Pharmaceutical composition for tumor immunotherapy
A drug and tumor technology, applied in the field of drug combination of tumor immunotherapy, can solve the problem of patients unable to respond to immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
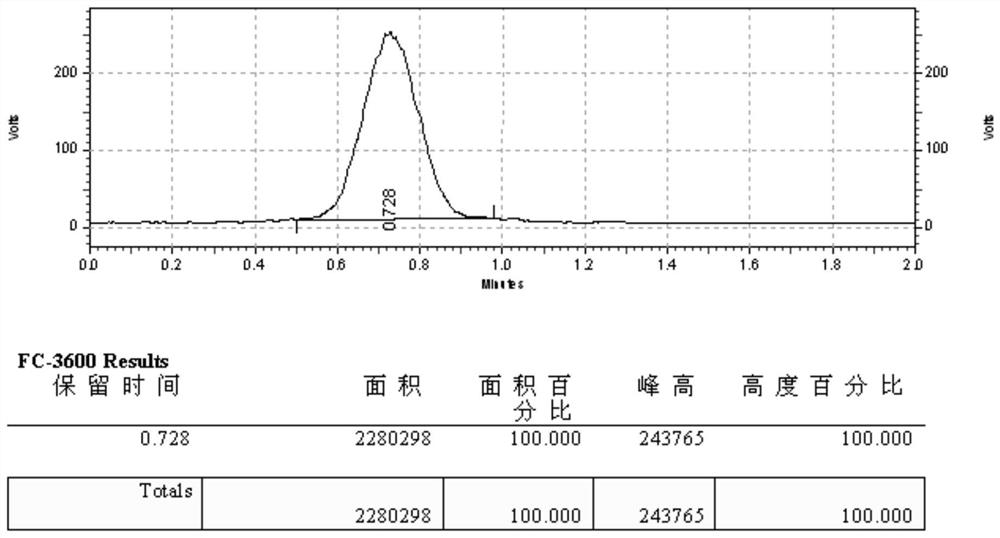
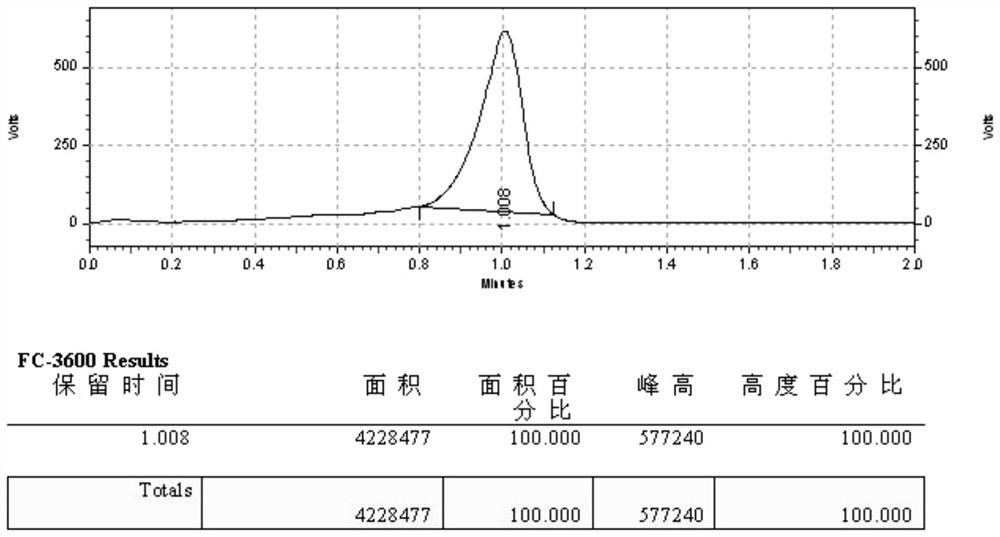
[0057] 1.[ 18 F] Synthesis of FDG
[0058] [ 18 F] FDG was synthesized by the IBA automation module. Based on the premise of trifluoromannose, the method of alkali hydrolysis chemical synthesis. Step 1 is the nucleophilic fluorination reaction: the cyclotron produces [ 18 F]F - , under helium transport, [ 18 F]F - Adsorbed onto the QMASep-Pak anion exchange column, under the action of a vacuum pump, take a mixture of potassium carbonate (6mg / mL) dissolved in water and phase transfer catalyst K2.2.2 (20mg / mL) dissolved in acetonitrile ,Will[ 18 F]F - Eluted into the reaction vial. [ 18 F]F - The nucleophilic activity of ions is one of the key factors affecting the reaction yield, so it is very important to remove water in the reaction system. Dry [ 18 F]F - , catalyst amino polyether (K2.2.2) to chelate potassium ions in the system, so that [ 18 F]F - It is exposed to increase its nucleophilic activity (activation of fluoride ions), followed by a nucleophilic fl...
Embodiment 2
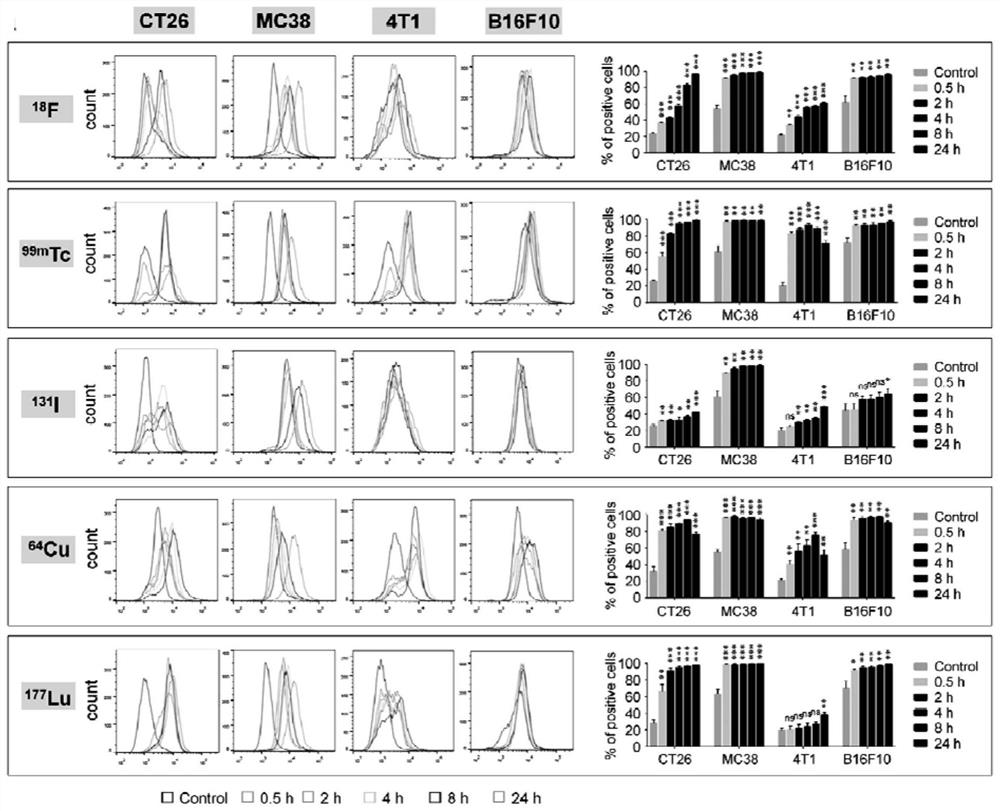
[0066] The following is the determination of the impact of different nuclides on the expression of PD-L1 and the markers synthesized by the method of the above-mentioned Example 1[ 18 F]FDG and 99m Description of the distribution and therapeutic effect of Tc-RGD in vivo:
[0067] 1. Flow cytometry to detect the effect of different nuclides on the expression of PD-L1
[0068] CT26, MC38, 4T1 and B16F10 tumor cells were plated in six-well plates overnight, and 740kBq of radionuclide Na was added to each well 18 F, The cells of the control group were added with an equal volume of normal saline, and the cells of the experimental group and the control group were cultured in different incubators to ensure that the control group was not affected. After incubation at different time points (0.5h, 2h, 4h, 8h and 24h), the cells were collected and washed twice with ice-cold PBS, then stained with anti-PD-L1 antibody (abcam, ab238697), and restained overnight Fluorescent secondary anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com