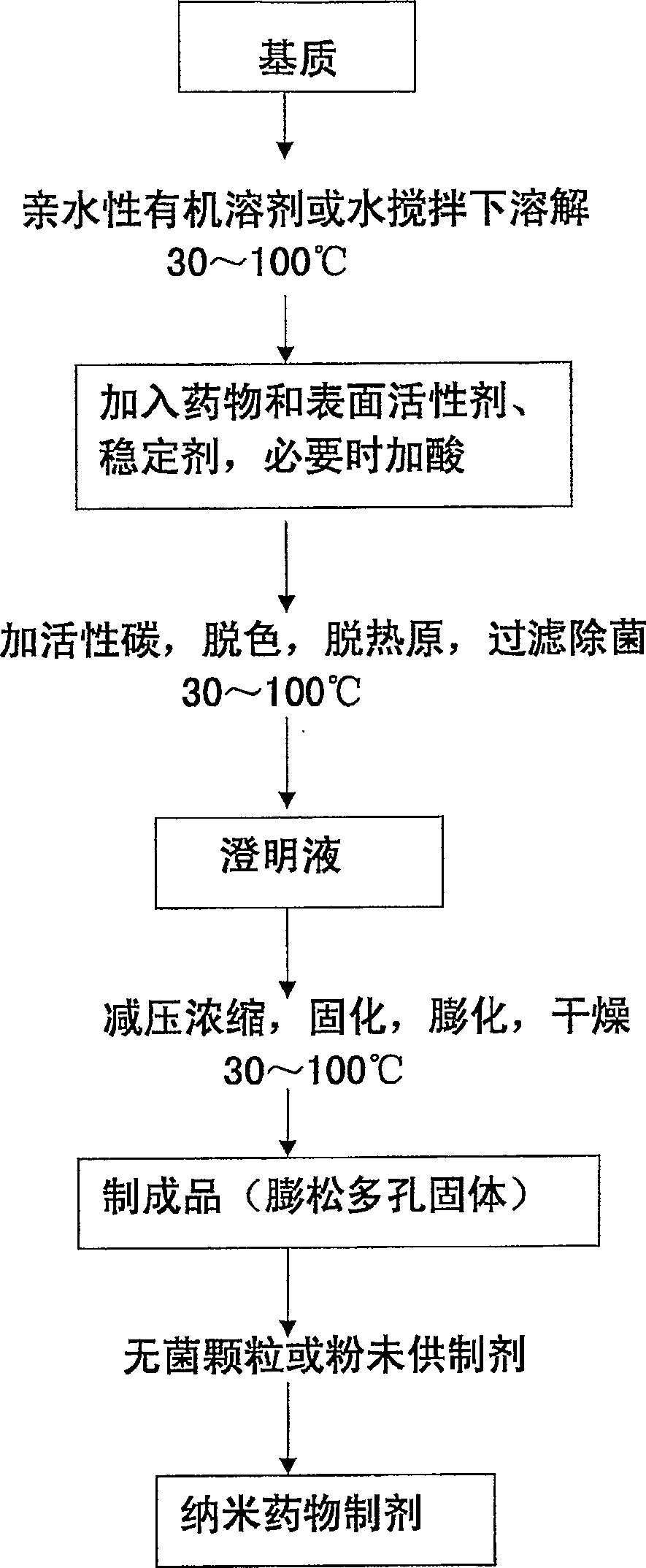
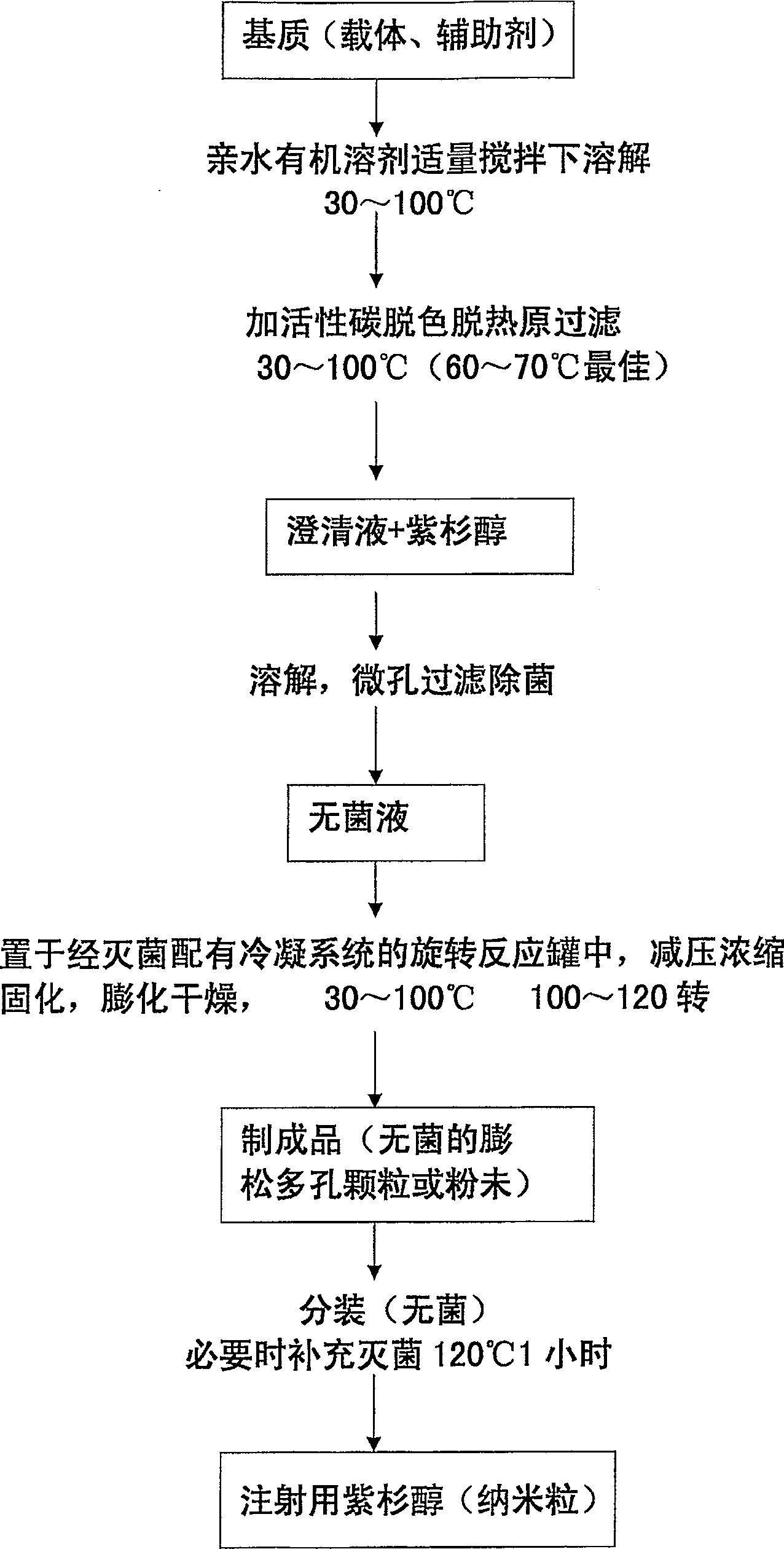
Solid nano-medicine and preparing method thereof
A technology of nano-medicine and medicine, which is applied in the direction of nano-medicine, nanotechnology, nanotechnology, etc., and can solve problems that do not meet the requirements of drug safety and industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1. Exemplary raw material formula
[0088] 1. Paclitaxel for injection Specification: 30mg / 2.5g
[0089] Matrix formula: Hydroxypropyl Beta Cyclodextrin 60 Phospholipids 8 Tween 80 9 The main parameters: Drug loading% 1.2
[0090] 2. Artemether for injection Specification: 60mg / 2.2g
[0091] Matrix formula: Hydroxypropyl Beta Cyclodextrin 31.5 Phospholipids 3 Tween 80 1.5 The main parameters: Drug loading% 2.7
[0092] 3. Dihydroartemisinin for injection Specification: 40mg / 1.5g
[0093] Matrix formula: Hydroxypropyl Beta Cyclodextrin 31.5 Phospholipids 3 Tween 80 3 The main parameters: Drug loading% 2.7
[0094] 4. Busulfan Specification: 2mg
[0095] Matrix formula: Hydroxypropyl Beta Cyclodextrin 17 Phospholipids 1.7 Tween 80 0.75 The main parameters: Drug loading% 5.1
[0096] 5. Nimodipin...
Embodiment 2
[0113] Preparation of paclitaxel for injection
[0114] In the preparation, the materials used are: hydroxypropyl beta cyclodextrin (for injection) Hydroxypropy-β-Cyclodextrin for Injection; soybean lecithin (for injection) Soya Phospholide for Injection; auxiliary agent polysorbate 80 Polysorbate-80 (Tween80); Povidone K 30 , Polydone K 30 or K 15 (PVP K 30 or K 15 ); and small molecule dextran, etc.
[0115] The formula for preparing paclitaxel for injection (nanoparticles) 30mg / 2.5g (branch) is:
[0116]
[0117] According to routine determination of the drug loading % of the present invention: 1.19.
[0118]Adjusting the formula to change the ratio of the matrix and the auxiliary agent can affect the solubility of the drug and control the particle size of the particles within a certain range to meet the requirements of the drug.
[0119] The matrix (carrier) and auxiliary agents in the formula are physiologically compatible, safe and easy to purchase. Wherein, p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com