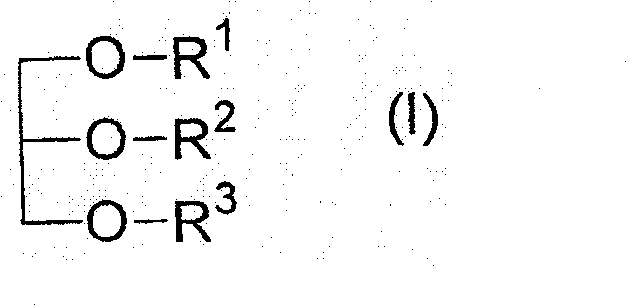
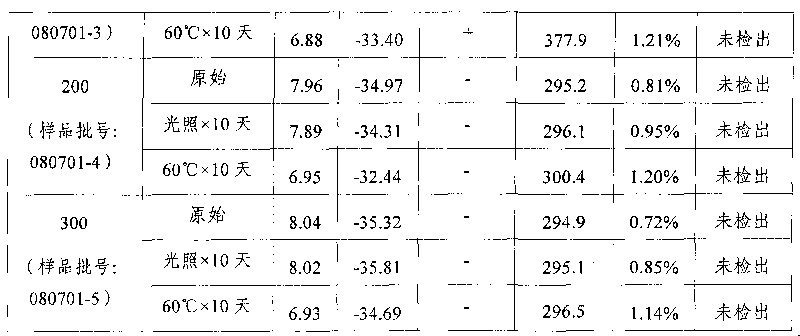Patents
Literature
239 results about "Fat emulsion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Uses for fat emulsion. Fat emulsions are used as dietary supplements for patients who are unable to get enough fat in their diet, usually because of certain illnesses (eg, parenteral nutrition-associated cholestasis) or recent surgery. Fats are used by the body for energy and to form substances needed for normal body functions.
Fat emulsion providing improved health and taste characteristics in food
InactiveUS20120053251A1Low in fatHigh emulsionBiocideEdible oils/fats with reduced calorie/fat contentFiberAntioxidant
The embodiments relate to fat emulsion structures based on both an aqueous and non-aqueous glycerin component as the primary aqueous component in which the fat emulsion can create a wide range of viscosities that mimic fat structures similar to cream, or all the way to hardened fat structures like Trans Fat. The fat emulsion can be added to a wide group of foods that use a monosaccharide or disaccharide as the basis for its sweetener component, can lower the sugar content of foods, can improve mouth feel while lowering the fat content in high fat foods, can add a balance of dietary fats and fiber to foods, and can add antioxidant content to food products.
Owner:ANTIOXIDANT SUPERFOODS
Fat emulsion injection of 'Xingnaojing' and its preparing process
InactiveCN1628779AHigh purityFully extractedMammal material medical ingredientsEmulsion deliveryHas active ingredientEmulsion
Disclosed is a fat emulsion injection of 'Xingnaojing' and its preparing process, which comprises using supercritical extraction technology and macroscopic adsorption resin technology for isolating and purifying the water soluble and fat soluble parts in the medicinal materials including musk, cape jasmine, and curcuma root, finally utilizing emulsion technology to obtain the end product.
Owner:JIANGSU QINGJIANG PHARMA
Fat emulsion containing docetaxel and its preparing method
InactiveCN1709236ASolve the existing technology deficiencies of low solubilityImprove securityOrganic active ingredientsDiseaseVegetable oil
The present invention relates to an infatmul containing docetaxel and its preparation method. Said preparation can be directly used for intravenous injection. At the same time of that it is used as medicine for curing tumor disease said preparation also can provide nutrients for patient. The weight volume concentration range of docetaxel contained by said preparation is 0.1-1.0 mg / ml, and its specification is that 1-500 mg of active component docetaxel is contained. Said infatmul composition includes docetaxel, vegetable oil, phospholipids and injection water, at the same time the components of glycerin and group emulsion, etc. can be added, in which the optimum group emulsion is poloxamer 188. Besides, said invention also provides the concrete steps of its preparation method and its application in preparation of medicine for resisting tumor.
Owner:CISEN PHARMA
Superregulated long-cycled lipid emulsion carrying medicine reagent for mainline
ActiveCN101032467AImprove stabilityImprove bioavailabilityEmulsion deliveryOil/fats/waxes non-active ingredientsRetention periodAntioxidant
The present invention relates to high stability medicine carrying fat emulsion preparation for intravenous injection and its preparation process. The high stability medicine carrying fat emulsion preparation consists of: clinically effective liposoluble medicine, oil with medicinal effect or as medicine solvent, lecithin as the surfactant of the emulsion, polyethylene glycol phospholipid derivative as the emulsion stabilizer and emulsifier to increase the half life of the fat emulsion in blood, oleic acid or oleate as the emulsion stabilizer and emulsifier, vitamin E as the antioxidant, and complexing agent for controlling metal ion. The fat emulsion preparation of the present invention has long retention period in blood, increased passive medicine targeting function, high stability, raised bioavailability of the liposoluble medicine and raised clinical therapeutic effect.
Owner:XIAN LIBANG PHARMA
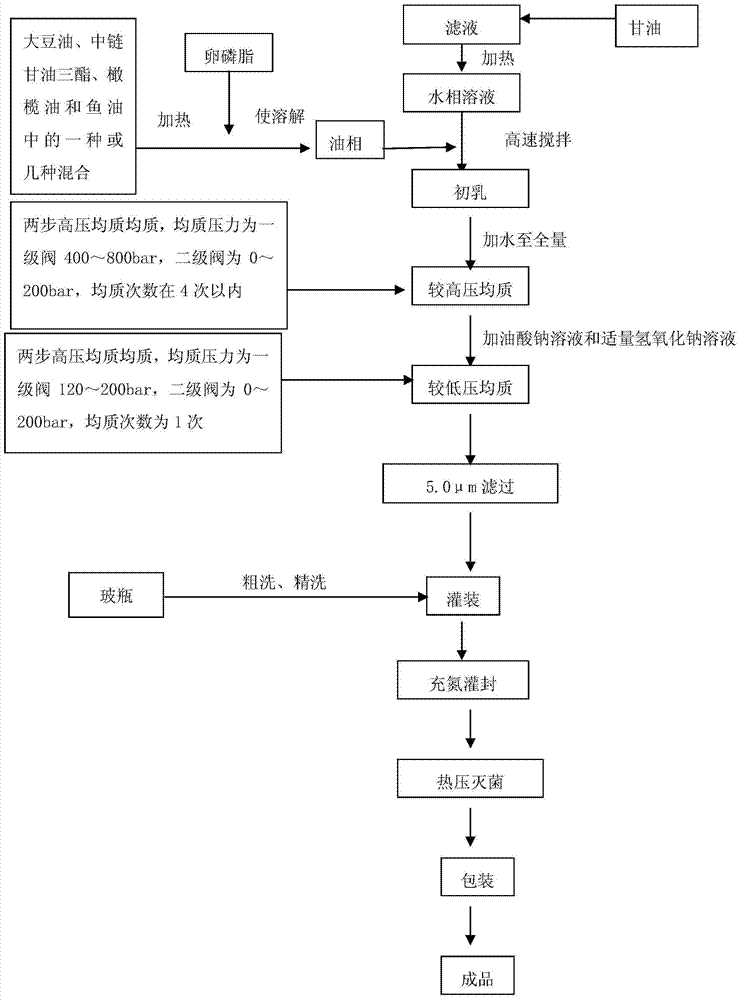
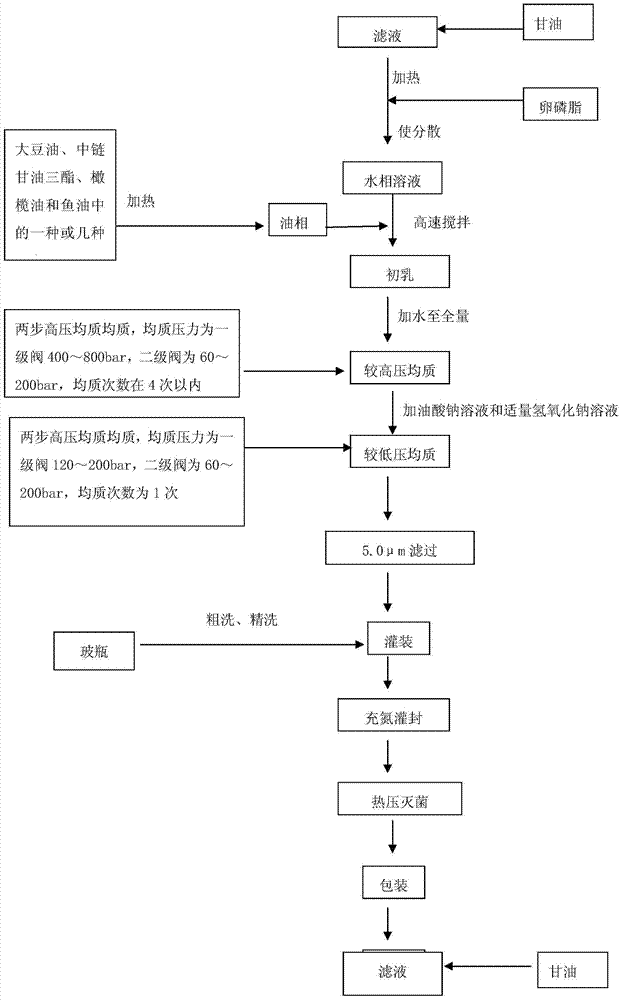
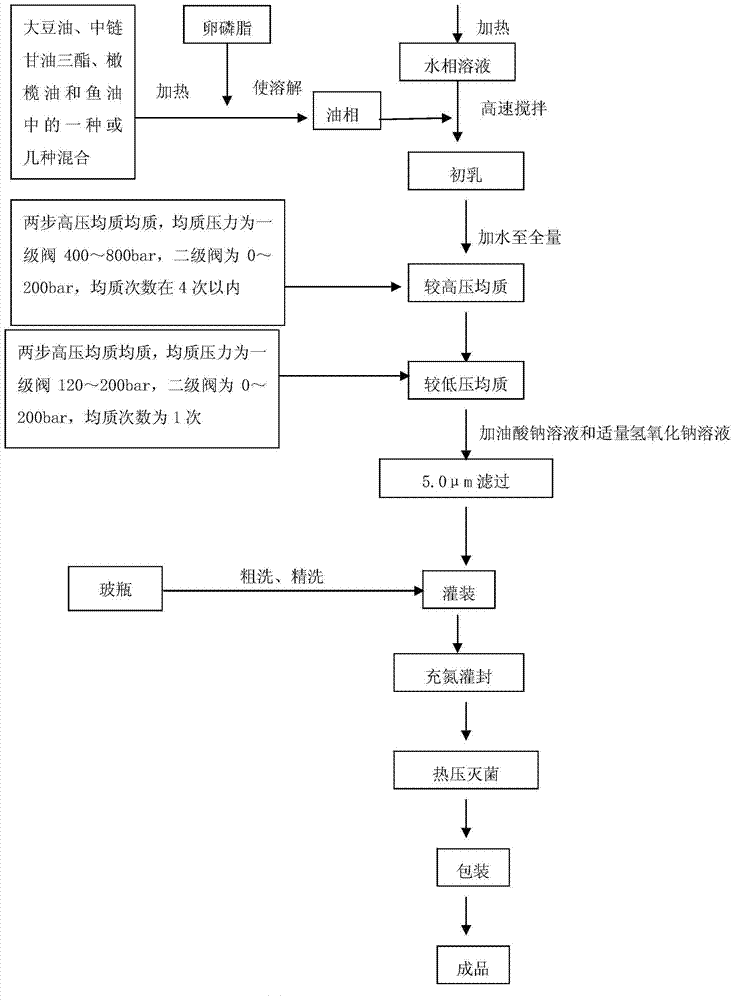
Fat emulsion injection liquid containing soybean oil, medium chain triglyceride, olive oil and fish oil and method for preparing the same
InactiveCN1965806AAvoid exhaustionImprove the outcome of standard clinical treatmentsOrganic active ingredientsMetabolism disorderYolkFish oil
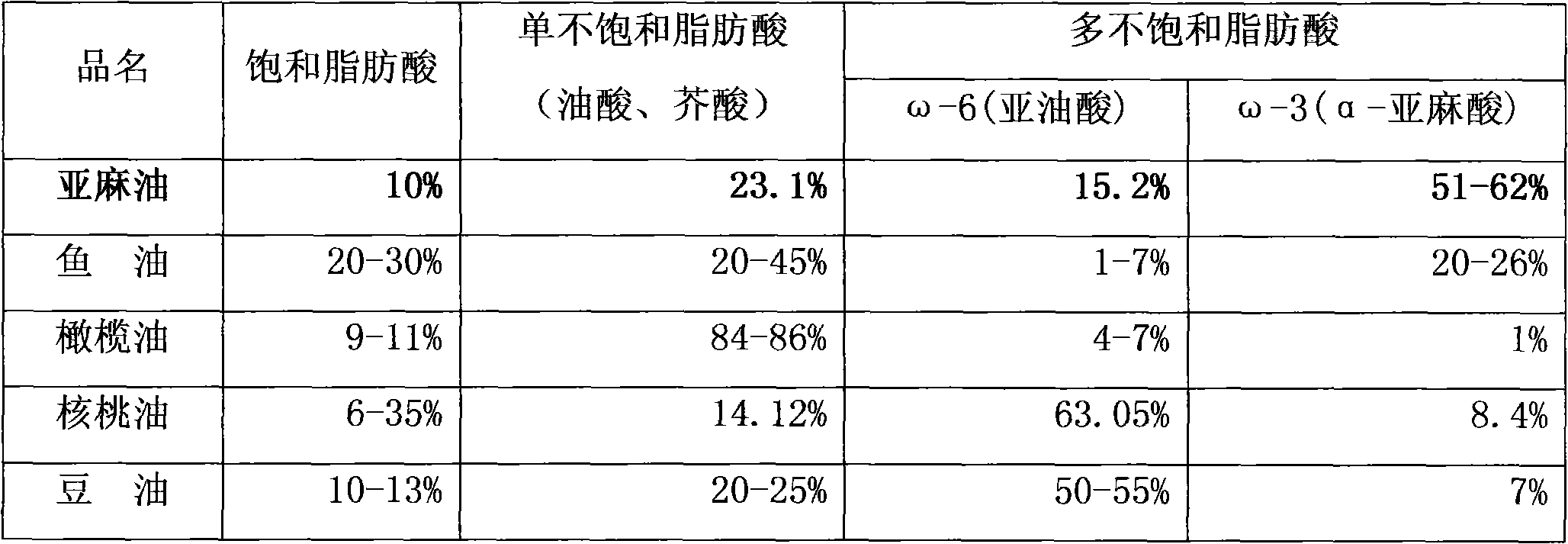
The invention relates to an intralipid injection which contains soya oil, middle chain triglyceride, olive oil, and fish oil, wherein it comprises 48-72g.L soya oil, 48-72g / L middle chain triglyceride, 40-60g / L olive oil, and 24-36g / L fish oil, 108-162 mg / L dl-alpha-tocofecol, 9.6-14.4g / L lipovitellin, 22.5-27.5g / L pure glycerin, 240-360mg / L sodium oleate, 18-22mg / L caustic soda, and 1L injection water. The ratio between w-6 and w-3 aliphatic acid is 3.0-2.2:1. The invention optimizes and balances the aliphatic acid mode.
Owner:费森尤斯卡比华瑞制药有限公司
Taxanes medicine preparation for intravenous injection and preparation method thereof
ActiveCN101288642AGood biocompatibilityHigh tolerance in vivoOrganic active ingredientsSolution deliveryDrugs solutionDocetaxel
The invention relates to the technical field of medicine, which is a preparation of a taxane drug for intravenous drug delivery, consisting of two parts of drug solution and an emulsion. The drug solution is composed of paclitaxel or docetaxel, a pH regulator and a solvent for injection, wherein, the solvent for injection is an organic solvent; the emulsion comprises a fat emulsion and is composed of oil for injection, an emulsifier, an antioxidant, an isotonic regulator, a stabilizer, a pH regulator and water for injection. When in use, the drug solution can be added and evenly mixed in the emulsion for direct intravenous drip according to the clinical drug dosage and can also be firstly added in the emulsion with the volume that is not less than 5 times of the volume of the drug solution according to the clinical drug dosage and then added with a certain amount of physiological saline or glucose injection for intravenous drip. The preparation of the invention does not contain solubilizer and has the advantages of little toxicity, safety, effectivity, stability and economy. The fat emulsion can also be taken as a nutrition replenisher for a patient, thus achieving better treatment effects. The physiological saline or the glucose injection can also replace a certain amount of the emulsion, so the storage and the transportation are convenient, and the preparation is more economical.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Fat emulsions for inhalational administration
The object of the present invention is to provide a pharmaceutical composition optimized for the administration of a drug, particularly a drug which is only sparingly soluble in water, by way of inhalation.The present invention is a fat emulsion for inhalational administration, or a lyophilized composition thereof, which is an o / w fat emulsion comprising fat emulsion particles essentially composed of an oil component, an emulsifying agent and a drug as dispersed in water, characterized in that the average particle diameter of said fat emulsion particles lies within the range of 5~100 nm.With the aid of a suitable inhaler, the inhalant of the invention readily yields a mist of aerosol particles fine enough to reach the alveolus; the inhalant is well amenable to size control of the aerosol particles.
Owner:NIPPON SHINYAKU CO LTD
Vegetable oil fatty milk oral or injection preparation
The present invention discloses a vegetable oil fat emulsion oral or injection preparation. Its main composition includes medicinal vegetable oil, lecithin as emulsifier, refined soybean oil or other refined oil as oil-soluble diluting agent and glycerine as isoosmotic regulating agent. It can reduce irritation of medicine and cosolvent to blood vessel, and can raise medicine effect.
Owner:XIAN LIBANG PHARMA TECH
Phosphatide composition of active skull cap components and its prepn process and prepn
The present invention discloses phosphatide composition of active skullcap components and its preparation. The active skullcap components are prepared through extracting and separating skullcap root, and contain baicalin or wogonin over 50 %. The active skullcap components have low water solubility and fat solubility, solubility increasing with raised solution pH value, and easy chemical degradation in alkaline condition, so that the active skullcap components can not be prepared into injection and oral preparation directly. By means of phosphatide composition technology, the present invention improves the water dispersivity and lipophilicity of the active skullcap components, so as to prepare oral preparation with high bioavailability, freeze dried powder for injection, fat emulsion and mucous membrane administration preparation.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
Disinsection composition with synergistic action
InactiveCN101380358AAvoid side effectsQuality assuranceMetabolism disorderPharmaceutical non-active ingredientsSide effectAntioxidant
The invention belongs to the technical field of medicine and relates to purple perilla seed oil fat emulsion injecta being rich in omega-3 fatty acid and a preparation method thereof. The purple perilla seed oil fat emulsion injecta comprises 5 to 30 percent of refined purple perilla seed oil, 0.5 to 7.5 percent of an emulsifying agent, 1.4 to 3.6 percent of glycerin, 0 to 0.5 percent of EDTA-2Na, 0 to 0.5 percent of an antioxidant, 0.1 percent of hydrochloric acid or sodium hydroxide with the pH being 6.0 to 8.5 and the residual amount of water for injection. The purple perilla seed oil fat emulsion injecta has good stability and can avoid side effect caused by the metabolism of fat emulsion which is rich in omega-3 fatty acid. The injecta of the invention is the fat emulsion injecta with natural refined purple perilla seed oil, which can be sent for high-temperature sterilization and provide human bodies with energy to keep the factors needed by brain and nerve functions. The injecta has the functions of anti-thrombus and antiatheroscloresis, canceration prevention, inhabitation of tumour cells transfer and inhabitation of abnormalism symptoms.
Owner:SHENYANG PHARMA UNIVERSITY
GLP-1 derivative and preparation thereof absorbable via mucous membrane
InactiveUS7291594B2Good clinical applicabilityImprove absorption ratePeptide/protein ingredientsMetabolism disorderDipeptidyl peptidaseFat emulsion
A GLP-1 derivative is provided including an amino acid sequence of GLP-1 (7-35) having deletion, substitution and / or addition of one or more amino acids and having Waa-(Xaa)n-Yaa (in which Waa is Arg or Lys, Xaa is Arg or Lys, n is an integer of 0 to 14, and Yaa is Arg, Arg-NH2, Lys, Lys-NH2 or Hse) added to the C-terminus of the peptide having a GLP-1 activity. These derivatives are highly absorbable via a mucous membrane. The GLP-1 derivative can be conferred with resistance to dipeptidyl peptidase IV by substituting amino acid 8 in its GLP-1 amino acid sequence with Ser, or with resistance to trypsin by substituting amino acids 26 and 34 with Gln and Asn, respectively.The absorption efficiency of the GLP-1 derivatives via mucous membranes can be further improved by preparing a composition using a charge-regulated fat emulsion regulated to be negatively charged thereon.
Owner:SANWAKAGUKU KENKYUSHO CO LTD
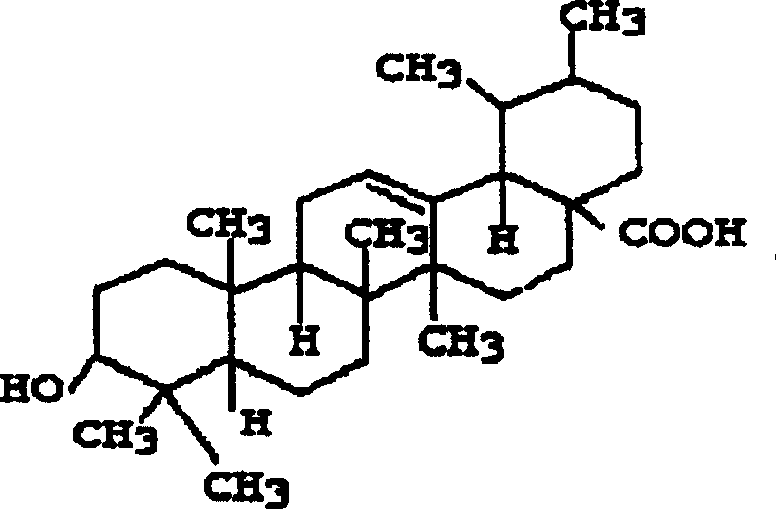
Ursolic acid fat emulsion injection and its prepn
InactiveCN1771968AQuick effectAvoid first pass effectAntibacterial agentsOrganic active ingredientsFat emulsionActive component
The present invention relates to one kind of ursolic acid fat emulsion injection and its preparation. The fat emulsion consists of ursolic acid as active component and medicinal supplementary material, and consists of ursolic acid 0.01-0.15 wt%, oil for injection 5-30 wt%, emulsifier 1-9 wt%, solubilizer 1-6 wt%, isosmotic agent 2-2.5 wt%, and antioxidant 0.01-0.03 wt%, except water for injection. The present invention has simple preparation process, and the injection may be used clinically in intravenous injection, and has high bioavailability, high stability, raised treating effect, slow releasing and targeting effect, and energy providing effect.
Owner:广东天之骄药物开发有限公司
Fat emulsion for injection and its preparing process
A fatty emulsion for injection is prepared from purified soybean oil, refined lecithin, anhydrous glycerine, sodium hydroxide and water for injection through heating said purified soybean oil to 70-90 deg.C, dissolving refined lecithin in it while stirring to obtain oil phase, dissolving anhydrous glycerine in the water for injection while stirring to obtain water phase, slowly adding said oil phase to said water phase while stirring, and stirring for 10 mins to obtain early emulsion. Its advantages are high safety and high energy density.
Owner:费森尤斯卡比华瑞制药有限公司
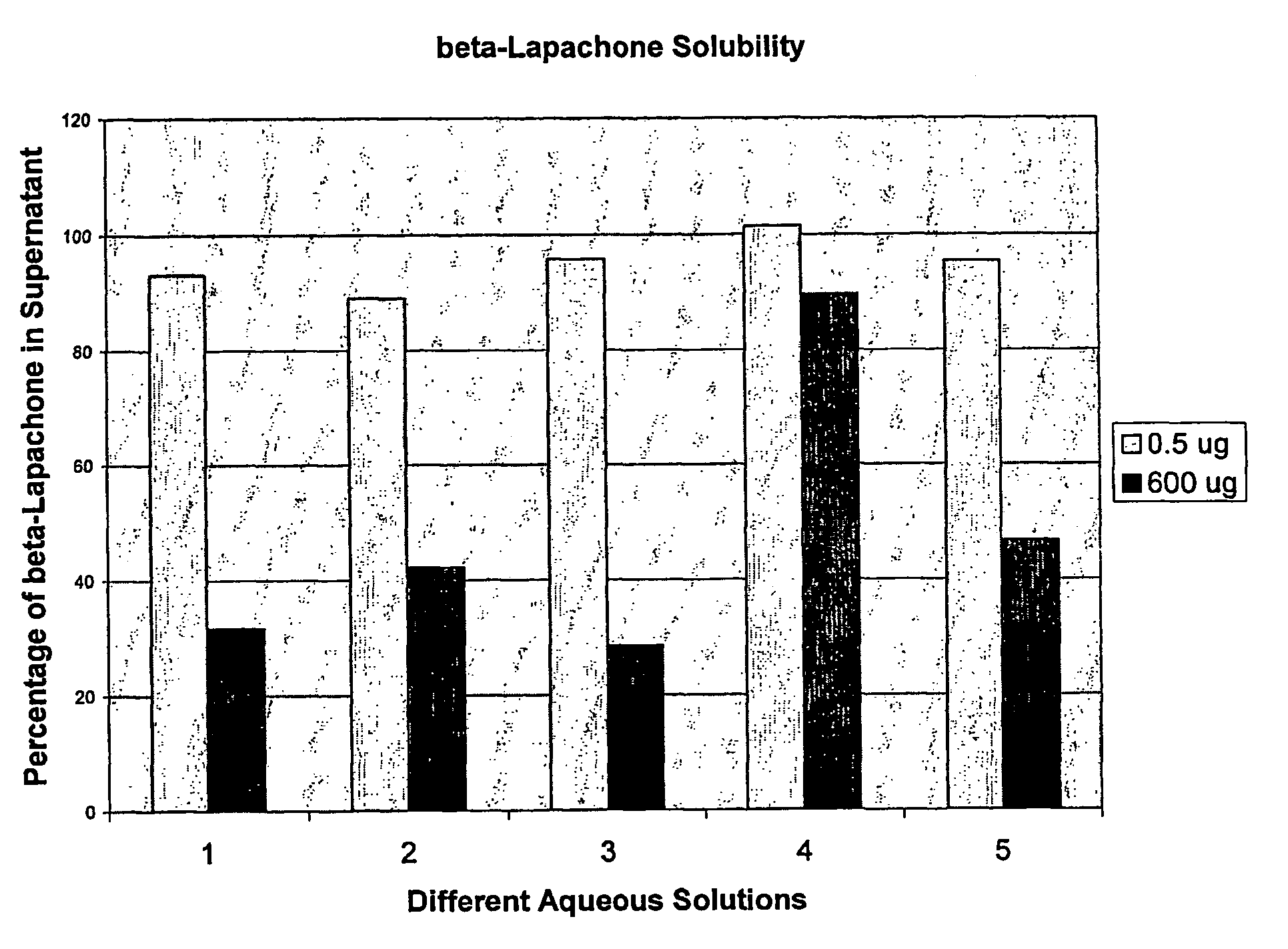
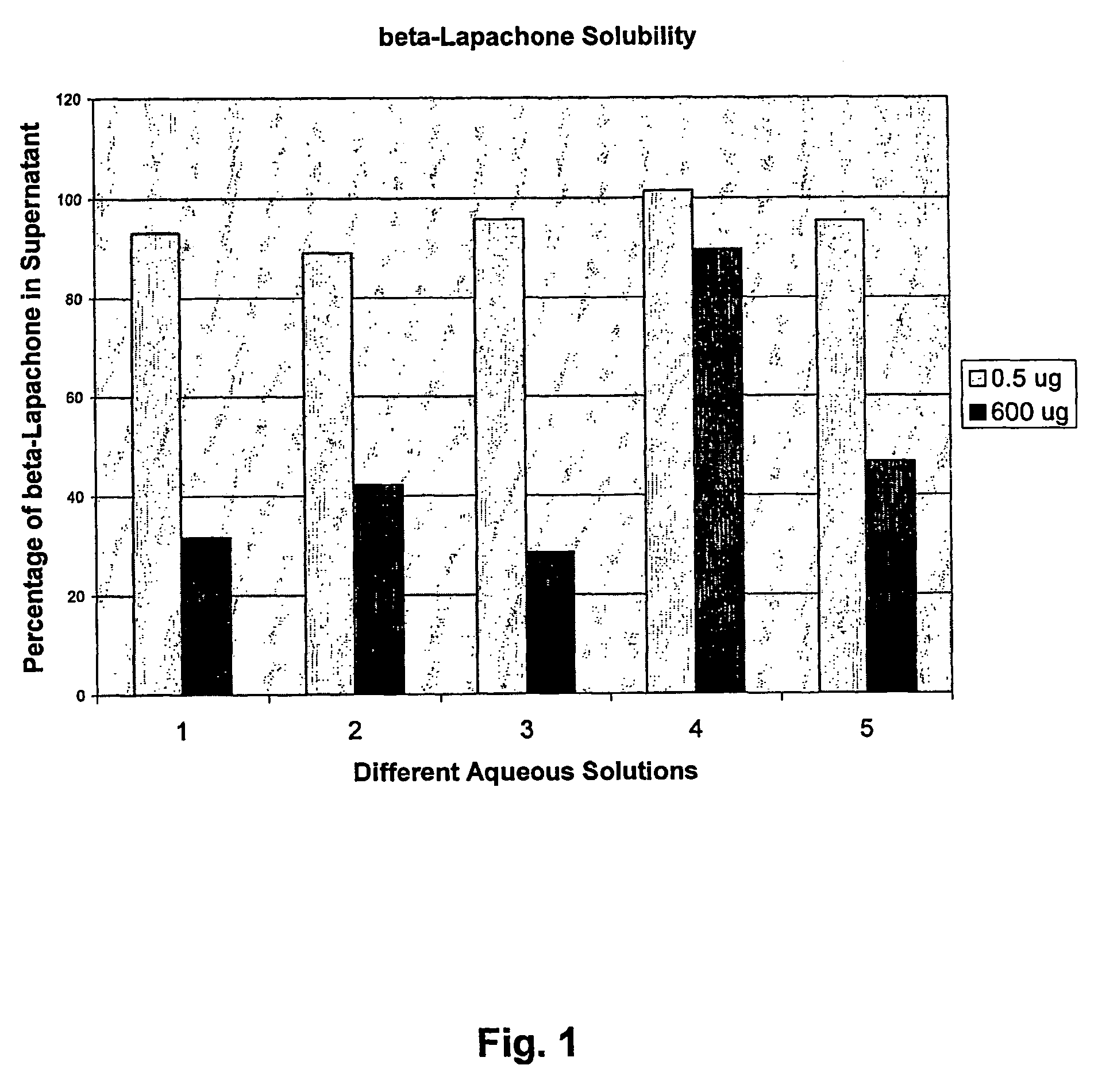
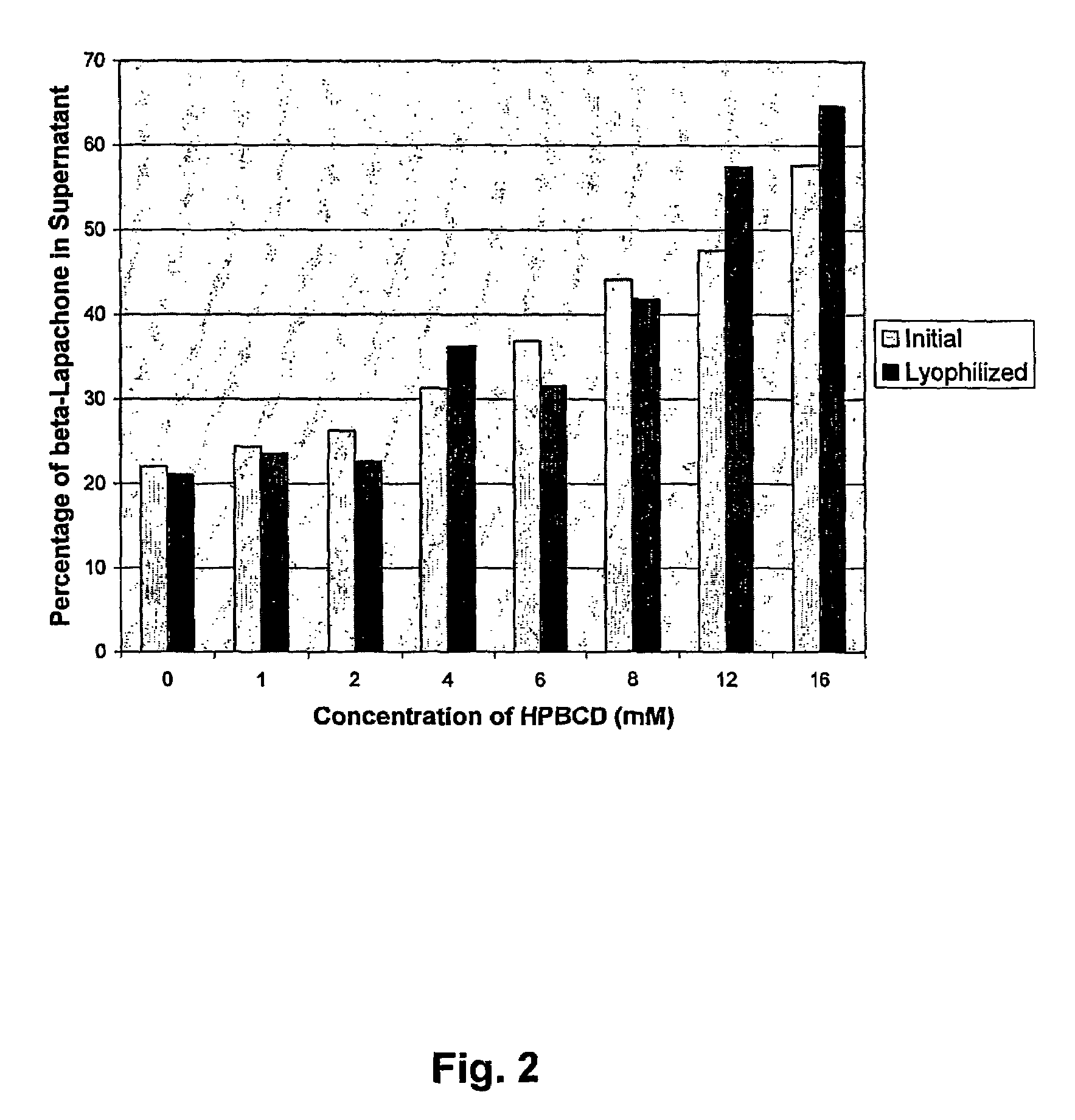
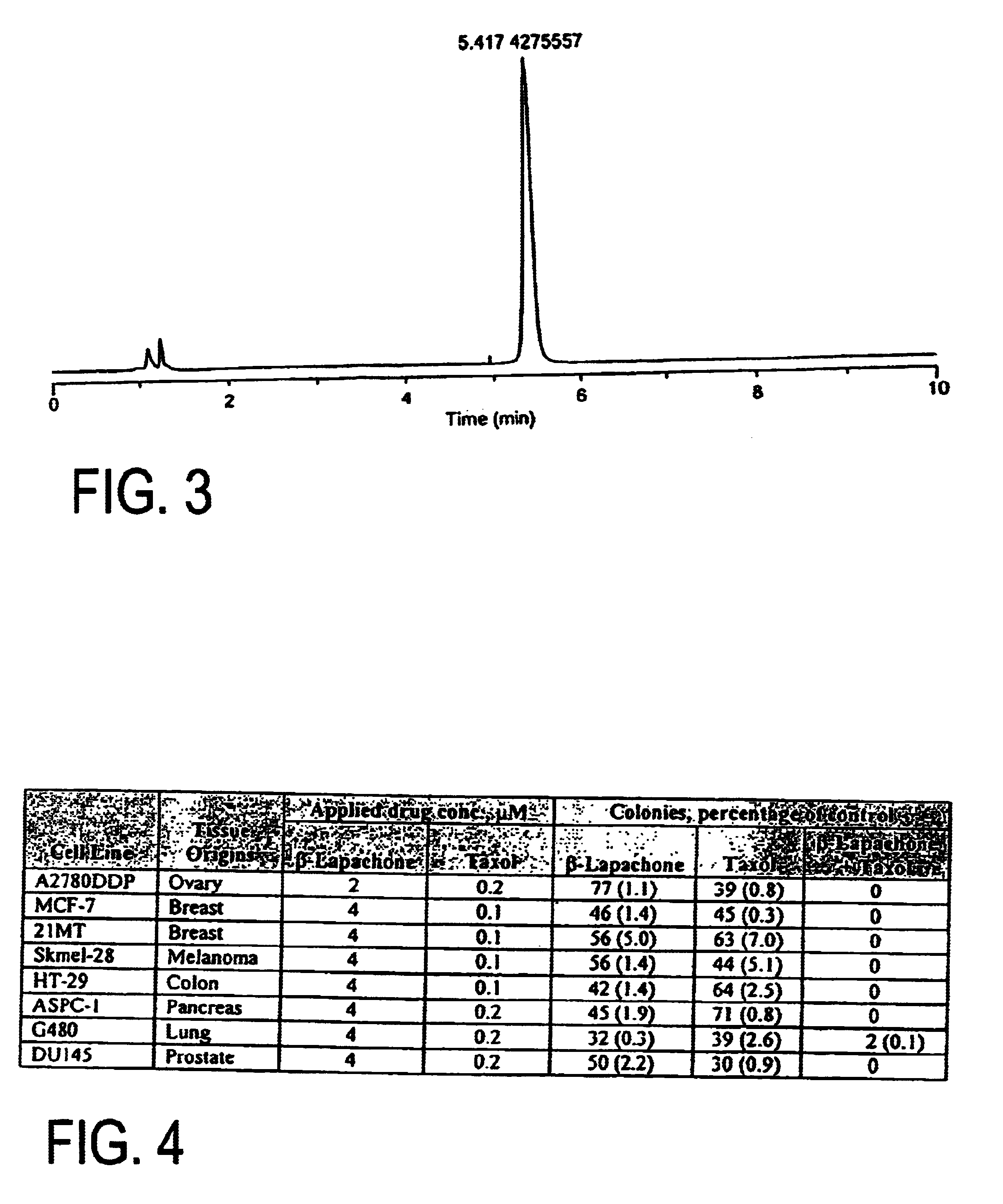
Pharmaceutical compositions containing beta-lapachone, or derivatives or analogs thereof, and methods of using same
Beta-lapachone, which is poorly soluble in most pharmaceutically acceptable solvents, has demonstrated significant antineoplastic activity against human cancer lines. The present invention overcomes this significant limitation by teaching novel pharmaceutical compositions comprising a therapeutically effective amount of Beta-lapachone, or a derivative or analog thereof, and a pharmaceutically acceptable solubilizing carrier molecule, which may be at water-solubilizing carrier molecule such as hydroxypropyl-β-cyclodextrin, or an oil-based solubilizing carrier molecule, for enhancing the solubility of Beta-lapachone in aqueous solution. The therapeutically effective amount of Beta-lapachone, or a derivative or analog thereof, may be complexed with the pharmaceutically acceptable solubilizing carrier molecule in aqueous solution. The novel pharmaceutical compositions may be administered with a second anticancer agent or in combination with radiation therapy. A formulation of Beta-lapachone or a derivative or analog thereof, complexed with a pharmaceutically acceptable solubilizing carrier molecule, wherein the complex can be freeze-dried and when subsequently reconstituted in aqueous solution is substantially soluble is also disclosed. Emulsions of Beta-Lapachone in a pharmaceutically acceptable fat emulsion vehicle are also provided. Also disclosed are methods for treating cancer by administering to a patient the novel pharmaceutical compositions and formulations. Pharmaceutical kits are also provided.
Owner:ARQULE INC
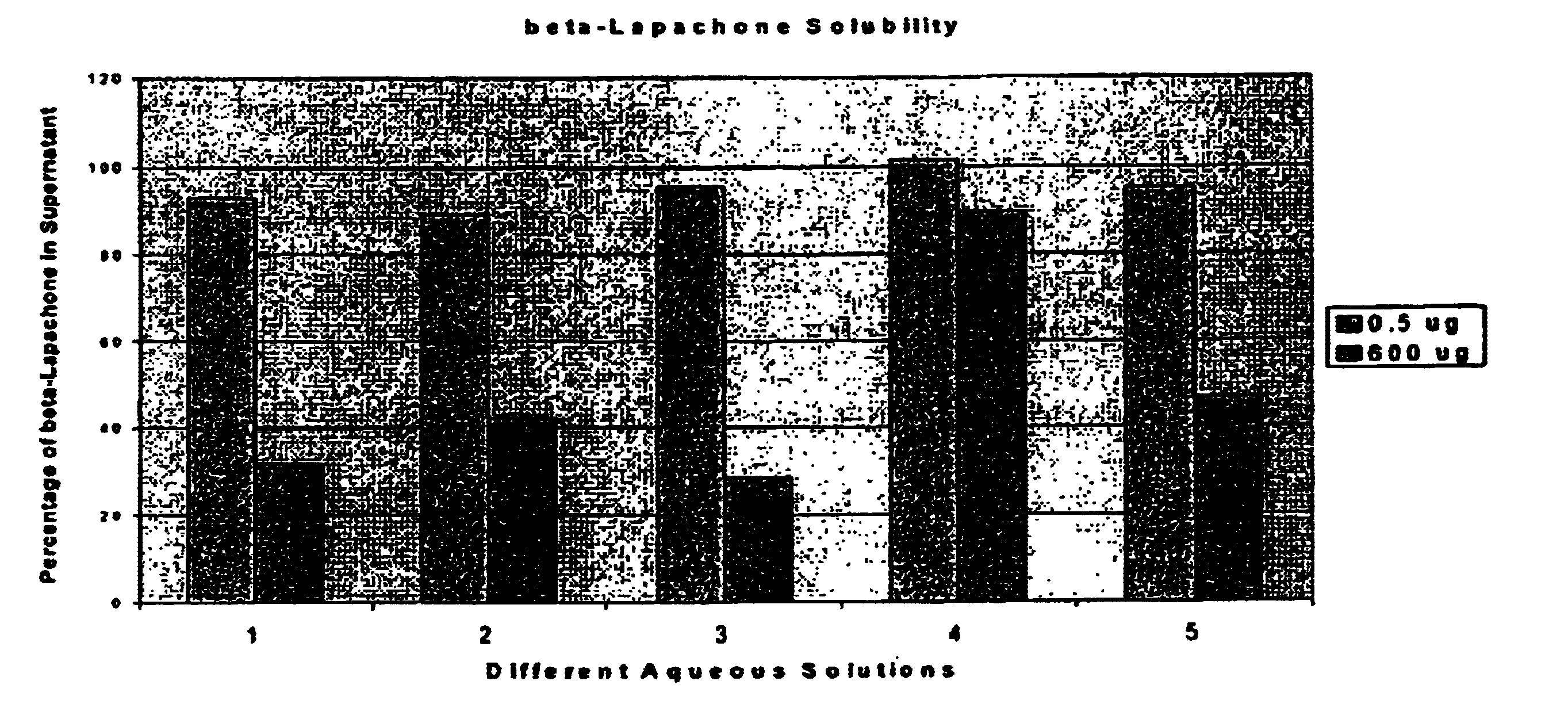
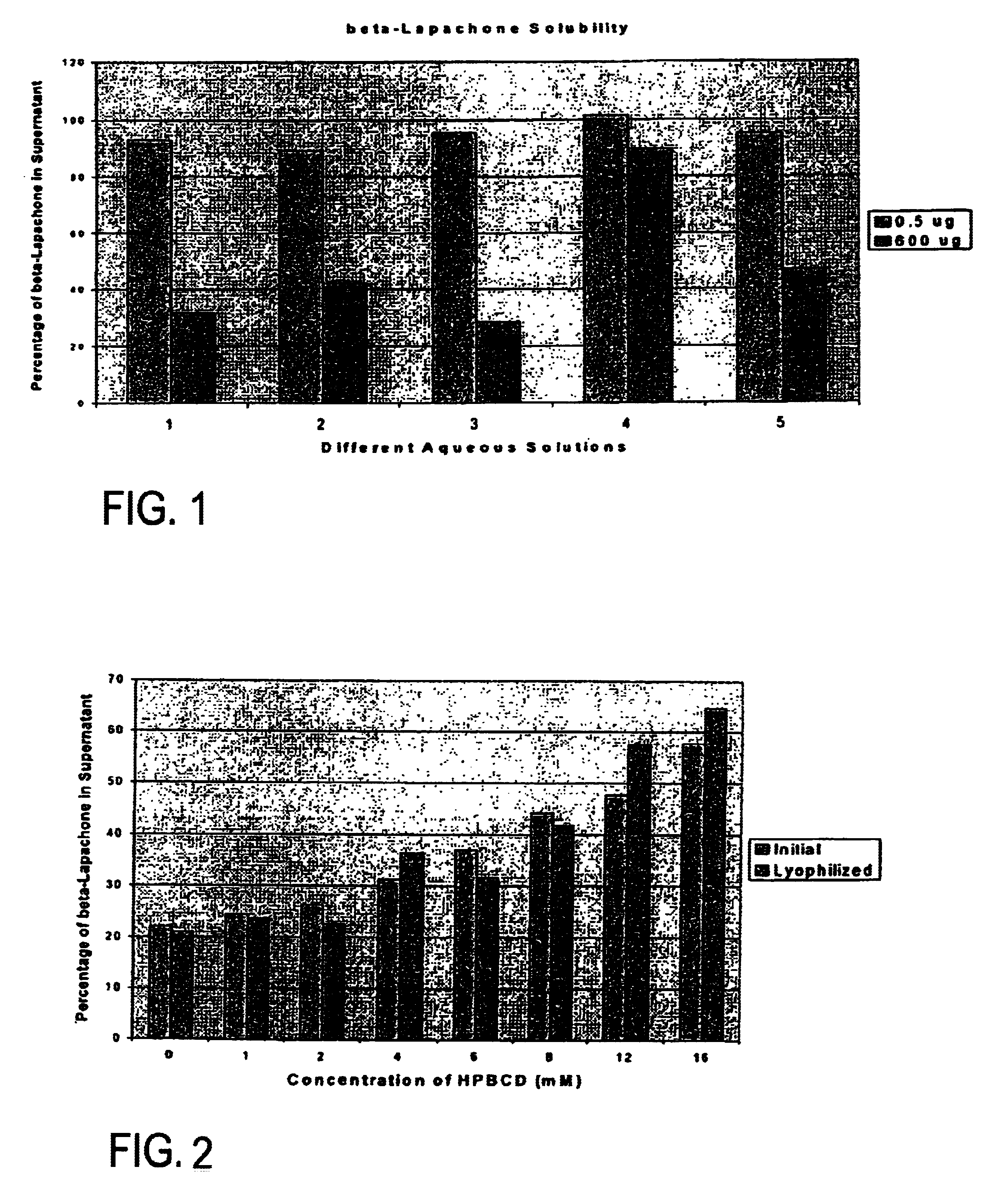
Pharmaceutical compositions containing beta-lapachone, or derivatives or analogs thereof, and methods of using same
Beta-lapachone, which is poorly soluble in most pharmaceutically acceptable solvents, has demonstrated significant antineoplastic activity against human cancer lines. The present invention overcomes this significant limitation by teaching novel pharmaceutical compositions comprising a therapeutically effective amount of Beta-lapachone, or a derivative or analog thereof, and a pharmaceutically acceptable solubilizing carrier molecule, which may be at water-solubilizing carrier molecule such as hydroxypropyl-β-cyclodextrin, or an oil-based solubilizing carrier molecule, for enhancing the solubility of Beta-lapachone in aqueous solution. The therapeutically effective amount of Beta-lapachone, or a derivative or analog thereof, may be complexed with the pharmaceutically acceptable solubilizing carrier molecule in aqueous solution. The novel pharmaceutical compositions may be administered with a second anticancer agent or in combination with radiation therapy. A formulation of Beta-lapachone or a derivative or analog thereof, complexed with a pharmaceutically acceptable solubilizing carrier molecule, wherein the complex can be freeze-dried and when subsequently reconstituted in aqueous solution is substantially soluble is also disclosed. Emulsions of Beta-Lapachone in a pharmaceutically acceptable fat emulsion vehicle are also provided. Also disclosed are methods for treating cancer by administering to a patient the novel pharmaceutical compositions and formulations. Pharmaceutical kits are also provided.
Owner:COPHARMA DEV +1
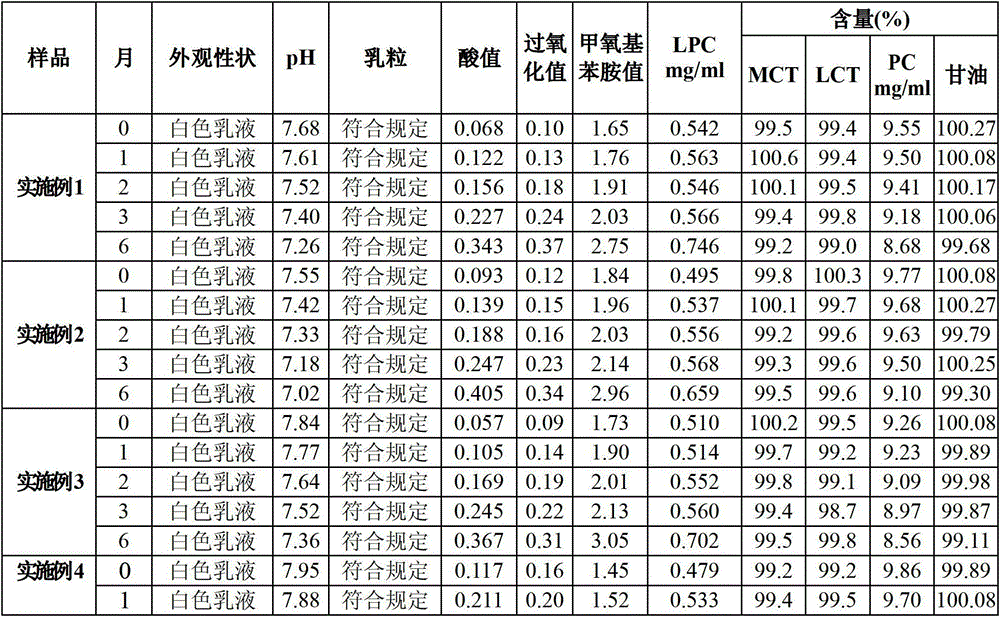
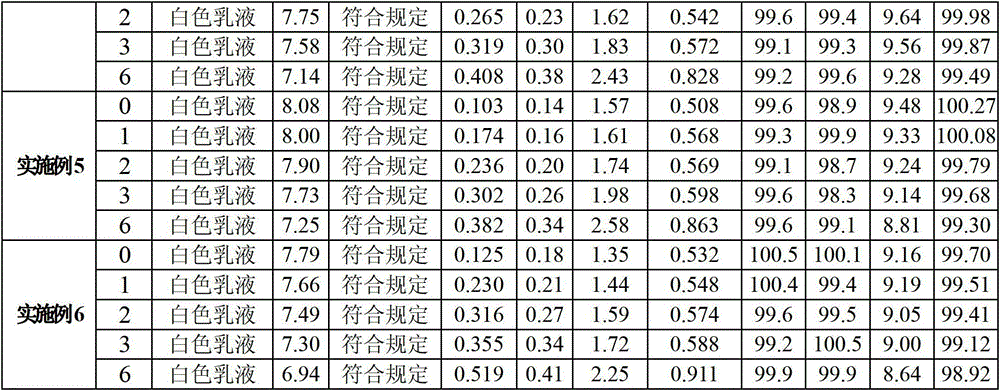
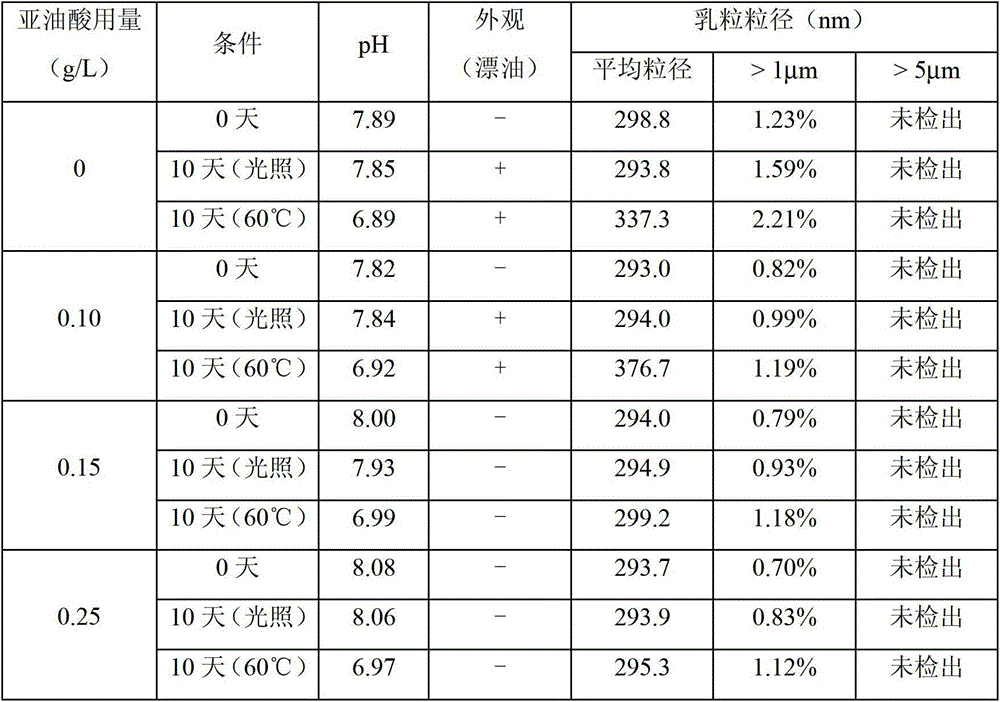
Medium/long-chain fat emulsion injection pharmaceutical composition and preparation method thereof
ActiveCN103330734ASimple production processImprove stabilityOrganic active ingredientsMetabolism disorderFat emulsionFiltration
The invention relates to a medium / long-chain fat emulsion injection pharmaceutical composition with improved stability and a preparation method thereof. The composition contains soybean oil for injection, medium-chain triglyceride for injection, lecithin for injection, glycerol for injection, linoleic acid for injection and water for injection. The preparation method comprises the steps of oil phase preparation, water phase preparation, initial emulsion preparation, homogenization, filter membrane filtration, filling, sterilization and the like. In the medium / long-chain fat emulsion injection pharmaceutical composition preparation process, the coemulsifier linoleic acid is added to optimize the preparation technique so as to enhance the emulsifying capacity of the system and reduce and stabilize the emulsion particle size, thereby overcoming the defects of floating oil, enlarged emulsion particle and the like in the prior art; and the invention reduces the number of times of homogenization, enhances the productivity, lowers the methoxyaniline value of the product, and improves the stability and safety of the composition.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Medicinal composition fat emulsion injection containing eucalyptol, limonene and alpha-pinene and preparation method
ActiveCN101590033AActive ingredients are clearImprove bioavailabilityMetabolism disorderRespiratory disorderYolkGlycerol
The invention aims to develop medicinal composition fat emulsion injection containing eucalyptol, limonene and alpha-pinene and a preparation method. The preparation comprises the following components in percentage by weight: 0.0128 to 0.0320 percent of eucalyptol, 0.0084 to 0.0210 percent of limonene, 0.0028 to 0.0070 percent of alpha-pinene, 10 to 30 percent of soybean oil for injection, 1.0 to 1.5 percent of yolk lecithin for injection or 0.8 to 1.5 percent of soybean phospholipids for injection, 2.0 to 2.5 percent of glycerol for injection, and water for injection added to 100 milliliters. The preparation method has the characteristics of strong controllability of production quality, and definite treatment effect of a product. The invention fills up the blank of the medicinal composition fat emulsion injection preparation containing the eucalyptol, the limonene and the alpha-pinene.
Owner:BEIJING GRAND JOHAUM PHARMA CO LTD
Fat emulsion providing taste masking for active health and wellness ingredients
InactiveUS20140080906A1Improve liquidityHealthier unsaturated fatsOrganic active ingredientsBiocideFiberAntioxidant
The embodiments relate to fat emulsion structures based on both an aqueous and non-aqueous glycerin component as the primary aqueous component in which the fat emulsion can create a wide range of viscosities that mimic fat structures similar to cream, or all the way to hardened fat structures like Trans Fat. The fat emulsion can be added to a wide group of foods that use a monosaccharide or disaccharide as the basis for its sweetener component, can lower the sugar content of foods, can improve mouth feel while lowering the fat content in high fat foods, can add a balance of dietary fats and fiber to foods, and can add antioxidant content to food products. The fat emulsion also can be used as a taste masking composition to mask the taste of unpalatable active ingredients or unpalatable components that may be added to the emulsion.
Owner:ANTIOXIDANT SUPERFOODS
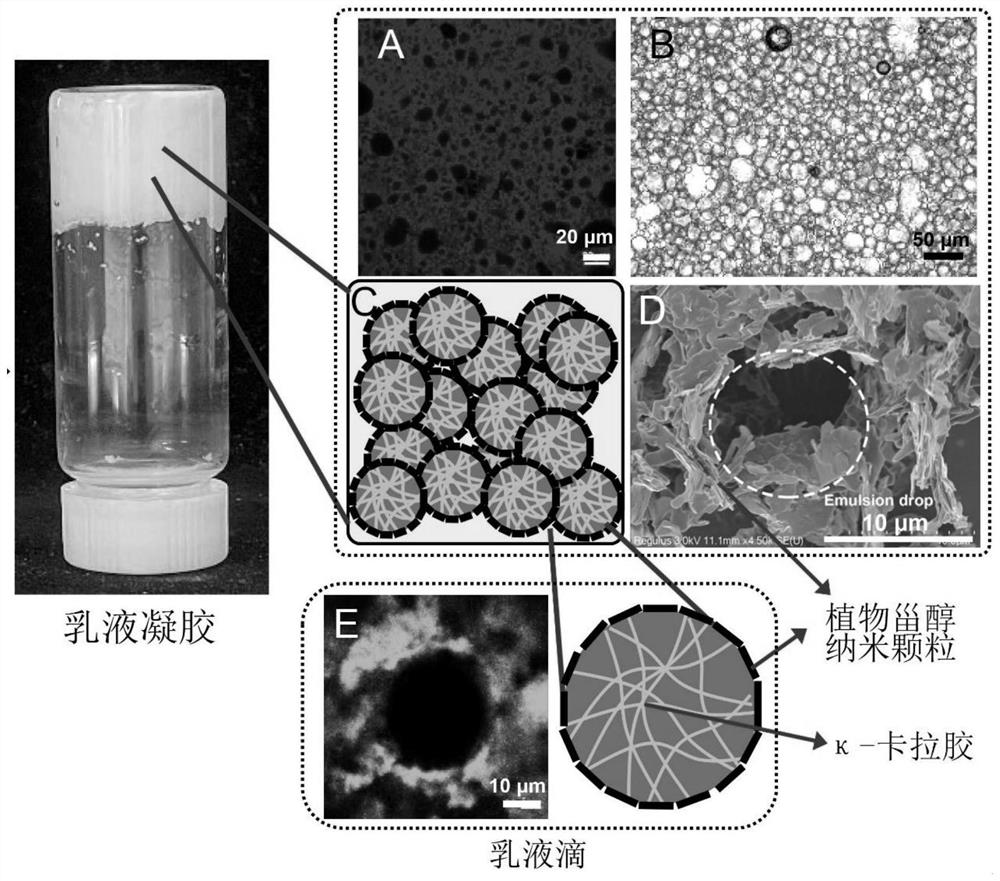
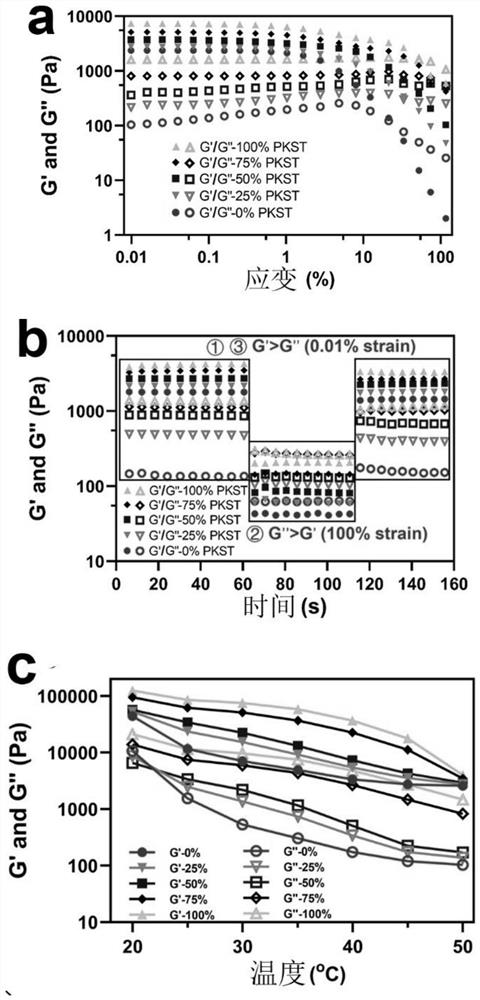
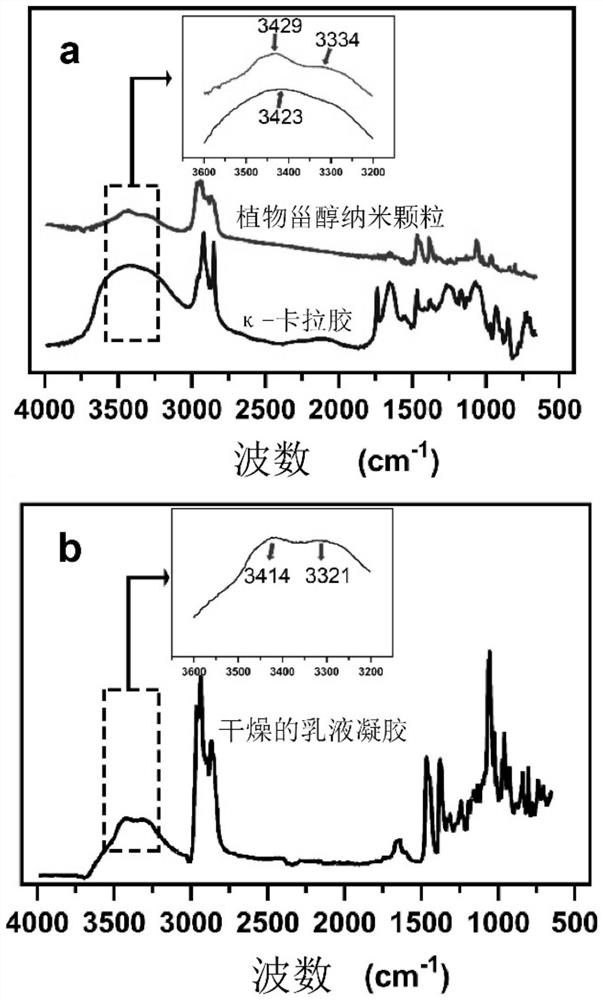
Dual-network zero-trans-fat-like emulsion gel capable of 3D/4D printing and preparation method of dual-network zero-trans-fat-like emulsion gel
ActiveCN114468062AImprove thixotropyGood shaping effectAdditive manufacturing apparatusCocoaBiotechnologyVegetable oil
The invention discloses dual-network zero-trans fat-like emulsion gel capable of 3D / 4D printing and preparation, and belongs to the technical field of healthy grease and food processing. The method for preparing the emulsion gel comprises the following steps: (1) adding a hydrophilic colloid into hot water containing emulsifier nanoparticles, and dissolving to obtain an aqueous solution; wherein the mass concentration of the nanoparticles in the aqueous solution is 0.5-15%; (2) dissolving oil-soluble small molecules in the heated vegetable fat, and uniformly mixing to obtain an oil solution; or mixing and heating a plurality of vegetable oils and fats to obtain mixed oil and fat; and (3) mixing the aqueous solution in the step (1) and the oil solution or the mixed grease in the step (2) according to a volume ratio of 1: 1-9: 1, homogenizing and emulsifying to obtain the emulsion gel. The emulsion gel provided by the invention can partially or completely replace traditional fat in chocolate, ice cream, non-dairy cream and other foods, and has the characteristics of zero trans-low saturated fatty acid nutrition and health.
Owner:JIANGNAN UNIV
Middle/long chain triglyceride flurbiprofen axetil injection and preparation method thereof
InactiveCN102552133AApplicable useTo achieve the therapeutic effect of anti-inflammatory and analgesicOrganic active ingredientsAntipyreticCritically illSide effect
The invention provides anti-inflammatory and analgesic flurbiprofen axetil injection and a preparation method thereof. A mixture of 0.1-10 percent (w / v) of flurbiprofen axetil, 10-20 percent (w / v) of middle chain triglyceride (MCT) and 10-20 percent (w / v) of long chain triglyceride (LCT) is contained in the prescription (wherein the proportion of MCT and LCT is 4:1-1:4). The preparation method comprises the following steps: taking 1-5 percent (w / v) of lecithin as an emulsifying agent, taking 1-5 percent (w / v) of glycerol as an isotonic agent, finally adding disodium hydrogen phosphate, citric acid and water for injection and adjusting the pH value to 4-7 to prepare flurbiprofen axetil fat emulsion injection through colostrums, homogeneity and sterilization. The injection has the beneficial effects that the anti-inflammatory and analgesic effect is taken more quickly; the accumulation of fat of a human body can be reduced, and fatty tissues and liver load can be reduced; and the injection is more suitable for critically ill patients and people with poor liver functions and has lower toxic and side effects.
Owner:GUANGDONG JIABO PHARM CO LTD
Clevidipine butyrate fat emulsion injection and preparation process thereof
The invention provides a clevidipine butyrate fat emulsion injection for intravenous injection. The clevidipine butyrate fat emulsion injection is prepared from an active ingredient, namely clevidipine butyrate and excipients, namely oil for injection, an emulsifier, an isoosmotic adjusting agent, a pH value regulator and water for injection. The fat emulsion injection for the intravenous injection is developed by taking the clevidipine butyrate as the active ingredient and adding some specific kinds of excipients in a specific proportion by the preparation process; and each milliliter of clevidipine butyrate fat emulsion injection contains 0.5 or 1.0mg of the clevidipine butyrate.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Hippophae rhamnoides seed oil fatty milk injection and technique of preparing the same
The invention discloses a medicament injection, which particularly relates to an injection which contains sea-buckthorn seed oil fat emulsion and a process for preparation. The sea-buckthorn seed oil fat emulsion of the invention is prepared according to the following components and proposition, 5 to 30g sea-buckthorn seed oil for injecting in each 100ml injection, 0.5 to 7.5g emulsifier, 1.5 to 3.6g isotonic regulator, 0.001 to 0.2g anti-oxidant, hydrochloric acid solution or sodium hydrate solution whose PH value is adjusted from 6.5 to 9.5, and the other are water for injecting. The proportions of the sea-buckthorn seed oil fat emulsion injection w-6 and w-3 are proper, and the stability is good, and the invention can not only provide heat energy for human bodies to replenish essential fatty acid for human bodies, but also the invention has the functions of improving body immunity, prompting tissue repair, anti-inflammatory anti-radiation and anti-mutation, and inhibiting cancer cell to diffuse. The invention particularly has a promoting effect to physical rapid restoration of postoperative patients, patients with large areas of burn, and cancer patients who have radiotheraphy and chemotherapy.
Owner:SHENYANG PHARMA UNIVERSITY
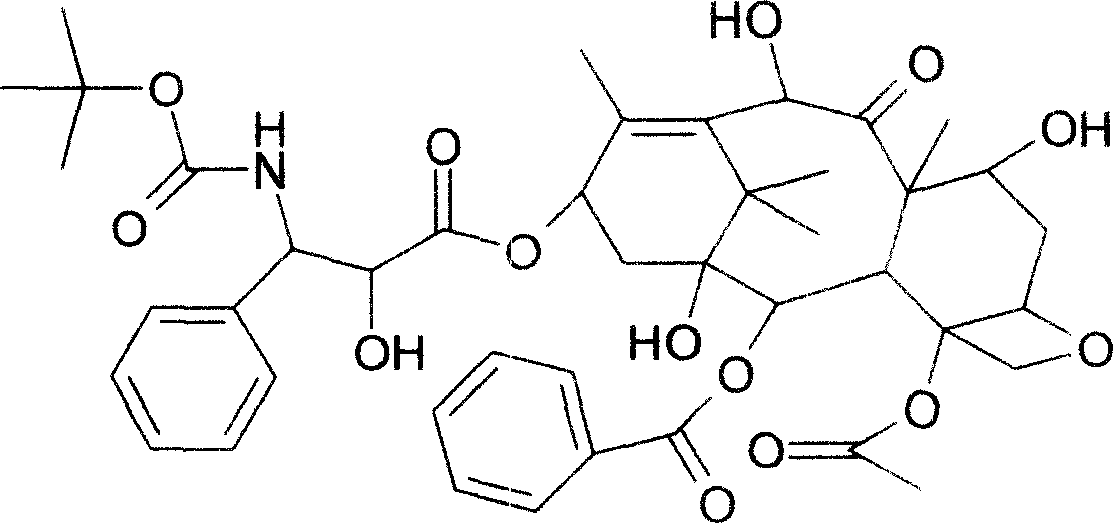
Compound taxol and its derivative docetaxel fat emulsion and preparation method
InactiveCN101006997APro-apoptosisGood anticancer effectOrganic active ingredientsEmulsion deliverySolubilityVegetable oil
The invention relates to complex paclitaxel and its derivates docetaxel intralipid which includes the following ingredients: paclitaxel or docetaxel, vegetable oil, solubilizing agent, lecithin, glycerine, and water for injection at a ratio of 0.5-10:10-100:10-100:10-20:20-25:700-950. The preparing method includes the following steps: stirring with high speed homogenating machine or ultrasonic oscillating to get the protogala; preparing the complex paclitaxel intralipid with high pressure homogenizer. The preparation is intralipid in O / W type which packages paclitaxel or docetaxel into the compound oil phase. The compound oil phase has good solubility for paclitaxel or docetaxel which has prevented the phenomenon of precipitation after diluting the emulsion; the ingredients of compound oil has the function of coordinated antitumous effect; it can also release the injecting irritative response, haemolysis and hypersensitiveness; it has the function of targeting which has increased the drug action.
Owner:董英杰
Fat emulsion injection and preparation method thereof
ActiveCN104739764AReduce wide distribution problemsReduce adverse reactionsMetabolism disorderEmulsion deliveryFat emulsionFish oil
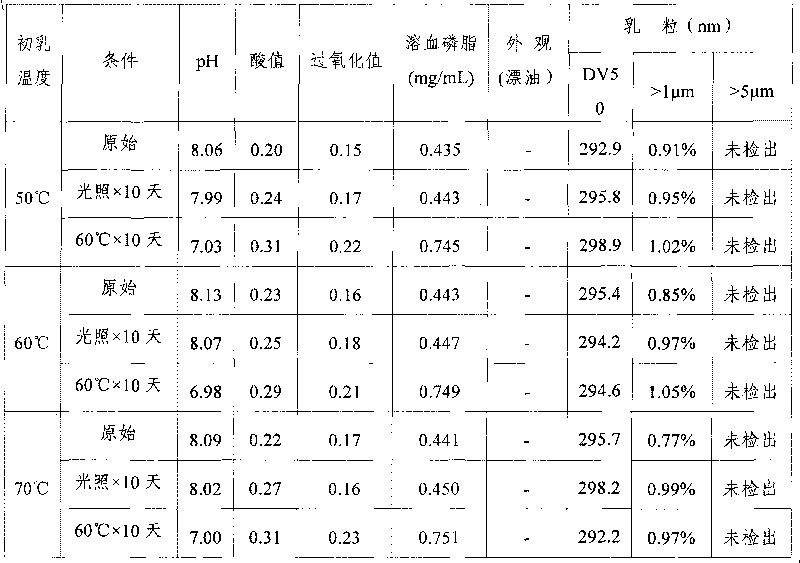
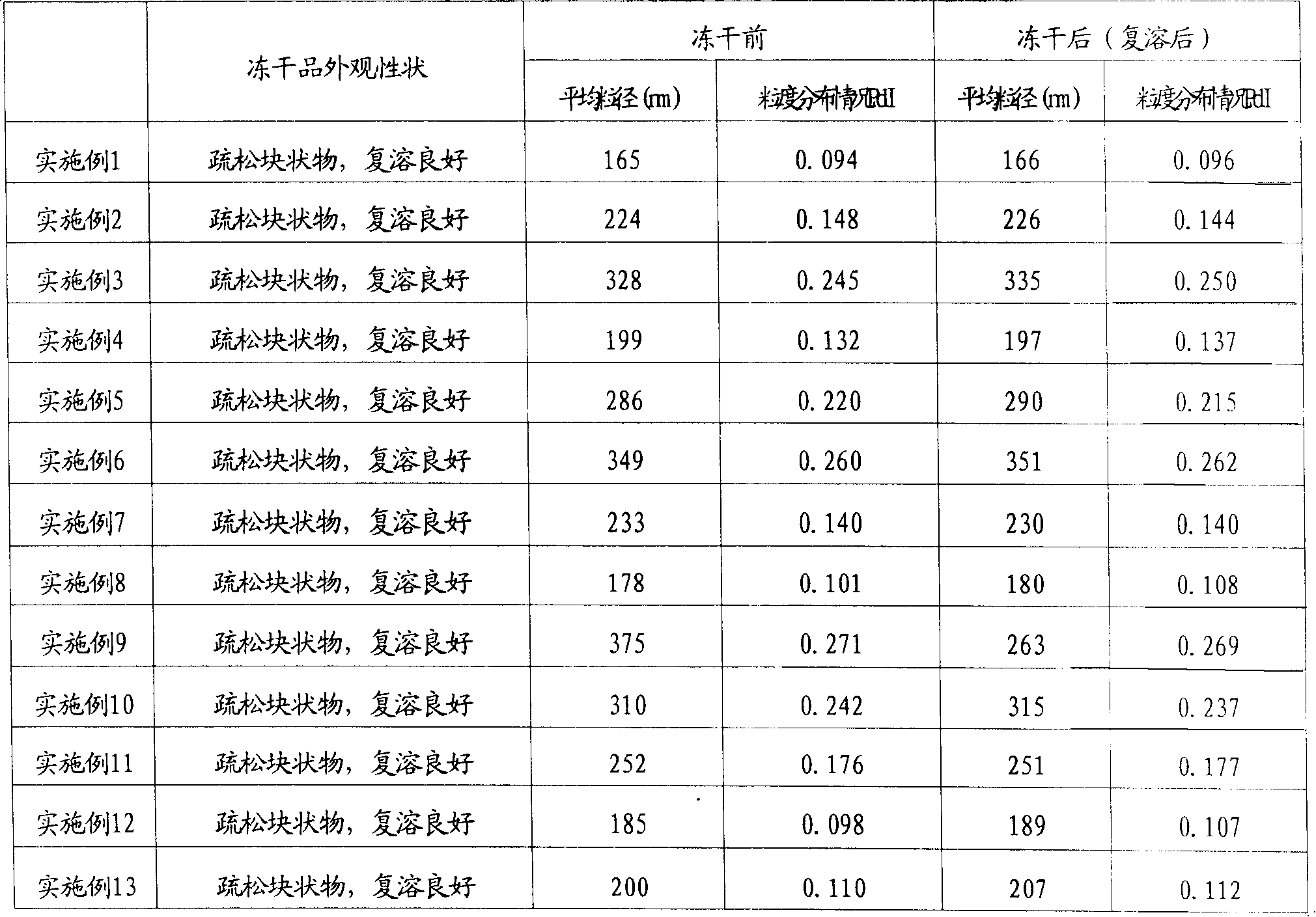
The invention provides a fat emulsion injection. The fat emulsion injection is prepared from an oil phase, an aqueous phase, a stabilizer and a pH regulator, wherein the oil phase contains one or more of soybean oil, medium chain triglyceride, olive oil and fish oil; in the fat emulsion injection, emulsion particles with particle size more than or equal to 0.1 micron account for more than 80% and emulsion particles with particle size more than or equal to 1.0 micron account for 0%. The invention also provides a method for preparing the fat emulsion injection. The invention can better relieve the problem of relatively narrow emulsion particle distribution, enhance the uniformity of the emulsion particles and improve the stability of the fat emulsion injection; and the adverse reaction of the emulsion agent is reduced and administration safety is enhanced.
Owner:SICHUAN KELUN PHARMA CO LTD
Fat emulsion for artificially feeding seriously ill intensive care patients
InactiveCN102215839AAntibacterial agentsNervous disorderCritical illness polyneuropathyCritically ill
The present invention relates to a pharmaceutical preparation for the prophylaxis and treatment of critical illness polyneuropathy (CIP) and critical illness myopathy (CIM). The invention further relates to an isotonic fat emulsion comprising at least one triglyceride that comprises at least one fatty acid group having an odd number of carbon atoms, wherein the fatty acid group comprises a carbon chain having 5 to 15 carbon atoms.
Owner:B BRAUN MEDICAL
Fat emulsion injection and production method thereof
ActiveCN101700225AImprove defects such as floating oil and enlarged milk particlesSmall particle sizeOrganic active ingredientsMetabolism disorderFat emulsionOil phase
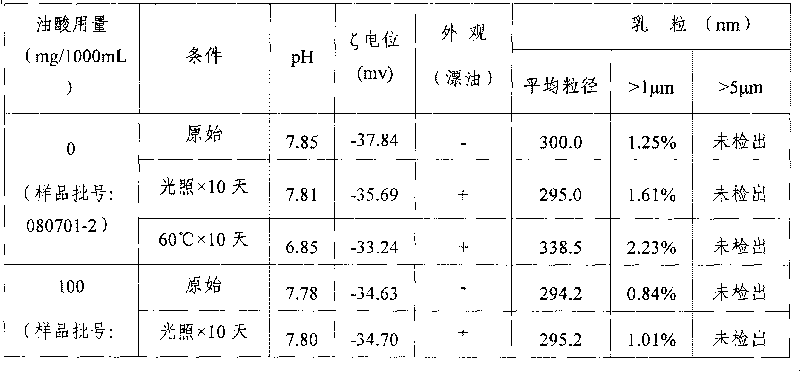
The invention relates to a fat emulsion injection and a manufacture method thereof. As the fat emulsion injection contains oleic acid, the fat emulsion injection can provide enough and stable emulsion particles when used as the auxiliary emulsifier so as to overcome the defects of oil floating, enlarged emulsion particles and the like of the emulsion. The method for manufacturing the fat emulsion injection adjusts the production technology in the production process by adding oleic acid in oil phase so as to improve the emulsifying capability, reduce the grain size of the emulsion particles, improve the stability of emulsion particles, reduce the value of methoxyaniline, reduce the number of homogenization, and improve the production yield.
Owner:ANHUI FENGYUAN PHARM CO LTD
Linseed oil fat emulsion oral solution, beverage and manufacturing method thereof
The invention provides a linseed oil fat emulsion oral solution, a beverage and a manufacturing method thereof. The linseed oil fat emulsion oral solution is prepared by raw materials including refined linseed oil, refined lecithin, glycerol, bacteria-free pure water, antioxidant, stabilizer, pH regulator, flavoring agent, spice and the like. The manufacturing process comprises the steps that: under the protection of nitrogen, the refined linseed oil and the refined lecithin are dissolved by heating into oil phase liquid; the glycerol, the antioxidant, the stabilizer, the flavoring agent, the spice and the bacteria-free pure water are dissolved by mixing into water phase liquid, the oil phase liquid is slowly added into the intensely-stirred water phase liquid in order to form colostrum liquid by means of high-speed shearing, pH of the colostrum liquid is regulated to the range from 6.5 to 8.5 by a little pH regulator, the colostrum liquid is then emulsified by a high pressure homogenizer and filtered by a microporous membrane filter, and the linseed oil fat emulsion oral solution containing 1%-50% of linseed oil is obtained after filling, sealing, microwave sterilization and heating sterilization. The linseed oil fat emulsion oral solution is used for the field of healthy food, the linseed oil fat emulsion oral solution with 5-50% of high concentration can be used as nutritious healthcare beverage; and the linseed oil fat emulsion oral solution with 1-20% of low concentration can be used as dining and popular beverage.
Owner:王京南
Glossy ganoderma spore oil fat emulsion
ActiveCN1616074AQuick effectFully absorbedUnknown materialsEmulsion deliverySporeNutrition supplementation
The present invention relates to tumor treating medicine, and is a kind of fat emulsion with spore oil extracted from glossy ganoderma as one Chinese medicinal material. The medicine contains glossy ganoderma spore oil 2.0-8.0 g, fatty oil 3.0-18.0 g, emulsifier 0.8-10 g, and isosmotic agent 0.5-6.0 g except injection water in each 100 ml. The medicine has the antitumor effect of glossy ganoderma spore oil and the nutrients replenishing effect of the fatty oil, and is suitable for salvage of serious tumor patient and post-operational nourishing. It may be used in arterial injection and intravenous injection to result in fast acting.
Owner:GUANGZHOU HANFANG PHARMA
Nimodipine lyophilized emulsion for injection and preparing method thereof
InactiveCN101199522AAvoid stimulationAvoid harmOrganic active ingredientsPowder deliveryFreeze-dryingNimodipine
The invention relates to nimodipine lyophilization dry emulsion for injection. Before freeze-dried or reconstituted, according to percentage concentration per 1000 ml of fat emulsion, the lyophilization dry emulsion contains 0.001 percent to 0.2 percent of nimodipine, 0.5 percent to 30 percent of oiliness solvent, 0.1 percent to 5 percent of emulsifier, 5 percent to 40 percent of the freeze-drying protective agent and 0.1 percent to 10 percent of isotonic regulator. The invention also relates to a preparation method of nimodipine lyophilization dry emulsion. The invention has the advantages that ethanol is avoided to decrease irritation; the product can be mixed with any proportion of water for injection, sodium chloride solution, glucose solution, blank fat emulsion or other aqueous solution without phenomena of precipitation or crystallization; in addition, compared with the fat emulsion, the lyophilization dry emulsion is more helpful to improve the stability of nimodipine and excipient of the nimodipine, thereby lowering the requirements of production, transportation and storage conditions and prolonging the period of validity.
Owner:YAOPHARMA CO LTD +1
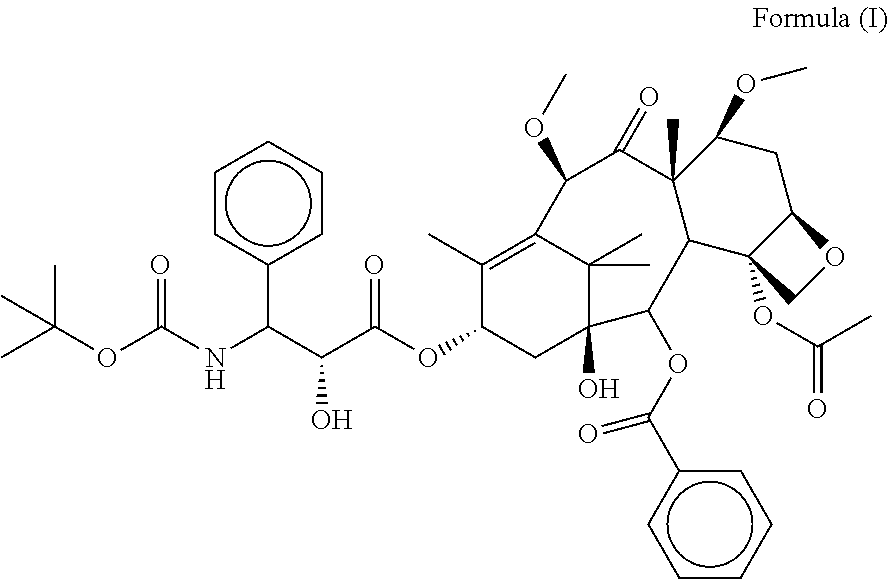
Cabazitaxel fat emulsion, and preparation method and use thereof
InactiveUS20180153848A1Excellent long-term storage stabilityExcellent resolubilityOrganic active ingredientsPharmaceutical non-active ingredientsCabazitaxelMedicine
Provided in the present invention is a cabazitaxel fat emulsion injection, wherein the cabazitaxel fat emulsion injection contains cabazitaxel, a medium chain triglyceride for injection, and lecithin. Also provided in the present invention are the method for preparing the cabazitaxel fat emulsion injection and the use thereof in preparing a drug for treating prostate cancer.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com