Cardiac marker quality control serum preparation method
A quality control substance and substance technology, which is applied in the field of the preparation of myocardial marker substance control substances, can solve the problems such as the unoptimistic current situation of myocardial marker quality control, the inability of product performance to meet clinical requirements, the unstable structure of myocardial markers, and the like. The effect of promotion and development, good clinical practicability, and complete quality control testing items
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of high, medium and low levels of myocardial marker substance control products
[0020] The first step is to take 1000ml of serum raw material, which comes from commercial normal human serum or hormone-free serum or lipid-free human serum, and the following indicators are negative after testing: HBsAg, HCV-Ab, HIV-Ab, TP ;
[0021] Reconstitute the serum and return it to room temperature, then bathe in water at 40-60°C for 1-3 hours; after returning to normal temperature, the serum background test results should meet the following requirements: CK<200U / L, CK-MB quality<0.6ng / ml, CK-MB activity<25U / L, MYO<45 ng / ml, cTnI<0.3ng / ml, cTnT<0.2ug / L, BNP<100pg / ml, NT-proBNP<300pg / ml, CRP<0.5mg / L , H-FABP<5ng / ml, HCY<15μmol / L, Dig<0.2ng / ml;
[0022] In the second step, the serum after the first water bath is removed by a filter membrane with a pore size of 0.22 μm-0.65 μm to obtain a quality control matrix solution;
[0023] The third step is to add posi...
Embodiment 2
[0026] The performance measurement of the quality control product prepared in embodiment 2 embodiment 1
[0027] 1. Detection value
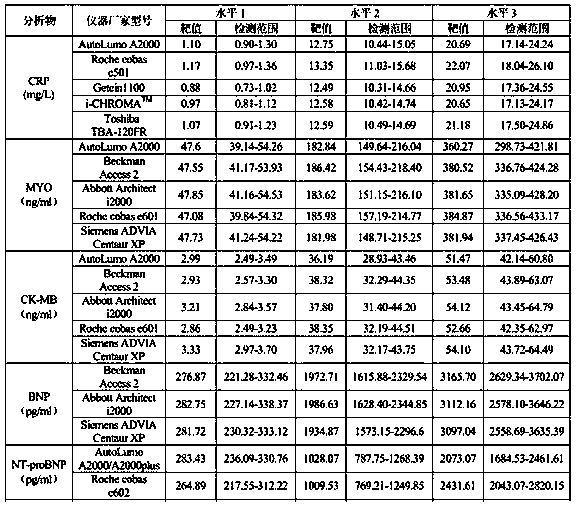
[0028] Use the corresponding kit to detect each level of the quality control product on the claimed detection system, and the results are shown in Table 1 below.
[0029] Table 1
[0030]
[0031]
[0032] The data in Table 1 show that the above results are all within the given range. It proves that the detection results of each detection system are consistent.
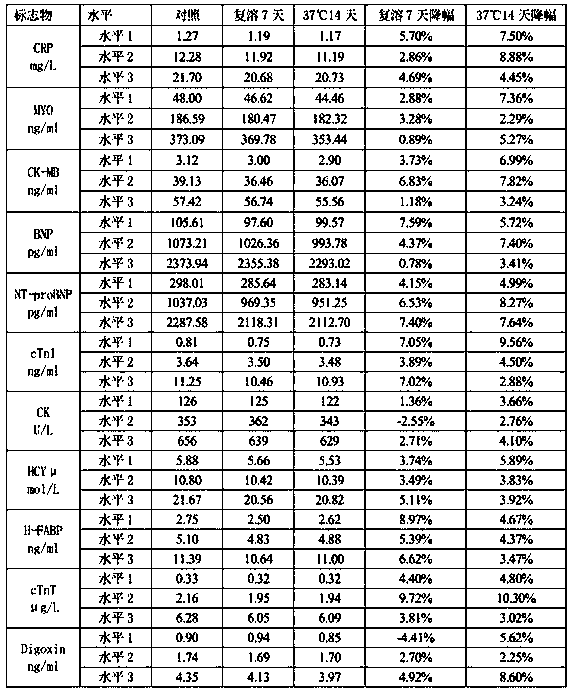
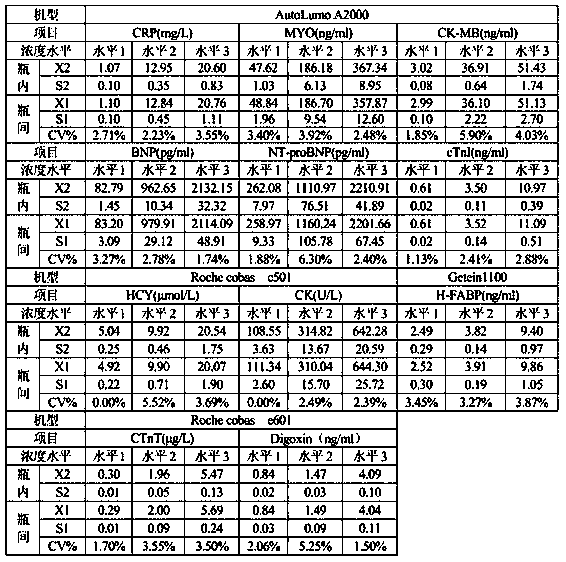
[0033] 2. Uniformity
[0034] Take 10 bottles of quality control products of each level with the same batch number, test each bottle of quality control products once with the corresponding kit, and calculate the average value of the 10 test results ( ) and standard deviation S 1 ;
[0035] In addition, use 1 bottle of the above-mentioned 10 bottles of quality control products for 10 consecutive tests, and calculate the average value of 10 test results ( ) and standard dev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com