Patents
Literature
108 results about "EGFR Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
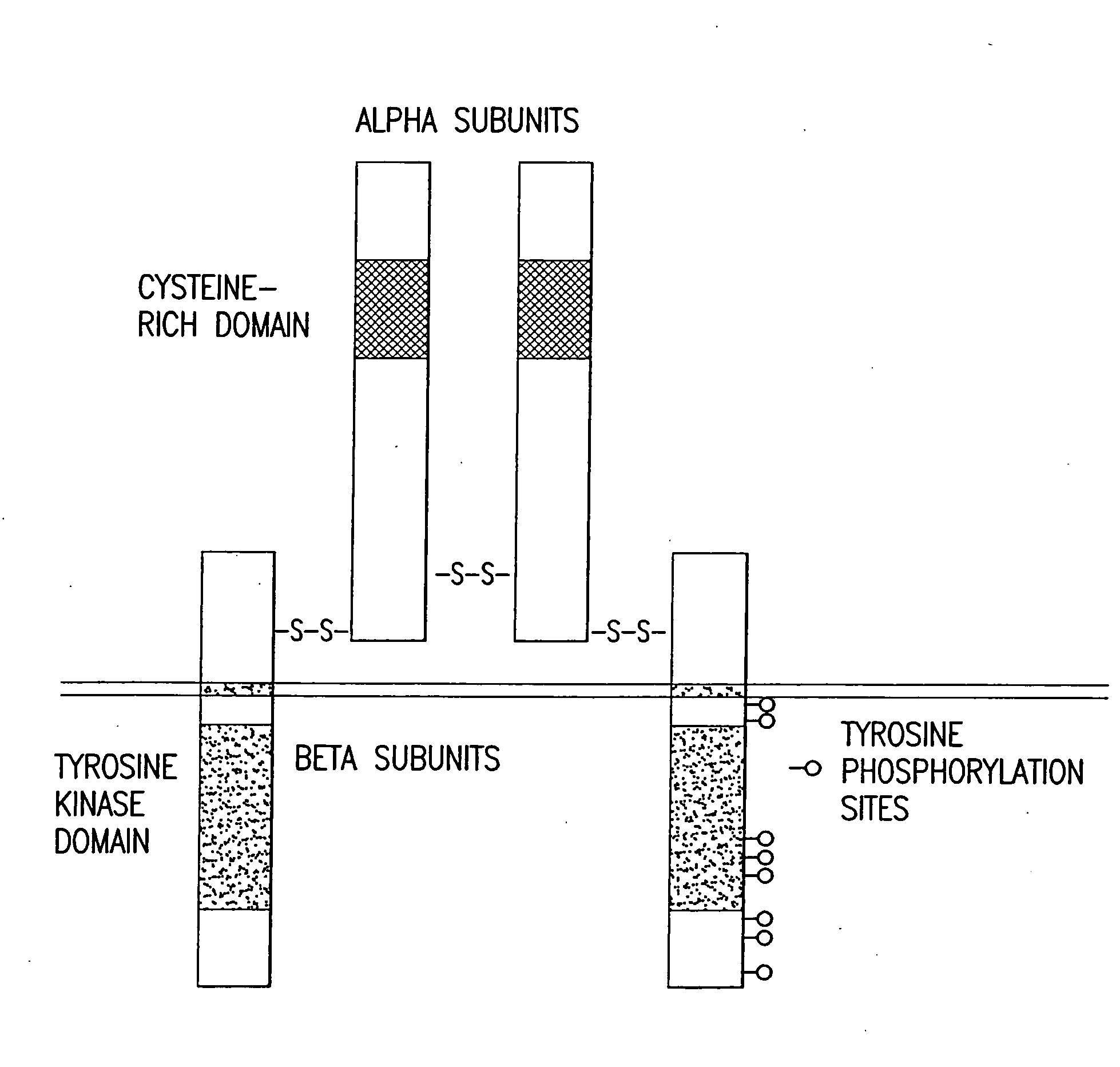
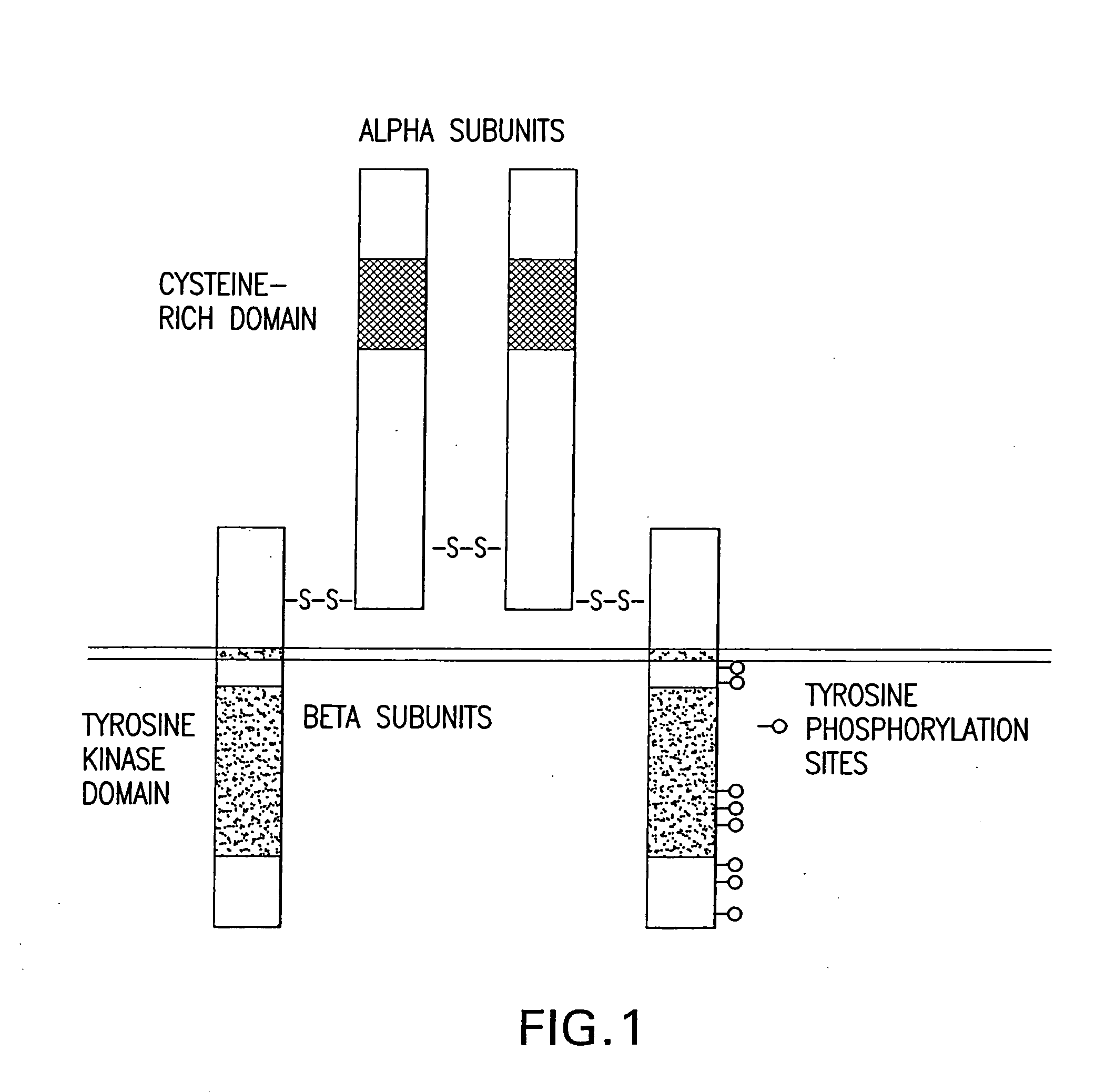
Any immunoglobulin that recognizes epidermal growth factor receptor protein.
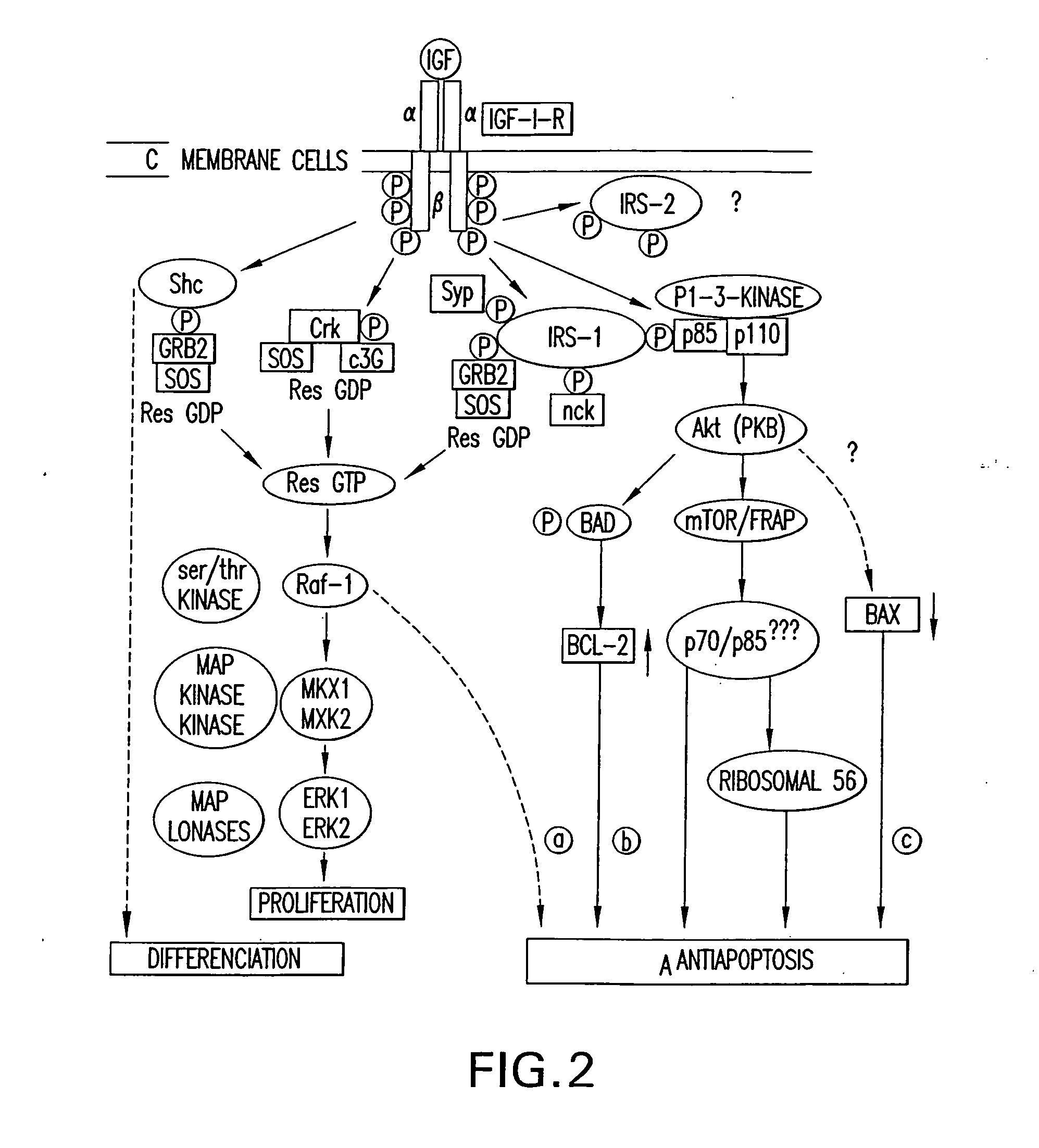
Novel anti-IGF-IR antibodies and uses thereof
InactiveUS20080193445A1Stimulate interestPromote secretionMicrobiological testing/measurementImmunoglobulins against growth factorsDiseaseAnticarcinogen
The present invention relates to novel antibodies capable of binding specifically to the human insulin-like growth factor I receptor IGF-IR and / or capable of specifically inhibiting the tyrosine kinase activity of said IGF-IR receptor, especially monoclonal antibodies of murine, chimeric and humanized origin, as well as the amino acid and nucleic acid sequences coding for these antibodies. The invention likewise comprises the use of these antibodies as a medicament for the prophylactic and / or therapeutic treatment of cancers overexpressing IGF-IR or any pathology connected with the overexpression of said receptor as well as in processes or kits for diagnosis of illnesses connected with the overexpression of the IGF-IR receptor. The invention finally comprises products and / or compositions comprising such antibodies in combination with anti-EGFR antibodies and / or compounds and / or anti-cancer agents or agents conjugated with toxins and their use for the prevention and / or the treatment of certain cancers.
Owner:INST DE RECH PIERRE FABRE +1
Solid forms of anti-EGFR antibodies
InactiveUS7960516B2Improve stabilityHigh purityPowder deliveryFrom normal temperature solutionsAdjuvantDissolution
The invention relates to solid forms of antibodies against the EGF receptor, in particular precipitates and crystals of monoclonal antibodies against the EGF receptor, particularly preferably of Mab C225 (cetuximab) and Mab h425 (EMD 72000), which result in biologically active antibody protein through dissolution or suspension in aqueous medium, obtainable by precipitation of the antibody and / or one of its variants and / or fragments dissolved or suspended in aqueous medium by means of a precipitation reagent. The invention furthermore relates to pharmaceutical preparations comprising at least one solid form of above-mentioned antibodies in precipitated non-crystalline, precipitated crystalline or in soluble or suspended form, and optionally excipients and / or adjuvants and / or further pharmaceutical active ingredients, and to a process for the preparation of solid forms of anti-EGFR antibodies according to the invention.
Owner:MERCK PATENT GMBH
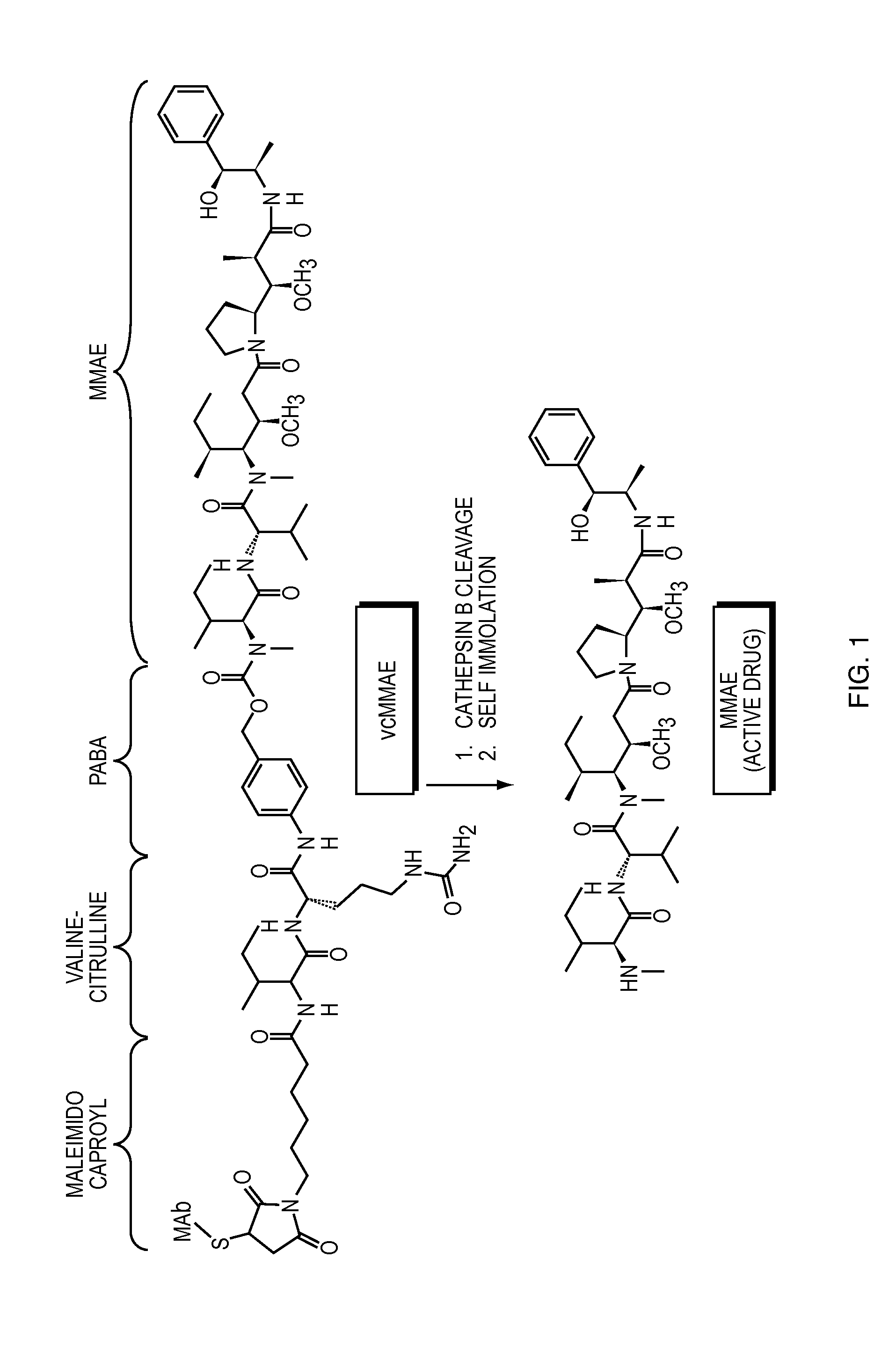
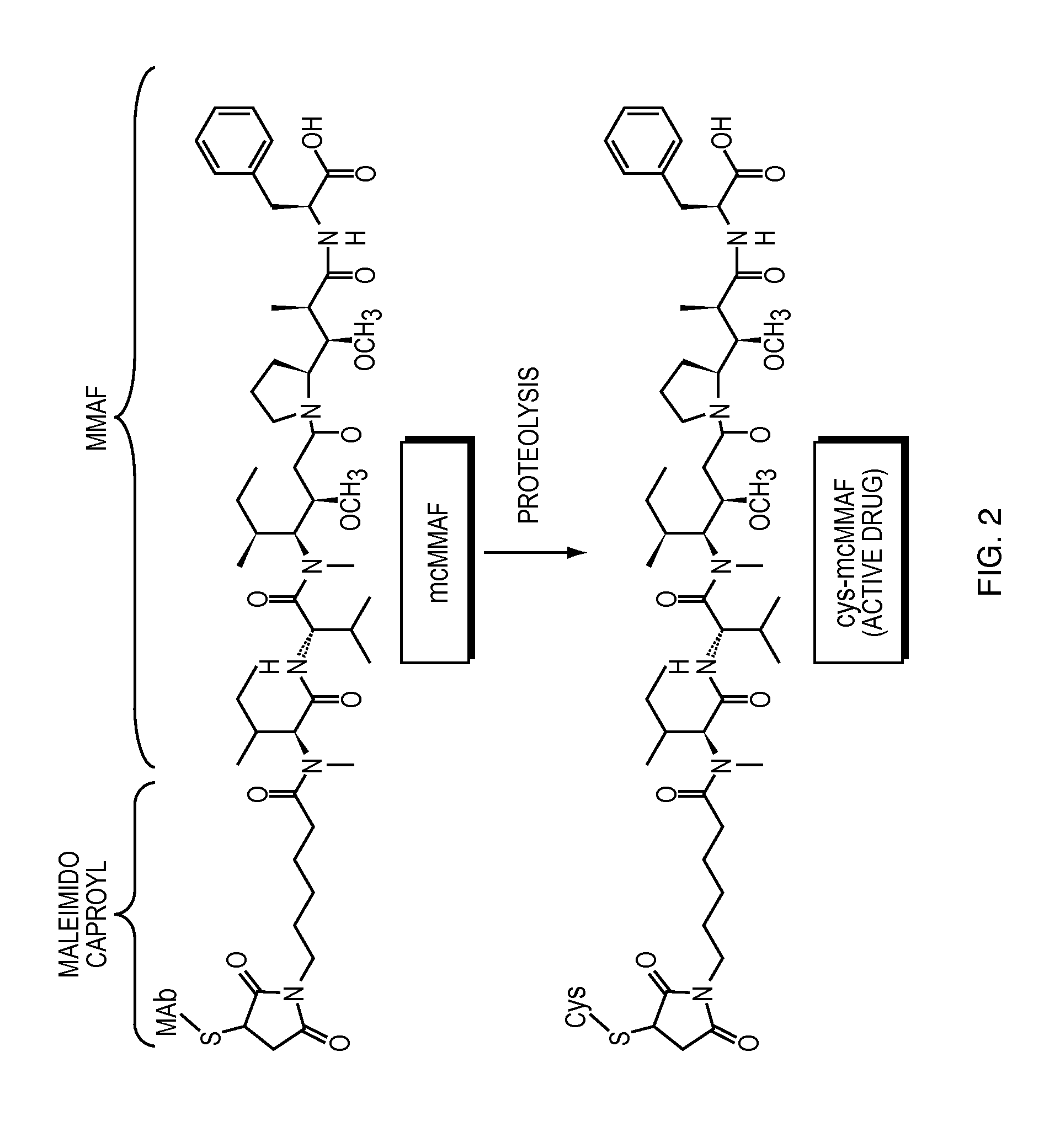
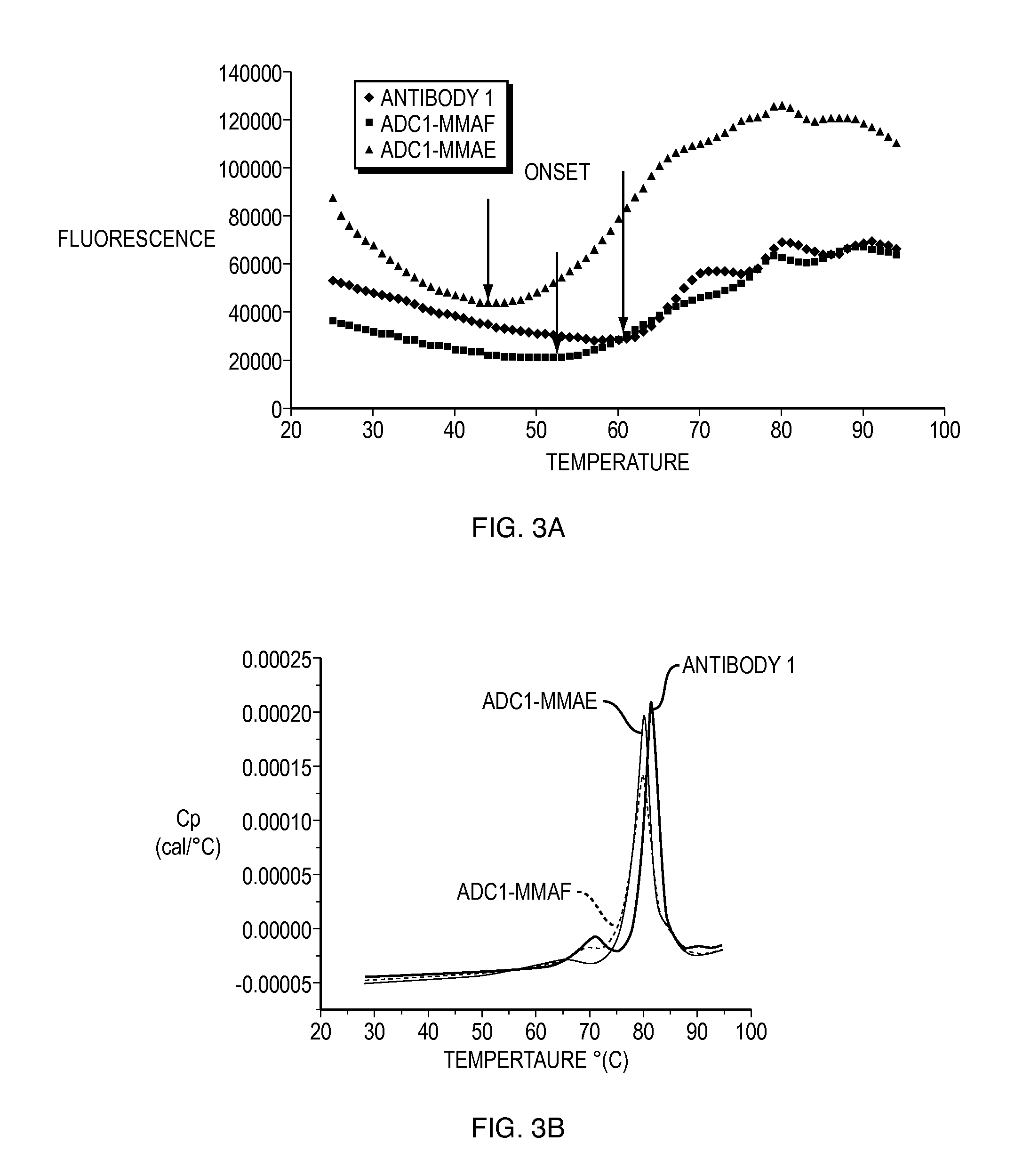
Anti-egfr antibody drug conjugate formulations
The invention provides a stable formulation comprising an anti-EGFR antibody drug conjugate (ADC), including an anti-EGFR antibody, e.g., antibody 1, conjugated to an auristatin, e.g., MMAF, histidine, a sugar, and a surfactant.
Owner:ABBVIE INC +1
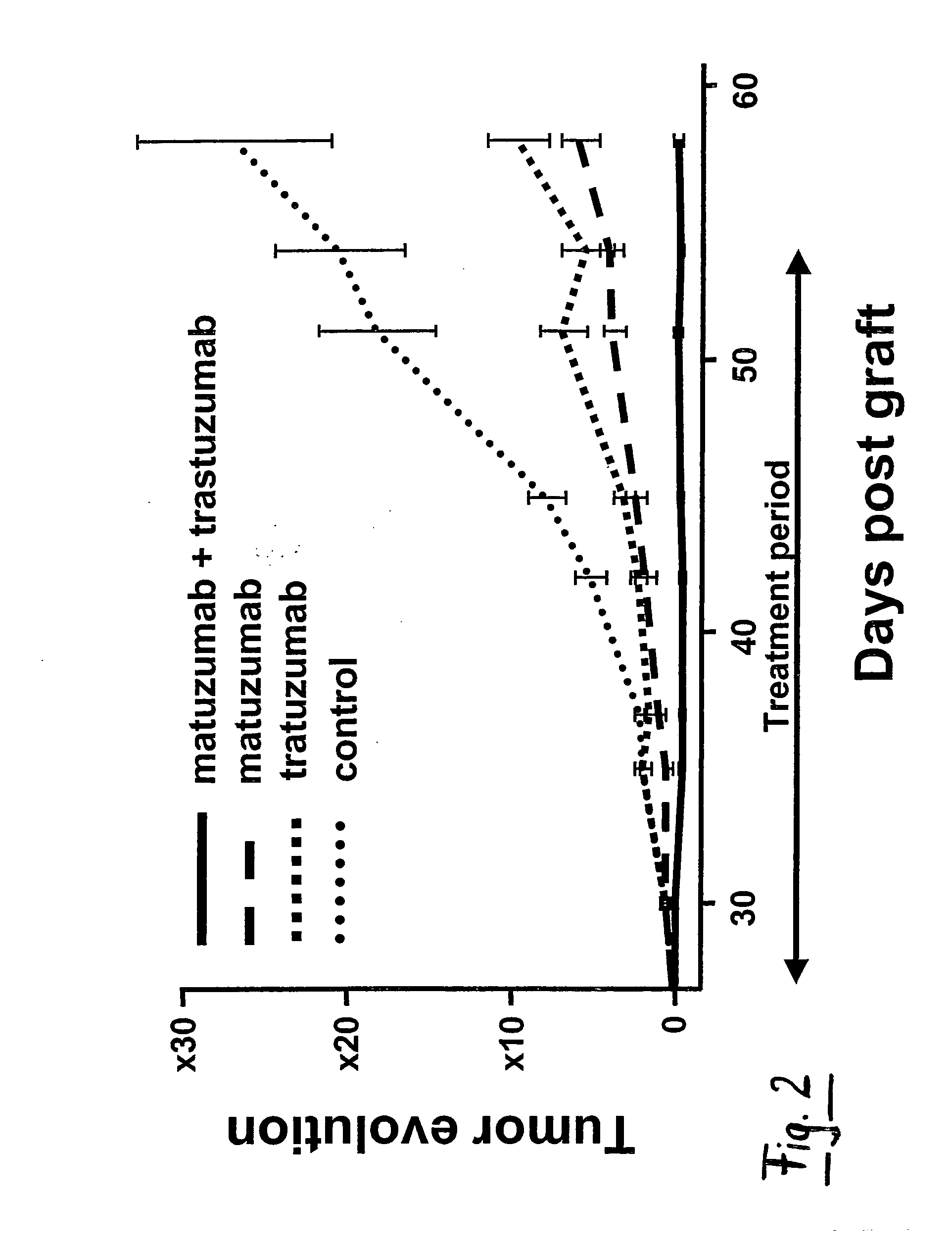
Combination Therapy Using Anti-EGFR and Anti-HER2 Antibodies
InactiveUS20090214541A1Good curative effectPrevent dimerizationAntibody ingredientsImmunoglobulinsEGFR AntibodyAnti her2
The invention relates to the combined use of anti-EGFR antibodies and anti-Her2 antibodies for the treatment of cancer, especially suitable for cancer expressing high levels of the EGFR type and low levels of HER2. The invention refers in particular monoclonal antibody “trastuzumab” (HERCEPTIN®) directed against the HER2 receptors the efficacy of which can be significantly increased in vivo when combined with monoclonal antibody “matuzumab” (hmAB 425, EMD 72000) directed against EGF receptors. The combination treatment is suitable for patients suffering from cancer having said receptor profile, preferably pancreatic cancer.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
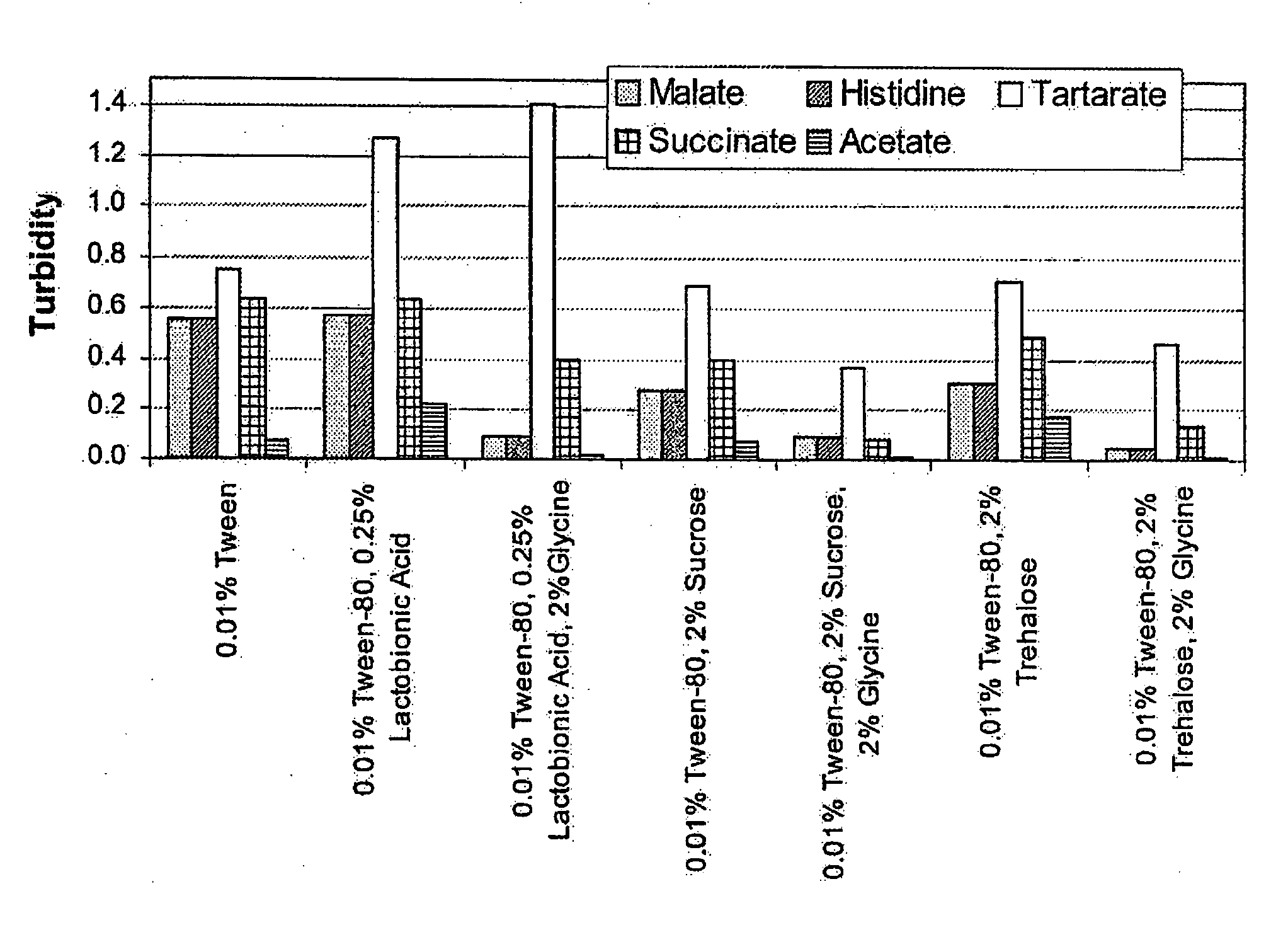
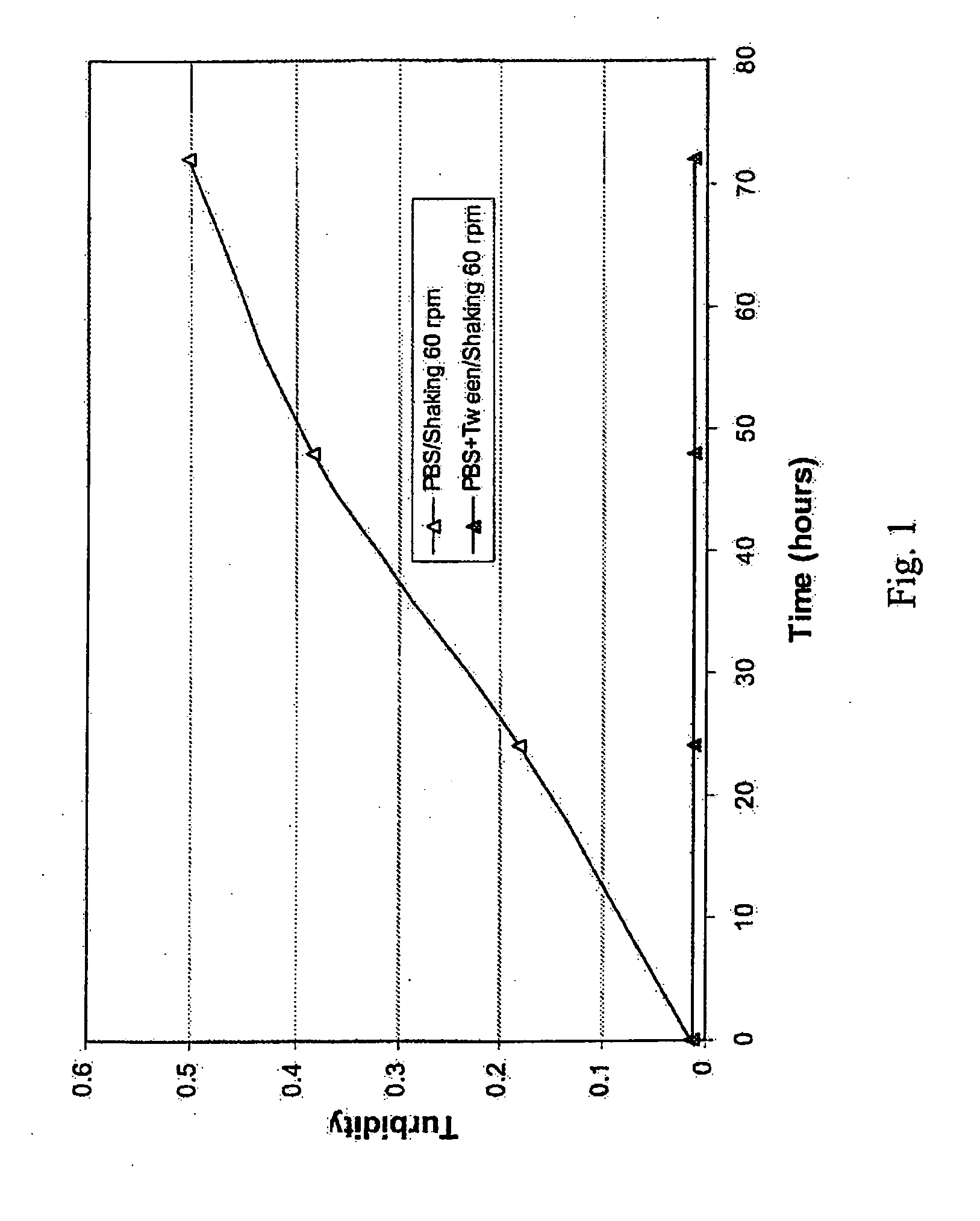
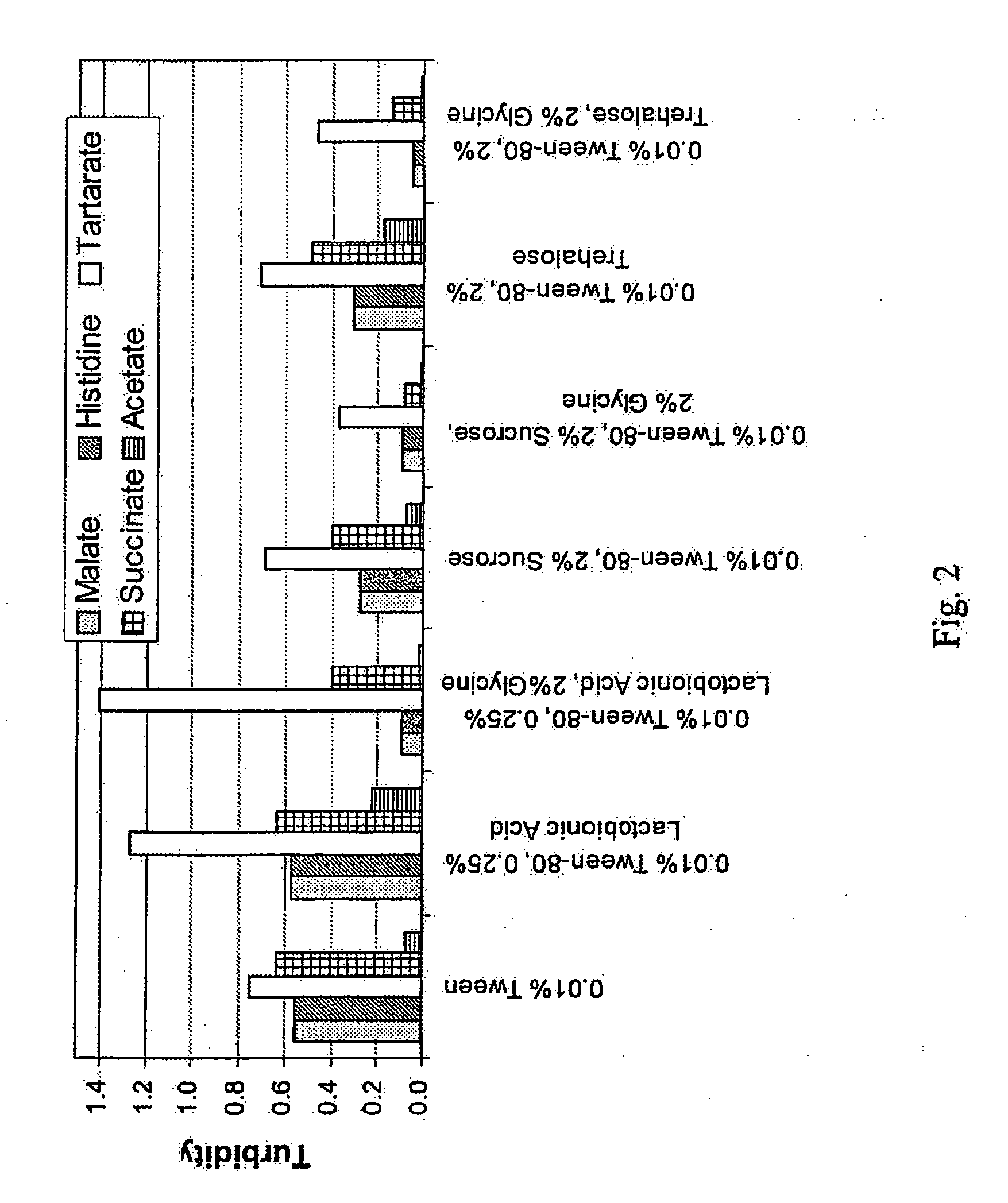
Lyophilized formulations of anti-egfr antibodies
In one embodiment, the present invention provides a stable lyophilized formulation comprising an anti-EGFR antibody, preferably cetuximab; lactobionic acid; and a buffer, preferably histidine. In one preferred embodiment, the present invention provides a stable lyophilized formulation comprising about 50 mg / mL to about 140 mg / mL of ERBITUX?, about 0.125% lactobionic acid, about 25 mM histidine buffer at a pH of about 6.0, about 0.005% Tween 80, and about 1.875% glycine.
Owner:IMCLONE SYSTEMS
Method of Treatment of Tumors That Are Resistant to EGFR Therapies by EGFR Antibody Cytotoxic Agent Conjugate
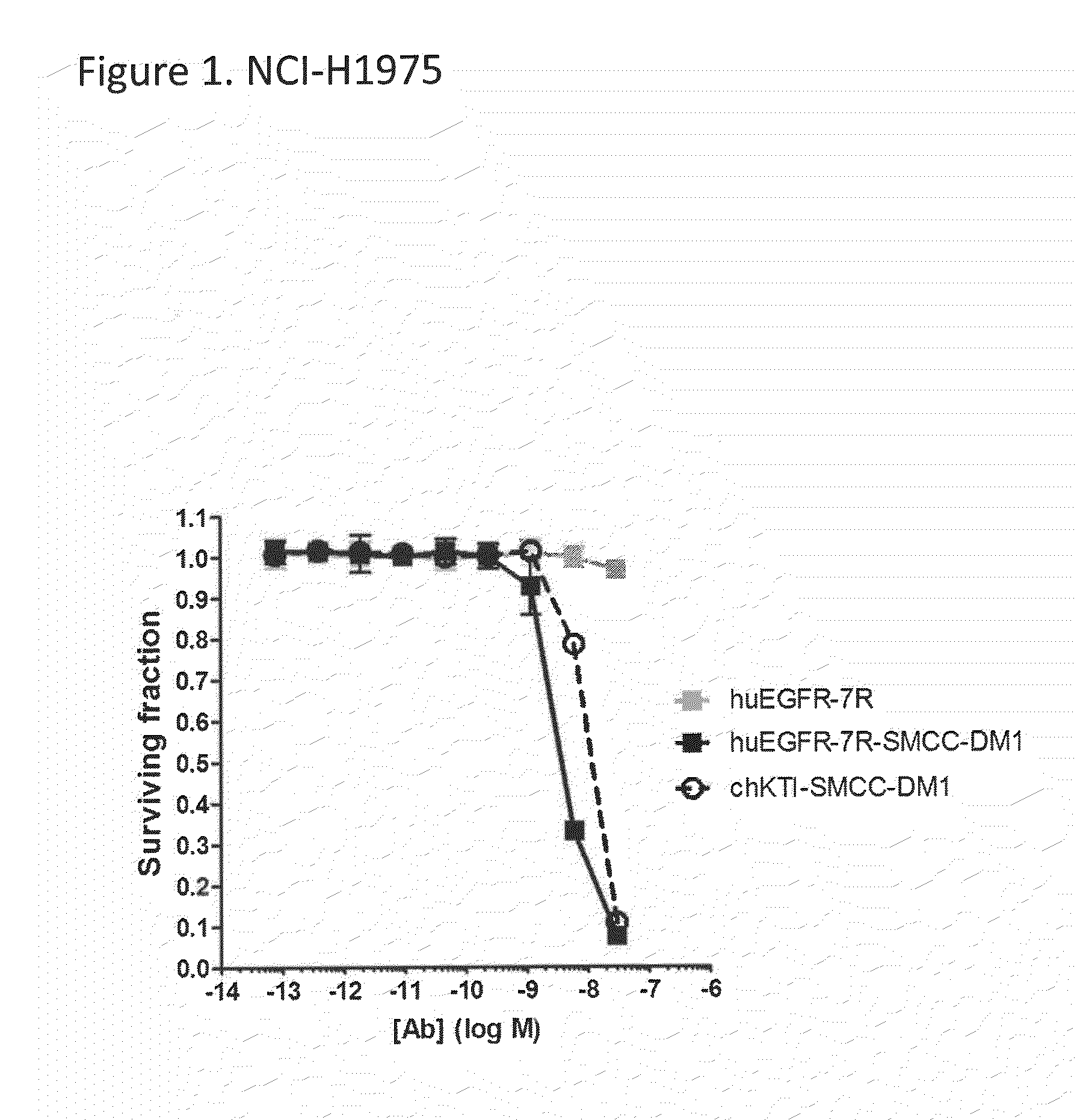
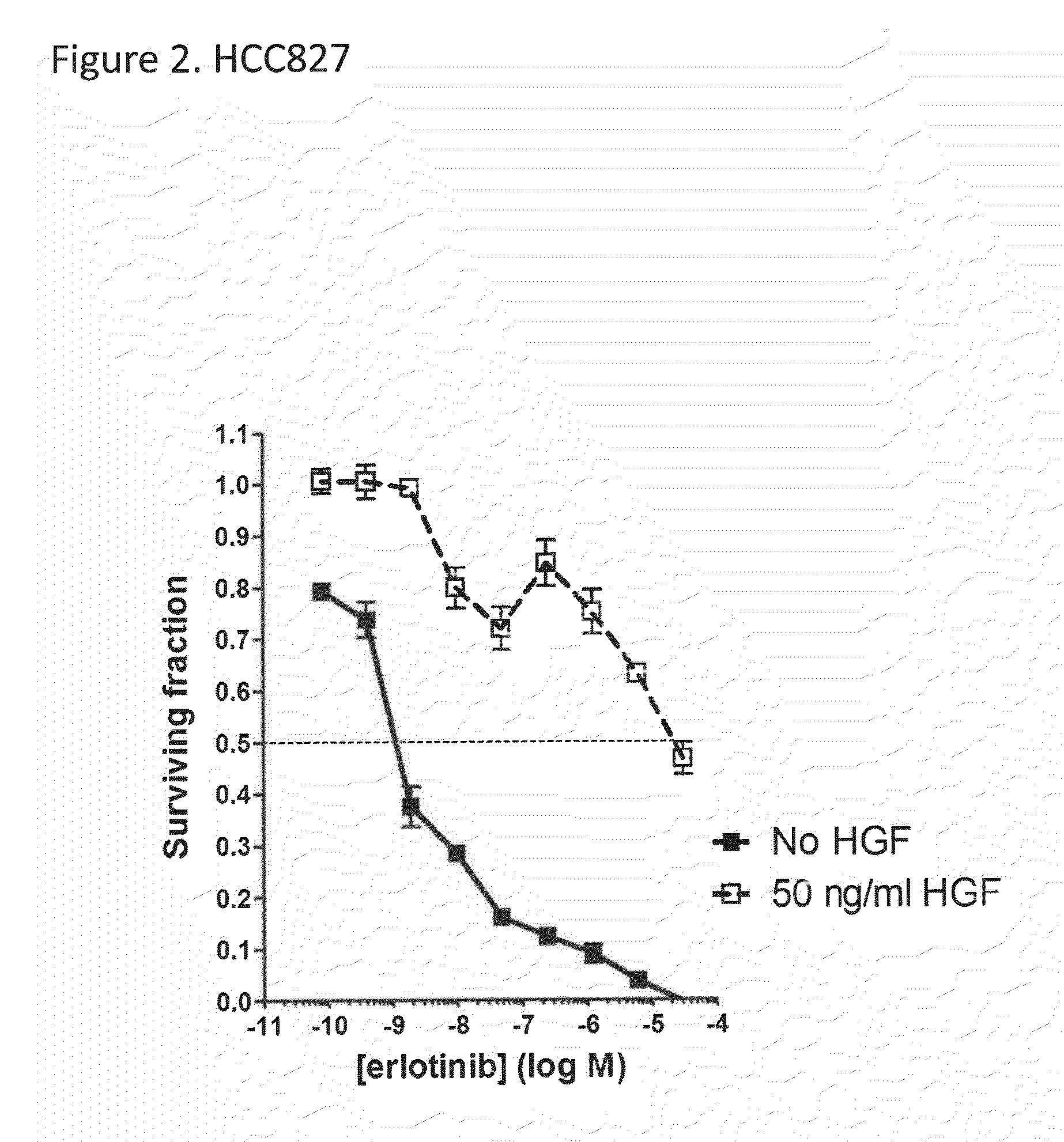
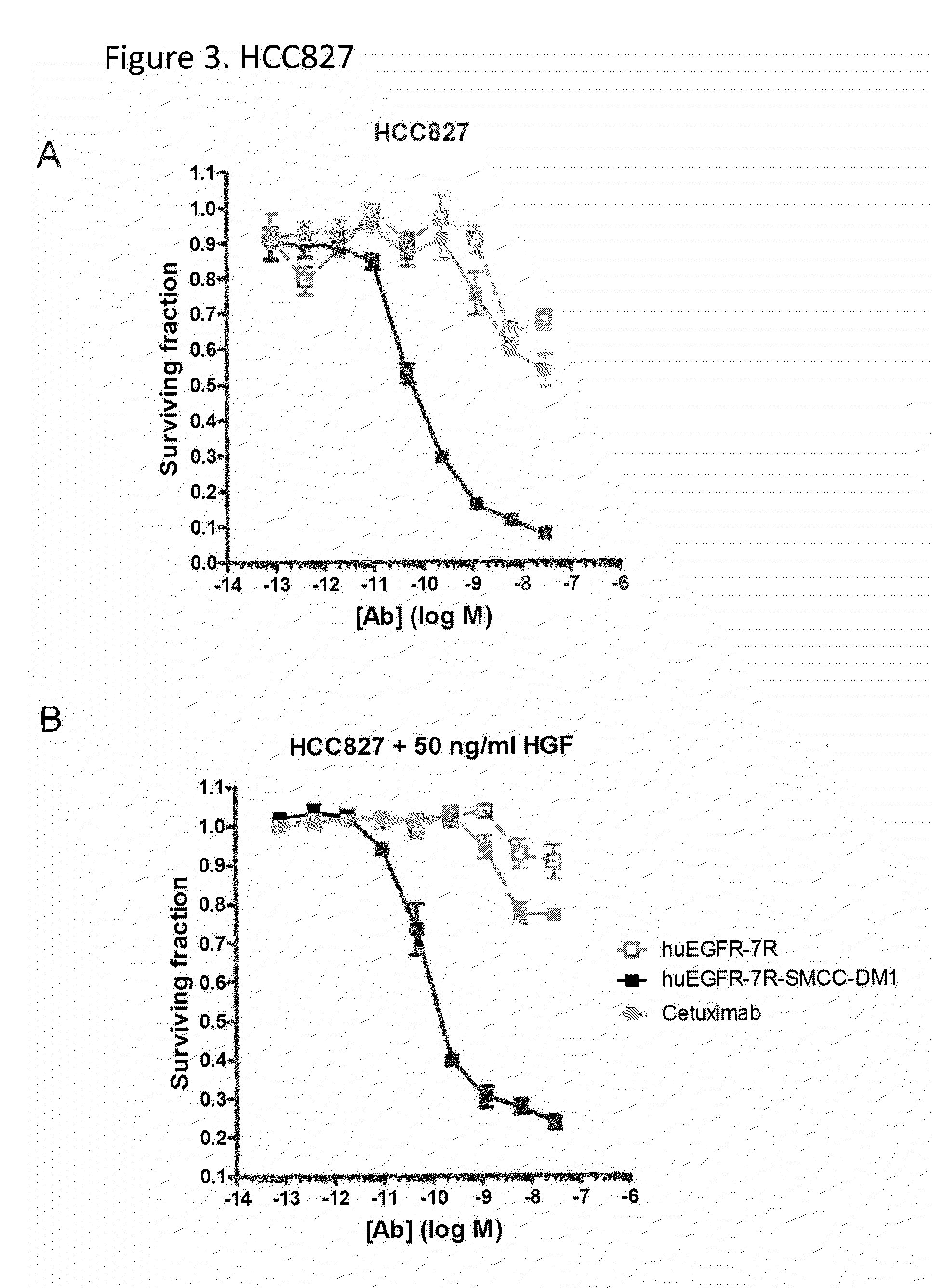
The present invention relates to the identification that EGFR antibody immunoconjugates are effective in inhibiting the growth of tumor cells that have developed EGFR and / or ALK resistance mechanisms. Methods of administering the EGFR antibody immunoconjugates to patients having resistant tumor cells is also disclosed.
Owner:IMMUNOGEN INC
Tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor)
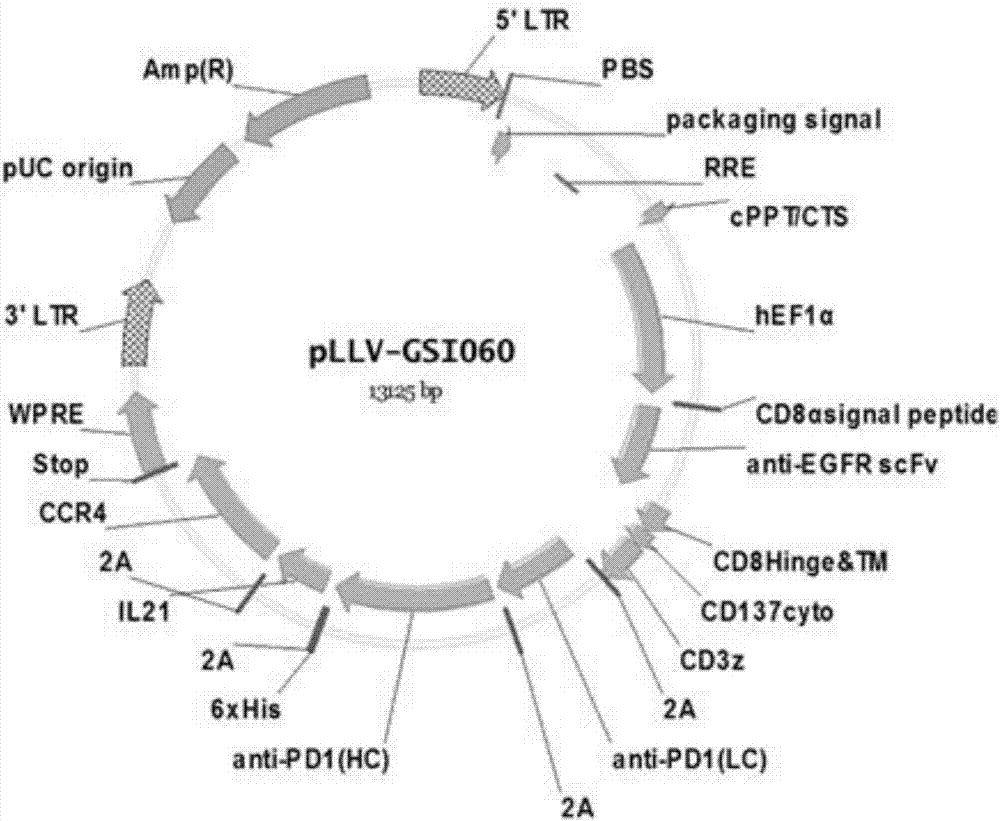
InactiveCN107034235AIncrease lethalityImprove homingGenetically modified cellsMammal material medical ingredientsTumor targetEGFR Antibody
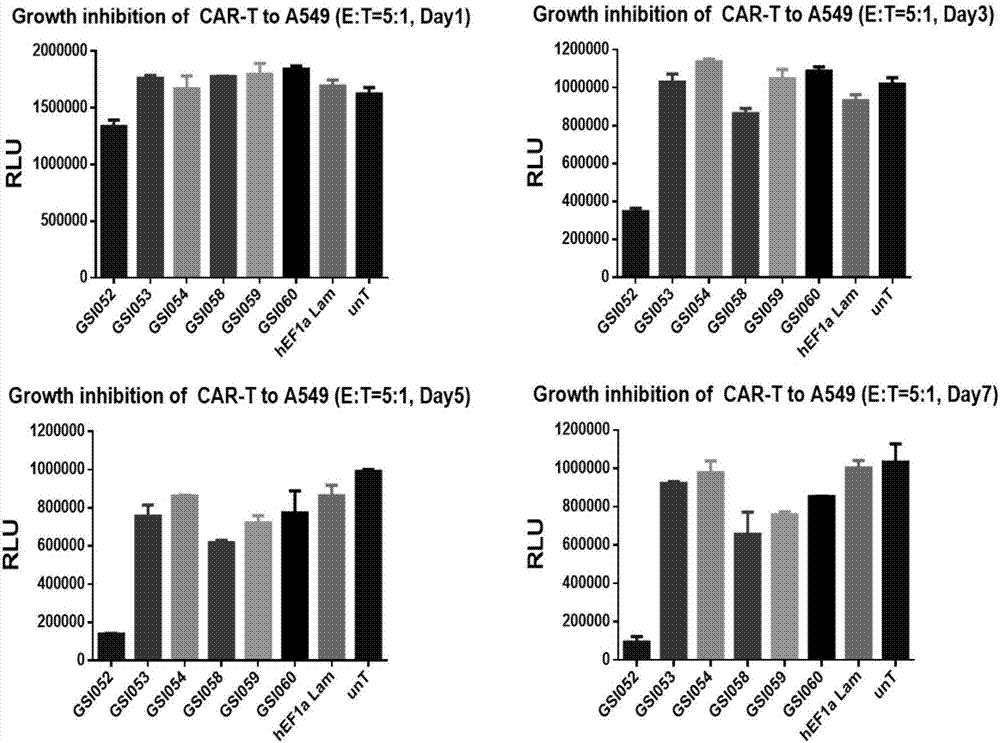
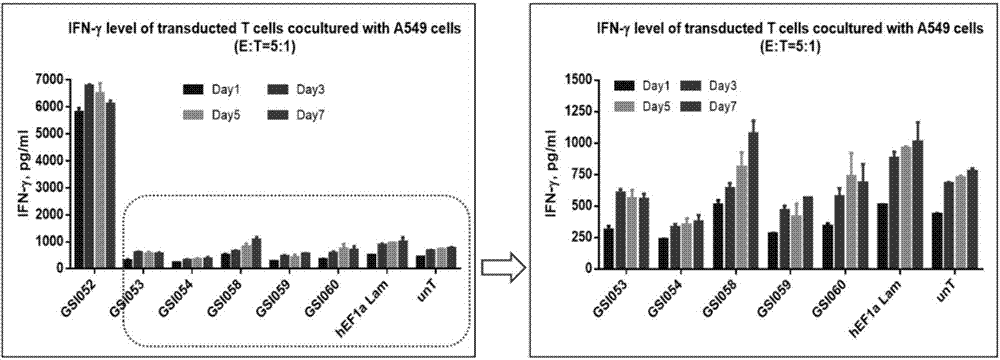
The invention discloses a tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor) and also discloses a plasmid vector for implementing the method. In combination with fourth-generation CAR-T (chimeric antigen receptor T) cells targeting at PD-1 and EGFR, a lentiviral vector is used as a CAR-T vector basic structure, and truncated EGFR antibody is selected as a CAR core to give tumor targeted enrichment to play, tumor-killing effect is given to play in conformity with overexpressed immune checkpoint inhibitor PD-1 monoclonal antibody; by constructing the fourth-generation CAR-T vector for co-expressing various regulatory factors such as IL21, CCR4 and Bcl2, the killing, homing and persistent proliferating abilities of CAR-T cells are improved. EP-CAR T (esophageal papilloma chimeric antigen receptor T) cells are treated by transducing patient's autologous T-lymphocytes in vitro, amplifying suitably and transducing back to the patient's body via autologous transfusion, and no reports on similar designs of CAR-T cells are provided at present.
Owner:尹荣
Targeted adriamycin-loaded magnetic nanoparticles and preparation method and application
InactiveCN106729773AGood target recognition abilityExcellent photothermal killing functionOrganic active ingredientsEnergy modified materialsEGFR AntibodyMagnetite Nanoparticles
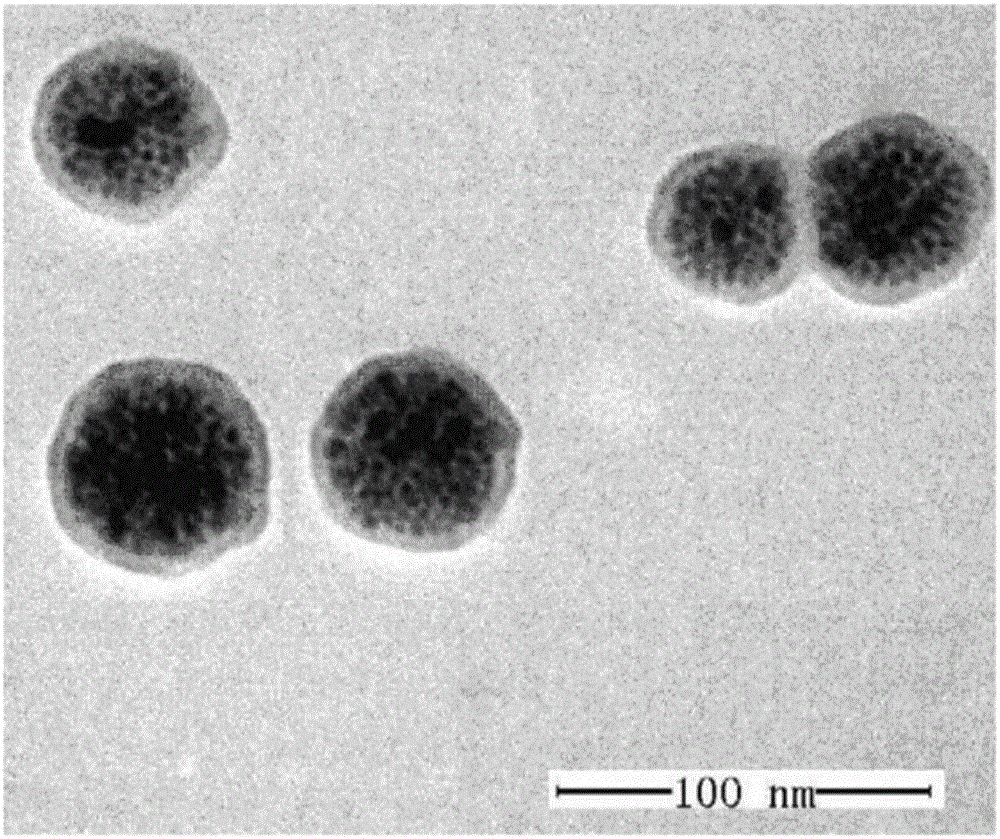
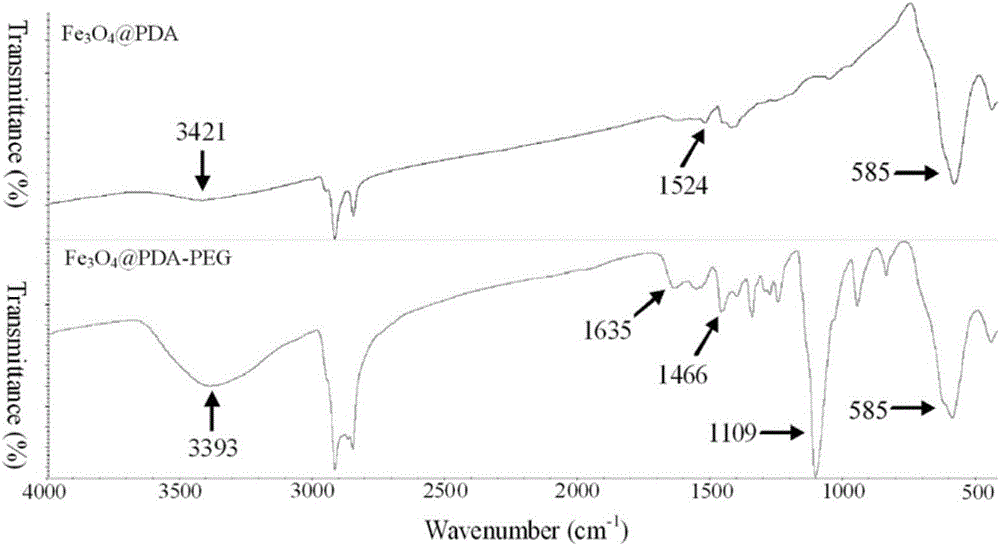
The invention provides a targeted adriamycin-loaded magnetic nanoparticles and a preparation method and application, and belongs to the magnetic nanoparticles and preparation thereof. The targeted adriamycin-loaded magnetic nanoparticles are formed by taking the magnetic Fe3O4 nanoparticles formed by using dopamine to wrap as a core, and an EGFR antibody covalently coupled on the surface of the magnetic Fe3O4 nanoparticles through 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride or N-hydroxysuccinimide, and adriamycin non-covalently coupled to the surface of the nanoparticles. The nanoparticles provided by the invention have good target recognition capacity to the colon cancer cell DLD-1, can effectively enter the internal of the DLD-1 cell, has excellent photo-thermal killing ability on the DLD-1 cell, can be used for the photo-thermal treatment and medicine chemotherapy of the colon cancer cell, and has a good magnetic resonance imaging function.
Owner:JILIN UNIV
Target quaternary ammonium salt cationic polymer lipid gene carrier, preparation method and application thereof
InactiveCN102234658ARich varietyHigh transfection efficiencyGenetic material ingredientsMacromolecular non-active ingredientsPositive controlQuaternary ammonium cation
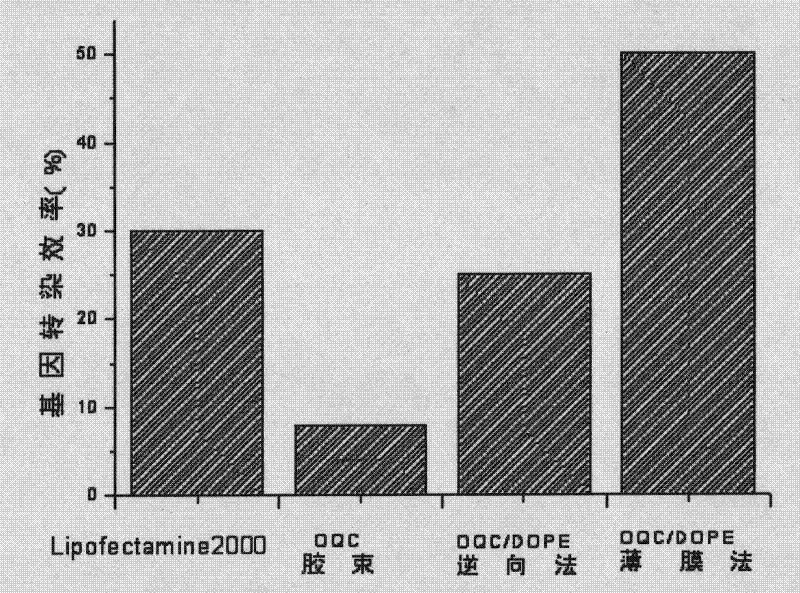
The invention discloses a target quaternary ammonium salt cationic polymer lipid gene carrier, a preparation method and an application thereof. The target quaternary ammonium salt cationic polymer lipid genetic carrier is characterized in that: a polymeric quaternary ammonium salt and lipid are adopted for preparing a quaternary ammonium salt cationic polymer lipid genetic carrier according to a mass ratio, wherein the mass ratio of the polymeric quaternary ammonium salt to the lipid is 0.05-20:1; then a assembly method or a modification method is adopted for modifying to prepare a folic acid or EGFR antibody modified cationic polymer lipid gene carrier. Results of gene transfection experiments show that: gene transfection efficiencies of the target quaternary ammonium salt cationic polymer lipid gene carrier in 293T cells and NIH-3T3 cells are the same as the gene transfection efficiencies of positive control lipofectamine of lipofectamine<TM>2000 in the 293T cells and the NIH-3T3 cells; the gene transfection efficiencies of the EGFR antibody modified cationic polymer lipid genetic carrier in liver cancer Huh-7 cells and breast cancer MCF-7 cells are higher than the gene transfection efficiencies of the lipofectamine<TM>2000 in the liver cancer Huh-7 cells and the breast cancer MCF-7 cells. The cationic polymer lipid genetic carrier system provided by the present invention has good biocompatibility and low cytotoxicity, and can be as an excellent non-viral gene delivery carrier.
Owner:SHANGHAI INST OF ONCOLOGY
Paclitaxel immune nano liposome and preparation method and application thereof
InactiveCN102379848AOrganic active ingredientsMacromolecular non-active ingredientsCancer cellMedicine
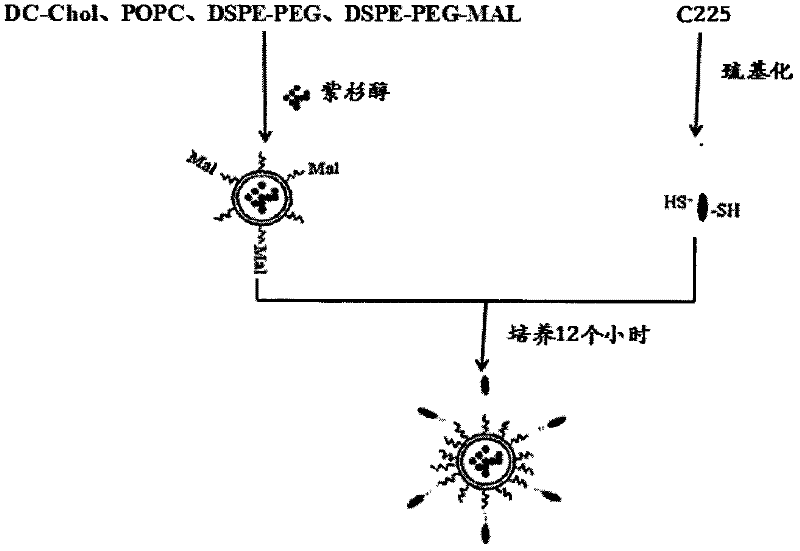
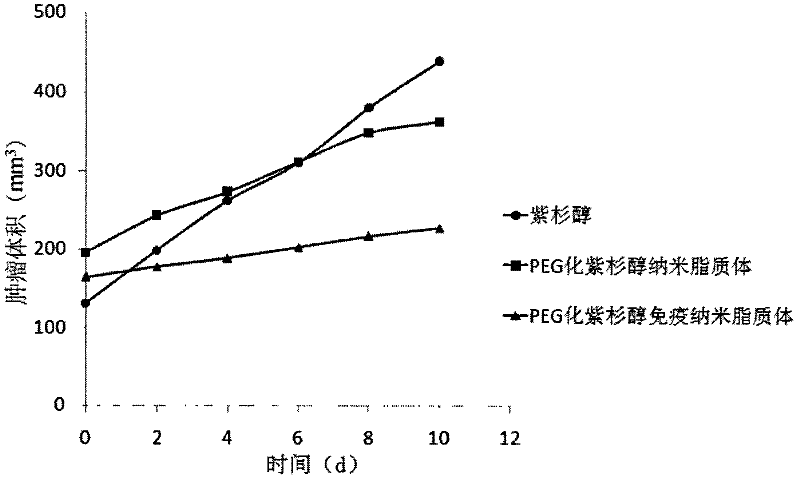
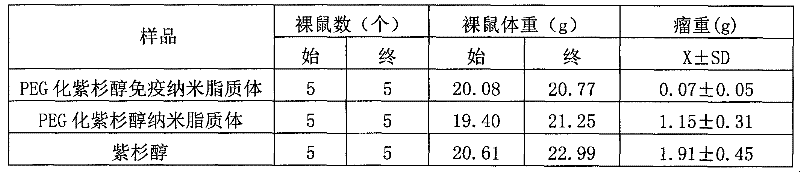
The invention belongs to the field of biomedicine, and particularly discloses a PEG (polyethylene glycol) gylation paclitaxel immune nano liposome and a preparation method and application thereof. Paclitaxel is wrapped in the liposome, and the outside of the liposome is connected with an anti-EGFR (epidermal growth factor receptor) antibody. The paclitaxel immune nano liposome wrapped with the paclitaxel inside and externally connected with the anti-EGFR antibody has the remarkable advantages of fine biological target property and remarkable cancer cell suppressing effect, and can be used for treating EGFR positive tumors.
Owner:TIANJIN GOALGEN BIOTECH
Nucleotide sequence, method and kit for detecting exons 12, 13 mutation of human K-ras gene
ActiveCN102010894AMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesFluorescence
The invention relates to a nucleotide sequence, a method and a kit for detecting exons 12, 13 mutation of a human K-ras gene. The nucleotide sequence for detecting the exons 12, 13 mutation of the human K-ras gene comprises any one nucleotide sequence shown from SEQ ID NO:1 to SEQ ID NO:10, wherein two ends of the nucleotide sequence shown in the SEQ ID NO:3 are labeled with JUP (Junction Plakoglobin) fluorescence groups, and two ends of the nucleotide sequences shown in the SEQ ID NO:4 to the SEQ ID NO:10 are labeled with MAR fluorescence groups. The kit of the invention can be used for instructing the clinical medication of Erbitux and other EGFR (Epithelial Growth Factor Receptor) antibody medicines.
Owner:上海源奇生物医药科技有限公司 +1
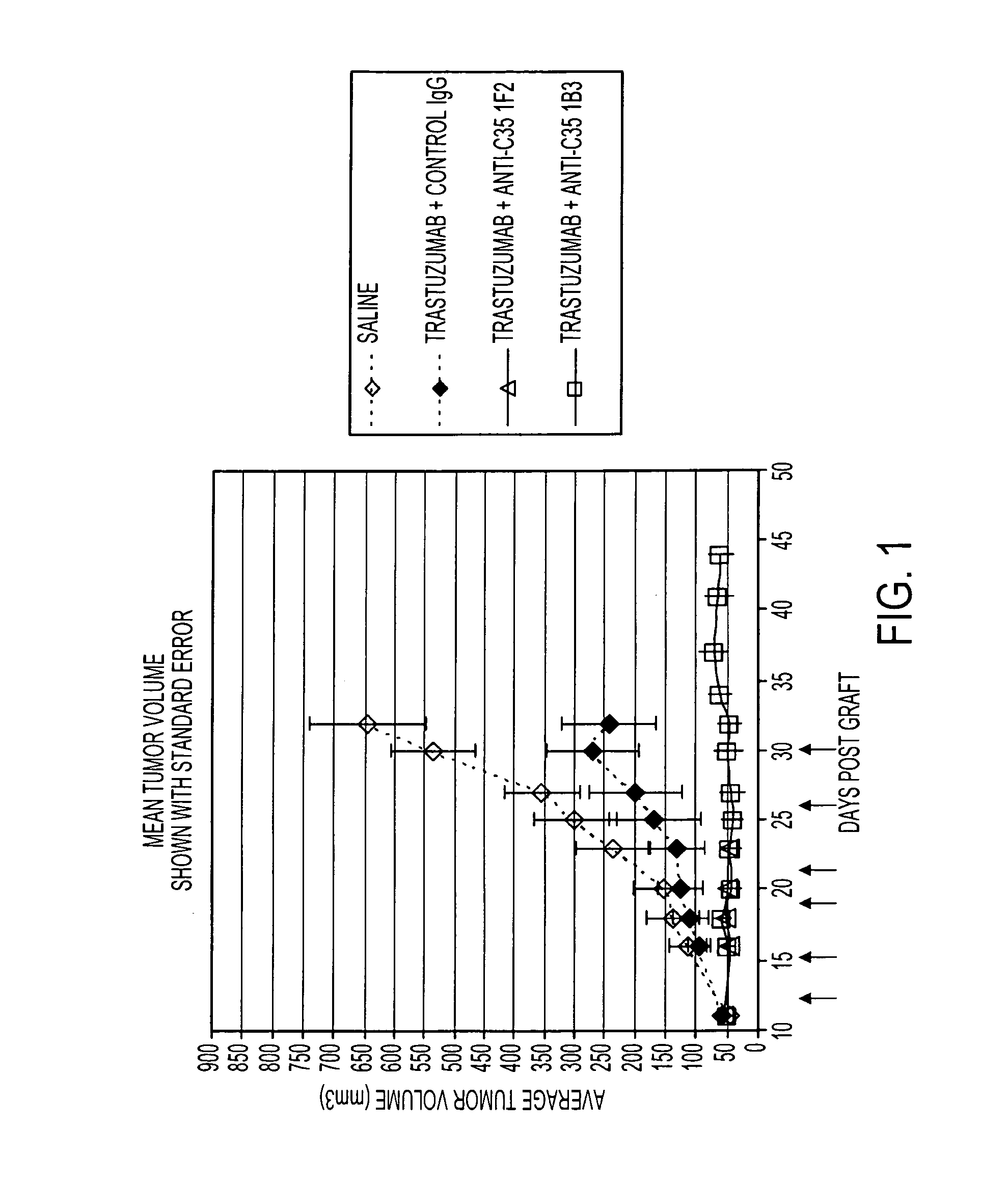
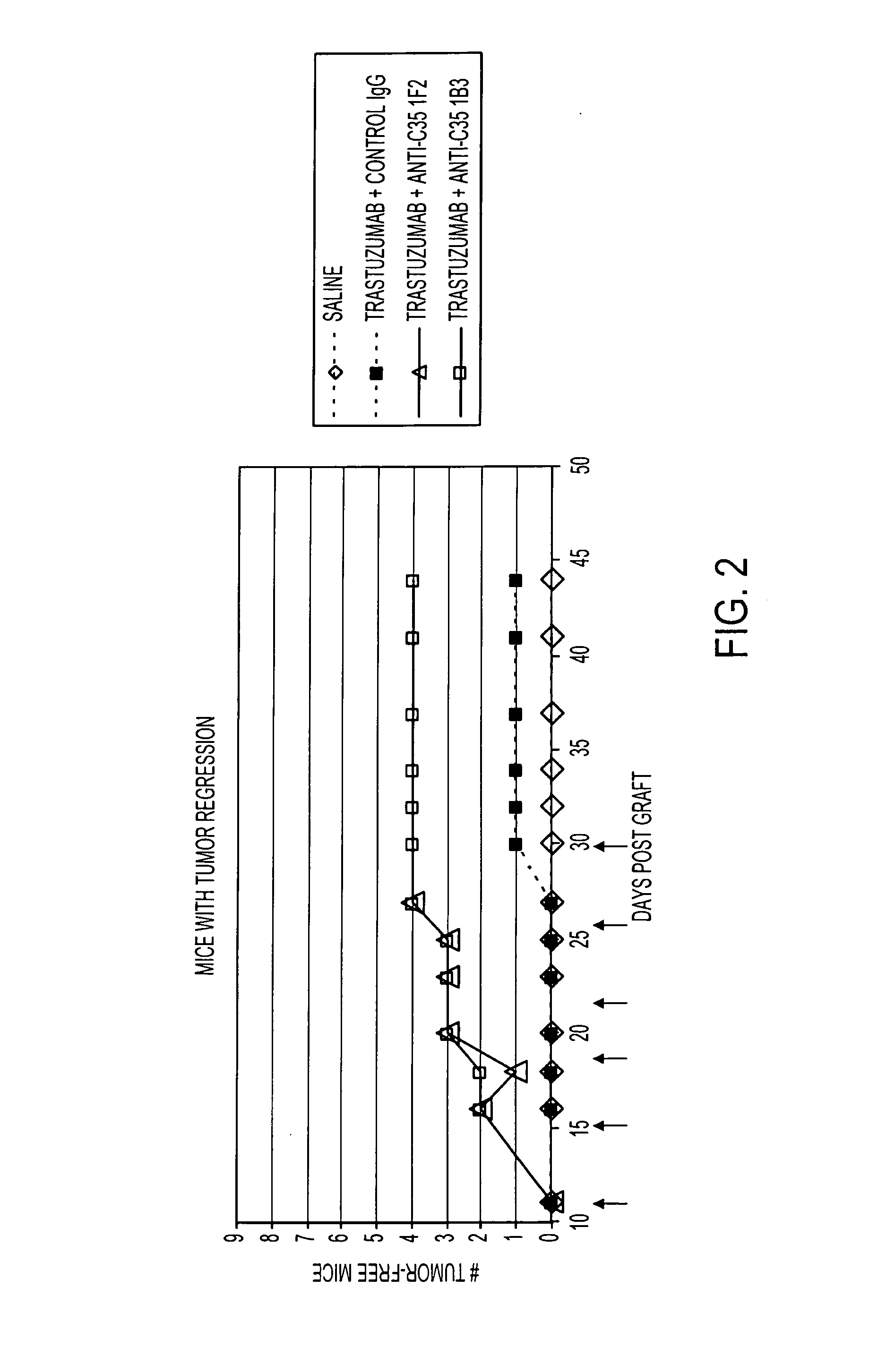
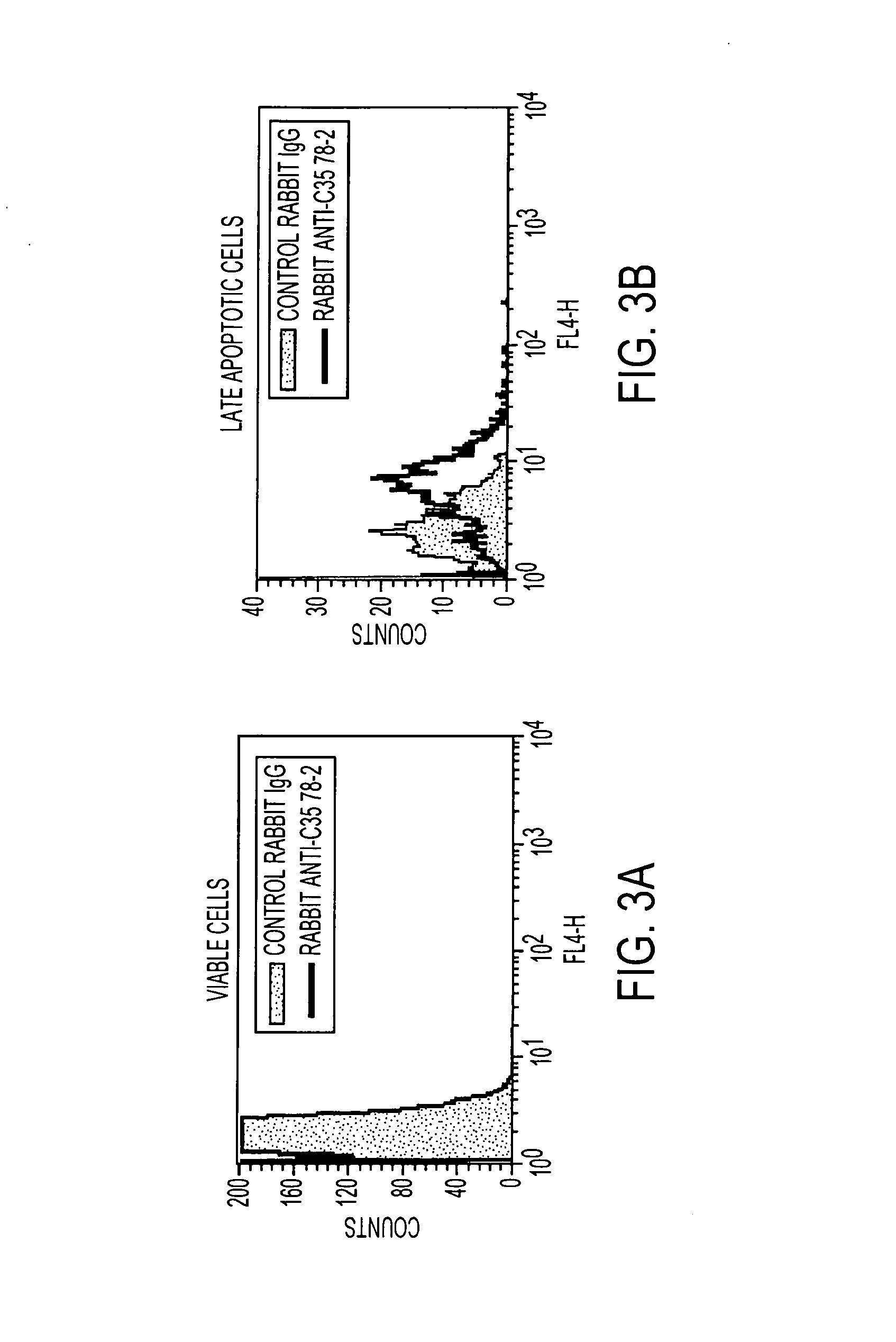
Anti-C35 antibody combination therapies and methods
ActiveUS8637026B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCancer cellEGFR Antibody
The present invention is directed to methods of killing cancer cells, the methods comprising administering at least one C35 antibody and either at least one HER2 or at least one EGFR antibody. In some embodiments, the antibodies are administered with a therapeutic agent. The present invention is further directed to C35, HER2 and EGFR antibodies useful in these methods.
Owner:VACCINEX
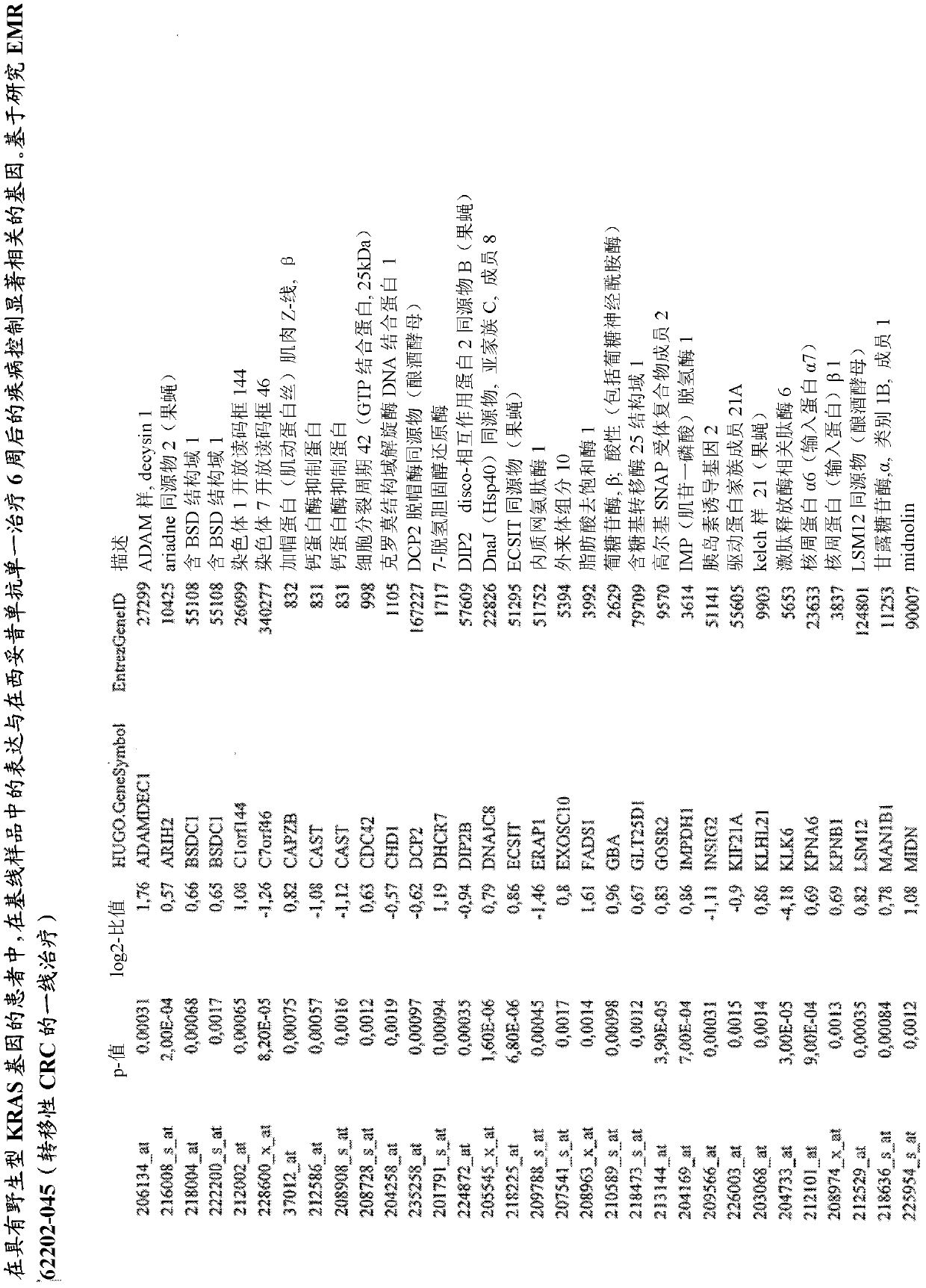
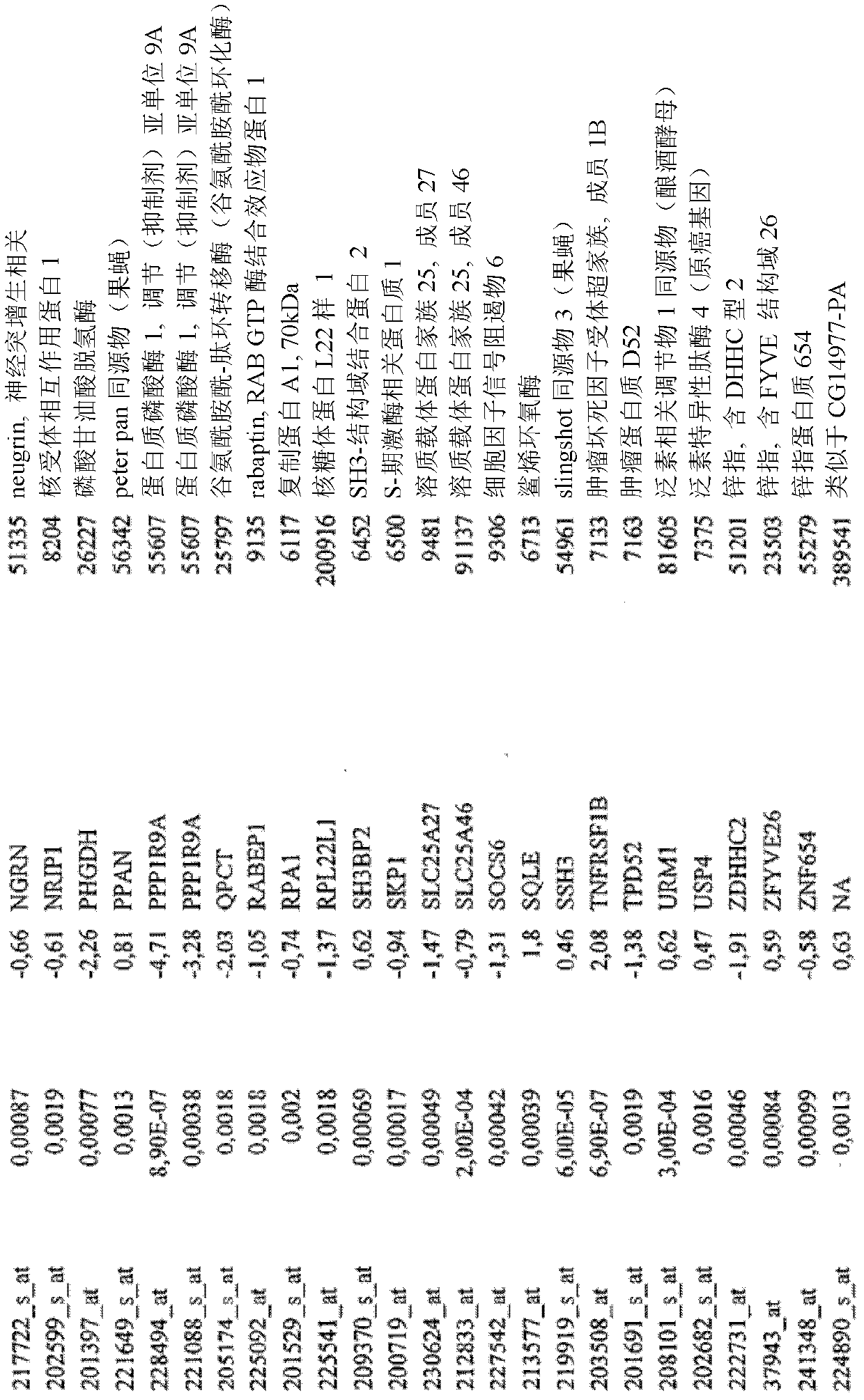
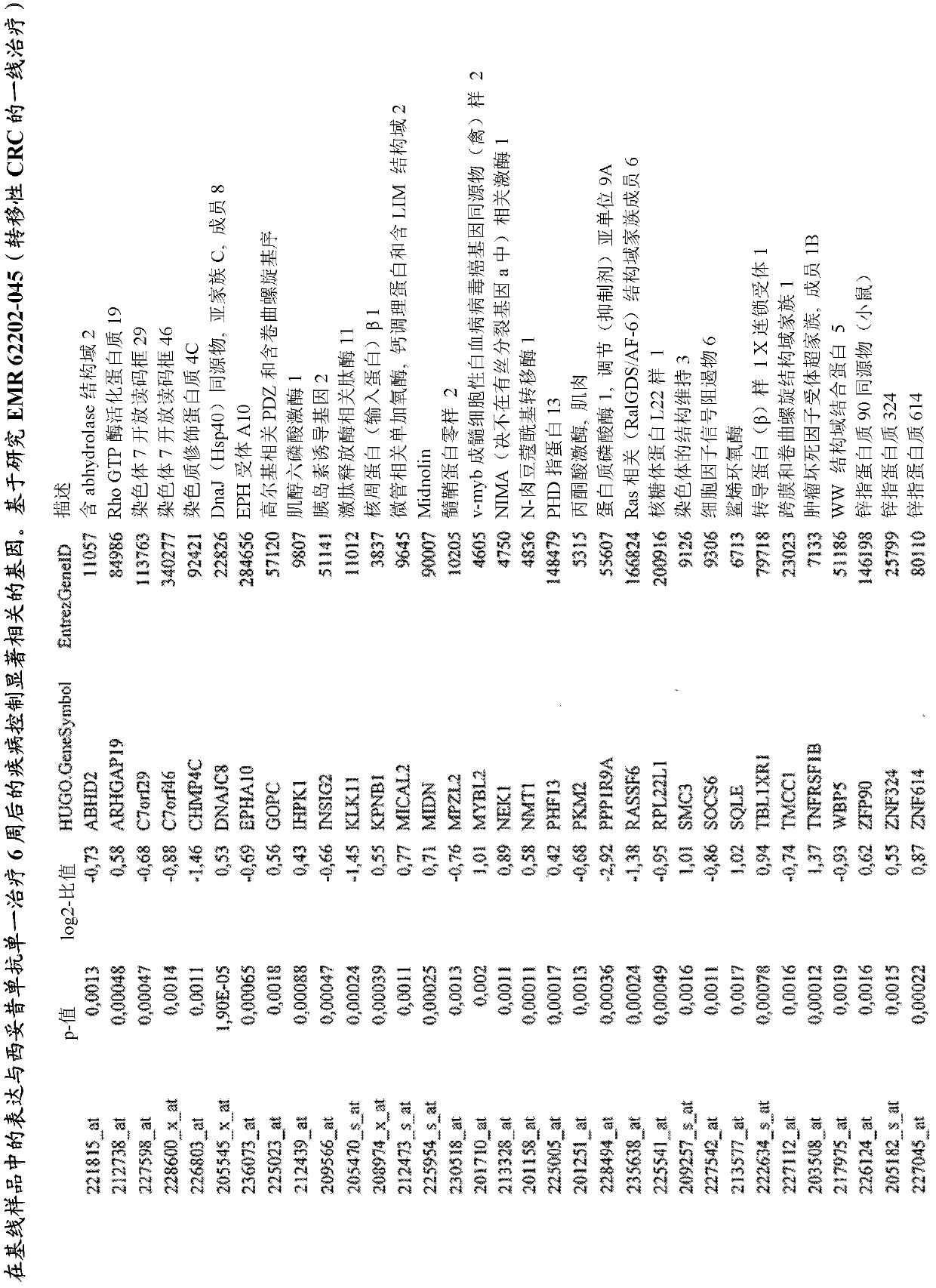
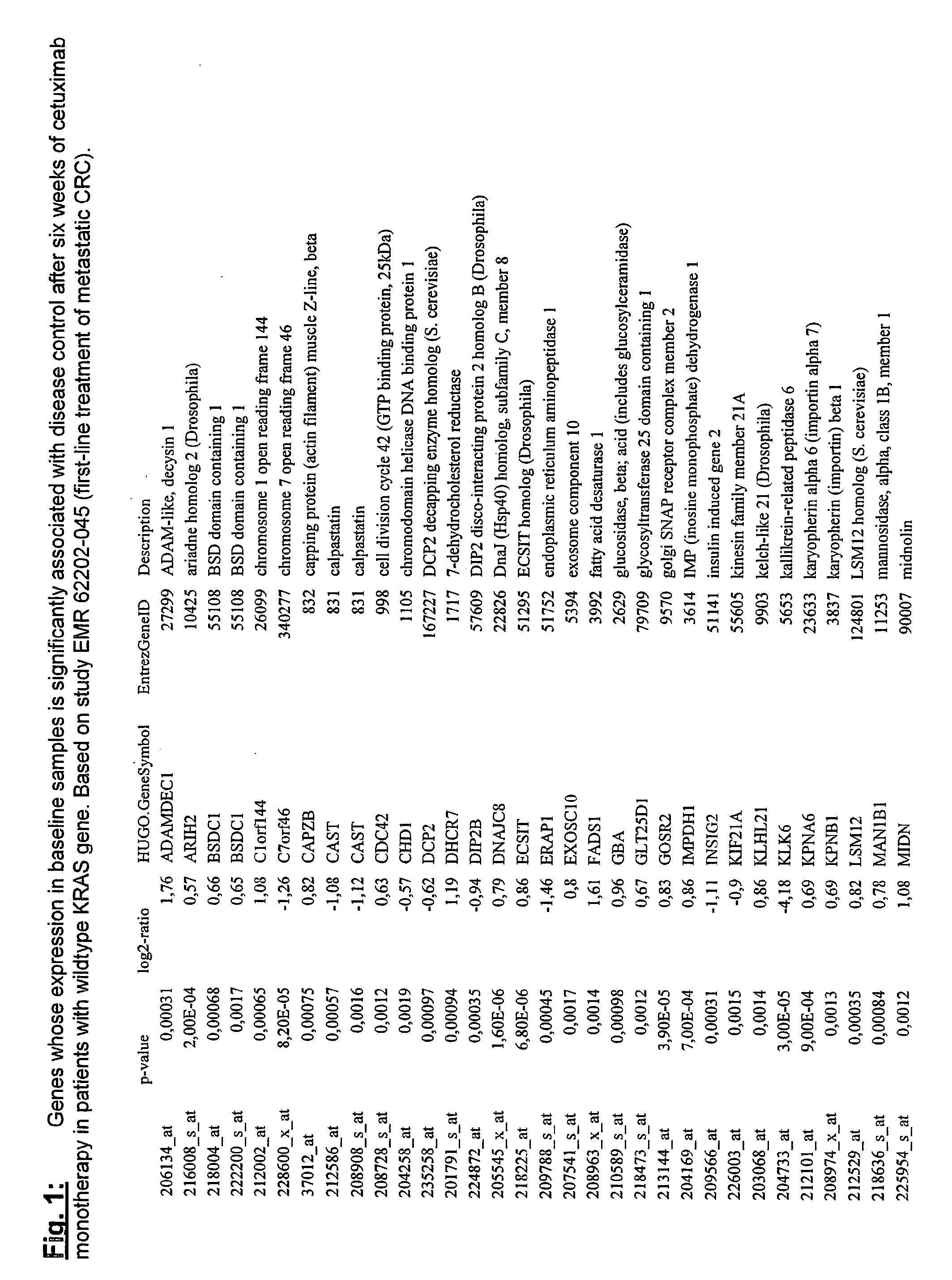
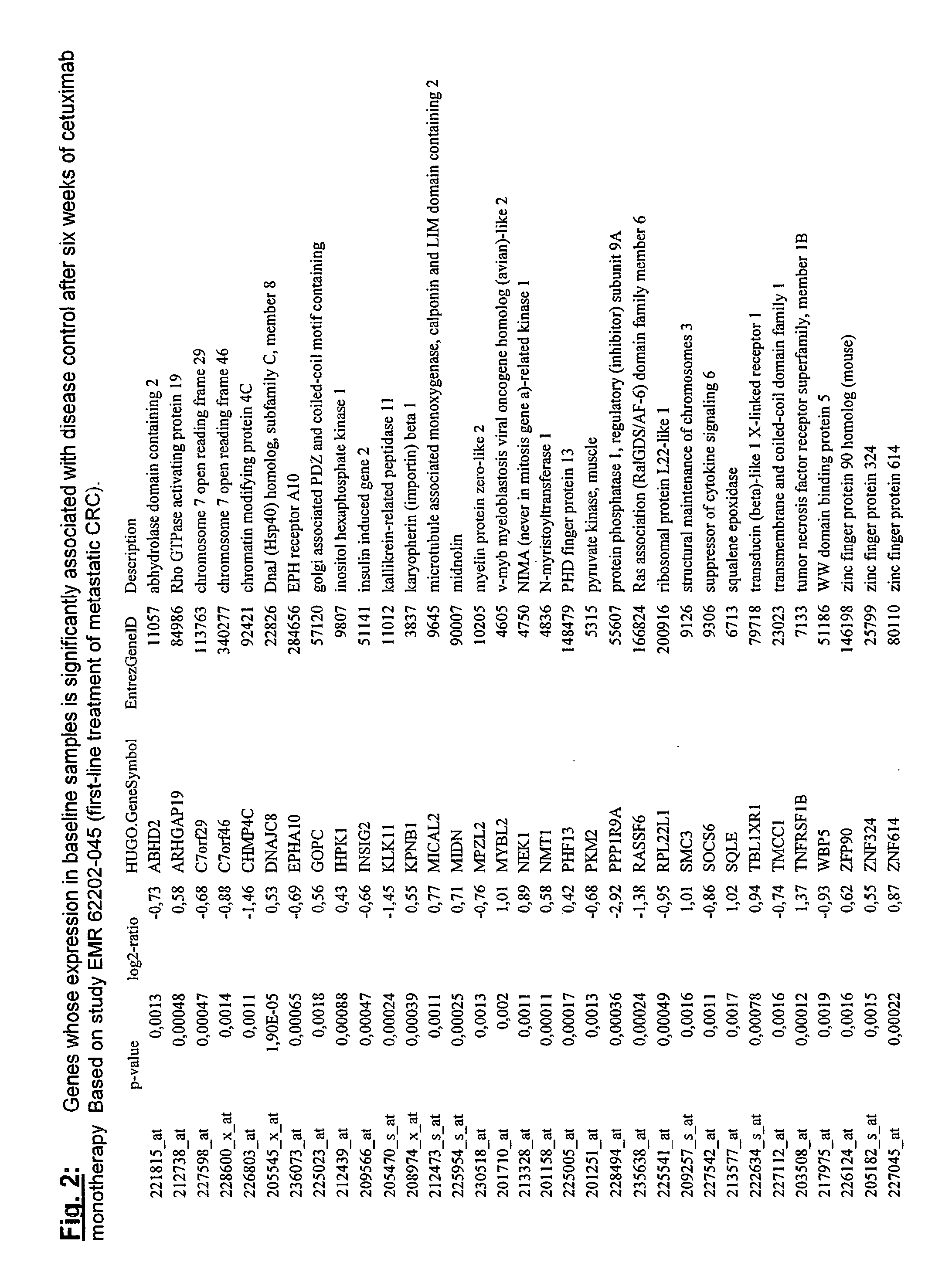
Biomarkers and methods for determining efficacy of anti-egfr antibodies in cancer therapy
The invention relates to biomarkers based on gene expression products and methods for determining the efficacy of anti-EGFR antibodies in the treatment of EGFR expressing cancer. The invention is further related to the prediction of sensitivity or resistance of a patient suffering from EGFR expressing cancer to the treatment of said patient with a specific anti- EGFR antibody. The invention is preferably related to the identification of respective biomarkers that allow a better prediction of the clinical outcome of the treatment with anti- EGFR antibodies in patients with KRAS wild-type tumors. In this context, the invention especially relates to anti-EFGR antibody c225 / cetuximab (Erbitux TM ) and its use in patients suffering from colorectal Cancer (CRC).
Owner:MERCK PATENT GMBH
Anti-cancer treatments with Anti-egfr antibodies having a low fucosylation
InactiveUS20160068609A1Good curative effectEffective treatmentAntibody ingredientsPharmaceutical non-active ingredientsFucosylationSide effect
The present invention pertains to the field of cancer therapy using anti-cancer antibodies. The medical use of anti-EGFR antibodies having improved glycosylation characteristics, in particular a reduced fucosylation, is provided which show anti-cancer efficacy and an improved adverse side effect profile.
Owner:GLYCOTOPE GMBH
Novel anti-epidermal growth factor receptor antibody, preparation method and application thereof
InactiveCN104910275AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsBinding siteEGFR Antibody
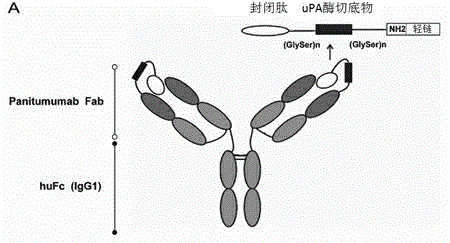
The invention discloses a novel anti-epidermal growth factor receptor EGFR antibody, a preparation method and application thereof. The novel whole human source anti-EGFR monoclonal antibody Pan-P provided by the invention is IgG1 type antibody, and has ADCC effect. Also, the antigen-binding site of the antibody provided by the invention contains a section of blocking peptide with tumor-specific protease cleavage site. The antibody is an enzyme-mediated specific binding antibody, has specific binding effect to tumors, and can reduce skin toxicity caused by general anti-EGFR antibodies.
Owner:TAIZHOU MABTECH PHARM CO LTD
HER2, EGFR, EpCAM and MUC1 multiple antibody immunomagnetic bead and preparation method thereof
ActiveCN106366197AAvoid damageStrong specificityInorganic material magnetismCarrier-bound/immobilised peptidesMedicineMicrosphere
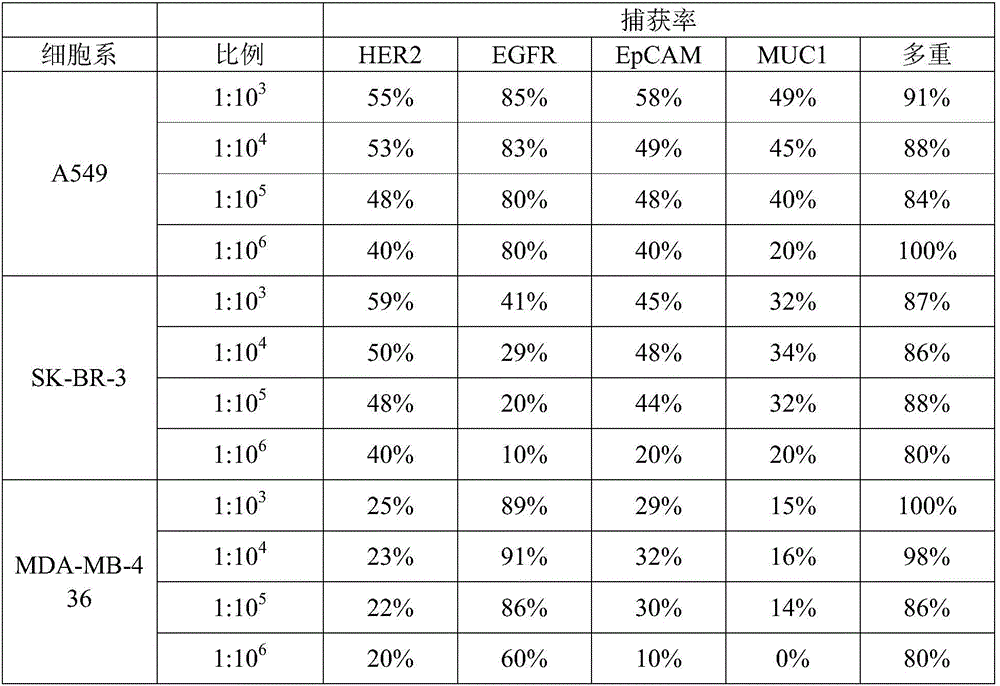
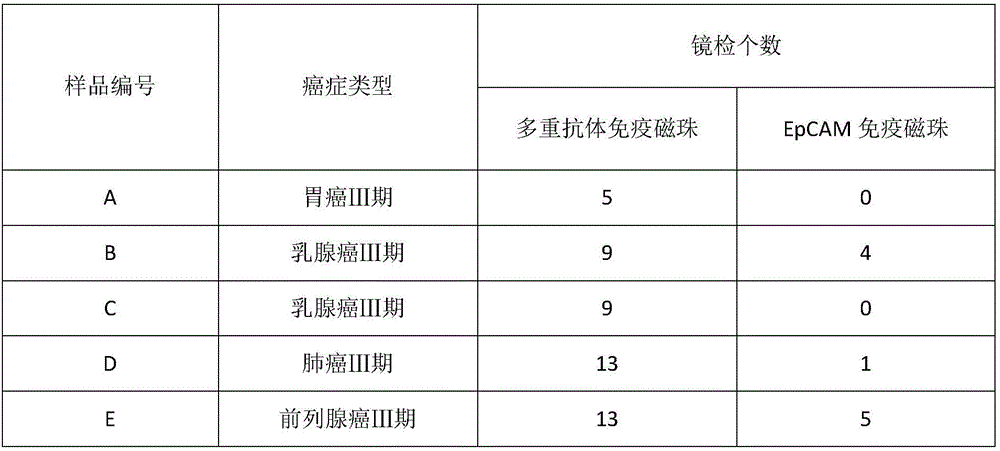
The invention provides a HER2, EGFR, EpCAM and MUC1 multiple antibody immunomagnetic bead and a preparation method thereof. The multiple antibody immunomagnetic bead includes a HER2 immunomagnetic bead, an EGFR immunomagnetic bead and an EpCAM immunomagnetic bead, and a HER2 antibody, an EGFR antibody and an EpCAM antibody are respectively coupled with magnetic microspheres to obtain the HER2 immunomagnetic bead, the EGFR immunomagnetic bead and the EpCAM immunomagnetic bead. The multiple antibody immunomagnetic bead is good in specificity and sensitivity, rapid in magnetic response, short in concentration time and high in capturing efficiency when being used for capturing CTCs (circulating tumor cells). The immunomagnetic bead is stable in nature, small in particle size, good in magnetic response and dispersibility, simple in preparation method and high in practicability.
Owner:SHANGHAI MAJORBIO BIO PHARM TECH
Method of producing an antibody to epidermal growth factor receptor
InactiveUS20060154334A1Animal cellsSugar derivativesEGFR AntibodyEpidermal Growth Factor Receptor Antibody
The present invention is directed to a method of producing an antibody to Epidermal Growth Factor Receptor (EGFR). The method includes producing transformed cells that express EGFR antibodies, culturing the transformed cells, harvesting the transformed cells to collect the EGFR antibodies, and purifying the EGFR antibodies.
Owner:IMCLONE SYSTEMS
EGR-EGFR complex
InactiveUS7514240B2Efficient reasonable productionProductionPeptide/protein ingredientsAnalogue computers for chemical processesEpitopeAntiendomysial antibodies
Owner:JAPAN SCI & TECH CORP +2
Fusion protein based on anti-EGFR single-chain antibody and arginine nonamer, and applications of fusion protein
ActiveCN103910799AReduce sizeGuaranteed targetingImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSingle-Chain AntibodiesArginine
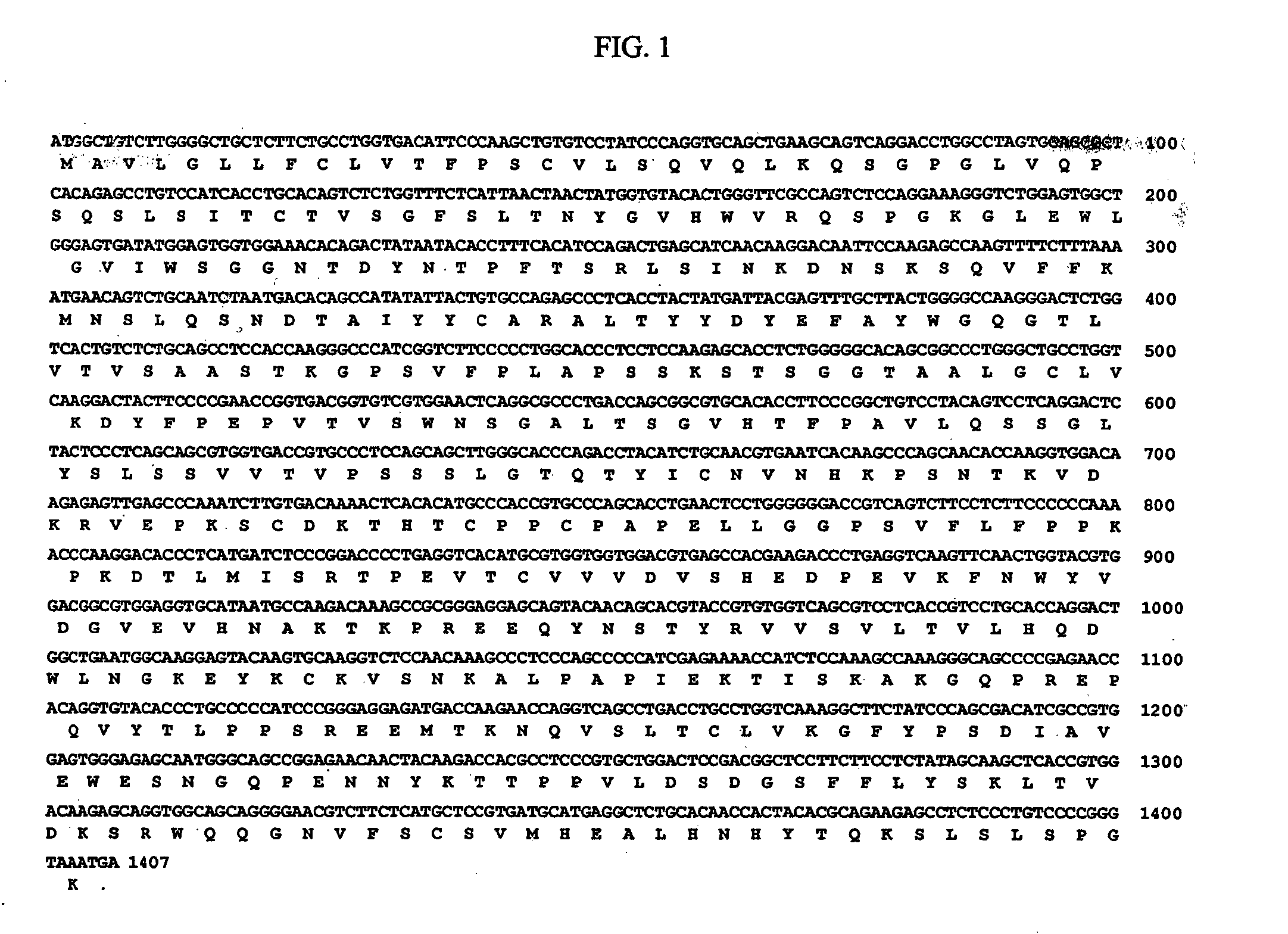
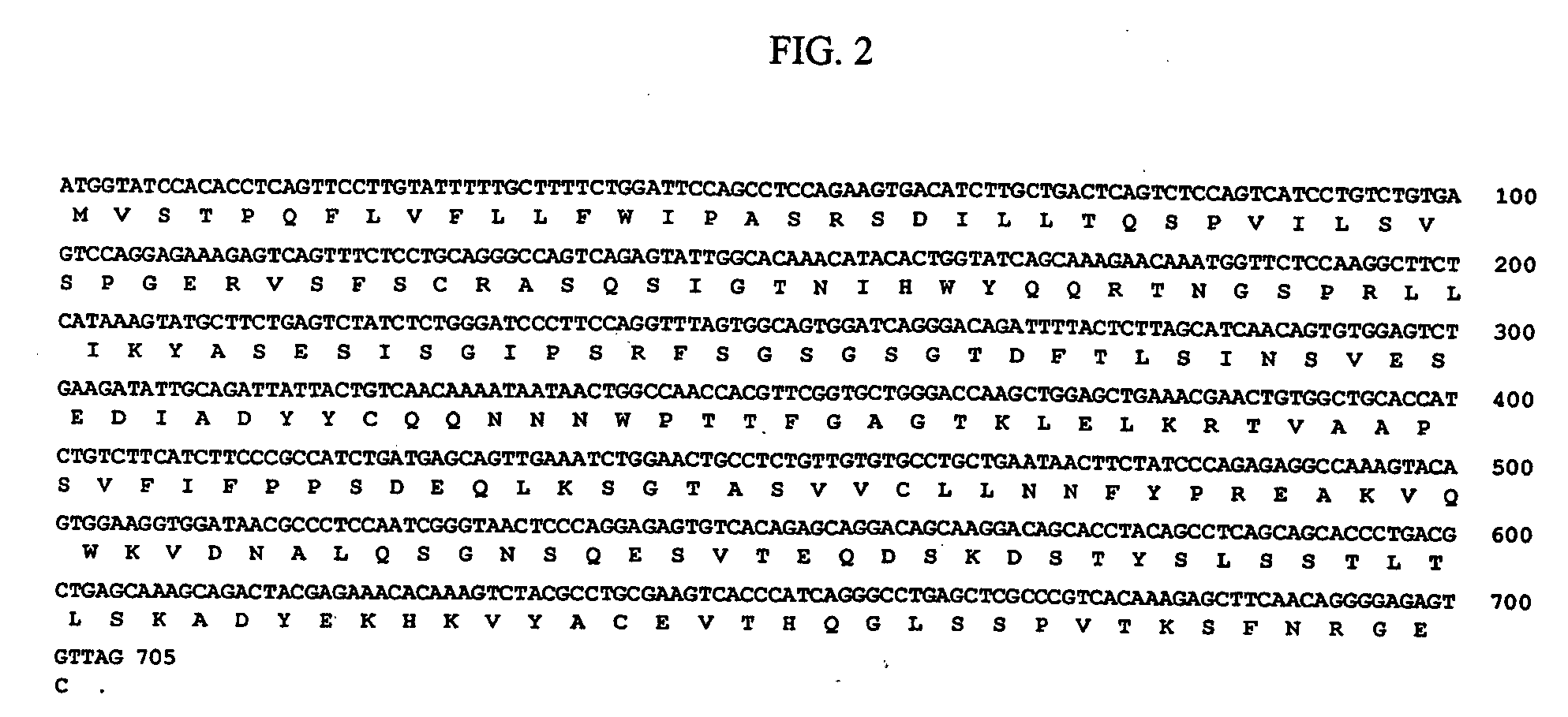
The invention discloses a fusion protein based on anti-EGFR single-chain antibody and arginine nonamer, and applications of the fusion protein. The fusion protein comprises a heavy chain variable region and a light chain variable region which are connected via connection peptides; the terminal of the light chain variable region is connected with the arginine nonamer; and amino acid sequence of the arginine nonamer is represented by SEQ ID No.3. According to the fusion protein, the heavy chain variable region and the light chain variable region of the anti-EGFR single-chain antibody are connected via the selected connection peptides, so that specificity and affinity of combination of the antibody with EGFR are maintained, and targeting performance of the constructed fusion protein on EGFR is ensured; the size of the fusion protein is reduced, so that on the one hand construction, expression and purification of the fusion protein are convenient, and cost is reduced, and on the other hand, combination of the fusion protein on EGFR is ensured, demands on molecular size and antibody activity during cellular internalization are ensured, and feasibility and accuracy of the combination of the fusion protein on EGFR and targeting tumor cellular internalization are ensured.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Humanized EGFR antibodies
InactiveCN103025760AEliminate side effectsHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsEGFR AntibodyAntigen binding
The present invention pertains to humanized anti-EGFR antibodies having antigen binding properties similar to those of the murine or chimeric anti-EGFR antibody from which they are derived. In particular, the present invention is directed to humanized anti-EGFR antibodies which are useful in the treatment of cancer.
Owner:GLYCOTOPE GMBH
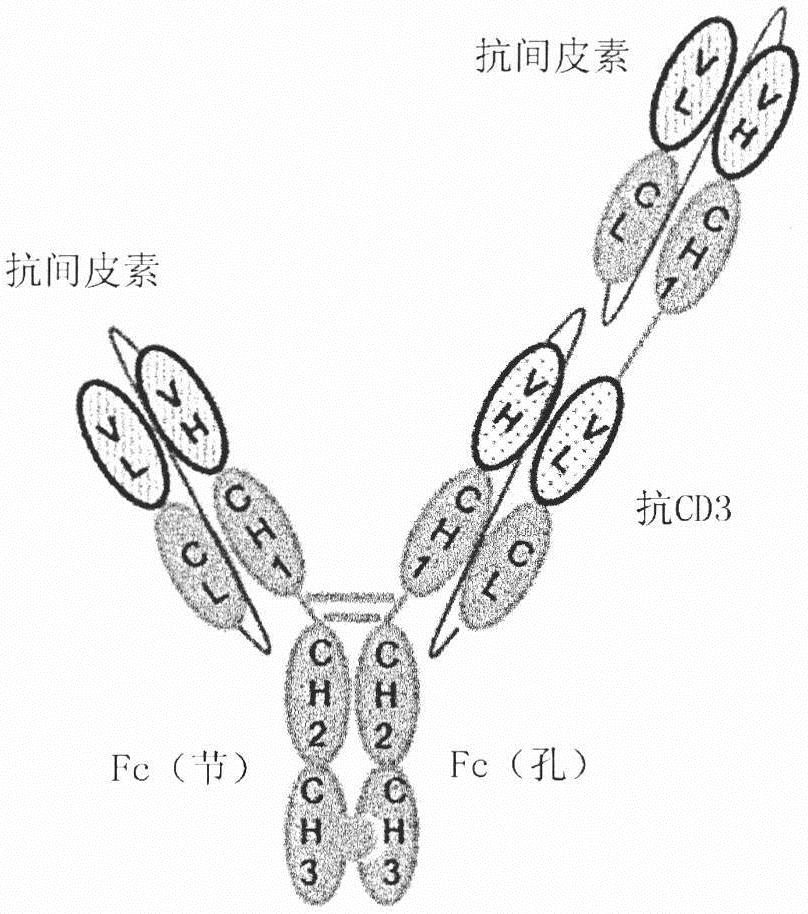
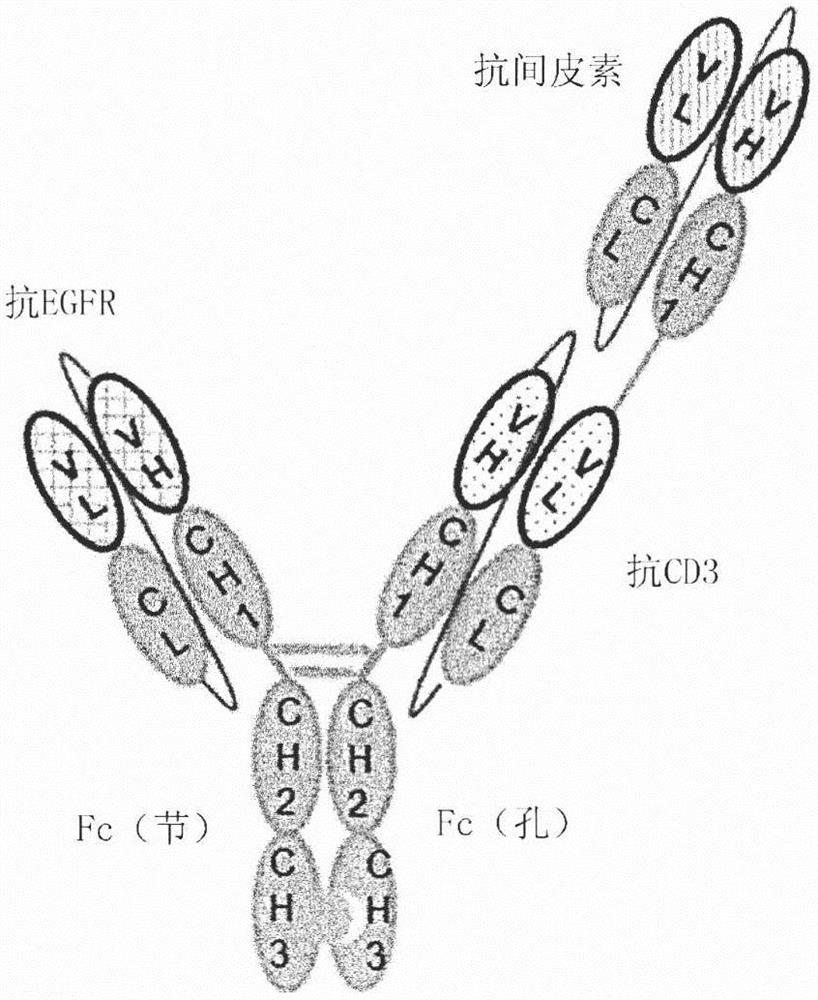
Fusion protein comprising Anti-mesothelin antibody, Anti-cd3 antibody or Anti-egfr antibody, bispecific or trispecific antibody comprising same, and uses thereof
PendingCN113874396AHigh yieldHigh purityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAnti-Mesothelin AntibodyAntiendomysial antibodies
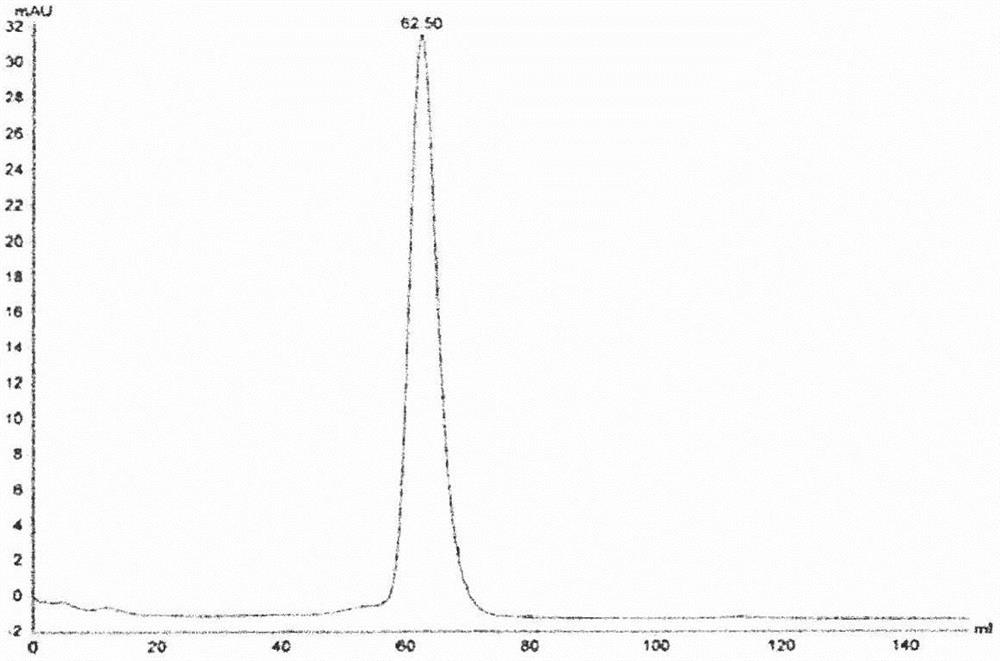
The present invention relates to: a fusion protein comprising a fragment of an anti-mesothelin antibody, an anti-CD3 antibody or an anti-EGFR antibody; a bispecific antibody that is specific to mesothelin and CD3; a trispecific antibody that is specific to mesothelin, CD3 and EGFR; and uses thereof. The bispecific or trispecific antibody according to the present invention can be prepared in a high yield and with high purity, and has excellent tumor killing and growth inhibitory effects, and thus can be effectively used in cancer treatment.
Owner:GREEN CROSS CORP THE +1
Biomarkers and methods for determining efficacy of Anti-egfr antibodies in cancer therapy
The invention relates to biomarkers based on gene expression products and methods for determining the efficacy of anti-EGFR antibodies in the treatment of EGFR expressing cancer. The invention is further related to the prediction of sensitivity or resistance of a patient suffering from EGFR expressing cancer to the treatment of said patient with a specific anti-EGFR antibody. The invention is preferably related to the identification of respective biomarkers that allow a better prediction of the clinical outcome of the treatment with anti-EGFR antibodies in patients with KRAS wild-type tumors. In this context, the invention especially relates to anti-EFGR antibody c225 / cetuximab (Erbitux®) and its use in patients suffering from colorectal Cancer (CRC).
Owner:MERCK PATENT GMBH
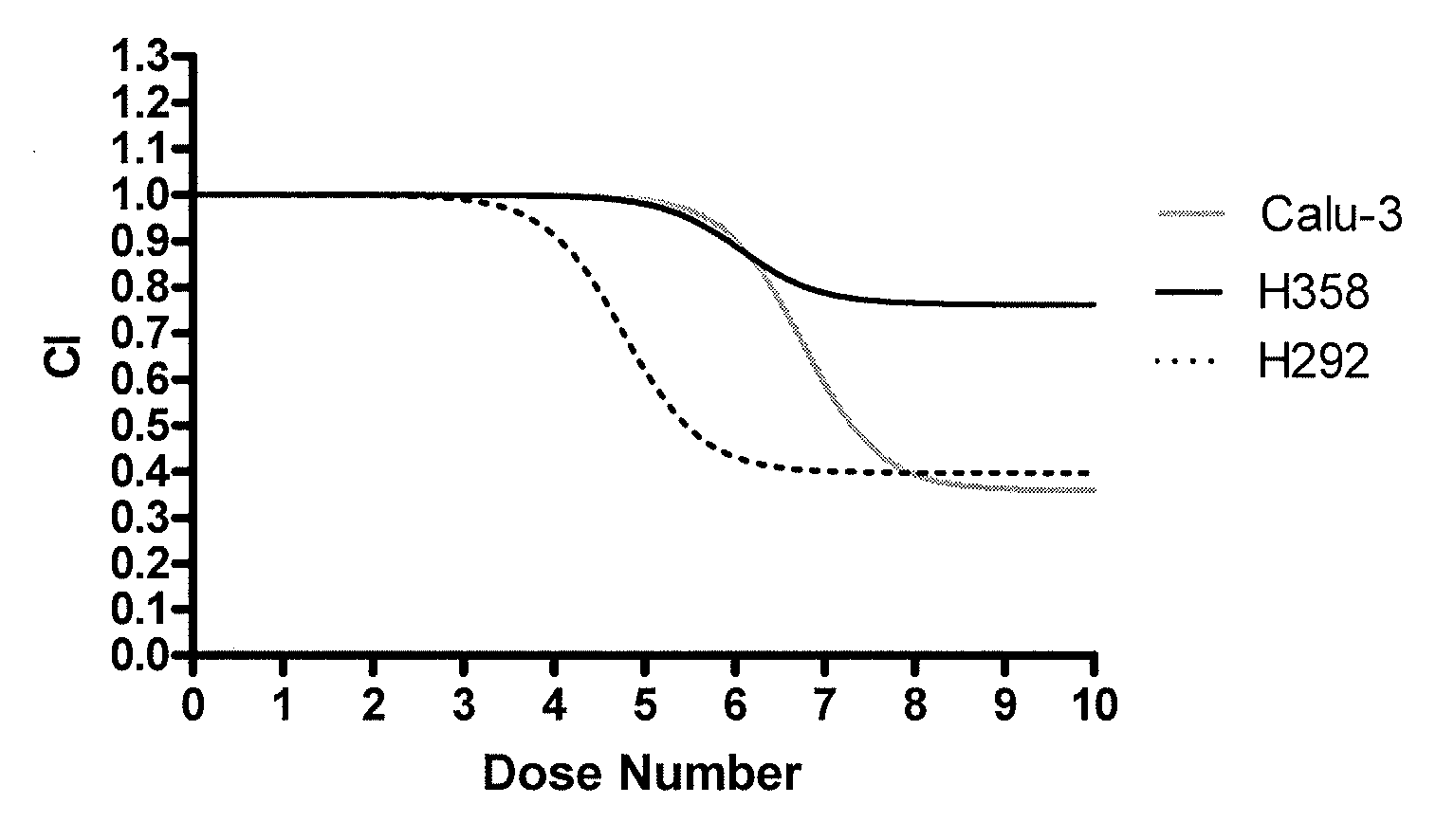
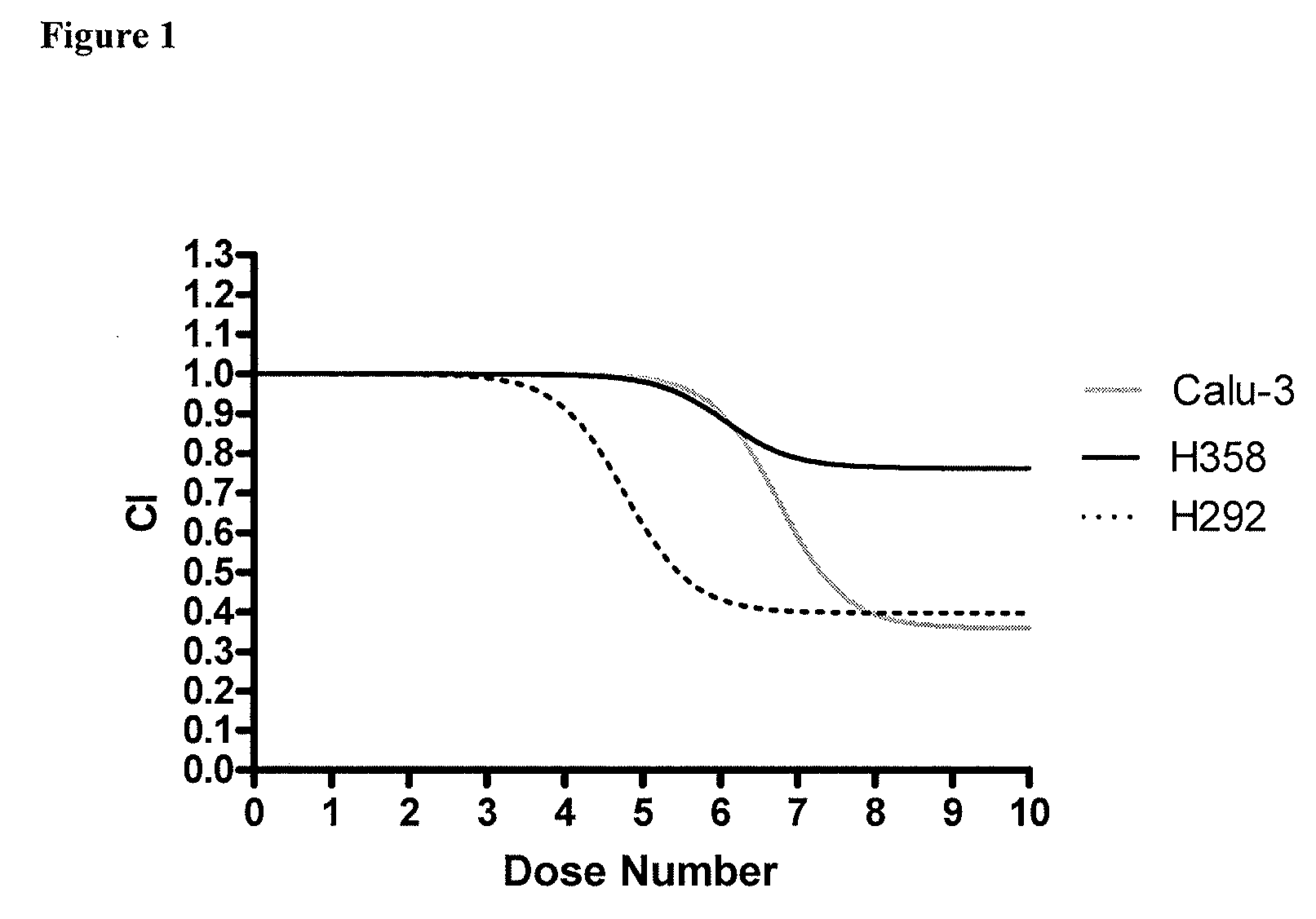
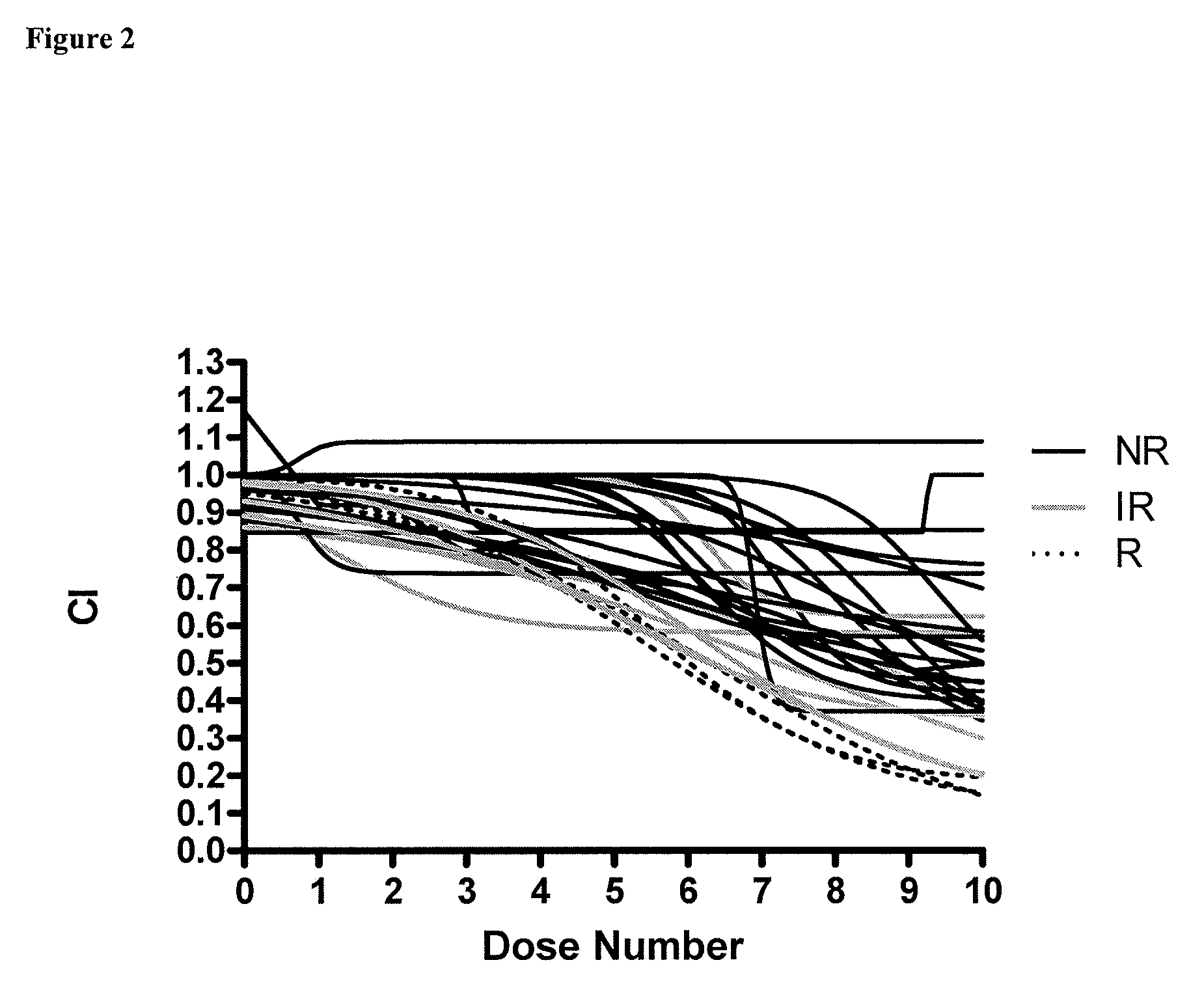
Methods for predicting a patient's response to EGFR inhibitors
The present invention provides methods for individualizing chemotherapy for cancer treatment, and particularly for evaluating a patient's responsiveness to one or more epidermal growth factor receptor (EGFR) inhibitors prior to treatment with such agents. Particularly, the invention provides an in vitro chemoresponse assay for predicting a patient's response to a monoclonal EGFR antibody, such as cetuximab. The method generally comprises culturing malignant cells from a patient's specimen (e.g., biopsy specimen), contacting the cultured cells with a monoclonal EGFR antibody that is a candidate treatment for the patient, and evaluating the cultured cells for a response to the drug. In certain embodiments, monolayer(s) of malignant cells are cultured from explants prepared by mincing tumor tissue, and the cells of the monolayer are suspended and plated for chemosenstivity testing. The in vitro response to the drug as determined by the method of the invention is correlative with the patient's in vivo response upon receiving the monoclonal EGFR antibody during chemotherapeutic treatment (e.g., in combination with other standardized or individualized chemotherapeutic regimen).
Owner:PRECISION THERAPEUTICS
Method for detecting cell PD-L1 protein expression in body fluid sample and special reagent thereof
The invention discloses a method for detecting a cell PD-L1 protein expression in a body fluid sample and a special reagent thereof. The method comprises the steps that a prepared immunomagnetic particle mixture and tumor circulating cells in the body fluid sample are combined and placed in an immune magnetic particle trap for capturing; and the captured tumor cells are stained by a PD-L1 immunohistochemical staining reagent to evaluate the PD-L1 protein expression, wherein the immunomagnetic particle mixture is coated with at least one of anti-human EpCAM immunomagnetic particles, anti-humanICAM-1 immunomagnetic particles, anti-human EGFR antibody immunomagnetic particles, anti-human folate receptor immunomagnetic particles, anti-human CD10 immunomagnetic particles, anti-human CD19 immunomagnetic particles, anti-human CD20 immunomagnetic particles and anti-human CD33 immunomagnetic particles. The method has the advantages of simple operation, high sensitivity, and easy detection result interpretation.
Owner:NINGBO MEIJING MEDICAL TECH
EGFR antibody having specific collagen binding ability, and application thereof in spinal cord injury repairing
ActiveCN107857817APromote differentiationInhibition formationImmunoglobulinsTissue regenerationNeurophysinsMedicine
Owner:DUBU BIOSCIENCES
Reagent kit for detecting non-small cell lung cancer on basis of liquid biopsy
ActiveCN106990080ARemove cleanHigh enrichment efficiencyPreparing sample for investigationFluorescence/phosphorescenceFluorescenceEGFR Antibody
The invention provides a reagent kit for detecting non-small cell lung cancer on the basis of liquid biopsy. The reagent kid comprises staining enhancement solution for enhancing staining effects and specific ligands with fluorescent staining markers. The specific ligands comprise EGFR (epidermal growth factor receptor) antibodies, CD45 antibodies and folate ligands; the staining enhancement solution comprises surfactants with the concentration of 0.001-1 mg / mL. The reagent kit has the advantages that target cells can be effectively enriched by the reagent kit, and whether the enriched target cells come from early-stage patients suffering from the non-small cell lung cancer or not can be verified; the CTC (circulating tumor cell) detection sensitivity can be improved by means of double-tumor-marker detection, and the detection accuracy further can be guaranteed by means of CEP8 detection; the staining effects can be enhanced by the staining enhancement solution, the diversified ligands with the fluorescent staining markers can be combined with the surfaces of the target cells, accordingly, the surfaces of the target cells can be stained, the good staining effects can be realized, fluorescence is intense, and boundaries are clear.
Owner:上海美吉医学检验有限公司
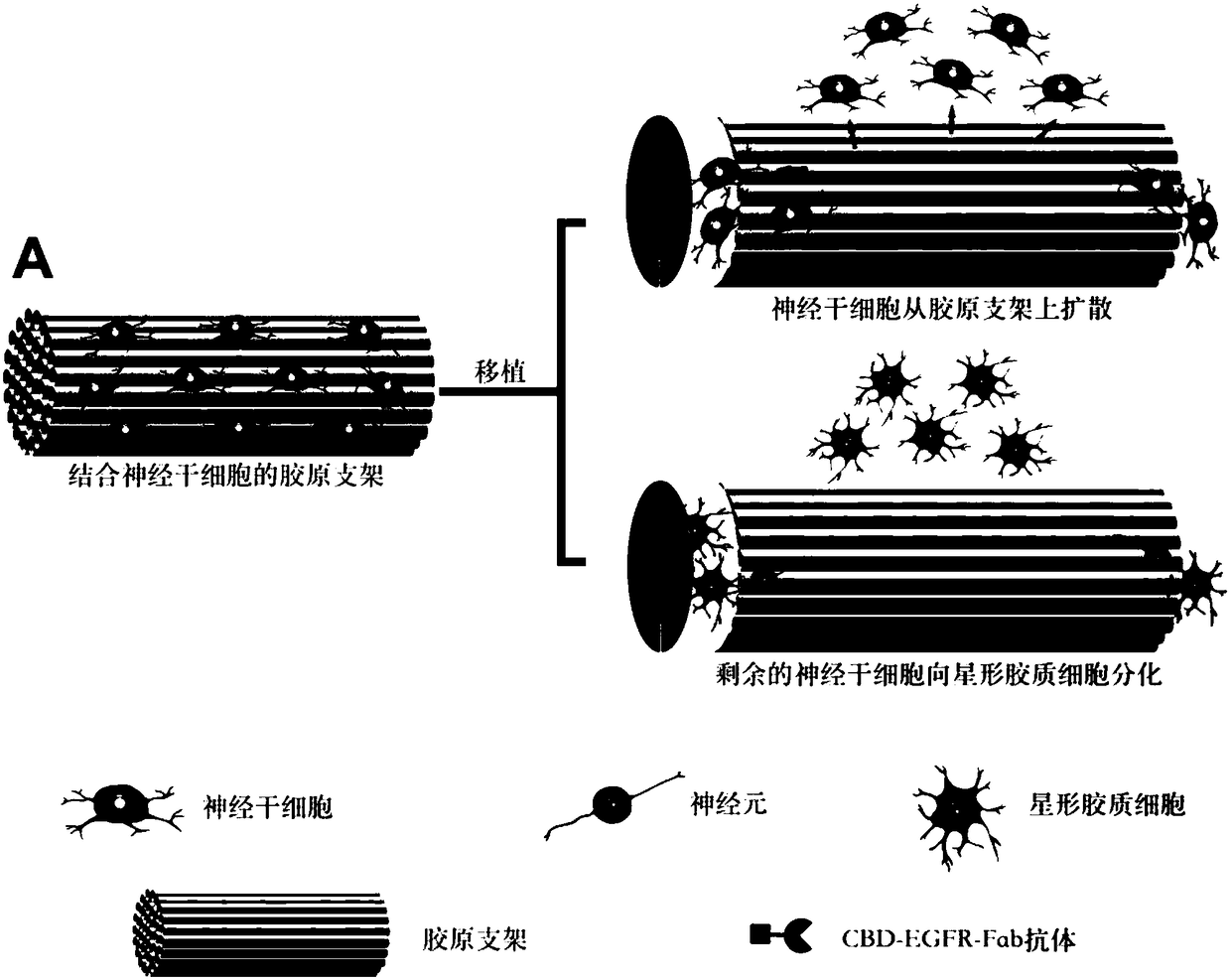
Dual-functional collagen scaffold material as well as preparation method and application thereof in spinal cord injury repair
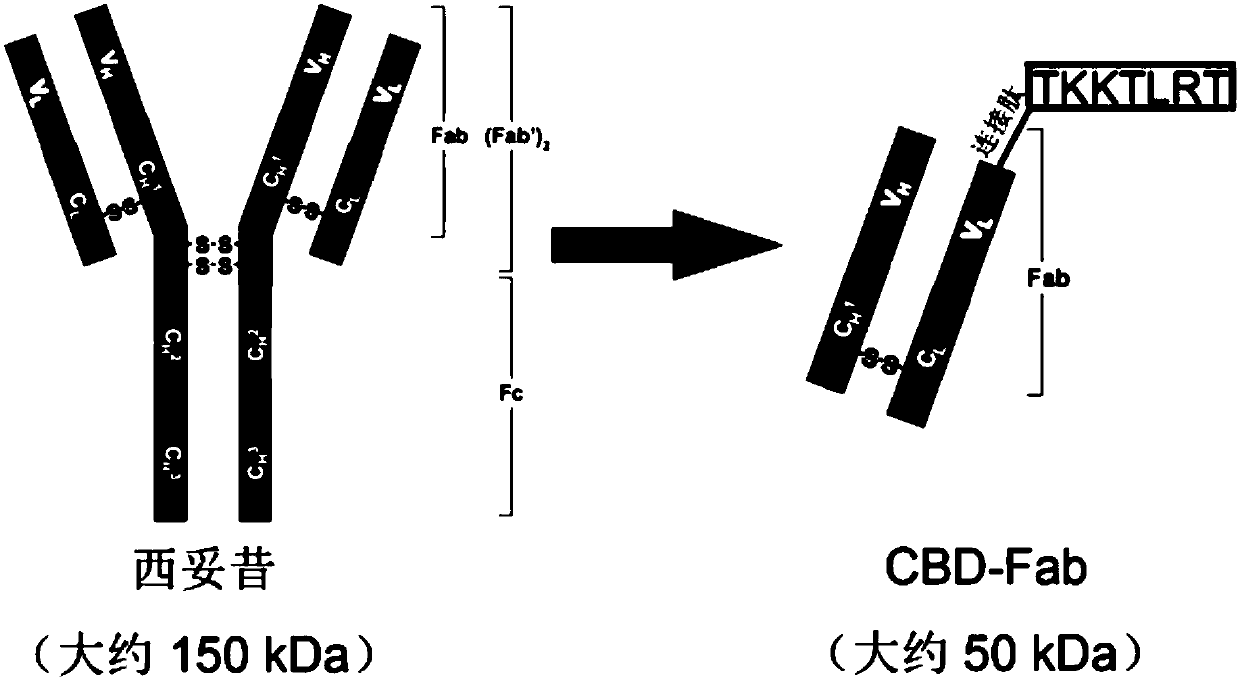
ActiveCN108144120AImprove bindingRescue differentiationTissue regenerationProsthesisMyelinEGFR Antibody
The invention discloses a dual-functional collagen scaffold material as well as a preparation method and an application thereof in spinal cord injury repair. The dual-functional collagen scaffold material consists of a nerve regeneration scaffold and a CBD-EGFR-Fab antibody which is bonded with the nerve regeneration scaffold, wherein the CBD-EGFR-Fab antibody comprises a collagen bonding area anda Fab area of an EGFR antibody which is linked to the collagen bonding area. According to the dual-functional collagen scaffold material provided by the invention, in vitro, exogenous neural stem cell bonding can be promoted and differentiation of inhibited neural stem cells towards neurons with the presence of myelin protein can be rescued, and in vivo, spread of the exogenous neural stem cellsfrom a transplantation site can be effectively relieved and a neuronal circuit can be reconstructed by promoting the differentiation of the neural stem cells towards functional neurons, so that the recovery of motor functions after spinal cord injury can be finally promoted.
Owner:DUBU BIOSCIENCES
Combinations of irs/stat3 dual modulators and Anti-cancer agents for treating cancer
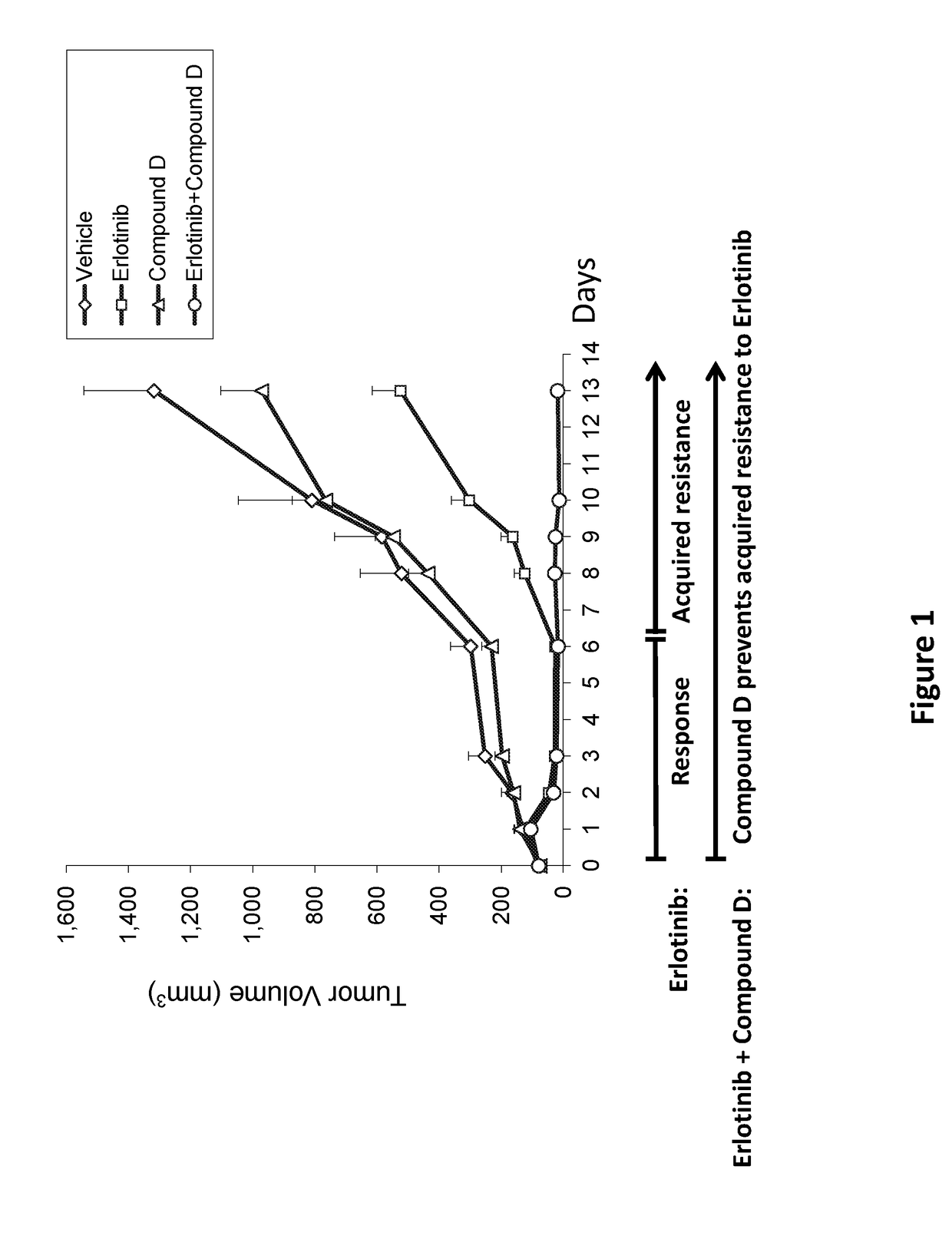
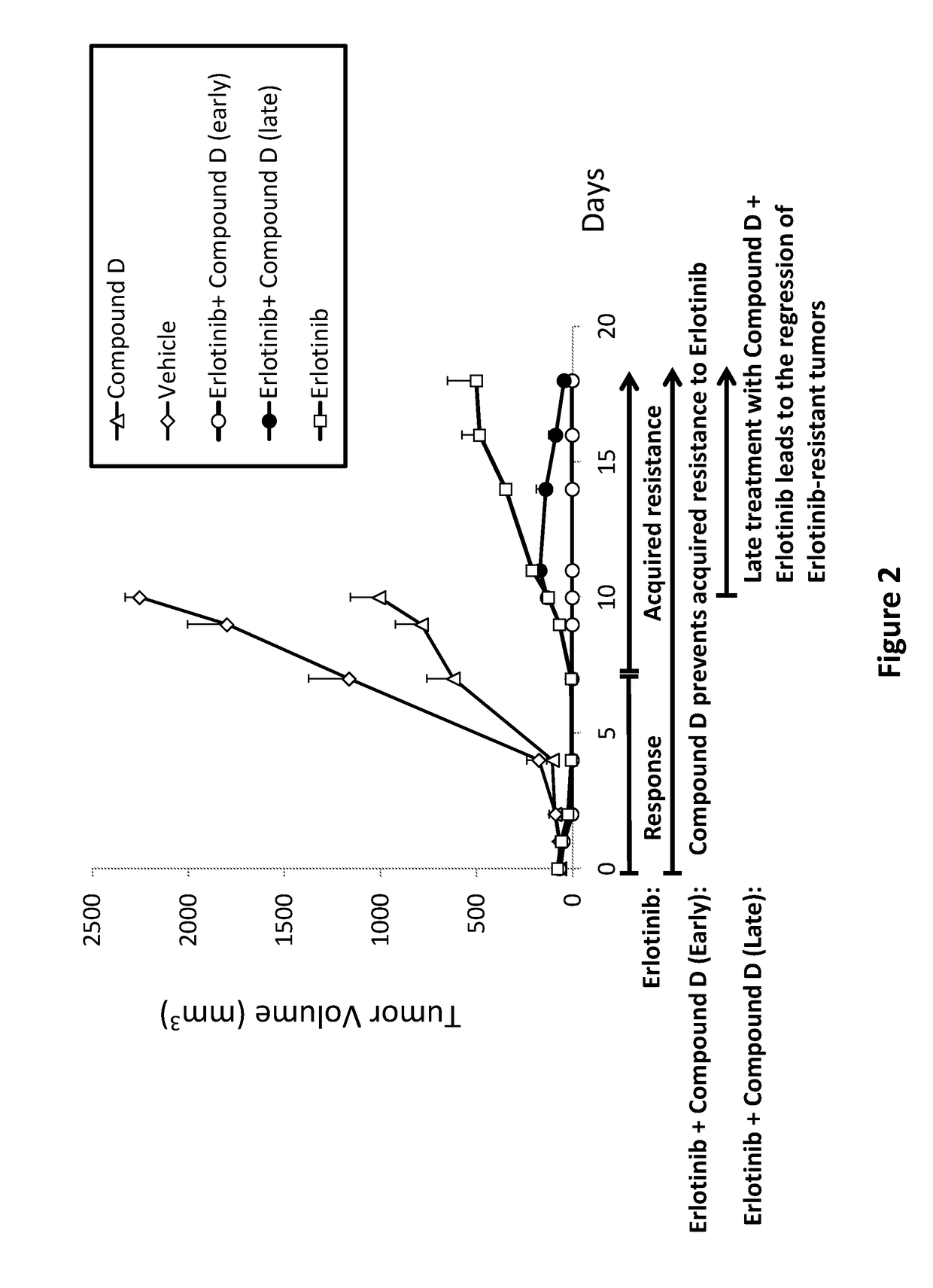
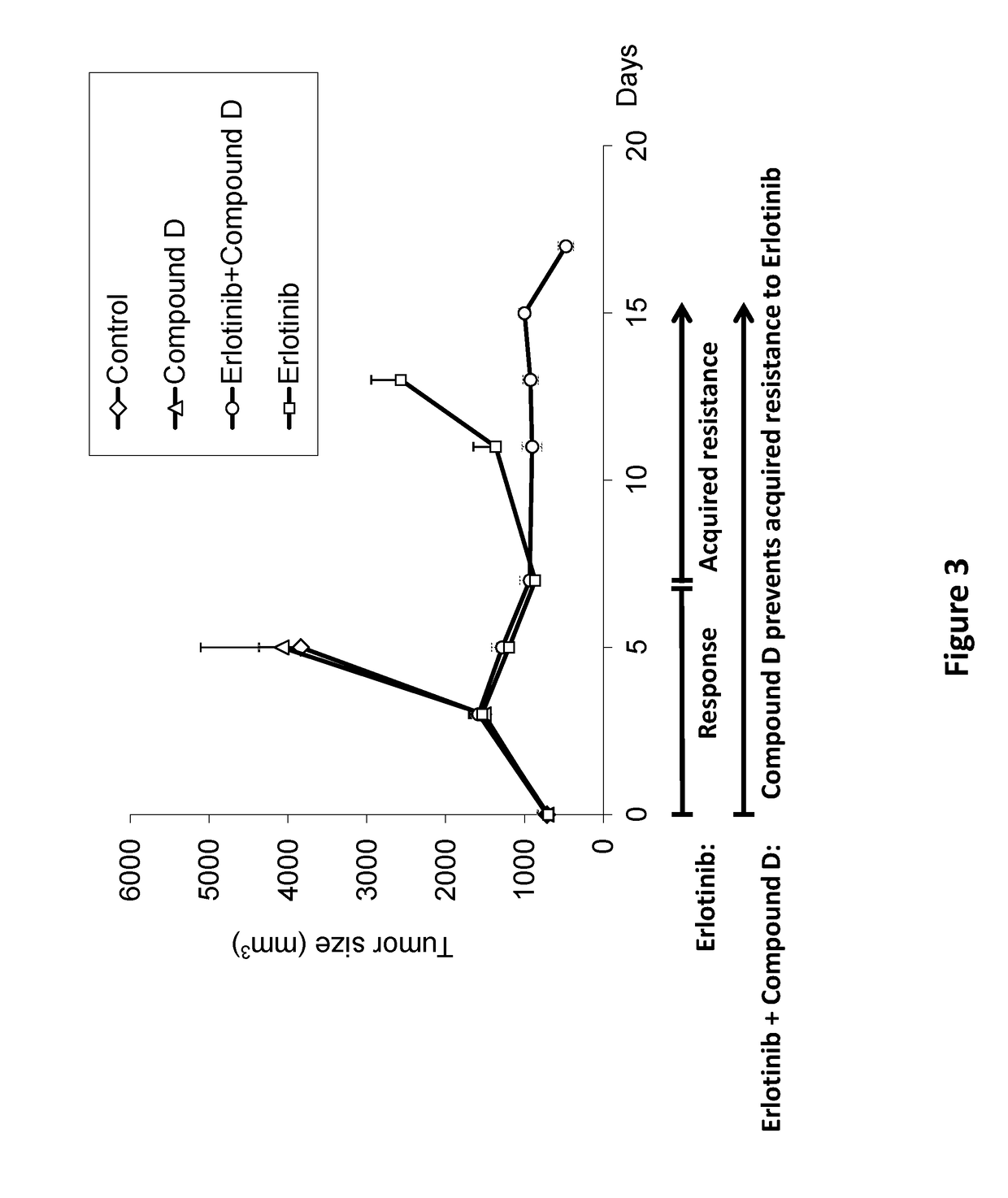
ActiveUS20180028475A1Preventing and delaying tumor recurrenceAvoid resistanceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAcquired resistanceImmunotherapeutic agent
Provided is a treatment of cancer using a dual modulator of Insulin Receptor Substrate (IRS) and signal transducer and activator of transcription 3 (Stat3), as well with other agents. The combination can be used to treat a tumor that has developed resistance to an EGFR inhibitor, EGFR antibody, mTOR inhibitor, MEK inhibitor, mutated B-Raf inhibitor, chemotherapeutic agents, and certain combinations thereof, or to prevent acquired resistance of a tumor to any of said inhibitors or agents, or to prevent tumor recurrence following cease of treatment with any of said inhibitors or agents or a combination thereof. The subject matter further provides a treatment of cancer using combination therapy comprising a dual modulator of IRS and Stat3, in combination with an immunotherapy agent. The combination can be used to sensitize a tumor to immunotherapy.
Owner:TYRNOVO LTD
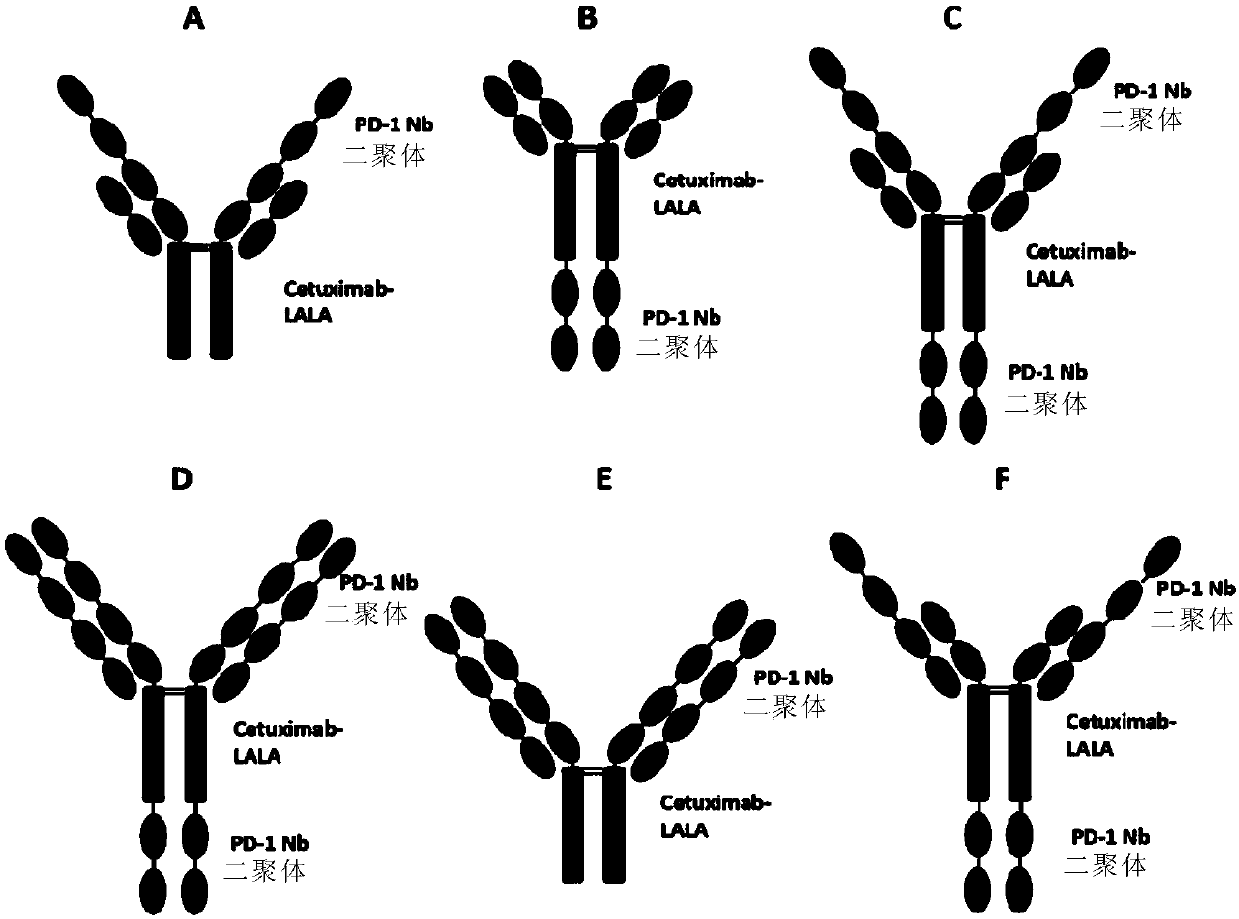
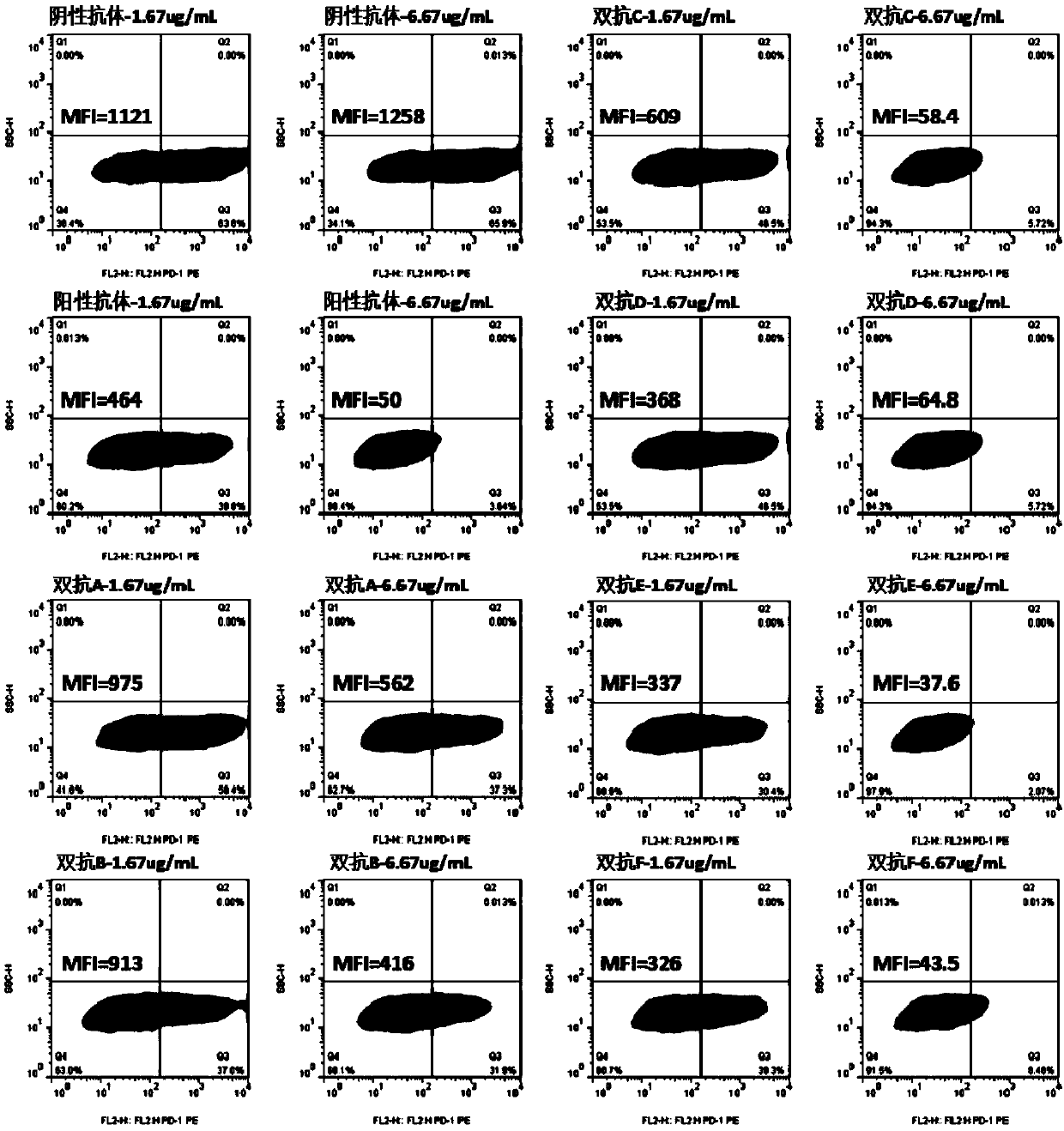
Anti-PD-1/EGFR bispecific antibody and application thereof
The invention provides an anti-PD-1 / EGFR bispecific antibody and an application thereof, and particularly provides the bifunctional antibody, which comprises: (a) an anti-EGFR antibody; and (b) an anti-PD-1 nanobody in a divalent form linked to the anti-EGFR antibody. The bifunctional antibody can be combined with EGFR and PD-1 at the same time, and therefore the treatment effect on EGFR positivetumor cells (especially malignant tumor cells) is achieved.
Owner:SHANGHAI NOVAMAB BIOPHARM CO LTD
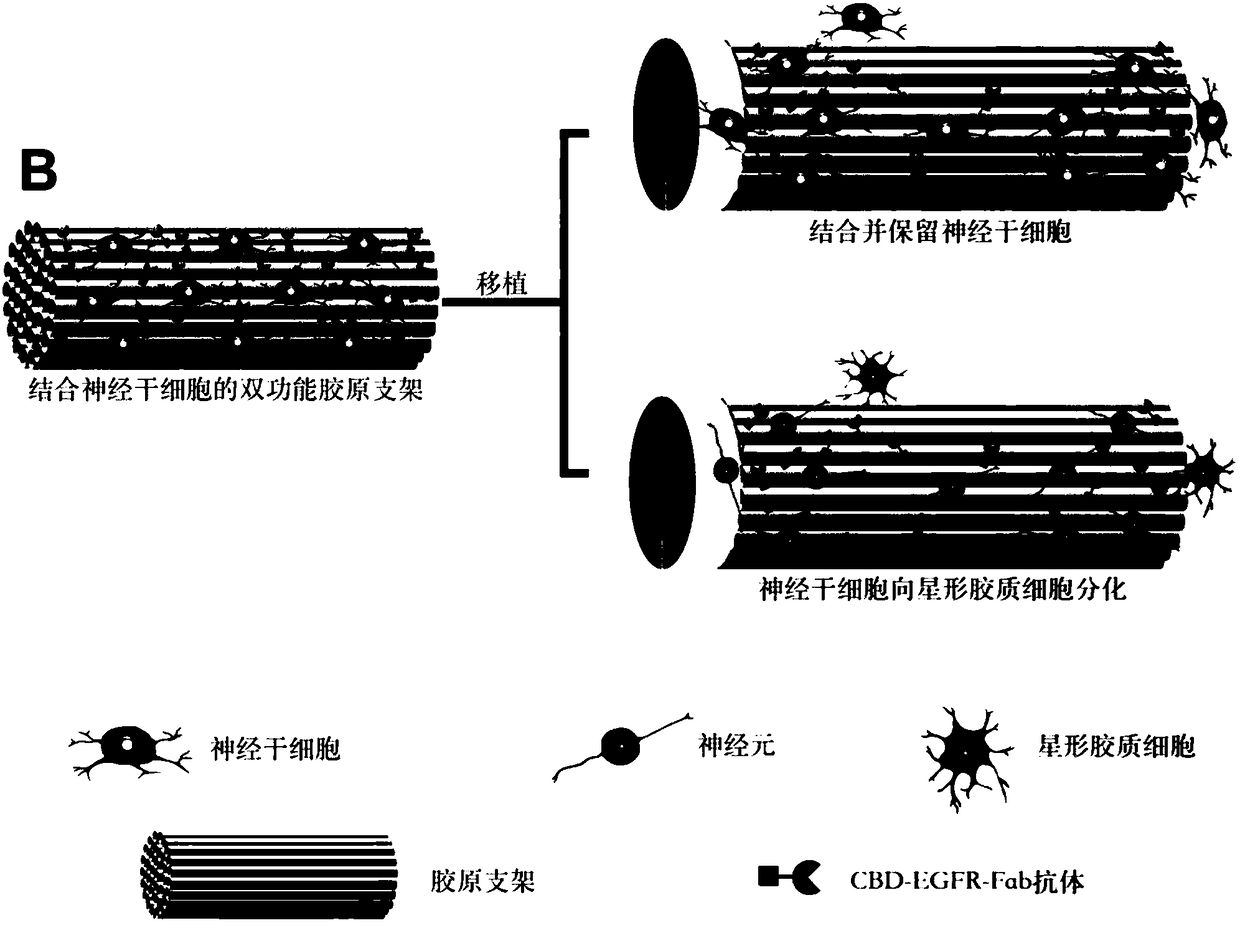
Double-modified collagen scaffold and application of double-modified collagen scaffold in product for repairing of spinal cord injury
ActiveCN109106981APromote differentiationPromote growthTissue regenerationProsthesisCollagen scaffoldEGFR Antibody
The invention discloses a double-modified collagen scaffold and an application of the double-modified collagen scaffold in a product for repairing of spinal cord injury. The double-modified collagen scaffold comprises a collagen scaffold material, and an EGFR antibody and microtubule stabilizing molecules modified on the collagen scaffold material. The EGFR antibody cetuximab and the microtubule stabilizing molecule paclitaxel are combined to modify the linear ordered collagen scaffold material, which can effectively antagonize the inhibitory effect of myelin-related inhibitory molecules on nerve regeneration by blocking the activation of an EGFR signaling pathway, the collagen scaffold promotes the generation of more neurons in the spinal cord injury position, is beneficial to the myelination of nerve fibers, and can also promote the regeneration of axons by stabilizing the structure of microtubules, reduces the deposition of inhibitory scar components in a injured area, blocks the formation of inhibitory scars, and reconstructs the spinal cord neural circuit, and ultimately achieves better behavioral recovery after spinal cord injury.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com