Novel anti-epidermal growth factor receptor antibody, preparation method and application thereof
An antibody, a new technology, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody, anti-tumor drug, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Construction of Pan
[0054] Use genetic engineering technology to synthesize the gene sequence of SEQ ID NO: 5 (which expresses the amino acid sequence described in SEQ ID NO: 6), connect it to the expression vector, and transfect it into the host cell CHO, screen for high-expression clones, and carry out mass culture, Expression, separation and purification to obtain the antibody Pan.
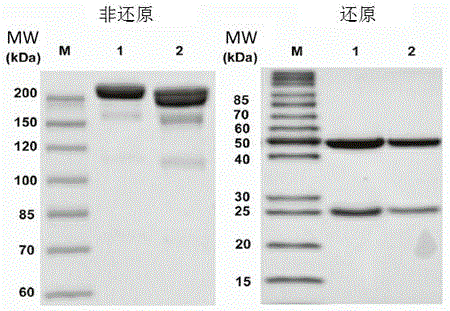
[0055] The molecular weight of Pan was determined by polyacrylamide gel electrophoresis (SDS-PAGE) ( figure 1 ). Under non-reducing conditions, a band of Pan was observed at about 200kD.
[0056] And both SDS-PAGE and size-exclusion chromatography (SEC) showed that its molecular size was larger than that of panitumumab before modification. As previously reported, IgG2 panitumumab has two bands of similar molecular size, suggesting that it may be composed of structural isomers. Under reducing conditions, both Pan and panitumumab produced heavy and light chains of approxi...
Embodiment 2
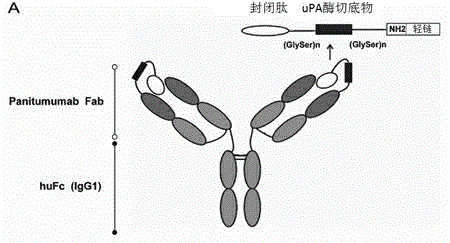
[0057] Example 2: Construction of Pan-P and digestion in vitro
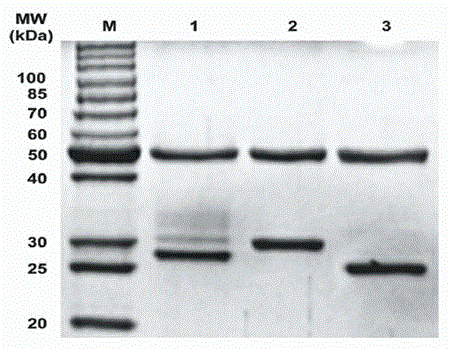
[0058] We used the previously described probody technology to develop Pan-P with enzymatic cleavage activity on the basis of Pan. as in figure 2 The indicated peptides were fused to the light chain of Pan as described in . Its sequence composition is as follows: blocking peptide (IYPPLLRTSQAM), substrate peptide (LSGRSDNH) and serine-glycine linking peptide (GSSGGSGGSGGSG). It has been previously identified that our chosen blocking peptide specifically binds panitumumab but not cetuximab. Urokinase-type plasminogen activator (uPA) is a protease highly expressed in many tumors. In the development of prodrugs (Prodrugs) in recent years, it has been widely used to convert the inactive state of prodrugs into an active state at the tumor site. Therefore, we linked the cleavage site specifically recognized by uPA (LSGRDNH) to the blocking peptide via a serine-glycine linker peptide. To determine whether Pan-P has...
experiment example 1
[0060] Experimental example 1: Activity comparison of Pan
[0061] Competition binding assay to determine the relative binding activities of Pan and Pani. The experimental process is as follows: EGFR-Fc (1 μg / mL) dissolved in PBS (PH=6.84) was coated on a 96-well microplate (Nunc), and the PBS containing 10% skimmed milk powder was used for 1 hour closed. Next, biotin-labeled panitumumab F(ab')2 (1:200) and unlabeled panitumumab or Pan diluted in PBS were mixed and incubated at 37°C for 60 minutes. Then, streptavidin-conjugated HRP (1:1000) was added and incubated at 37 °C for 30 min. The detection method is the same as the EGFR binding ELISA (enzyme-linked immunosorbent assay) test. Curves were fitted using a four-parameter model of the S-curve. see results Figure 5 , the results showed that Pan and Pani had similar relative binding activities, with average EC50 values of 1.849 μg / ml and 2.112 μg / ml, respectively.
[0062] The growth inhibitory effect of Pan on A431...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com