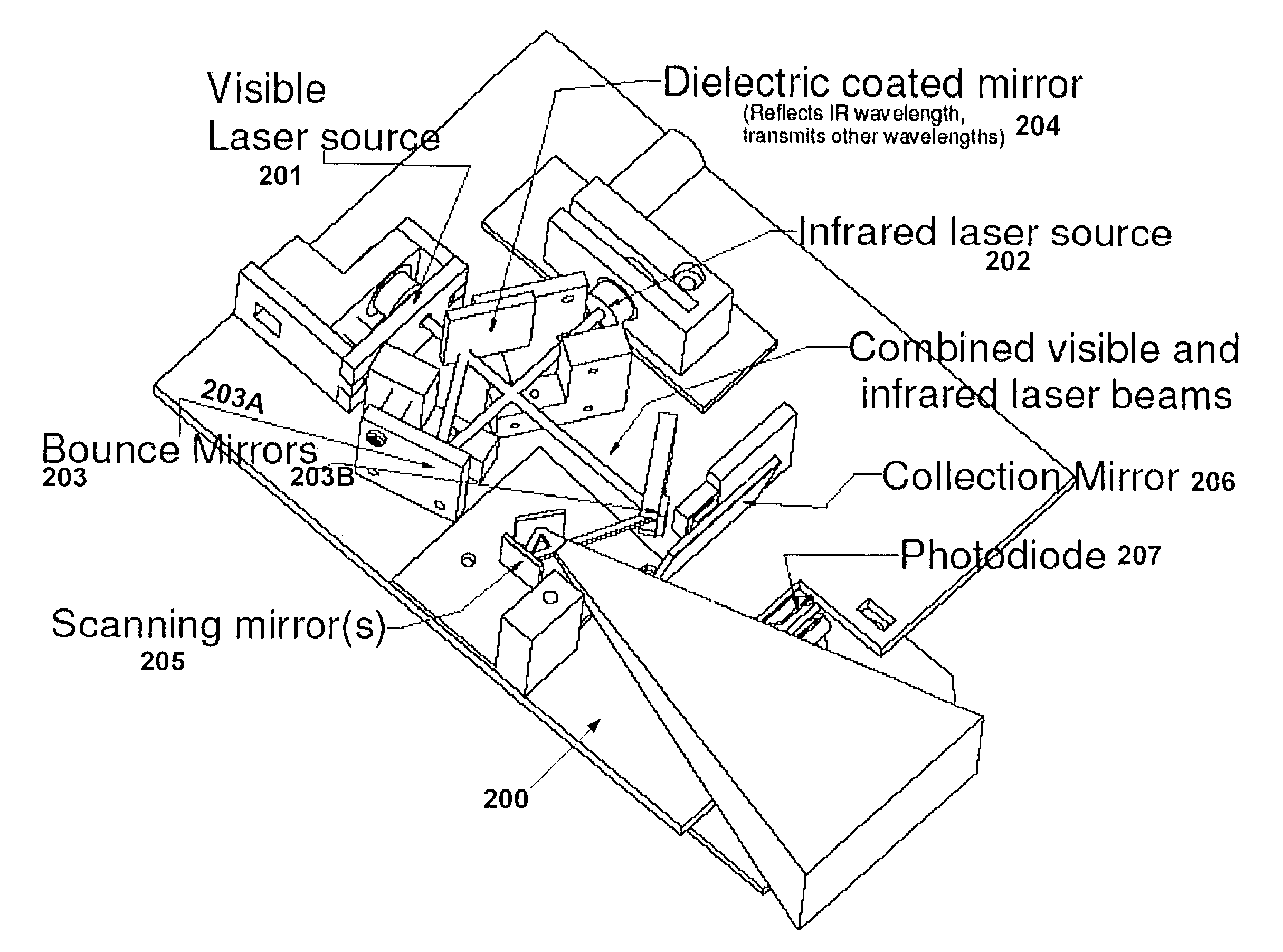
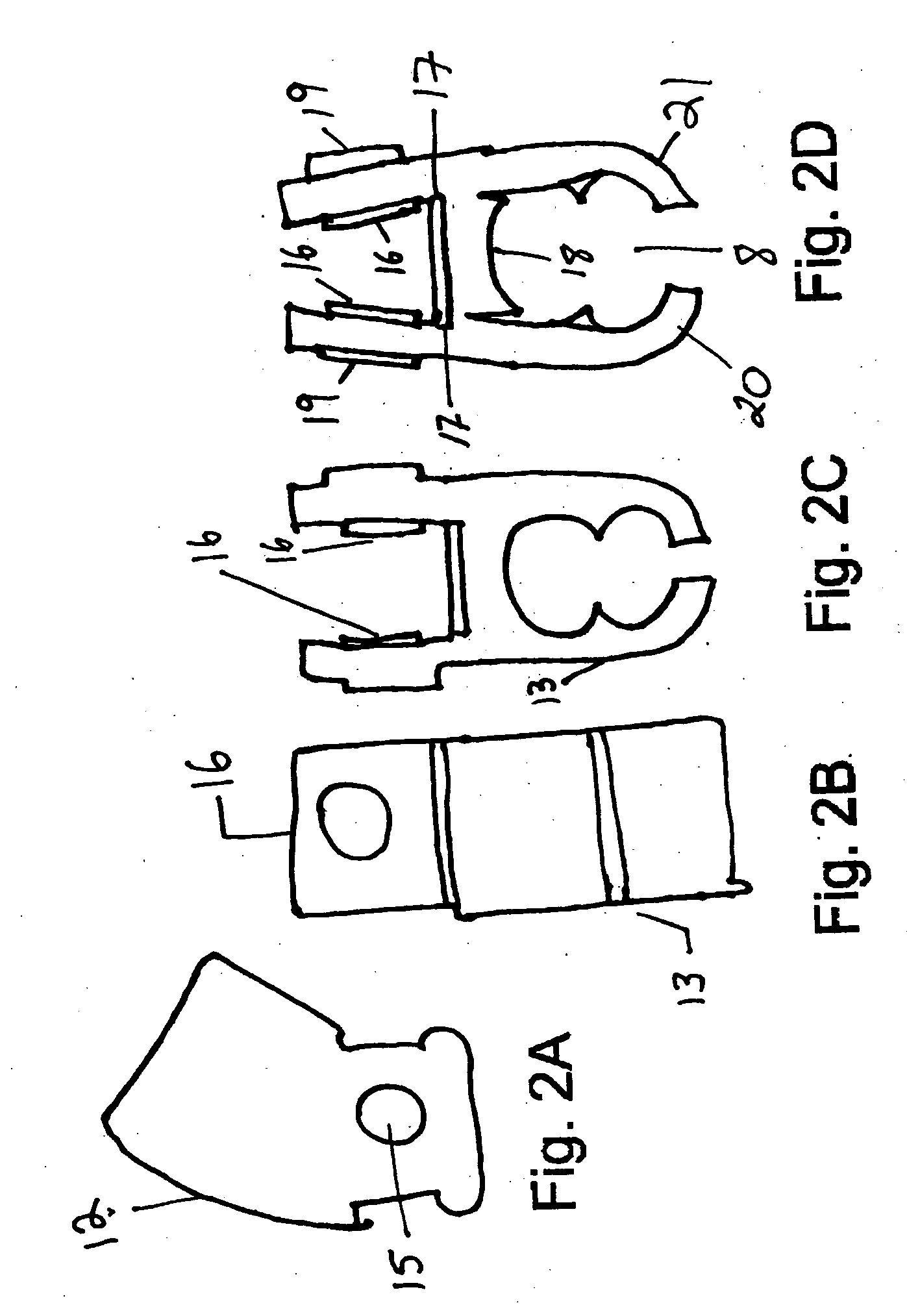
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test, and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
Scanned laser vein contrast enhancer
Owner:ACCUVEIN
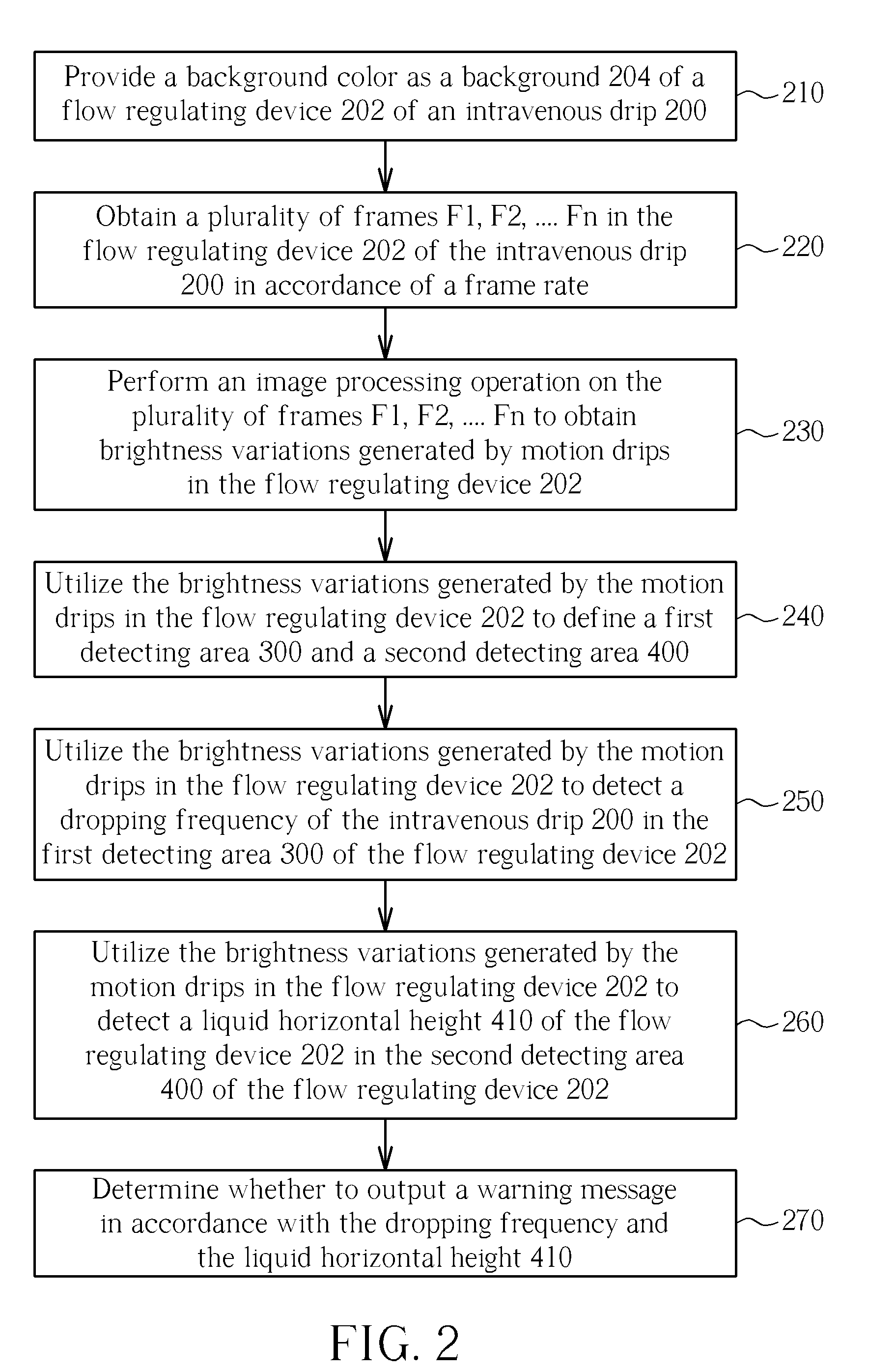
Intravenous drip monitoring method and related intravenous drip monitoring system
InactiveUS20110144595A1Improve efficiencyLow costMedical devicesFlow monitorsImaging processingMonitoring system
The present invention provides an intravenous drip monitoring method and related intravenous drip monitoring system. The intravenous drip monitoring method comprises: providing a background color as a background of a flow regulating device of an intravenous drip; obtaining a plurality of frames in the flow regulating device of the intravenous drip in accordance of a frame rate; performing an image processing operation on the plurality of frames to obtain brightness variations generated by motion drips in the flow regulating device; utilizing the brightness variations generated by the motion drips in the flow regulating device to detect a dropping frequency of the intravenous drip in a first detecting area of the flow regulating device; and utilizing the brightness variations generated by the motion drips in the flow regulating device to detect a liquid horizontal height of the flow regulating device in a second detecting area of the flow regulating device.
Owner:PRIMAX ELECTRONICS LTD
Novel pharmaceutical compositions administering n-0923
The invention relates to a pharmaceutical composition for administering the dopamine agonist N-0923 in depot form. The invention makes available for the first time a depot form of N-0923, which achieves a therapeutically significant plasma level over a period of at least 24 hours after administration to a patient. As a result of poor oral bio-availability and the short plasma half-life, N-0923 was previously administered either by an intravenous drip or by transdermal systems. Preferred embodiments of said invention are oily suspensions, containing the active ingredient N-0923 in a solid phase, in addition to anhydrous pharmaceutical preparations of N-0923.
Owner:UCB SA
Refillable device with counting means
InactiveUS7080642B2Increase equipment costSimple mechanical structureRespiratorsMedical devicesNeedle Free InjectionMedical device
A refillable medical device comprising a base unit (4) adapted to be engaged with a refill unit (2), the device comprising means for counting the number of different refill units which are engaged with the base unit (4). The device may be in the form of a dry powder or pressurised aerosol inhaler, needleless injector, intravenous drip system etc. The device may comprise means to disable the device after a predetermined number of refill units have been used with the base.
Owner:3M INNOVATIVE PROPERTIES CO
Scanned laser vein contrast enhancer
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test, and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
Scanned laser vein contrast enhancer
Owner:ACCUVEIN
Micro vein enhancer
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test, and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
Paclitaxel submicron emulsion using lipid composite as middle carrier
ActiveCN101396343AOvercome the shortcomings that are not conducive to making liquid formulationsHigh drug loadingOrganic active ingredientsEmulsion deliveryLipid formationFreeze-drying
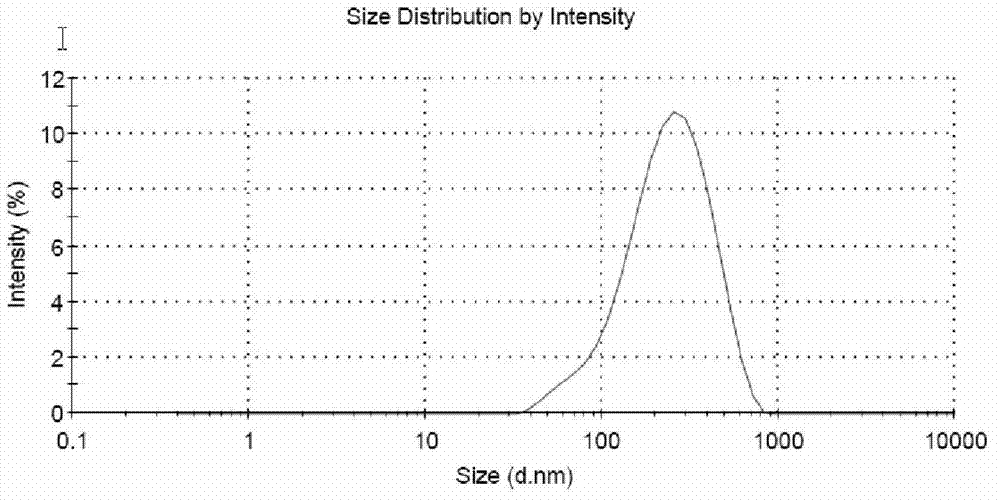
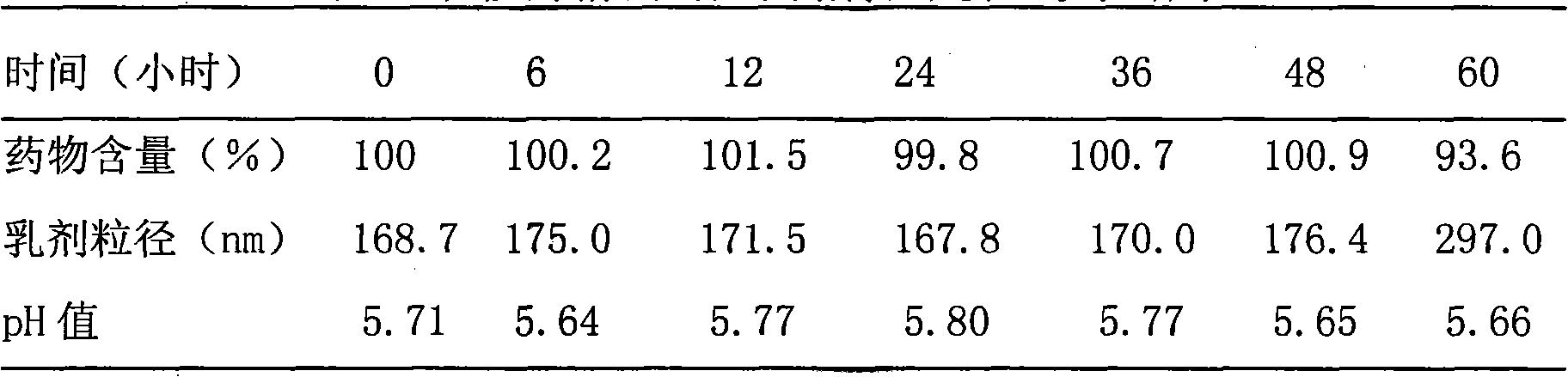
The invention discloses a paclitaxel submicron emulsion with a lipid compound as an intermediate carrier. With the paclitaxel lipid compound as the intermediate carrier, the paclitaxel is solved in oil phase, and water phase, emulsifier and auxiliary emulsifier are added. Emulsion droplet grain diameter is less than 600nm; the proportion of the oil phase and the water phase is 5 to 95 to 35 to 65; and the drug loading quantity is 0.25mg / ml to 5mg / ml if counted according to the paclitaxel. The prepared submicron emulsion has high drug loading quantity, is resistant for autoclaving and has stable quality after long time storage. The submicron emulsion can be made into intravenous drip transfusion directly, as well as into dry emulsion by a freeze drying technology, and when being used, the submicron emulsion is added with physiological saline or glucose to be diluted into intravenous drip.. The submicron emulsion uses nontoxic refined plant oil as the oil phase and phospholipid as the emulsifier, drug is coated in the oil phase, and thereby the submicron emulsion reduces the irritation and the adverse reaction of the paclitaxel preparation and has good safety.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
Refillable device with counting means
InactiveUS20030205227A1Increase equipment costSimple mechanical structureRespiratorsMedical devicesNeedle Free InjectionMedical device
A refillable medical device comprising a base unit (4) adapted to be engaged with a refill unit (2), the device comprising means for counting the number of different refill units which are engaged with the base unit (4). The device may be in the form of a dry powder or pressurised aerosol inhaler, needleless injector, intravenous drip system etc. The device may comprise means to disable the device after a predetermined number of refill units have been used with the base.
Owner:3M INNOVATIVE PROPERTIES CO
Cabazitaxel drug composition and preparation method thereof
ActiveCN103393632AMeet treatment needsImprove stabilityOrganic active ingredientsGranular deliveryZeta potentialMass ratio
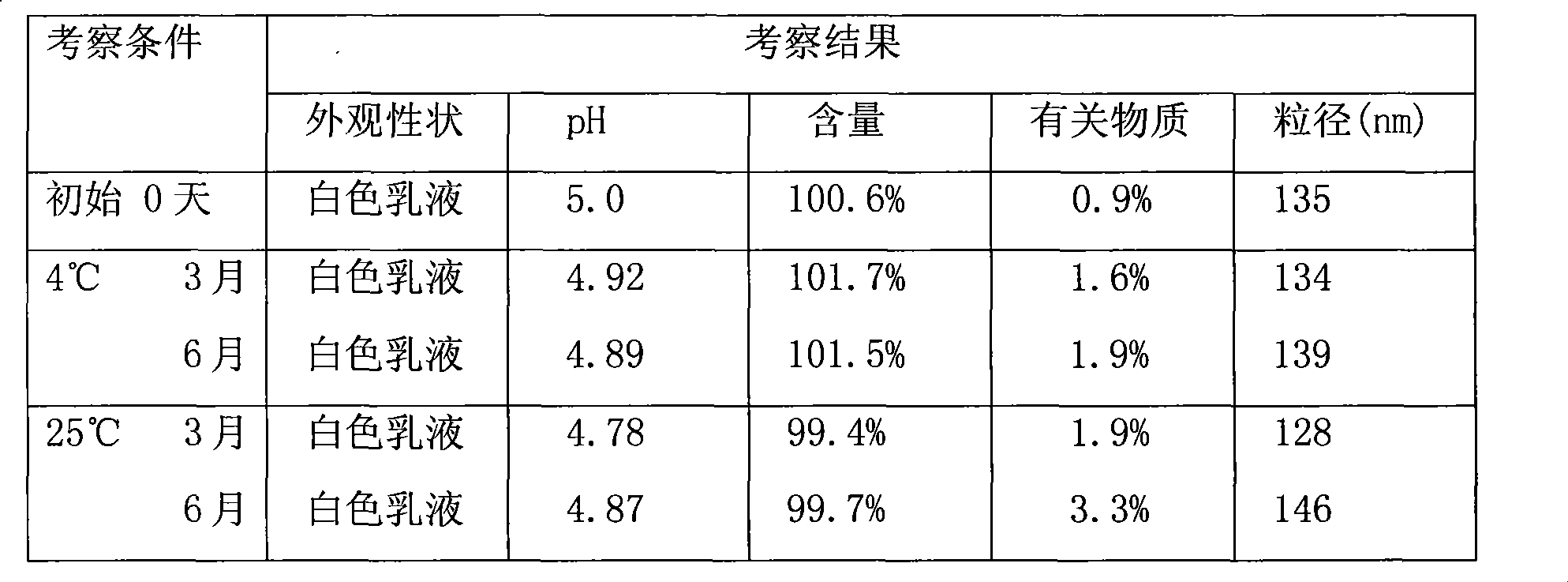
The invention provides a drug composition of cabazitaxel and a pharmaceutically acceptable biological carrier and a preparation method thereof. The cabazitaxel drug composition is actually a nanoparticle colloid dispersing system containing cabazitaxel. Cabazitaxel is encapsulated in a polymer shell made of proteins or is associated with the proteins by way of association to form nanoparticles, wherein the mass ratio of the cabazitaxel to the proteins is 1:(8-15); the pH value ranges from 5.0 to 7.0; the average diameter of the particles is not more than 200nm; the Zeta potential ranges from minus 10mv to minus 30mv; and the particles can be subjected to sterile filtration. The composition can be prepared by a high-pressure homogenating method or a protein denaturation and renaturation method. The composition prepared by the invention can be transformed to re-dispersable cakes or powder, can maintain stability for at least 48 hours at 37 DEG C after being re-dispersed in an aqueous medium, and can meet the requirements of intravenous drip therapy.
Owner:QILU PHARMA HAINAN
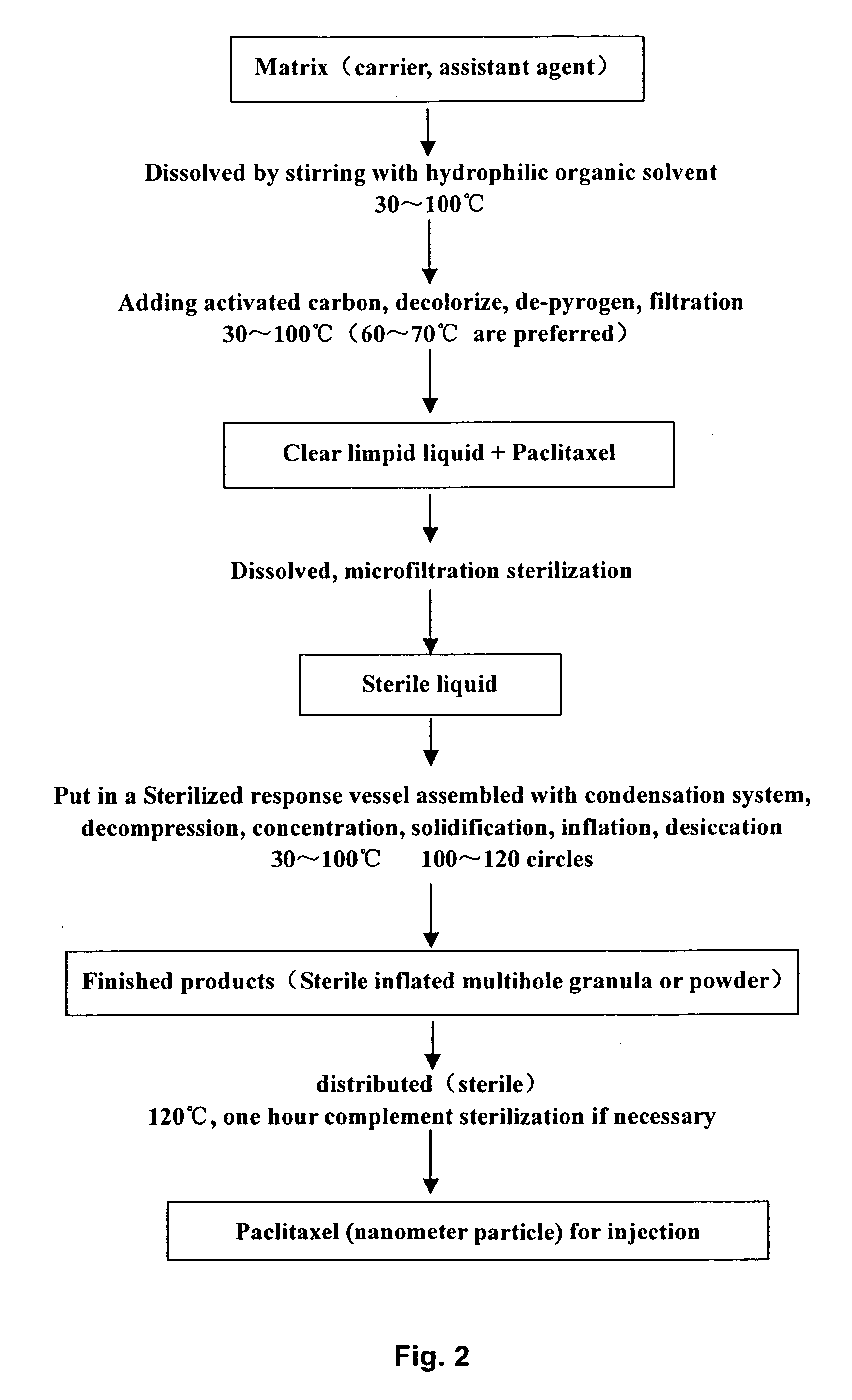
Taxanes medicine preparation for intravenous injection and preparation method thereof
ActiveCN101288642AGood biocompatibilityHigh tolerance in vivoOrganic active ingredientsSolution deliveryDrugs solutionDocetaxel
The invention relates to the technical field of medicine, which is a preparation of a taxane drug for intravenous drug delivery, consisting of two parts of drug solution and an emulsion. The drug solution is composed of paclitaxel or docetaxel, a pH regulator and a solvent for injection, wherein, the solvent for injection is an organic solvent; the emulsion comprises a fat emulsion and is composed of oil for injection, an emulsifier, an antioxidant, an isotonic regulator, a stabilizer, a pH regulator and water for injection. When in use, the drug solution can be added and evenly mixed in the emulsion for direct intravenous drip according to the clinical drug dosage and can also be firstly added in the emulsion with the volume that is not less than 5 times of the volume of the drug solution according to the clinical drug dosage and then added with a certain amount of physiological saline or glucose injection for intravenous drip. The preparation of the invention does not contain solubilizer and has the advantages of little toxicity, safety, effectivity, stability and economy. The fat emulsion can also be taken as a nutrition replenisher for a patient, thus achieving better treatment effects. The physiological saline or the glucose injection can also replace a certain amount of the emulsion, so the storage and the transportation are convenient, and the preparation is more economical.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
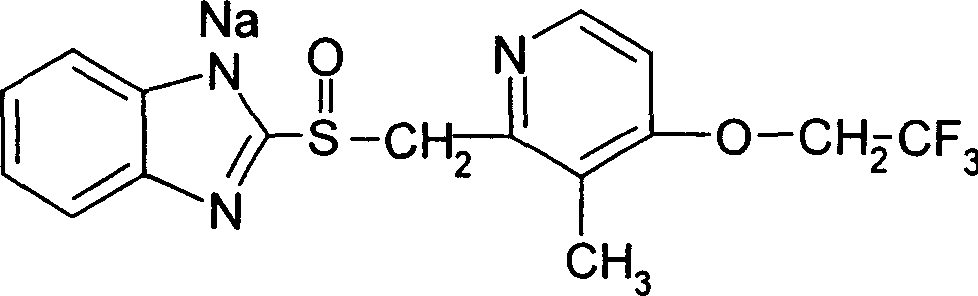
Pharmaceutical liquid composition containing pyridone derivative
A pharmaceutical liquid composition containing the Pirfenidone in a very high concentration of more or less 25% by weight can be obtained by dissolving the Pirfenidone in diethylene glycol monoethyl ether. Even when the liquid medicinal compositions are stored for a long period of time, the Pirfenidone will not be recrystallized with a good chemical and physical stability. Furthermore, the liquid compositions are little irritating to the wounds on the mucous membrane of the skin and suitable for the manufacture of pharmaceutical formulations to be administered either via the oral, percutaneous, nasal or vaginal routes or by means of spray, patch, inhalation, injection or intravenous drip.
Owner:KDL
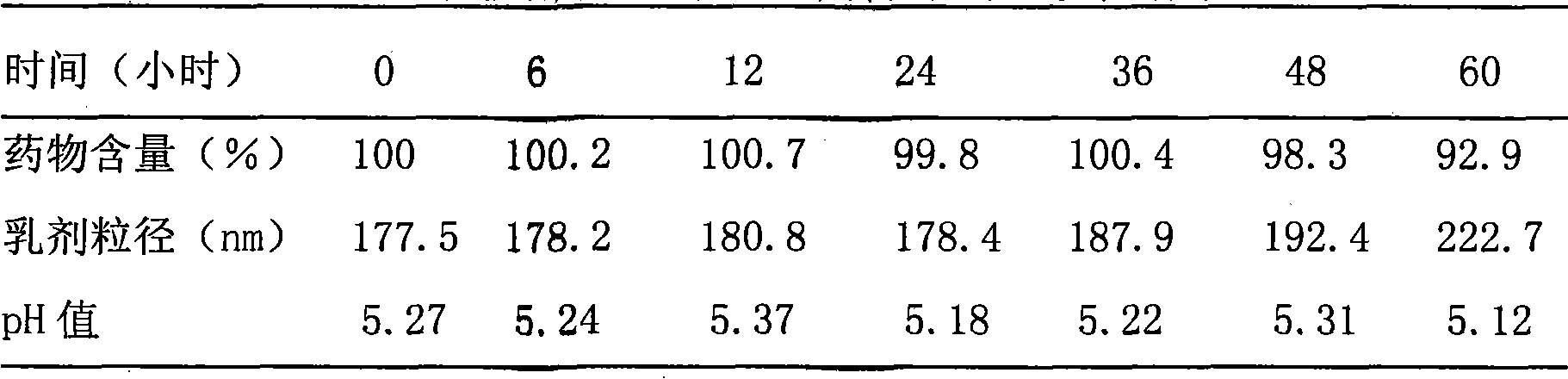
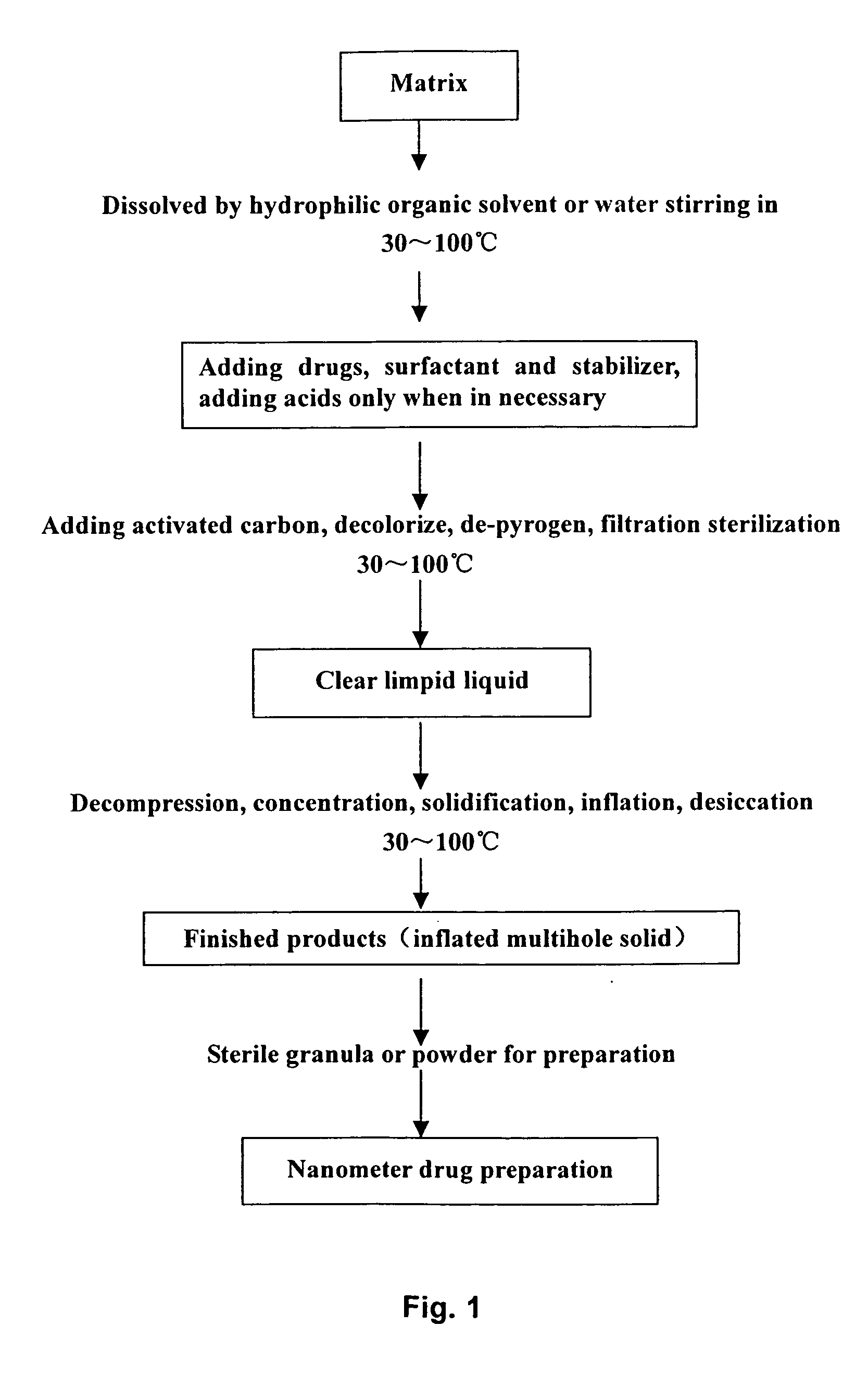
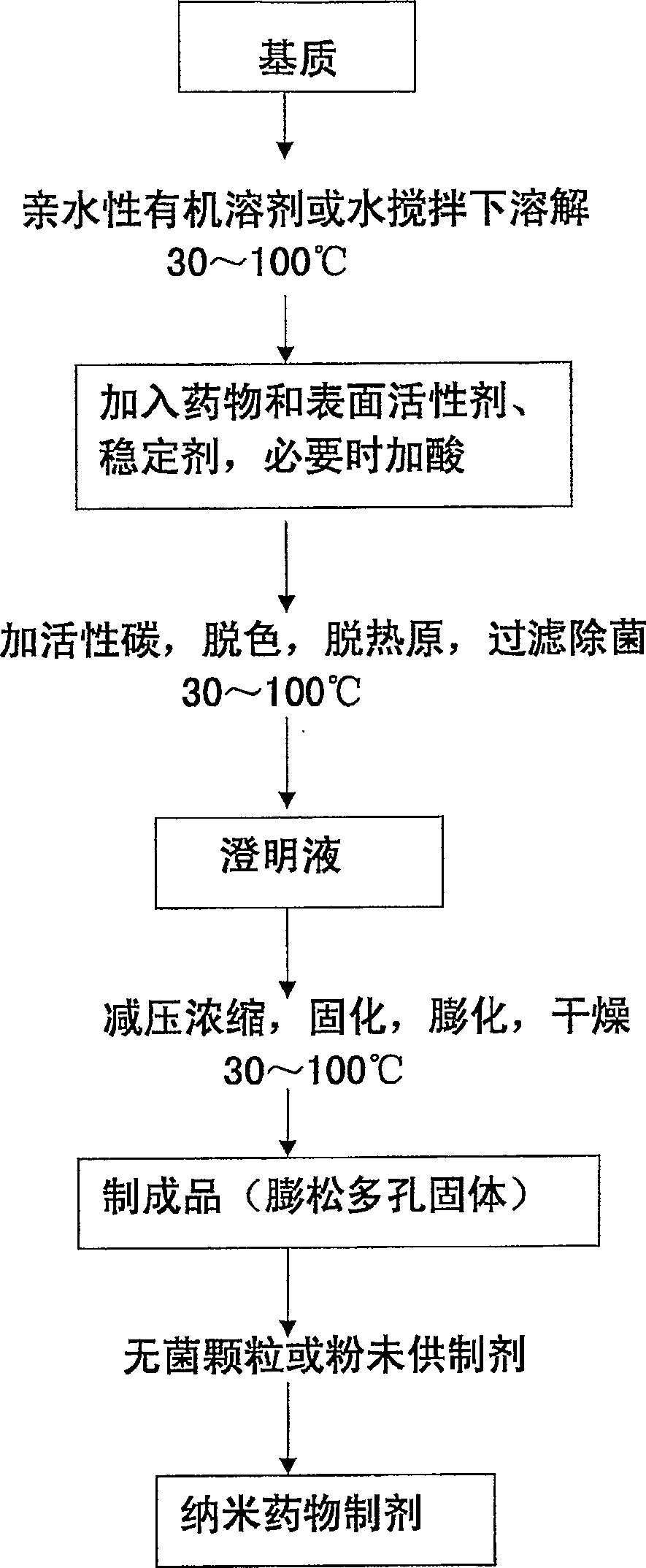
Solid nano pharmaceutical formulation and preparation method thereof
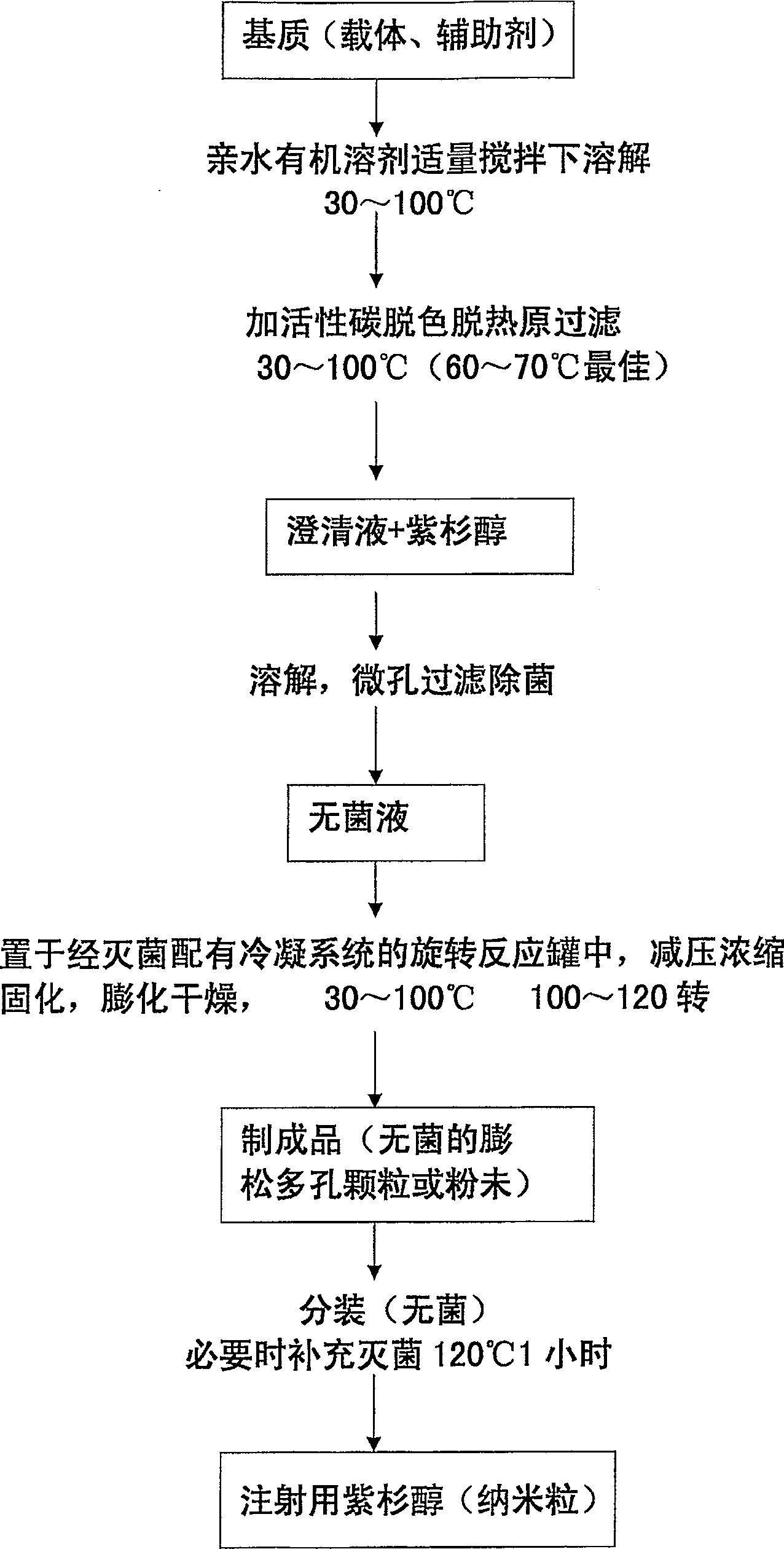
A method of preparing low water-soluble medicine into solid nanometer pharmaceutical formulation is disclosed. According to the characters of molecular aggregates such as supramolecular chemical micelles and vesicles, the formulation, which based on the hydroxypropyl-beta-cyclodextrin and phospholipid, is prepared under the condition of hyperthermia sterilization and decompression. Such nanometer formulation is sterile particle or powder with loose porosity. For directly intravenous use, the formulation has targeting activity, sustained release and long circulating characters. While as a solid oral product, it is fast-release, fast-effective, and improved bioavailability characters, and is readily melted in mouth. The formulation utilizes secure accessories, traditional equipments and methods, thus, it is suited to be used and manufactured widely. Also disclosed is intravenous formulation of anticancer paclitaxel, which characterized that there has no polyoxyethylenated castor oil in it. Such intravenous formulation is nonallergic so that it has higher security and efficiency compared to present commercially available paclitaxel formulations.
Owner:LIU YUNGING +3
Micro vein enhancer
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test, and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
Micro vein enhancer
ActiveUS20070161908A1Reduce in quantityEasy to operateImage analysisDiagnostics using lightVeinBlood test
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test, and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
Scanned Laser Vein Contrast Enhancer
The present invention is a Miniature Vein Enhancer that includes a Miniature Projection Head. The Miniature Projection Head may be operated in one of three modes, AFM, DBM, and RTM. The Miniature Projection Head of the present invention projects an image of the veins of a patient, which aids the practitioner in pinpointing a vein for an intravenous drip, blood test and the like. The Miniature projection head may have a cavity for a power source or it may have a power source located in a body portion of the Miniature Vein Enhancer. The Miniature Vein Enhancer may be attached to one of several improved needle protectors, or the Miniature Vein Enhancer may be attached to a body similar to a flashlight for hand held use. The Miniature Vein Enhancer of the present invention may also be attached to a magnifying glass, a flat panel display, and the like.
Owner:ACCUVEIN
Edaravone-containing injection
InactiveCN101732247AReduce the amount of infusionSuitable for useOrganic active ingredientsInorganic non-active ingredientsHigh concentrationEdaravone Injection
The invention relates to an edaravone-containing injection, which belongs to the field of pharmaceutical preparation. The edaravone-containing high-concentration injection consists of 1 to 5 percent (w / v) of edaravone, 5 to 25 percent (v / v) of 1,2-propanediol, 5 to 15 percent (v / v) of ethanol and water for injection, and has a pH of 4.5 to 5.5. The edaravone-containing injection in clinical use is added to sodium chloride infusion solution for the intravenous drip of patients, increases the population of applicable patients, and is applicable to the patients with acute cerebral infarction.
Owner:湖南万健康品生物科技有限公司
Intravenous drip set controller with flusher
ActiveUS20090312719A1Simple and efficient flushing mechanismFacilitate an easy, efficient and regular flushing procedureDiaphragm valvesEngine diaphragmsLine tubingEngineering
Owner:IM HIGH TECH LTD
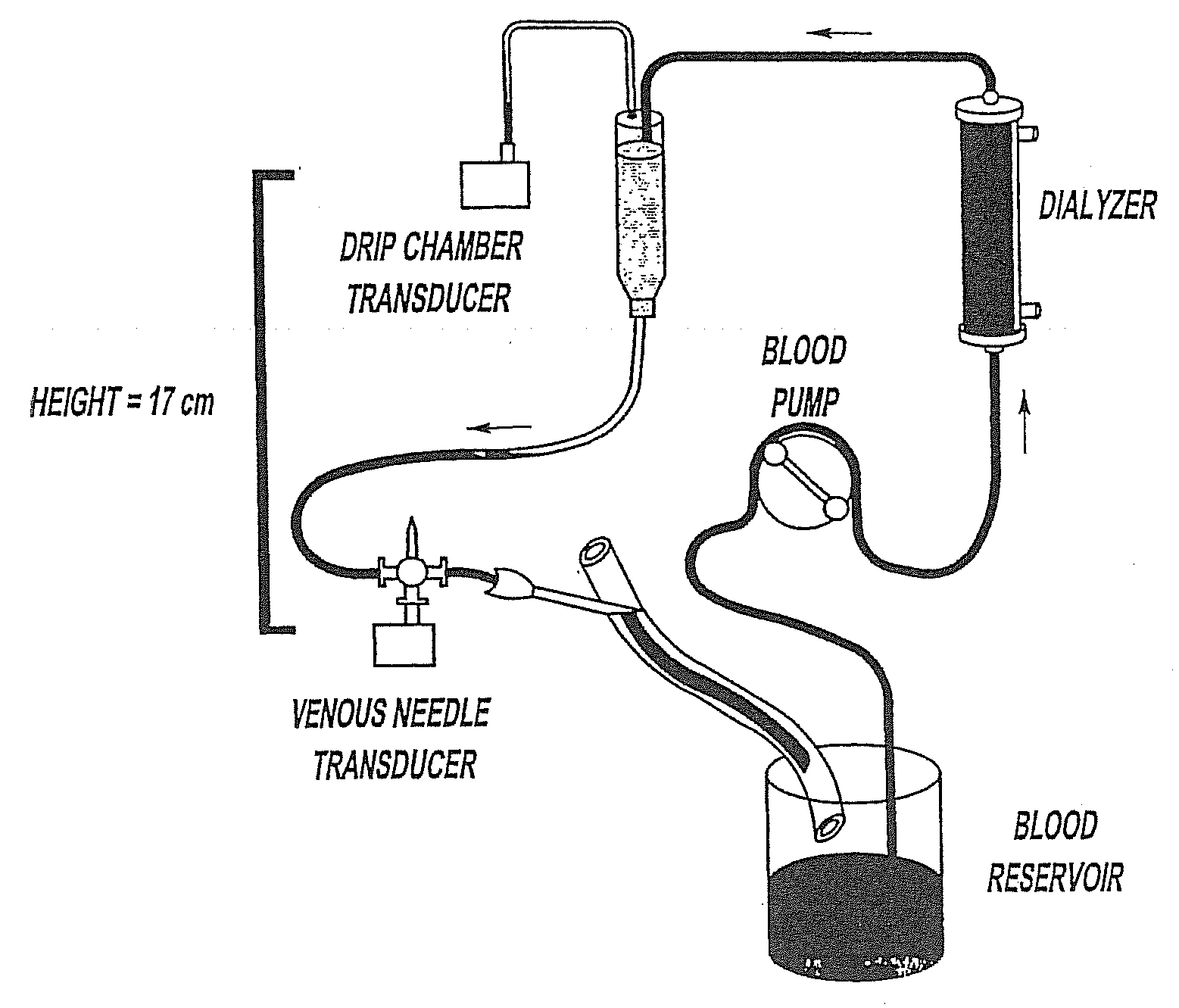
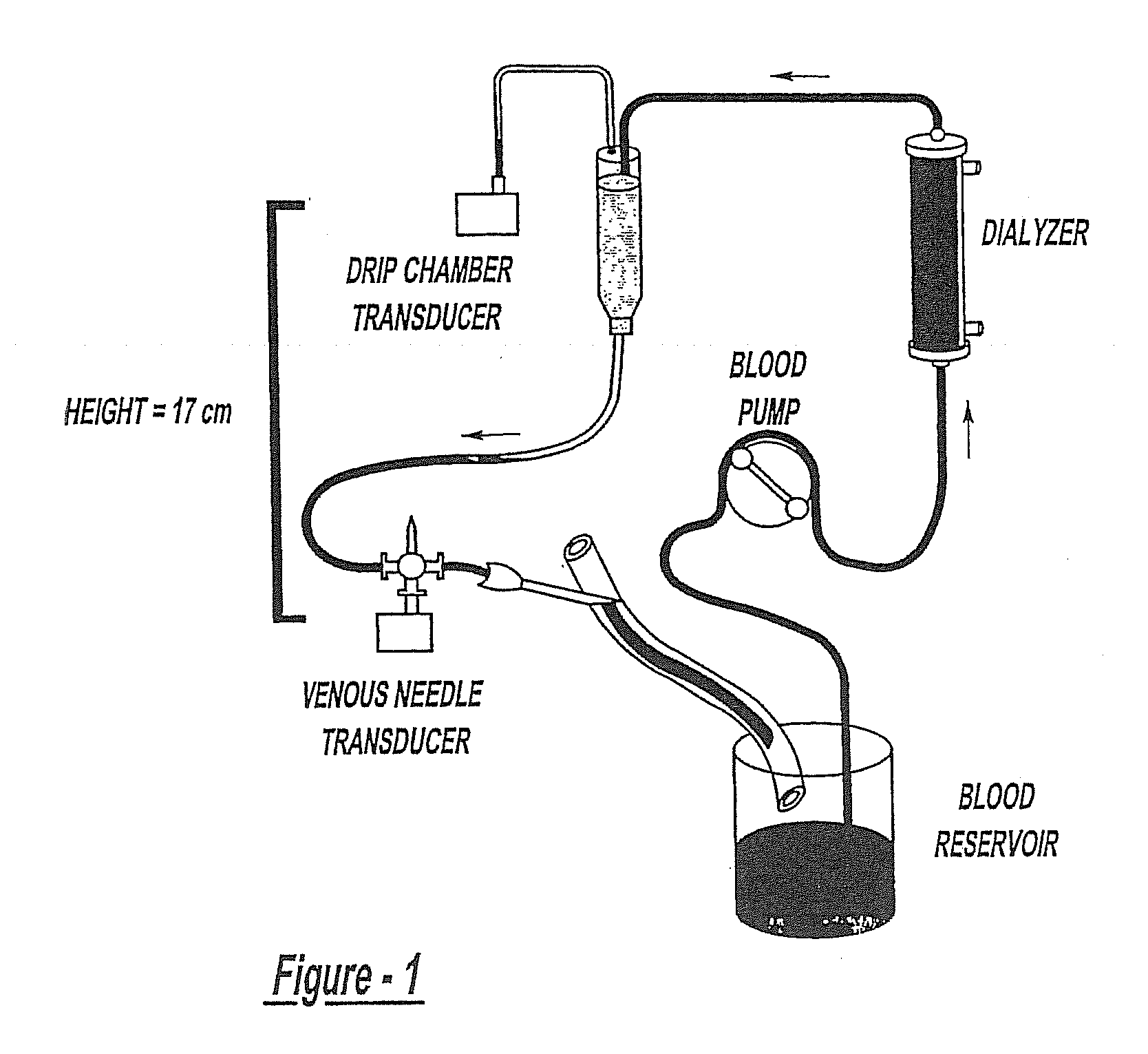
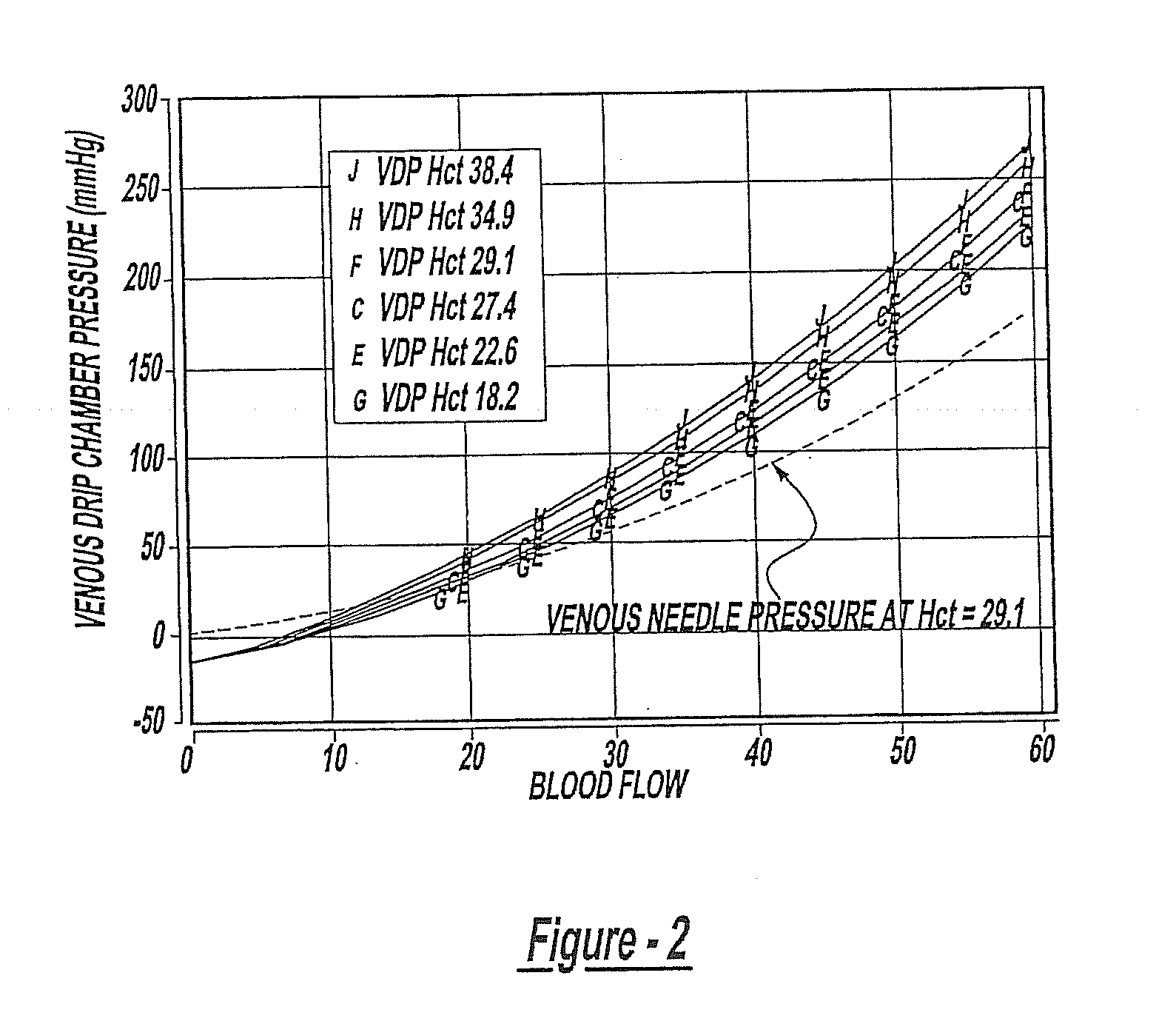
Method of Monitoring Dislodgement of Venous Needles in Dialysis Patients
A method of detecting a dislodged needle in a hemodialysis procedure includes measuring venous drip pressure of the dialysis machine of a patient undergoing hemodialysis, analyzing the venous drip pressure and deriving intravascular blood pressure at a location of venous needle insertion into the patient, comparing the derived intravascular blood pressure to a standard, repeating the measuring, analyzing and deriving, and comparing steps and, if the intravascular blood pressure is within a specified range of the standard, determining that a needle has been dislodged in the hemodialysis procedure. A method of alerting the patient and medical personnel of a dislodged needle in a hemodialysis procedure includes detecting a drop in intravascular pressure derived from measured venous drip pressure, determining that a needle is dislodged, and alerting medical personnel of the dislodged needle.
Owner:HENRY FORD HEALTH SYST
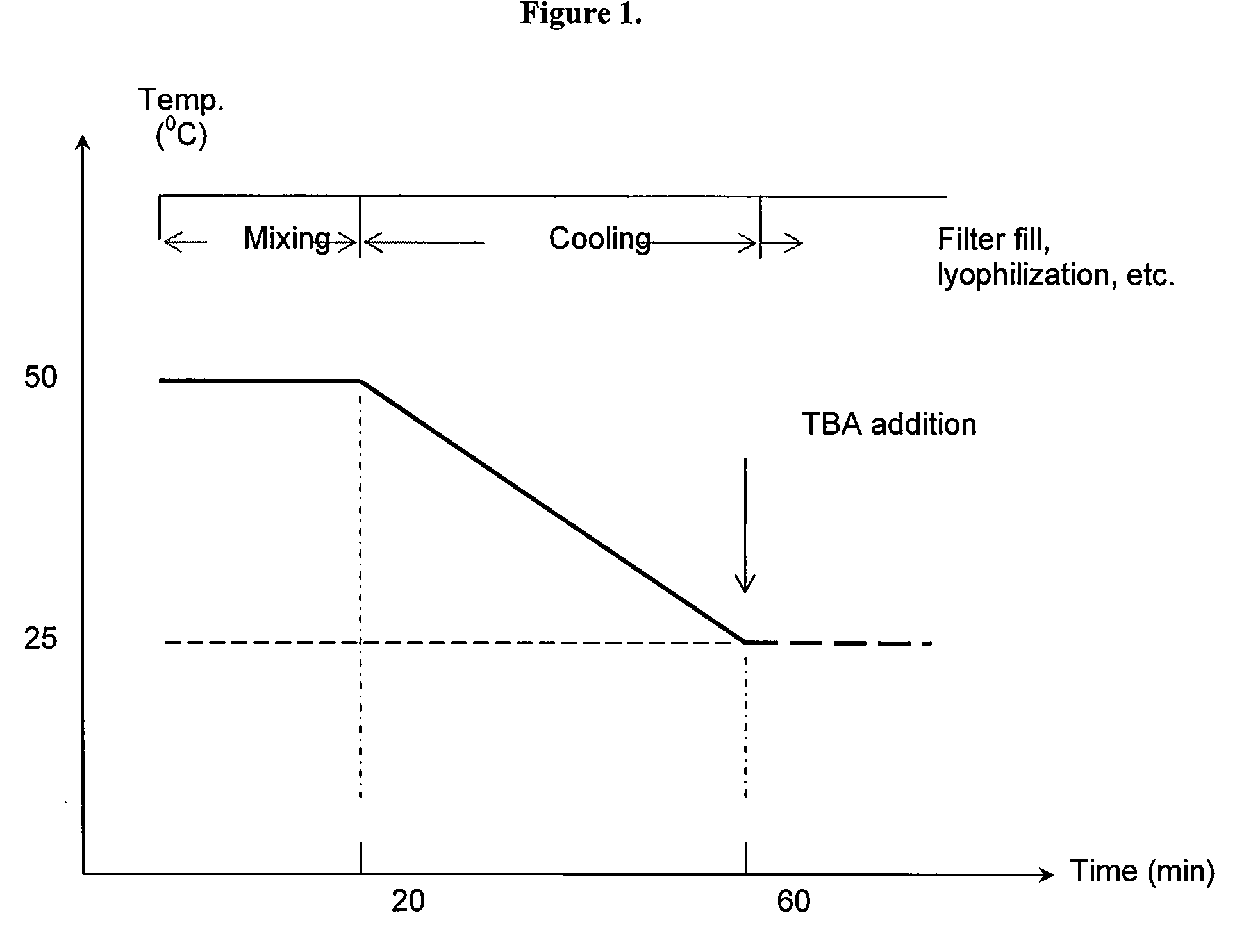
Co-Solvent Compositions and Methods for Improved Delivery of Dantrolene Therapeutic Agents
The present invention provides for methods of using tert-butyl alcohol (TBA) co-solvent systems in the formulation and production of a pharmaceutical agent with low solubility. The present invention also provides for pharmaceutical compositions made using the novel co-solvent system. In one embodiment, the invention provides for a method of making dantrolene sodium (DS) formulation for intravenous use (DS-IV). This instantaneous reconstitution of the DS-IV product constitutes a significant improvement in the pharmacotherapy of patients undergoing malignant hyperthermia during surgery.
Owner:US WORLDMEDS
Culture method, application and combined preparation of hypoxia mesenchymal stem cell
InactiveCN101792737AHigh activityImprove survival rateOrganic active ingredientsMammal material medical ingredientsCell massTherapeutic effect
The invention discloses a culture method, application and a combined preparation of a hypoxia mesenchymal stem cell. By adopting an umbilical or placenta mesenchymal stem cell and combining the means of transfusing compound amino acid injection and the like, the vitality, the implantation survival rate and the treating effect of the mesenchymal stem cell in vivo can be improved; and in order to avoid the possibility of conglutination, conglomerating and cell mass embolism of the mesenchymal stem cell in blood vessel, the matching of saline containing heparin during treatment can be carried out by a mode of intravenous injection or intravenous drip. With more than three times of treatment, physical conditions of curee can be remarkably improved and objective indicators such as immunity, blood fat, blood sugar and the like can be remarkably improved.
Owner:晏泽
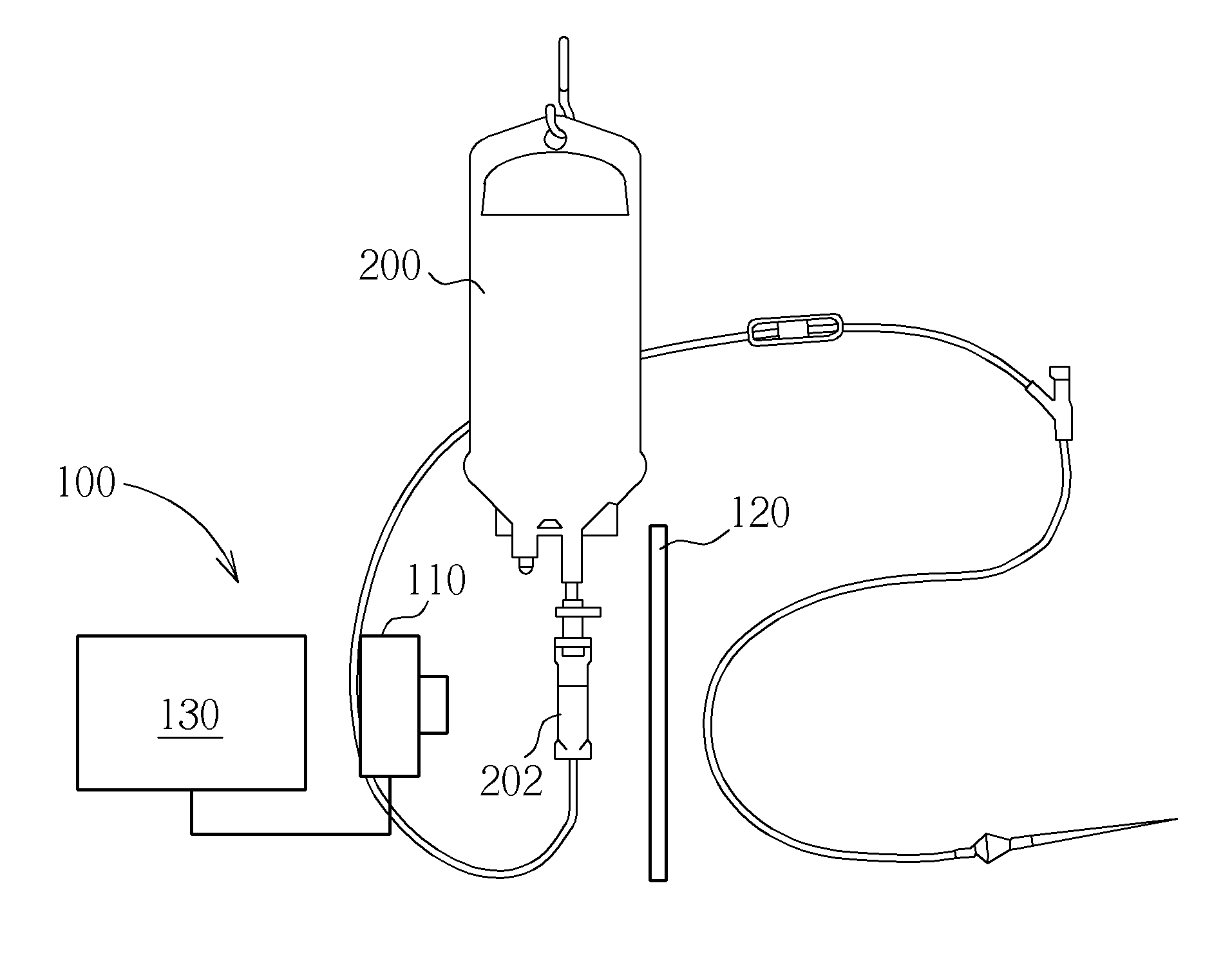
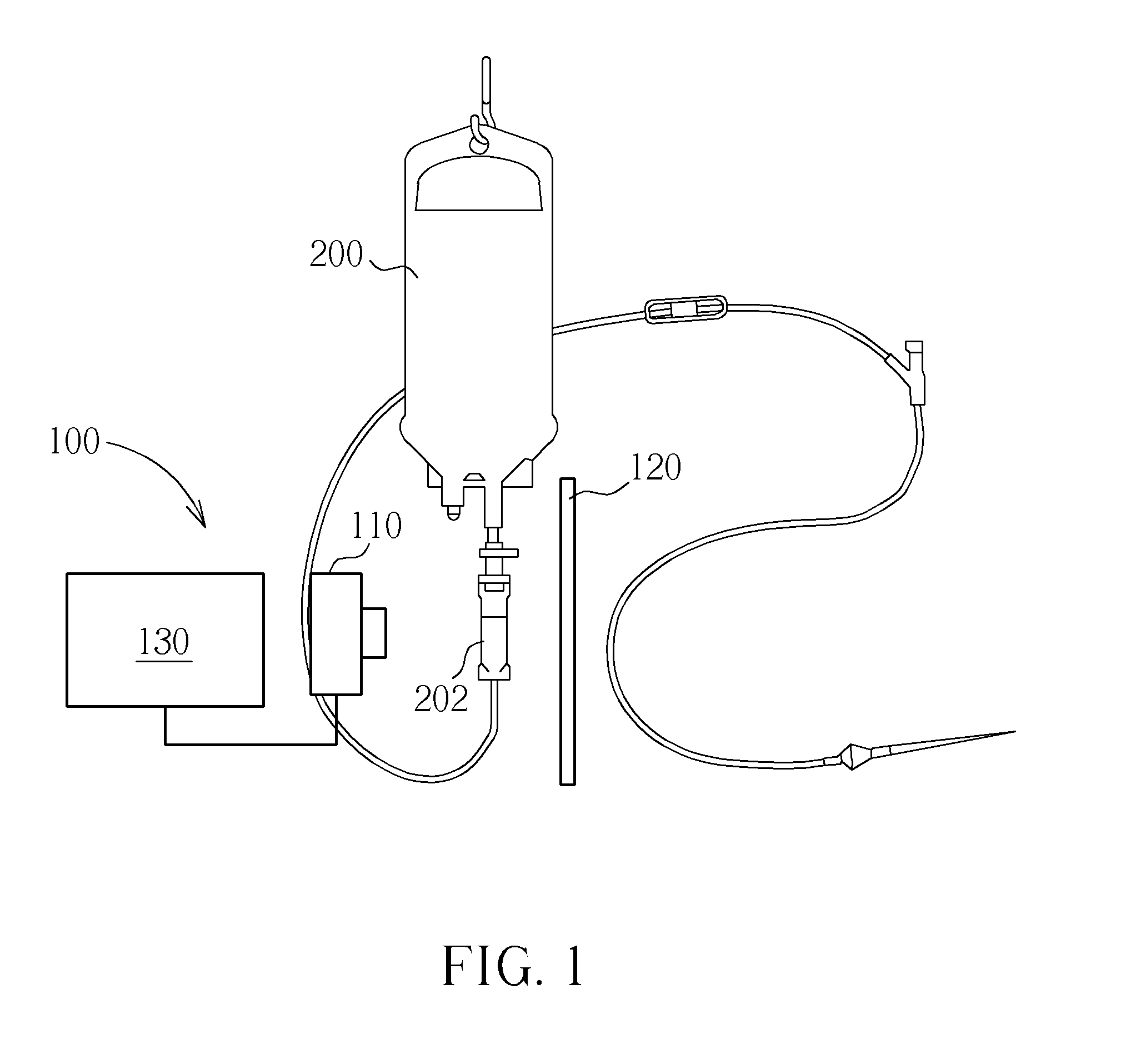
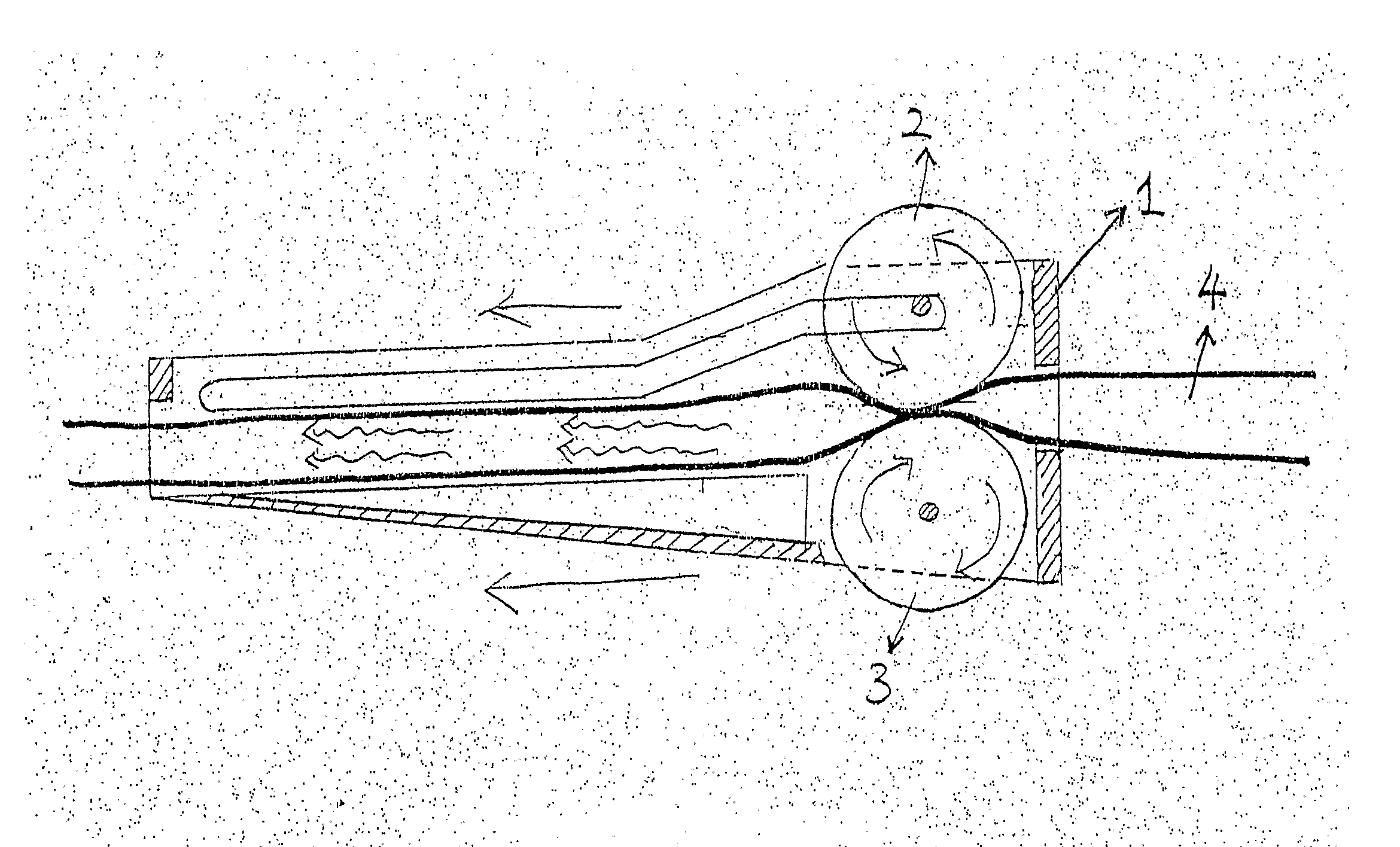
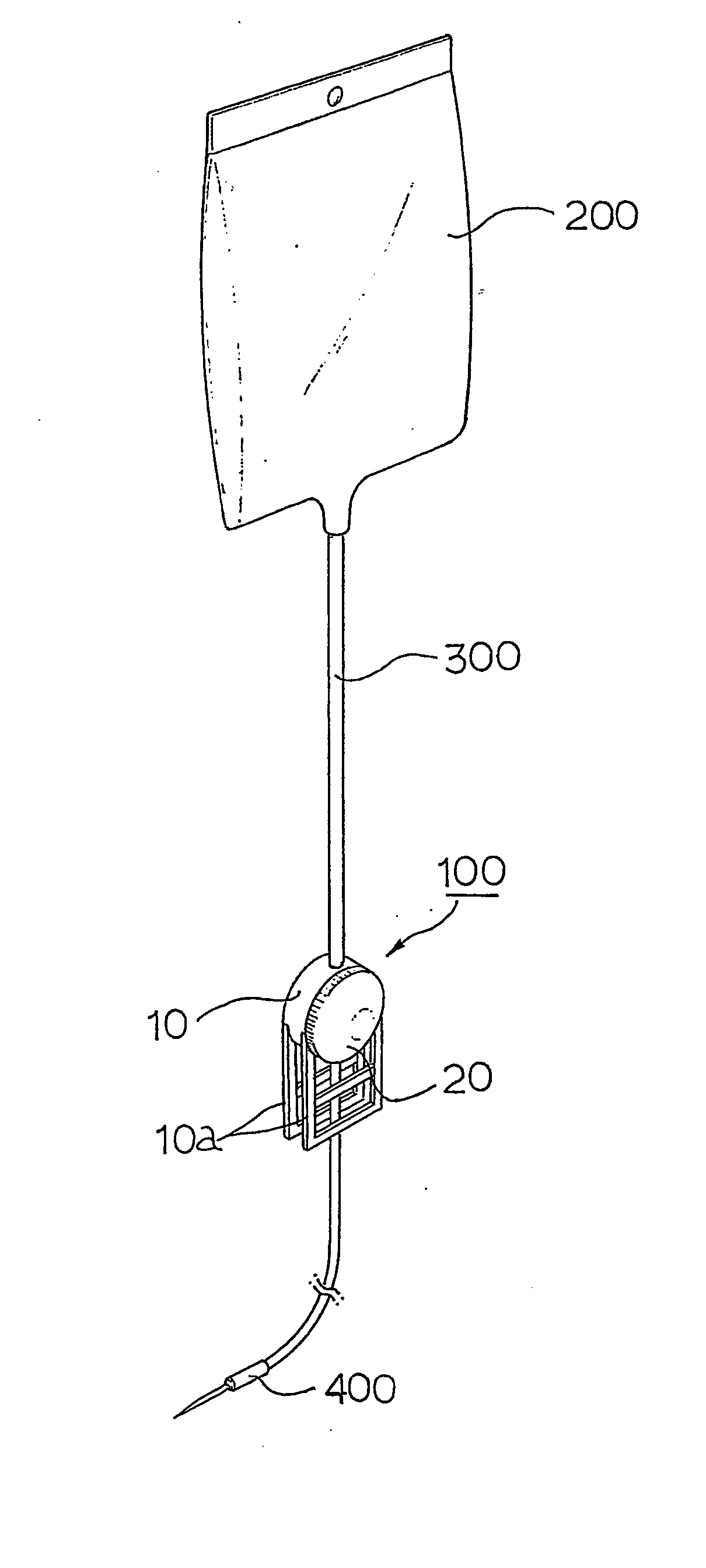
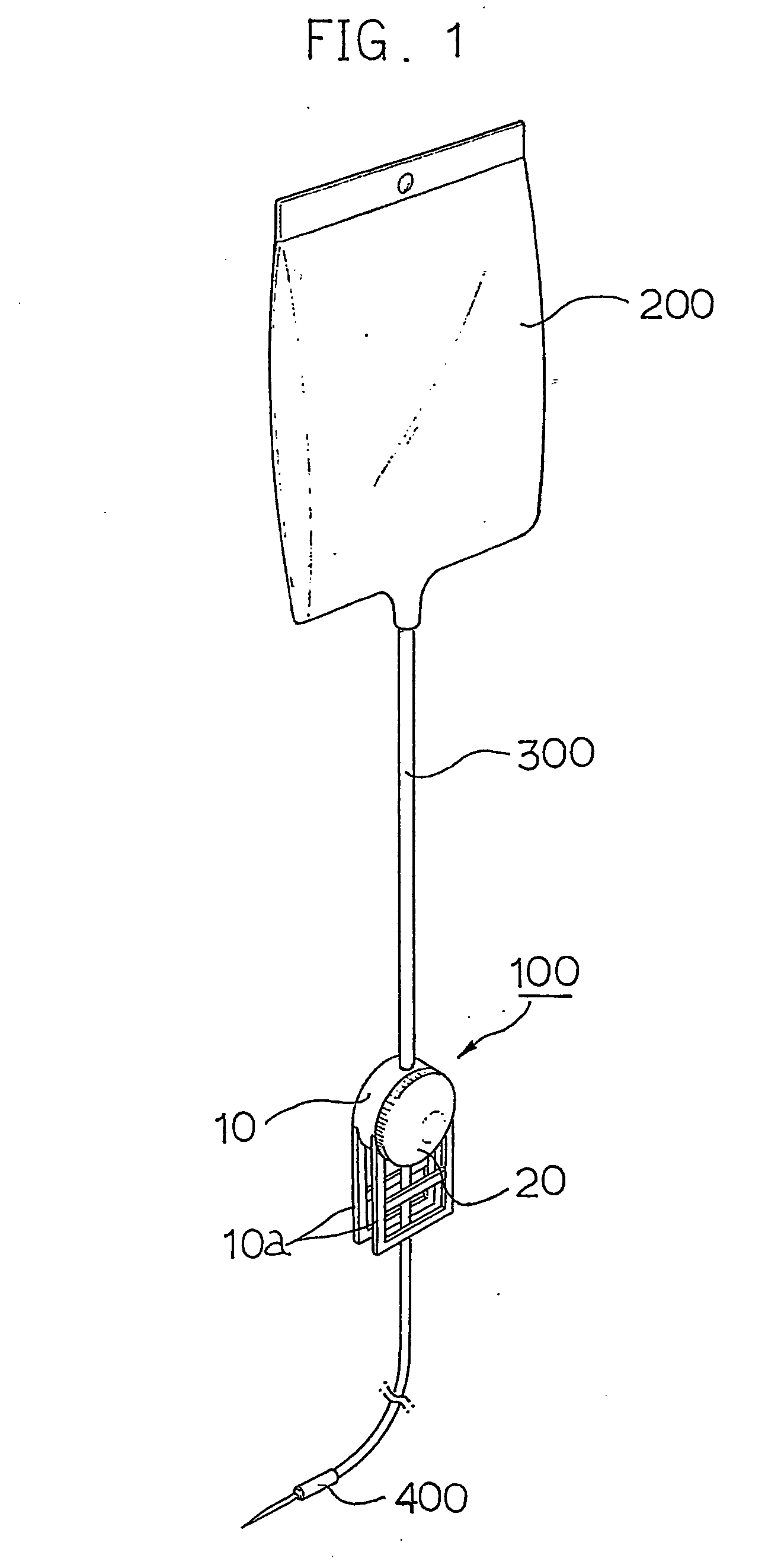
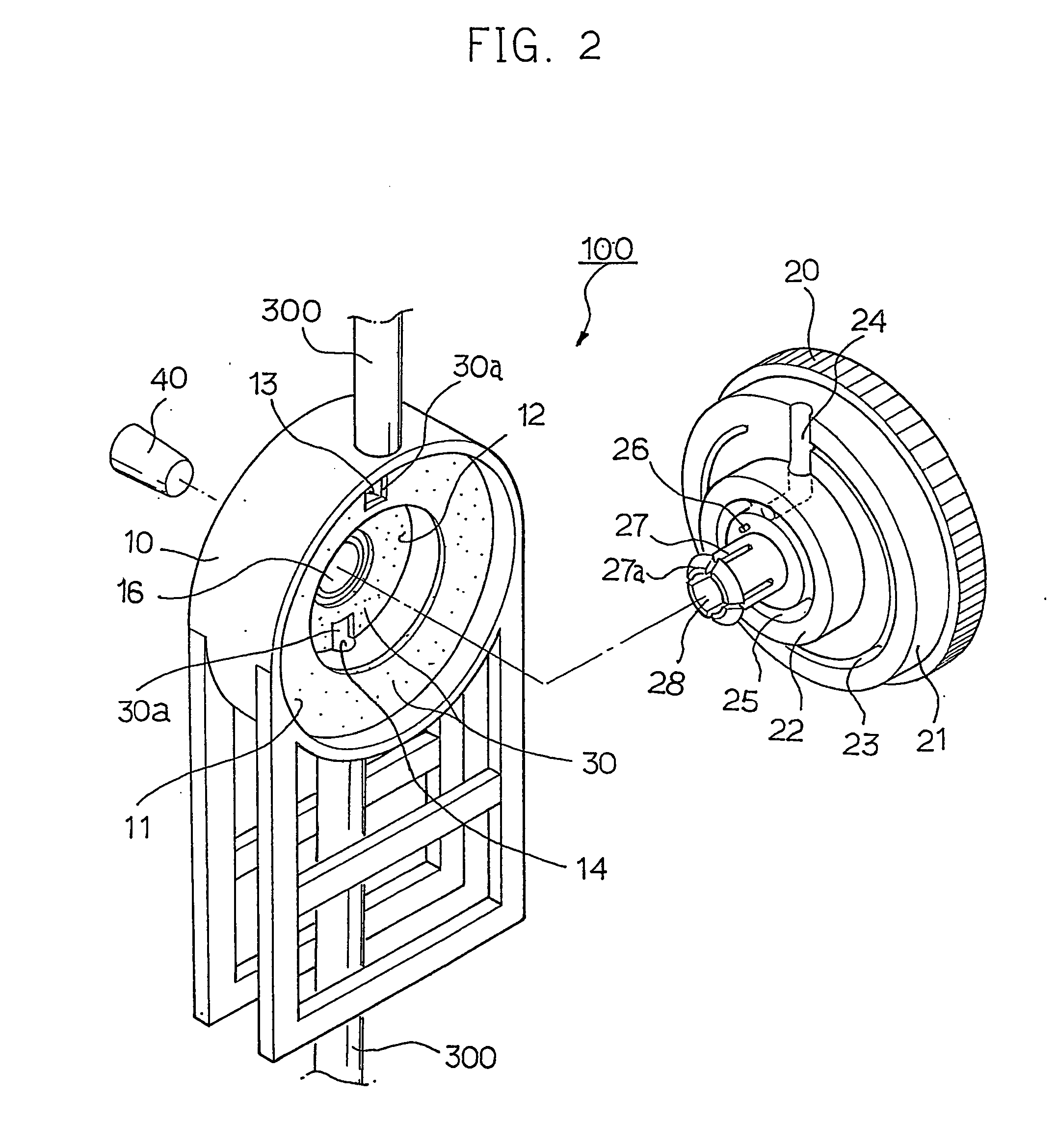
Device for regulating flow rate of intravenous medical solution during injection
The object of this invention is to provide a device for regulating the flow rate of an intravenous medical solution during an injection using an intravenous drip unit. The device has a housing (10) mounted to a hose (300) to allow the flow of the solution from a solution container (200) into the vein, and a control member (20) rotatably assembled with the housing to regulate the flow rate of the solution. The housing has first and second seats (11, 12) communicating with inlet and outlet ports, and the control member has first and second bosses (21, 22) seated in the first and second seats. An arc-shaped solution path (23), a radial channel (24) and an annular groove (25) are formed on the control member to regulate the flow rate of the solution in accordance with an adjusted angle of the control member relative to the fixed housing.
Owner:MAINTECH
Freeze dried Lansoprazole sodium injection and its prepn process
InactiveCN1810244AAvoid destructionImprove efficacyOrganic active ingredientsPowder deliveryDiseaseFreeze-drying
The freeze dried Lansoprazole sodium sdinjection has Lansoprazole sodium as active component and mannitol or meglumine as excipient in the weight ratio of 15-30 g to 5-50 g. The preparation process of the freeze dried Lansoprazole sodium injection includes the following steps: dissolving Lansoprazole sodium in 15-30 g in injection water of 200-400 ml, dissolving mannitol or meglumine as excipient in 5-50 g in injection water of 100-300 ml, mixing the two kinds of obtained solutions and adding injection water to 2000-3000 ml, adding active carbon in 0.5-1.5 g, filtering with microporous filter membrane, packing in vial, freeze drying under bacteria-free condition, and pressing cap. The freeze dried Lansoprazole sodium injection is used clinically in intravenous drip or intramuscular injection to treat peptic ulcer and other diseases.
Owner:JINZHOU JIUTAI PHARML CHINA
Intravenous drip automatic monitoring system
InactiveCN103800968AAffect restNo manual processing requiredIntravenous devicesFlow controlDrugs solutionMonitoring system
The invention discloses an intravenous drip automatic monitoring system, and belongs to the field of medical apparatus and instruments. The intravenous drip automatic monitoring system aims to solve the problems that an existing device for generating radially polarized beams and azimuthally polarized beams is complex in structure and high in cost. The intravenous drip automatic monitoring system comprises an upper computer, k nurse terminals and m*k intravenous drip control devices. When each patient is on an intravenous drip and needs to be infused with n sets of drug solutions in sequence, the n sets of drug solutions are placed in n sets of drug solution containers respectively, and the n sets of drug solution containers are communicated with an intravenous drip pipe through n-1 three-way valves; each intravenous drip control device is used for monitoring the intravenous drip state of the corresponding patient and sending the intravenous drip finishing state of the patient to the upper computer and the corresponding nurse terminal which is in charge of the intravenous drip control device; each nurse terminal is in charge of monitoring the m intravenous drip control terminals, and all the intravenous drip control terminals communicate with the upper computer. The intravenous drip automatic monitoring system has the advantages that nurses can be automatically informed of the intravenous drip state, blood return can be automatically avoided, special accompanying relatives are not needed, and rest of the patients is not affected.
Owner:HARBIN BO QIANG ROBOT TECH CO LTD
Solid nano-medicine and preparing method thereof
A method of preparing low water-soluble medicine into solid nanometer pharmaceutical formulation is disclosed. According to the characters of molecular aggregates such as supramolecular chemical micelles and vesicles, the formulation, which based on the hydroxypropyl-beta-cyclodextrin and phospholipid, is prepared under the condition of hyperthermia sterilization and decompression. Such nanometer formulation is sterile particle or powder with loose porosity. For directly intravenous use, the formulation has targeting activity, sustained release and long circulating characters. While as a solid oral product, it is fast-release, fast-effective, and improved bioavailability characters, and is readily melted in mouth. The formulation utilizes secure accessories, traditional equipments and methods, thus, it is suited to be used and manufactured widely. Also disclosed is intravenous formulation of anticancer paclitaxel, which characterized that there has no polyoxyethylenated castor oil in it. Such intravenous formulation is nonallergic so that it has higher security and efficiency compared to present commercially available paclitaxel formulations.
Owner:刘 云清 +3
Ibuprofen injection composite and preparation method thereof
ActiveCN101966147AImprove stabilityMeet the needs of clinical medicineOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
The invention discloses an ibuprofen injection composite which can obviously improve the quality stability of products and meet the clinical compatibility demand and a preparation method thereof to overcome the defect of the traditional ibuprofen injection. The injection is prepared by mixing ibuprofen and cosolvent according to a molar ratio of 1: 1.001-2, and the pH value of the injection is 7.5-9.0. The ibuprofen injection prepared through the method has obviously improved tolerance capability to high temperature and strong light and better stability and no irritant to vessels, and can effectively meet the requirement of the clinical intravenous drip. Moreover, the preparation method of the invention has the advantages of simple process and good stability of the prepared product.
Owner:四川阳光润禾药业有限公司
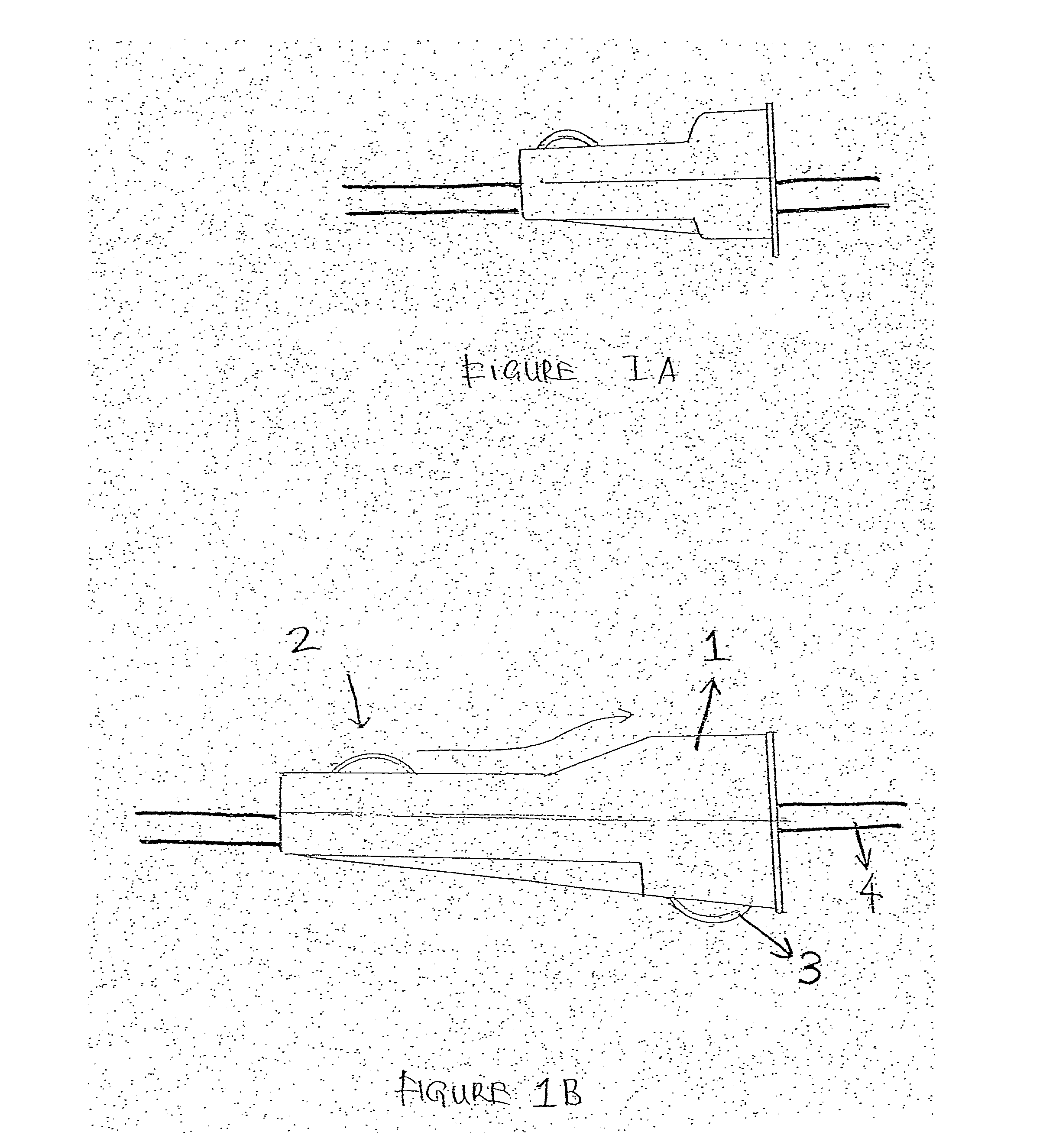
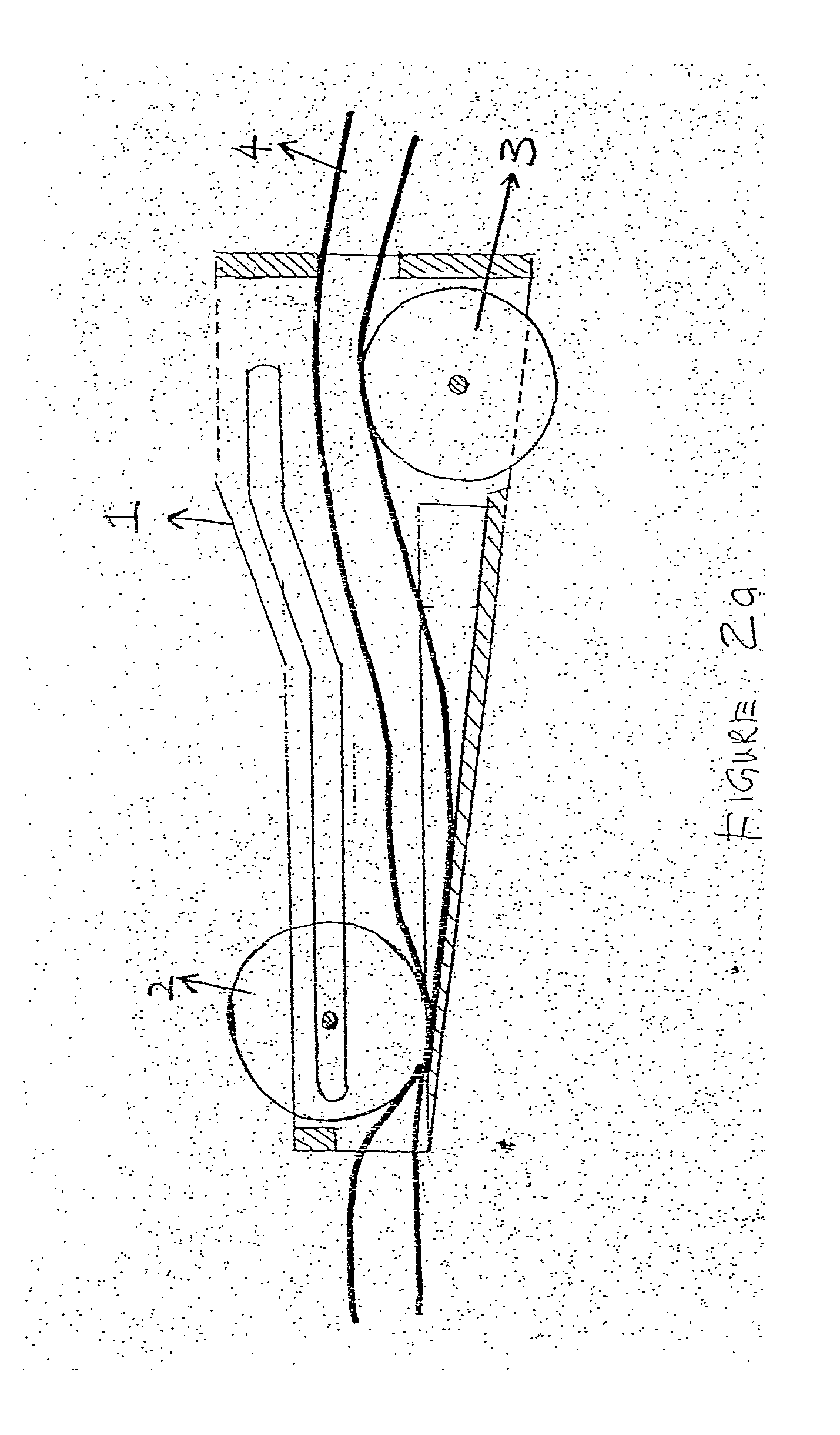
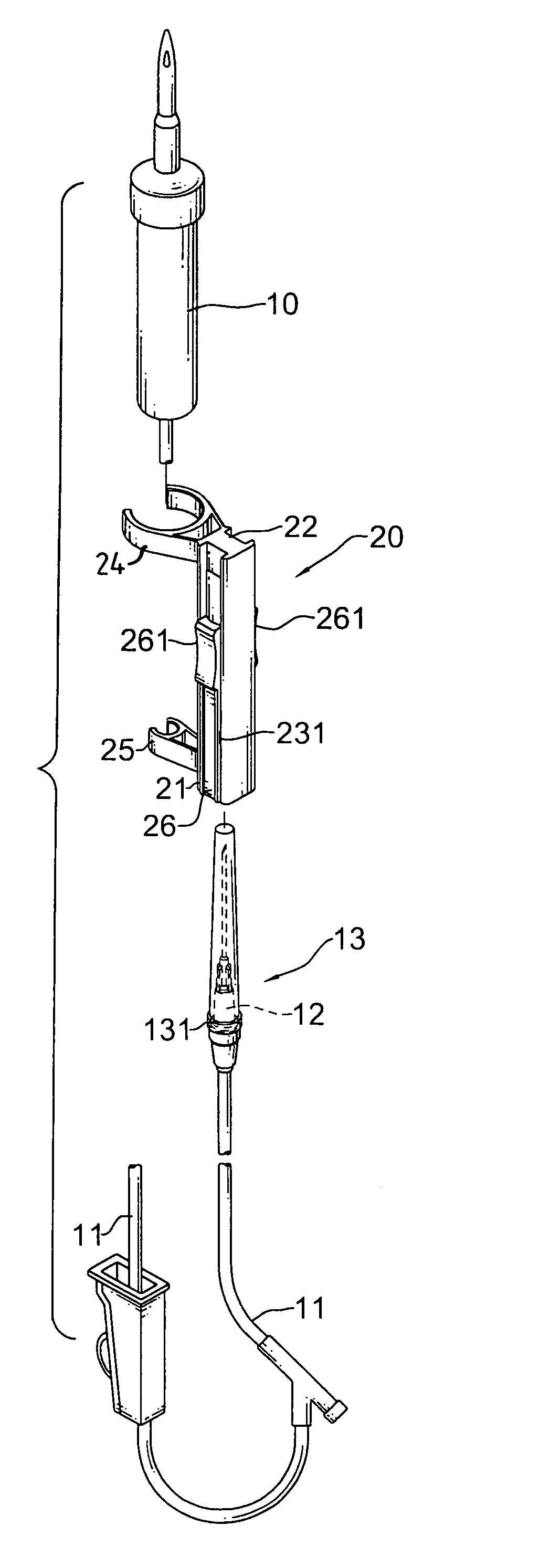
Safety receptacle for a needle of an intravenous drip assembly
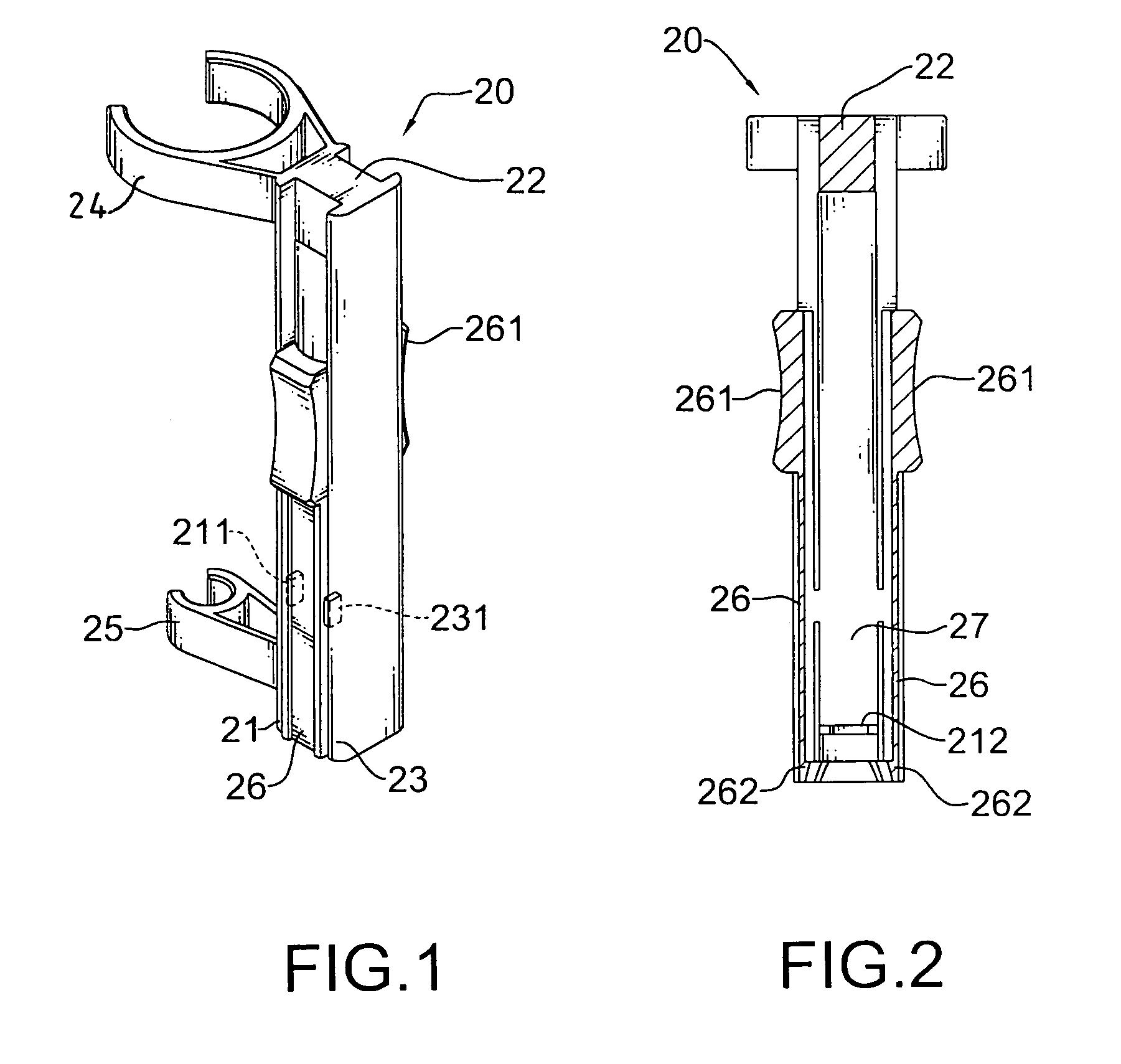
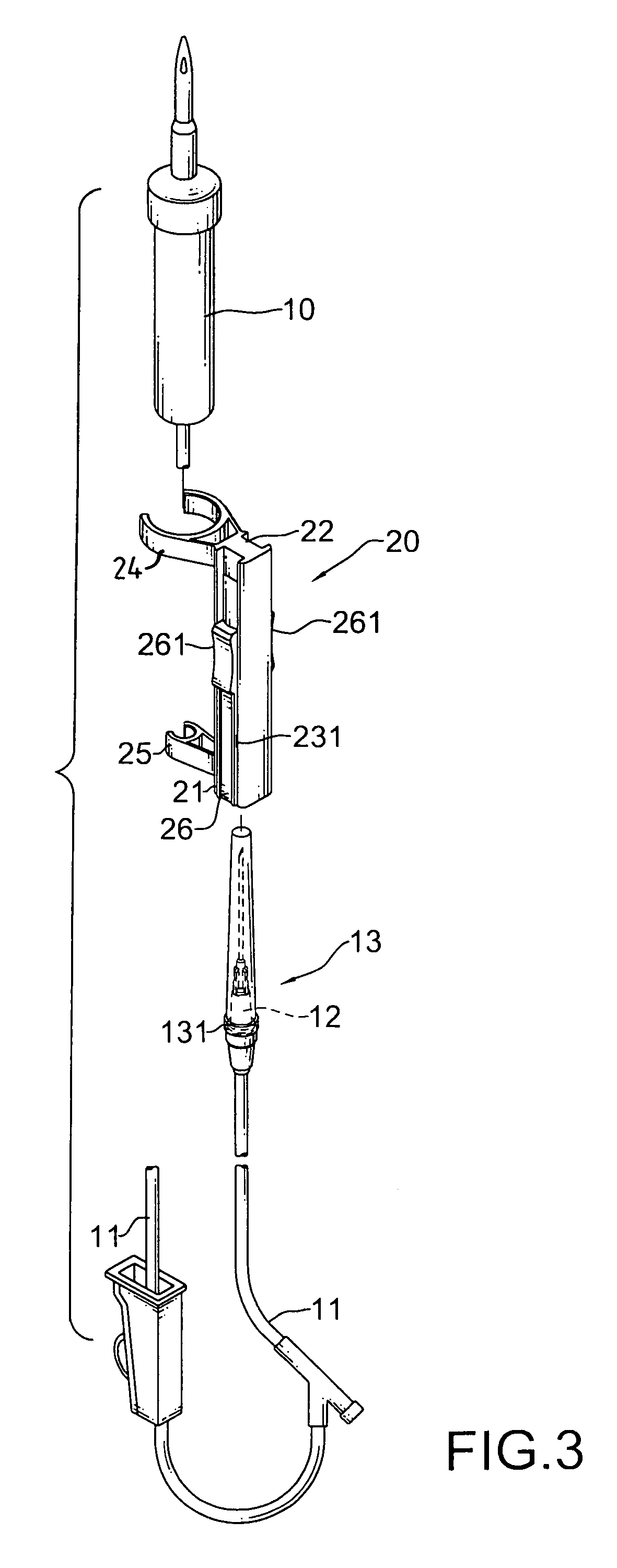
The safety receptacle is used with an intravenous drip assembly having an intravenous drip, a needle and a tip protector and has a front bracket, a connecting bracket, a rear bracket, at least one C-clip and two clipping sheets. The C-clip is formed on the front bracket to hold the intravenous drip. The clipping sheets are mounted pivotally between the front and rear brackets. The needle can be held in and removed out from the tip protector. The tip protector can be held in the safety receptacle by the clipping sheets. When the clipping sheets are pivoted, the tip protector is released and can be removed from the safety receptacle.
Owner:BIOTOP TECH SHANGHAI
Single intraveneous drip component illumination device
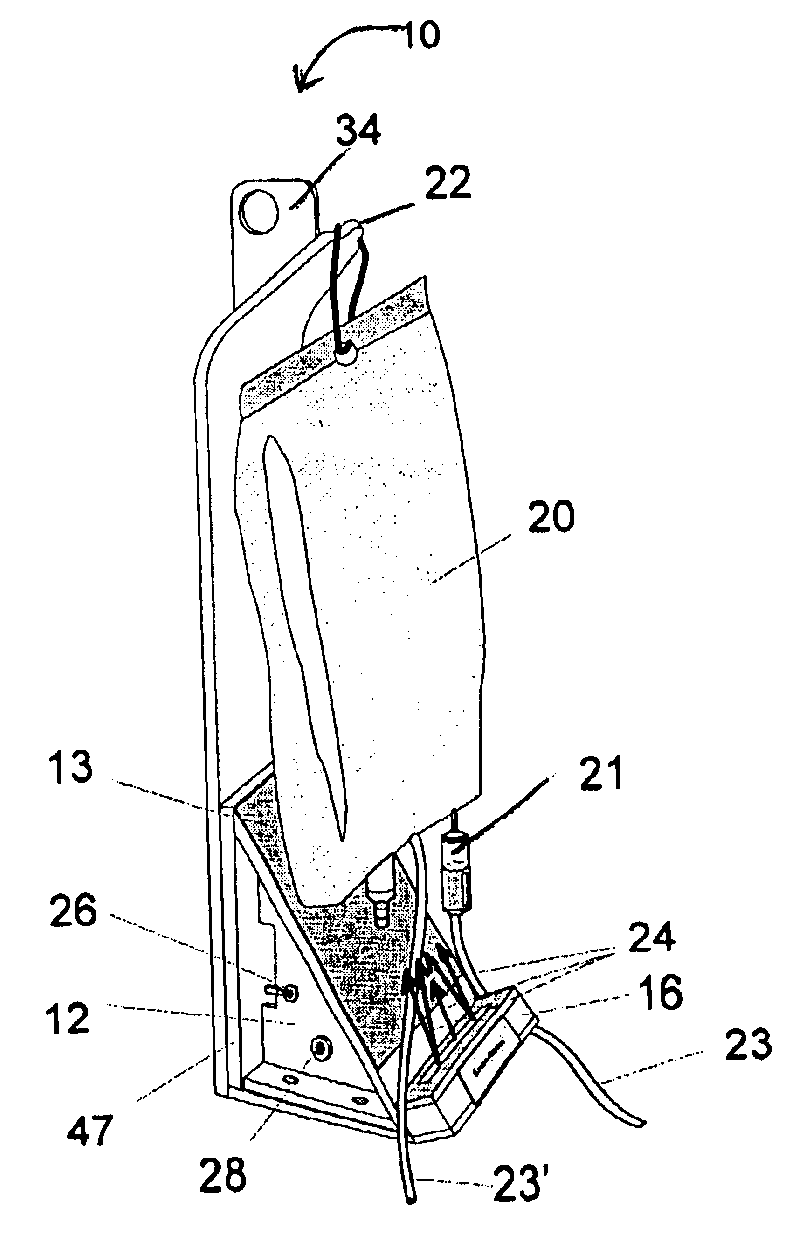
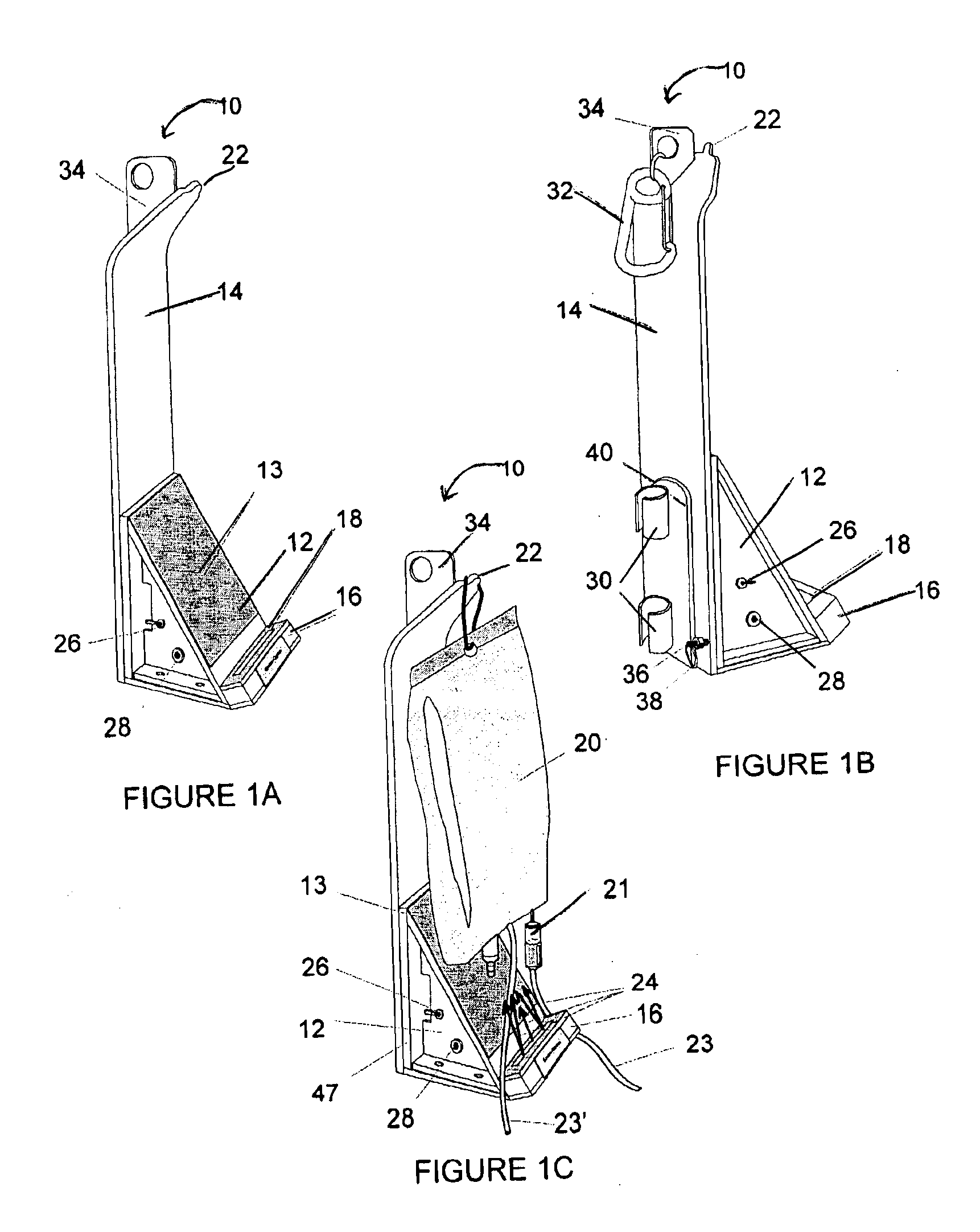
A single intravenous drip components illumination device containing a source of light that can illuminate IV bags or bottles, drip chambers and tubing. The directed light of the light source provides adequate lighting for use of the invention in unlit or dimly lit settings with minimum lateral scattering. The invention is powered by an external AC or DC power supply and / or by batteries mounted internally to the base of the invention.
Owner:EMBO OPTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com