Paclitaxel submicron emulsion using lipid composite as middle carrier
A technology of lipid complexes and intermediate carriers, which is applied in the fields of drug combination, emulsion delivery, and medical preparations containing active ingredients, etc. problems, to achieve the effect of reducing allergic reactions and toxicity, easy industrialized large-scale production, and stable storage quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
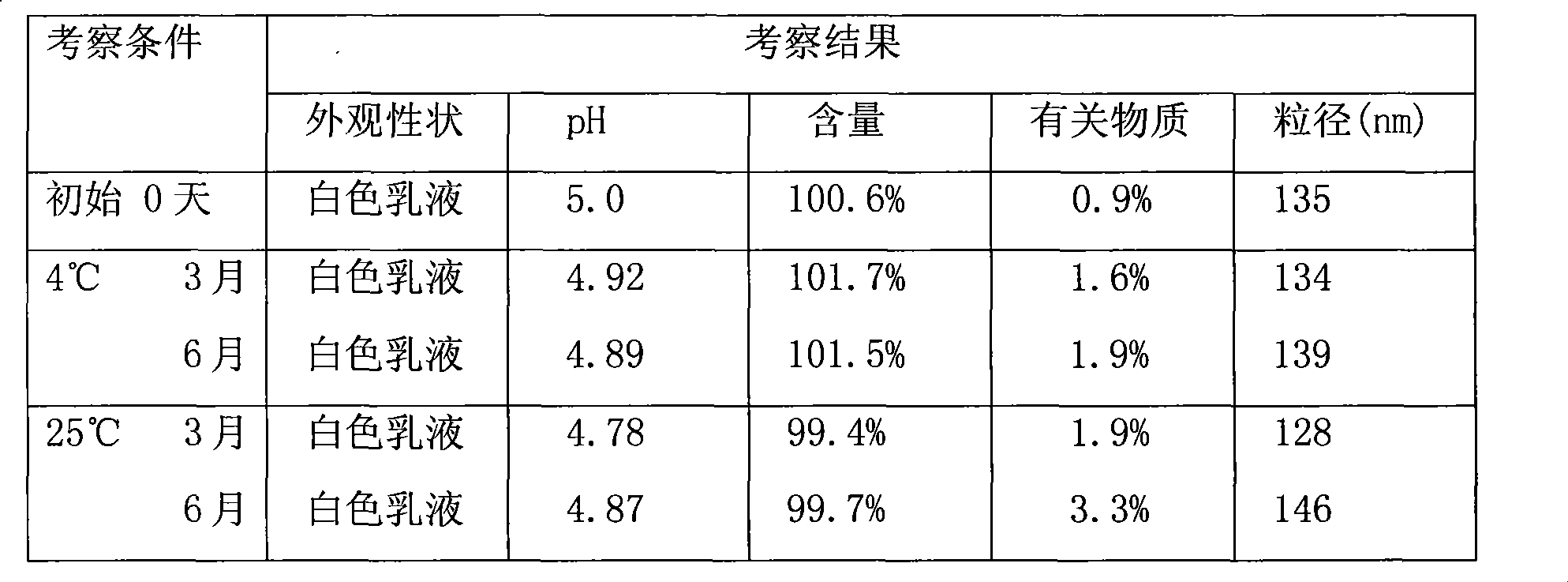
Examples
preparation example 1
[0039] Preparation Example 1: Paclitaxel-egg yolk phospholipid complex
[0040] Take 0.4g of paclitaxel and 2.4g of egg yolk lecithin, add 100ml of tetrahydrofuran, compound at 40°C for 1 hour, remove the tetrahydrofuran by rotary evaporation, and dry in vacuum for more than 12 hours (20-30°C) to obtain the drug-loaded egg yolk phospholipid complex. Pack and store in the refrigerator.
preparation example 2
[0041] Preparation Example 2: Paclitaxel-soybean phospholipid complex
[0042] Take 1g of paclitaxel and 6g of soybean lecithin, add 250ml of acetone preheated to 40°C, compound at 40°C for 1 hour, remove the solvent by rotary evaporation, and dry in vacuum for more than 12 hours (20-30°C), to obtain drug-loaded soybean lecithin compound Items, airtightly packaged, stored in the refrigerator.
preparation example 3
[0043] Preparation Example 3: Paclitaxel-Cholesterol Complex
[0044] Take 2g of paclitaxel and 4g of cholesterol, add 750ml of acetone preheated to 40°C, compound at 40°C for 2 hours, remove the solvent by rotary evaporation, and dry in vacuum for more than 12 hours (20-30°C) to obtain the drug-loaded cholesterol-lipid compound Items, airtightly packaged, stored in the refrigerator.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com