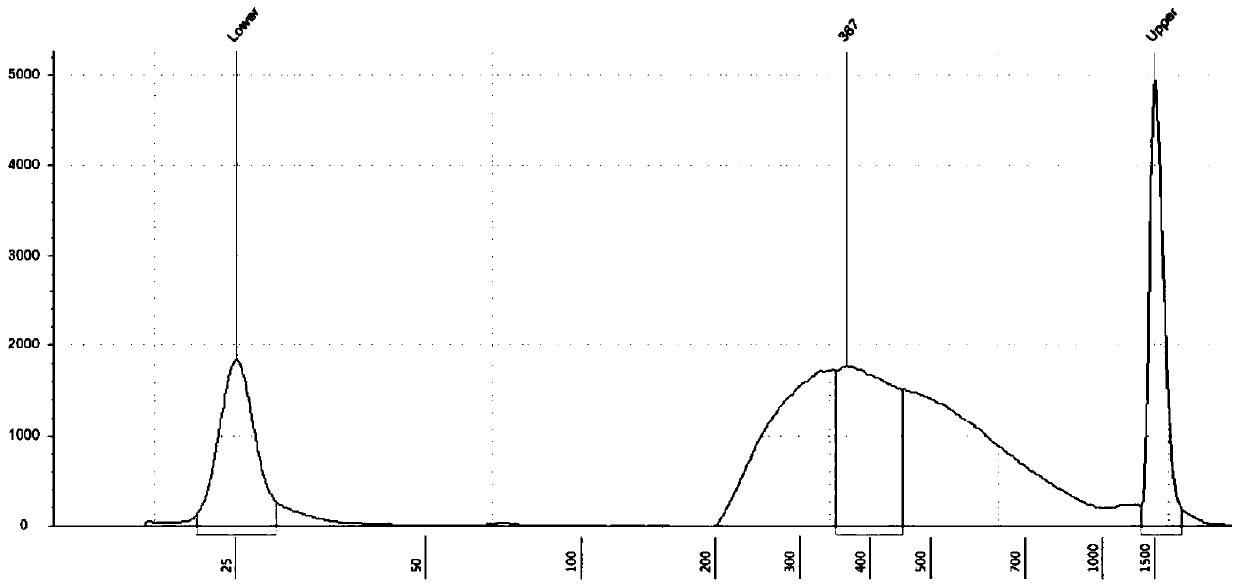
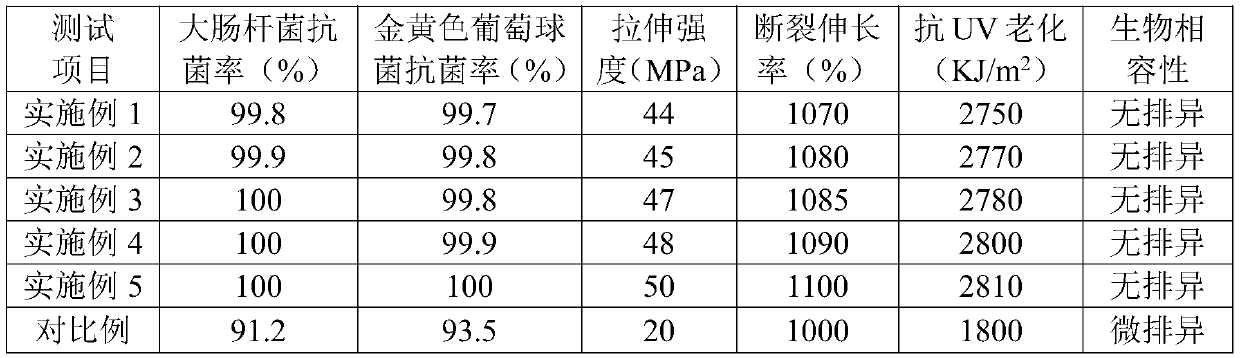
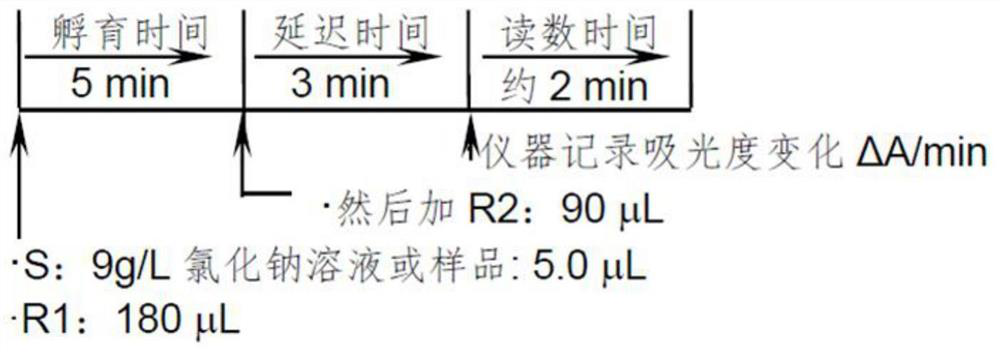
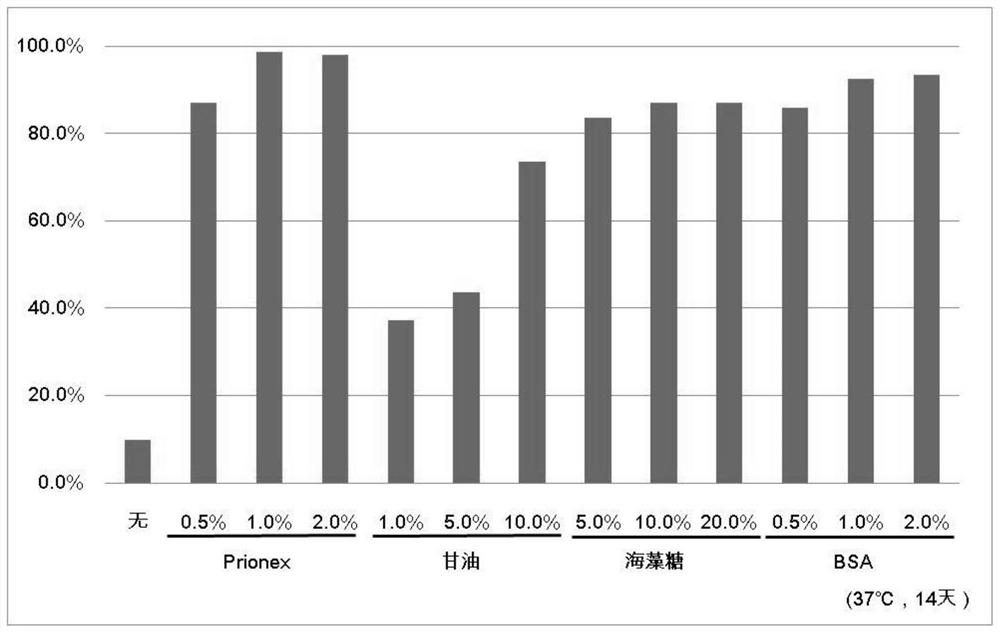
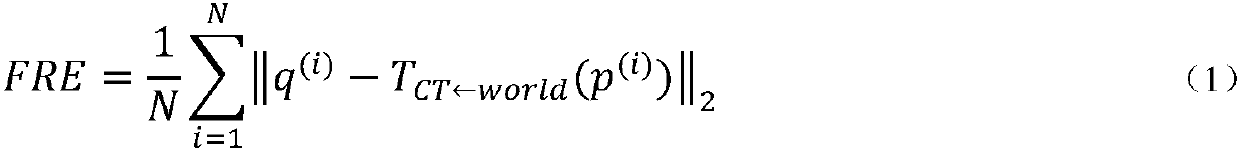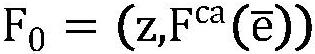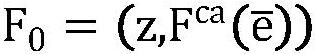Patents
Literature
85results about How to "Good clinical applicability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
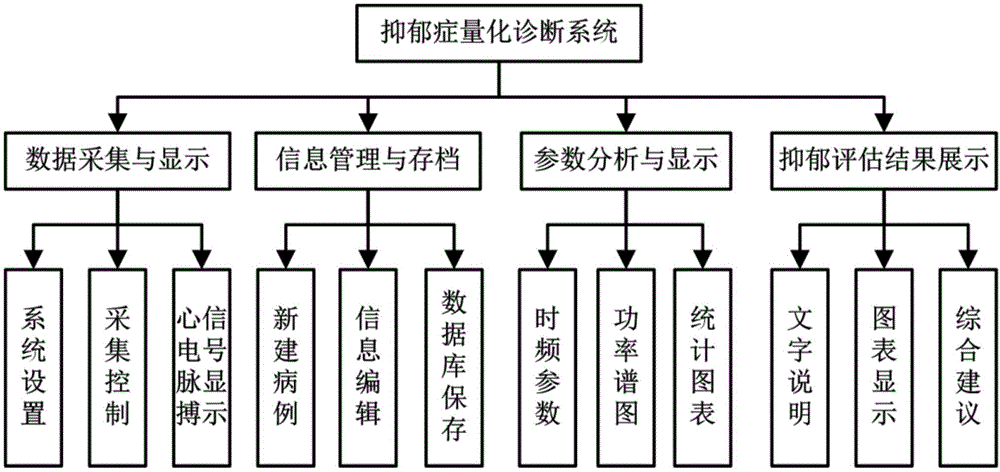
Depression evaluating system and method based on heart rate variability analytical method
ActiveCN104127194AAvoid subjectivityAvoid variabilitySensorsPsychotechnic devicesPerinatal DepressionPeak value
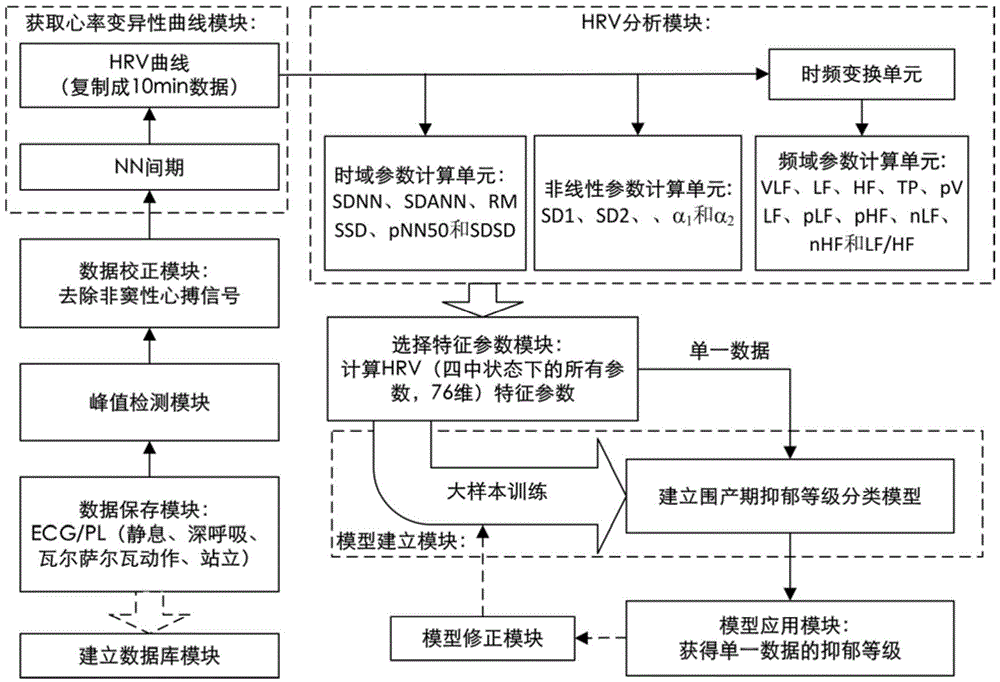
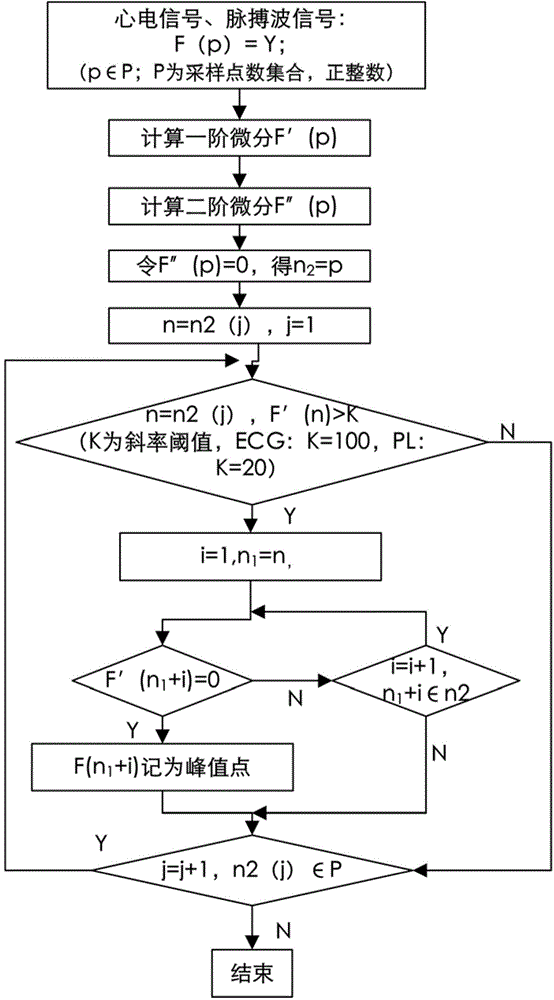
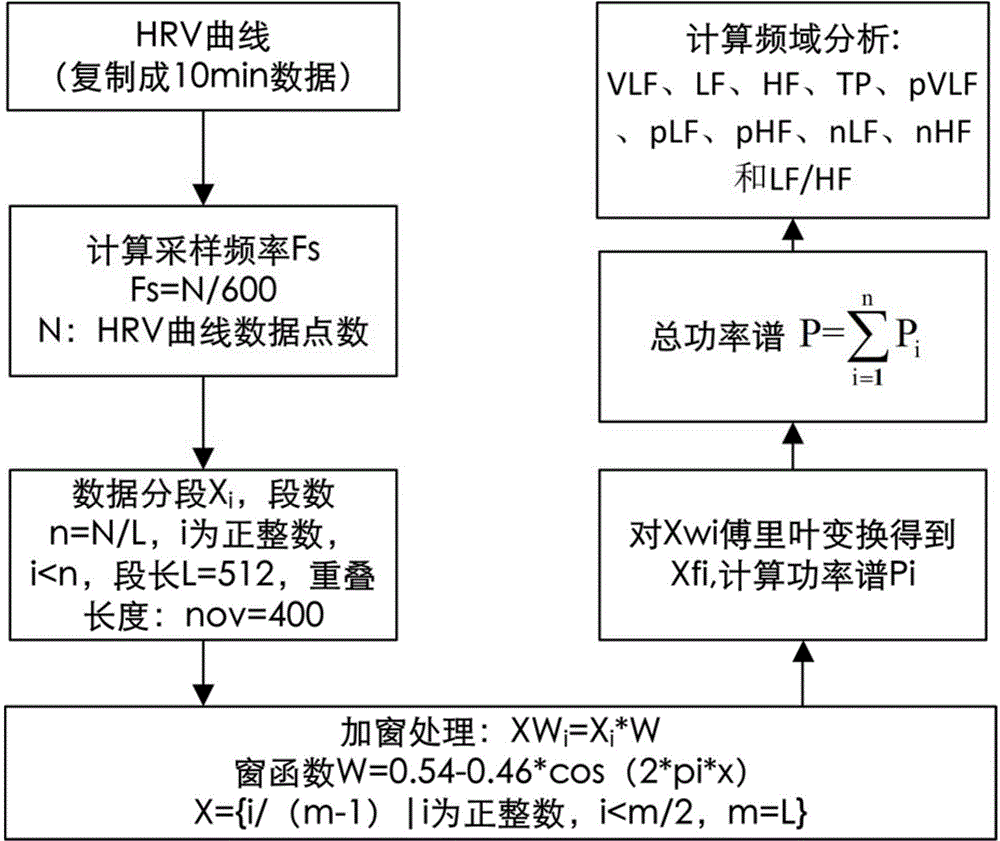
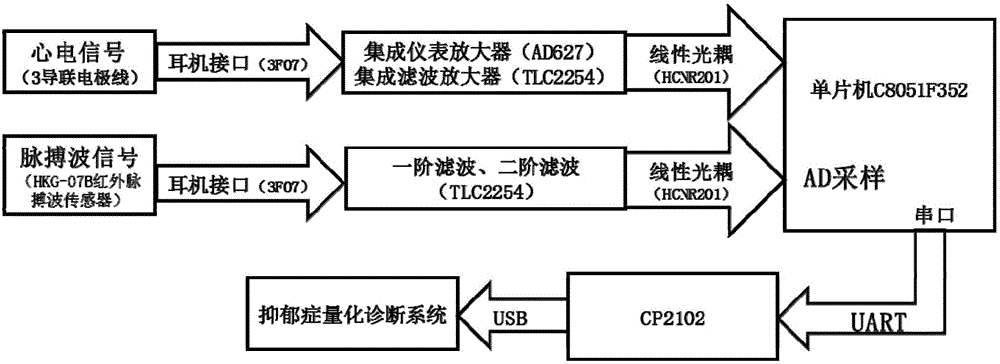
The invention discloses a depression evaluating system and method based on a heart rate variability analytical method, particularly a depression evaluating system and method in a perinatal period. The depression evaluating system comprises a data storage module used for storing electrocardio and pulse wave signals, a peak detecting module used for acquiring a peak point sequence of recording data, a data correcting module used for acquiring sinus beat NN interval sequence, a heart rate variability curve acquiring module used for acquiring a heart rate variability curve, an HRV analyzing module conducting time domain analysis, frequency domain analysis and nonlinear analysis, a feature parameter selecting module used for selecting a feature parameter from HRV parameters, a modeling module used for obtaining a perinatal period depression classification model, and a model applying module used for inputting the data of a testee into the classification module to obtain a depression degree. By means of the depression evaluating system and method based on the heart rate variability analytical method, degree quantitative evaluation of perinatal period depression is achieved, a scientific research method of the depression based on the technical field of physiological information examination is enriched, a test is simple and practicable, medical resources can be effectively saved, and good clinical practicality is achieved.
Owner:SOUTH CHINA UNIV OF TECH +1
Clinical acupuncture evidence-based decision support system and application method thereof
InactiveCN102902871ARapid human-computer interaction platformHigh clinical applicabilitySpecial data processing applicationsLiterature evaluationTreatment decision making
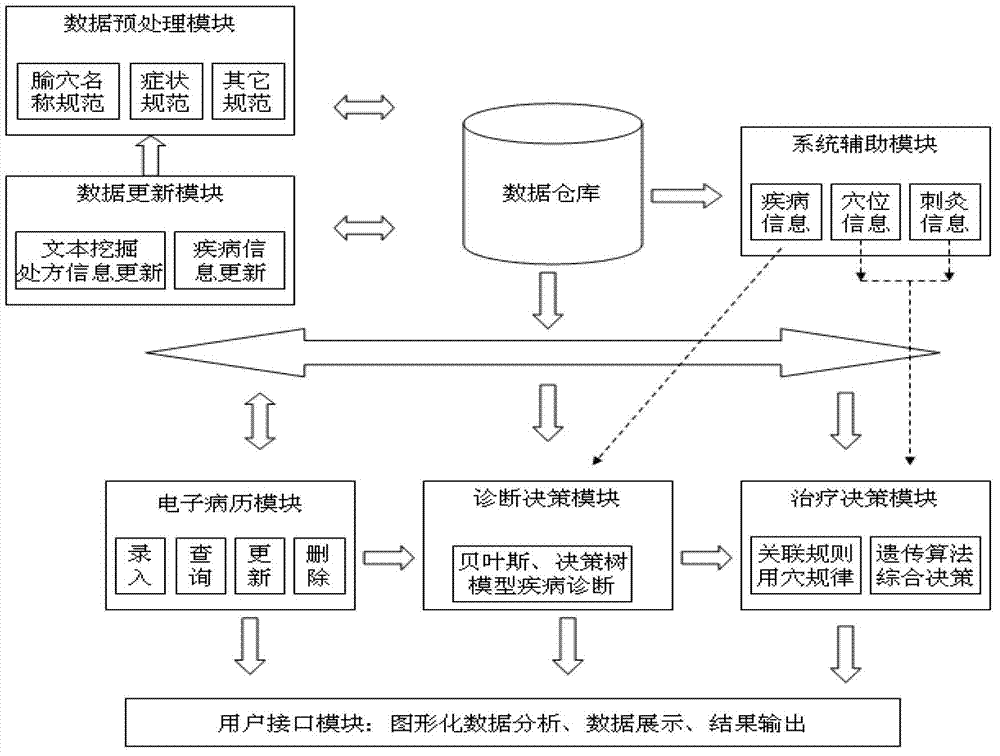
The invention discloses a clinical acupuncture evidence-based decision support system and an application method thereof. The system comprises a data preprocessing module, a data importing and updating module, an electronic medical record module, a diagnosis decision-making module, a treat decision-making module, a system auxiliary module, a result output module and a databank. According to the system, decision-making mechanisms of the evidence-based medicine are blended into a decision-making scheme of a CDSS (Clinical Decision Support System), the evidence utilization is used as the core, an electronic medical record technique is embedded into a clinical acupuncture diagnosis and treat process so as to integrate an evidence-based databank and a data mining model, a clinical acupuncture evidence-based auxiliary support system in which the evaluation, storage, analysis and utilization of acupuncture research evidences are integrated is established, and a convenient and rapid man-machine interaction platform is provided for the effective utilization of the acupuncture research evidences. With the adoption of the system, the requirements of doctors in the overall clinical diagnosis and treat process are met well, and the alternative thinking process of diagnosis and treat is effectively supported, so that a treat scheme which is proposed finally is an optimal scheme obtained from literature evaluation, so that the clinical acupuncture evidence-based decision support system has relatively high clinical adaptation.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Evaluating system and evaluating method of depressive disorder degree quantization
ActiveCN104127193AAvoid subjectivityAvoid variabilitySensorsPsychotechnic devicesNervous systemData treatment
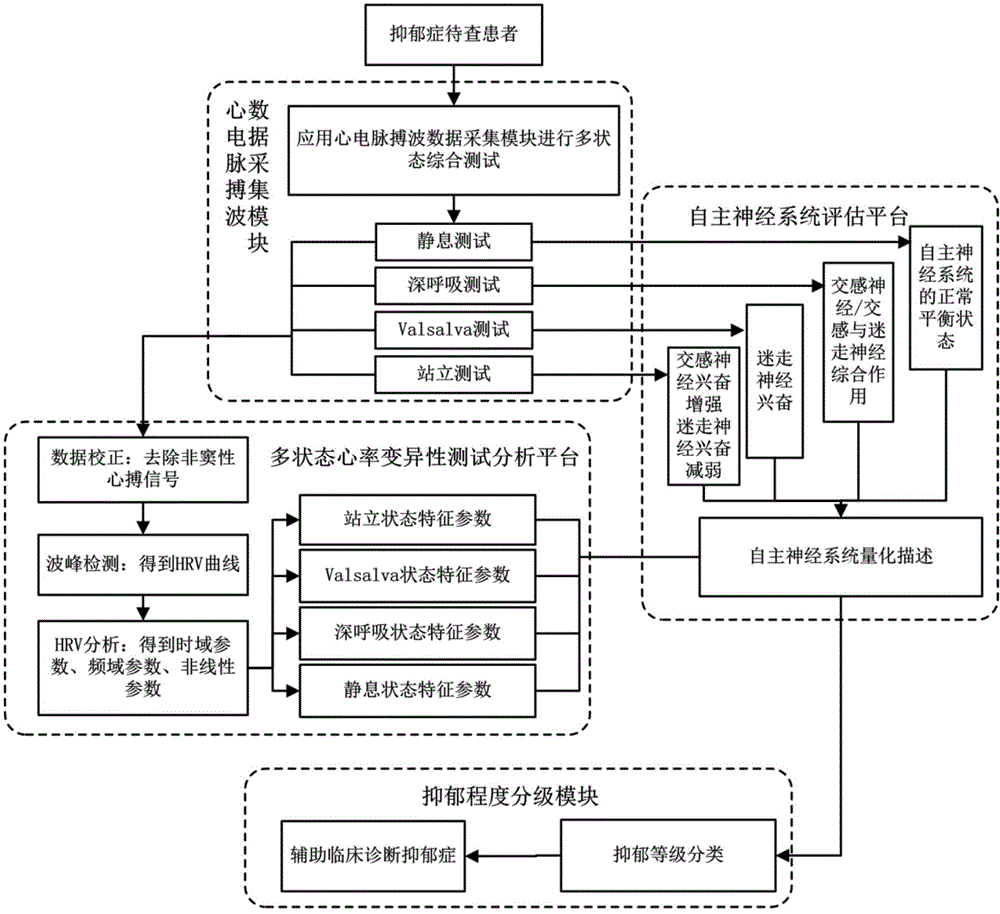
The invention discloses an evaluating system of depressive disorder degree quantization. The system comprises an electrocardio and pulse wave integral detection device, a data transmission device and a data processing platform. The invention further discloses an evaluating method of depressive disorder degree quantization. The method comprises the following steps that firstly, human physiological information under different states is acquired through a multi-state comprehensive test platform; secondly; HRV characteristic parameters under the different states are obtained on the basis of the heart rate variability analysis principle; thirdly, the function balance states of sympathetic nerves and pneumogastric nerves in the automatic nervous system are evaluated; fourthly, a depressive disorder degree quantization evaluation model is built, and the depressive disorder degree level of a tested person is evaluated fast and objectively. The system and method belong to the technical field of computer-aided diagnosis, depressive disorder degree quantization evaluation is achieved, the blank of the technical field of depressive disorder inspection is filled up, and the system and method are easy and convenient to implement, save medical resources and have the good clinic practicality.
Owner:SOUTH CHINA UNIV OF TECH +1
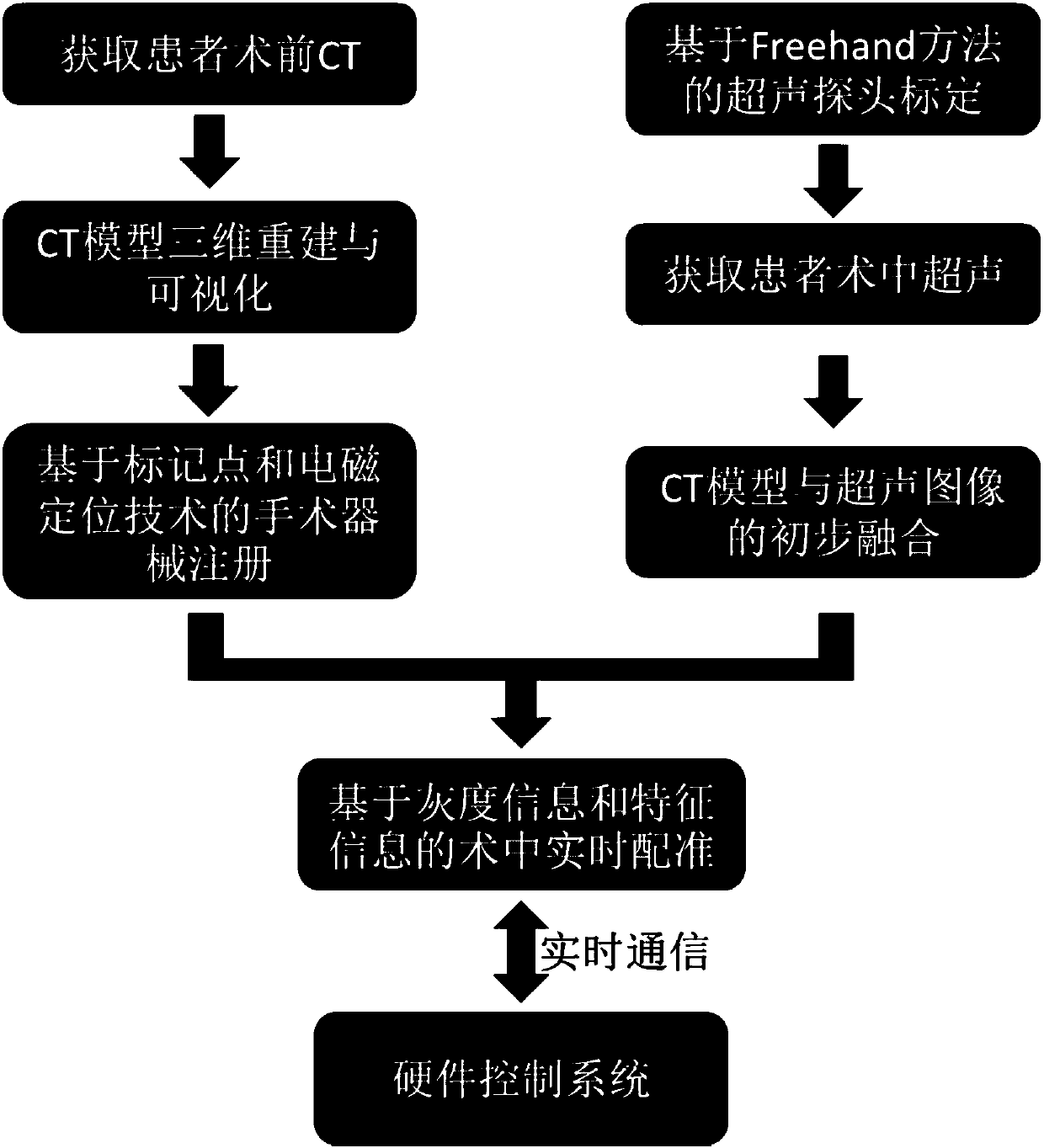
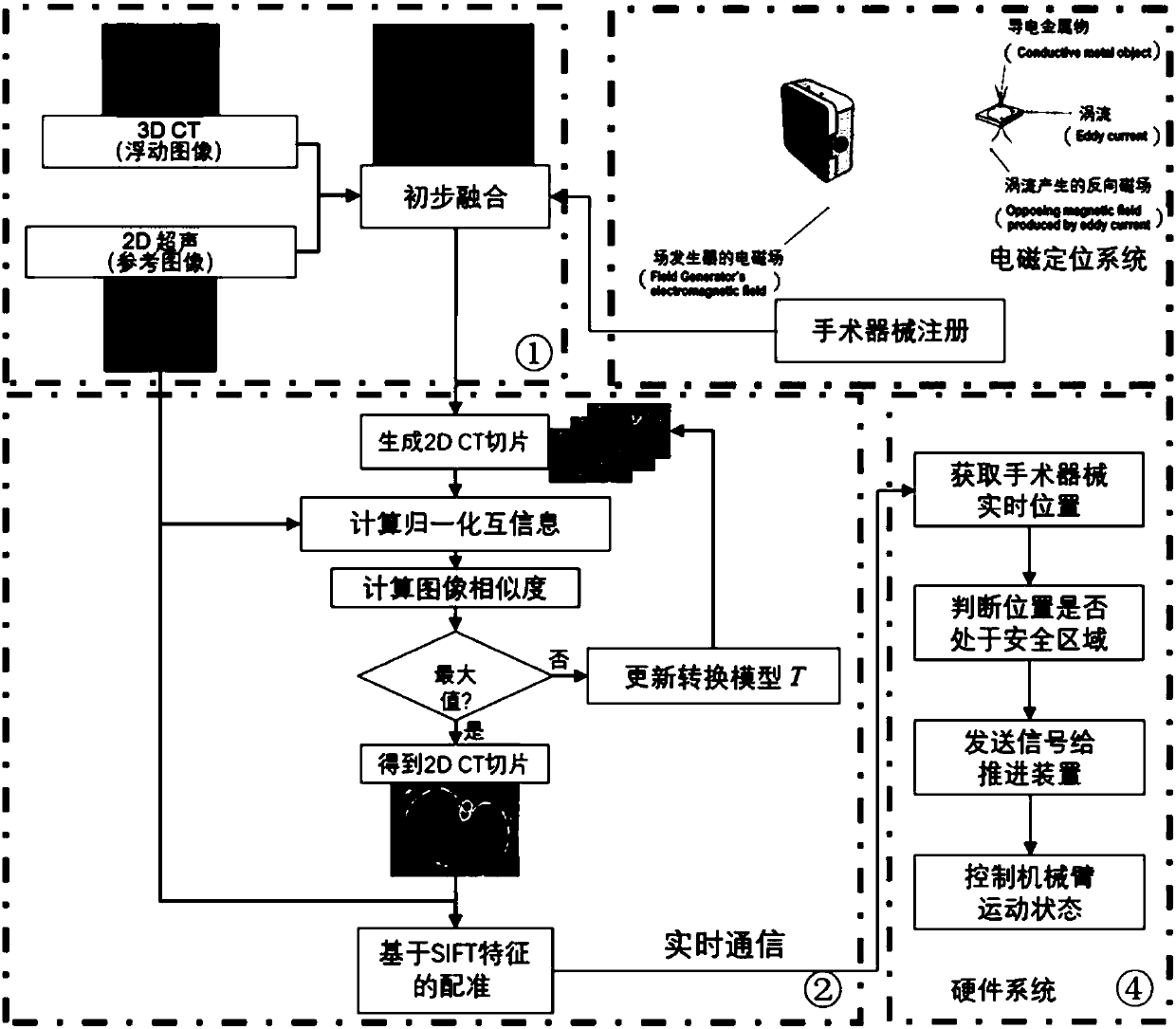
Surgical navigation simulation method based on electromagnetic positioning technology and intraoperative image guidance
InactiveCN108420529ARealize real-time trackingSafe intraoperative real-time guidanceSurgical navigation systemsComputer-aided planning/modellingThree dimensional ctComputation complexity
The invention relates to a surgical navigation simulation method based on an electromagnetic positioning technology and intraoperative image guidance. The surgical navigation simulation method comprises the steps that 1, a preoperatively shot CT image of a patient is obtained; 2, the preoperatively shot CT image of the patient is reconstructed into a three-dimensional CT model; 3, registration andvisualization of surgical instruments are achieved; 4, the electromagnetic positioning technology is utilized to achieve real-time tracing of the surgical instruments under an image coordinate system; 5, an ultrasonic probe is calibrated, an ultrasonic image is obtained during operation in real time, and preliminary fusion between the three-dimensional CT model and the ultrasonic image is achieved; 6, by utilizing grey information and characteristic information of the ultrasonic image and adopting a mutual information method and an automatic SIFT characteristic extraction method, real-time registering of the preoperative three-dimensional CT model and the intraoperative ultrasonic image in the surgical process is performed; 7, simulation control of surgical robot movements is achieved. Compared with the prior art, the surgical navigation simulation method has the advantages of high navigation precision, low calculation complexity, good stability and reliability, convenient achievement, low cost, good clinic applicability and the like.
Owner:SHANGHAI JIAO TONG UNIV
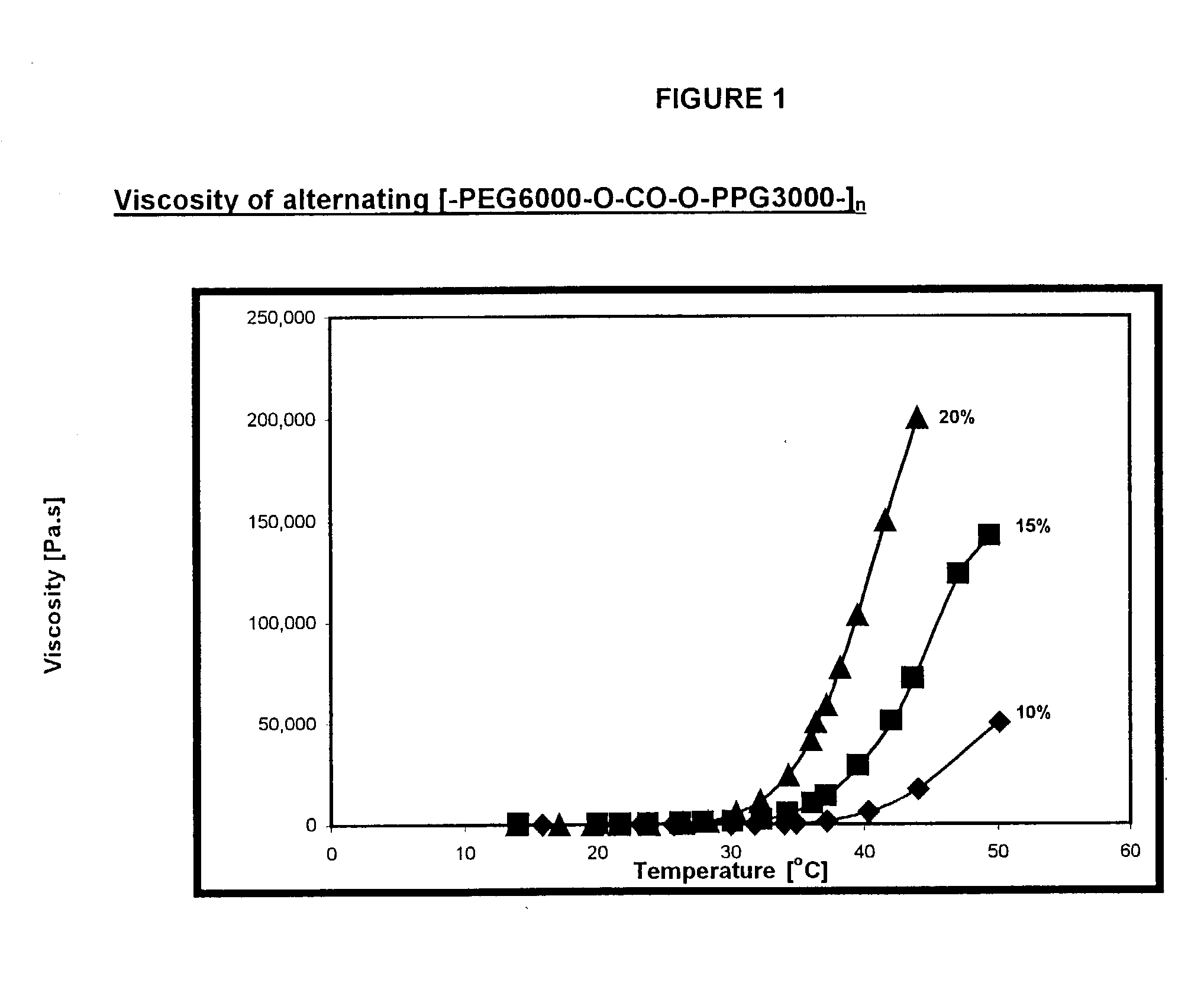
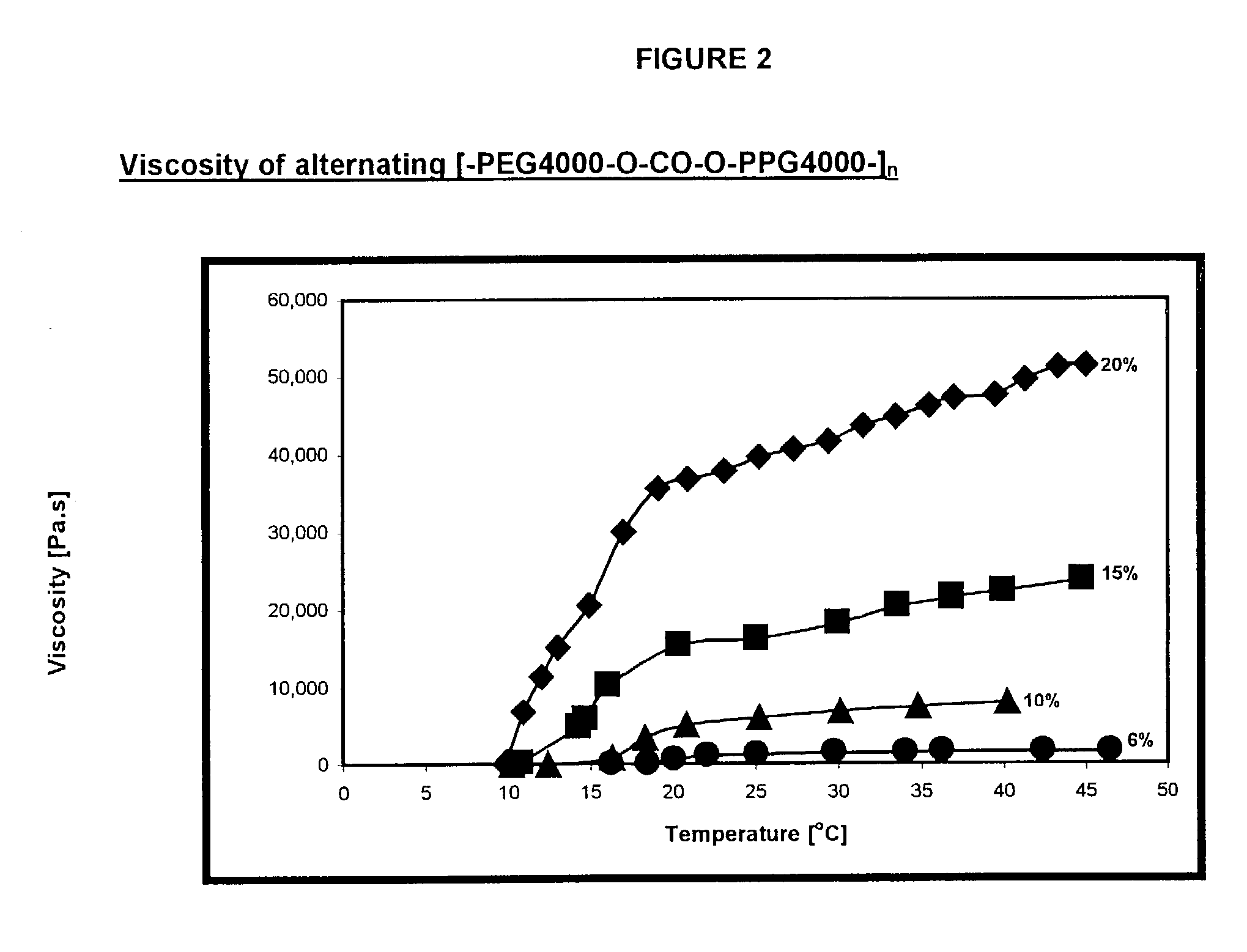
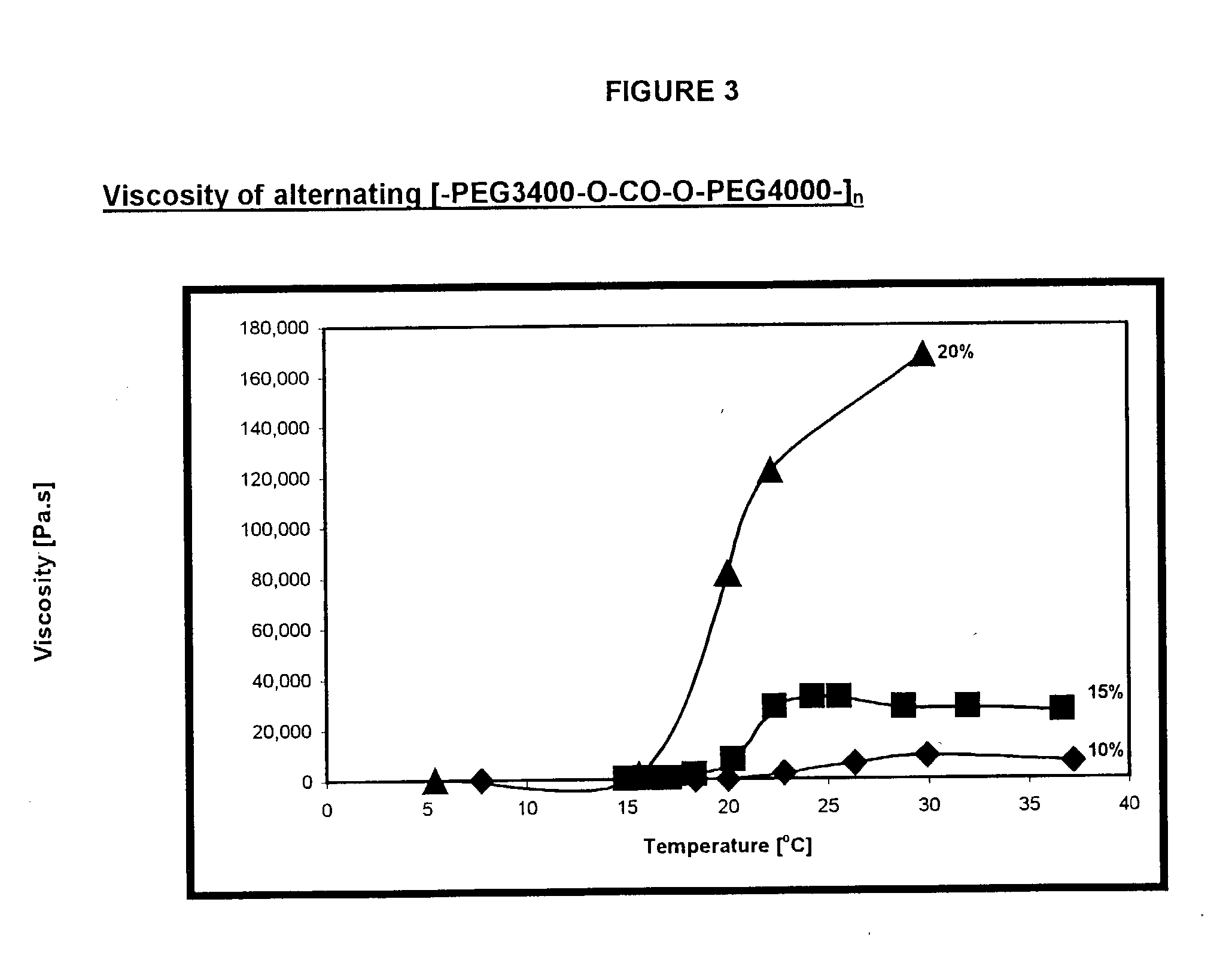
Novel reverse thermo-sensitive block copolymers
InactiveUS20030082235A1Improve initial liquidityHigh viscosityPowder deliveryPharmaceutical non-active ingredientsPolymer sciencePolymer chemistry
The invention provides a responsive polymeric system, comprising: a polymeric responsive component capable of undergoing a transition that results in a sharp increase in viscosity in response to a change in temperature at a predetermined body site; wherein the polymeric component comprises hydrophilic and hydrophobic segments covalently bound within the polymer component, by at least one chain extender or coupling agent, having at least 2 functional groups; wherein the hydrophilic and hydrophobic segments do not display Reverse Thermal Gelation behavior of their own at clinically relevant temperatures and; wherein the viscosity of the polymeric component increases by at least about 2 times upon exposure to a predetermined trigger.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
GLP-1 derivative and preparation thereof absorbable via mucous membrane
InactiveUS7291594B2Good clinical applicabilityImprove absorption ratePeptide/protein ingredientsMetabolism disorderDipeptidyl peptidaseFat emulsion
A GLP-1 derivative is provided including an amino acid sequence of GLP-1 (7-35) having deletion, substitution and / or addition of one or more amino acids and having Waa-(Xaa)n-Yaa (in which Waa is Arg or Lys, Xaa is Arg or Lys, n is an integer of 0 to 14, and Yaa is Arg, Arg-NH2, Lys, Lys-NH2 or Hse) added to the C-terminus of the peptide having a GLP-1 activity. These derivatives are highly absorbable via a mucous membrane. The GLP-1 derivative can be conferred with resistance to dipeptidyl peptidase IV by substituting amino acid 8 in its GLP-1 amino acid sequence with Ser, or with resistance to trypsin by substituting amino acids 26 and 34 with Gln and Asn, respectively.The absorption efficiency of the GLP-1 derivatives via mucous membranes can be further improved by preparing a composition using a charge-regulated fat emulsion regulated to be negatively charged thereon.
Owner:SANWAKAGUKU KENKYUSHO CO LTD
Trypsin micropore plate type magnetic granule chemoluminescence immunoassay measuring kit and preparation method thereof
InactiveCN101545912AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceBiological testingPolyclonal antibodiesBlood serum
The invention discloses a trypsin micropore plate type magnetic granule chemoluminescence immunoassay measuring kit and a preparation method thereof. The kit comprises: (1) a trypsin calibration sample, (2) magnetic granules coated by a trypsin monoclonal antibody, (3) another monoclonal antibody or a polyclonal antibody marked by alkaline phosphatase (ALP), (4) a micropore plate, (5) washing solution and (6) chemoluminescence substrate solution on which the enzyme acts. The preparation method for preparing the kit comprises the steps of preparing the calibration sample, preparing the magnetic granules coated by the trypsin antibody, marking the trypsin antibody with the alkaline phosphatase (ALP), preparing the washing solution and preparing the chemoluminescence substrate solution. The kit can carry out batch measurement and is suitable for batch clinic blood serum screening.
Owner:北京科美东雅生物技术有限公司
Dissociate human chorionic gonadotrophin beta-subunit magnetic particle chemiluminescence quantitative assay kit and its preparation method
InactiveCN103076458AGuaranteed SensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingImmune profilingChromogenic Substrates
The invention relates to the medical field of immunoassay, and concretely provides a dissociate human chorionic gonadotrophin beta-subunit magnetic particle chemiluminescence quantitative assay kit having the advantages of simplicity, rapidness, high sensitivity, wide linear range and good stability, and its preparation method. The method combines a magnetic particle immune separating technology to apply an enzymatic chemiluminescence substrate on the basis of enzyme-linked immunosorbent assay, and allows an optical signal generated by the detection of the luminescence substrate to substitute a chromogenic substrate in enzyme immune assay, so the method has the advantages of substantially improved sensitivity, simple operation and wide practicality, can be applied to open semi-automatic chemiluminescence detectors, can also be applied to full-automatic measure systems, and can realize batch and fast detection, low use cost, and easy popularization and application.
Owner:BEIJING LEADMAN BIOCHEM
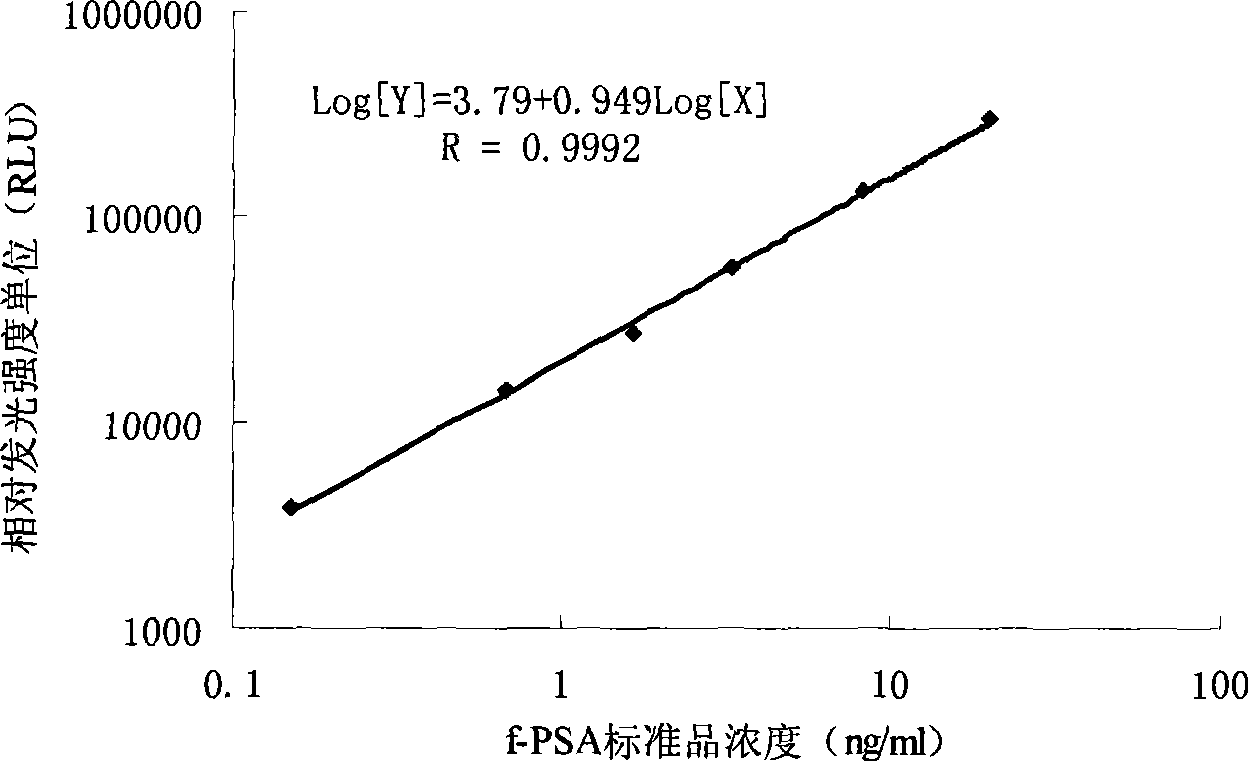
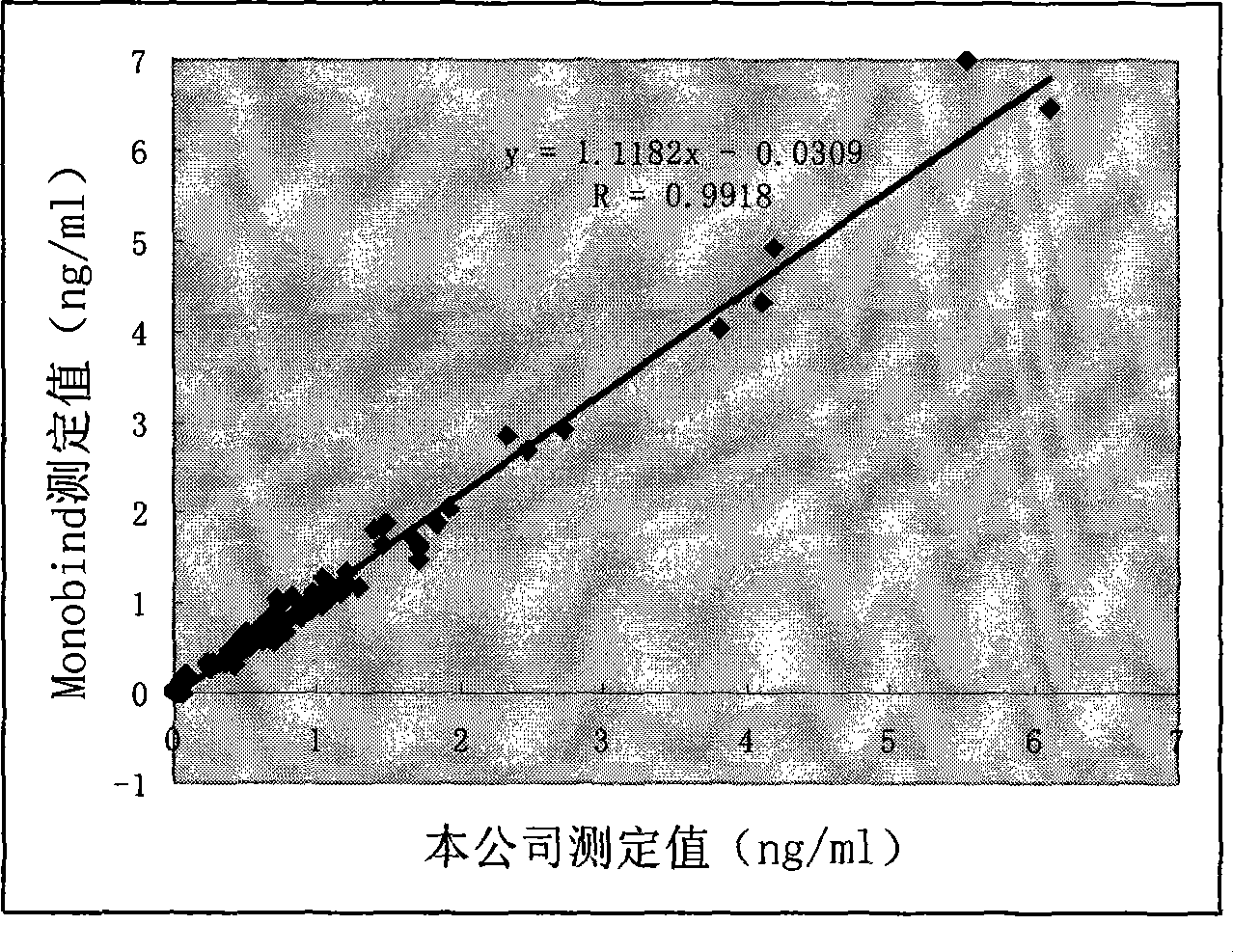
Free prostate gland specificity antigen chemiluminescence immune analysis determination reagent kit and preparing method thereof
InactiveCN101377500AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceBiological testingSolid phasesAntigen
The invention relates to the medical field of immunoassay, more specially, the invention provides a chemiluminescent immunoassay detection kit for free prostate specific antigens and a preparation method thereof. The kit of the invention comprises: 1) free prostate specific antigen calibrators, 2) solid-phase vectors which are coated by prostate specific antigen monoclonal antibodies, 3) anti-free prostate specific antigen antibodies which are marked by enzyme and 4) chemiluminescent substrate solutions. Further, the preparation method of the kit according to the invention comprises the following steps: 1) preparing the calibrators, 2) coating the solid-phase vectors with the free prostate specific antigen monoclonal antibodies, 3) marking the anti-free prostate specific antigen antibodies with the enzyme, 4) packaging the calibrators, the enzyme markers and chemiluminescent substrate solutions, and 5) assembling finished products. The kit of the invention has the advantages of convenience, rapidness, sensitivity, stability, and the like.
Owner:北京科美东雅生物技术有限公司
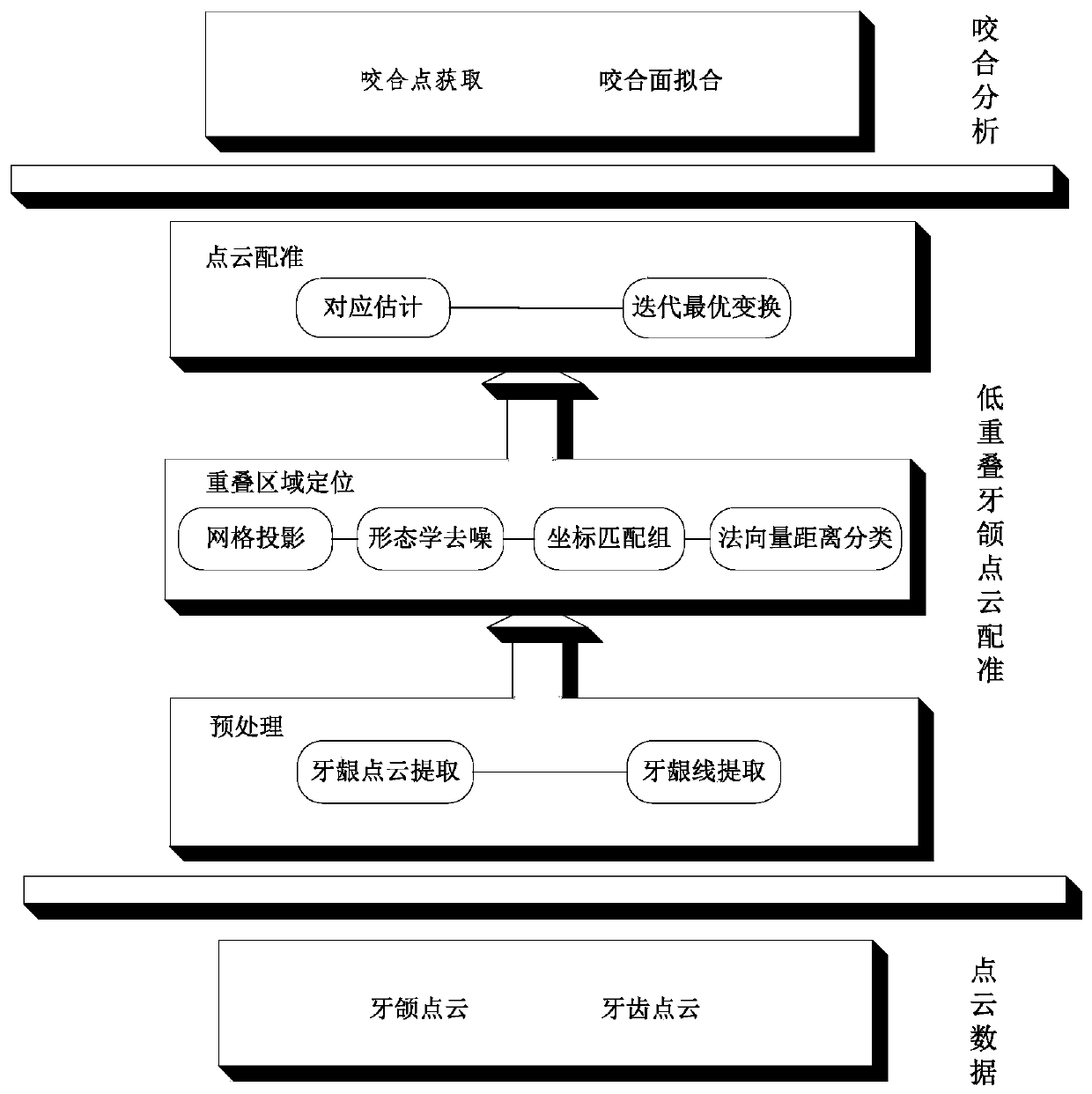
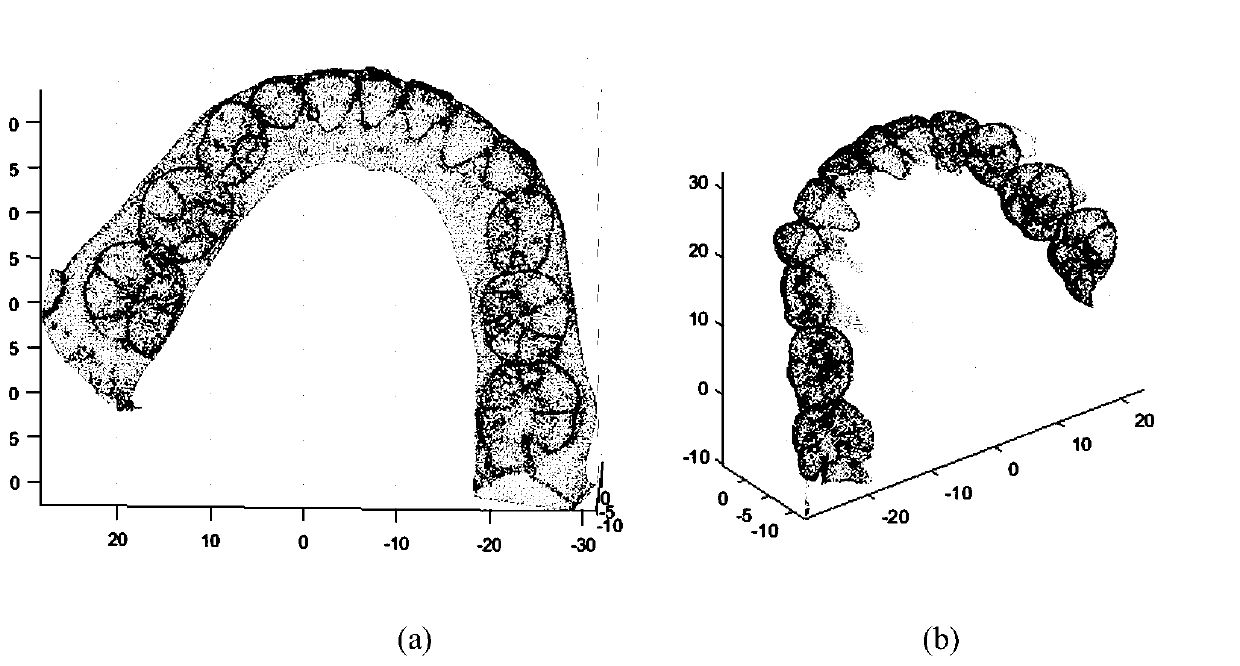
Tooth occlusion analysis system based on point cloud spatial characteristics
ActiveCN110276758AReduce the impactImprove the efficiency of efficacy evaluationImage enhancementImage analysisPoint cloudComputer science
The invention discloses a tooth occlusion analysis system based on point cloud spatial characteristics. The system comprises a data acquisition unit, a preprocessing unit, an overlapping region positioning unit, a point cloud registration unit and an occlusion analysis unit; the data acquisition unit is used for acquiring tooth jaw point cloud data and tooth point cloud data of the same case in different periods; the preprocessing unit is used for extracting and obtaining auxiliary point cloud data based on the tooth jaw point cloud data and the tooth point cloud data; the overlapping region positioning unit determines an overlapping region according to the tooth jaw point cloud data, the tooth point cloud data and the auxiliary point cloud data; the point cloud registration unit completes registration of dental jaw point cloud data of the same case in different periods based on the overlapped area; and the occlusion analysis unit performs tooth occlusion analysis based on the registered tooth jaw point cloud data. According to the method, the influence of low overlapping rate and noise on the matching effect can be reduced to the maximum extent, and the registration precision is greatly improved.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA +1
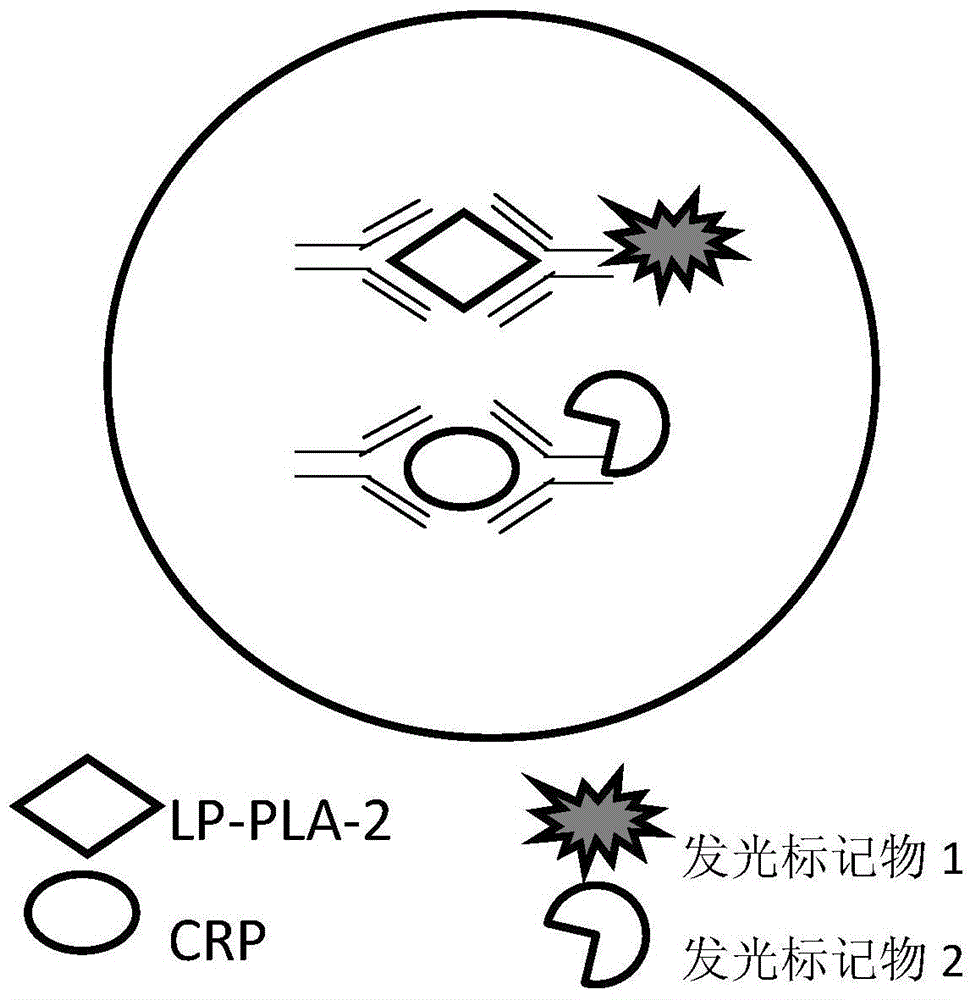
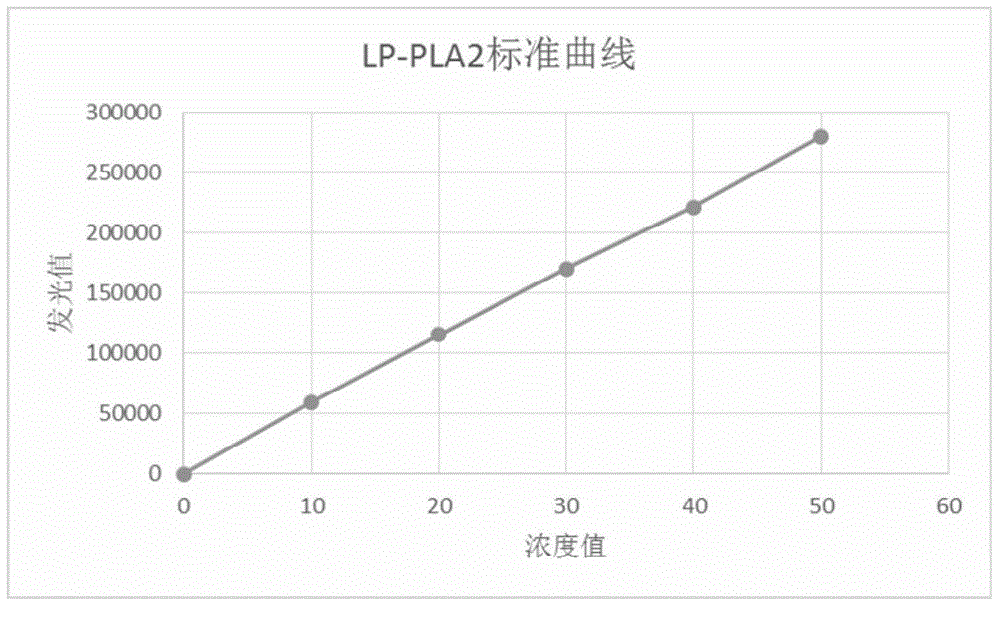
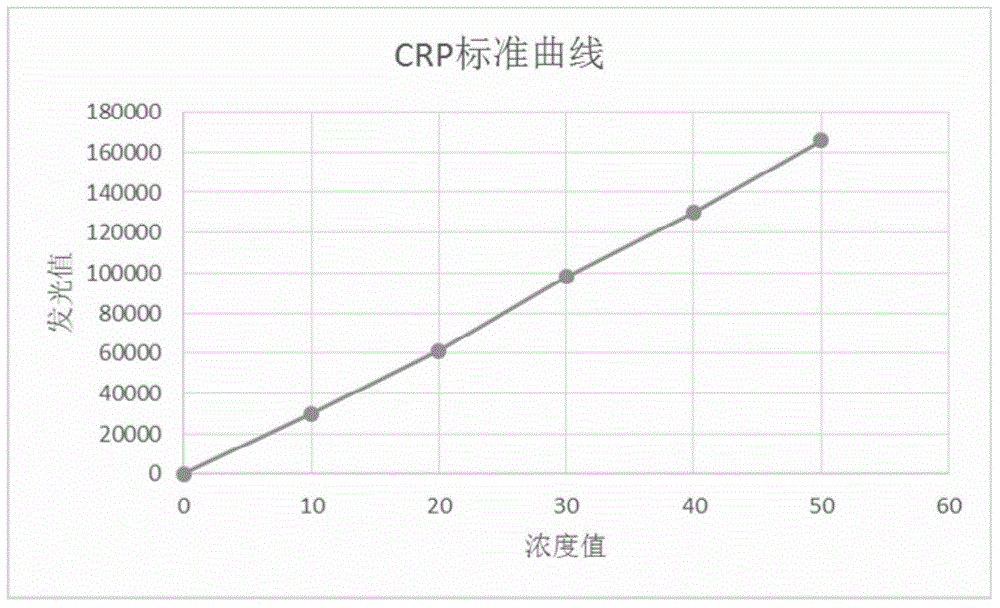
Kit of detecting the content of Lp-PLA2 and CRP on the basis of chemiluminescence, and method and application thereof
InactiveCN104820102AGuaranteed SensitivityEasy to operateBiological testingSeparation technologyMicroparticle
The invention discloses a kit of detecting the content of Lp-PLA2 and CRP on the basis of chemiluminescence, wherein the kit comprises: magnetic particles coated by an anti-fluorescein isothiocyanate polyclonal antibody, an Lp-PLA2 monoclonal antibody marked by fluorescein isothiocyanate, a CRP monoclonal antibody marked by fluorescein isothiocyanate, an Lp-PLA2 monoclonal antibody marked by alkaline phosphatase, a CRP monoclonal antibody marked by alkaline phosphatase, a chemiluminescent substrate liquid with alkaline phosphatase catalytic luminescence, a diluent liquid, a cleaning agent, an Lp-PLA2-series standard substance, and a CRP-series standard substance. The kit of detecting the content of Lp-PLA2 and CRP is used in a reaction mode of a double antibody sandwich method, so that the technical principle of chemiluminescent detection with a magnetic particle immune-separation technology is effectively utilized, by that the content of the Lp-PLA2 and the CRP in human serum or blood plasma samples are quantitatively measured and detection sensitivity is ensured. The kit is low in pre-treatment requirement on samples, is simple and reliable in the pre-treatment of the samples, can quickly detection large batches of samples in high throughput and is convenient to operate and produce.
Owner:南京格耀生物科技有限公司
Quantitative gastrin-releasing peptide precursor kit, as well as preparation method and detection method thereof
InactiveCN104089949ALow costHigh sensitivityChemiluminescene/bioluminescenceQuality controlTrue positive rate
The invention relates to a quantitative gastrin-releasing peptide precursor (ProGRP) kit and a detection method thereof. A method comprises seven steps of preparing a magnetic separation reagent, preparing an enzyme reactant, preparing a reaction enhancer, preparing a calibration substance diluent, preparing a calibration substance and a quality control substance, preparing a cleaning concentrate liquid and preparing a substrate solution. According to the quantitative gastrin-releasing peptide precursor kit and the detection method thereof, the sensitivity and the specificity are relatively high, the detection result obtaining time is relatively short, the operation mode is relatively simple, cell lung cancers (SCLC) and non small cell lung cancer (NSCLC) can be distinguished, and the kit has the important significance on early diagnosis on lung cancers.
Owner:JIANGSU FLON BIOTECH
Antigen stimulant and kit for detecting mycobacterium tuberculosis infection, and application of antigen stimulant
ActiveCN104597239AIncreased sensitivityImprove featuresMicrobiological testing/measurementBiological testingMycobacterium InfectionsStimulant
The invention provides an antigen stimulant for detecting mycobacterium tuberculosis infection, and a kit comprising the antigen stimulant. The invention also provides an application of the antigen stimulant in reagents for detecting mycobacterium tuberculosis infection. The antigen stimulant comprises at least one polypeptide or analogues thereof in polypeptides shown as the sequences 1-11 in a sequence table, wherein the polypeptides respectively come from tuberculosis specific antigen polypeptides ESAT-6 and tuberculosis specific antigen polypeptides CFP-10. According to the antigen stimulant provided by the invention, peripheral blood T lymphocytes of tuberculosis infection patients can be effectively stimulated to generate IFN-gamma, so that the tuberculosis infection can be diagnosed at high sensitivity and high specificity, and the influence on BCC inoculation or other underlying diseases can be avoided.
Owner:SUN YAT SEN UNIV +1
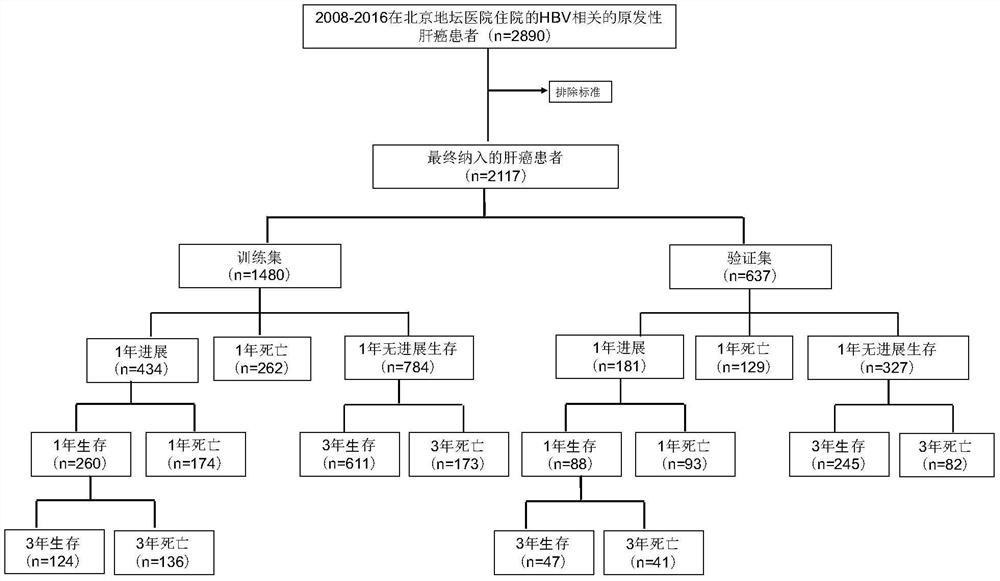
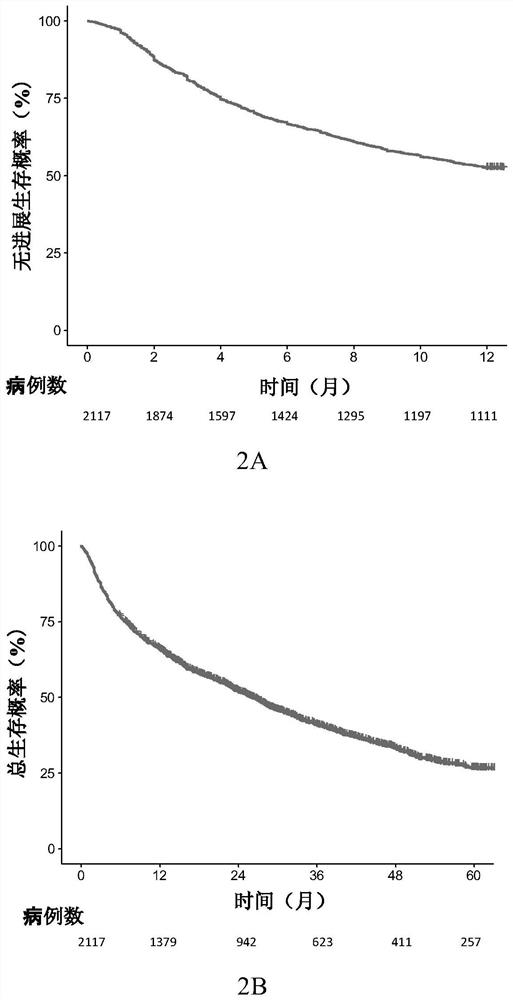
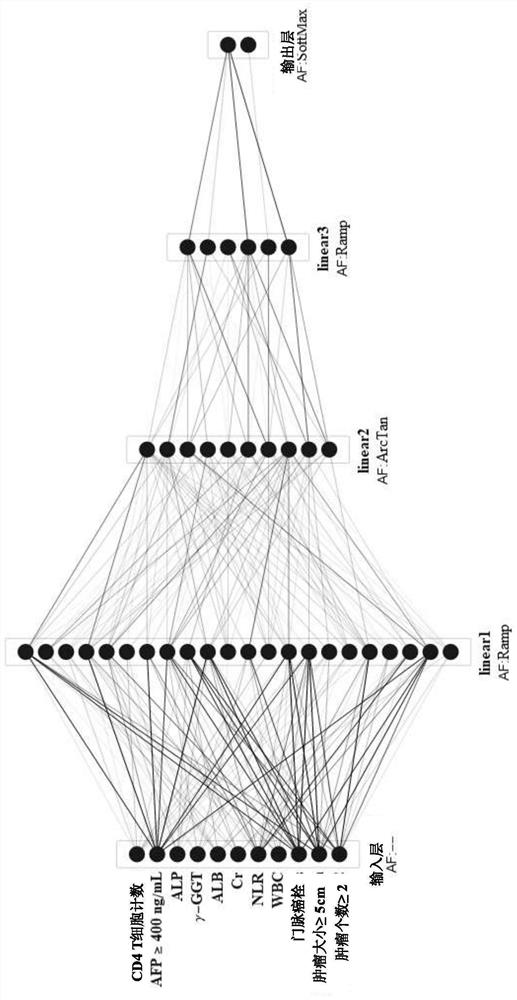
System for judging prognosis conditions of liver cancer patients based on artificial neural network model
ActiveCN112017791AProgress helpsProgress aids assessmentMedical data miningEpidemiological alert systemsOncologyArtificial neural network model
The invention provides a device for judging prognosis conditions of liver cancer patients based on an artificial neural network model. The device comprises: (1) an input module configured to input tumor feature information of a to-be-discriminated liver cancer patient; (2) a prognosis discrimination module which is set to comprise an artificial neural network model used for calculating the prognosis condition of the to-be-discriminated liver cancer patient based on the input information; and (3) an output module, wherein the output module is set to output the obtained prognosis condition. Theinvention also provides a method for grouping prognosis conditions of liver cancer patients by adopting the device.
Owner:BEIJING DITAN HOSPITAL CAPITAL MEDICAL UNIV
Glp-1 derivatives and transmicosal absorption preparations thereof
InactiveUS20060194720A1High absorptionImprove absorptionPeptide/protein ingredientsMetabolism disorderPeptideChemistry
Owner:SANWAKAGUKU KENKYUSHO CO LTD
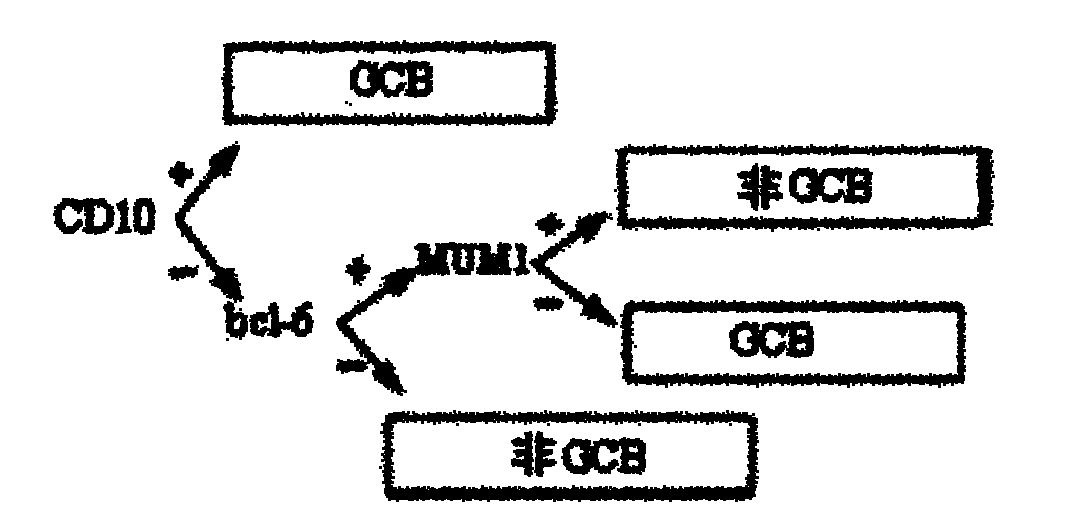
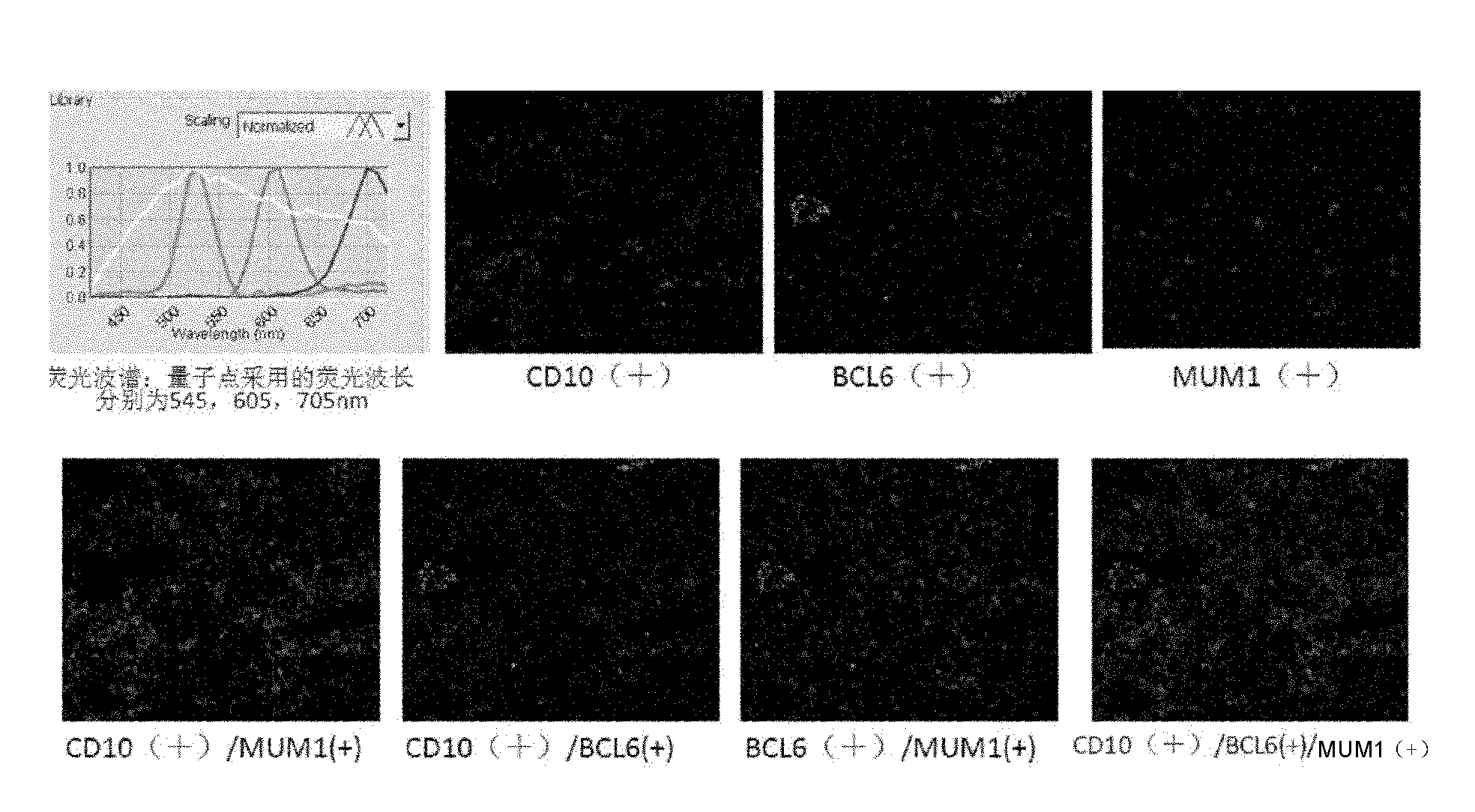
Molecular pathological classification method of diffuse large B-cell lymphomata, kit and application thereof
InactiveCN101968491AReduce medical expensesImprove work efficiencyBiological testingMedical expensesRegimen
The invention discloses a molecular pathological classification method of diffuse large B-cell lymphomata, which use three quantum dot markers of different wavelengths as fluorescent probes to detect the expression of CD10, Bcl-6 and MUM1 proteins in a biological sample. The invention also discloses a kit for the molecular pathological classification of diffuse large B-cell lymphomata and an application thereof. The molecular pathological classification method of diffuse large B-cell lymphomata obviously improves the working efficiency, and can provide a rational regimen for patients with diffuse large B-cell lymphomata, thereby being beneficial to saving medical expenses.
Owner:SHANGHAI BIOCHIP +1
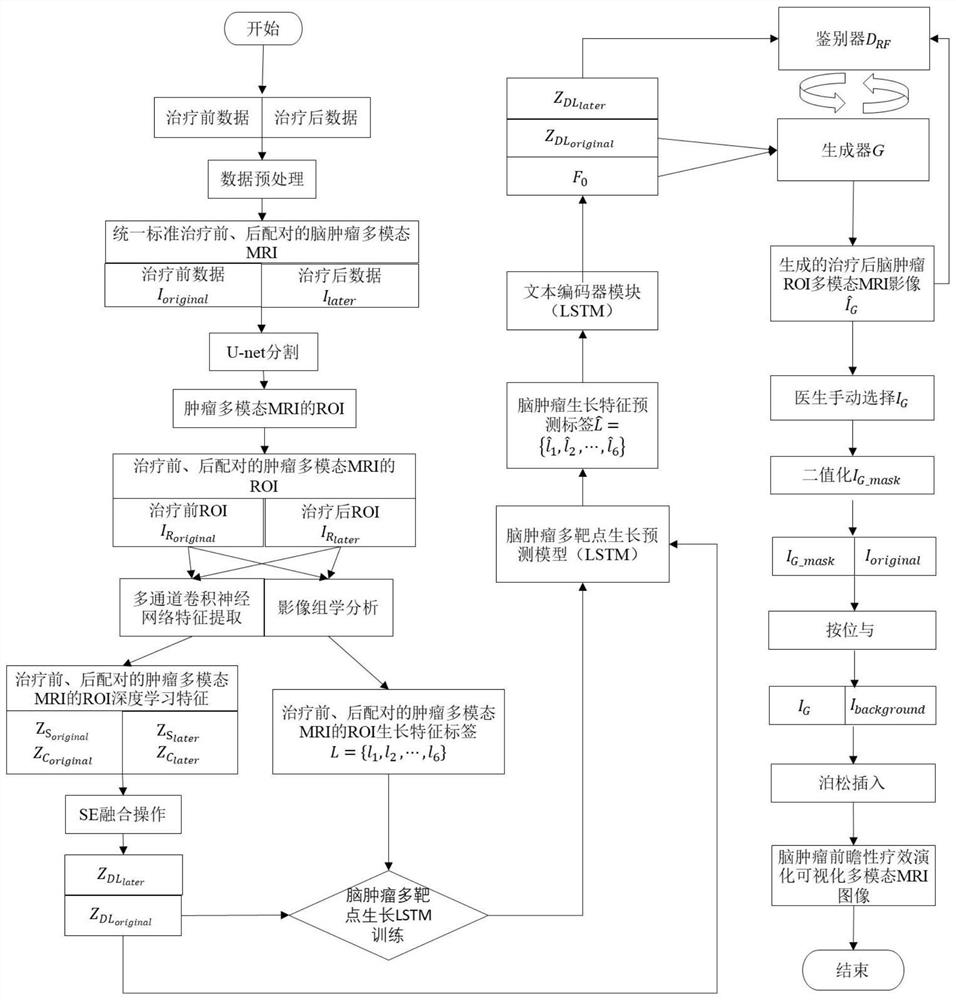
Brain tumor multi-target auxiliary diagnosis and prospective treatment evolution visualization method and system
PendingCN112365980AChange the way of communicationIntuitive efficacy evaluation resultsImage enhancementImage analysisBiologyVisualization methods
The invention provides a brain tumor multi-target auxiliary diagnosis and prospective treatment evolution visualization method and system. The brain tumor multi-target auxiliary diagnosis and prospective treatment evolution visualization method comprises the steps: acquiring and preprocessing paired brain tumor multi-target multi-mode MRI data before and after treatment; carrying out tumor regionsegmentation on the preprocessed paired brain tumor multi-target multi-mode MRI data before and after treatment through a 3DU-net convolutional neural network, and obtaining a growth characteristic label L = {l1, l2, l3,..., ln} through an imaging omics method; and performing feature extraction on the sum through a multi-channel convolutional neural network and then performing SE fusion operationto obtain deep learning features and input the deep learning features into a prediction model to obtain a brain tumor multi-target growth prediction label, inputting the brain tumor multi-target growth prediction label into a trained prospective treatment visualization model to obtain a final brain tumor region-of-interest growth evolution image, and inserting the brain tumor region-of-interest growth evolution image into a non-brain tumor region Ibackground to complete a brain tumor prospective treatment visualization task. The method and system in the invention has higher clinical practicability.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Novel ophthalmic curcumin multi-core vesicle gel preparation and preparation method thereof
InactiveCN103040726ALow costImproved dwell timeSenses disorderAerosol deliveryGel preparationPolyethylene glycol
The invention relates to a novel ophthalmic curcumin multi-core vesicle gel preparation and a preparation method thereof. The preparation consists of the following medicaments: 0.5 to 2.5 milligrams of curcumin, 5 to 25 milligrams of polyethylene glycol monooleate or polyethylene glycol dioleate, 5 to 25 milligrams of polyethylene glycol monolaurate or polyethylene glycol dilaurate, 5 to 25 milligrams of poloxamer F68, 20 to 200 milligrams of soybean phospholipid or lecithin, and mixed solution of 0.1 to 0.7 mg / L of glycerophosphate solution and 0.01 to 0.05 mg / L of chitosan hydrochloride solution in a volume ratio of 1:1-1:10. By organically combining a multi-core vesicle preparation technology with thermo-sensitive gel, the medicament loading capacity is improved, the bioavailability is also improved, meanwhile, an effect of slow release is achieved, the blank of the ophthalmic curcumin multi-core vesicle gel preparation is filled, and a novel ophthalmic preparation with good treatment effect and low price is provided for clinic.
Owner:YANTAI UNIV
Acipenser sinensis VTG protein antigen and antibody, and preparation method and application thereof
ActiveCN106632643AEasy to trainRealize large-scale productionSerum immunoglobulinsImmunoglobulins against animals/humansAmpicillinEnzyme digestion
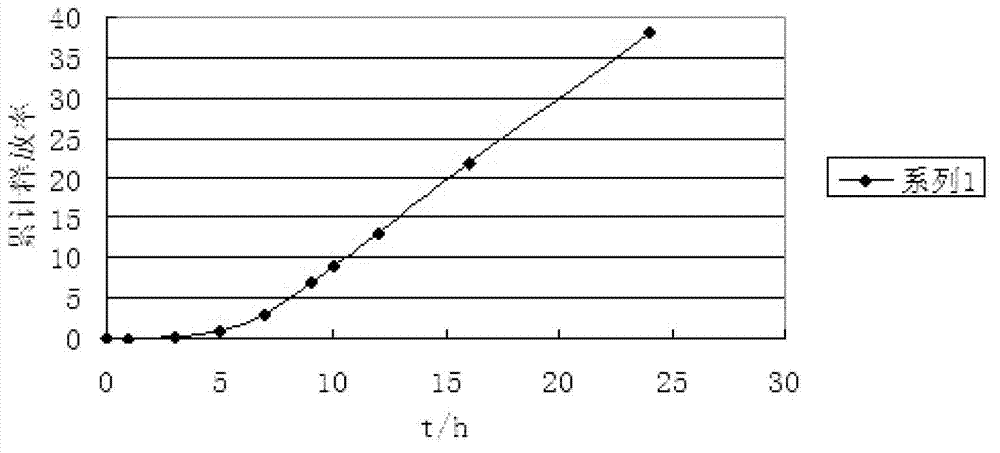
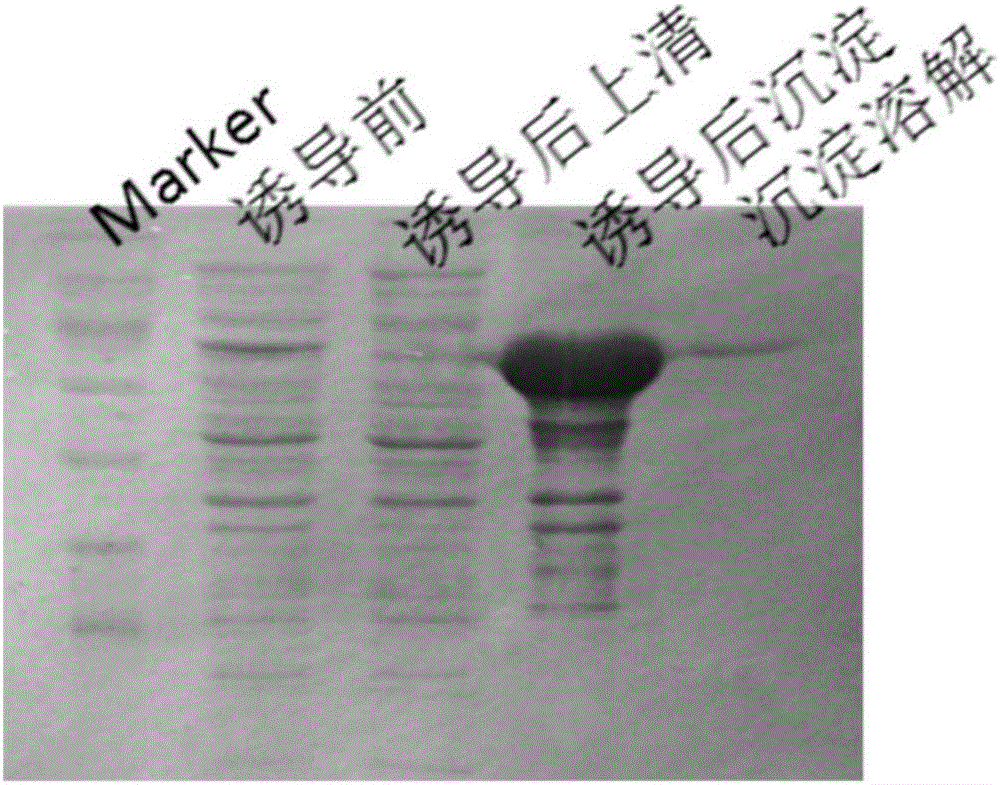
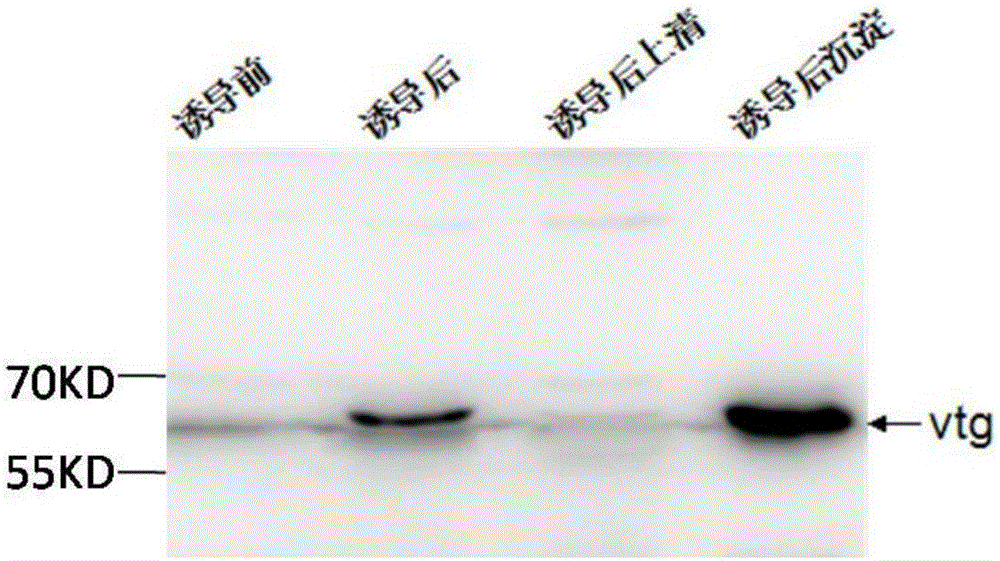
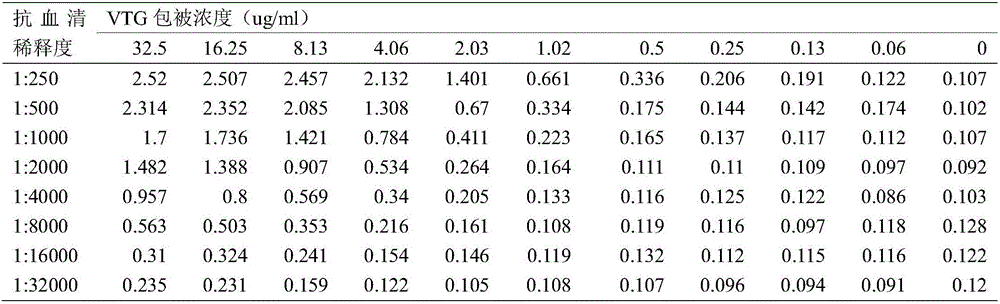
The invention discloses Acipenser sinensis VTG protein antigen and antibody, and a preparation method and an application thereof. The preparation method comprises the following steps: 1, preparation of a recombinant Acipenser sinensis VTG protein antigen: 1) designing an upstream primer and a downstream primer, and amplifying a protein domain truncated sequence; 2) subjecting a pMD-18T-VTG plasmid and a Pet32a (+) expression vector to enzyme digestion by using EcoRI and XhoI, then purifying a target fragment, connecting the pMD-18T-VTG plasmid and the Pet32a (+) expression vector into Pet32a (+)-VTG, converting the Pet32a (+)-VTG into DH5a, coating the DH5a onto an ampicillin containing LB culture medium, and carrying out culturing; 3) converting the Pet32a (+)-VTG plasmid into a BL21(DE3) competent cell, coating the BL21(DE3) competent cell onto an ampicillin containing LB solid culture medium, and carrying out culturing; and 4) subjecting collected mycelium precipitate to resuspension precipitation by using an 8M urea containing protein buffer solution, then carrying out filtering, and collecting a supernatant so as to obtain a recombinant protein; 2, obtainment of an Acipenser sinensis VTG protein antibody through immunization of Japanese rabbit with the recombinant Acipenser sinensis VTG protein antigen; and 3, application of the Acipenser sinensis VTG protein antigen and antibody. The Acipenser sinensis VTG protein antibody and the VTG protein antigen provided by the invention have the following advantages: the immunity is good; a titer can reach 1: 1000; detection results have high positive rate; and the clinical applicability is good.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
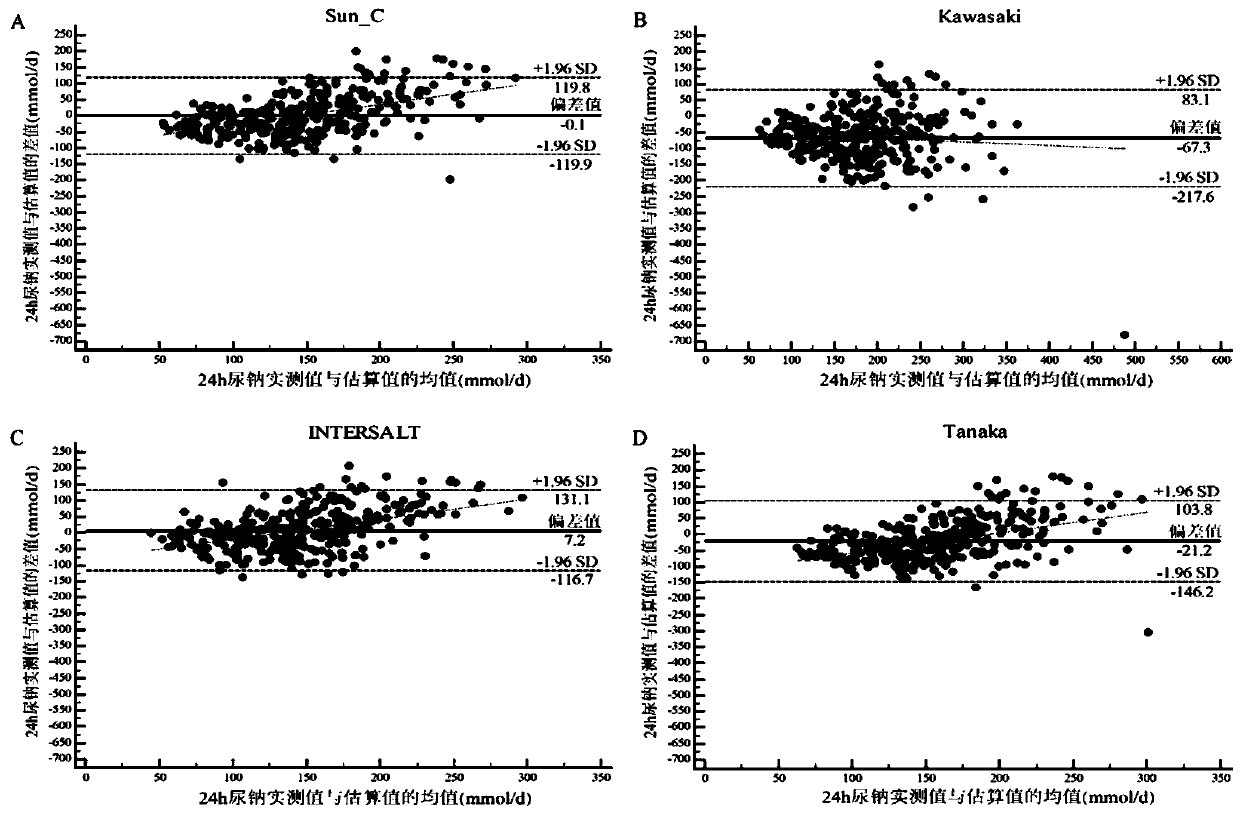
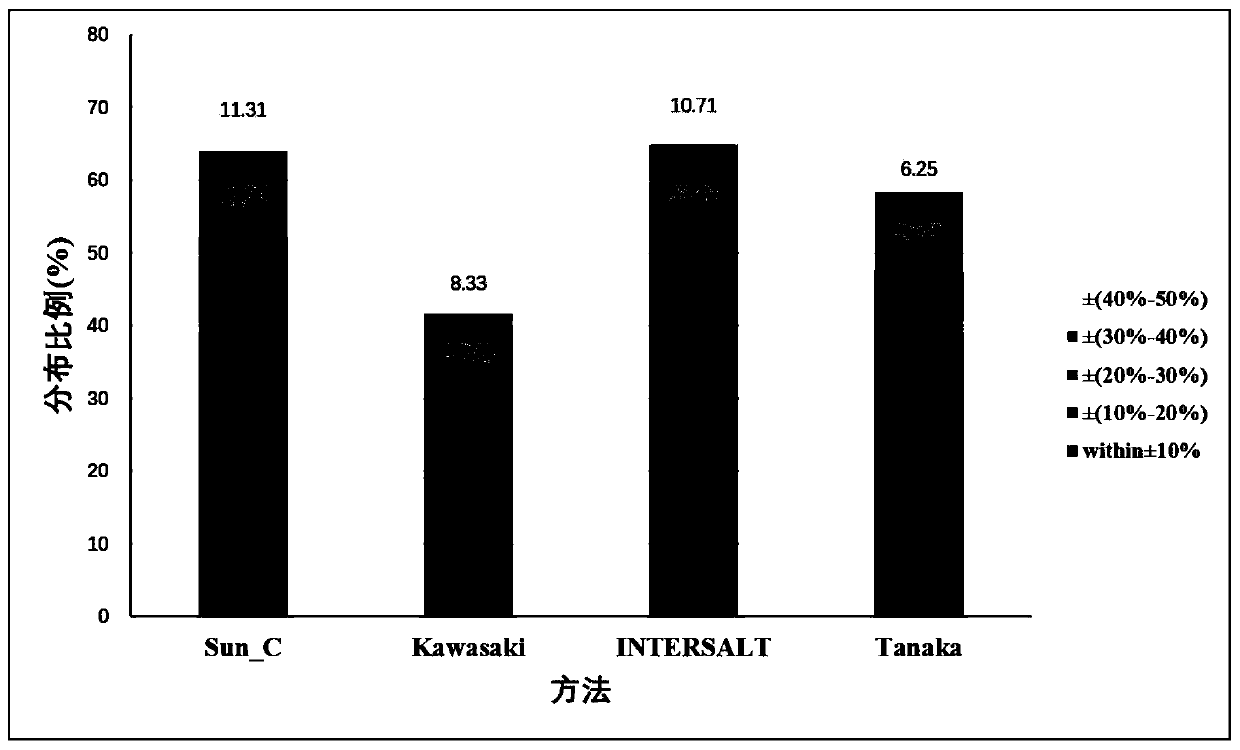
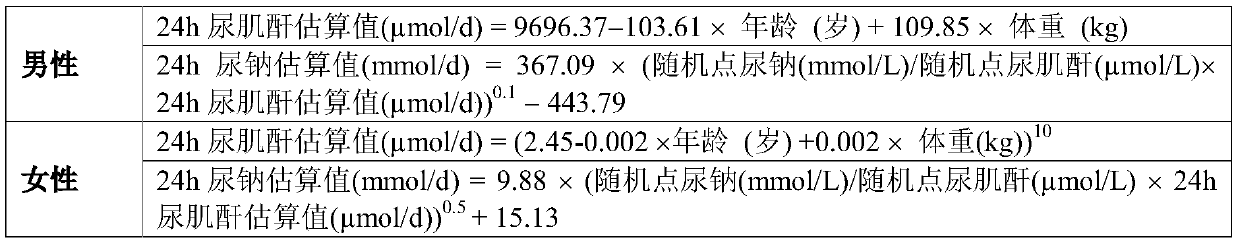
Method for evaluating daily salt content of hypertensive patient by random point urine
ActiveCN111508584AReduce the difficulty of operationIncrease the difficulty of clinical detectionHealth-index calculationMaterial analysis by electric/magnetic meansUrine sodiumHuman body
The invention discloses a method for evaluating daily salt content of a hypertensive patient by using random point urine, which comprises the following steps: 1, acquiring basic information of a hypertensive patient, the basic information comprising gender, age and weight; 2, measuring random point urine sodium and point urine creatinine values; 3, substituting the data into the following calculation formula according to the gender, obtaining a 24-hour urine creatinine estimated value according to age and weight data and then obtaining the 24-hour urine sodium estimated value of the hypertension patient by combining the random point urine sodium and point urine creatinine data; and 4, assuming that the table salt contains 100% of sodium chloride, and calculating the daily table salt intake(g / d) according to 96-99% of sodium chloride excretion of the kidney of the human body and the 24-hour urine sodium estimated value. According to the invention, the problem that the existing common formula has many clinical indexes is solved, the clinical detection difficulty is increased, the clinical applicability is reduced, and the application is relatively tedious.
Owner:孙宁玲 +1
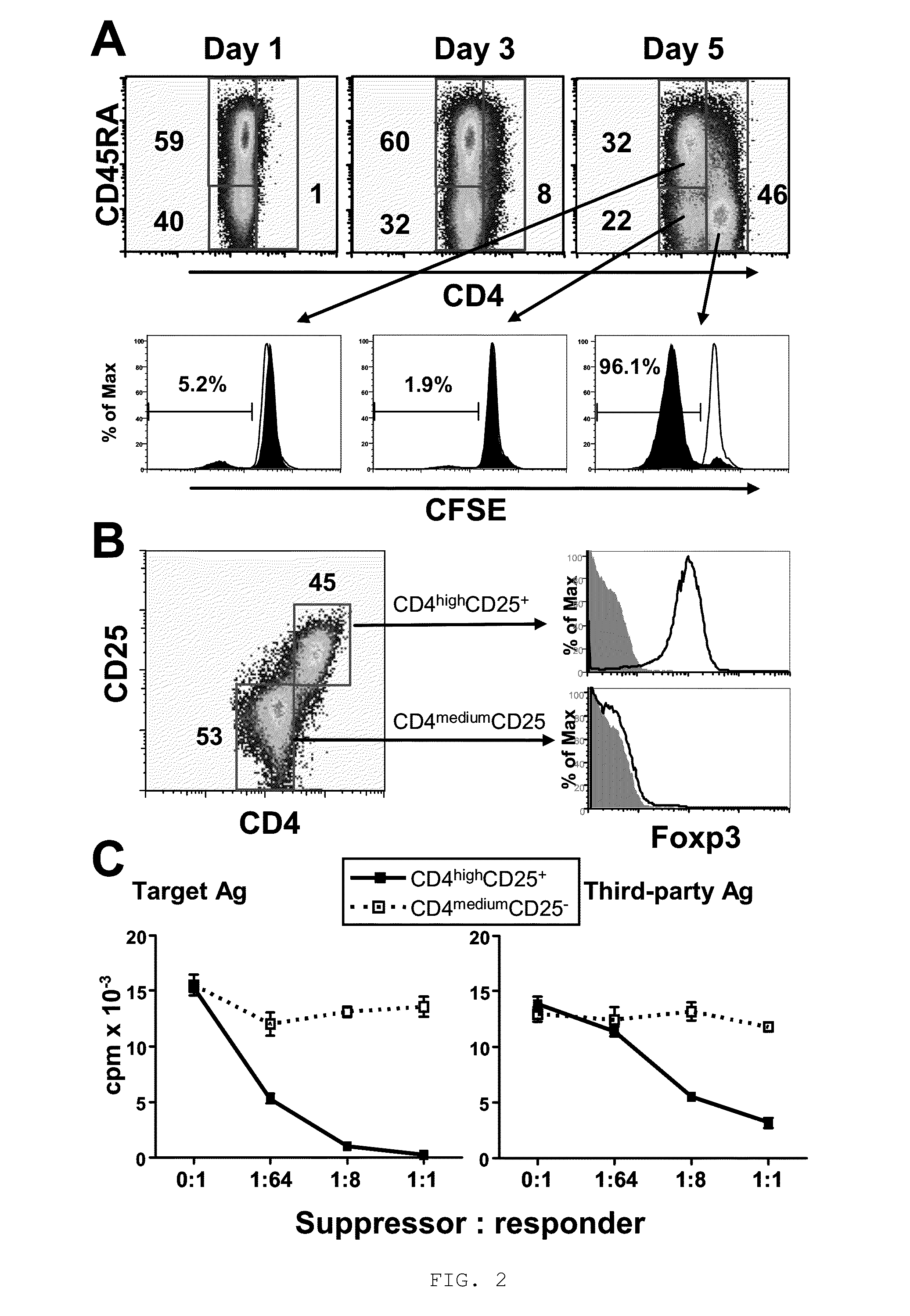
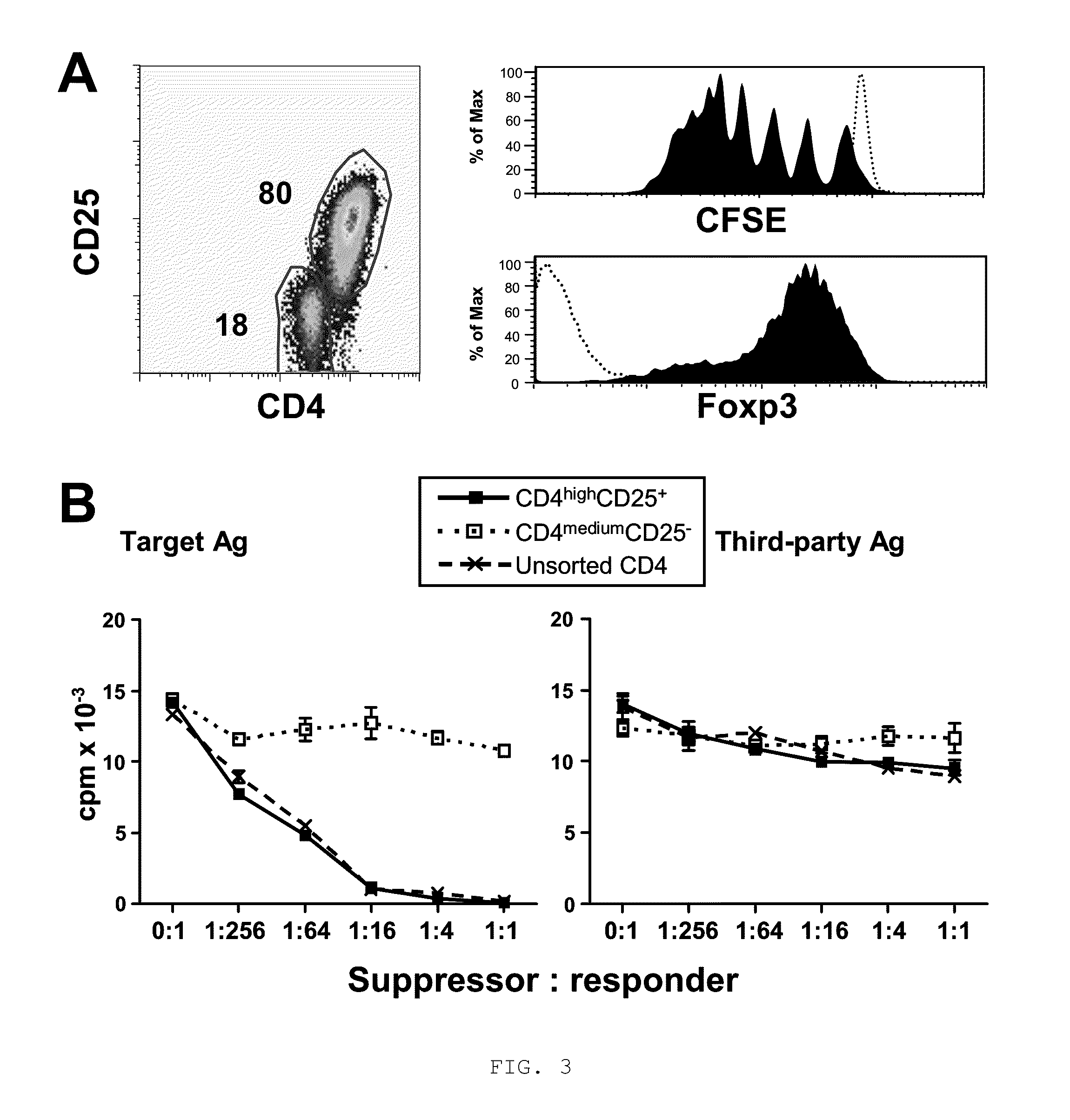
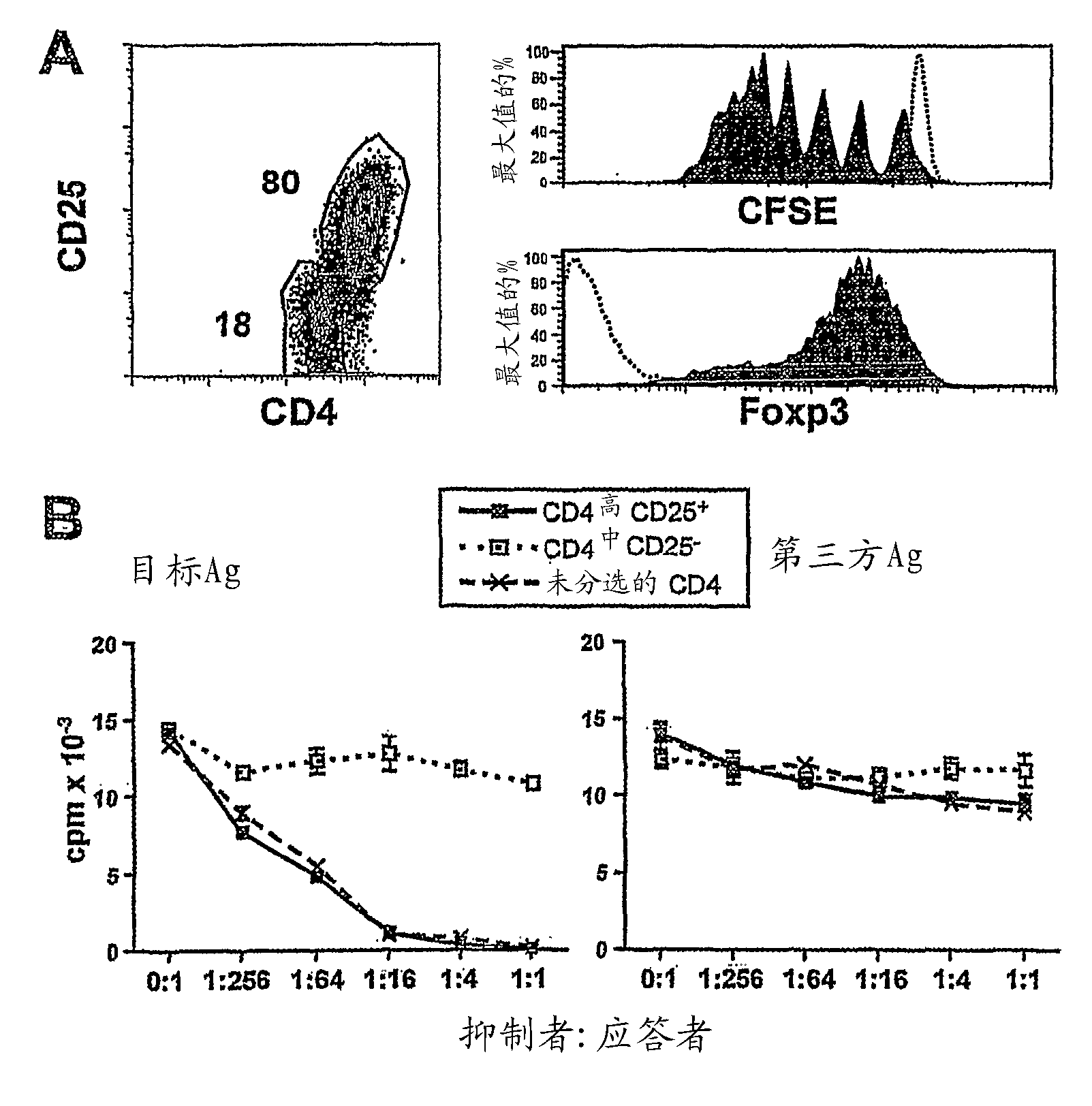
Method to induce and expand therapeutic alloantigen-specific human regulatory T cells in large-scale
ActiveUS8658159B2Improve developmentPreventing transplant allograft rejectionBiocideArtificial cell constructsRegulatory T cellCD4 antigen
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Construction method for human single cell mitochondrial high-throughput sequencing library and kit for library construction
ActiveCN111172157AEnabling ring-wide point mutationsEliminate distractionsMicrobiological testing/measurementLibrary creationPhosphorylationCell layer
The invention provides a construction method for a human single cell mitochondrial high-throughput sequencing library and a kit for library construction. The kit includes mitochondrial genome full-loop amplification primers, single cell lysate, terminal repaired, phosphorylated and 3' added adenylate components of high-throughput library construction, adaptors of high-throughput library construction and forward and reverse library amplification primers. The method realizes the high-throughput sequencing library construction of single or several cell mitochondrial genomes DNA in a mitochondrialgenome complete full-loop amplification based system, and high miss rates and high-proportion false positive site detection rates brought by conventional single cell amplification systems can be evaded, so that massive disadvantages in mitochondrial mutation detection can be got rid of at the single cell level, high sensitivity and accuracy detection can be realized; and as the method adopts themode of full-loop and full-length mitochondrial amplification, the large fragment duplication and deletion of mitochondrial DNA can be discovered while mutation sites are detected.
Owner:福州福瑞医学检验实验室有限公司
Medical care glove
The invention discloses a medical care glove. The medical care glove is prepared from, by weight, 10-15 parts of diatomite with the surface modified with hydantoin, 65-75 parts of fluorine-containingdouble-end amino silicon oil-based benzanilide-based polycondensate and 10-20 parts of polyamide thermoplastic elastomer, wherein the fluorine-containing double-end amino silicon oil-based benzanilide-based polycondensate is prepared through condensation polymerization of 4,4'-diamino benzanilide, 3-ethynylbenzoic acid, polyether amine and 4-azido-2,3,5,6-tetrachloride phthalic anhydride. The invention further discloses a preparation method of the medical care glove. The medical care glove has the advantages of being remarkable in antibacterial effect, good in biocompatibility, high in strength and excellent in comprehensive performance.
Owner:李新虹
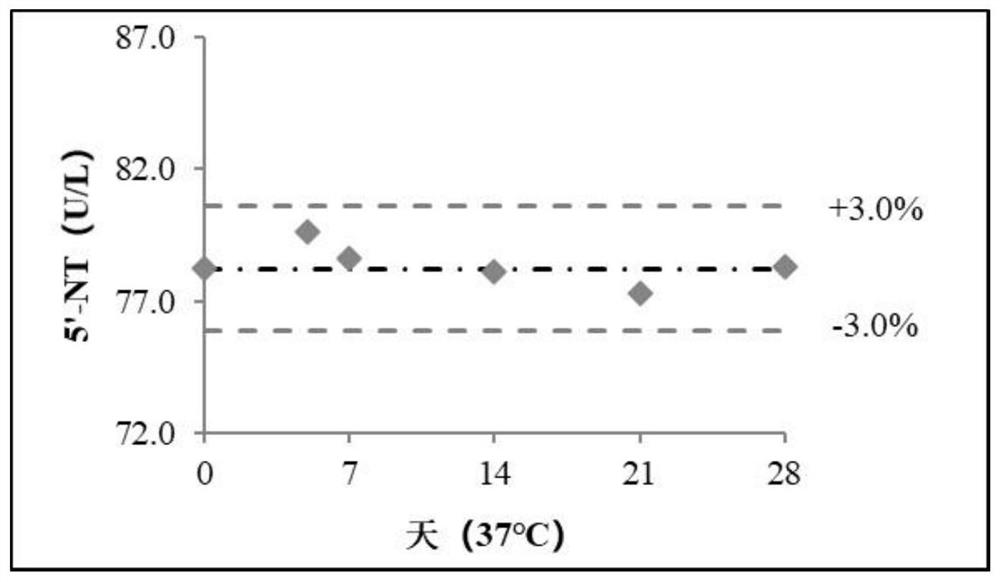
Liquid-stable 5 '-nucleotidase calibrator, detection kit and application thereof
PendingCN113584125AImprove stabilityOvercome the problem of poor stabilityMicrobiological testing/measurementBiological material analysisPeroxidaseNucleotidase
The invention discloses a liquid stable 5 '-nucleotidase calibrator. The calibrator comprises the following components: a buffer solution, a stabilizer, a preservative and 5'-nucleotidase. The invention also discloses a 5 '-nucleotidase detection kit. The kit comprises a 5'-nucleotidase R1 reagent and a 5 '-nucleotidase R2 reagent. The 5 '-nucleotidase R1 reagent is an enzyme reaction system and consists of a first buffer solution, a stabilizer, a first preservative, 4-aminoantipyrine, an enzyme activator, purine nucleoside phosphorylase (PNP), xanthine oxidase (XOD) and peroxidase (POD); the 5 '-nucleotidase R2 reagent is a chromogenic system and is composed of a second buffer solution, a second preservative, inosine-5'-disodium phosphate, beta-sodium glycerophosphate and a Trinder chromogenic substrate. The invention also discloses an application of the 5 '-nucleotidase detection kit in determination of 5'-nucleotidase.
Owner:DIASYS DIAGNOSTIC SYST SHANGHAI
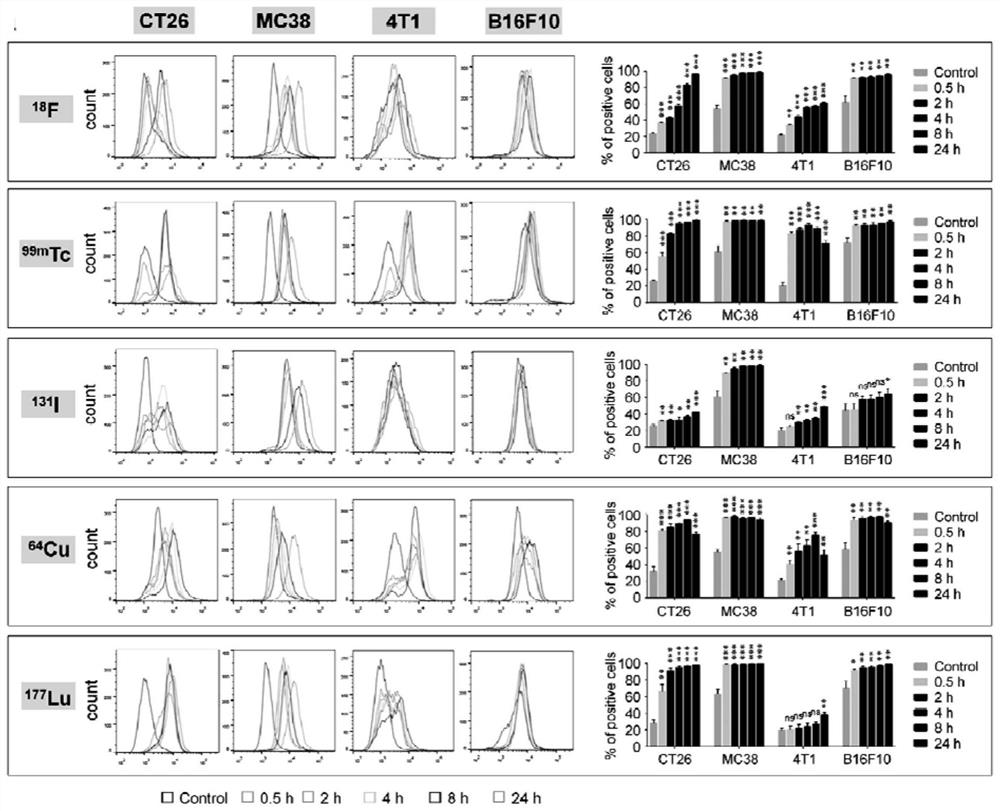
Pharmaceutical composition for tumor immunotherapy
ActiveCN111840585APromote infiltrationImproving the effect of immunotherapyRadioactive preparation carriersAntibody ingredientsPharmaceutical drugBiochemistry
The invention relates to a pharmaceutical composition for tumor immunotherapy, and provides a new application of radionuclide or a marker thereof, and the new application is an application of the radionuclide or the marker thereof in remodeling a tumor immunological microenvironment. The invention also provides a pharmaceutical composition for tumor immunotherapy of a human body or a mouse. The pharmaceutical composition is prepared from radionuclide or the marker thereof and an immune checkpoint inhibitor according to a specific ratio. The invention also provides a kit for tumor immunotherapy. The kit contains the pharmaceutical composition for tumor immunotherapy of human bodies or mice. According to the pharmaceutical composition provided by the invention, the tumor immune microenvironment can be remodeled while the tumor is detected through radionuclide or the marker thereof, so that the tumor growth speed is remarkably reduced through the immune checkpoint inhibitor, and the lifetime is prolonged. The pharmaceutical composition disclosed by the invention has a good synergistic effect, and the kit disclosed by the invention is convenient to use and easy to realize clinical popularization.
Owner:XIAMEN UNIV
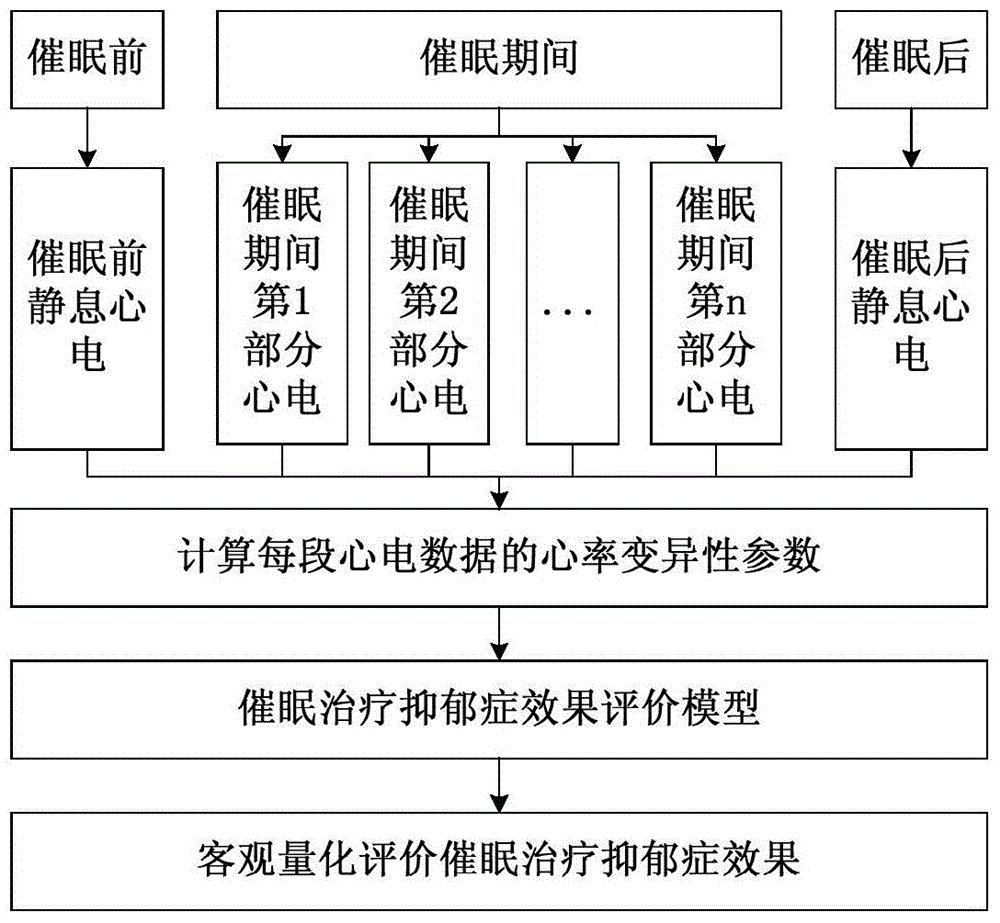
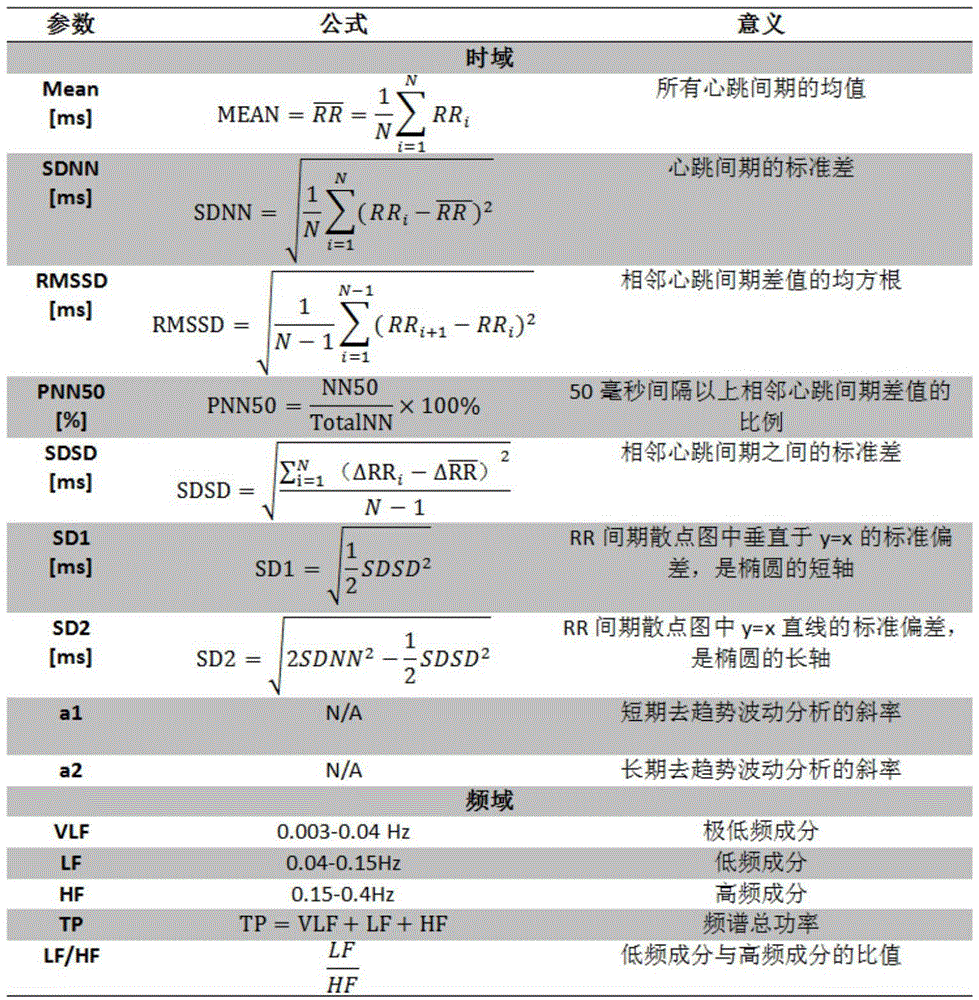
Heart rate variability based evaluation method for hypnotherapy effect of depression
InactiveCN104921720AAvoid subjectivityEnrich scientific research methodsSensorsMeasuring/recording heart/pulse rateRR intervalPulse rate
The invention discloses a heart rate variability based evaluation method for hypnotherapy effect of depression. The method comprises the steps of 1, collecting ECG data before hypnotherapy, during hypnotherapy and after hypnotherapy; 2, calculating the heart rate variability parameters of the three hypnotherapy stages; 3, building an evaluation model for the hypnotherapy effect of depression, inputting the heart rate variability parameters into the model, and then evaluating the hypnotherapy effect of depression through the model. According to the method, the hypnotherapy effect of depression is quantitatively evaluated according to the objective physiological parameters, and thus the objectivity of the treatment effect evaluation is avoided; the psychology and the neurosciences are combined, so as to enrich the method on studying hypnotherapy of the depression; the method has the advantage of clinical practicability.
Owner:SOUTH CHINA UNIV OF TECH
Vascular stent material and preparation method thereof
ActiveCN109289094AEasy to operateMild reaction conditionsSurgeryConjugated synthetic polymer artificial filamentsPolyesterInsertion stent
The invention discloses a preparation method of a vascular stent material. The preparation method comprises the following steps: (1) preparing chloromaleimide-terminated polycaprolactone, (2) preparing a copolymer, (3) modifying the copolymer with chitosan, (IV) synthesizing a furyl polyester condensation polymer, and (5) molding the stent. The invention also discloses the vascular stent preparedthrough the preparation method. The preparation method of the vascular stent material has the advantages of simplicity, easiness in implementation, and low preparation price; and the prepared vascularstent material has the advantages of good biocompatibility, excellent mechanical properties, excellent degradable performance, and safety and reliability in use.
Owner:湖南博隽生物医药有限公司
Method to induce and expand therapeutic alloantigen-specific human regulatory T cells in large-scale
ActiveCN102083966ASuppression of immune responseAvoid exclusionMammal material medical ingredientsBlood/immune system cellsRegulatory T cellT cell
Owner:VERSITECH LTD +1
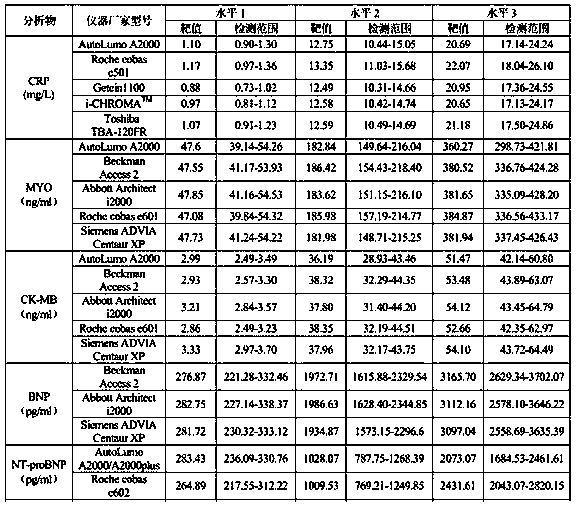
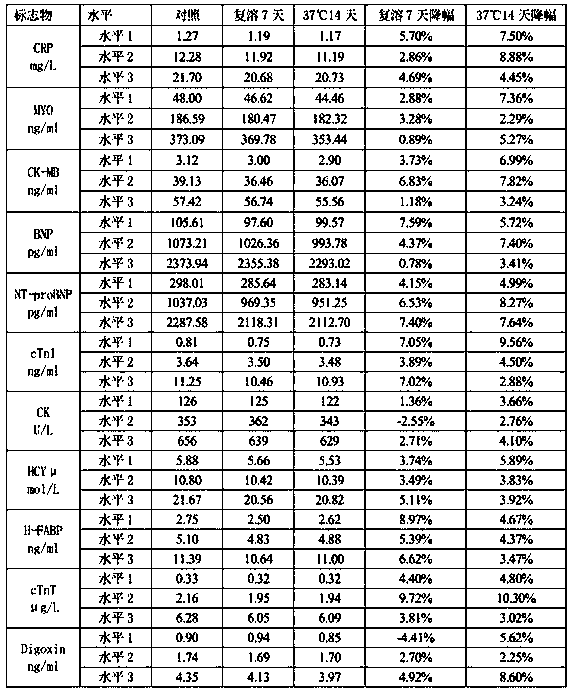
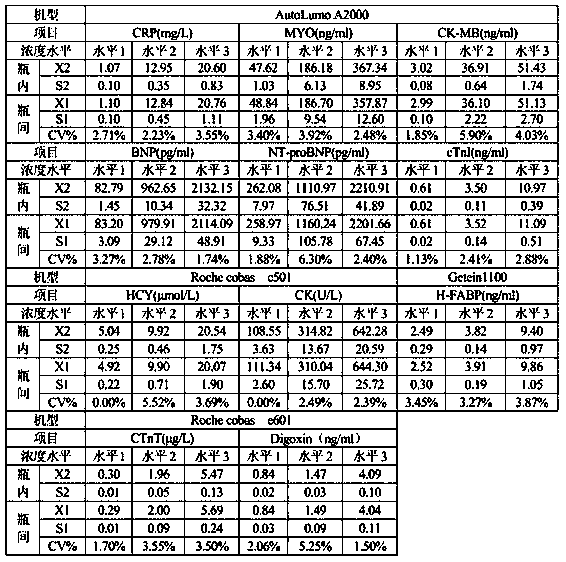
Cardiac marker quality control serum preparation method
InactiveCN109916682ALow viscosityReduce matrix effectPreparing sample for investigationFreeze-dryingBlood plasma
The invention discloses a cardiac marker quality control serum preparation method. Normal human serum is adopted as a substrate liquid, impurities are removed by a filter membrane after a warm bath for 1 to 3 hours, CK, CK-MB, cTnI, cTnT, CRP, BNP, MYO, NT-proBNP, HCY, H-FABP, Dig and other cardiac marker positive substances are added to the obtained quality control serum substrate liquid, qualitycontrol serum solutions with a high, a medium and a low concentration level are prepared, a proper amount of preservative, a protein protective agent and a stabilizer are added and are then mixed uniformly, debugging and determining are carried out, and after the detected result meets a quality standard, the solution is placed in a glass bottle for low temperature vacuum drying to be in a freeze-dried state and is saved at 2 to 8 DEH C. The quality control serum selects normal human serum / normal human plasma / de-hormone human serum / delipidated human serum as the substrate liquid, and the stable-performance multi-item cardiac marker quality control serum prepared according to actual clinical demands has low viscosity, quick redissolving, little substrate effects, excellent clinical applicability, thereby greatly facilitating clinical promotion and development.
Owner:郑州标源生物科技有限公司
A lysosome-targeted pH-sensitive nanoparticle and a preparation method and application thereof
ActiveCN109200021ASmall particle sizeGood dispersionOrganic active ingredientsPharmaceutical non-active ingredientsPh sensitive nanoparticlesLysosomal targeting
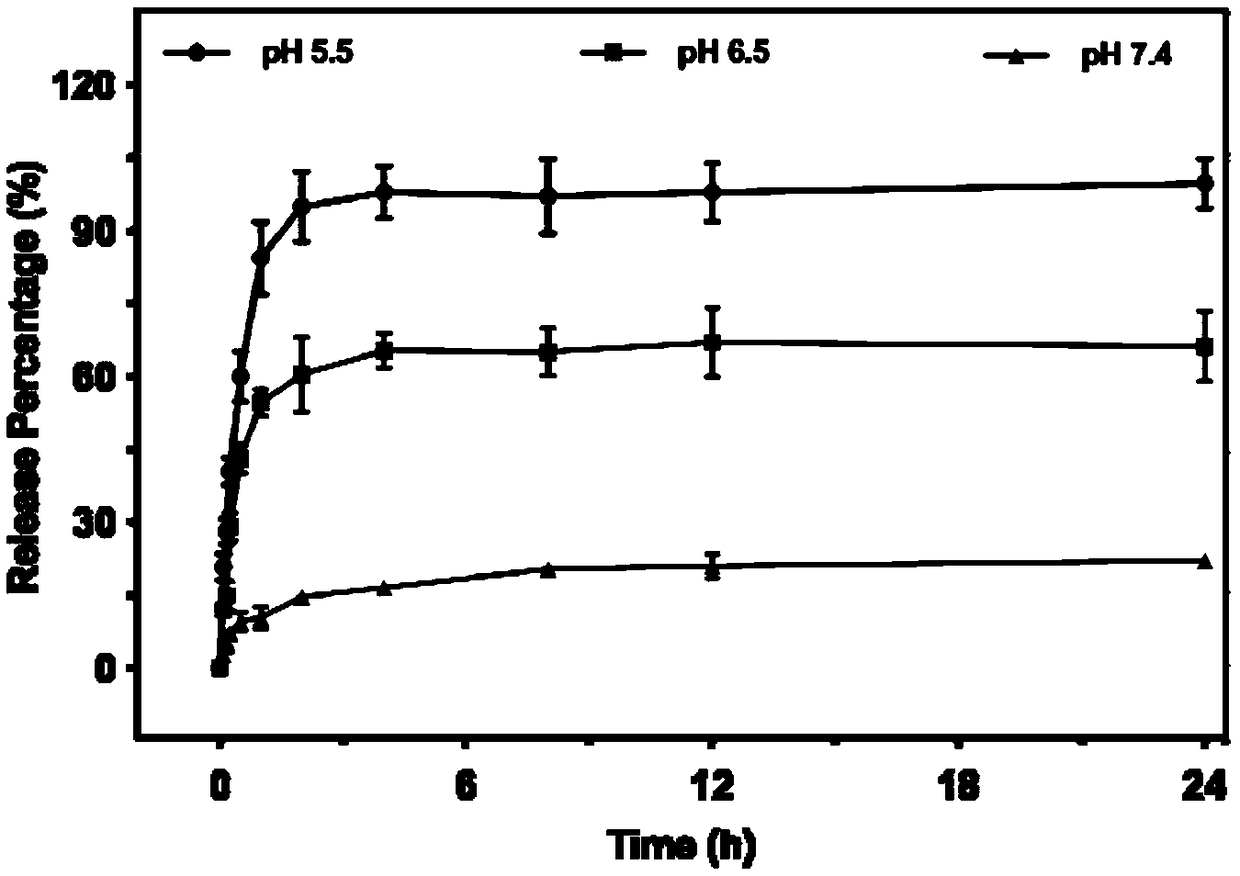
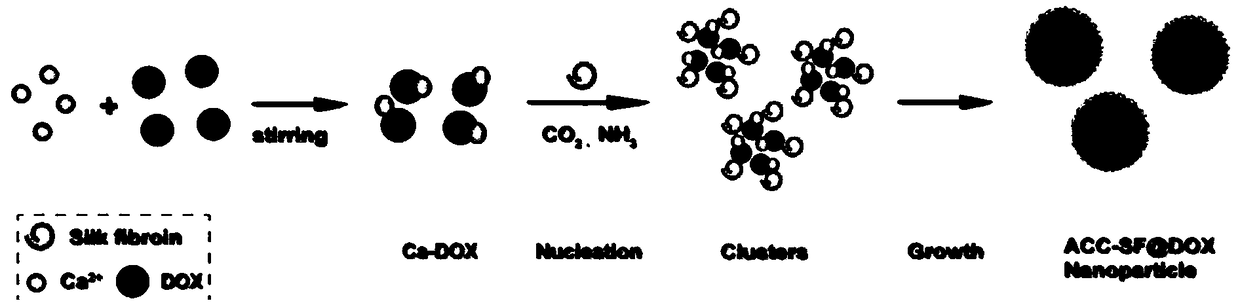
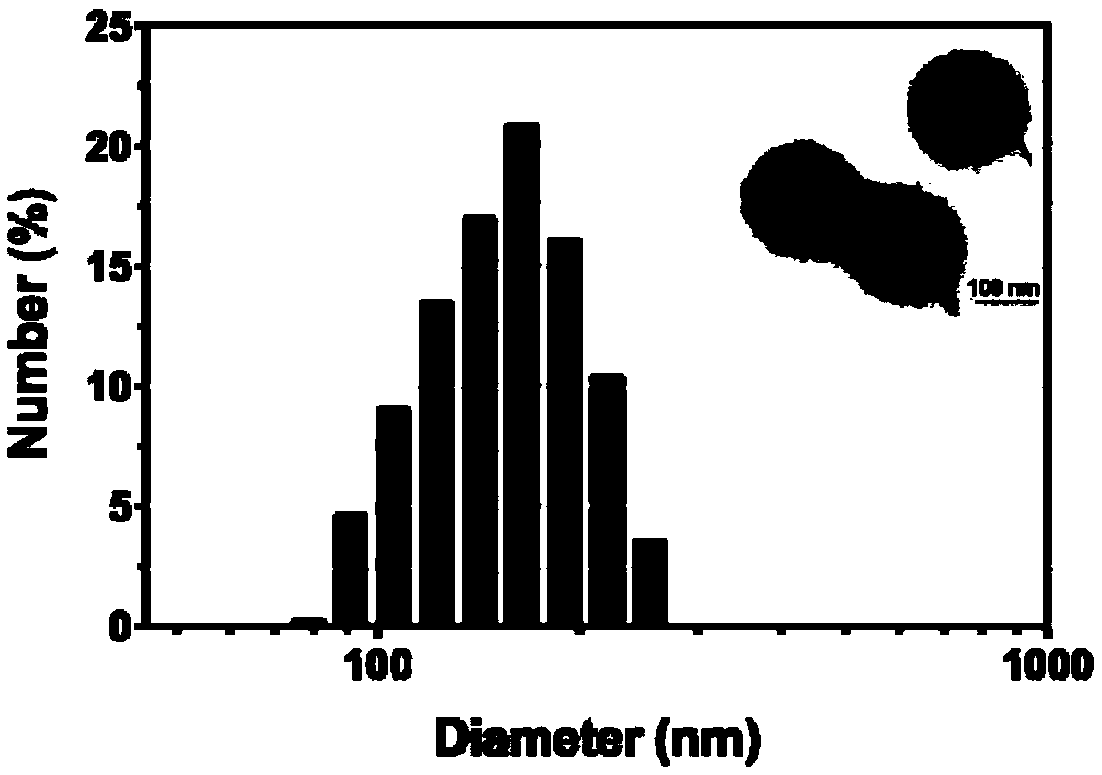
The invention relates to the technical field of biomedical technology, and discloses lysosome-targeted pH-sensitive nanoparticle, comprising adriamycin calcium complex Ca-DOX cluster formed by chelating calcium ions Ca<2+> with adriamycin DOX. Fibroin is attached to the surface of the adriamycin calcium complex Ca-DOX cluster. The invention can enable more nanoparticles to be swallowed into the lysine body by cells, the aggregation degree and effective drug loading of nanoparticles in tumor region are improved, and accelerated the decomposition of nanoparticles in lysosomes, so that more Ca<2+> and CO2 are released, adriamycin can be released from the lysosome and enter the cell nucleus to play an anti-tumor role, thus effectively improving the lethality of the tumor, achieving the effectof tumor treatment, and providing a new direction for the clinical treatment of tumor.
Owner:CHONGQING MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com