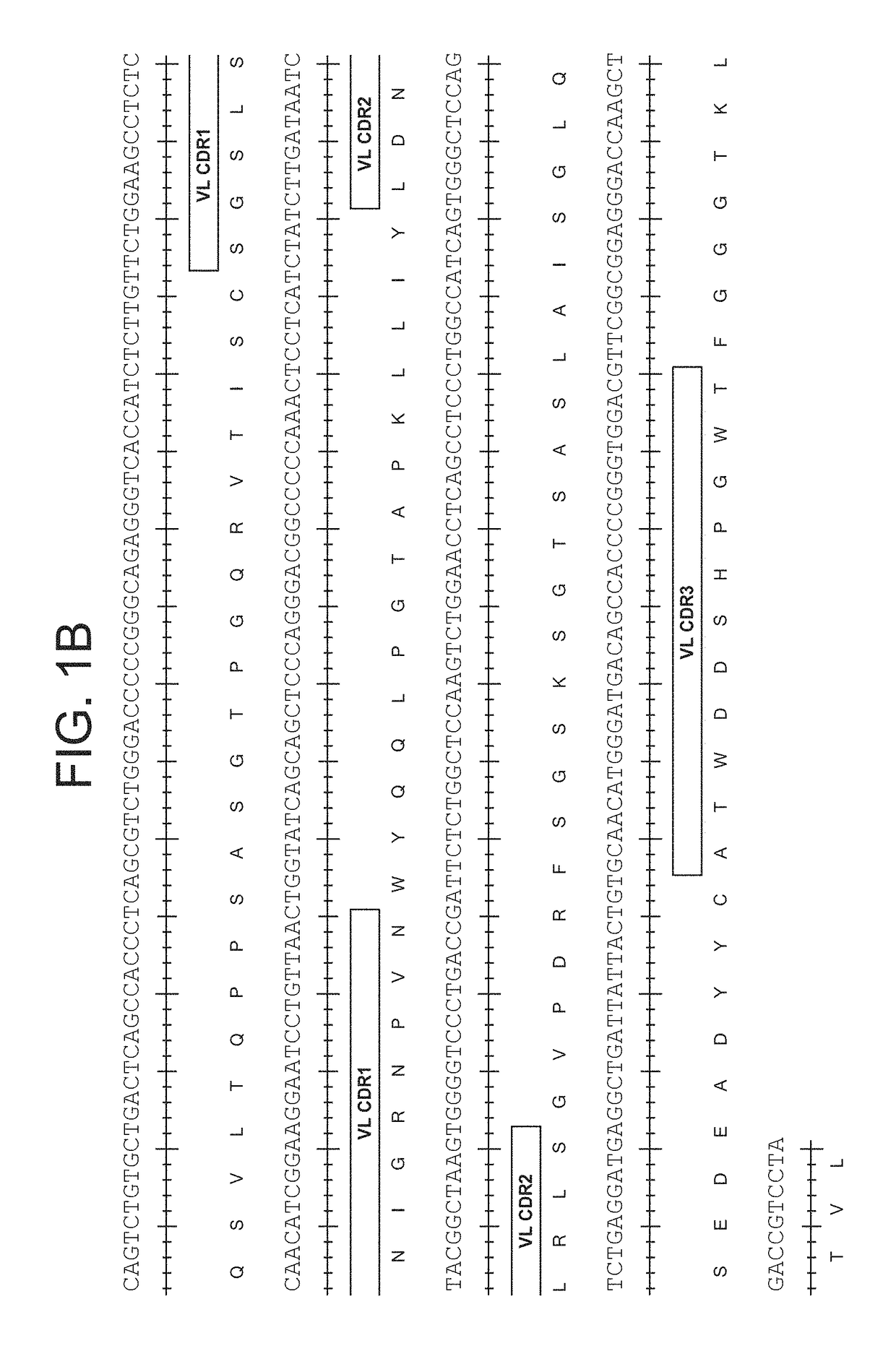
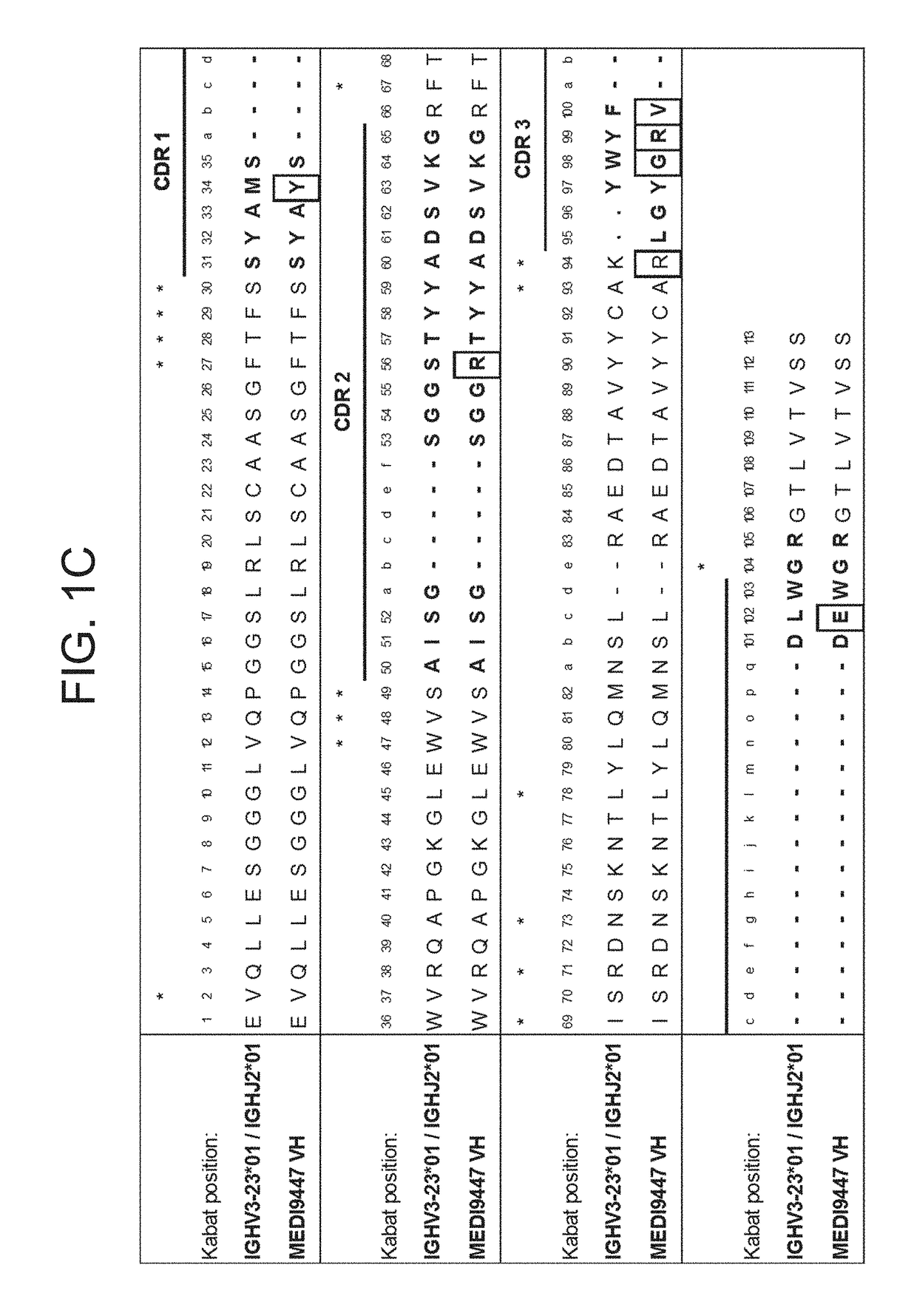
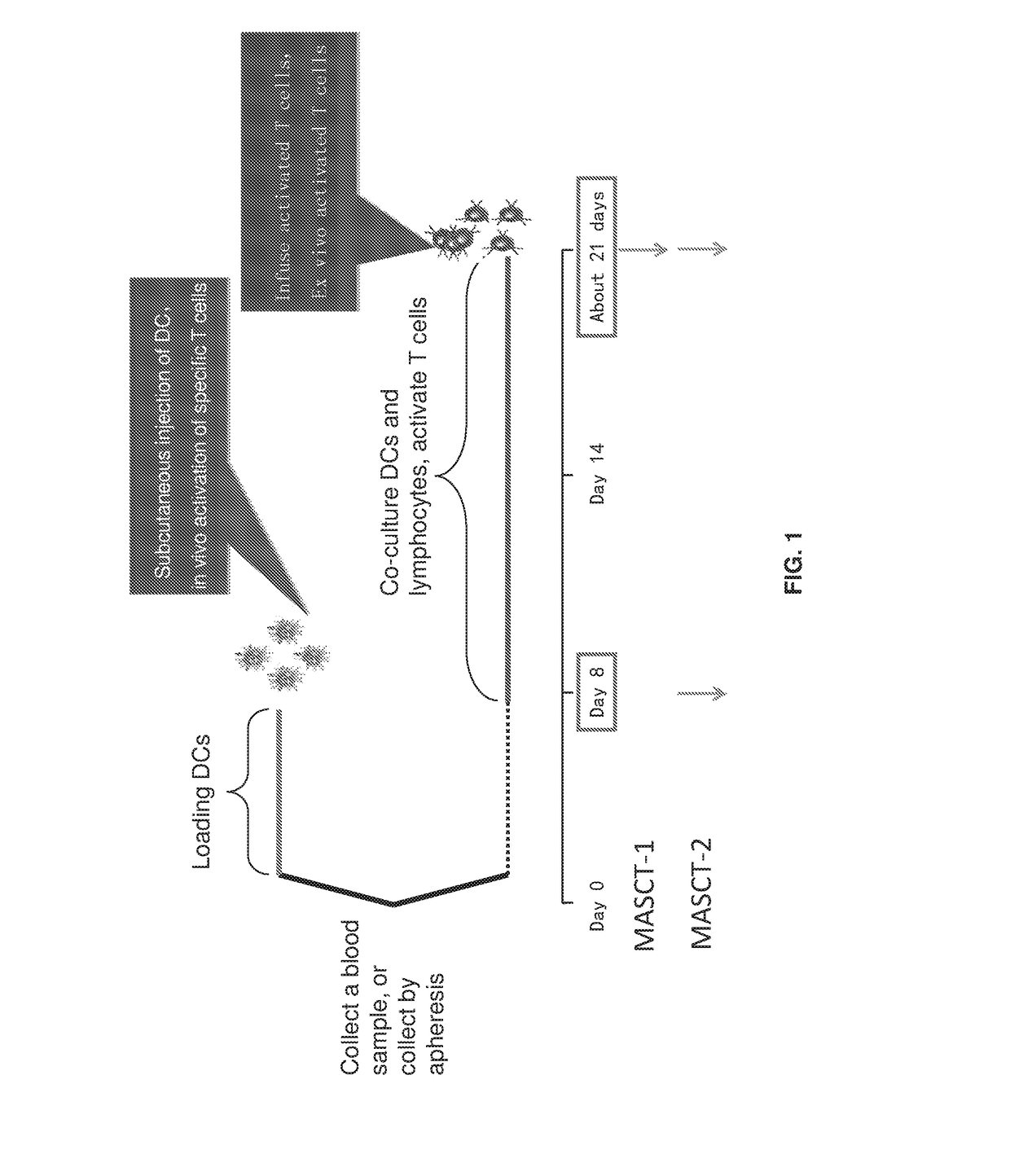
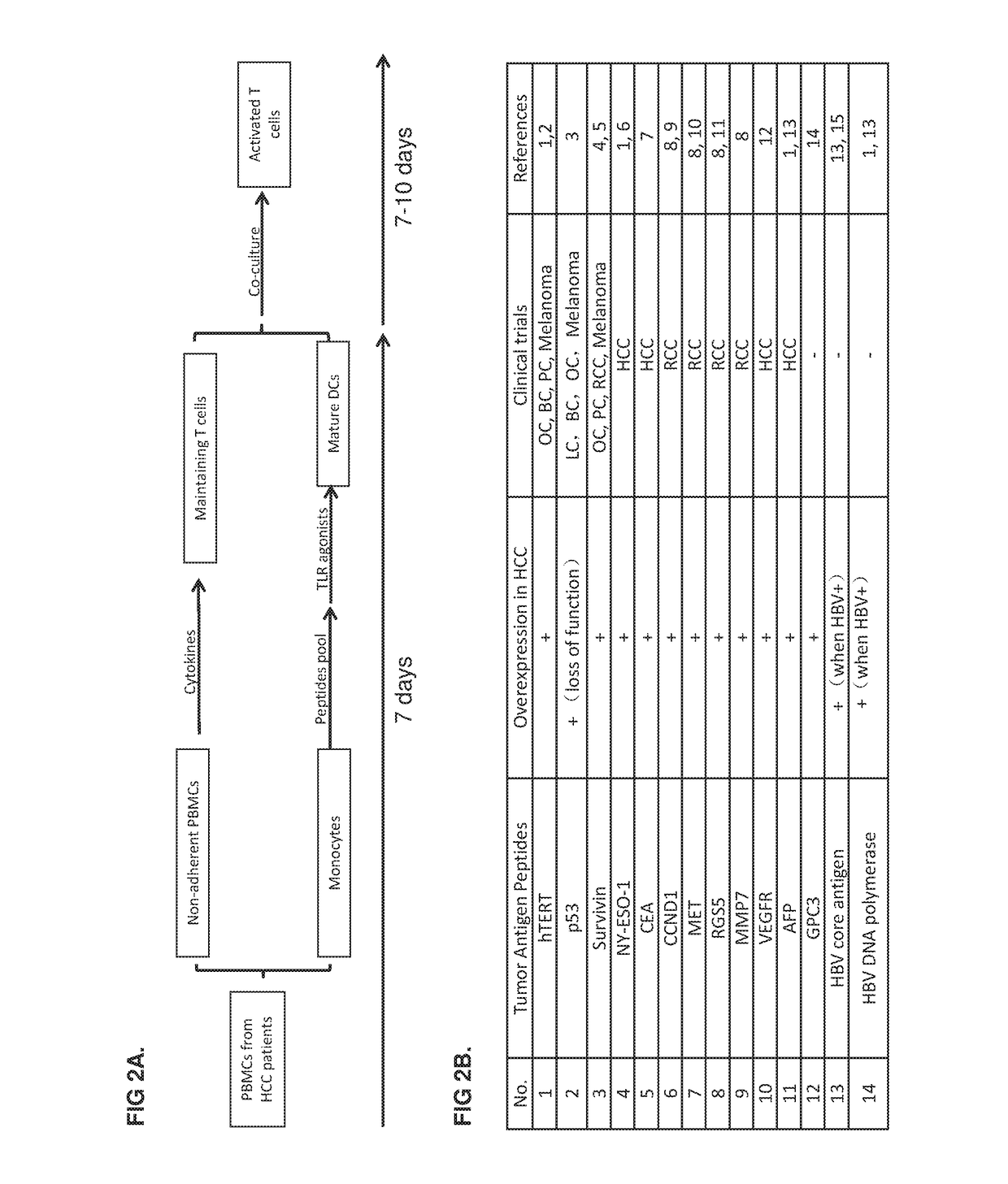
Patents
Literature
485 results about "Immune checkpoint inhibitors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Checkpoint inhibitor therapy is a form of cancer immunotherapy. The therapy targets immune checkpoints, key regulators of the immune system that when stimulated can dampen the immune response to an immunologic stimulus. Some cancers can protect themselves from attack by stimulating immune checkpoint targets.
Methods and compositions for treatment with synthetic nanocarriers and immune checkpoint inhibitors
Disclosed are synthetic nanocarrier compositions and immune checkpoint inhibitor compositions and related methods for administration to a subject.
Owner:SELECTA BIOSCI
Binding molecules specific for cd73 and uses thereof
ActiveUS20160194407A1Reduce proliferationEnhance immune responseAnimal cellsSugar derivativesDiseaseDrug conjugation
The present disclosure provides anti-CD73 binding molecules, e.g., antibodies and antigen binding fragments thereof. Also provided are pharmaceutical formulations comprising the disclosed compositions, and methods for the diagnosis and treatment of diseases associated with CD73-expression, e.g., cancer. Such diseases can be treated, e.g., by direct therapy with the anti-CD73 binding molecules disclosed herein (e.g., naked antibodies or antibody-drug conjugates that bind CD73), by adjuvant therapy with other antigen-binding anticancer agents such as immune checkpoint inhibitors (e.g., anti-CTLA-4 and anti-PD-1 monoclonal antibodies), and / or by combination therapies where the anti-CD73 molecules are administered before, after, or concurrently with chemotherapy.
Owner:MEDIMMUNE LTD
Predicting patient responsiveness to immune checkpoint inhibitors
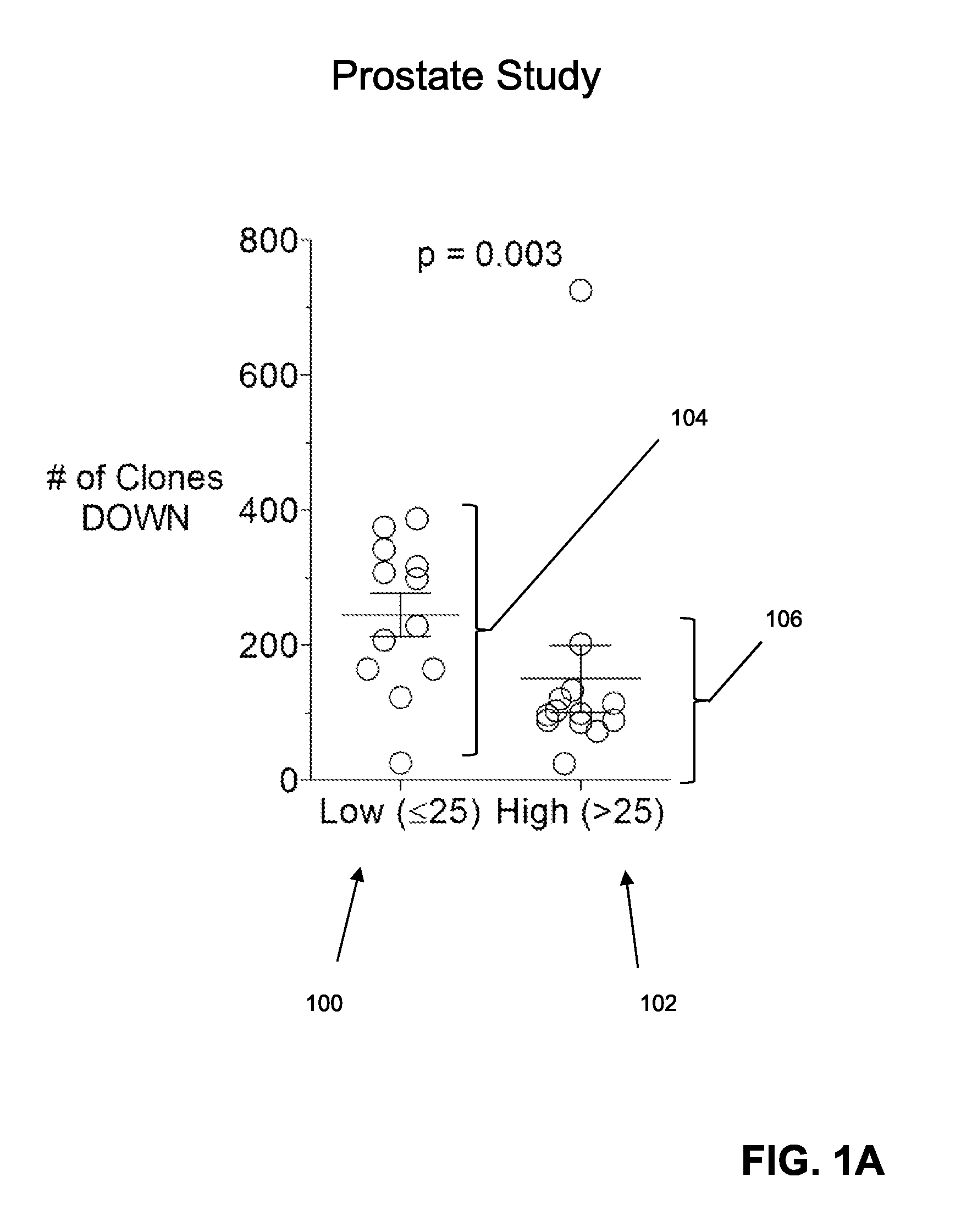
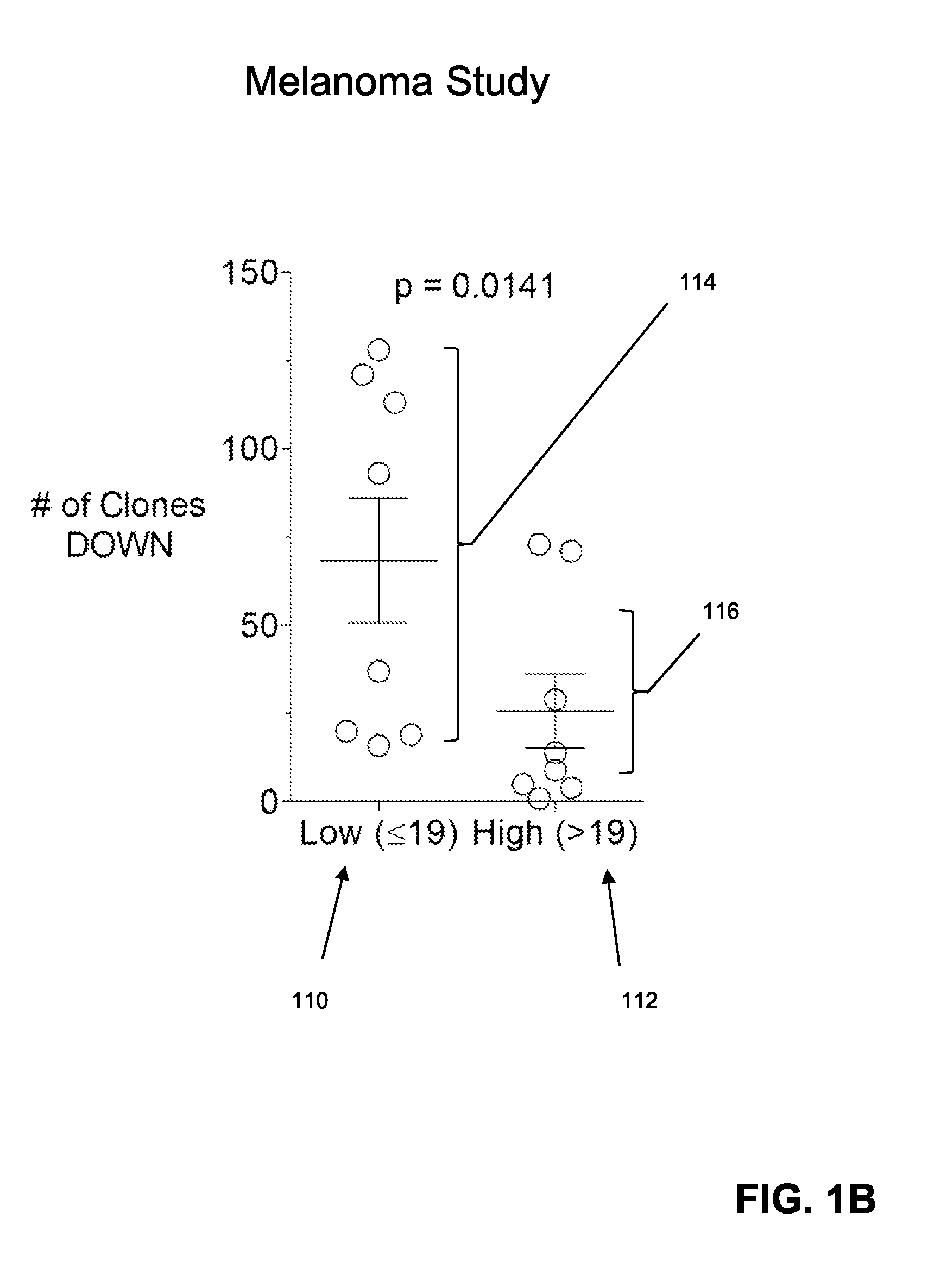
The invention is directed to a method of predicting clinical response of a patient to treatment of a cancer by an immune checkpoint pathway inhibitor, such as an anti-CTLA-4 or anti-PD-1 antibody binding compound. In one aspect the method comprises generating pre- and post-treatment clonotype profiles, determining a number of clonotypes that decrease in frequency between the first and second clonotype profiles, and predicting a lack of responsiveness in the patient to the treatment whenever the number of clonotypes that decrease in frequency is greater than a predetermined value.
Owner:ADAPTIVE BIOTECH +1
Tumor suppressor and oncogene biomarkers predictive of Anti-immune checkpoint inhibitor response
ActiveUS20170130271A1Prevent cancerMicrobiological testing/measurementBiological material analysisAbnormal tissue growthSuppressor
The present invention is based on the identification of novel biomarkers predictive of responsiveness to anti-immune checkpoint inhibitor therapies.
Owner:DANA FARBER CANCER INST INC +1
Tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor)
InactiveCN107034235AIncrease lethalityImprove homingGenetically modified cellsMammal material medical ingredientsTumor targetEGFR Antibody
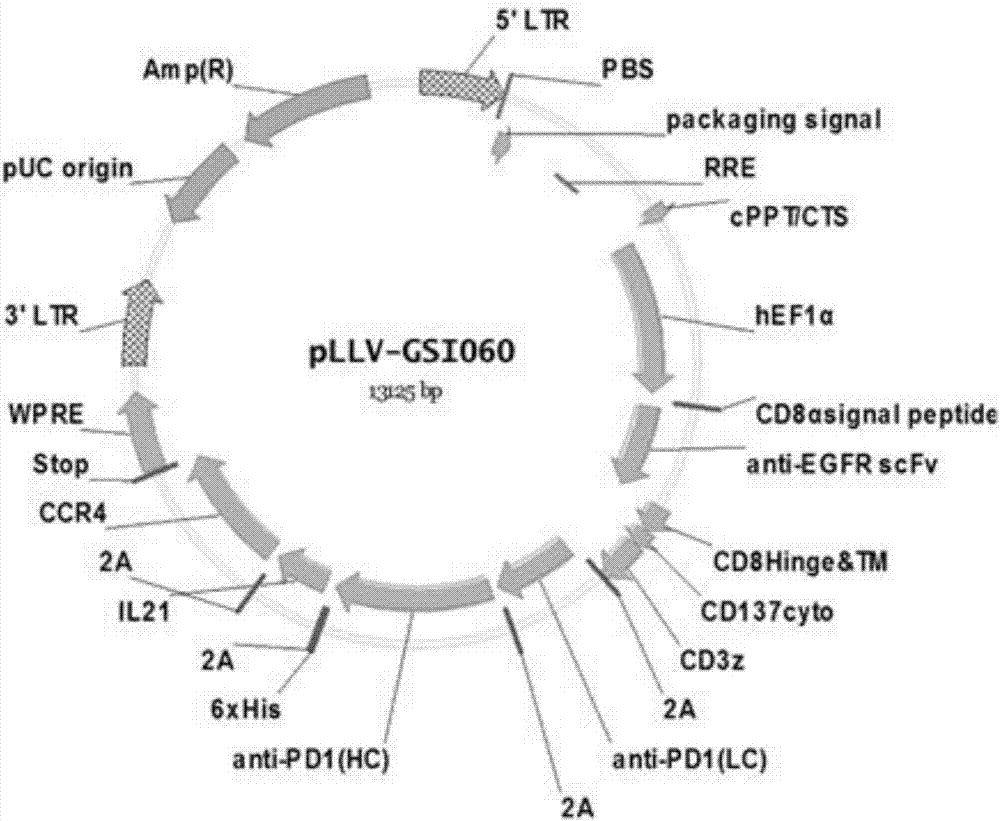
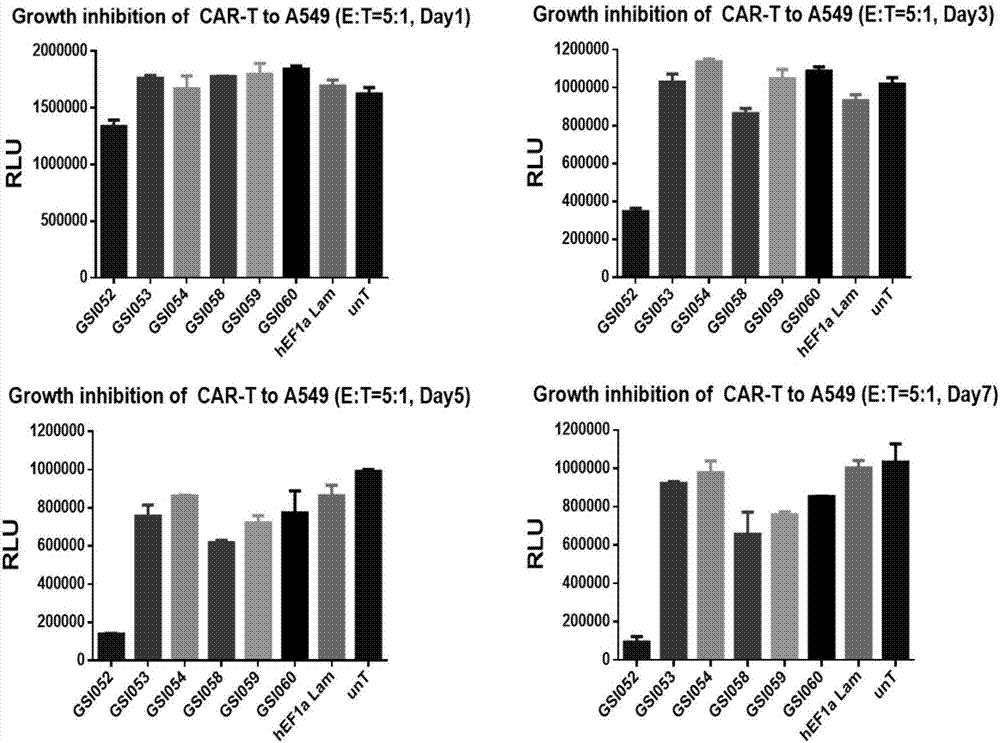
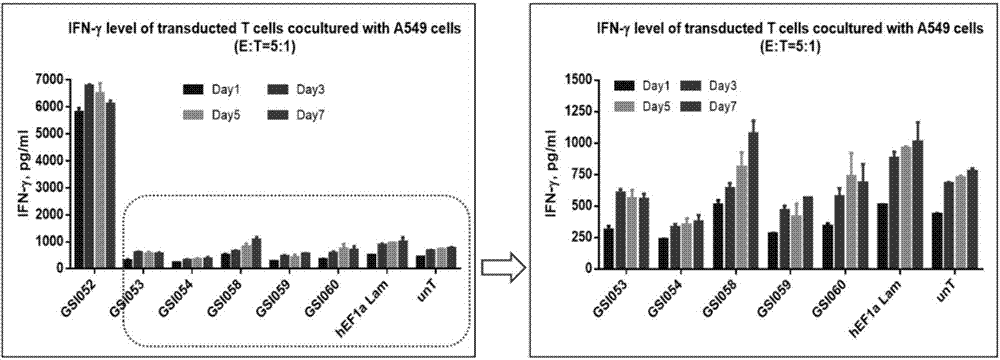
The invention discloses a tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor) and also discloses a plasmid vector for implementing the method. In combination with fourth-generation CAR-T (chimeric antigen receptor T) cells targeting at PD-1 and EGFR, a lentiviral vector is used as a CAR-T vector basic structure, and truncated EGFR antibody is selected as a CAR core to give tumor targeted enrichment to play, tumor-killing effect is given to play in conformity with overexpressed immune checkpoint inhibitor PD-1 monoclonal antibody; by constructing the fourth-generation CAR-T vector for co-expressing various regulatory factors such as IL21, CCR4 and Bcl2, the killing, homing and persistent proliferating abilities of CAR-T cells are improved. EP-CAR T (esophageal papilloma chimeric antigen receptor T) cells are treated by transducing patient's autologous T-lymphocytes in vitro, amplifying suitably and transducing back to the patient's body via autologous transfusion, and no reports on similar designs of CAR-T cells are provided at present.
Owner:尹荣
Method for predicting sensitivity of patients with NSCLC (non-small cell lung cancer) to immunotherapies
ActiveCN110305965AAccurate predictionAvoid blind medicationMicrobiological testing/measurementProteomicsOncologyBiomarker (petroleum)
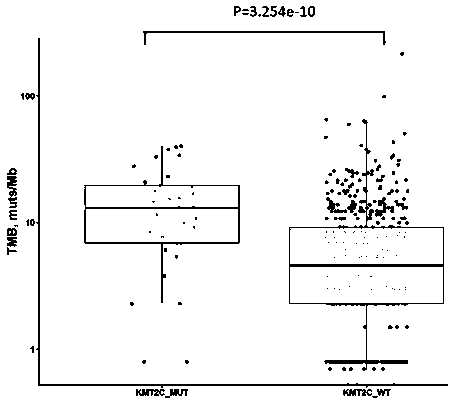
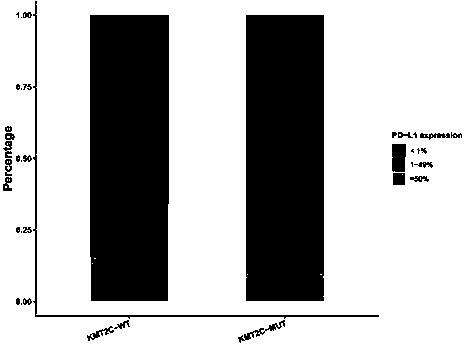
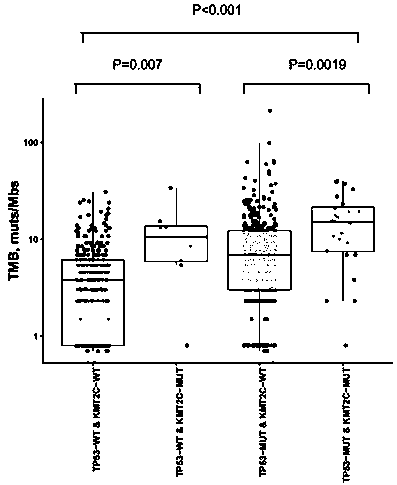
The invention discloses a method for predicting sensitivity of patients with a cancer (non-small cell lung cancer) to immunotherapies, such as immunotherapy using an immune checkpoint inhibitor, by using KMT2C and TP53 genes as biomarkers, and also discloses application of a reagent, which detects the biomarkers specifically, in the preparation of kits to predict the sensitivity of patients with acancer (non-small cell lung cancer) to immunotherapies, such as immunotherapy using an immune checkpoint inhibitor. Through the combinatory consideration of TP53 and KMT2C gene mutation states herein, groups sensitive to ICIs (immune checkpoint inhibitors) can be predicted accurately from patients with NSCLC (non-small cell lung cancer), blind medication is avoided, and economy of ICI therapy isimproved.
Owner:SHANGHAI ORIGIMED CO LTD
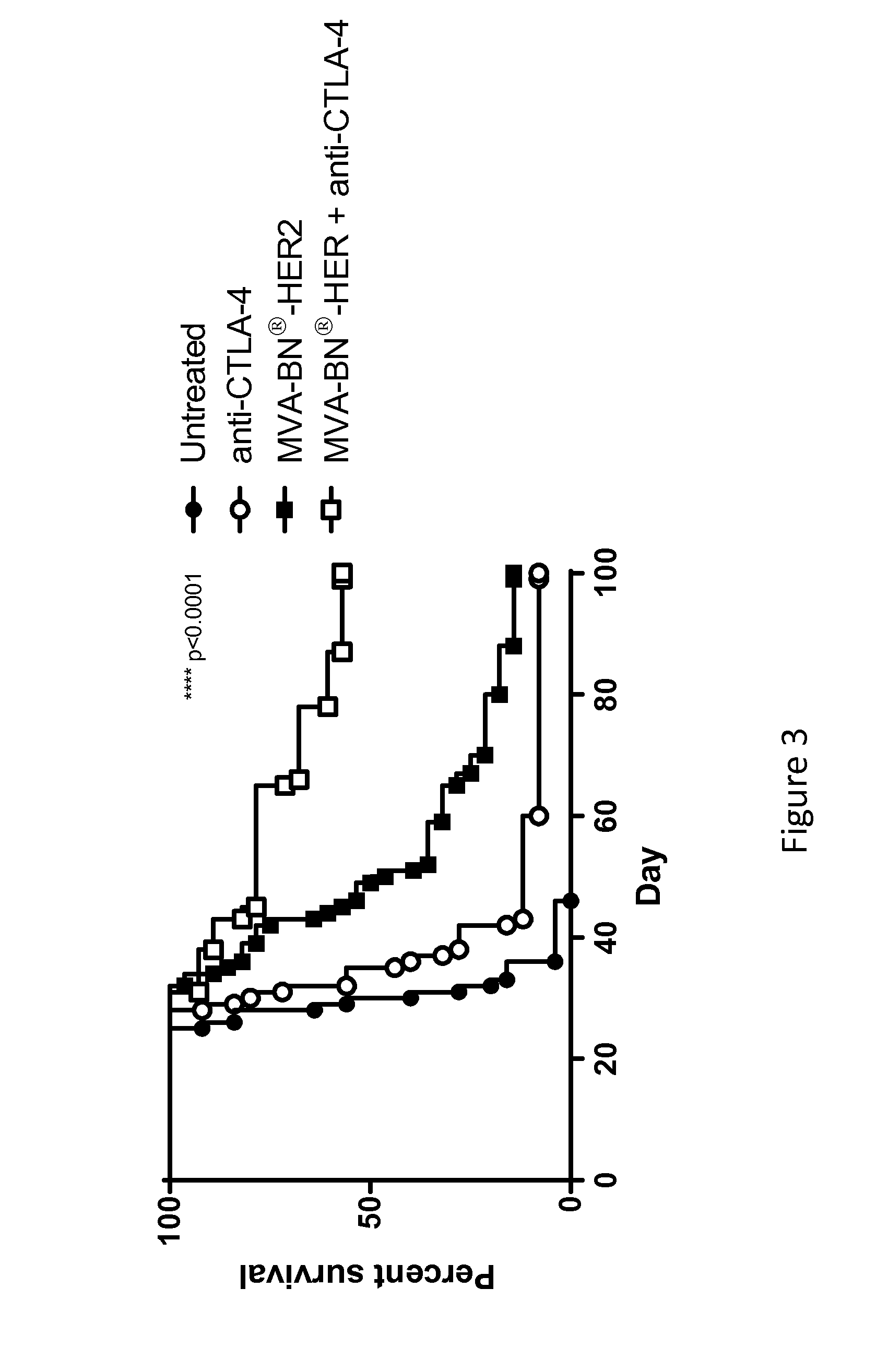
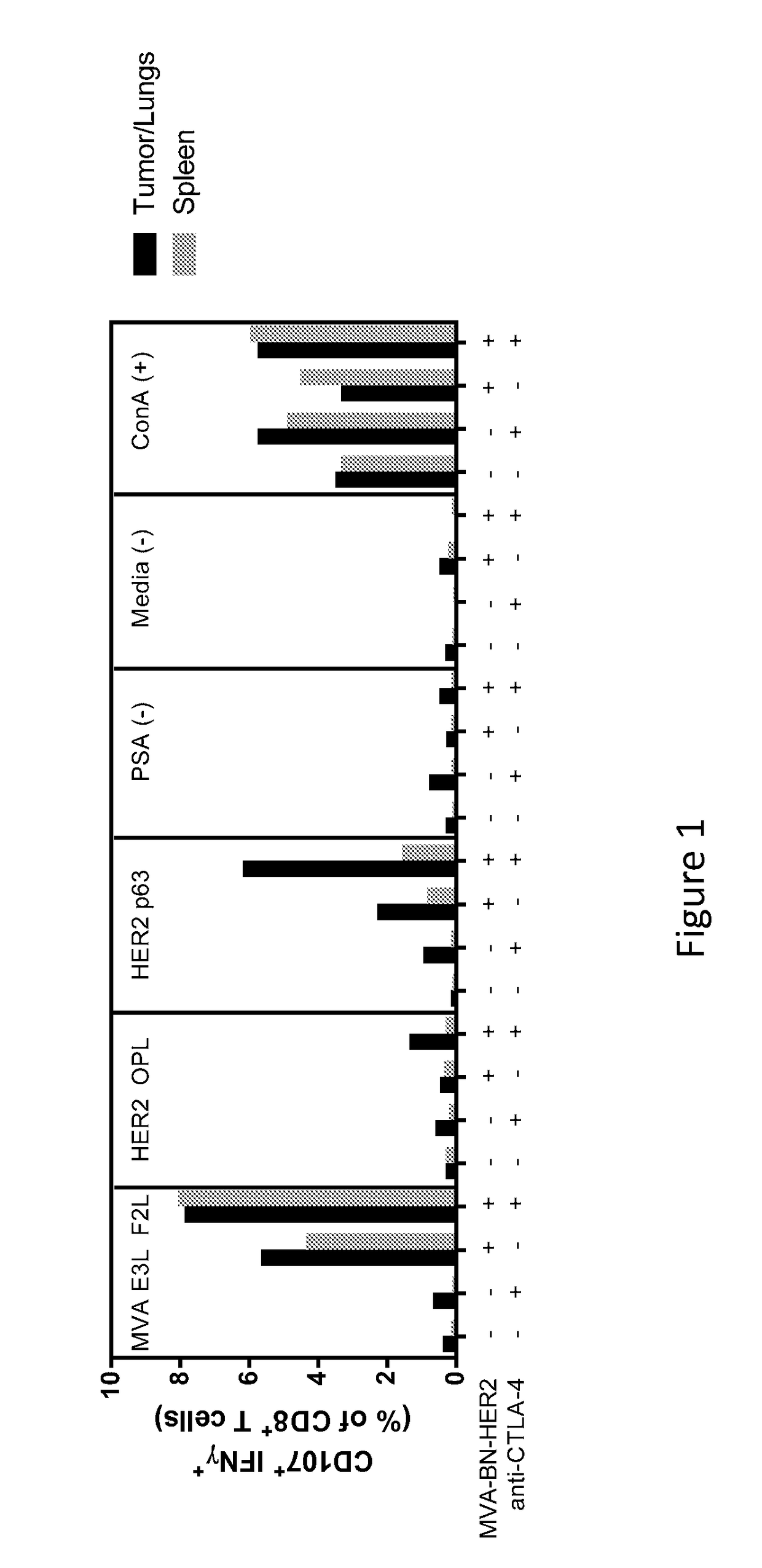
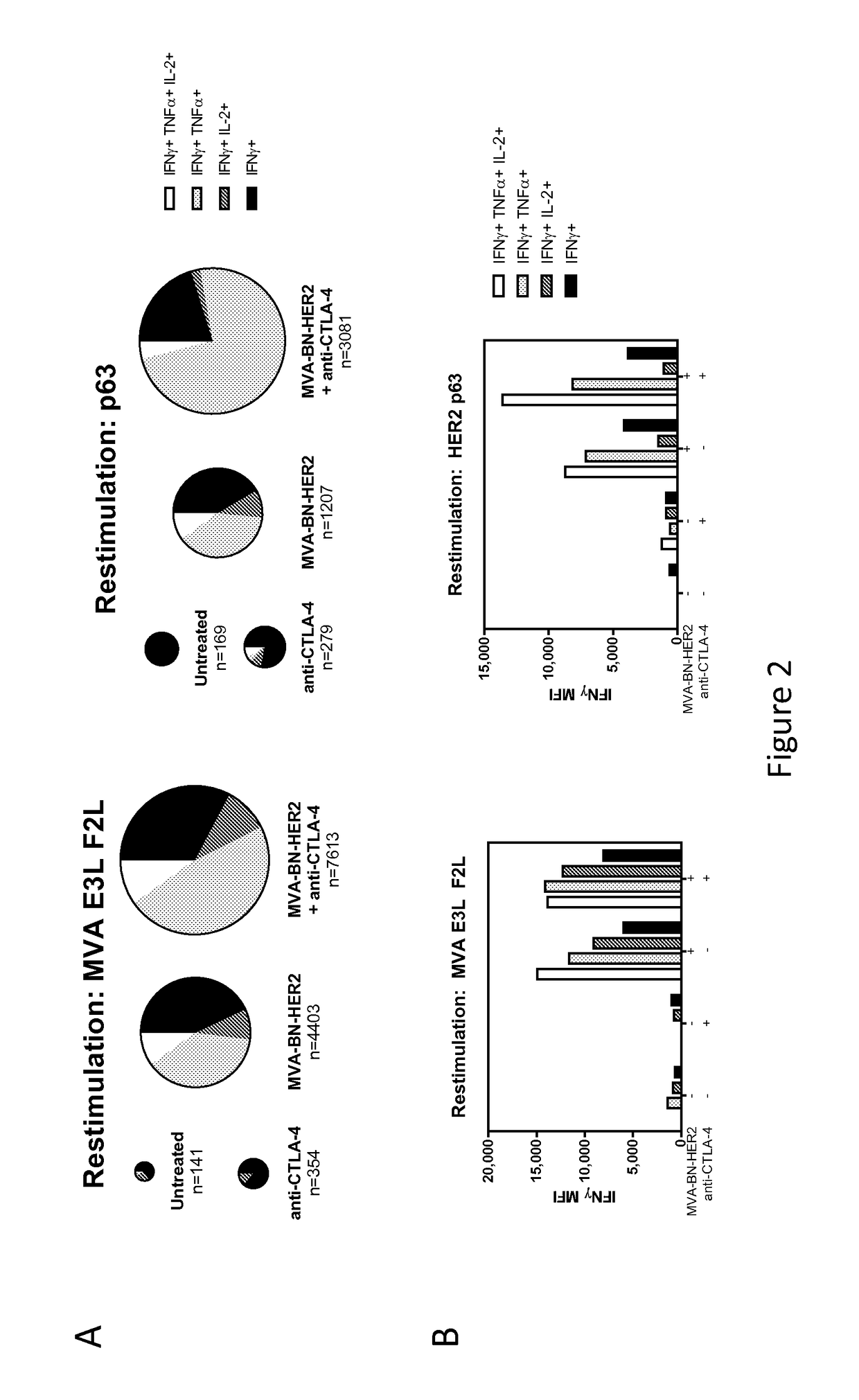
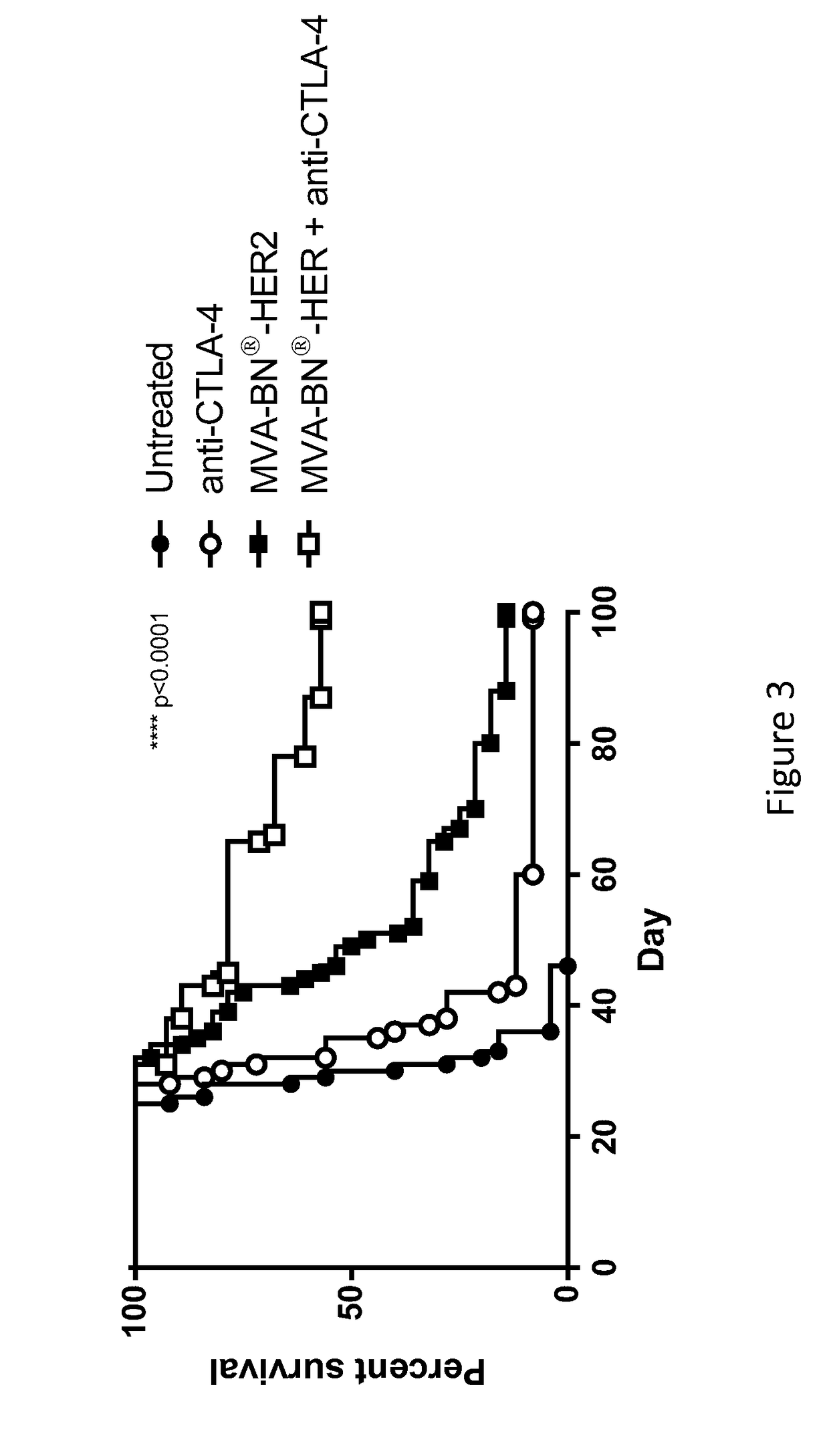
Combination Therapy for Treating Cancer with a Poxvirus Expressing a Tumor Antigen and an Antagonist and/or Agonist of an Immune Checkpoint Inhibitor
InactiveUS20160271239A1Antibody ingredientsCancer antigen ingredientsHuman cancerCombined Modality Therapy
The present disclosure encompasses therapies, compositions, and methods for treatment of a human cancer patient using a recombinant poxvirus encoding a tumor-associated antigen in combination with one or more agonists or antagonists of immune checkpoint inhibitors.
Owner:BAVARIAN NORDIC AS
Combination Therapy for Treating Cancer with a Poxvirus Expressing a Tumor Antigen and an Antagonist and/or Agonist of an Immune Checkpoint Inhibitor
InactiveUS20170266270A1Good treatment effectImprove combination effectViral/bacteriophage medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsOncologyTumor antigen
The invention relates to compositions, kits, and methods for cancer therapy using recombinant poxviruses encoding a tumor-associated antigen in combination with antagonists or agonists of immune checkpoint inhibitors.
Owner:BAVARIAN NORDIC AS
Therapeutic and diagnostic methods for cancer
PendingUS20190025308A1Prolonged progression-free survivalImprove survivalImmunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisImmune checkpoint inhibitorsPD-L1
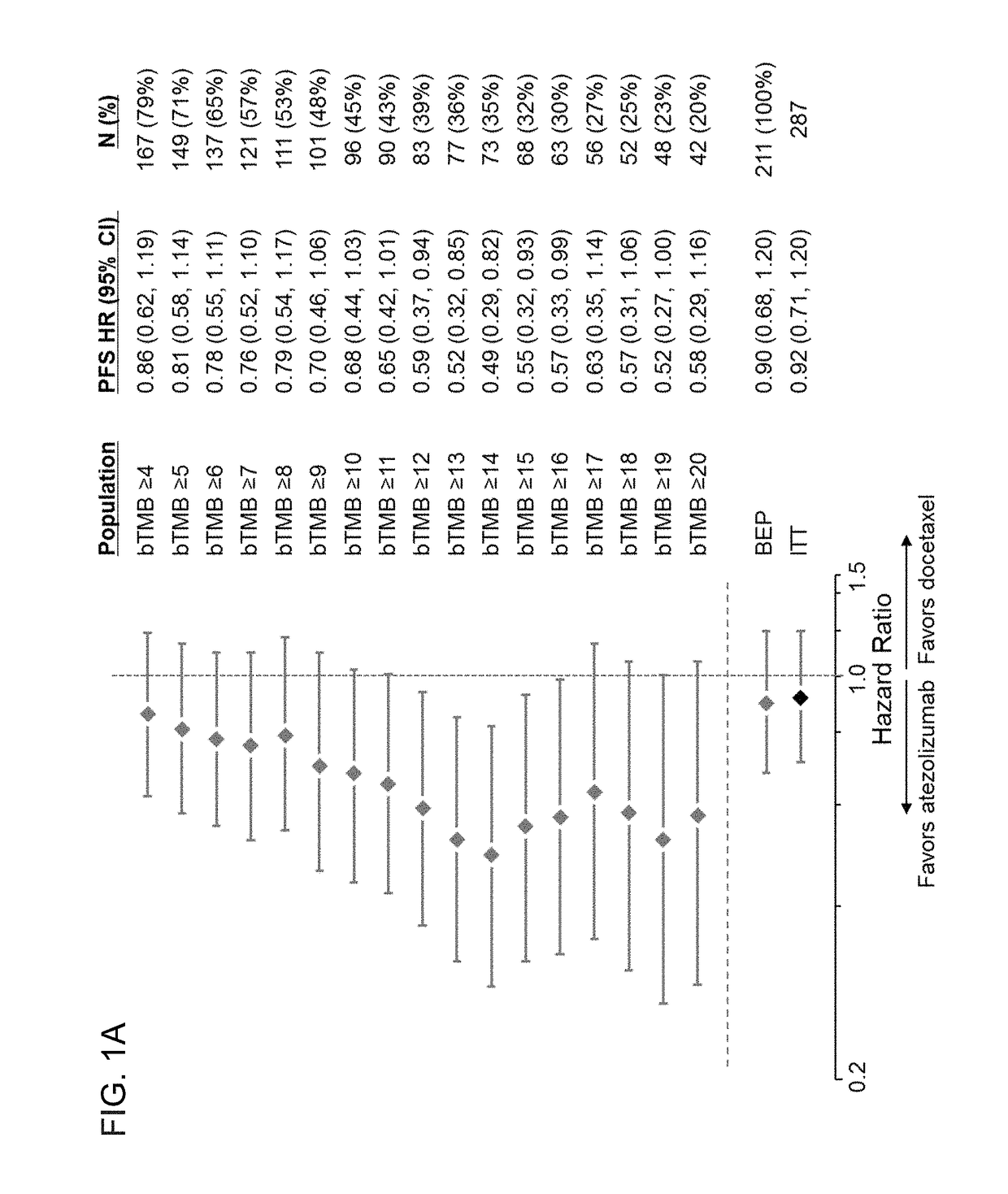
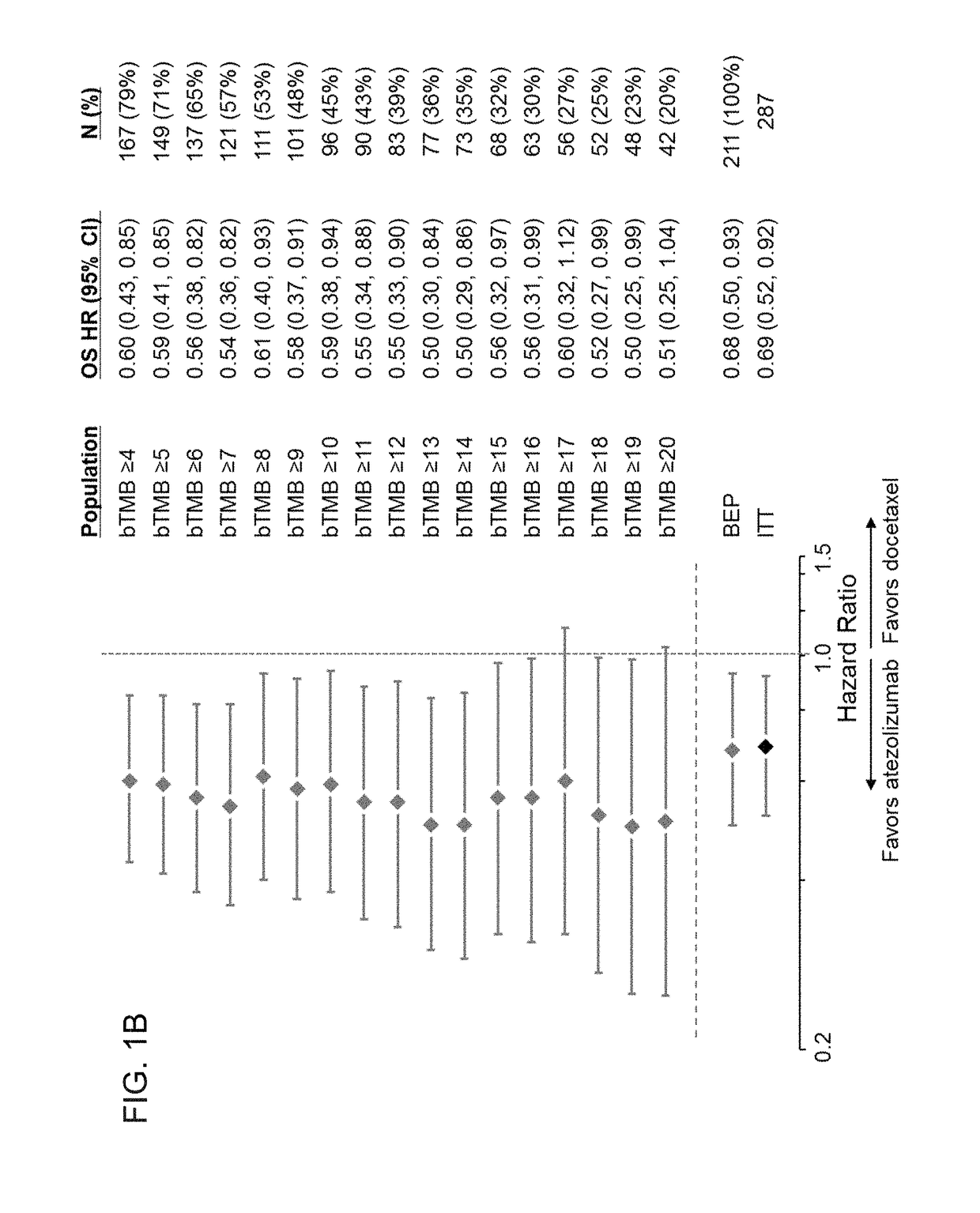
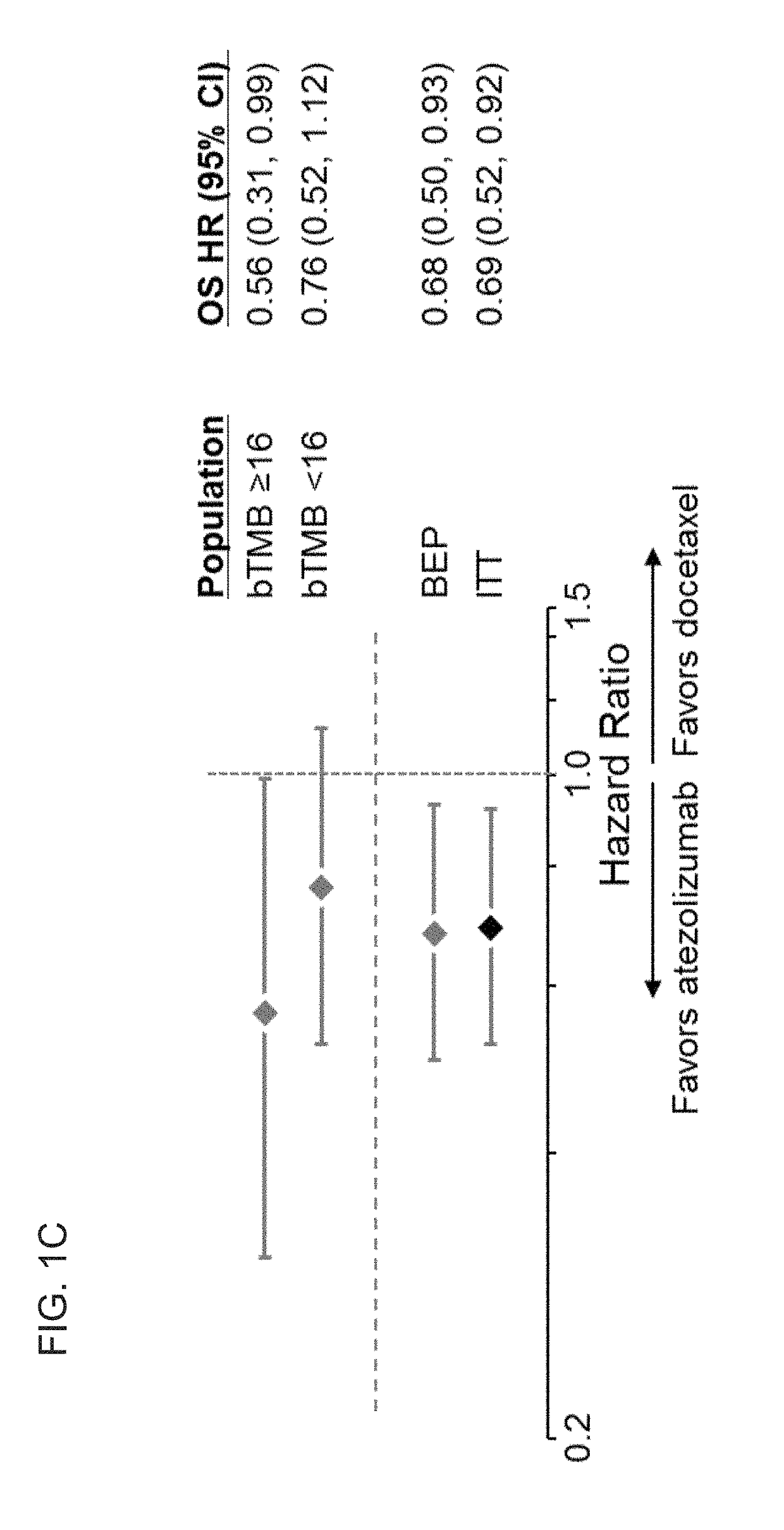
The present invention provides therapeutic, diagnostic, and prognostic methods for cancer. The invention provides methods of treating a cancer, methods of determining whether an individual having a cancer is likely to respond to a treatment including an immune checkpoint inhibitor (e.g., a PD-L1 axis binding antagonist), methods of predicting responsiveness of an individual having a cancer to a treatment including an immune checkpoint inhibitor (e.g., a PD-L1 axis binding antagonist), methods of selecting a therapy for an individual having a cancer, methods of providing a prognosis for an individual having a cancer, and methods of monitoring a response of an individual having a cancer, based on a blood tumor mutational burden (bTMB) score or a maximum somatic allele frequency (MSAF) from a sample (e.g., a whole blood sample, a plasma sample, a serum sample, or a combination thereof) from the individual.
Owner:FOUND MEDICINE INC
Binding molecules specific for CD73 and uses thereof
ActiveUS9938356B2Reduce proliferationEnhance immune responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAnticarcinogen
The present disclosure provides anti-CD73 binding molecules, e.g., antibodies and antigen binding fragments thereof. Also provided are pharmaceutical formulations comprising the disclosed compositions, and methods for the diagnosis and treatment of diseases associated with CD73-expression, e.g., cancer. Such diseases can be treated, e.g., by direct therapy with the anti-CD73 binding molecules disclosed herein (e.g., naked antibodies or antibody-drug conjugates that bind CD73), by adjuvant therapy with other antigen-binding anticancer agents such as immune checkpoint inhibitors (e.g., anti-CTLA-4 and anti-PD-1 monoclonal antibodies), and / or by combination therapies where the anti-CD73 molecules are administered before, after, or concurrently with chemotherapy.
Owner:MEDIMMUNE LTD
Methods of cancer treatment using activated t cells
ActiveUS20180078624A1Promote absorptionMammal material medical ingredientsCancer antigen ingredientsPopulationImmune checkpoint inhibitors
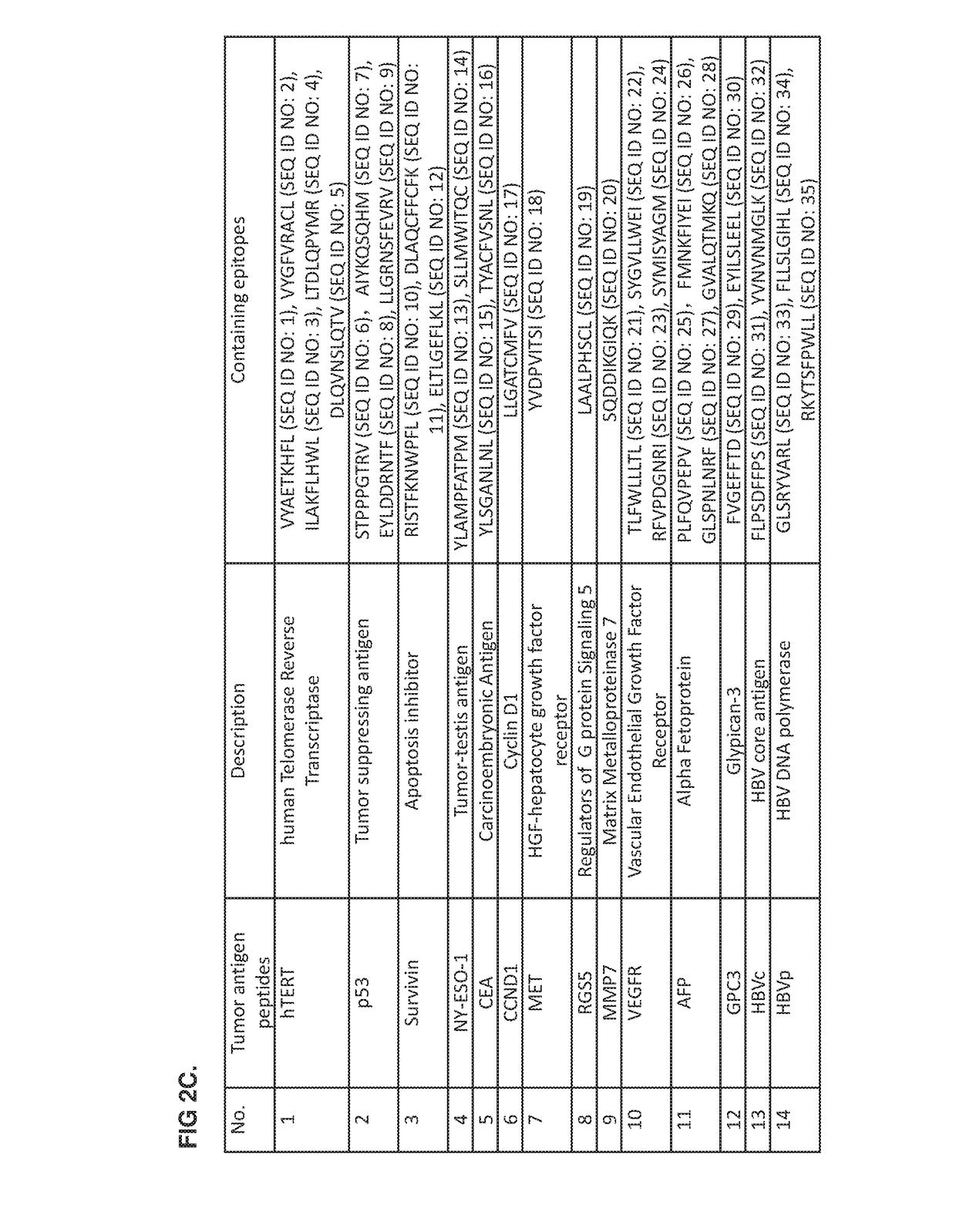
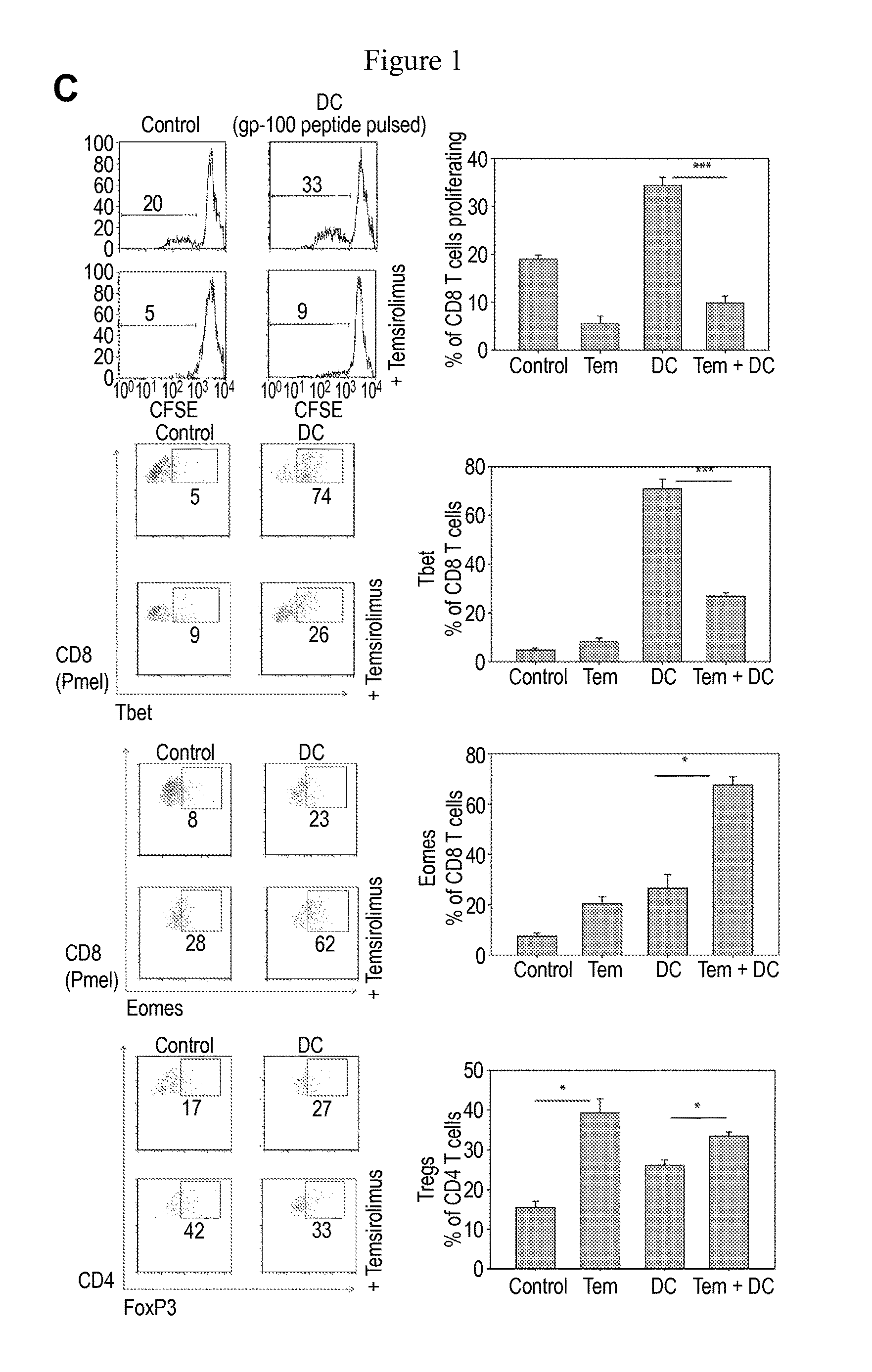
Provided is a method of treating a cancer in an individual using activated T cells or PBMCs induced by antigen presenting cells (such as dendritic cells) loaded with a plurality of tumor antigen peptides. The method may further comprise administration of the antigen presenting cells loaded with the plurality of tumor antigen peptides to the individual. The methods may be used singly or in combination with an immune checkpoint inhibitor. Also provided are precision therapy methods customized for the individual using neoantigen peptides or based on the mutation load in the tumor of the individual, methods of preparing the activated T cells, methods of monitoring the treatment, methods of cloning tumor-specific T cell receptors, an isolated population of cells comprising the activated T cells, and compositions and kits useful for cancer immunotherapy.
Owner:SYZ CELL THERAPY
Humanized PD-L1 monoclonal antibody and preparation method application thereof
InactiveCN109776678ARecovery functionPromote proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAnti-PD-L1 Monoclonal AntibodyPD-L1
The invention relates to the technical field of biomedical engineering, in particular to an anti-PD-L1 humanized rabbit monoclonal antibody, provides a novel humanized rabbit anti-PD-L1 monoclonal antibody, and discloses an amino acid sequence of the humanized rabbit monoclonal antibody, a corresponding expression vector, a host cell capable of expressing the antibody, and a production method of the antibody. Specially, the anti-PD-L1 monoclonal antibody is obtained through humanized transformation after a rabbit is immunized, has high expression quantity in mammalian cells, can be specially bound to PD-L1 protein in an extracellular region, has a significant ability of inhibiting ligand-receptor binding, blocks binding of PD1 and PD-L1, and restores the function of a T cell. The anti-PD-L1 humanized rabbit monoclonal antibody can serve as an immune checkpoint inhibitor, especially for the treatment, prevention or diagnosis of PD-L1 related cases such as the tumor.
Owner:ANHUI ANKE BIOTECHNOLOGY (GRP) CO LTD
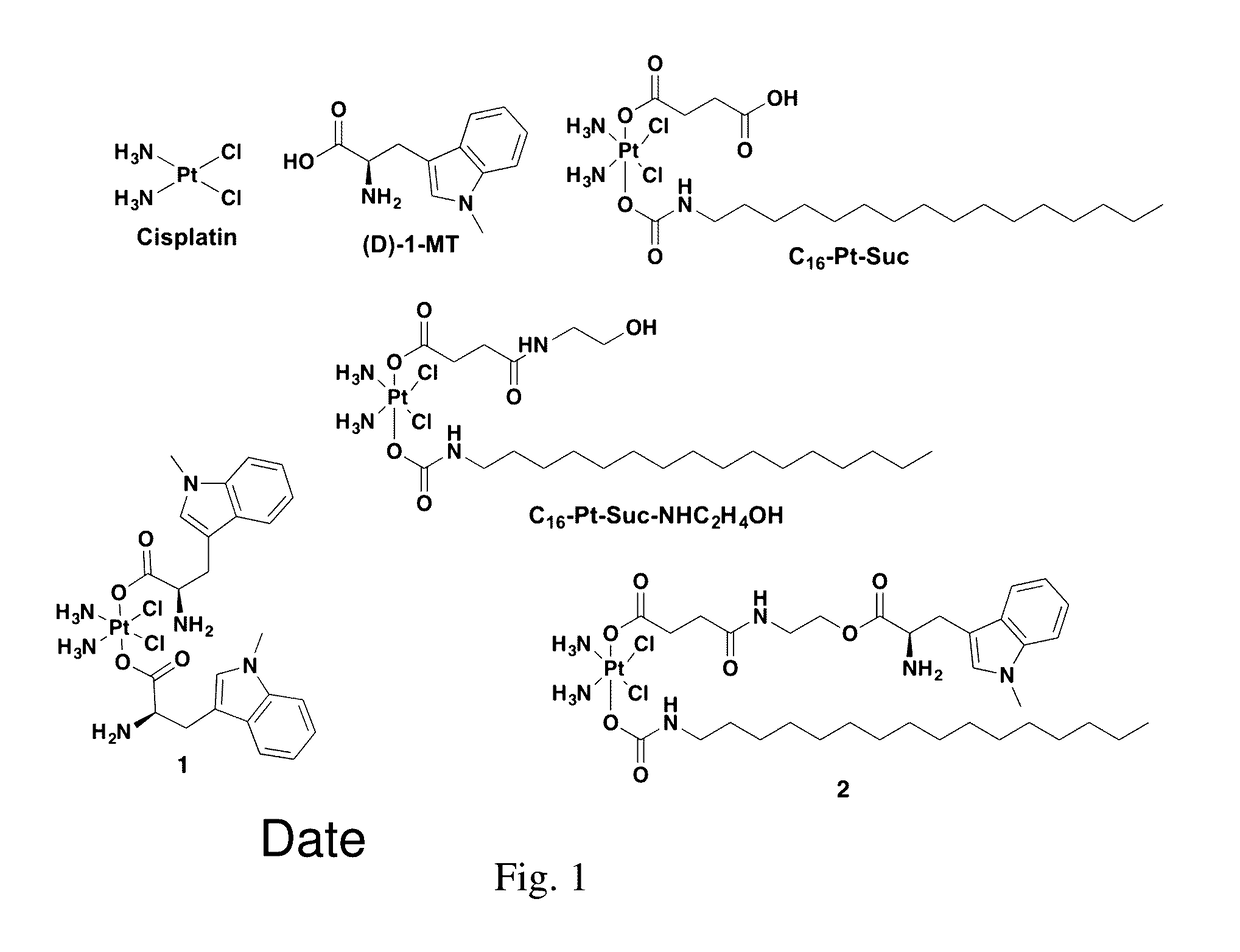
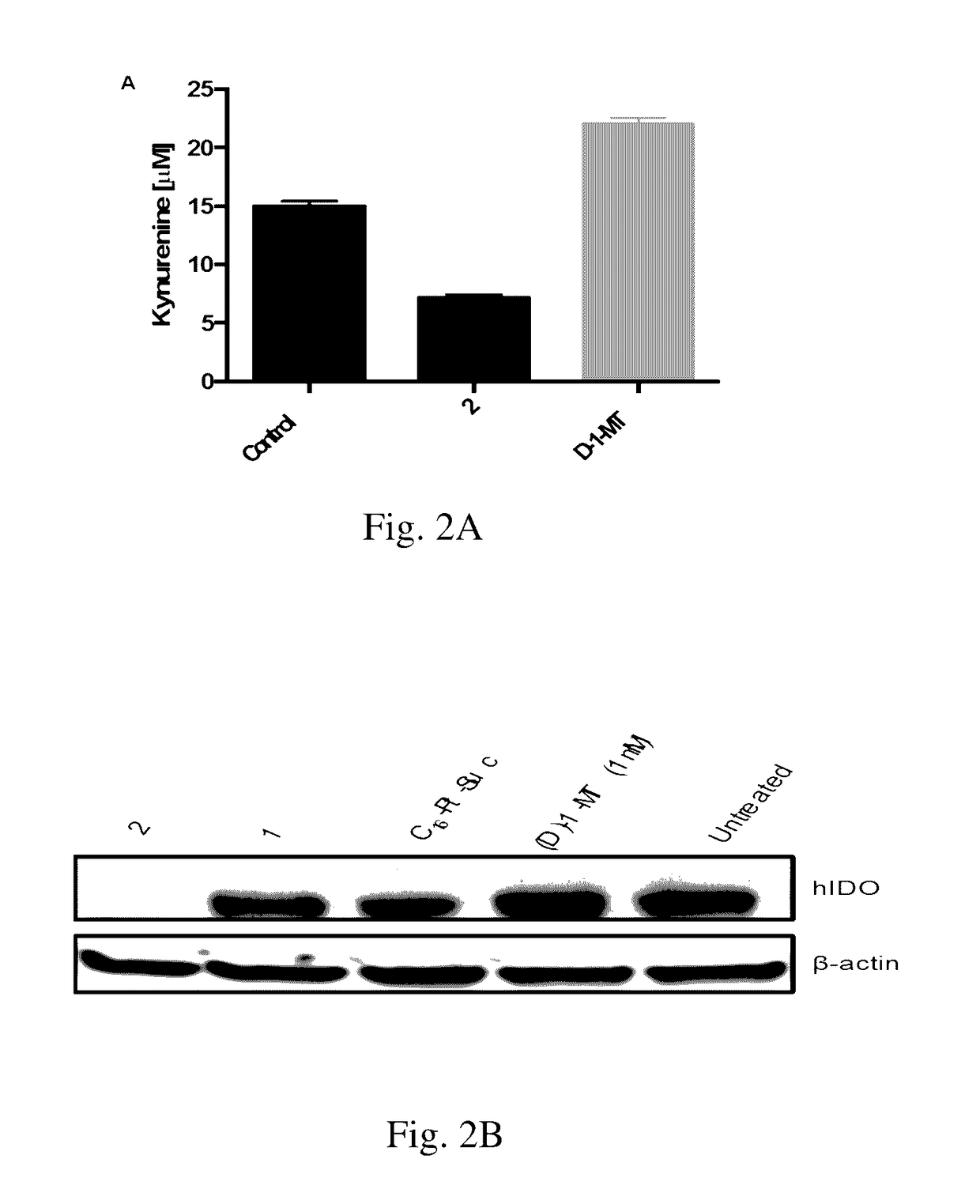
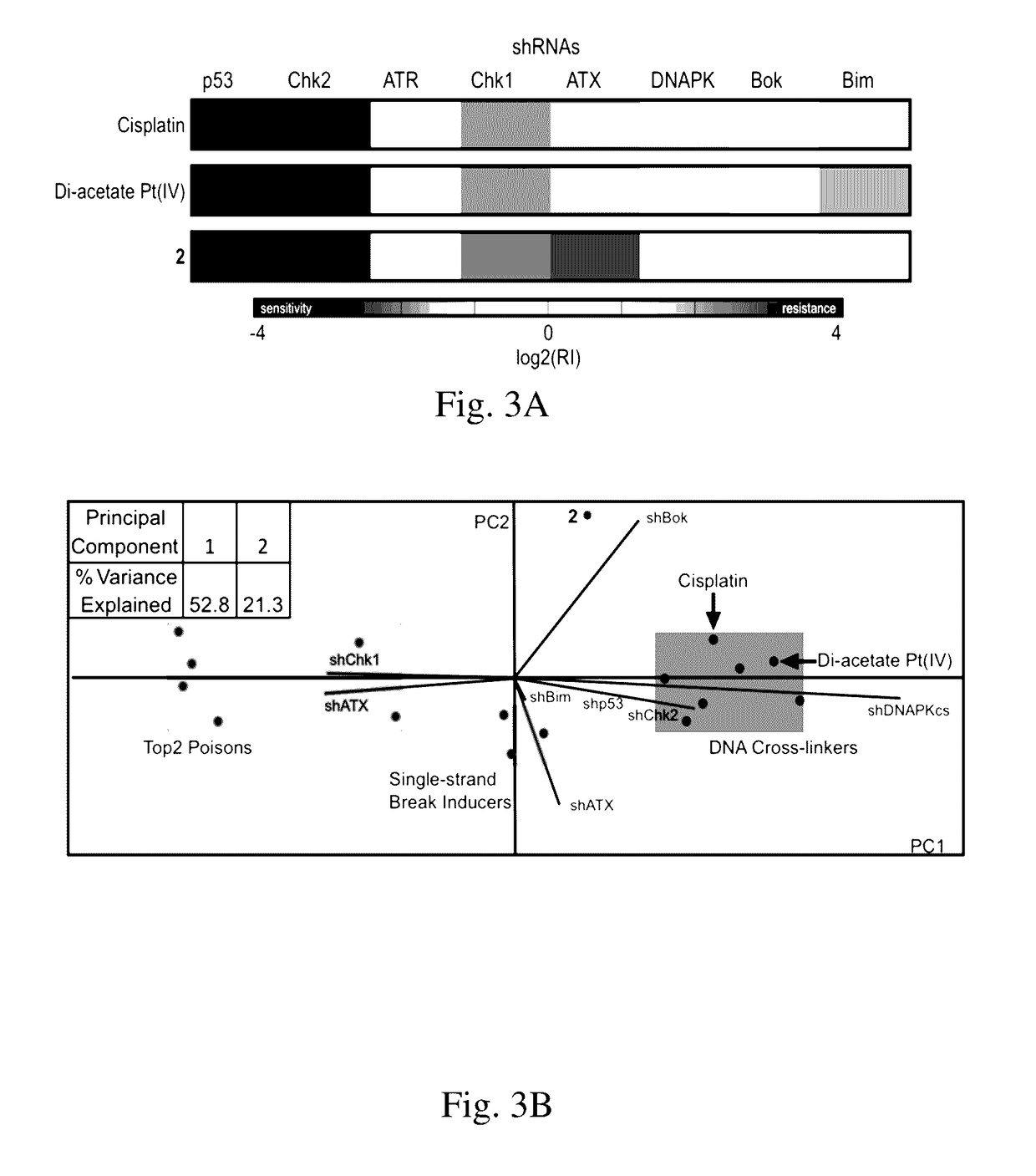
Complexes comprising a platinum compound and an immune checkpoint inhibitor and related methods
Complexes comprising a platinum compound and an immune checkpoint inhibitor, and related methods, are generally provided. In some embodiments, the immune checkpoint inhibitor is an inhibitor for indoleamine-2,3-dioxygenase.
Owner:MASSACHUSETTS INST OF TECH
Method for treating melanoma using a herpes simplex virus and an immune checkpoint inhibitor
Owner:AMGEN INC
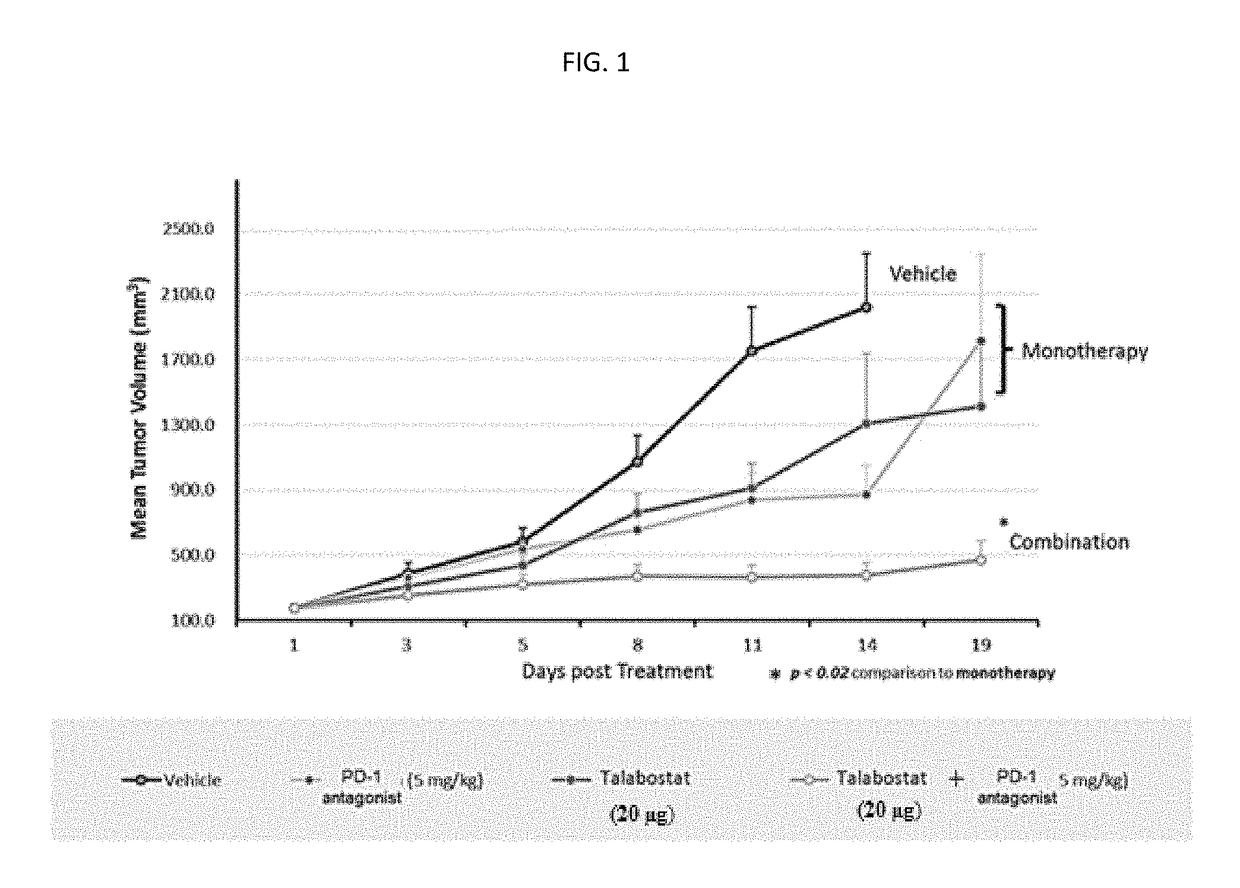
Novel approach for treatment of cancer using immunomodulation
ActiveUS20170266280A1Promote migrationStrong additive/synergisticPeptide/protein ingredientsBoron compound active ingredientsImmunomodulationsNeoplasm
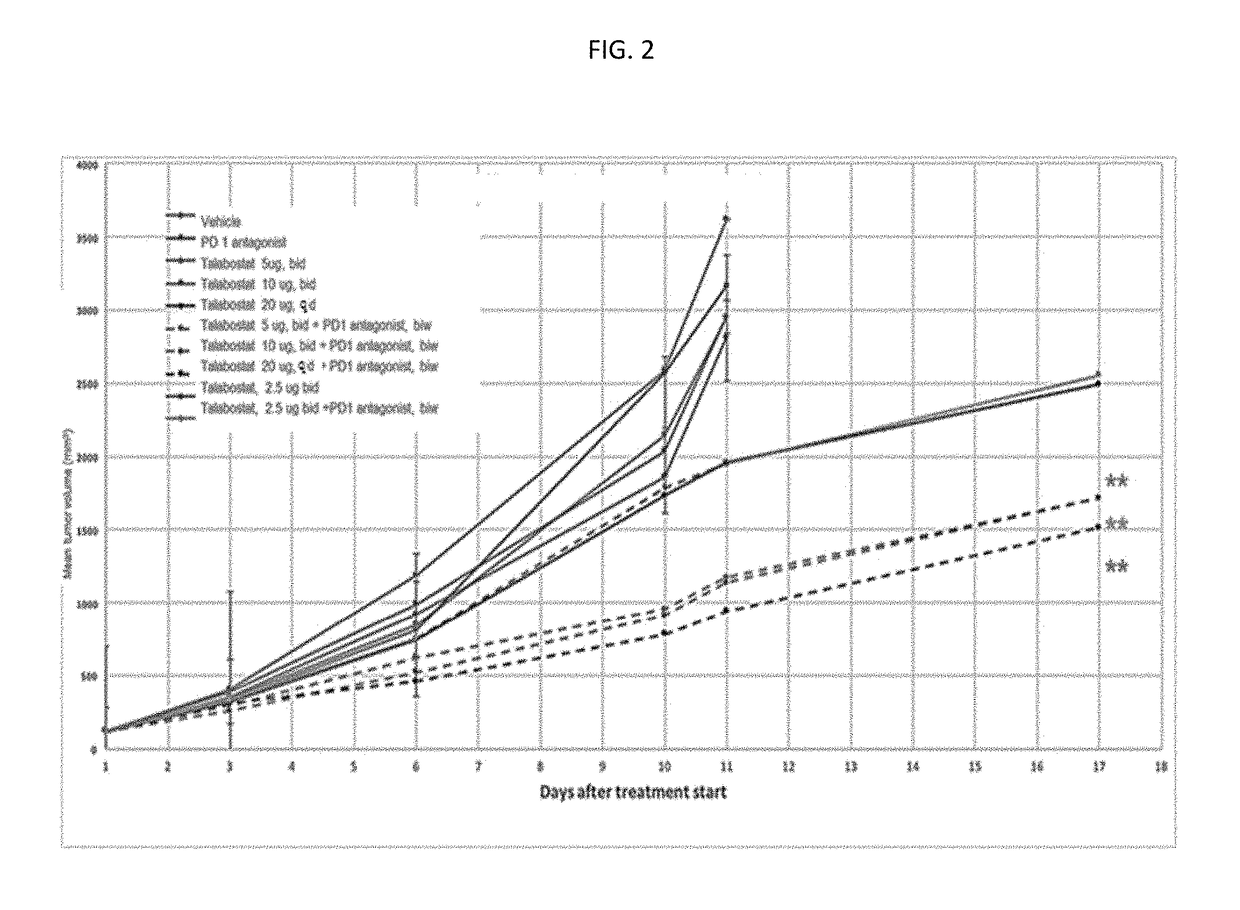
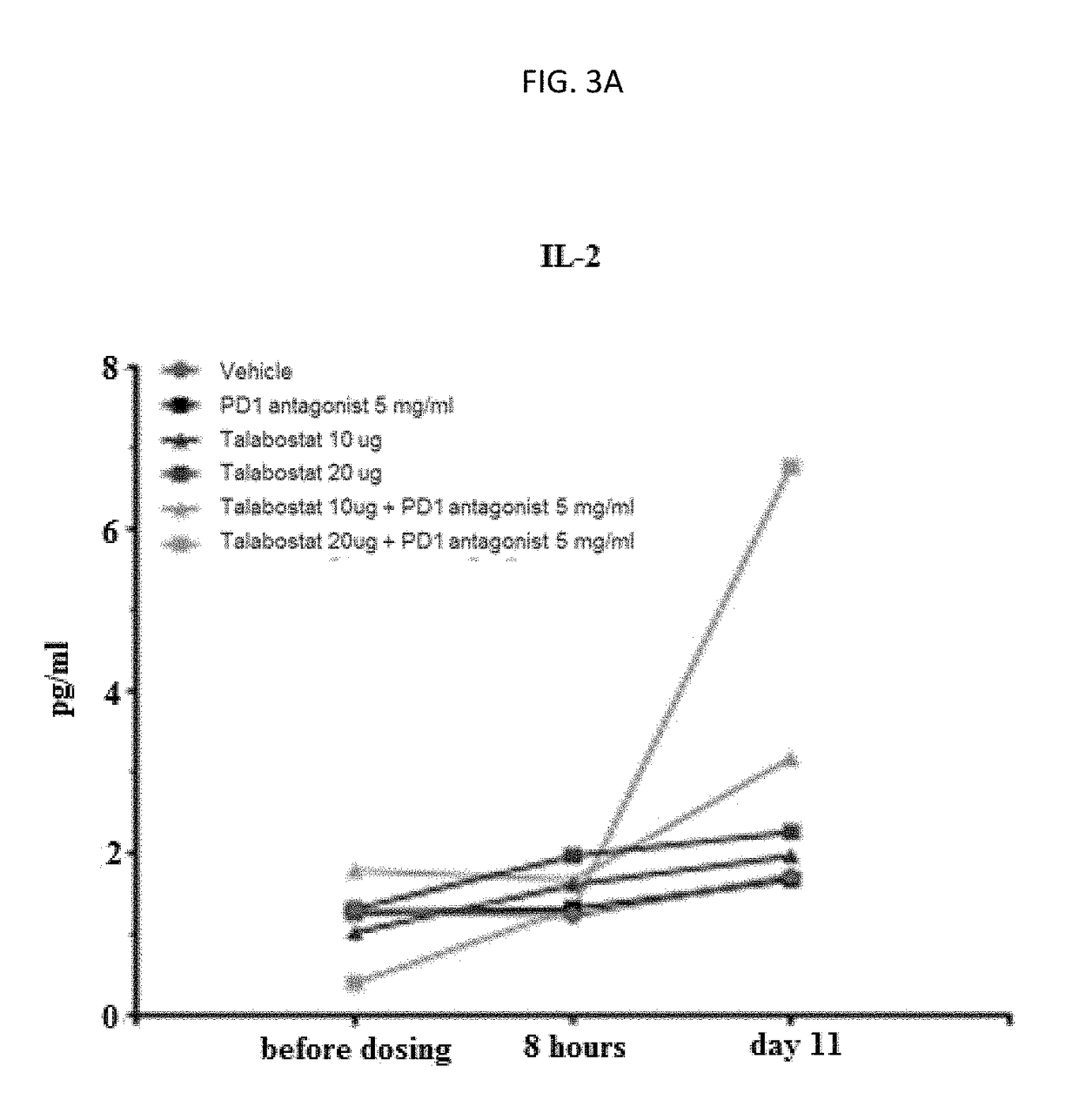
The present invention discloses a method of treating, preventing or ameliorating tumor growth by administering a therapeutic agent that selectively inhibits dipeptidyl peptidase including fibroblast activation protein and dipeptidyl peptidase 8 / 9 in combination with an immune checkpoint inhibitor. The method specifically discloses use of Talabostat in combination with an immune checkpoint inhibitor, its pharmaceutical composition and process of preparing such composition.
Owner:ONKOSXCEL THERAPEUTICS LLC
Methods and compositions for treating cancer and infectious diseases
ActiveUS20170007698A1Prevention and reduction of severityShorten the progressOrganic active ingredientsAntibody ingredientsInfectious DisorderImmune modulator
The invention relates to compositions comprising a CD4 lymphocyte depleting agent; and methods of using the compositions to treat, prevent, reduce the severity of and / or slow the progression of a condition in a subject. The invention also relates to use of combinations of a CD4 lymphocyte depleting agent and at least one additional agent to treat, prevent, reduce the severity of and / or slow the progression of a condition in a subject. The additional agent may be an immune check point inhibitor, an adoptive immune therapeutic, an immune adjuvant, or an immune modulating agent, or their combinations.
Owner:CEDARS SINAI MEDICAL CENT
Methods for treating cancer
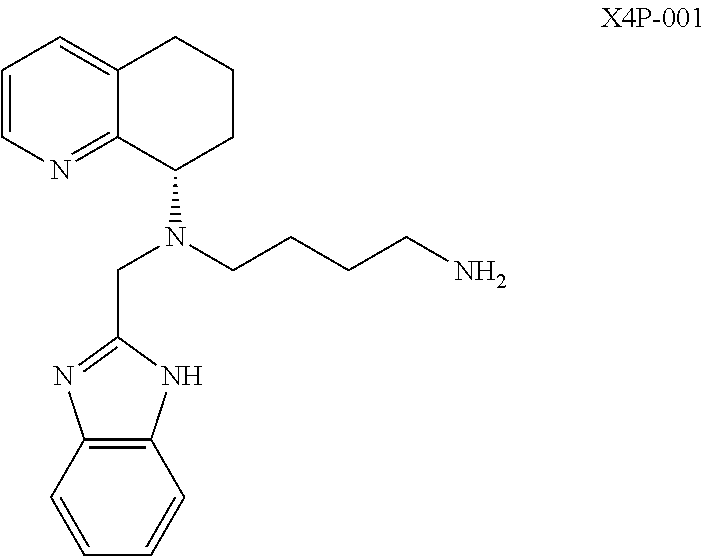
InactiveUS20190030023A1High densitySignificant comprehensive benefitsOrganic active ingredientsAntibody ingredientsDiseaseMetastatic melanoma
The present invention relates to methods of treating patients with advanced forms of cancer, such as metastatic melanoma and non-small cell lung cancer, in which X4P-001 is administered as monotherapy or in combination with immune checkpoint inhibitors, such as pembrolizumab. The methods demonstrate surprising results, including regression of disease, with comparatively little toxicity.
Owner:X4 PHARMA INC
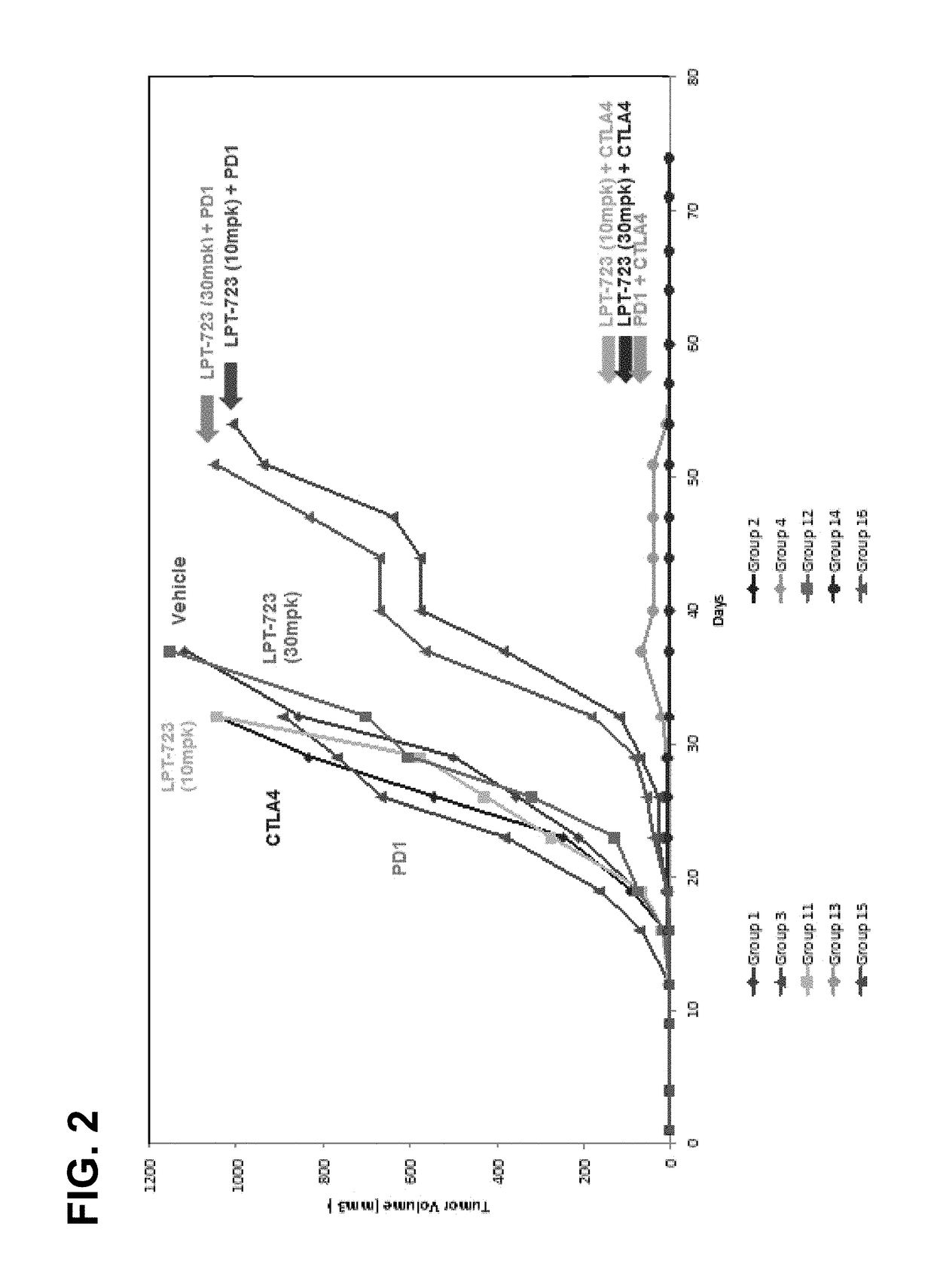
LPT-723 and immune checkpoint inhibitor combinations and methods of treatment
ActiveUS9861621B2Reduce MDSCsInhibit tumor growthImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseChemical compound
The present invention provides, inter alia, a composition containing a compound of formula (I):or a pharmaceutically acceptable salt thereof, optionally, in combination with at least one immune checkpoint inhibitor compound. Kits containing the composition, and methods of using the composition for ameliorating or treating the effects of a disease such as a cancer, in a subject, are also provided herein.
Owner:BIOMED VALLEY DISCOVERIES INC
Method of treating cancer with a multiple integrin binding Fc fusion protein
The present invention provides a method of treating cancer with an integrin-binding-Fc fusion protein alone or in combination with IL-2 and / or an immune checkpoint inhibitor. The invention also provides composition for use in such methods.
Owner:XYENCE THERAPEUTICS INC
Anti-PD-1 monoclonal antibody and application thereof
ActiveCN108314734AStrong specificityIncrease binding rateImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsNucleotidePD-L1
The invention relates to a novel anti-PD-1 monoclonal antibody and a variable region sequence of the antibody and belongs to the technical field of biology. The novel anti-PD-1 monoclonal antibody takes recombinant human PD-1 protein (rhEPD-1) as an immunogen for immunizing a BAL b / c mouse; spleen cells of the mouse are taken and are fused with myeloma cells sp2 / 0-Ag14 to obtain a hybridoma cell line capable of expressing the anti-PD-1 antibody; the cell line can stably secrete the single monoclonal antibody. According to the novel anti-PD-1 monoclonal antibody, variable region nucleotide andamino acid sequences of an H chain and an L chain of the anti-PD-1 monoclonal antibody are cloned at the same time. The anti-PD-1 monoclonal antibody provided by the invention can be specifically combined with PD-1 and the combination of the PD1 and PD-L1 is blocked; functions of T cells are recovered. The novel anti-PD-1 monoclonal antibody can be used as an immune checkpoint inhibitor and is used for treating cancer and infectious diseases.
Owner:CHINA PHARM UNIV
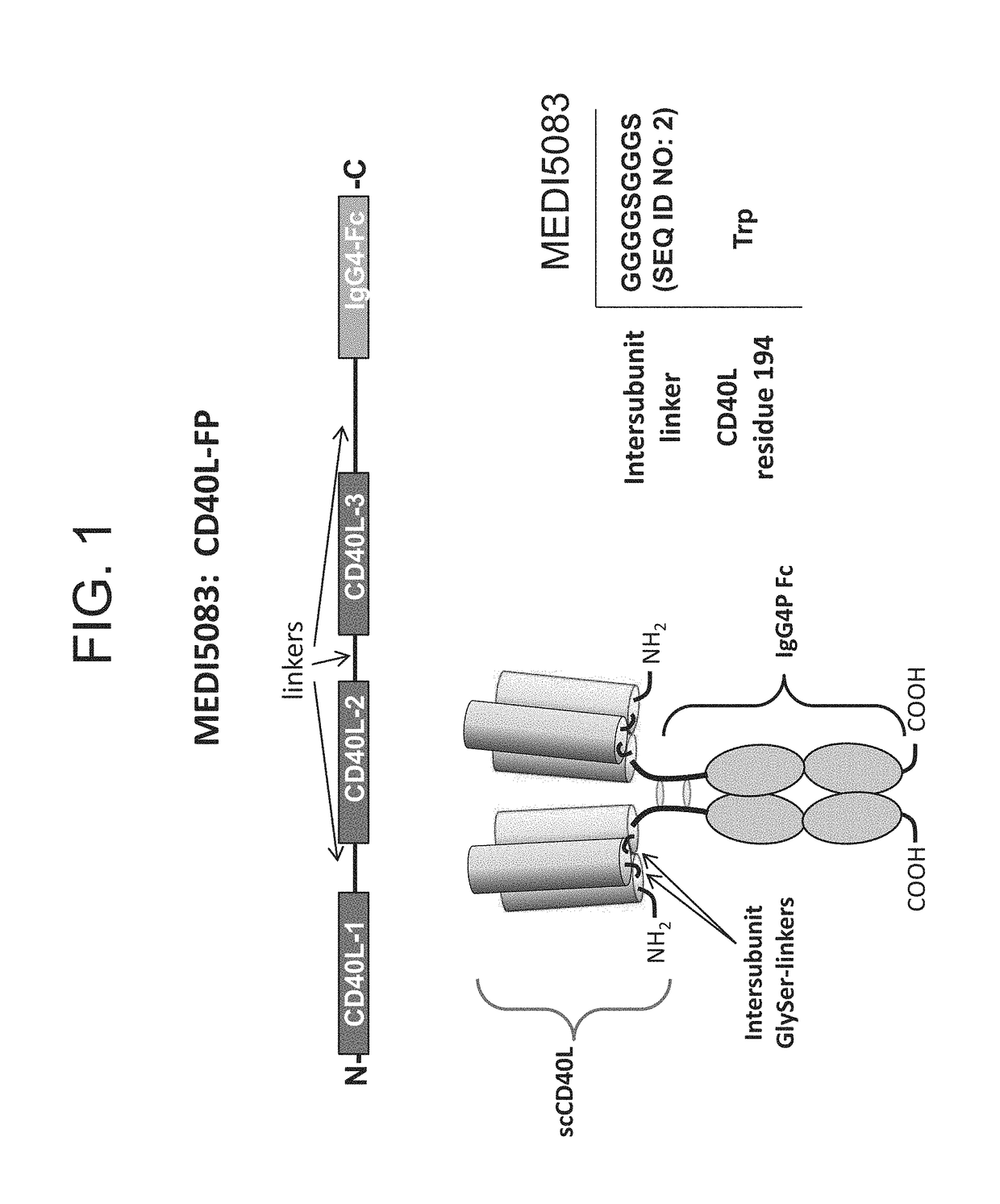
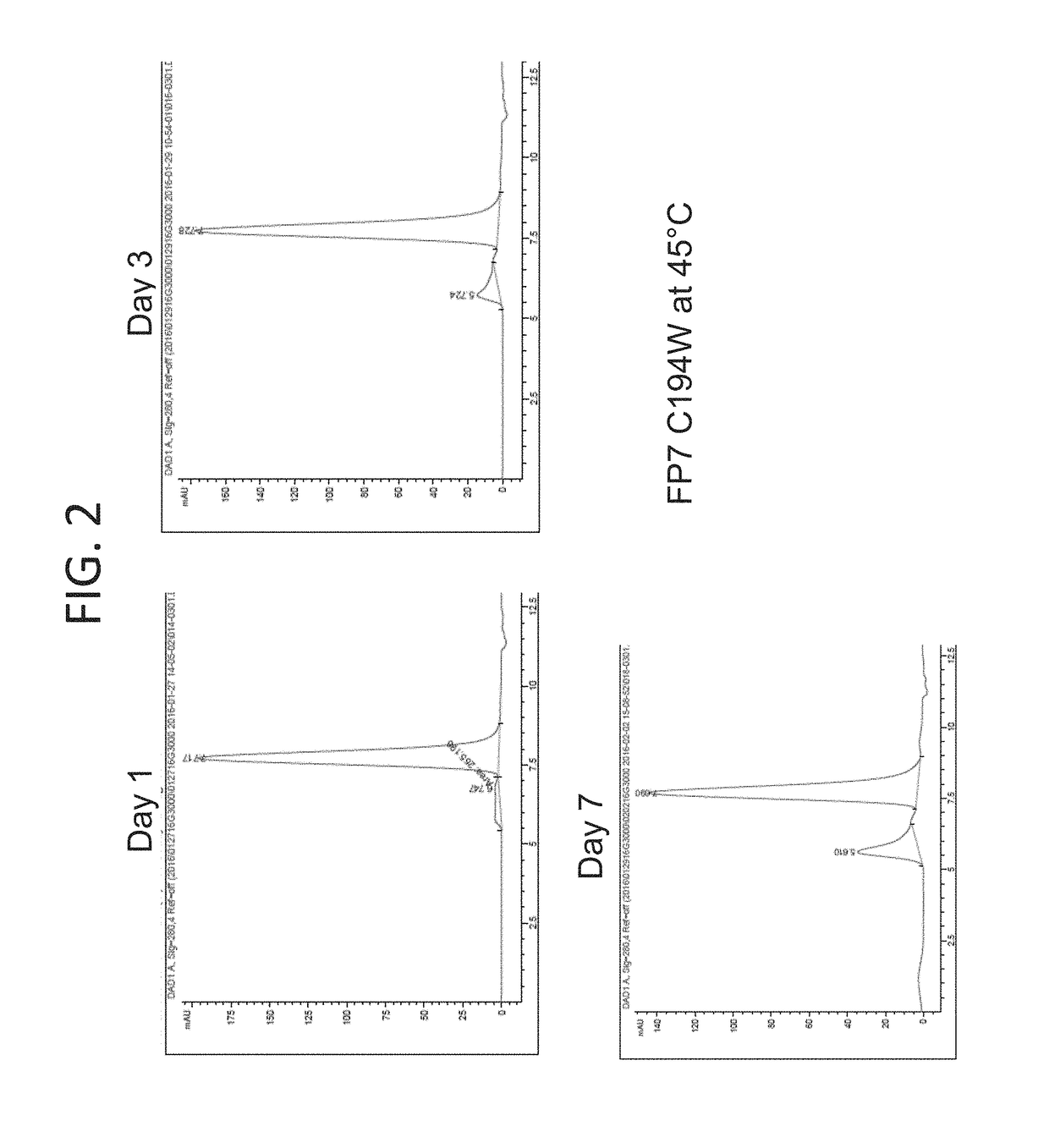
CD40L-Fc Fusion Polypeptides And Methods Of Use Thereof
ActiveUS20170327588A1Enhance immune responseReduce immunosuppressionPeptide/protein ingredientsAntibody mimetics/scaffoldsMedicinePD-L1
Provided herein is a CD40L-Fc fusion protein and methods of using the fusion protein in the treatment of cancer comprising administering the CD40L-Fc fusion protein or the CD40L-Fc fusion protein in combination with one or more immune checkpoint inhibitors (e.g., an anti-CTLA4 antibody, anti-PD-L1 antibody).
Owner:MEDIMMUNE LLC
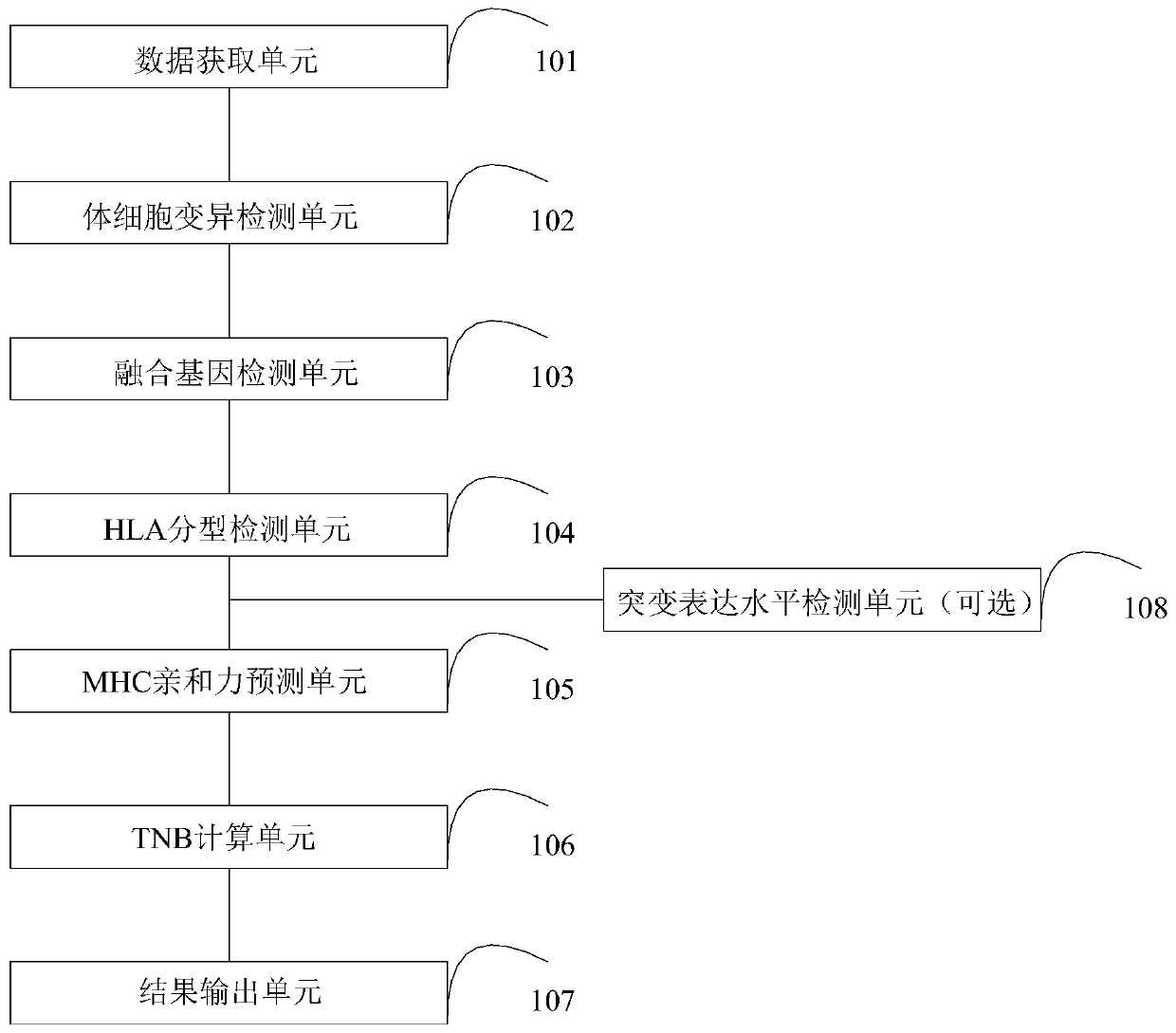
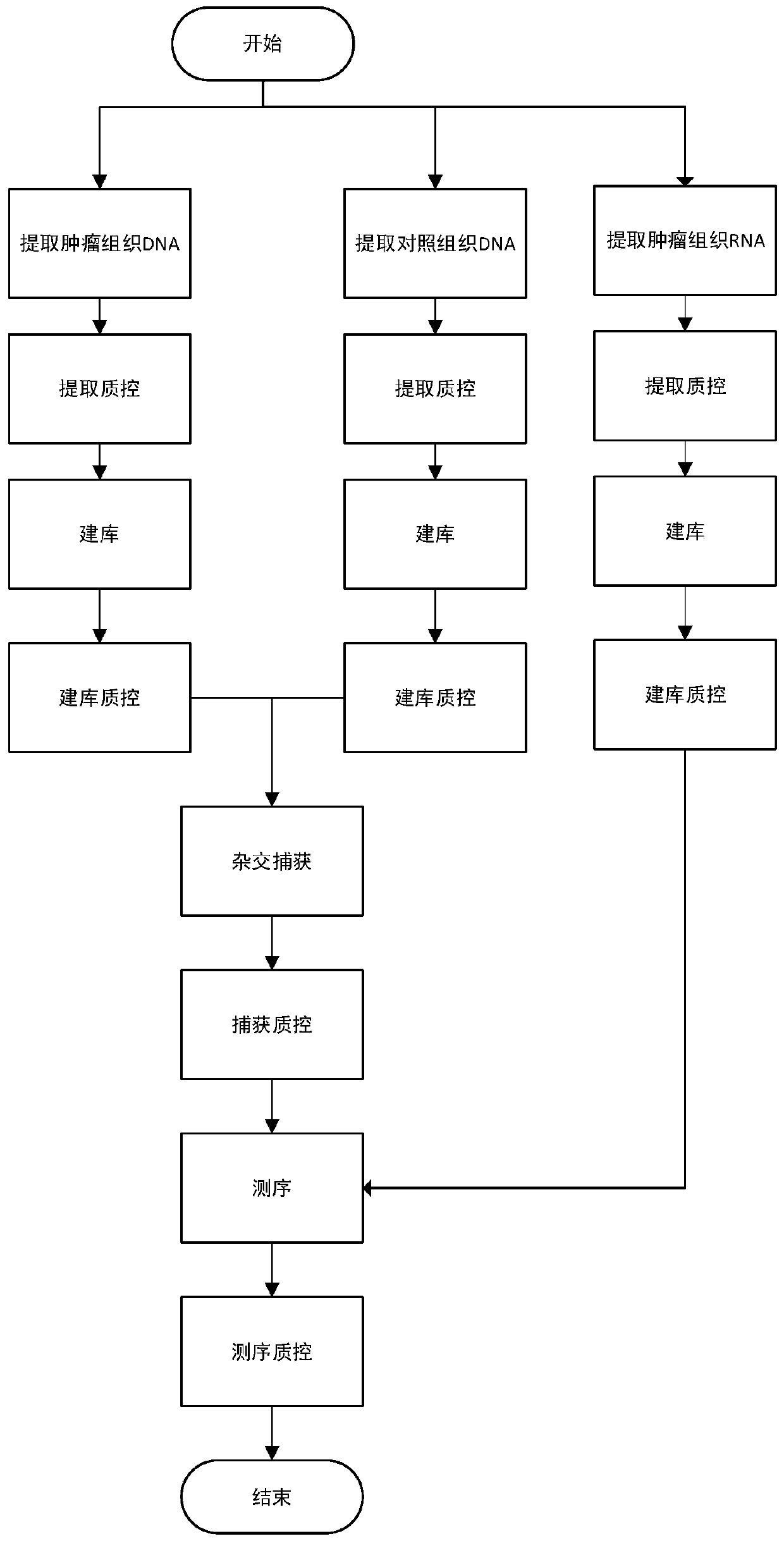
A tumor neoantigen load detecting device and a storage medium
InactiveCN109706065ASave the amount of sequencing dataLow costBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenSomatic cell
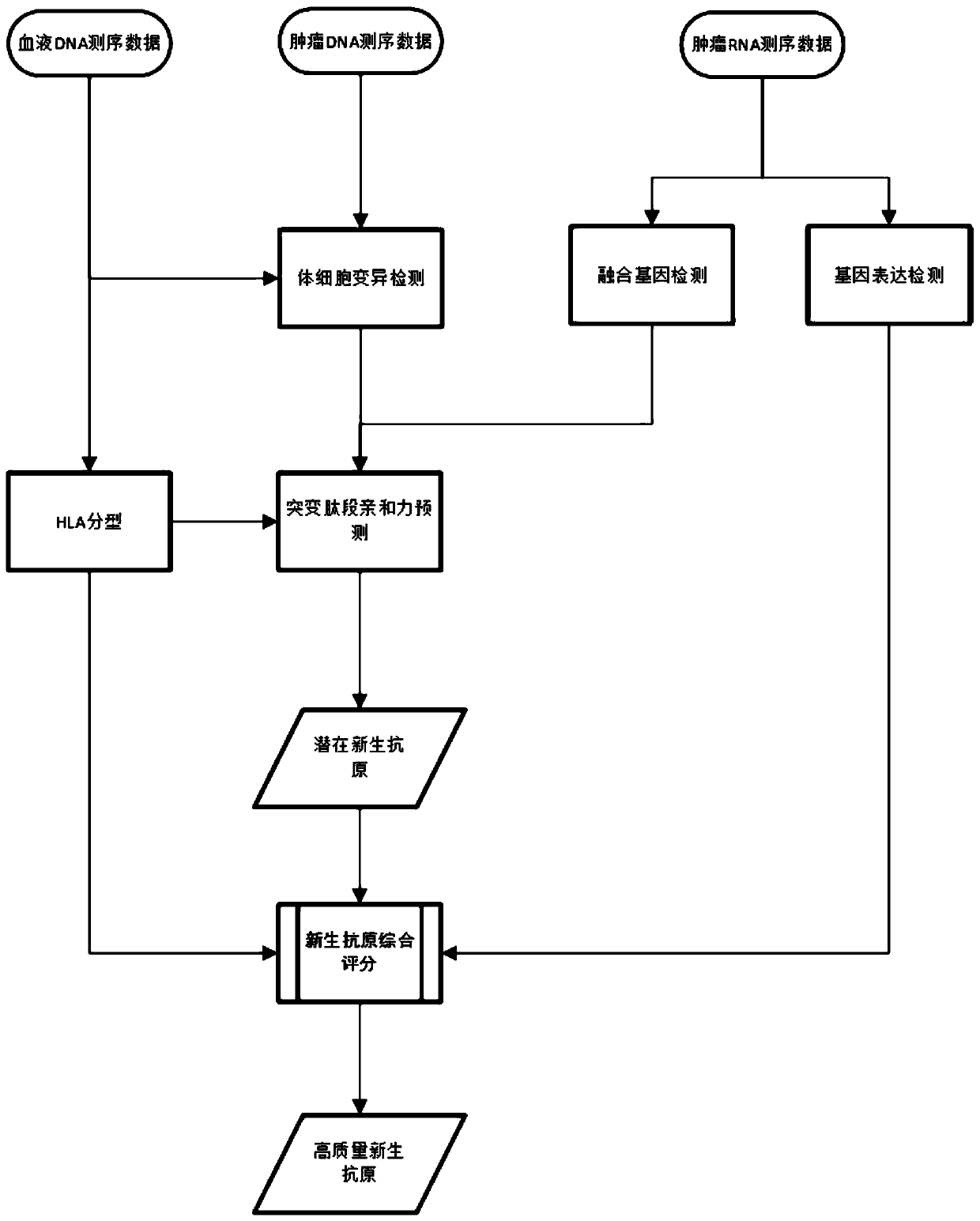
A tumor neoantigen load detecting device and a storage medium are provided. The detecting device includes a data acquisition unit used for acquiring sequencing data of target capturing zones of tumortissue DNA of a detection subject, control sample DNA, and tumor tissue RNA; a somatic cell mutation detecting unit used for mutation detection and annotation on the sequencing data of the tumor tissue DNA and the control sample DNA to obtain a mutant peptide segment; a fusion gene detecting unit used for subjecting the sequencing data of the tumor tissue RNA to fusion gene detection and annotation to obtain a mutant peptide segment; an HLA typing detection unit used for performing HLA typing on the sequencing data of the target capturing zones of the control sample DNA; an MHC affinity predicting unit used for predicting neoantigen for the mutant peptide segments according to an HLA typing detection result; a TNB calculating unit used for calculating TNB; and a result outputting unit usedfor outputting a TNB detection result. The device and the storage medium can be used for detecting and monitoring the tumor neoantigen load, and predicting curative effects of immune checkpoint inhibitors.
Owner:深圳裕策生物科技有限公司
Method for immunotherapy prognosis of non-small cell lung cancer (NSCLC) patients
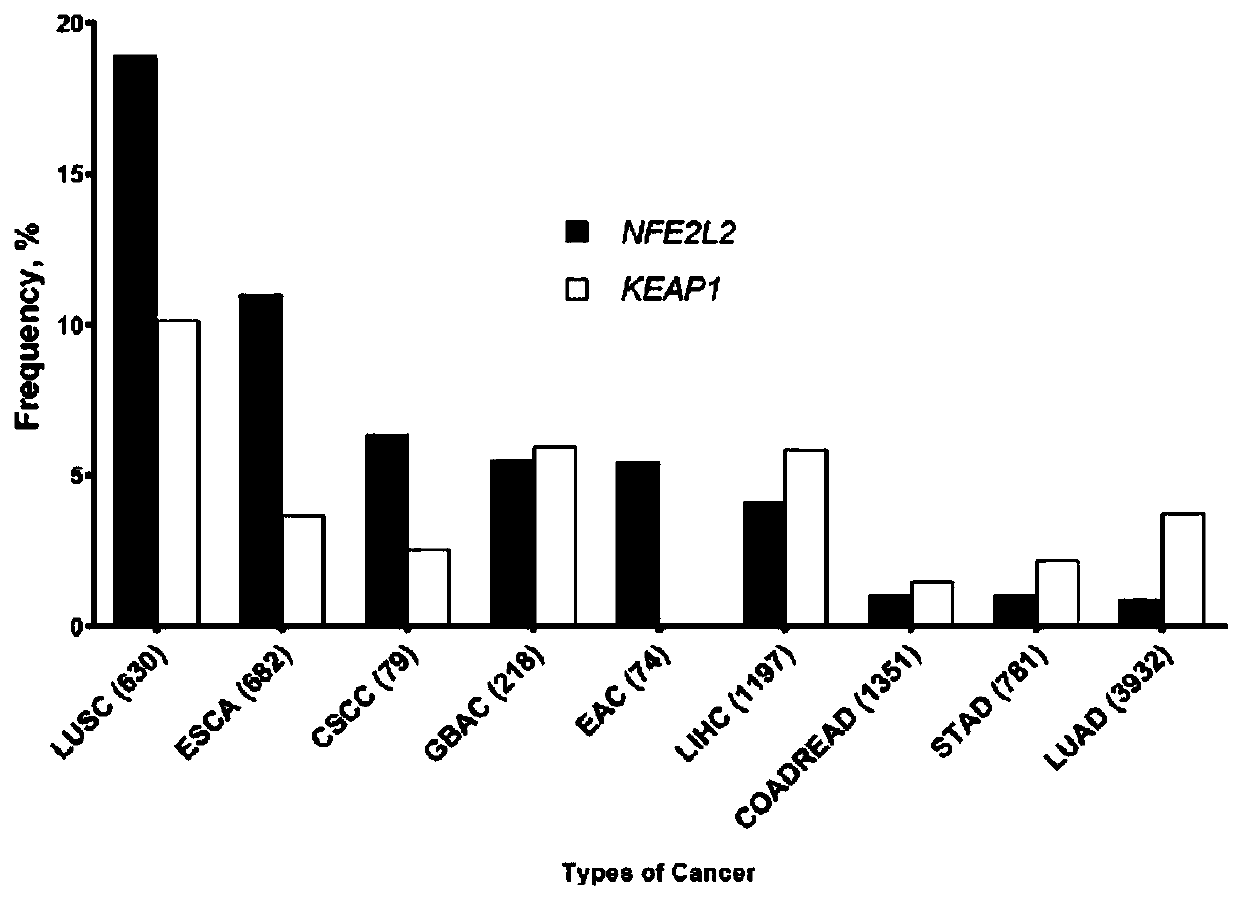
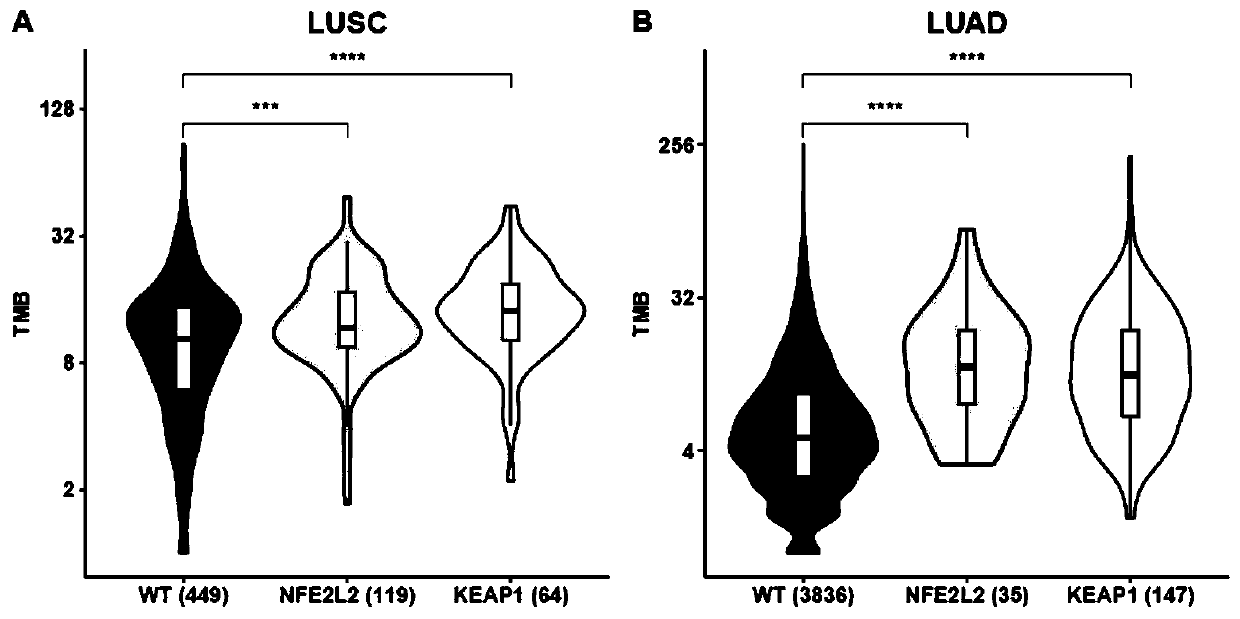
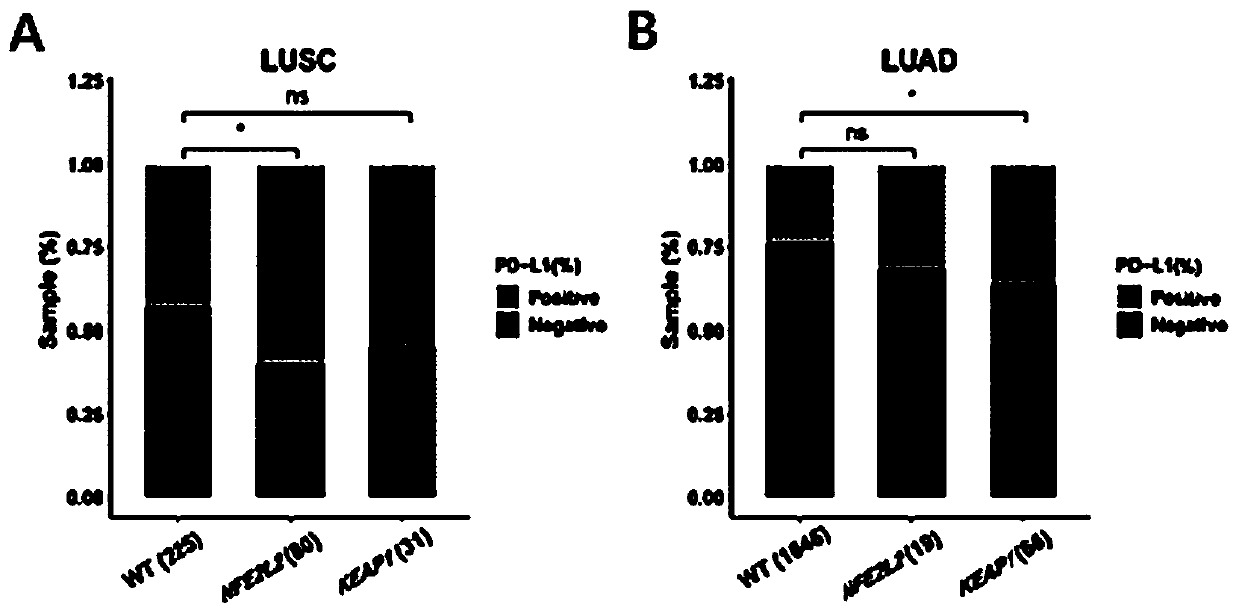
PendingCN111534585AHigh benefitMicrobiological testing/measurementSCLC - Small cell lung cancerKEAP1
The invention belongs to the technical field of immunotherapy prognosis, and in particular relates to a method for immunotherapy prognosis of non-small cell lung cancer (NSCLC) patients. The method uses a gene NFE2L2 and / or KEAP1 as a biomarker, whether the biomarker is mutated or not is detected, and thereby the prognosis of the sensitivity of NSCLC patients to immunotherapy, especially immunotherapy with immune checkpoint inhibitors (ICIs) is performed. In addition, the invention also provides a kit including biomarker detection reagents and an application of the kit. The method or the kit of the invention can be used to accurately predict the population sensitive to ICIs immunotherapy among NSCLC patients with NFE2L2 and / or KEAP1 mutations, and thereby the effectiveness and clinical benefit of immunotherapy for such patients are improved.
Owner:SHANGHAI ORIGIMED CO LTD
Method for reducing the likelihood of developing bladder or colorectal cancer in an individual human being
ActiveUS11026982B2Treatment complicationsAvoiding undesiredAntibacterial agentsOrganic active ingredientsPrevotellaMogibacterium diversum
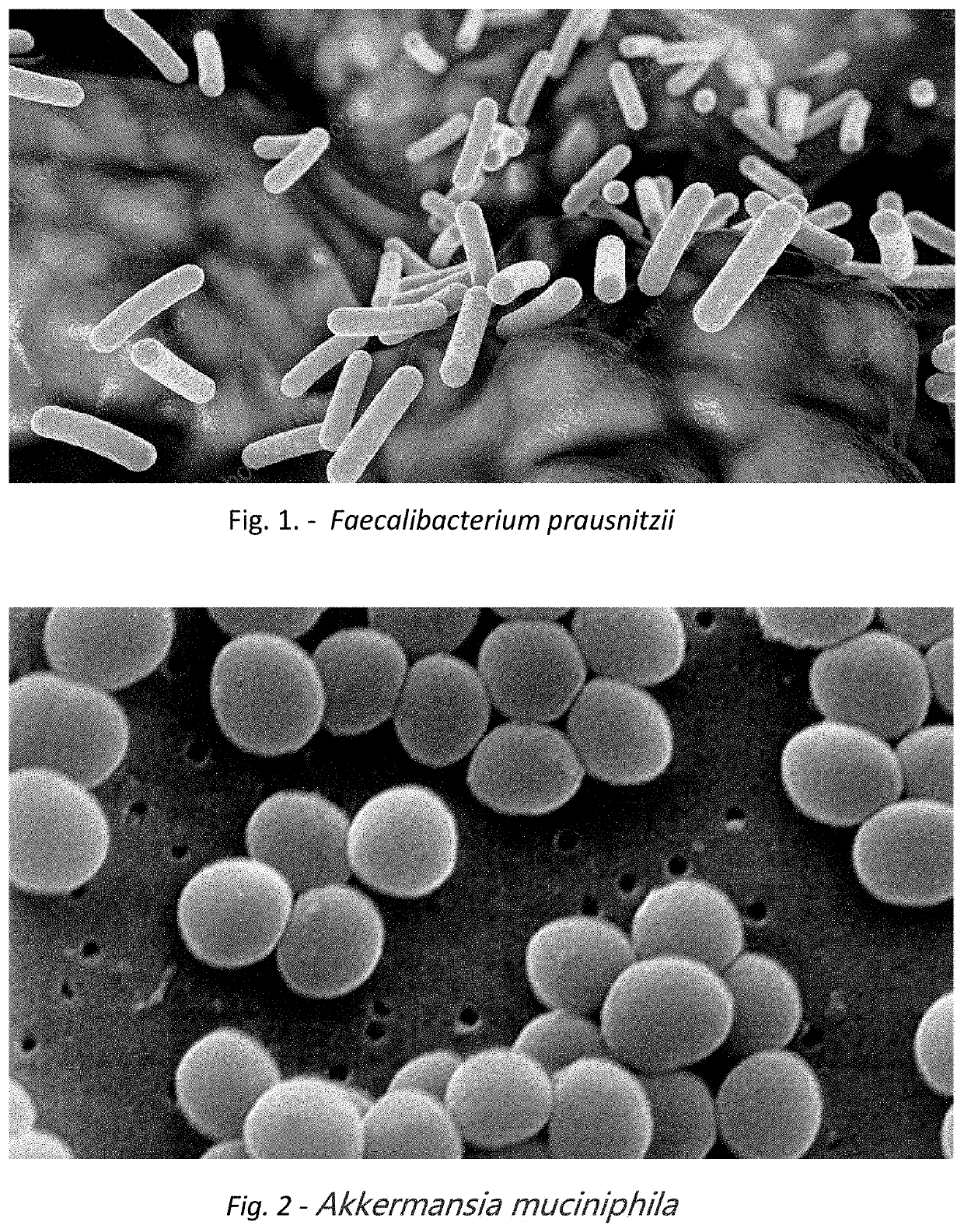
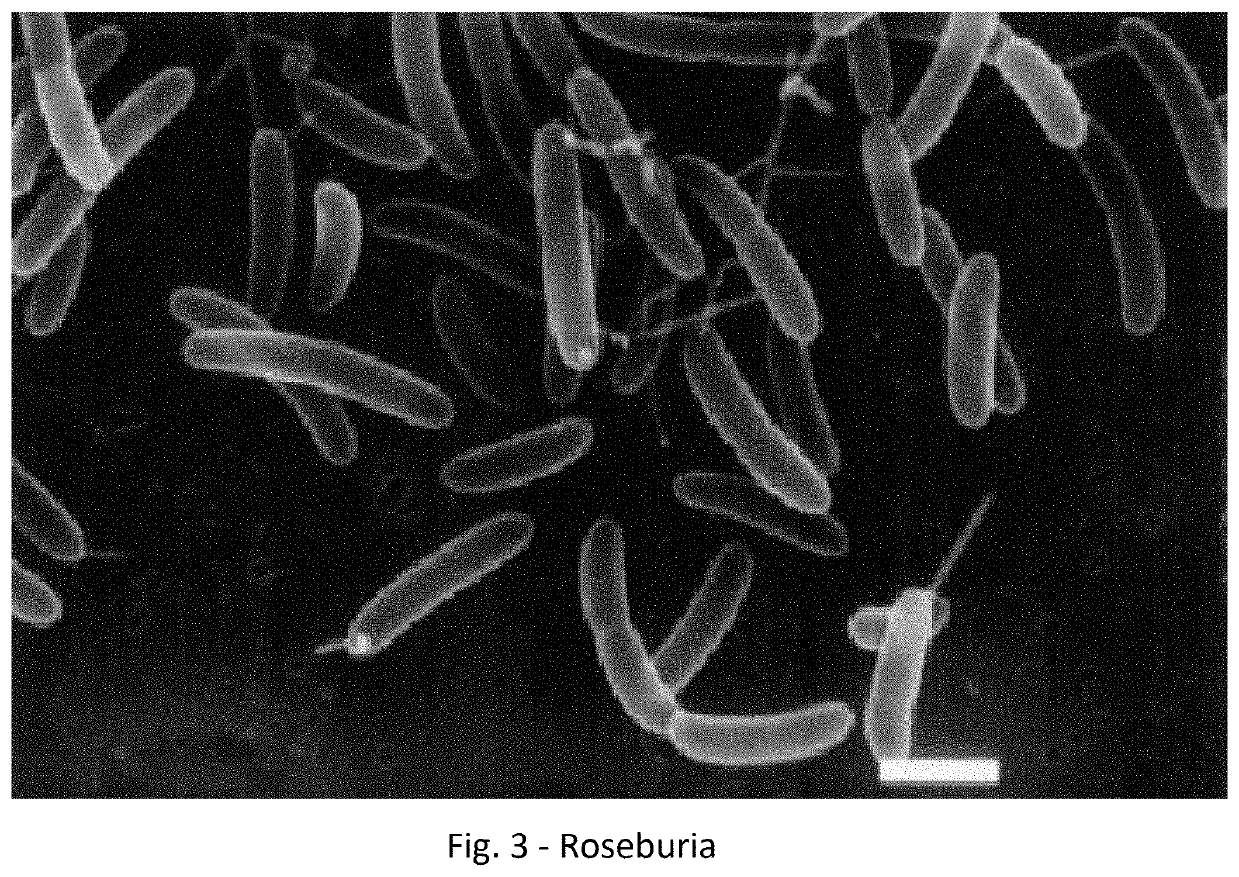
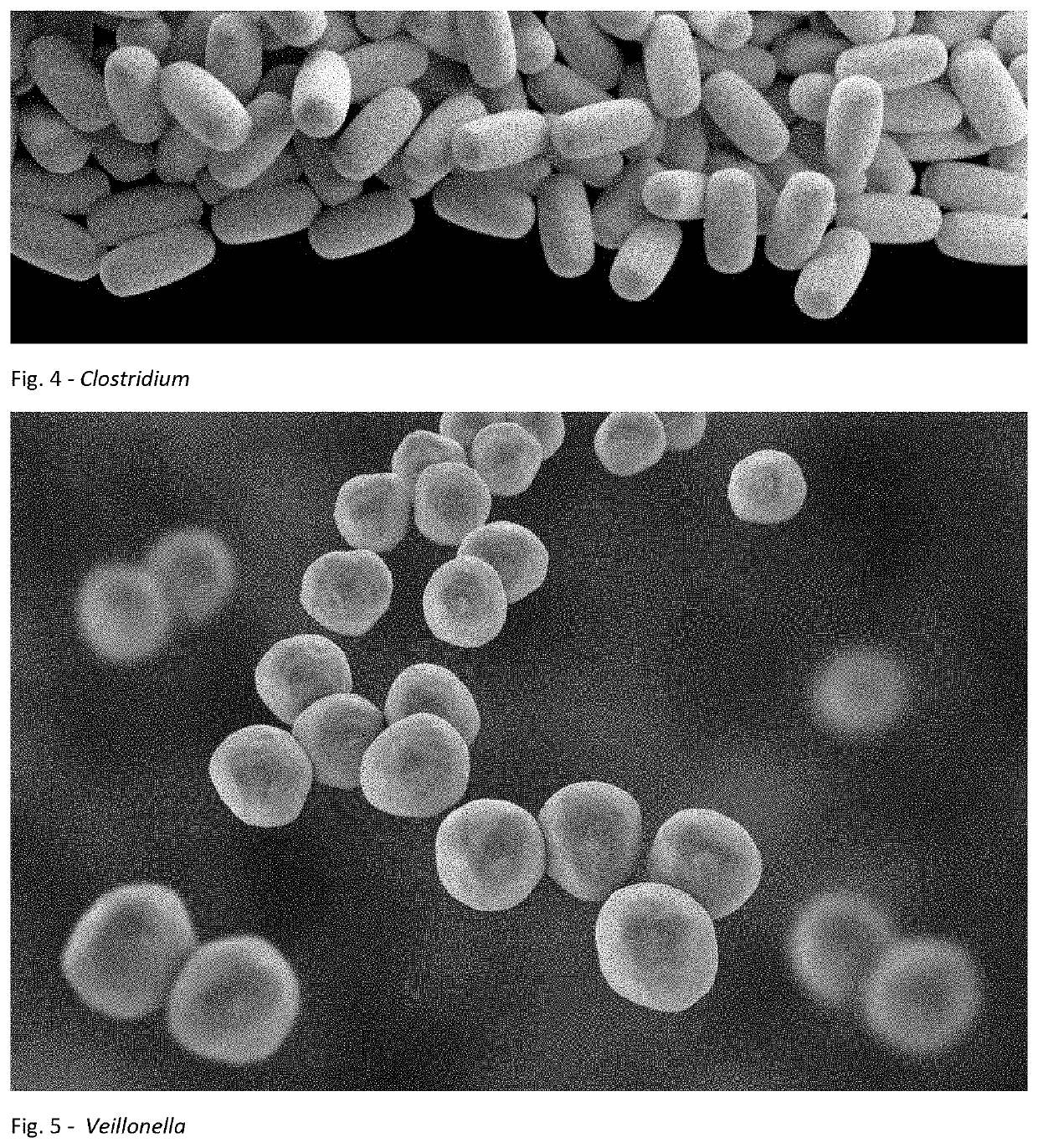
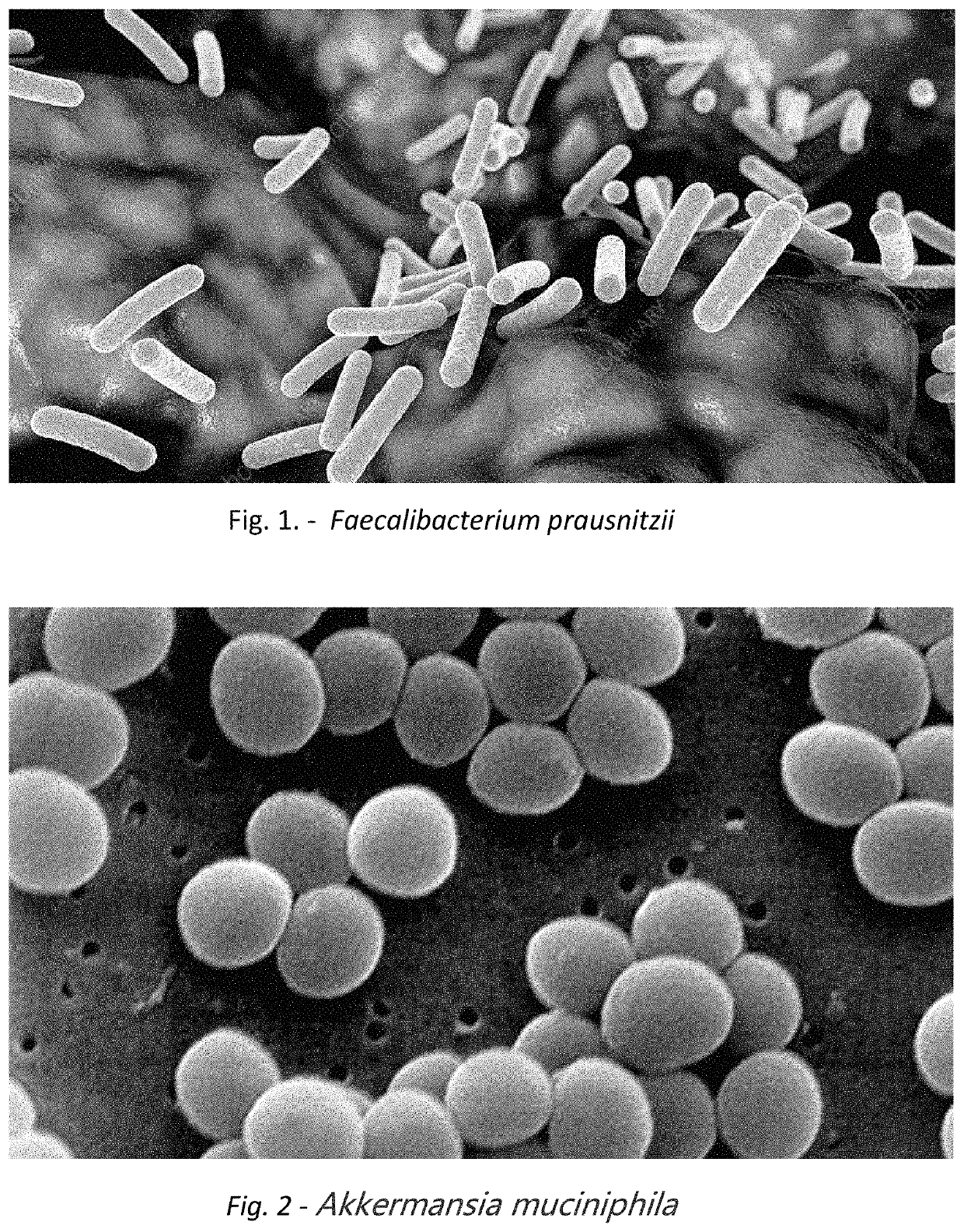
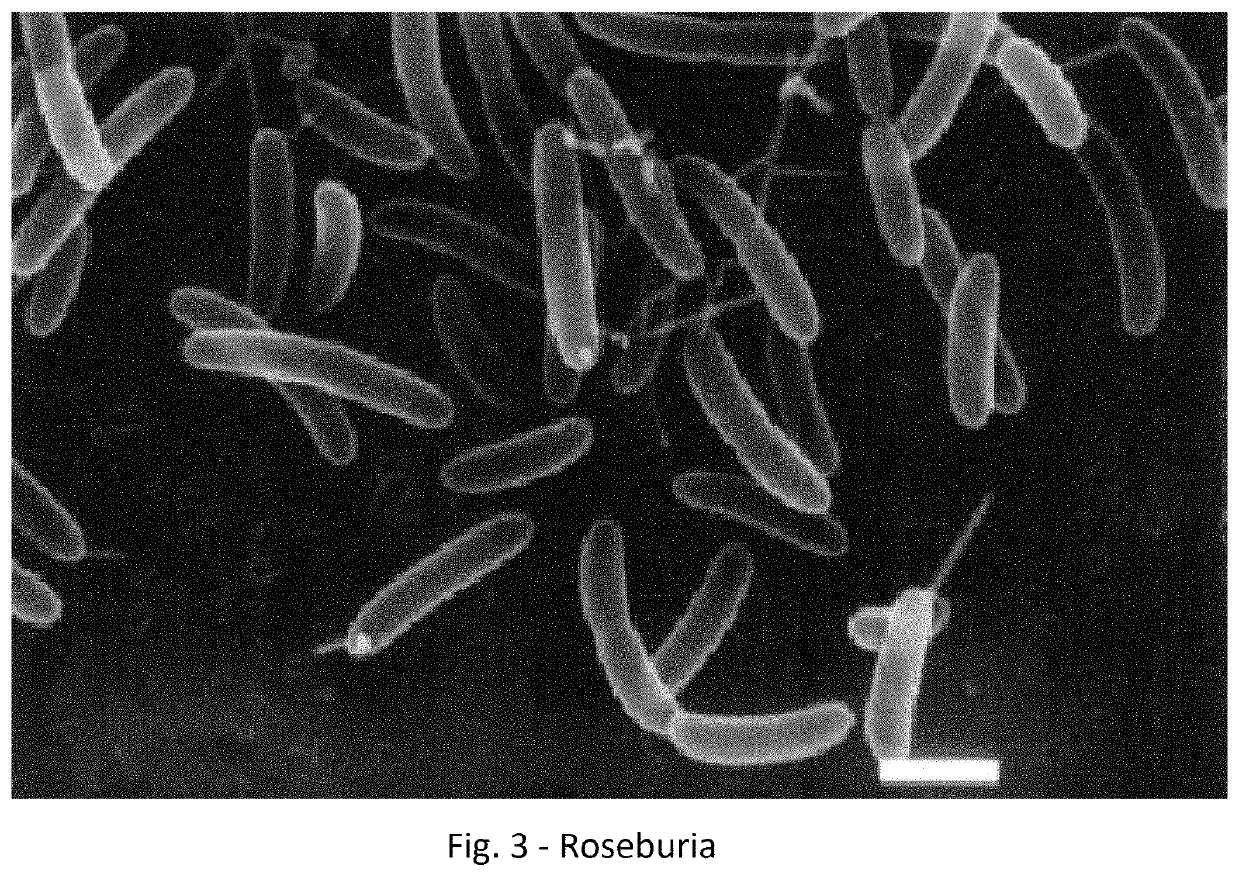
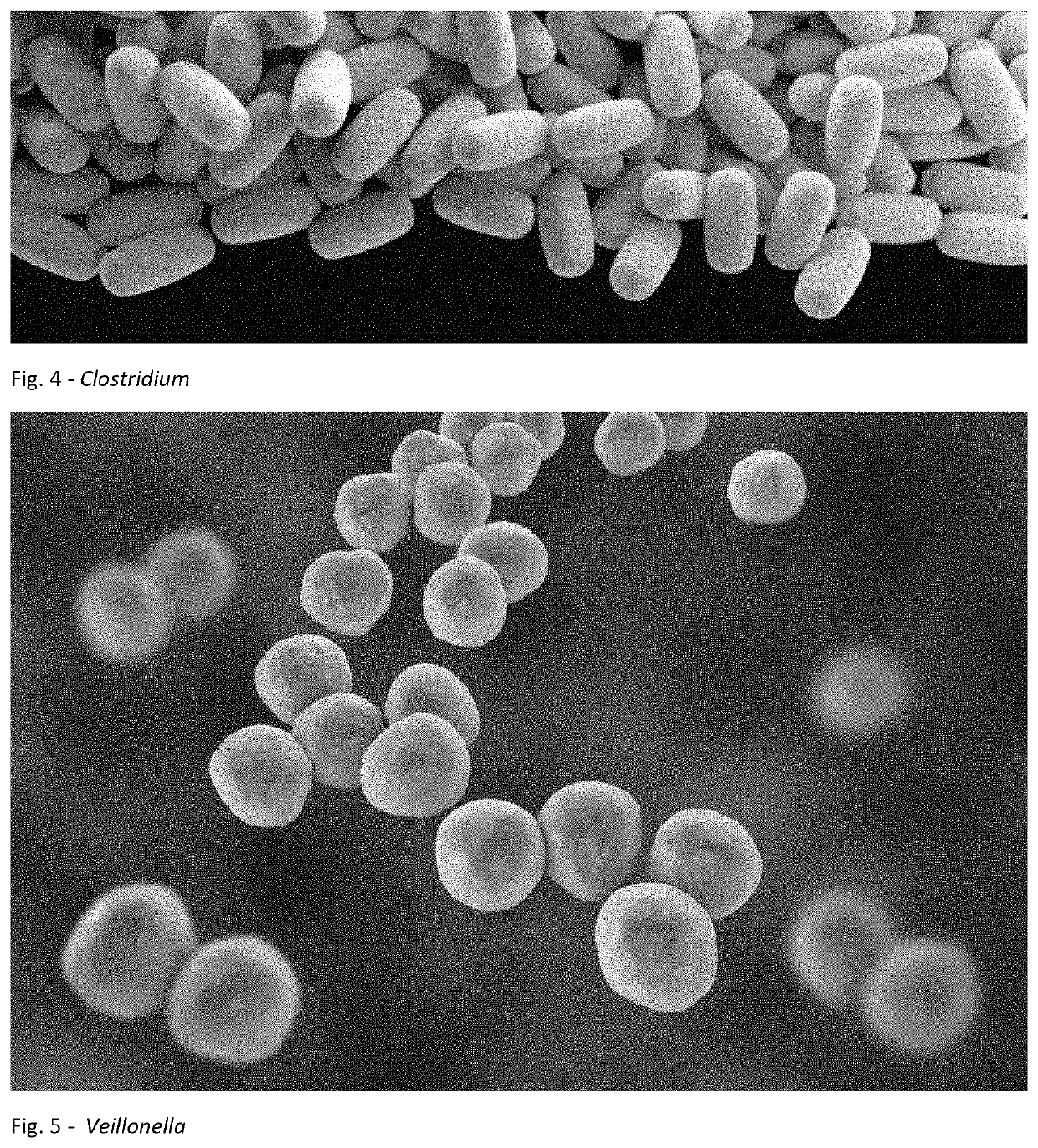
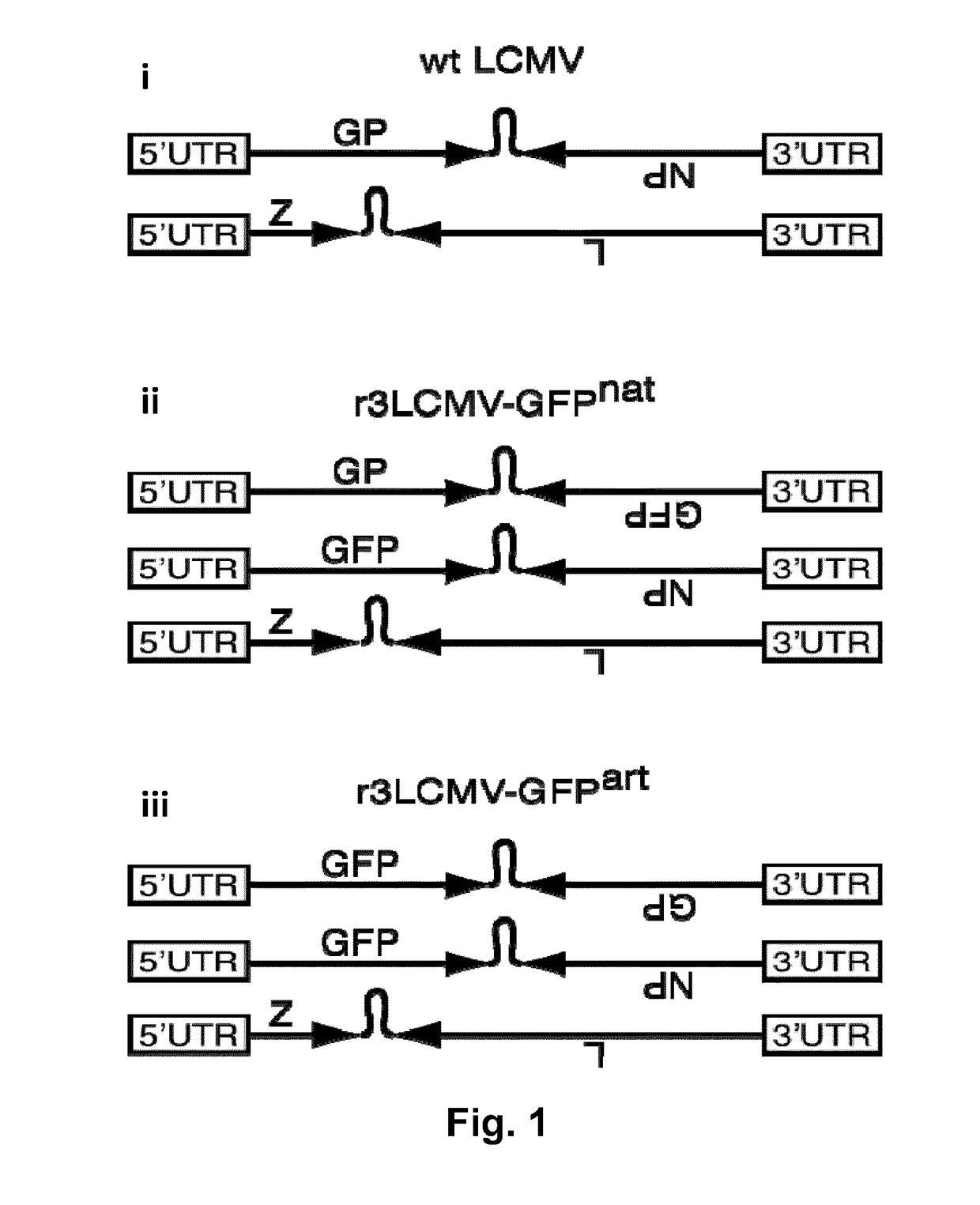
A method for treating an individual suffering from one of bladder cancer and colorectal cancer employs a CRISPR system to selectively kill or reduce the numbers of pathogenic bacteria within the individual and the individual is then administered an immune checkpoint inhibitor. In particular embodiments, the pathogenic bacteria is one of E. coli, Pseudomonas aeruginosa and Klebsiella bacteria, and the checkpoint inhibitor is selected from the group consisting of nivolumab, pembrolizumab, pidilizumab, AMP-224, AMP-514, STI-A1110, TSR-042, RG-7446, BMS-936559, MEDI-4736, MSB-0020718C, AUR-012 and STI-A1010. Further embodiments include enhancing the growth of a second bacteria in the individual, such bacteria including Akkermansia, Bacteroides, Bifidobacterium, Clostridium, Enterococcus, Fusobacterium, Lactobacillus, Propionibacterium, Ruminococcus, Veillonella, Prevotella, Escherichia and Streptococcus. Still other embodiments include increasing the levels of Roseburia and / or Faecalibacterium prausnitzii, in the individual's gut microbiome.
Owner:SEED HEALTH INC
Method for treating an individual suffering from a chronic infectious disease and cancer
ActiveUS11213552B2Treatment complicationsAvoiding undesiredAntibacterial agentsOrganic active ingredientsPseudomonasPrevotella
A method for treating an individual suffering from a chronic infectious disease and who has cancer employs a CRISPR system to selectively kill or reduce the numbers of pathogenic bacteria within the individual and the individual is then administered an immune checkpoint inhibitor. In particular embodiments, the pathogenic bacteria is one of E. coli, Pseudomonas aeruginosa and Klebsiella bacteria, and the checkpoint inhibitor is selected from the group consisting of nivolumab, pembrolizumab, pidilizumab, AMP-224, AMP-514, STI-A1110, TSR-042, RG-7446, BMS-936559, MEDI-4736, MSB-0020718C, AUR-012 and STI-A1010. Further embodiments include enhancing the growth of a second bacteria in the individual, such bacteria including Akkermansia, Bacteroides, Bifidobacterium, Clostridium, Enterococcus, Fusobacterium, Coprococcus, Lactobacillus, Propionibacterium, Ruminococcus, Veillonella, Prevotella, Escherichia and Streptococcus. The CRISPR system may include Cas9, Cpf1 and Cas3, and may be delivered using a bacteriophage.
Owner:SEED HEALTH INC
Arenavirus particles as cancer vaccines
ActiveUS20180344830A1Reduce distractionsViral antigen ingredientsCancer antigen ingredientsDiseaseNucleotide
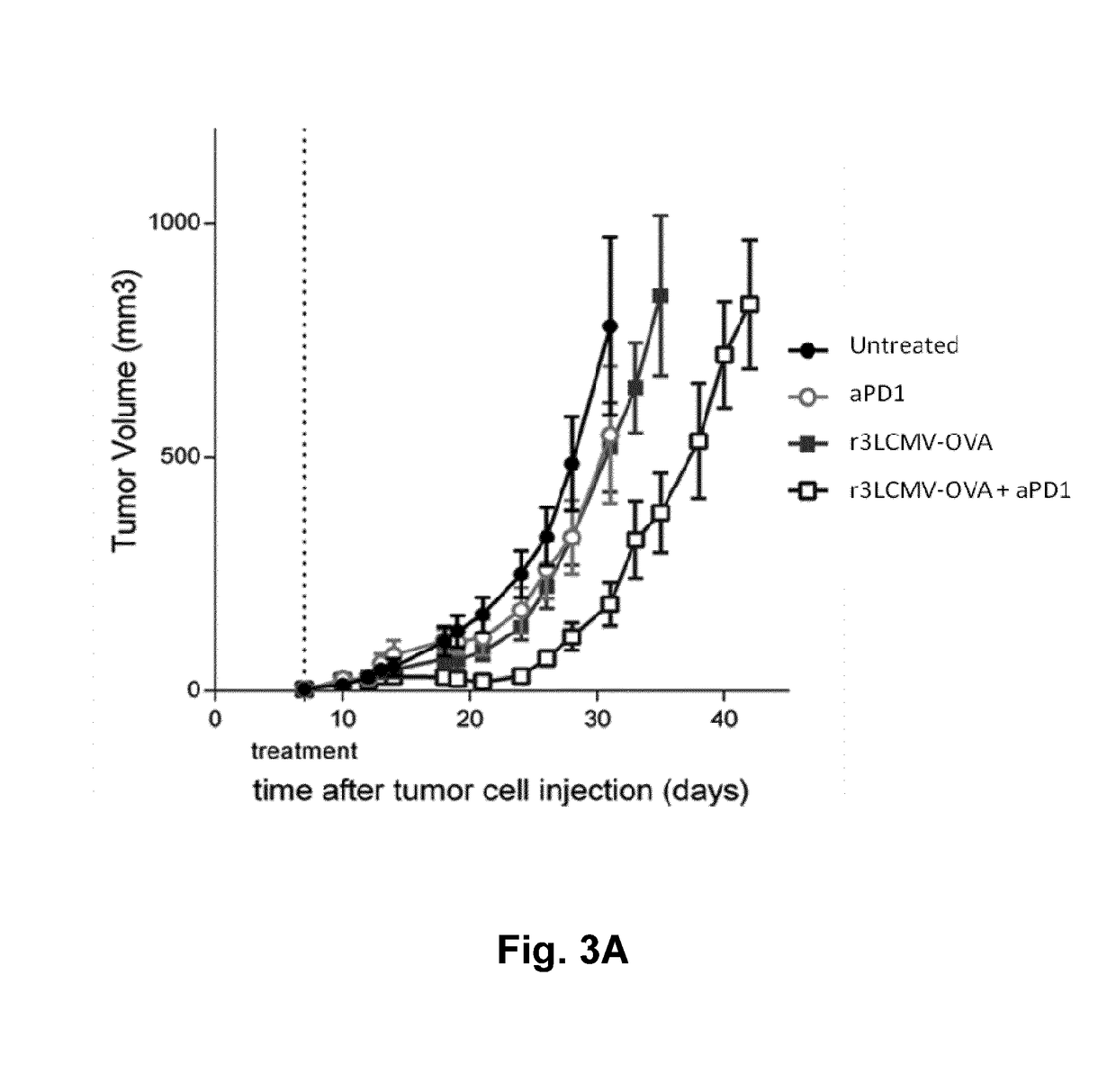
The present application relates generally to genetically modified arenaviruses that are suitable vaccines against neoplastic diseases, such as cancer. The arenaviruses described herein may be suitable for vaccines and / or treatment of neoplastic diseases and / or for the use in immunotherapies. In particular, provided herein are methods and compositions for treating a neoplastic disease by administering a genetically modified arenavirus in combination with an immune checkpoint inhibitor, wherein the arenavirus has been engineered to include a nucleotide sequence encoding a tumor antigen, tumor associated antigen or antigenic fragment thereof.
Owner:HOOKIPA BIOTECH GMBH
Gene combination and application thereof in preparation of reagent for predicting prognosis of patient treated by immune checkpoint inhibitor
ActiveCN110229894AImproved prognosisReduce financial burdenMicrobiological testing/measurementDNA/RNA fragmentationWilms' tumorBiology
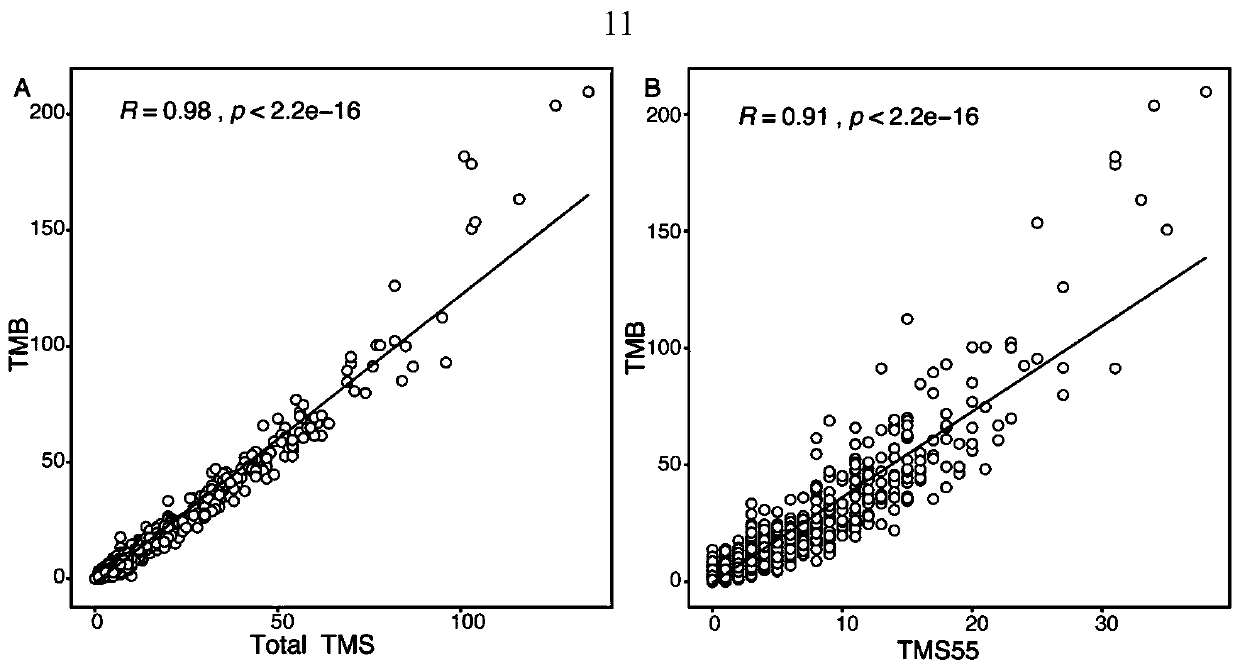
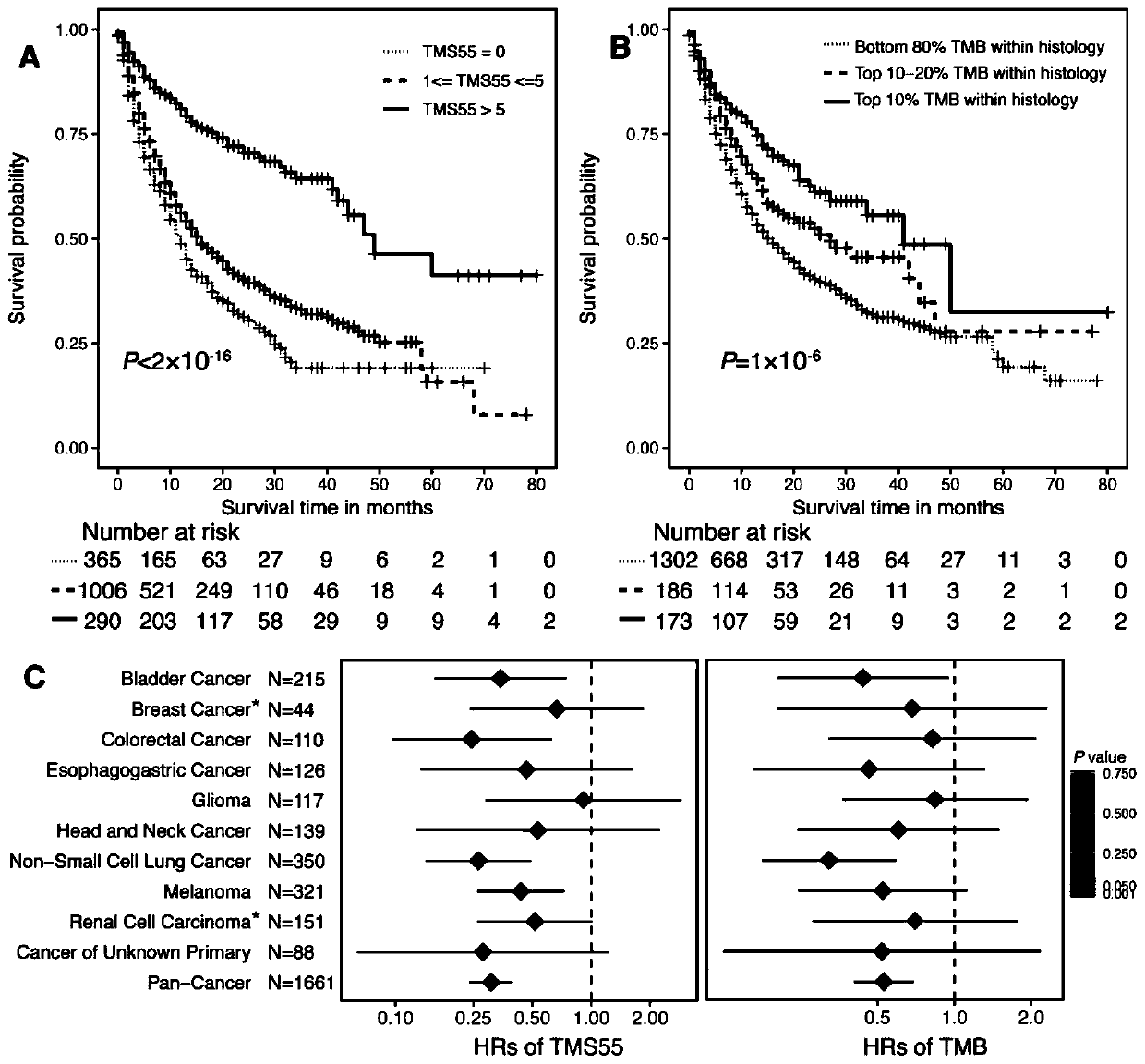
The invention provides a gene combination and application thereof in preparation of a reagent for predicting prognosis of a patient treated with an immune checkpoint inhibitor. Based on the combination of 55 genes, the invention develops a tumor mutation score TMS32055 method through the gene combination, and TMS is defined as the number of genes containing non-synonymous mutations in a specific gene combination. TMS 55 is divided into 3 groups: TMS 55 is equal to 0, TMS 55 is greater than or equal to 1 and is less than or equal to 5, and TMS 55 is greater than 5. Compared with a group that TMS 55 is equal to 0, the patients of groups that TMS 55 is greater than or equal to 1 and is less than or equal to 5 and TMS 55 is greater than 5 have better prognosis and longer overall survival time.The TMS55 based on the 55-gene combination has higher prediction efficiency than TMB in predicting the overall survival after immunotherapy, and has uniform cutoff value. Therefore, the TMS55 can beused for predicting the prognosis of patients receiving immune checkpoint inhibition treatment, and is easier to popularize and apply in clinical practice.
Owner:WUHAN UNIV
Multi-immunohistochemical analysis kit of lung cancer, and using method and application thereof
ActiveCN109596831AGood treatment effectStrong detection signalBiological testingMonoclonal antibodyImmunohistochemistry technique
The invention provides a multi-immunohistochemical analysis kit of lung cancer, and a using method and application thereof, and relates to the field of the multi-immunohistochemical technology. The multi-immunohistochemical analysis kit defines that the monoclonal antibody group comprises more than two types of a CD163 monoclonal antibody, a CD68 monoclonal antibody, a PDL1 monoclonal antibody, aCD8 monoclonal antibody, a PD1 monoclonal antibody and a CD57 monoclonal antibody. The multi-immunohistochemical analysis kit uses the human or animal in vitro tissue, which is given the immune checkpoint inhibitor, as the sample to test, so as to judge the lung cancer effect validity of the multi-immunohistochemical analysis kit. The invention further provides a using method of the multi-immunohistochemical analysis kit; the method can effectively mark not less than 6 types of immune checkpoints on the same one tissue; and the cross reaction does not exist among multiple marks.
Owner:ZHENYUE BIOTECHNOLOGY JIANGSU CO LTD
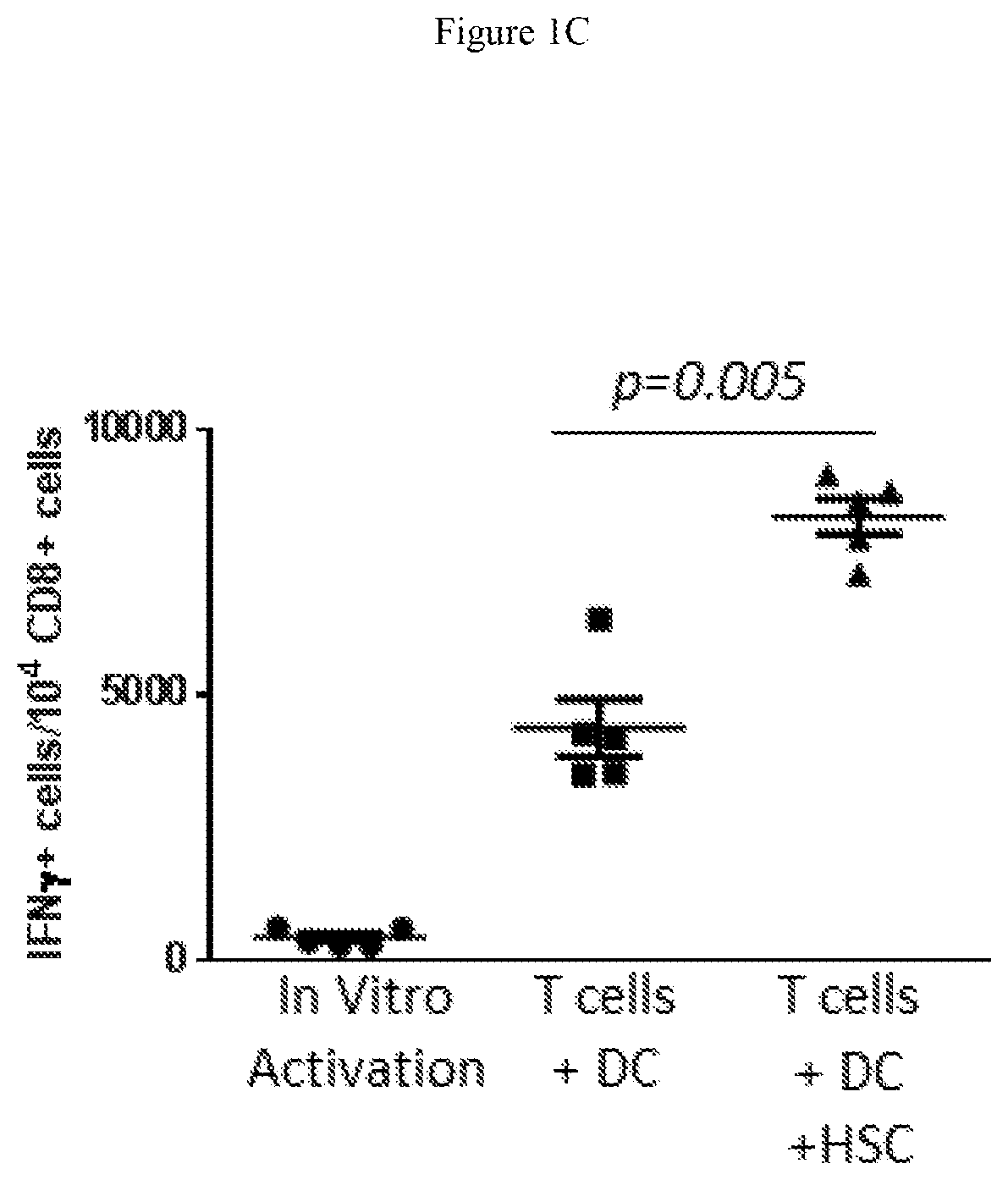
Hematopoietic stem cells in combinatorial therapy with immune checkpoint inhibitors against cancer
ActiveUS10660954B2Promote persistenceEasy SurvivalAntiviralsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseHematopoietic Stem Cell Mobilization
The novel synergistic combination of immune checkpoint blockade and hematopoietic stem cell transplantation and / or hematopoietic stem cell mobilization yield synergistic effects in disease therapy.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Combination tumor treatment with an integrin-binding-fc fusion protein and immune modulator
ActiveUS20180369329A1InducesOrganic active ingredientsPeptide/protein ingredientsIMMUNE STIMULANTSImmune modulator
The present invention provides a method of treating cancer with an integrin-binding-Fc fusion protein alone or in combination with IL-2 and / or an immune stimulant (i.e., an immune checkpoint stimulator), and / or an immune checkpoint inhibitor. The invention also provides composition for use in such methods.
Owner:XYENCE THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com