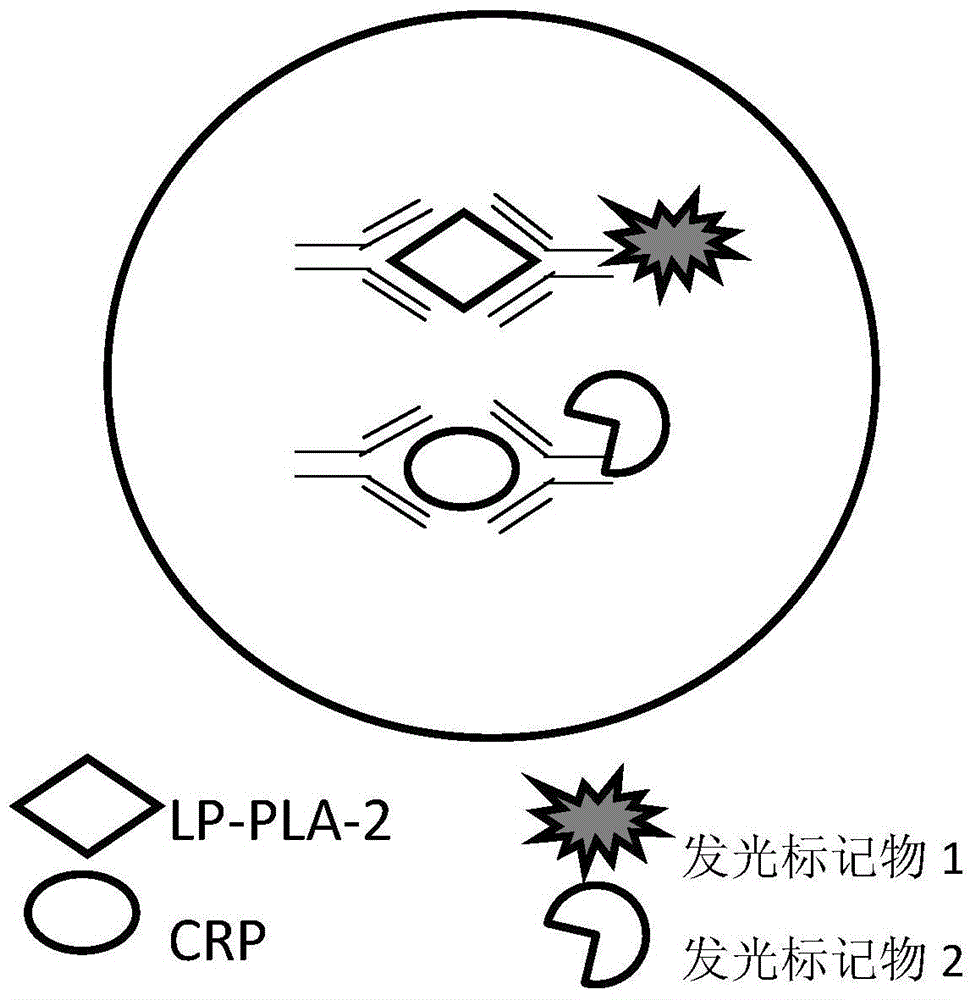
Kit of detecting the content of Lp-PLA2 and CRP on the basis of chemiluminescence, and method and application thereof
A chemiluminescence method and chemiluminescence technology, applied in the field of immunoassay, can solve the problems such as the inability to be widely used in clinical diagnosis and scientific research work, the low sensitivity of the ELISA method, and the difficulty in realizing full automation, and achieve a simple and reliable pretreatment process without radioactivity. contamination, the effect of ensuring sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of a kit for detecting Lp-PLA2 and CRP.
[0026] (1) Preparation of Lp-PLA2 and CRP calibrator:
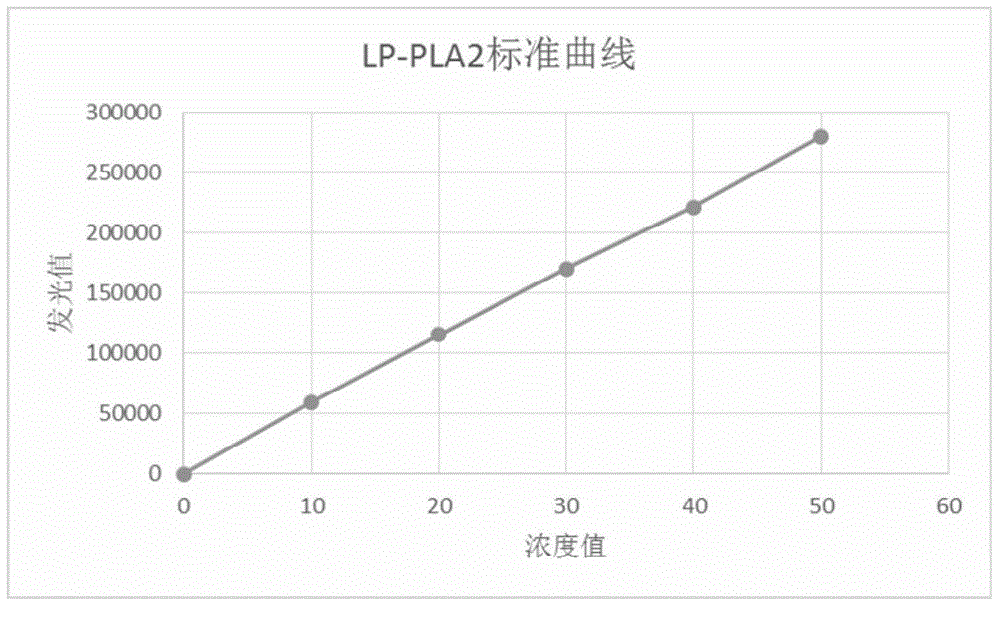
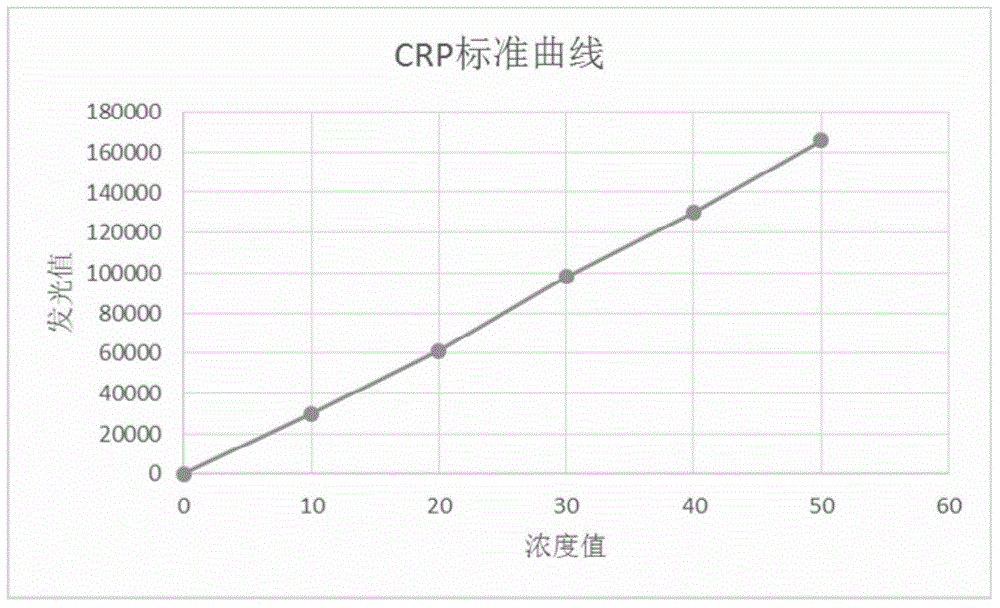
[0027] Dilute the pure Lp-PLA2 and CRP with pH=7.5 and 0.1M Tris-HCl buffer solution into six levels of lyophilized calibrator, and the target concentrations after reconstitution with pure water are 0, 0.25, 2, and 10 respectively. , 25 and 50 ng / ml. Wherein, the raw materials of the Lp-PLA2 and CRP calibrator are standard grade, and the purity is not lower than 99%.
[0028] (2) Preparation of magnetic particle solution coated with anti-fluorescein isothiocyanate (FITC) polyclonal antibody:
[0029]Apply a magnetic field to magnetic particles with a particle size of 1 μm, let them stand for 15 minutes, pour out the supernatant, wash 3 times with 25mM MES buffer solution with pH=4.7, and suspend with the buffer solution, the concentration is 50mg / mL; Add 2 mg of anti-FITC polyclonal antibody to the suspension, and mix uniformly at room temperature; ...
Embodiment 2
[0041] Example 2 Preparation of a kit for detecting Lp-PLA2 and CRP.
[0042] Basically the same as Example 1, the difference is that the magnetic particles coated with anti-fluorescein isothiocyanate polyclonal antibody are suspended in the diluent to prepare a 0.5 μg / mL magnetic particle solution; the fluorescein isothiocyanate-labeled Dissolve Lp-PLA2 monoclonal antibody in diluent to prepare 0.25 μg / mL FITC-labeled Lp-PLA2 monoclonal antibody solution; FITC-labeled CRP monoclonal antibody is dissolved in diluent Prepare 0.25 μg / mL fluorescein isothiocyanate-labeled CRP monoclonal antibody solution; alkaline phosphatase-labeled Lp-PLA2 monoclonal antibody is dissolved in diluent to prepare 0.25 μg / mL alkaline phosphatase-labeled Lp -PLA2 monoclonal antibody solution; alkaline phosphatase-labeled CRP monoclonal antibody was dissolved in diluent to prepare 0.25 μg / mL alkaline phosphatase-labeled CRP monoclonal antibody solution; chemiluminescence substrate solution was 0.1M, ...
Embodiment 3
[0043] Example 3 Detection of the kit prepared in the present invention (the kit prepared in Example 2).
[0044] 1. Sampling: Take 1ml of whole blood or serum;
[0045] 2. Sample testing:
[0046] The reagents in the kit should be equilibrated to room temperature after being taken out from storage conditions before being used for detection; before use, the magnetic particle solution coated with anti-FITC polyclonal antibody should be thoroughly mixed to ensure that the magnetic particles are evenly suspended, but a magnetic stirrer cannot be used Stir; cleaning solution: dilute the cleaning solution 15 times with deionized water and mix well; set up a 37°C water bath; put the chemiluminescence detector in the waiting state; prepare test tubes and mark them as needed.
[0047] Mix 50 μl of calibrator or sample to be tested with 50 μl of FITC-labeled Lp-PLA2 monoclonal antibody solution and ALP-labeled Lp-PLA2 monoclonal antibody solution, and incubate 50 μl of calibrator or s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com