Ibuprofen injection composite and preparation method thereof
A technology for injection preparation and injection, applied in the directions of drug combination, pharmaceutical formulation, drug delivery, etc., can solve problems such as unfavorable long-term storage and clinical use, increase in visible foreign bodies, poor long-term stability, etc., to improve stability and improve safety. Good performance and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
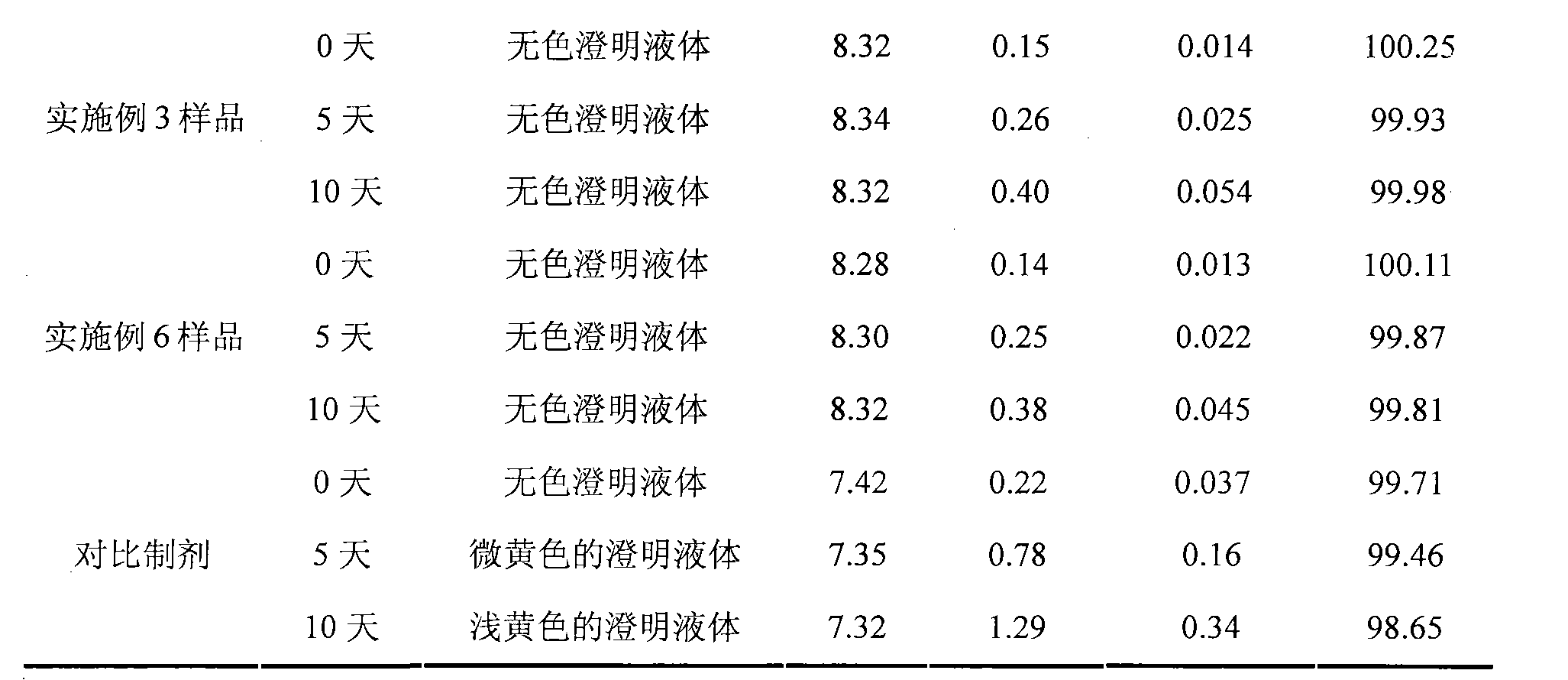
Embodiment 1
[0041] Each 1000 injection preparations contains the following ingredients:
[0042] Ibuprofen 100g
[0043] Lysine 72g
[0044] Add water for injection to 1000ml
[0045] Weigh lysine according to the prescription amount, add 600-900ml water for injection, stir to dissolve it, then add the prescribed amount of ibuprofen, stir continuously to dissolve it, measure the pH value of the solution to be about 7.3, and use sodium hydroxide solution Adjust the pH value of the solution to 7.5-8.5, add water for injection to 1000ml, stir and mix evenly; add activated carbon for needles with a total volume of 0.1% (W / V), heat and stir at 40-60°C for 20 minutes, filter and remove Carbon for standby; the above solution is finely filtered with 0.22 μm and 0.45 μm microporous membranes until the filtrate is clear. After the semi-finished product is qualified, it is subpackaged and filled in 1000 1ml ampoules, so that the content of the main drug in each ampule is 100 mg, sterilized by ste...
Embodiment 2
[0047] Each 1000 injection preparations contains the following ingredients:
[0048] Ibuprofen 200g
[0049] Histidine 155g
[0050] Add water for injection to 2000ml
[0051] Weigh histidine according to the prescription amount, add 1200-1800ml water for injection, stir to dissolve it, then add the prescribed amount of ibuprofen, stir continuously to dissolve it, measure the pH value of the solution to be about 7.2, and use sodium hydroxide solution Adjust the pH value of the solution to 8.0-9.0, add water for injection to 2000ml, stir and mix evenly; add activated carbon for needles with a total volume of 0.2% (W / V), heat and stir at 40-60°C for 20 minutes, filter and remove Carbon for standby; the above solution is finely filtered with 0.22 μm and 0.45 μm microporous membranes until the filtrate is clear. After the semi-finished product is qualified, it will be subpackaged and filled in 1000 2ml ampoules, so that the content of the main drug in each ampule is 200mg, ster...
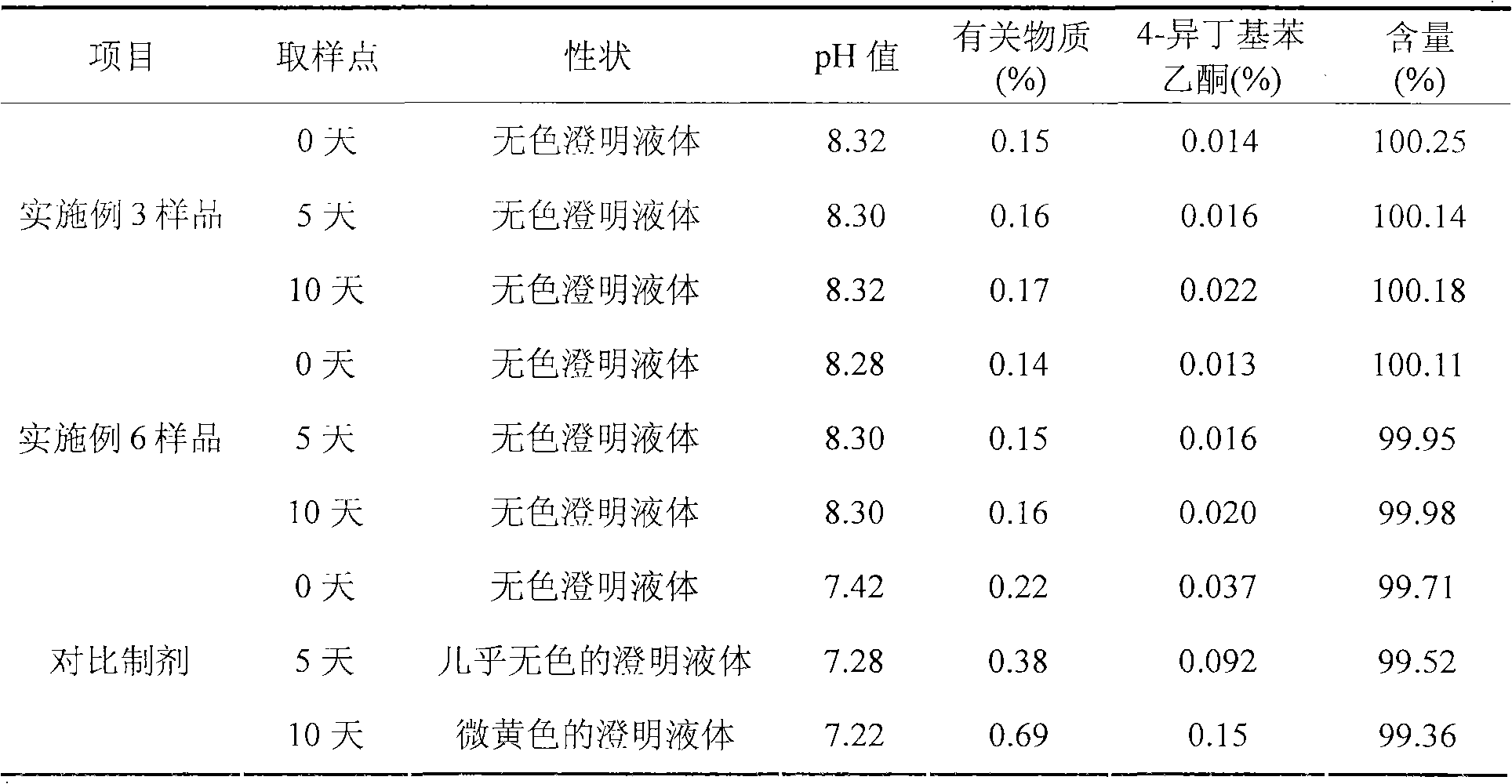
Embodiment 3
[0053] Each 1000 injection preparations contains the following ingredients:
[0054] Ibuprofen 400g
[0055] Arginine 354g
[0056] Add water for injection to 4000ml
[0057] Weigh arginine according to the prescription amount, add 2400 ~ 3600ml of water for injection, stir to dissolve it, then add the prescription amount of ibuprofen, stir continuously to dissolve it, measure the pH value of the solution to 7.8 ~ 8.8, add water for injection to 4000ml, stir and mix evenly; add activated carbon for needles with a total amount of 0.1% (W / V), heat and stir at 40-60°C for 20 minutes, filter and decarbonize for later use; fine the above solution with a 0.22 μm microporous membrane filter Filtrate until the filtrate is clear and the semi-finished product is qualified, sub-package, fill and seal in 1000 5ml ampoules, so that the content of the main drug in each ampule is 400mg, sterilize at 115°C for 30 minutes, leak detection, light inspection , get the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com