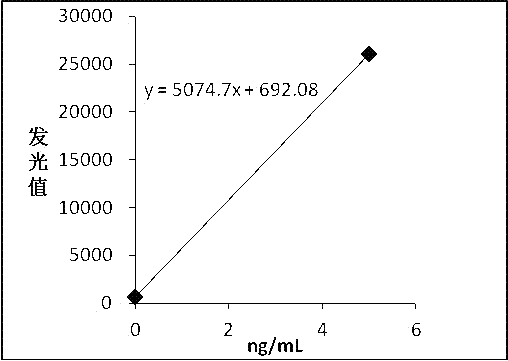Dissociate human chorionic gonadotrophin beta-subunit magnetic particle chemiluminescence quantitative assay kit and its preparation method
A chorionic gonadotropin and chemiluminescence technology, applied in the field of immunoassay medicine, can solve the problems of limited popularization and use, inability to be widely used in clinical diagnosis and scientific research, and achieve no radioactive contamination, simple, reliable, and specific sample pretreatment process. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of the kit for quantitative determination of free human chorionic gonadotropin β subunit magnetic particle chemiluminescence method of the present invention
[0041] 1. Preparation of F-hCGβ calibrator
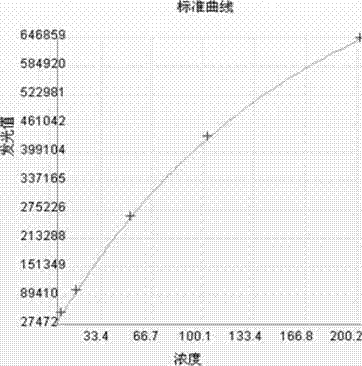
[0042] The pure F-hCGβ was diluted with newborn bovine serum into a calibrator, and divided into 6 bottles of 0 ng / mL, 5 ng / mL, 15 ng / mL, 50 ng / mL, 100 ng / mL, and 200 ng / mL.
[0043] 2. Preparation of magnetic particle solution coated with anti-FITC polyclonal antibody
[0044] Apply a magnetic field to magnetic particles with a particle size of 1 μm, let them stand for 15 minutes, pour out the supernatant, wash 3 times with 25mM MES buffer solution with pH=4.7, and suspend with the buffer solution, the concentration is 50mg / mL; Add 2 mg of anti-FITC polyclonal antibody to the suspension, and mix well at room temperature; prepare an EDC solution with a concentration of 10 mg / mL with deionized water, and add 1 mL of EDC solution with a concentratio...
Embodiment 2
[0054] Embodiment 2 The usage method of the kit of the present invention
[0055] 1. Sample requirements
[0056] Take 5.0ml of venous blood into a glass test tube without adding anticoagulant, let it stand at room temperature, and then separate the serum part by centrifugation (3000rpm×5min).
[0057] 2. Detection method
[0058] Reagents should be equilibrated to room temperature after being taken out from storage conditions before being used for detection; Magnetic separation reagents: Mix thoroughly before use to ensure that the magnetic particles are evenly suspended, and cannot be stirred with a magnetic stirrer; Cleaning concentrate: Dilute 15 times with deionized water , mix well; set up a 37°C water bath; please put the chemiluminescence detector in the standby state; prepare and mark the test tubes as needed.
[0059] The specific operation steps of using this kit to carry out the experiment according to the method of implementation 2 are as follows:
[0060] Add ...
Embodiment 3
[0061] Embodiment 3 The methodological test of the kit of the present invention
[0062] The test kit prepared in Example 1 is tested according to the conventional manufacturing and testing procedures in the art, and the results are as follows:
[0063] 1. Determination of kit precision
[0064] (1) Analytical internal precision
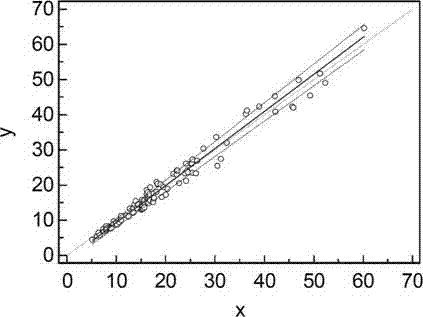
[0065] A batch of the kit prepared in Example 1 was used to measure three different concentrations of serum, namely low, medium and high, and 10 wells were measured in parallel, and the intra-assay coefficient of variation was 4.35% to 6.25%.
[0066] Table 1 Analytical Inner Precision Test
[0067] Determination of serum concentration (ng / mL) Measurement times Intra-analytical CV(%) 10.58 10 6.25 78.65 10 3.69 126.36 10 4.35
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com