Novel ophthalmic curcumin multi-core vesicle gel preparation and preparation method thereof
A gel preparation, curcumin technology, applied in the field of new curcumin ophthalmic multi-core vesicle gel preparation and its preparation, can solve reproductive vitreoretinopathy, pterygium, vernal catarrhal conjunctivitis, age Related cataracts, difficulty in achieving effective drug concentration, low water solubility of curcumin, etc., to achieve the effects of reducing eye irritation and side effects, avoiding drug oxidation, and improving stability and drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
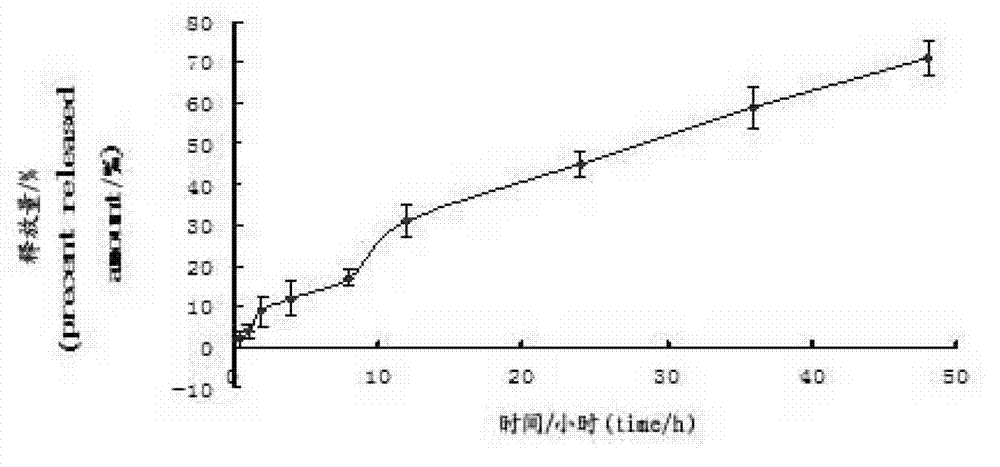
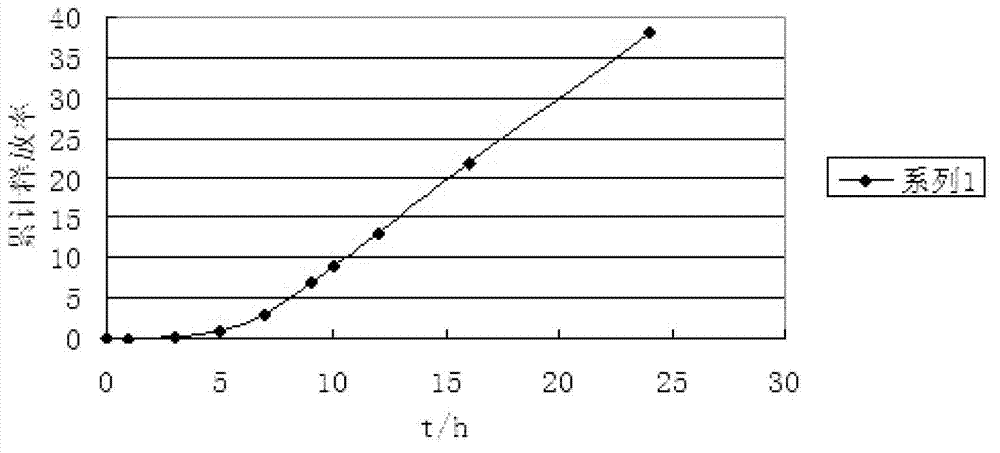
Image
Examples
Embodiment 1
[0037] 1) Precisely weigh 1mg of curcumin, 5-10mg of polyethylene glycol monooleate PEG400MO, 5-10mg of polyethylene glycol monolaurate PEG200ML, 20mg of poloxamer F68, put in a 50ml beaker, 90℃ Heated in a water bath for 10 minutes, and immediately placed in an ice-water bath for 10 minutes, the phase transition process of the raw material from solid to liquid and then to solid, to obtain drug-loaded curcumin nonionic surfactant vesicles;
[0038] 2) Precisely weigh 100mg of soybean lecithin, and make it into an aqueous solution with a concentration of 0.25g / ml by ultrasonic dispersion method;
[0039] 3) Mix the drug-loaded curcumin non-ionic surfactant vesicles obtained in step 1) with the soybean phospholipid aqueous solution obtained in step 2) at a volume ratio of 1:6, and sonicate for 20 minutes to obtain curcumin multi-core vesicles;
[0040] 4) At room temperature and under magnetic stirring, add the glycerol phosphate solution with a concentration of 0.56 mg / L dropwi...
Embodiment 2
[0061] 1) Precisely weigh 1 mg of curcumin, 5-10 mg of polyethylene glycol dioleate (PEG400DO), 5-10 mg of polyethylene glycol dilaurate (PEG200DL), 20 mg of poloxamer F68, and place them in a 50ml beaker. Heated in a water bath at 90°C for 10 minutes, and immediately placed in an ice-water bath for 10 minutes. The phase transition process of the raw material from solid to liquid and then to solid obtained drug-loaded curcumin nonionic surfactant vesicles;
[0062] 2) Precisely weigh 100mg of lecithin, and make it into an aqueous solution with a concentration of 0.25g / ml by ultrasonic dispersion;
[0063] 3) Mix the drug-loaded curcumin non-ionic surfactant vesicles obtained in step 1) with the soybean phospholipid aqueous solution obtained in step 2) at a volume ratio of 1:6, and sonicate for 20 minutes to obtain curcumin multi-core vesicles;
[0064] 4) At room temperature and under magnetic stirring, add the glycerol phosphate solution with a concentration of 0.56 mg / L drop...
experiment example 1
[0067] Experimental example 1 investigates curcumin vesicle encapsulation efficiency
[0068] Precisely pipette 0.1 mL of the vesicle suspension prepared in Example 1 and Example 2 into a 10 mL volumetric flask, break the emulsification with methanol and dilute to the mark with ethanol, and measure the absorbance as A 2 . Similarly, after passing the vesicles through a 0.22 μm microporous membrane, pipette 0.1 mL into a 10 mL volumetric flask, break the emulsion with methanol and dilute to the mark with ethanol, and measure the absorbance as A 1 .
[0069] Encapsulation efficiency of vesicles: EE%=(A1 after passing through the membrane / A2 without passing through the membrane)×100%
[0070] Table 1 Curcumin Vesicle Encapsulation Efficiency Results
[0071] Test No.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com