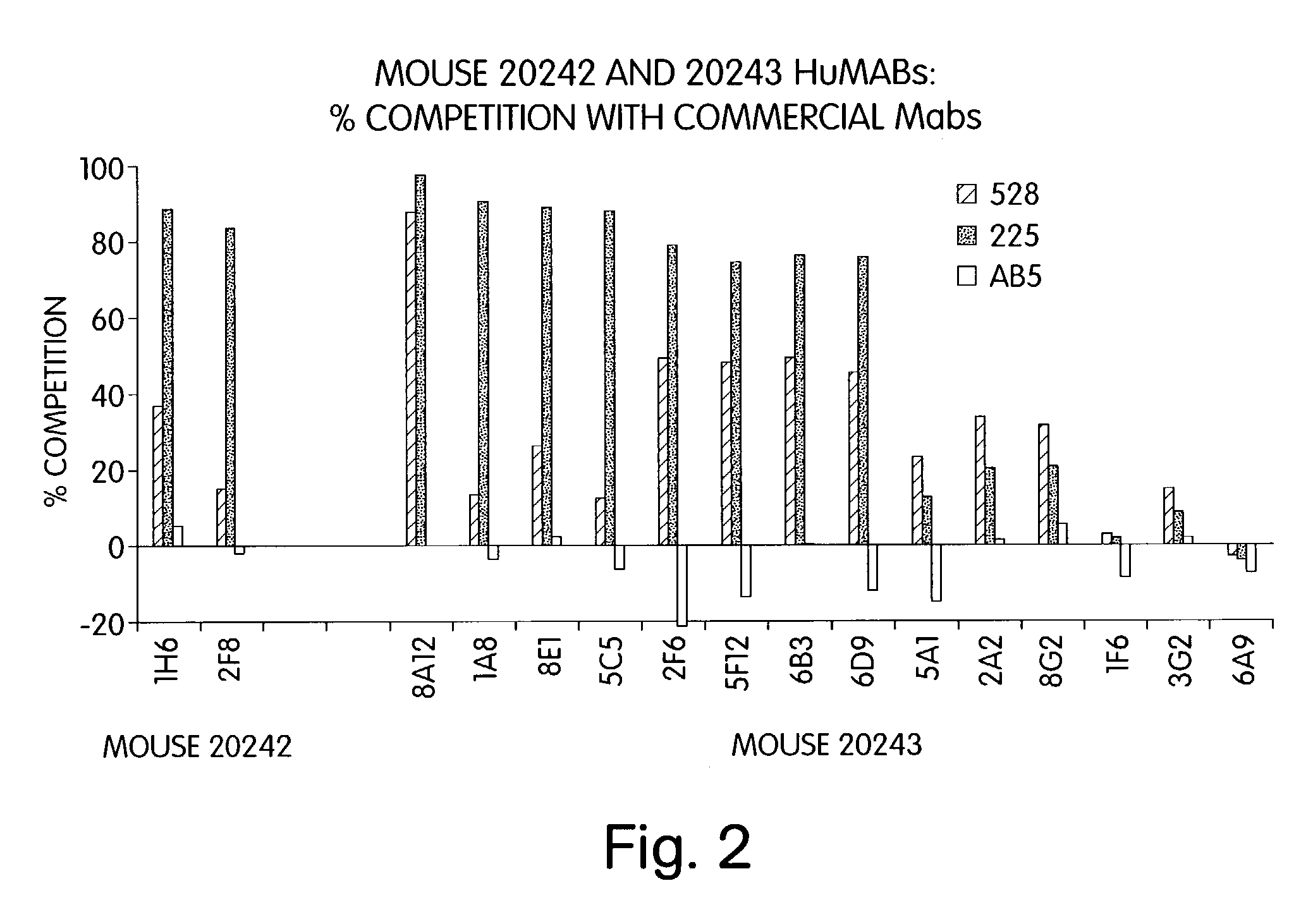
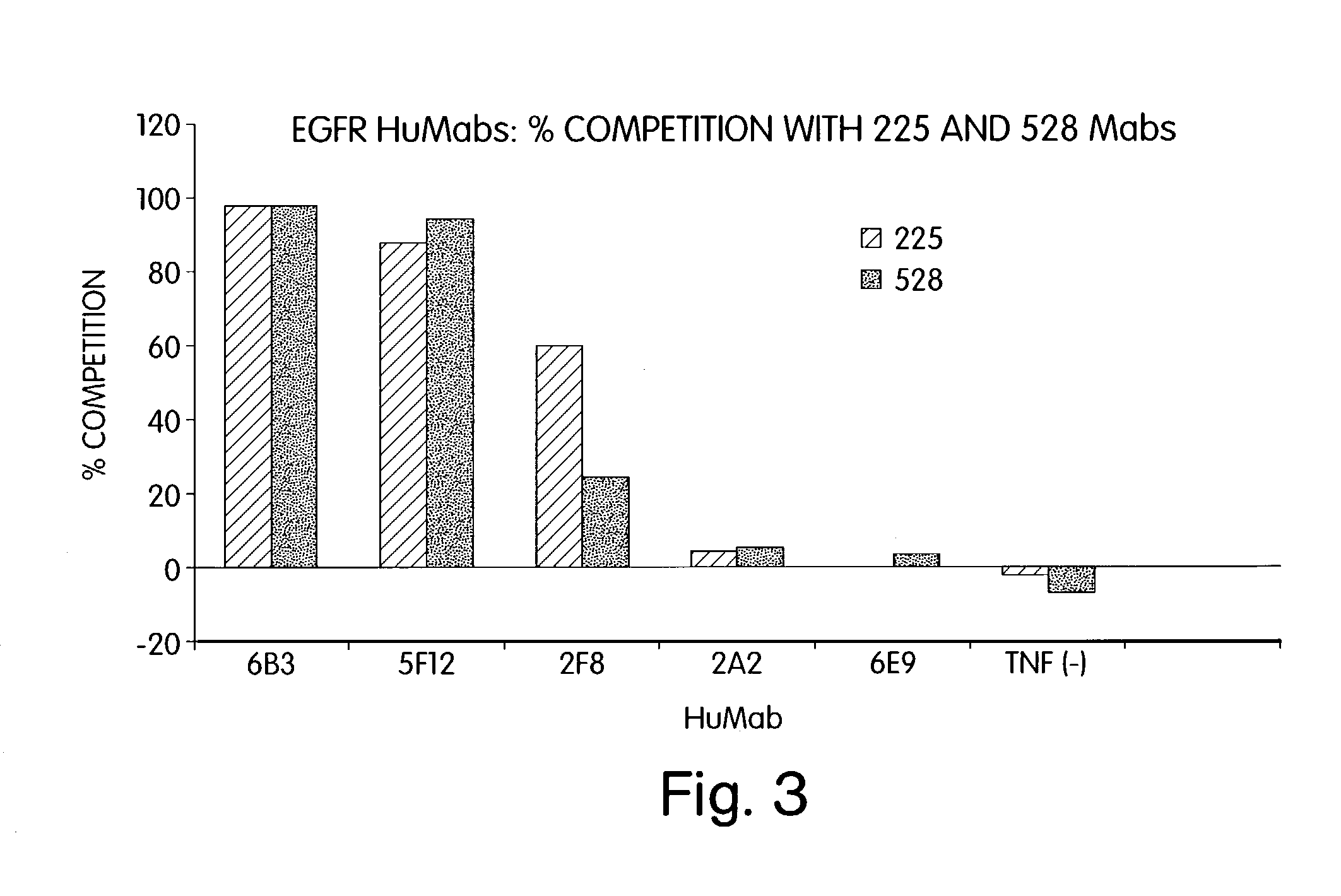
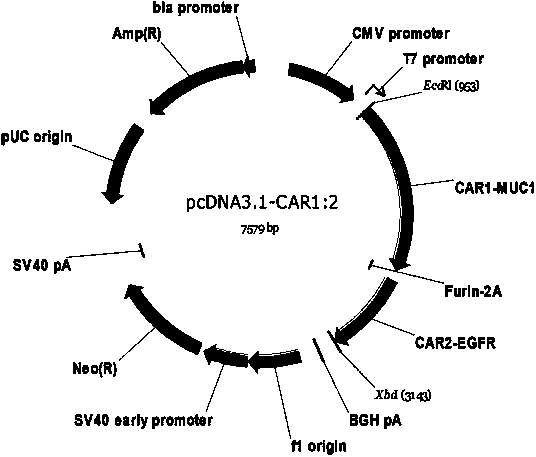
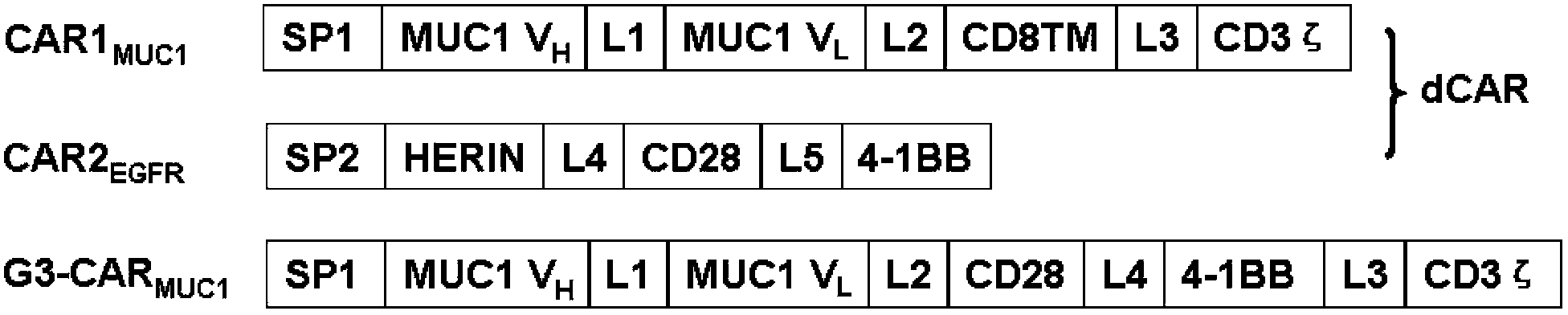
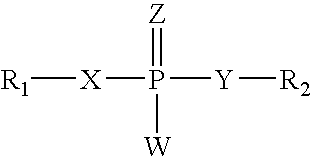Patents
Literature
816 results about "Epidermal growth factor receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
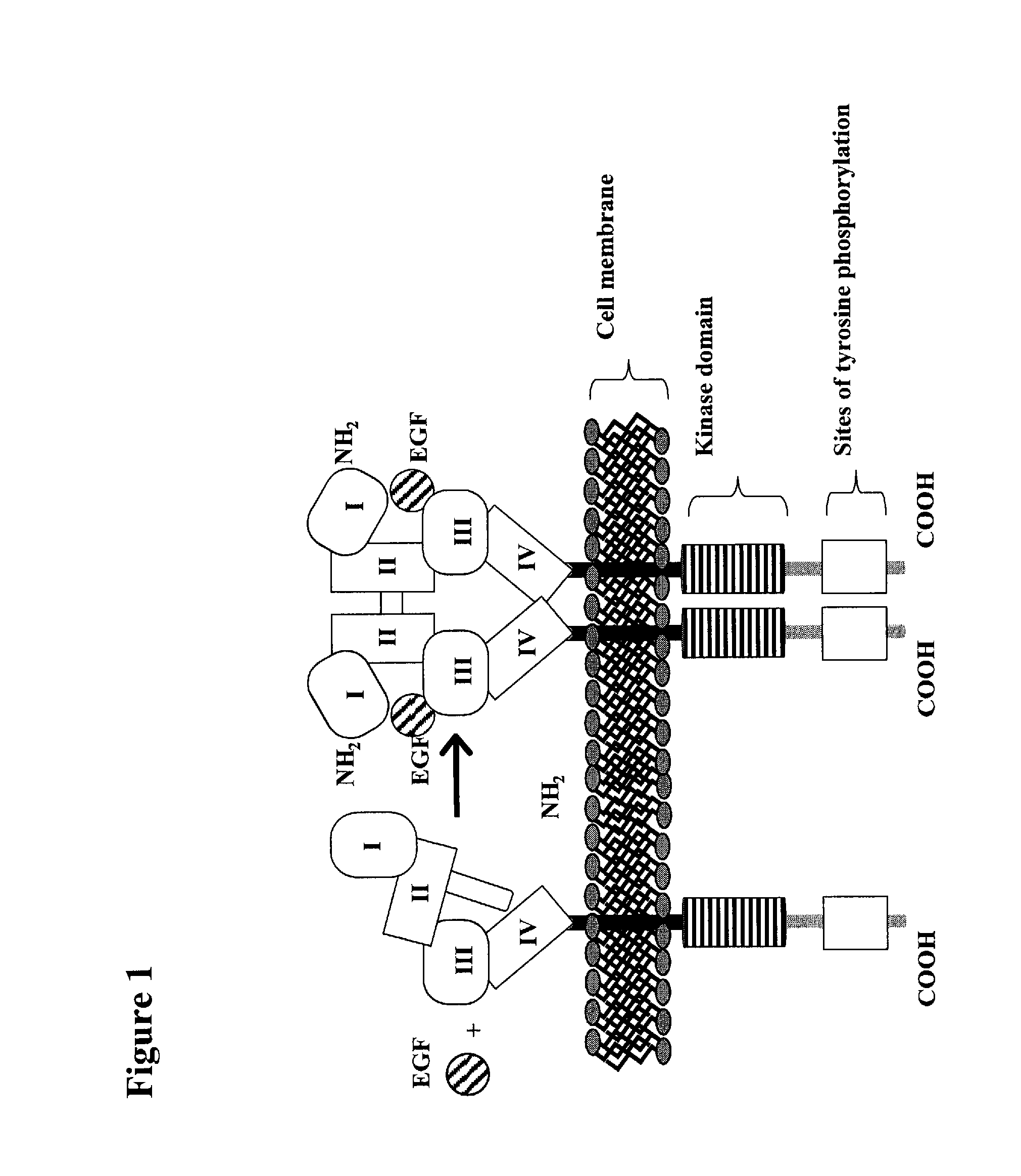
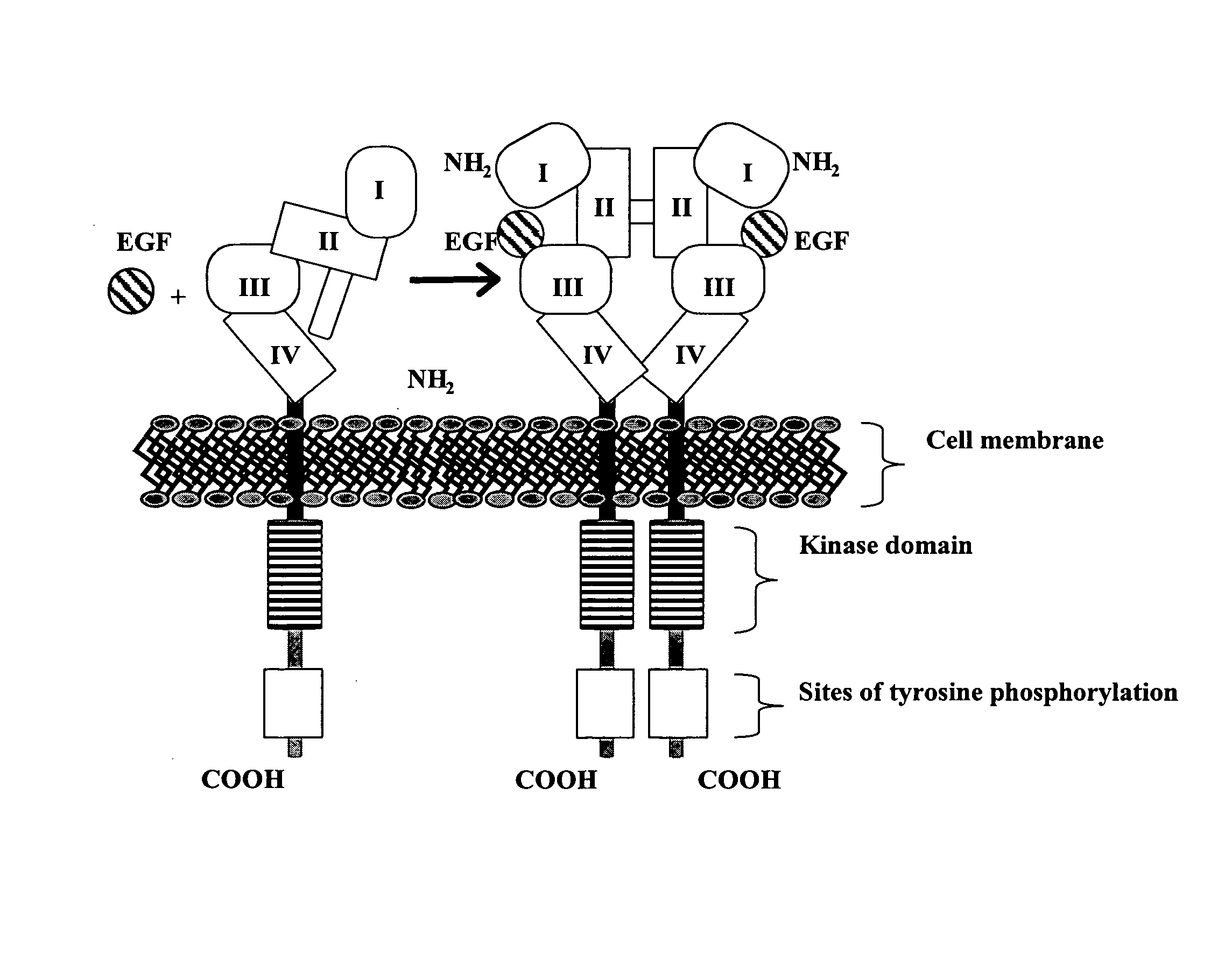
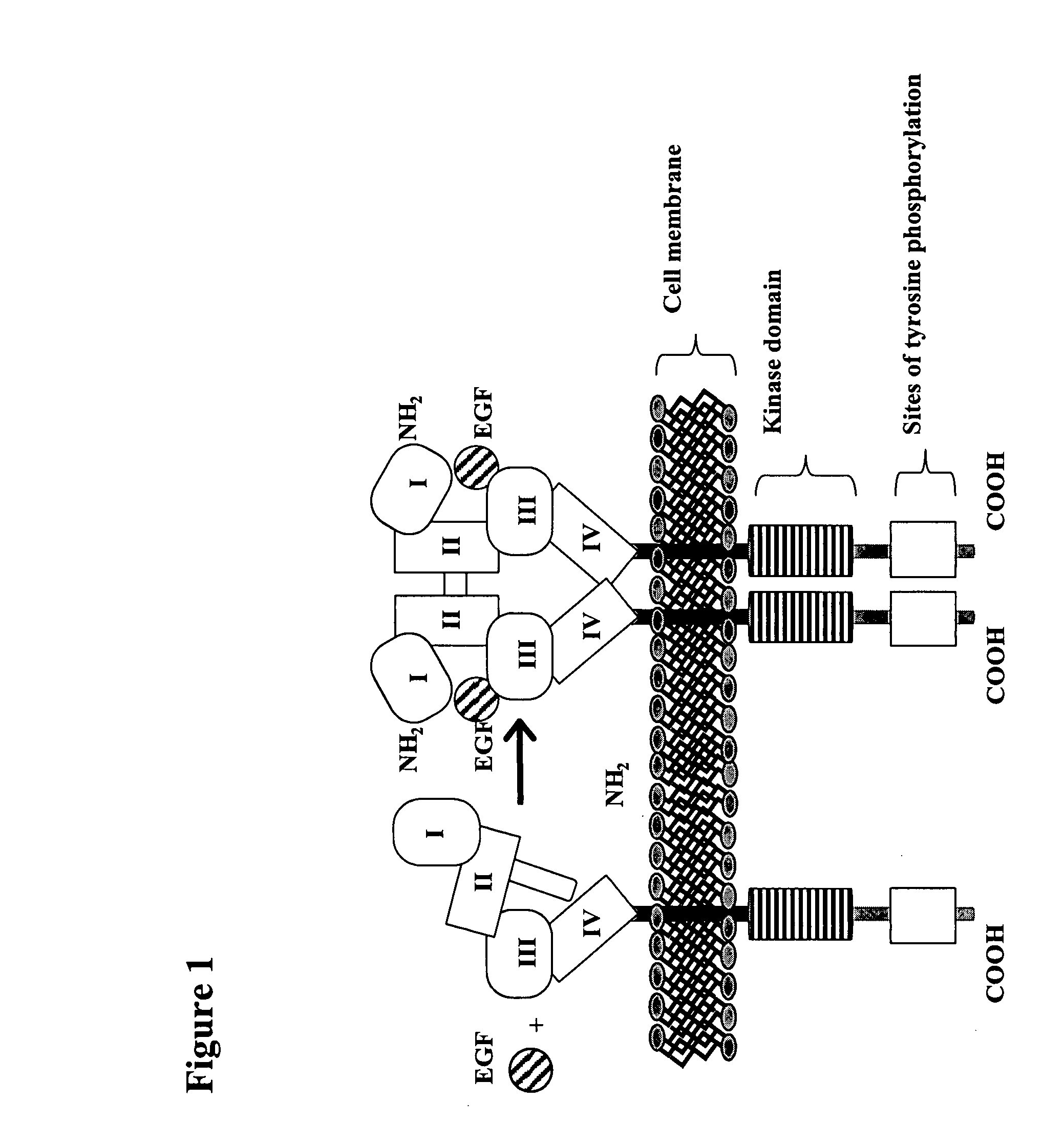
The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans) is a transmembrane protein that is a receptor for members of the epidermal growth factor family (EGF family) of extracellular protein ligands.
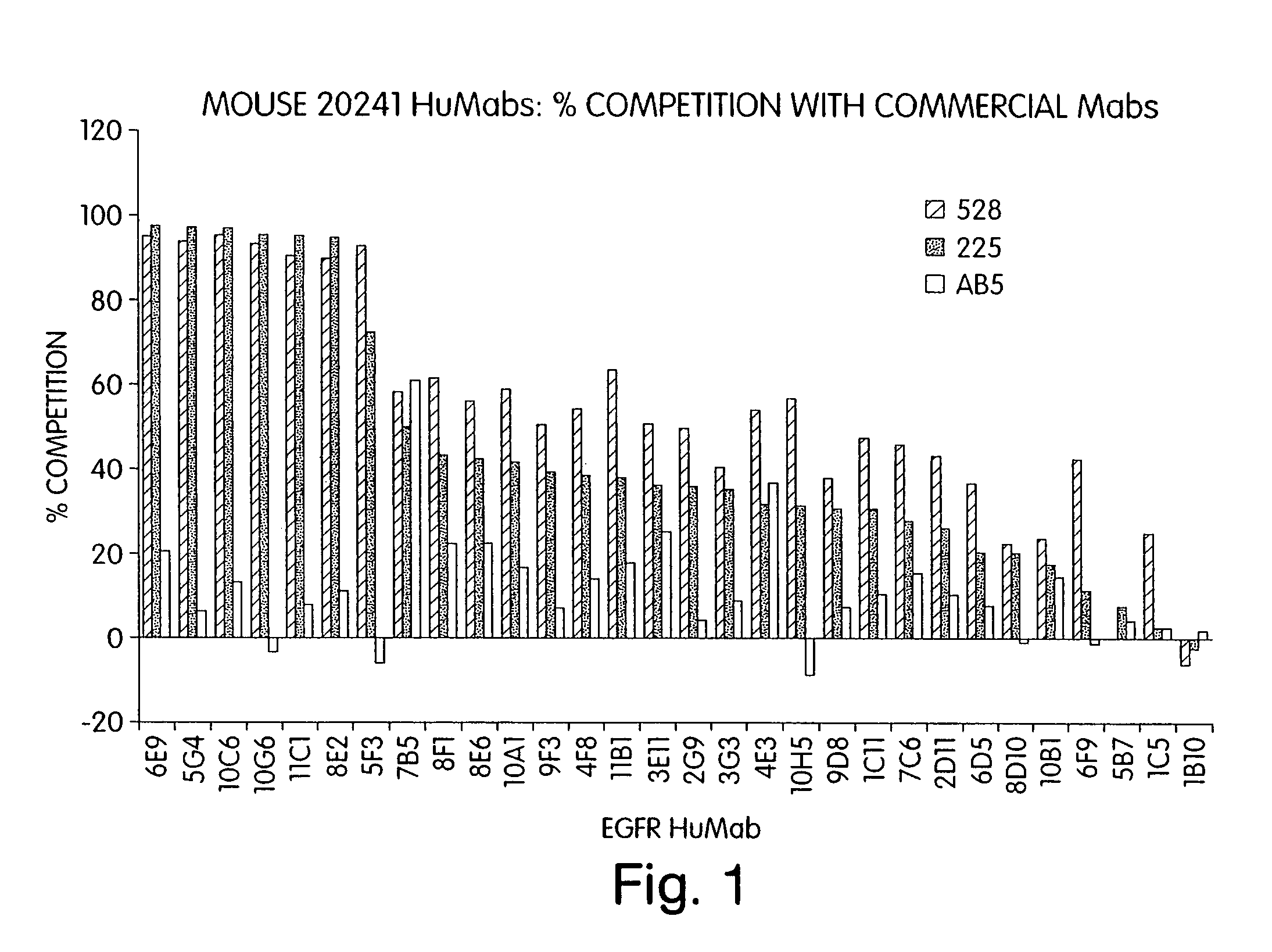
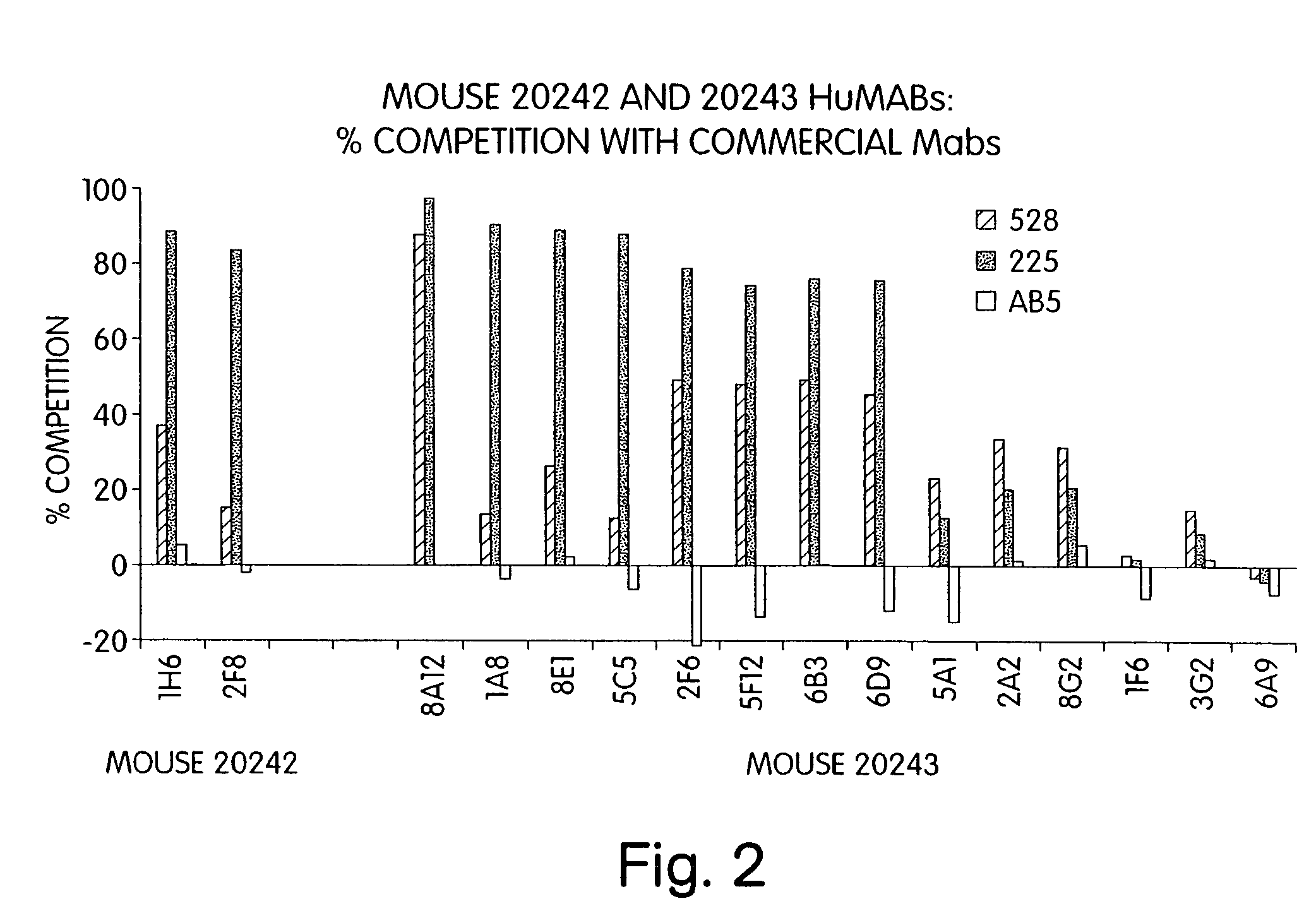
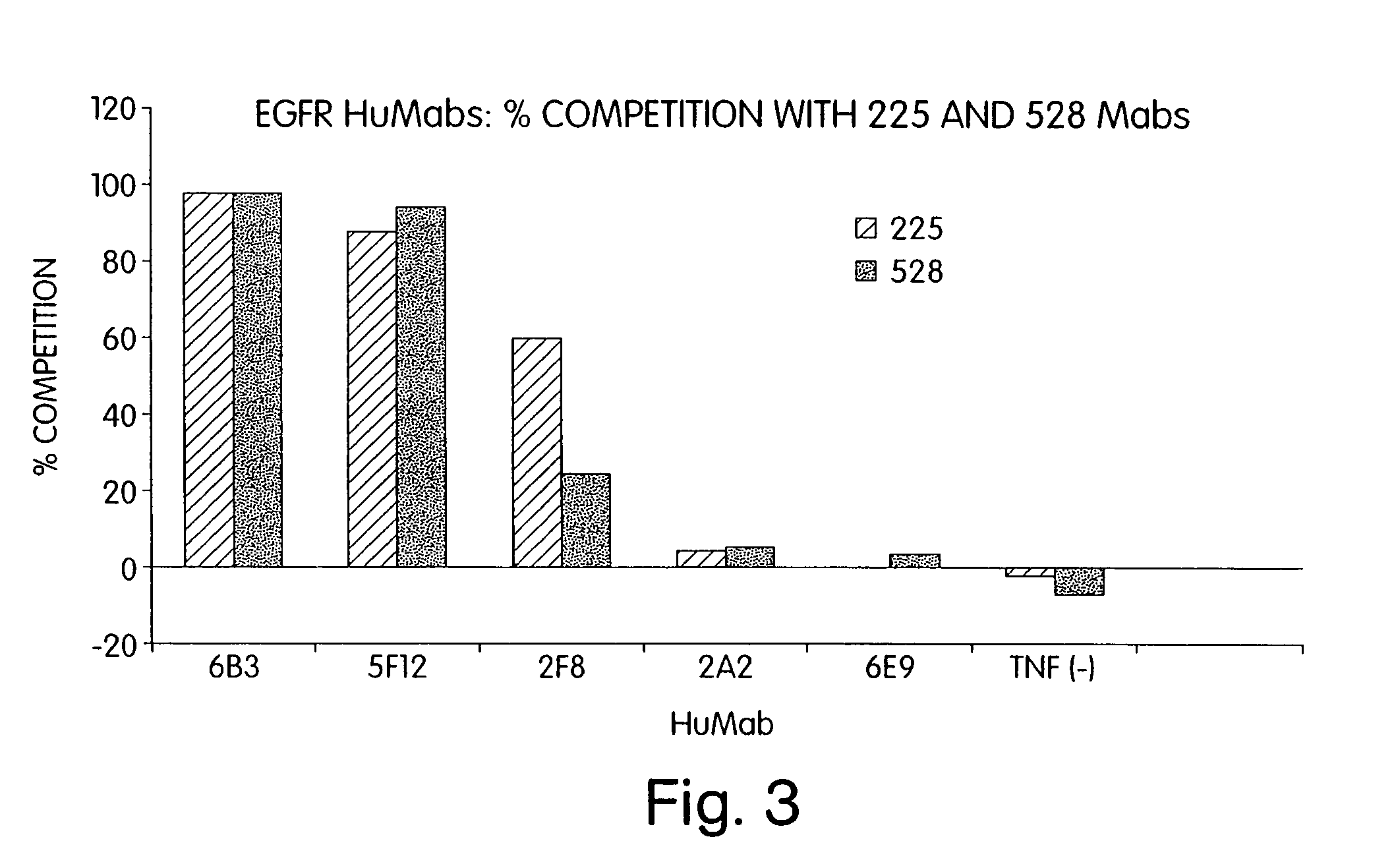
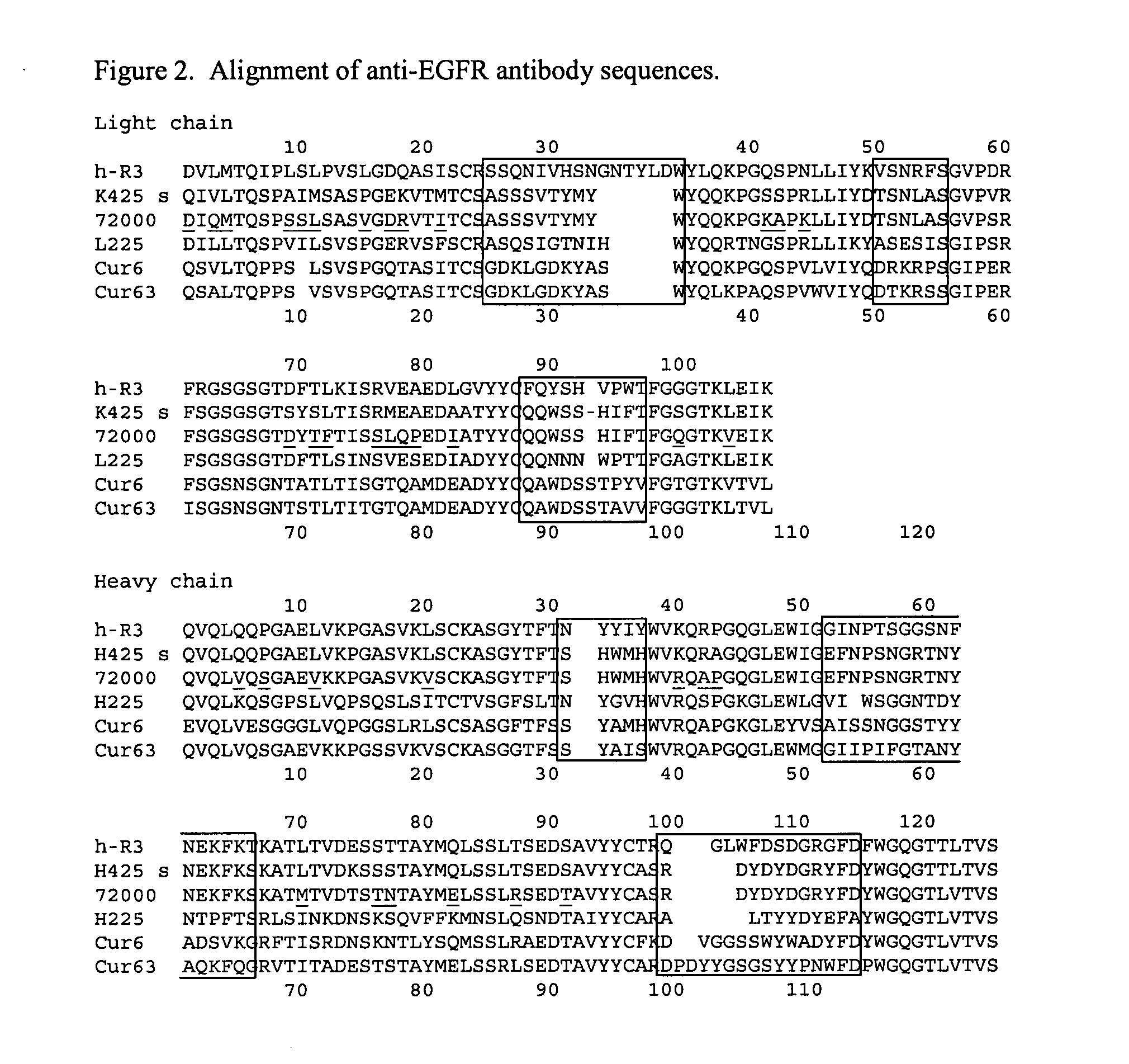
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
InactiveUS7247301B2Less immunogenicReduce adverse side effectsInorganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsV(D)J recombinationHuman epidermal growth factor receptor
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB INC
EGFR mutations
The present invention relates to mutations in Epidermal Growth Factor Receptor (EGFR) and methods of detecting such mutations as well as prognostic methods method for identifying a tumors that are susceptible to anticancer therapy such as chemotherapy and / or kinase inhibitor treatment. The methods involve determining the presence of a mutated EGFR gene or mutated EGFR protein in a tumor sample whereby the presence of a mutated EGFR gene or protein indicates the tumor is susceptible to treatment.
Owner:GENENTECH INC
Ligands that have binding specificity for VEGF and/or EGFR and methods of use therefor
InactiveUS20070003549A1Not effectiveExtended half-lifeNervous disorderAntipyreticVascular endothelial growth factorCancer therapy
Disclosed are ligands that have binding specificity for vascular endothelial growth factor (VEGF), for epidermal growth factor receptor (EGFR), or for VEGF and EGFR. Also disclosed are methods of using these ligands. In particular, the use of these ligands for cancer therapy is described.
Owner:DORMANTIS LTD
Optimized proteins that target the epidermal growth factor receptor
InactiveUS20050142133A1Efficient effector functionEffective functionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsBiochemistryProtein
The present invention relates to optimized proteins that target the Epidermal Growth Factor Receptor (EGFR), and their application, particularly for therapeutic purposes.
Owner:XENCOR INC
Antibody fragment-polymer conjugates and uses of same
Described are conjugates formed by an antibody fragment covalently attached to a non-proteinaceous polymer, wherein the apparent size of the conjugate is at least about 500 kD. The conjugates exhibit substantially improved half-life, mean residence time, and / or clearance rate in circulation as compared to the underivatized parental antibody fragment. Also described are conjugates directed against human vascular endothelial growth factor (VEGF), human p185 receptor-like tyrosine kinase (HER2), human CD20, human CD18, human CD11a, human IgE, human apoptosis receptor-2 (Apo-2), human tumor necrosis factor-α (TNF-α), human tissue factor (TF), human α4β7 integrin, human GPIIb-IIIa integrin, human epidermal growth factor receptor (EGFR), human CD3, and human interleukin-2 receptor α-chain (TAC) for diagnostic and therapeutic applications.
Owner:GENENTECH INC
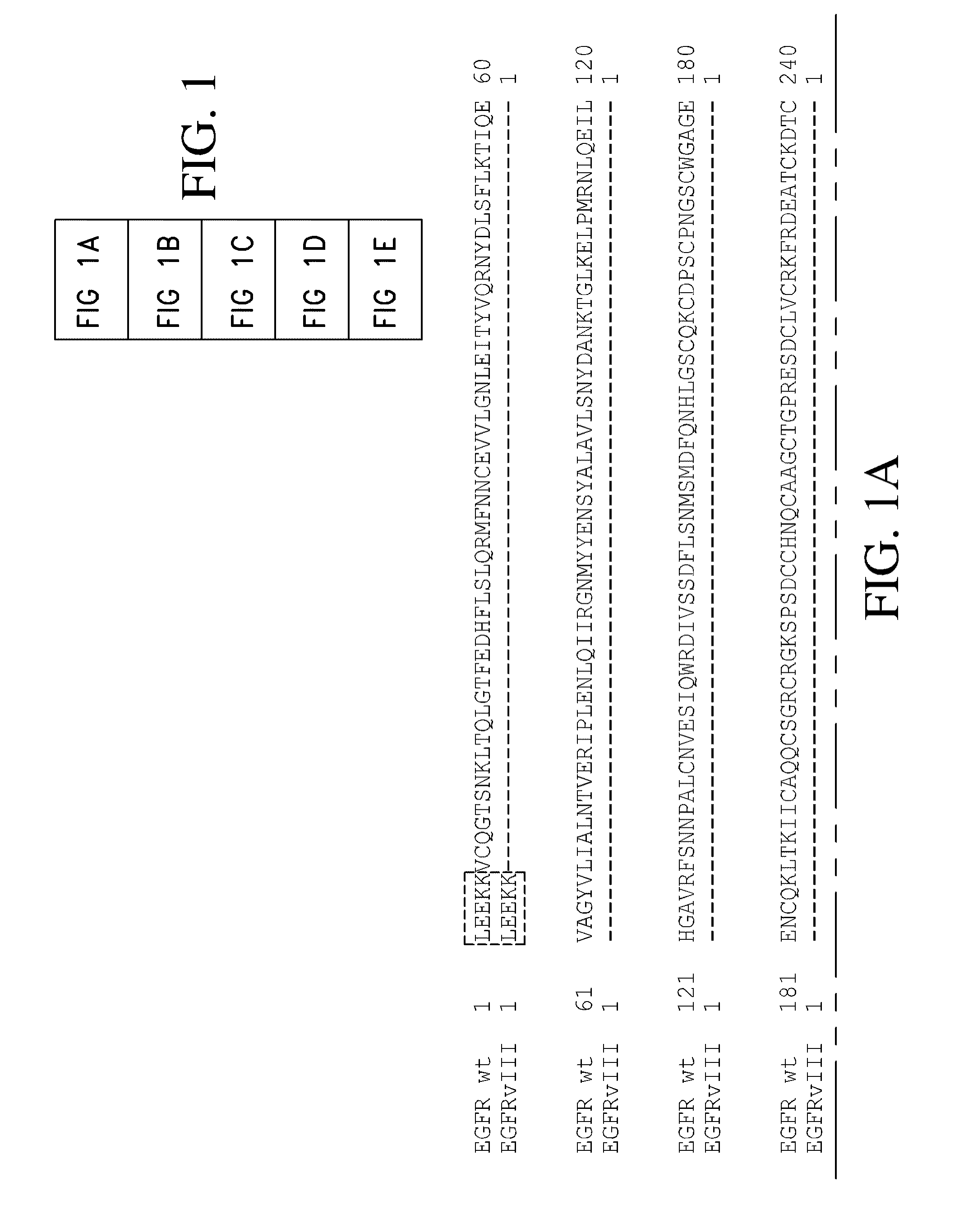
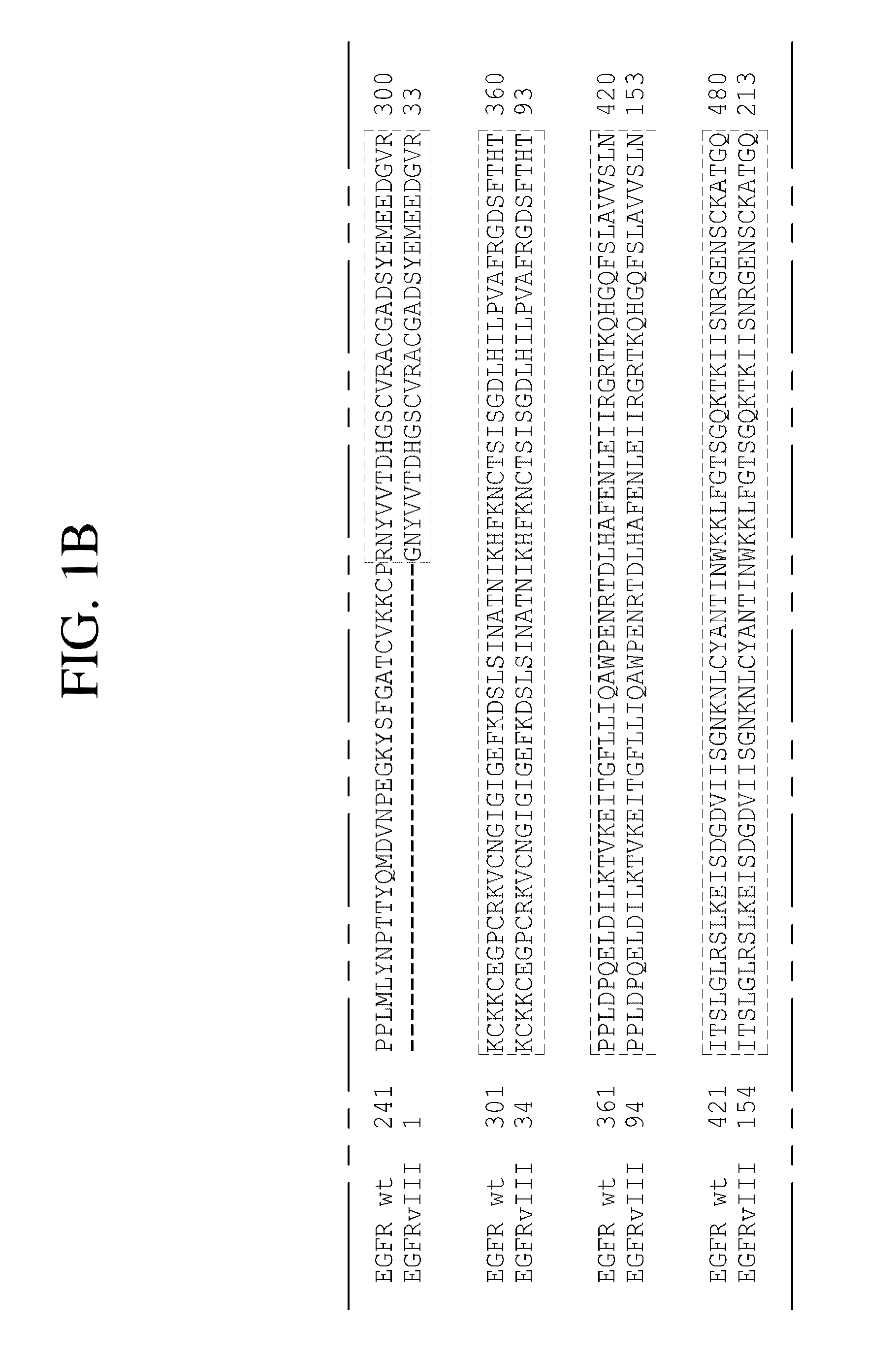
Antibodies directed to the deletion mutants of epidermal growth factor receptor and uses thereof
ActiveUS20090175887A1Inhibit cell proliferationEnzymologyNanomedicineMonoclonal antibodyHuman epidermal growth factor receptor
The present invention relates to novel antibodies, particularly antibodies directed against deletion mutants of epidermal growth factor receptor and particularly to the type III deletion mutant, EGFRvIII. The invention also relates to human monoclonal antibodies directed against deletion mutants of epidermal growth factor receptor and particularly to EGFRvIII. Diagnostic and therapeutic formulations of such antibodies, and immunoconjugates thereof, are also provided.
Owner:AMGEN FREMONT INC
Radiolabeled irreversible inhibitors of epidermal growth factor receptor tyrosine kinase and their use in radioimaging and radiotherapy
InactiveUS6562319B2BiocideOrganic chemistryPositron emission tomographyEpidermal growth factor receptor tyrosine kinase
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Radiolabeled irreversible inhibitors of epidermal growth factor receptor tyrosine kinase and their use in radioimaging and radiotherapy
InactiveUS20020128553A1BiocideOrganic chemistryPositron emission tomographyEpidermal growth factor receptor tyrosine kinase
Radiolabeled epidermal growth factor receptor tyrosine kinase (EGFR-TK) irreversible inhibitors and their use as biomarkers for medicinal radioimaging such as Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) and as radiopharmaceuticals for radiotherapy are disclosed.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
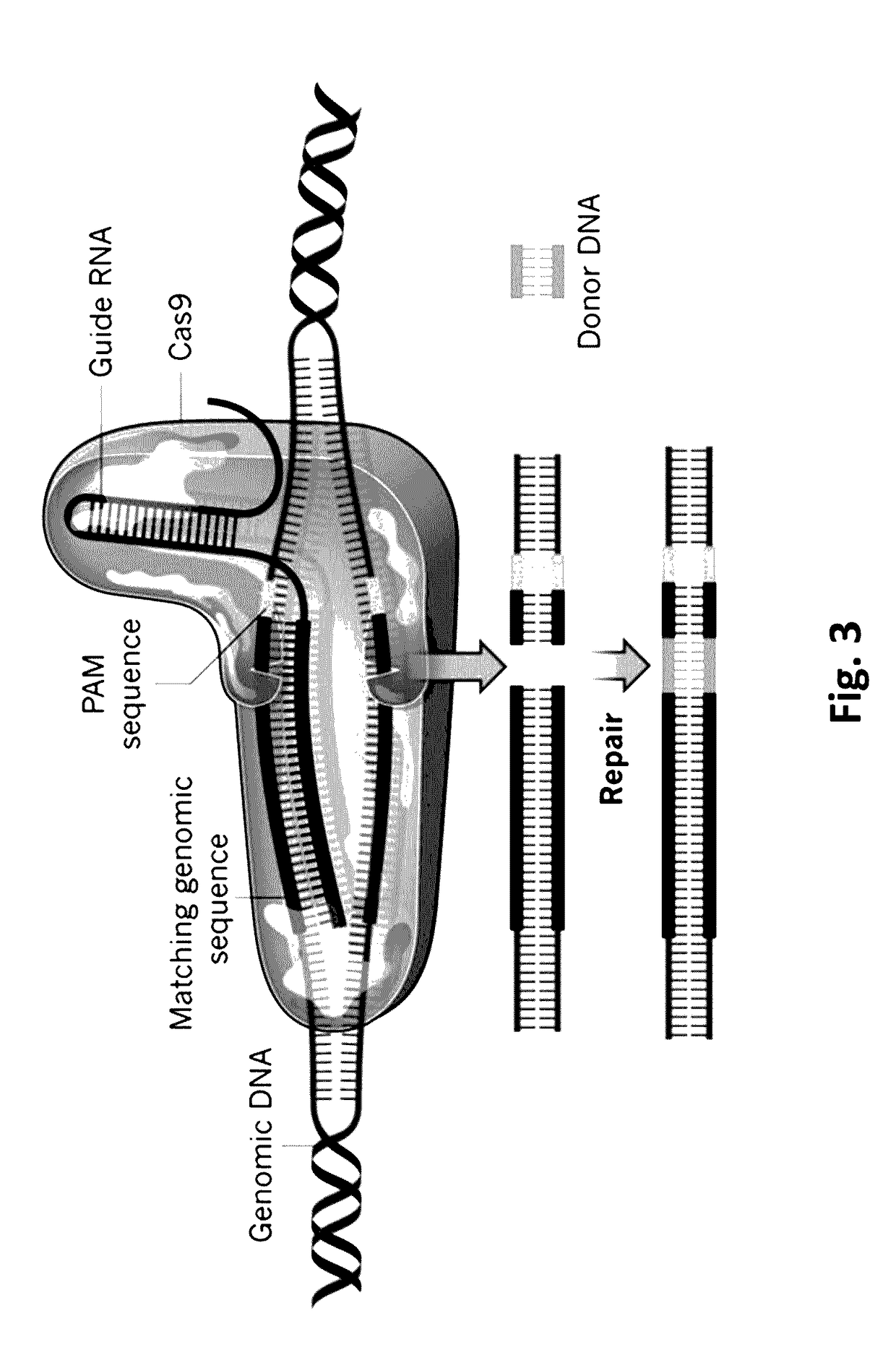
Crispr/cas-mediated genome editing to treat egfr-mutant lung cancer
The invention relates to a clustered regularly interspaced short palindromic repeats (CRISPR) / Cas guide RNA (gRNA) comprising a targeting domain that is complementary to human genomic Epidermal Growth Factor Receptor (EGFR) DNA, and a vector system including one or more packaged vector(s) including: (a) a first regulatory element operably linked to a gRNA, and (b) a second regulatory element operably linked to a nucleic acid encoding a Cas protein. Also disclosed are methods of altering a nucleic acid sequence encoding EGFR in a cell including contacting the cell with a vector system, methods of treating lung cancer, and methods of selectively inducing apoptosis in a cell including administering a gRNA to the cell.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
RNA interference mediated inhibition of epidermal growth factor receptor (EGFR) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050176024A1Improves various propertyImprove the immunityCompounds screening/testingSpecial deliveryDouble stranded rnaGene expression
This invention relates to compounds, compositions, and methods useful for modulating epidermal growth factor receptor (EGFR) (e.g., HER1, HER2, HER3, and / or HER4) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of EGFR gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (mRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of EGFR genes, including HER 1, HER2, HER3, and / or HER4. The small nucleic acid molecules are useful in the treatment and diagnosis of cancer.
Owner:SIRNA THERAPEUTICS INC
Anti-EGFRvIII scFvs with improved cytotoxicity and yield, immunotoxins based thereon, and methods of use thereof
InactiveUS7129332B2Improve bindingPeptide/protein ingredientsAntibody mimetics/scaffoldsComplementarity determining regionCytotoxicity
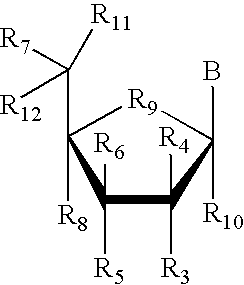
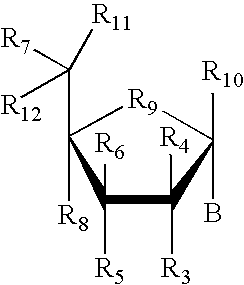
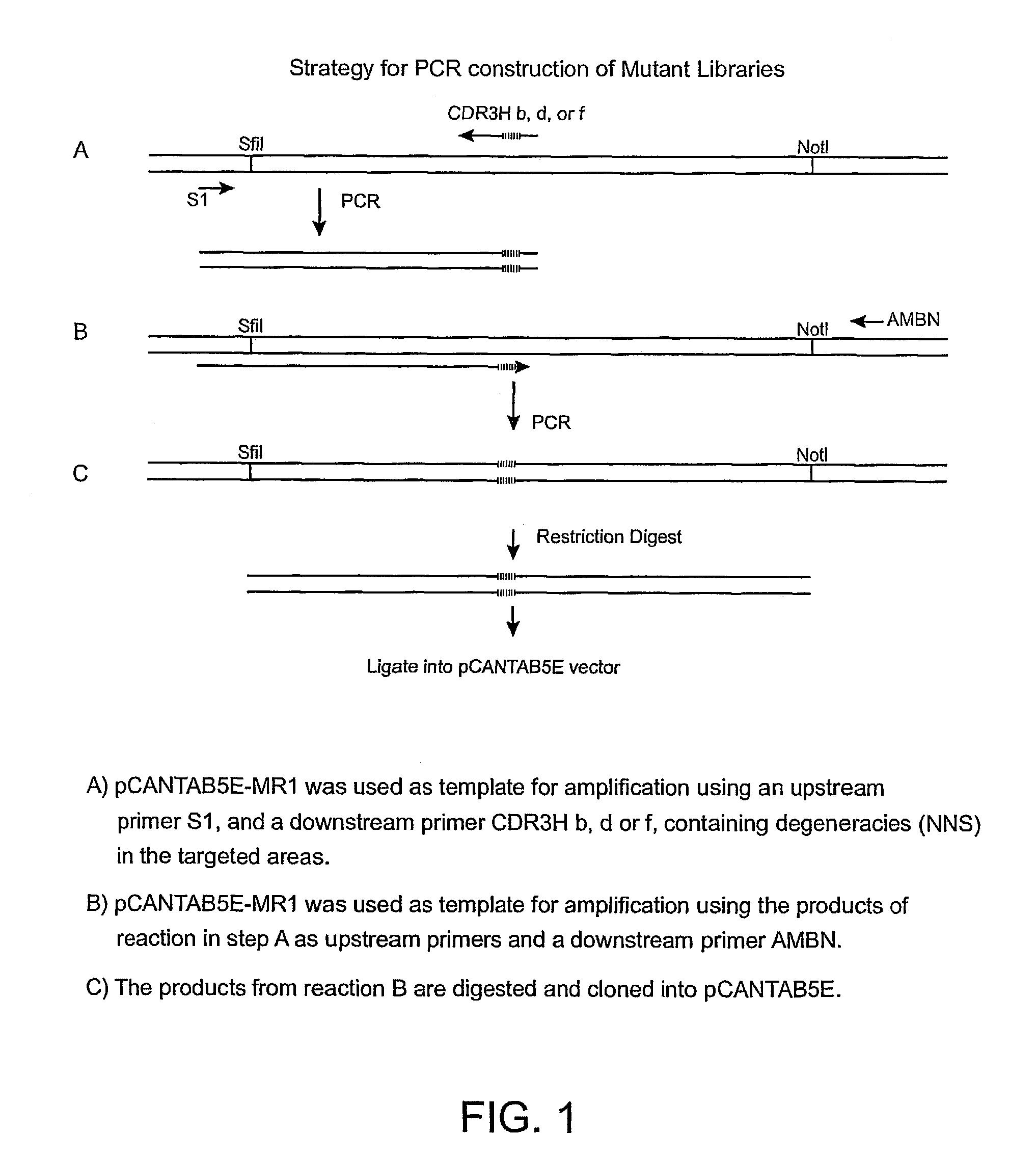
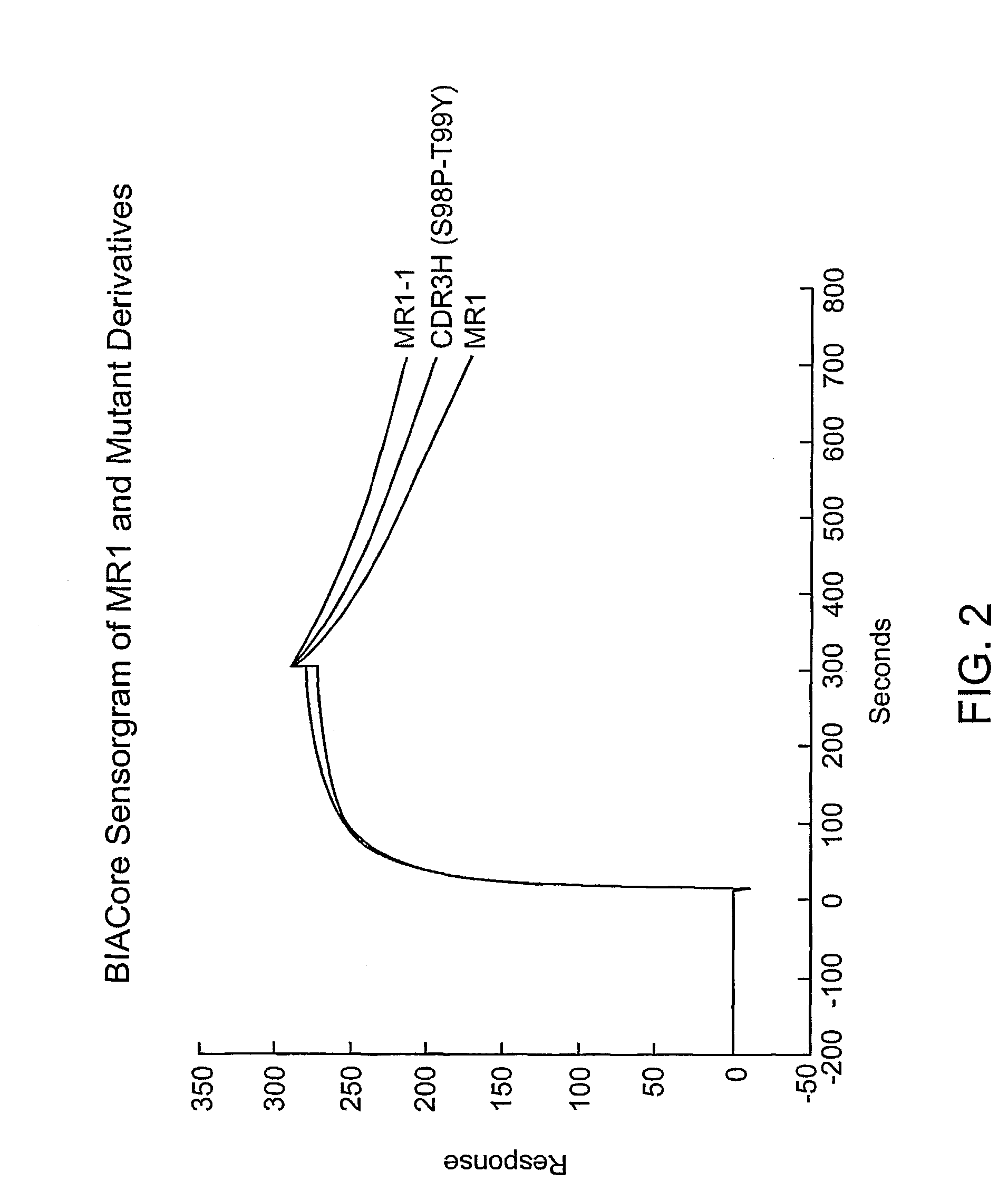
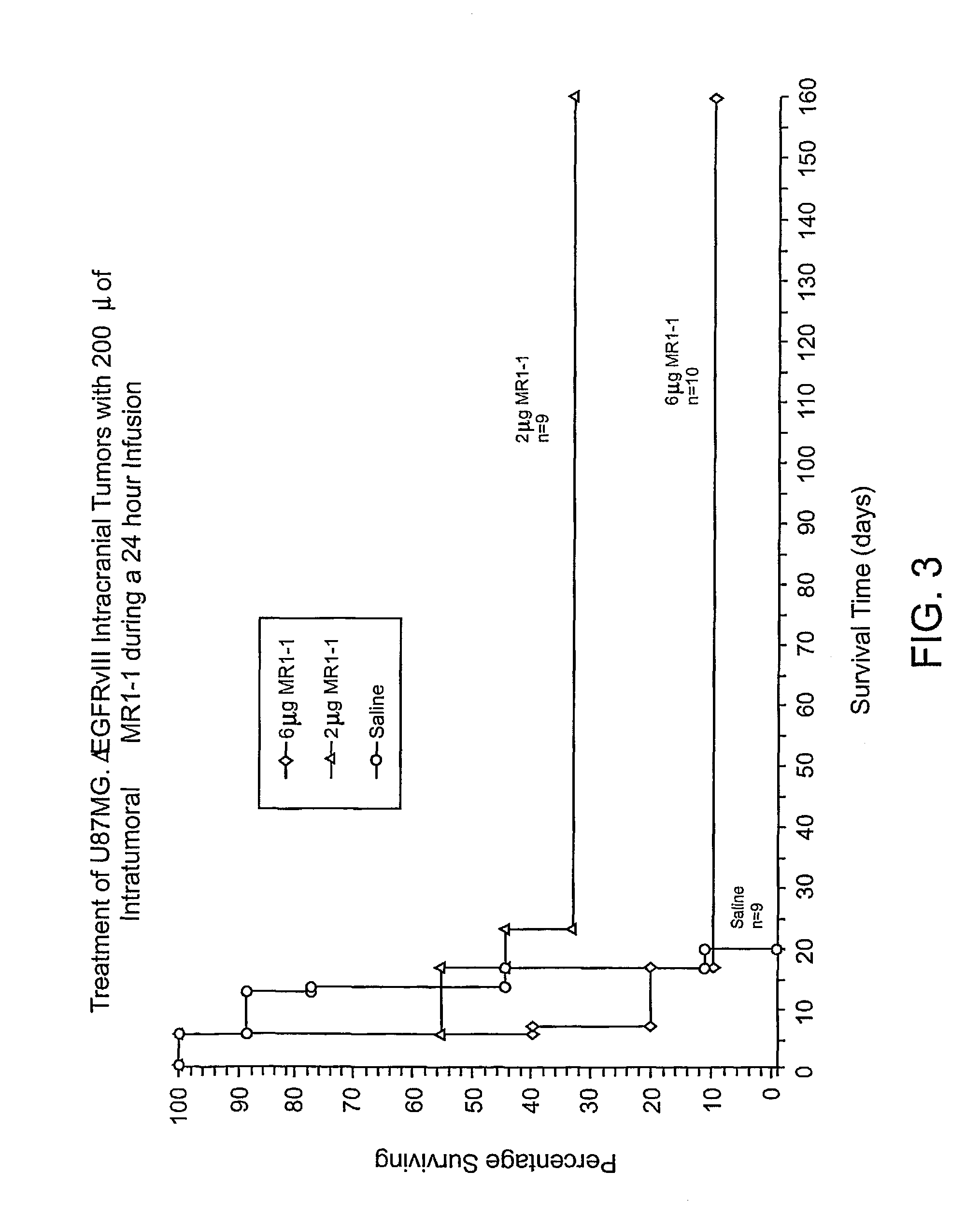
The invention provides antibodies to a mutant form of the epidermal growth factor receptor known as EGFRvIII found only or primarily on the surface of glioblastoma cells, and on cells of breast, ovarian and non-small cell lung carcinomas. The antibodies provided by the invention have the complementarity determining regions (“CDRs”) of the scFv designated MR1, but with mutations at positions 98 and 99 in the CDR3 of the heavy chain variable region and, optionally, in other CDRs. In particular, the invention provides an antibody, designated MR1-1, which mutates MR1 in the CDR3 of the VH and VL chains. The invention provides additional antibodies in which MR1 is mutated in the CDR1 and 2 of VH or VL, or both.
Owner:UNITED STATES OF AMERICA +2
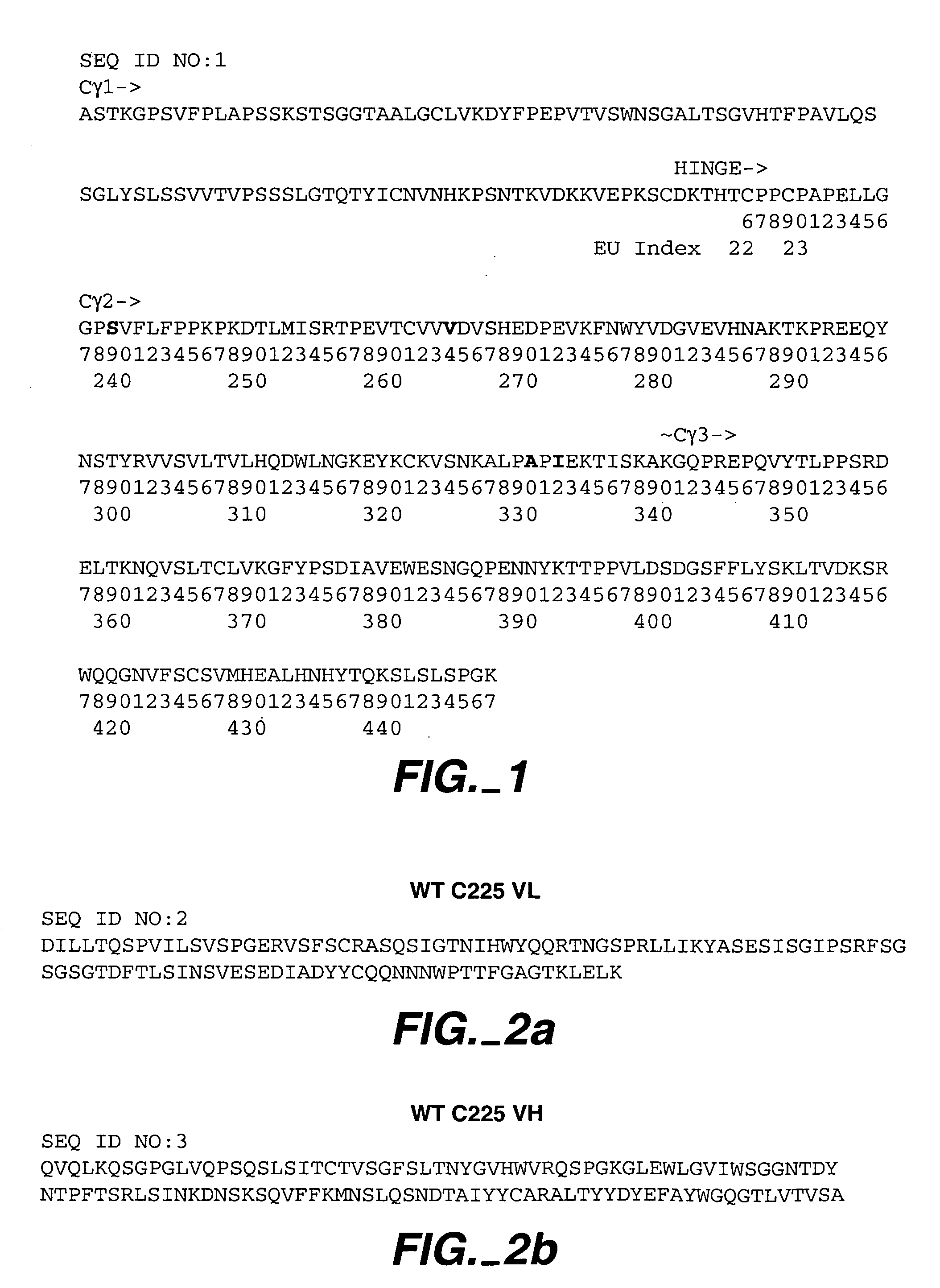
Human anti-epidermal growth factor receptor antibody
ActiveUS7598350B2Neutralize EGFRInhibit bindingSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsSingle-Chain AntibodiesHuman epidermal growth factor receptor
Owner:IMCLONE SYSTEMS
Treatment Of Tumors Expressing Mutant EGF Receptors
InactiveUS20090311803A1Reduces and prevents signalingShrink tumorAntibody ingredientsImmunoglobulinsAntibodyEGF Receptors
The invention discloses methods for identifying antibodies that reduce or prevent signaling by intact epidermal growth factor receptor (EGFR), or mutant EGFRs, such as EGFRvIII.
Owner:WAY JEFFREY C +4
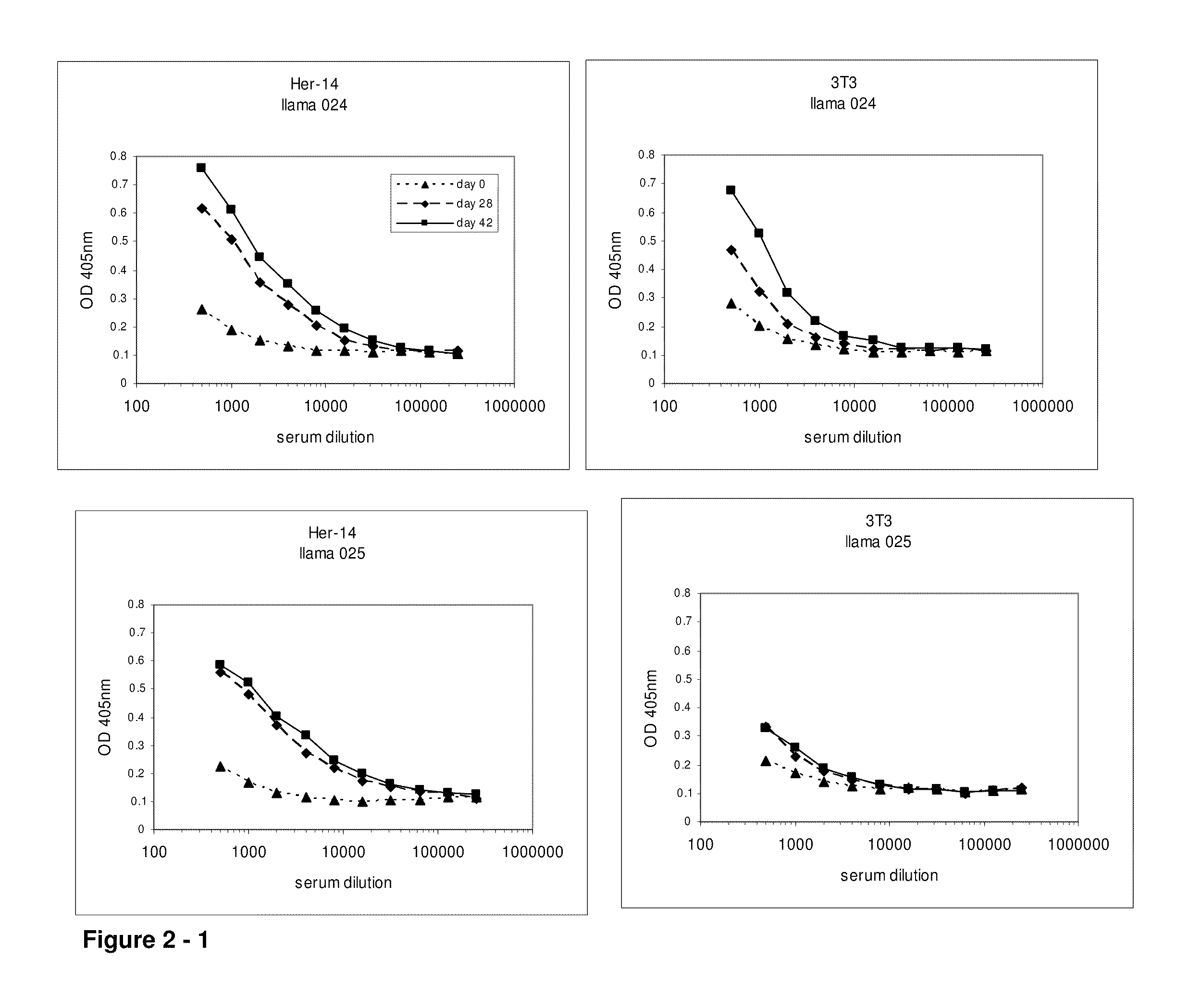
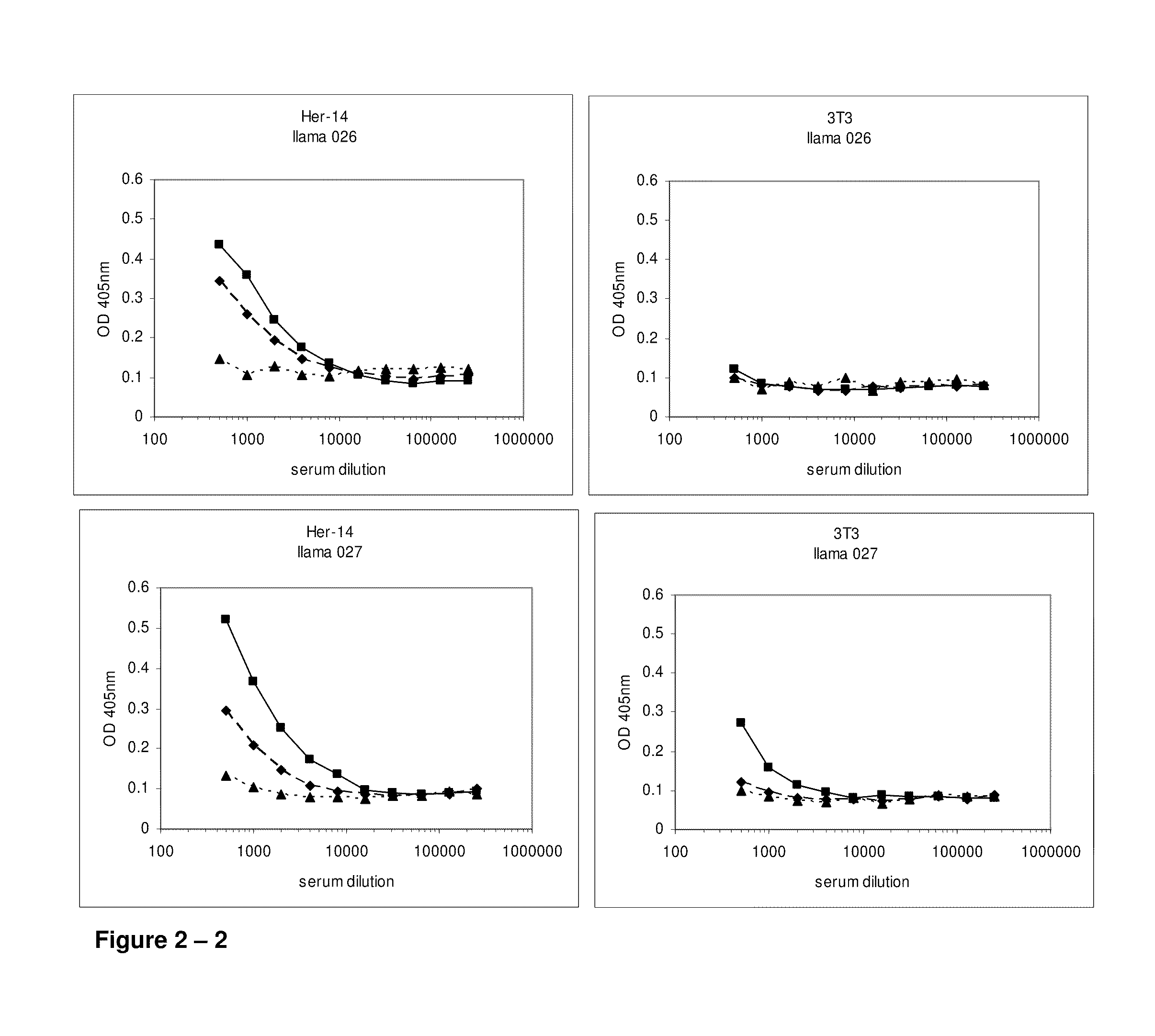
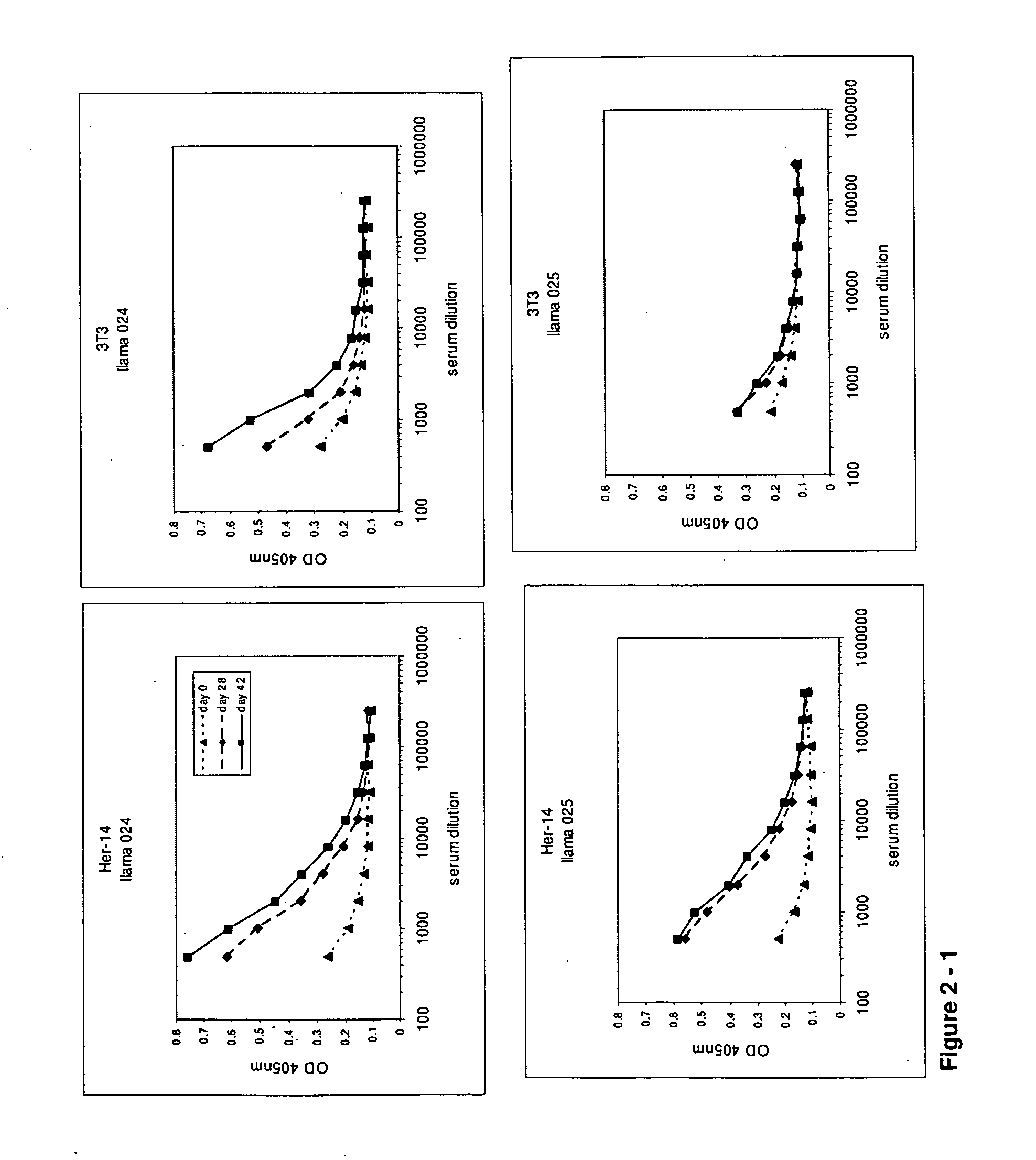
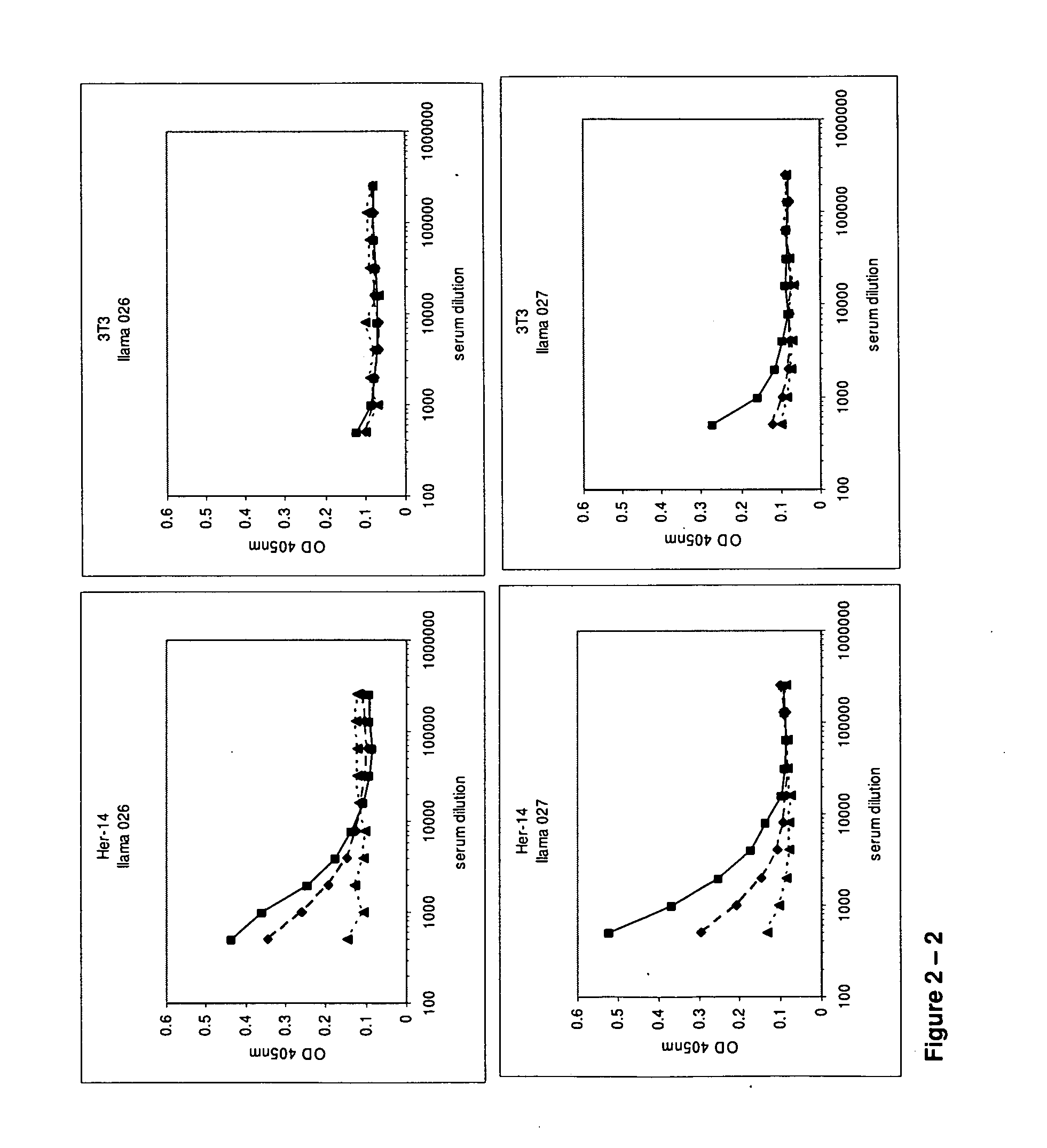
Camelidae single domain antibodies vhh directed against epidermal growth factor receptor and uses therefor
InactiveUS20060228355A1Improve breathabilityPreventing and alleviating symptomAnimal cellsSugar derivativesSingle-domain antibodyEpidermal growth factor receptor
The present invention relates to antibodies directed to Epidermal Growth Factor Receptor that are single domain antibodies Camelidae VHHs. It further relates to methods of of use of said polypeptides.
Owner:ABLYNX NV
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
InactiveUS7595378B2Less immunogenicReduce adverse side effectsAntibacterial agentsNervous disorderV(D)J recombinationDiagnostic methods
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB AS
Dual-signal independent chimeric antigen receptors (dsCAR) and uses thereof
ActiveCN103483452APromote proliferationHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsViral infectious disease
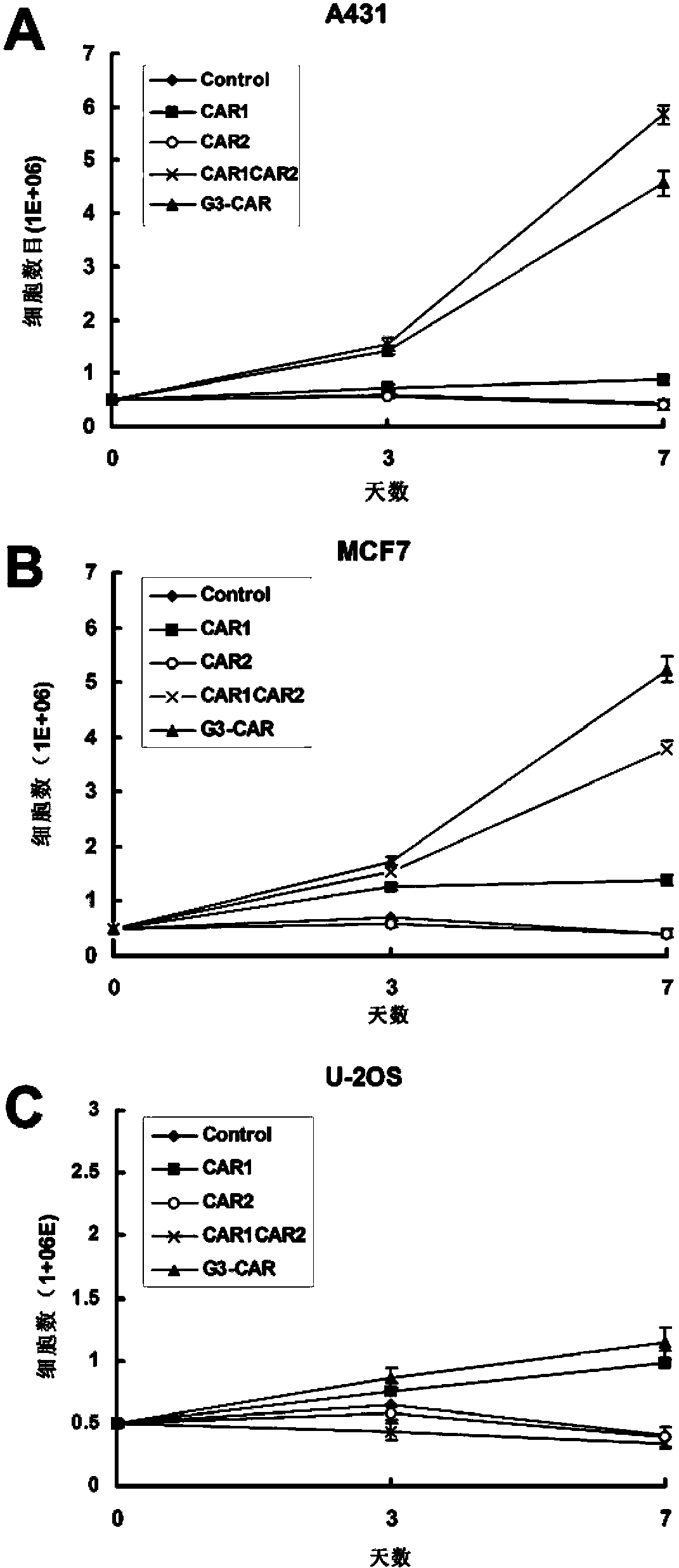
The invention relates to chimeric antigen receptors (CAR), particularly relates to dual-signal independent chimeric antigen receptors (dsCAR), and also relates to immune response cells of the dual-signal independent chimeric antigen receptors (dsCAR) and uses of the immune response cells in preparation of drugs for treatment of malignant tumor and virus infected diseases. In detail, the dual-signal independent chimeric antigen receptors (dsCAR) can respectively identify two different family antigens of tumor cells and can respectively transmit two T-cell-activation related signals. One of the CAR can transmit a first T-cell-activation related signal by combing a ligand of a tumor specific antigen or a tumor-associated antigen to decide T-cell killing specificity, and the other CAR can transmit a second T-cell-activation related signal by combing a ligand of a membrane receptor (such as EGFR (epidermal growth factor receptor) family protein) widely expressed by the tumor cells to promote T cell activation, proliferation and survival. The dual-signal independent chimeric antigen receptors (dsCAR) can avoid the potential safety problems on the basis of maintaining curative effects of second generation and third generation CAR.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
Treatment of tumors expressing mutant EGF receptors
InactiveUS20070274991A1Shrink tumorSlowing and preventing in size of tumorAntibody ingredientsImmunoglobulinsEpitopeWilms' tumor
The invention discloses methods of treatment of tumors that express oncogenic forms of the epidermal growth factor receptor (EGFR), such as EGFRvIII. The methods include testing of cancer patients for expression of EGFRvIII in their tumors, followed by treatment with a protein that contains antibody variable regions that recognize specific epitopes on the surface of the EGFR.
Owner:MASSACHUSETTS INST OF TECH +1
Treatment of refractory human tumors with epidermal growth factor receptor and HER1 mitogenic ligand (EGFRML) antagonists
InactiveUS20030202973A1Reduce HAMA responseMinimize side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman tumorHuman epidermal growth factor receptor
A method of inhibiting the growth of refractory tumors that are stimulated by mitogenic ligands of epidermal growth factor receptor in human patients, comprising treating the human patients with an effective amount of a mitogenic ligand antagonist.
Owner:DR GEORGE PIECZENIK
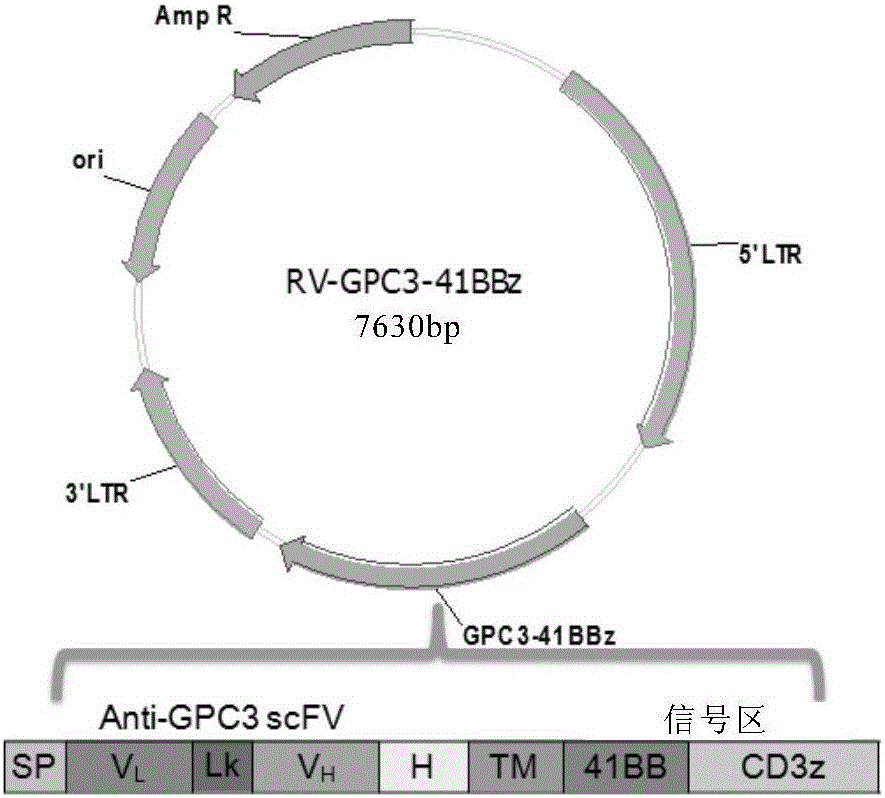
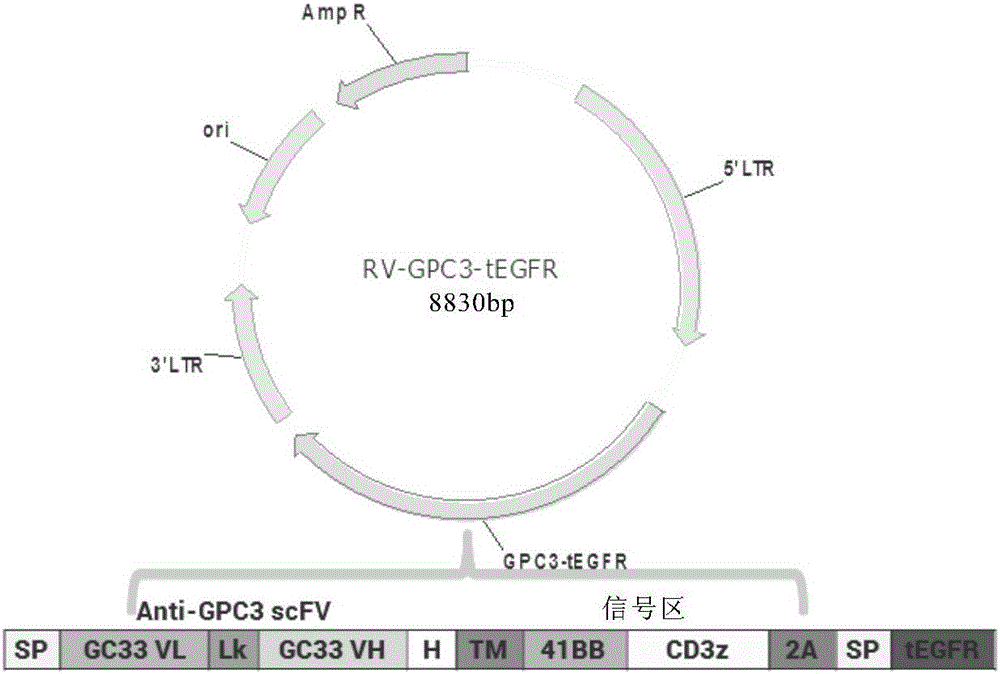
Chimeric antigen receptor of targeted GPC3 (Glypican 3) and application thereof
The invention relates to a chimeric antigen receptor of a targeted GPC3 (Glypican 3) and an application thereof. Specifically, the invention provides a polynucleotide sequence, wherein the polynucleotide sequence is selected from: polynucleotide sequences (1) containing a coding sequence of a CD8 antigen leading peptide, a coding sequence of an anti-GPC3 single-chain antibody, a coding sequence of a human CD8 alpha hinge region, a coding sequence of a human CD8 transmembrane region, a coding sequence of a human 41BB intracellular region, and a coding sequence of a human CD3 zeta intracellular region, and a coding sequence of an optional fragment of an EGFR (Epidermal Growth Factor Receptor) sequence containing an extracellular domain III and an extracellular domain IV, wherein the coding sequences are sequentially connected; and complementary sequences (2) of the polynucleotide sequences (1). The invention provides a CAR (chimeric antigen receptor) coded by the polynucleotide sequences and a T cell expressing the CAR. The prepared CAR-T cell has a function of strongly killing a specific tumor cell, and the kill effectiveness is more than 90% under the condition that the effector-target ratio is 20:1.
Owner:HRAIN BIOTECHNOLOGY CO LTD
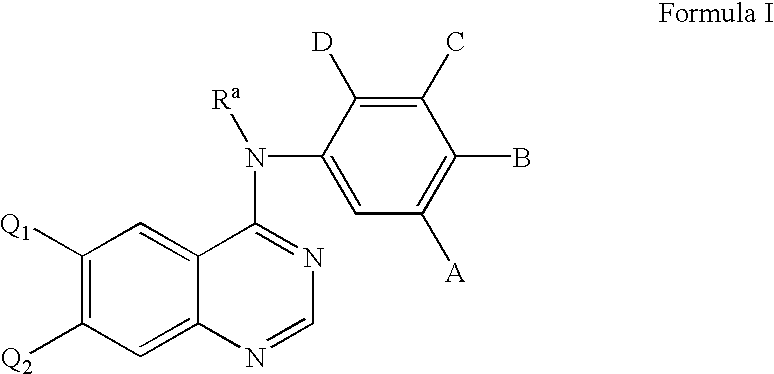
Amino-quinazoline derivative with antineoplastic activity and its salts
The present invention provides an amido quinazoline derivative which has recipient singal conductance for inhibition of epidermal growth factors with anti-tumor activity. The novel compounds with a structure identical to quinazoline has quite high activity for inhibition of tumor cells, in particular to the remarkable inhibition effects on the growth of tumor cells of EGFR high expression. And the effective inhibition concentration is 5 times higher than the medicine IRESSA on the market.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
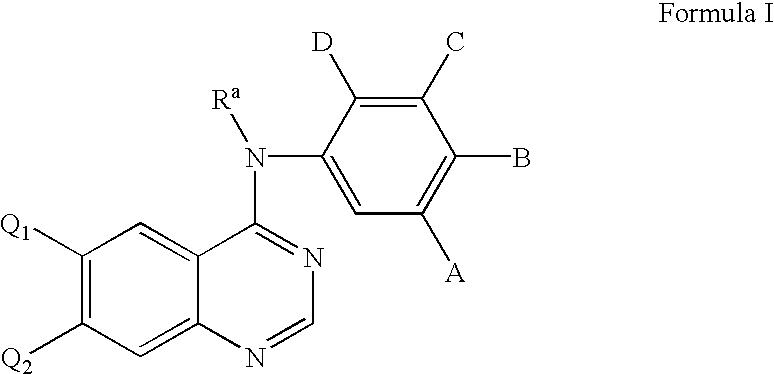
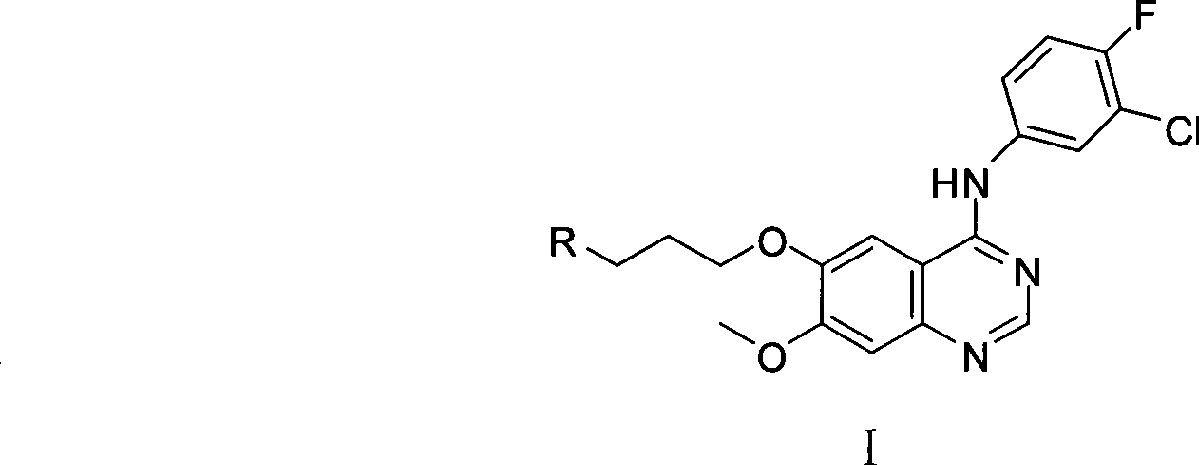
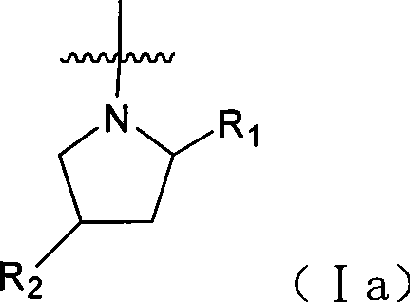
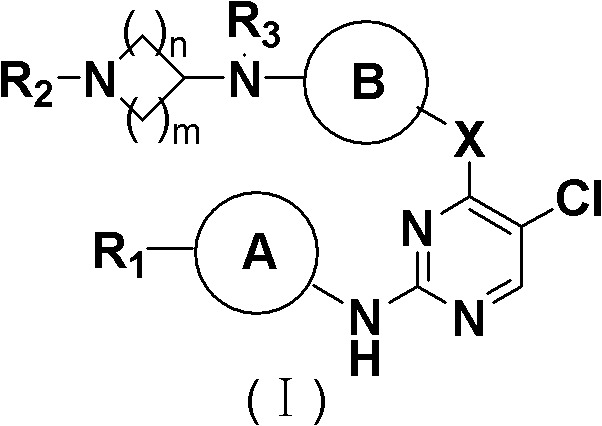
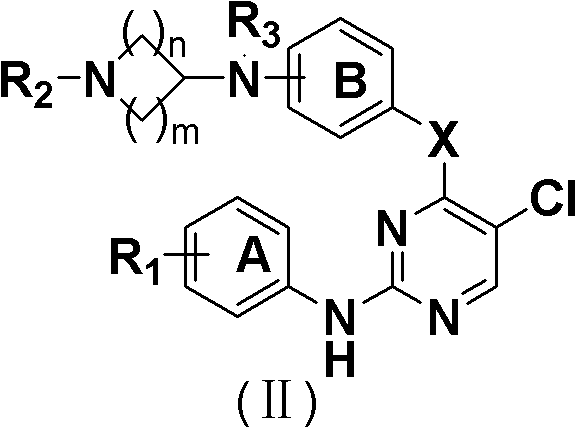
5-Chloropyrimidine compound and application of 5-Chloropyrimidine compound serving as epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor
ActiveCN103159742AHigh inhibitory strengthSmall toxicityNervous disorderOrganic chemistryEGFR Tyrosine Kinase InhibitorsHuman epidermal growth factor receptor
The invention discloses an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, and the structural formula of the inhibitor is shown as in the formula (I). The invention further discloses an application of any compound shown as in the formula (I) and pharmacy-acceptable salt of the compound. The invention further provides a medicine compound for curing, and the medicine compound comprises the compound shown as in the formula (I) and an EGFR modifier.
Owner:BEIJING HANMI PHARMA CO LTD
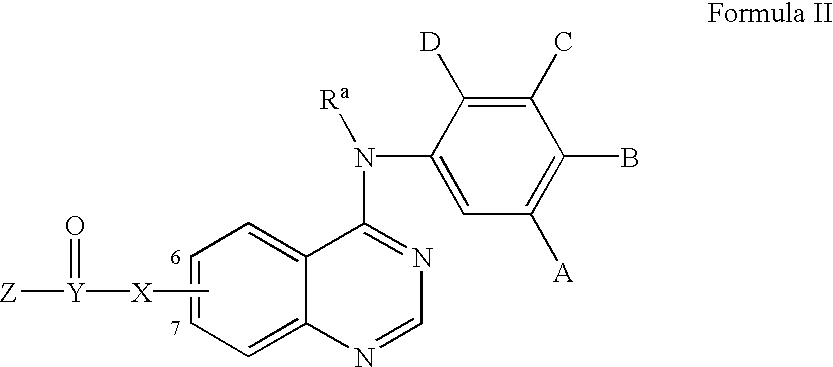
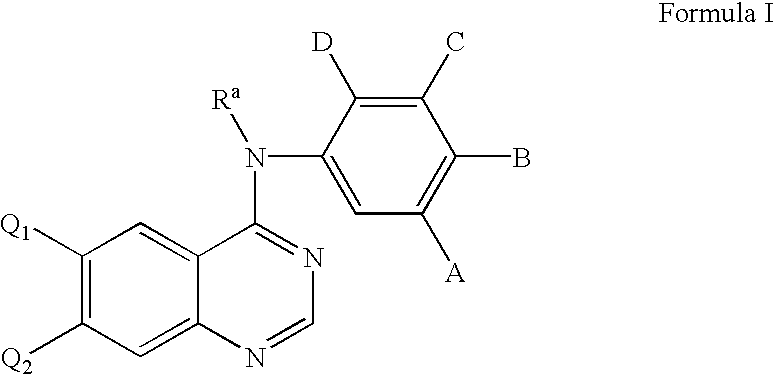
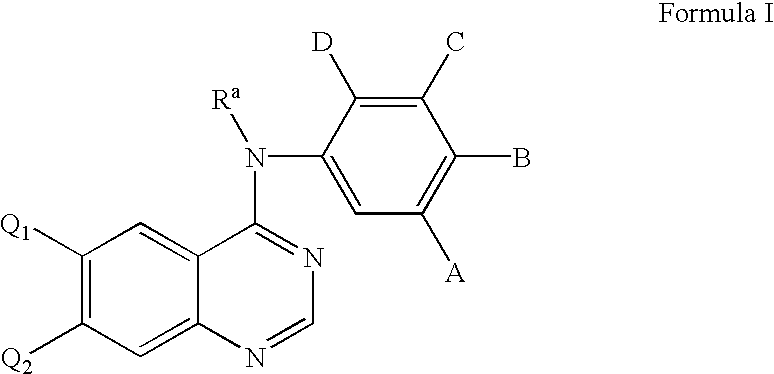
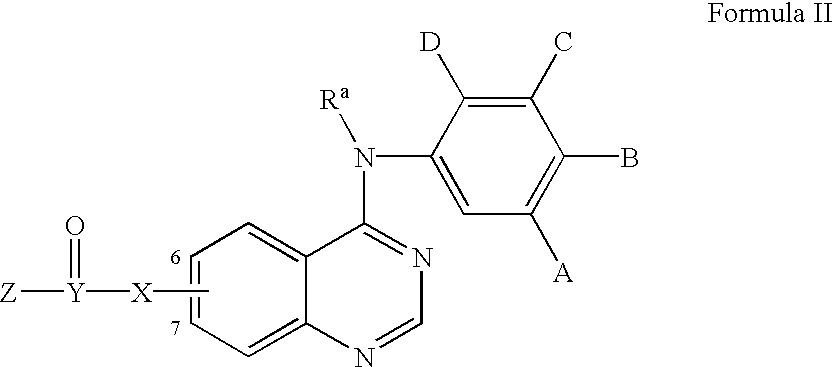
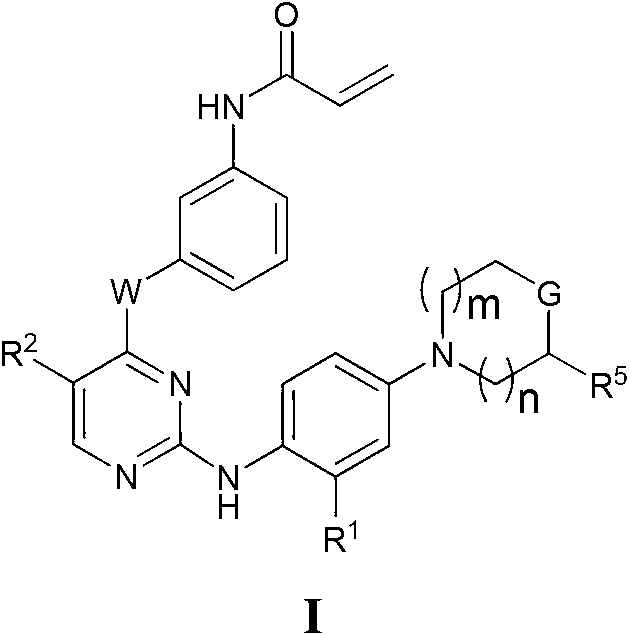
Heterocyclic compounds and uses thereof
Heterocyclic pyrimidine compounds that modulate mutant-selective epidermal growth factor receptor (EGFR) kinase activity are disclosed. Selectivity in inhibition of various mutant-EGFR is disclosed. Pharmaceutical compositions containing the pyrimidine derivatives, and methods of treating diseases associated with EGFR kinase activity comprising administration of the pyrimidine derivatives or pharmaceutical compositions containing the pyrimidine derivative, are described.
Owner:BRISTOL MYERS SQUIBB CO
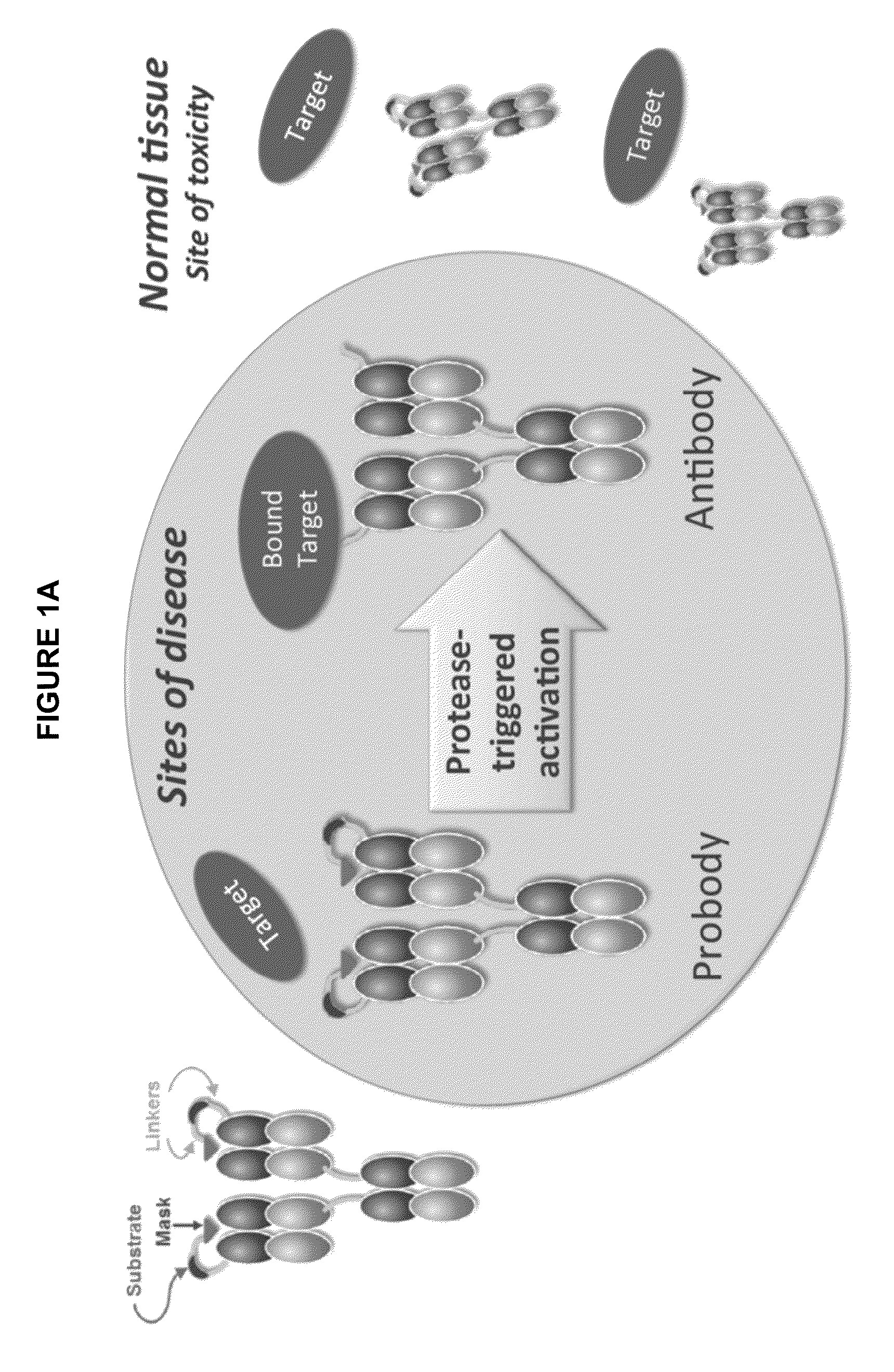
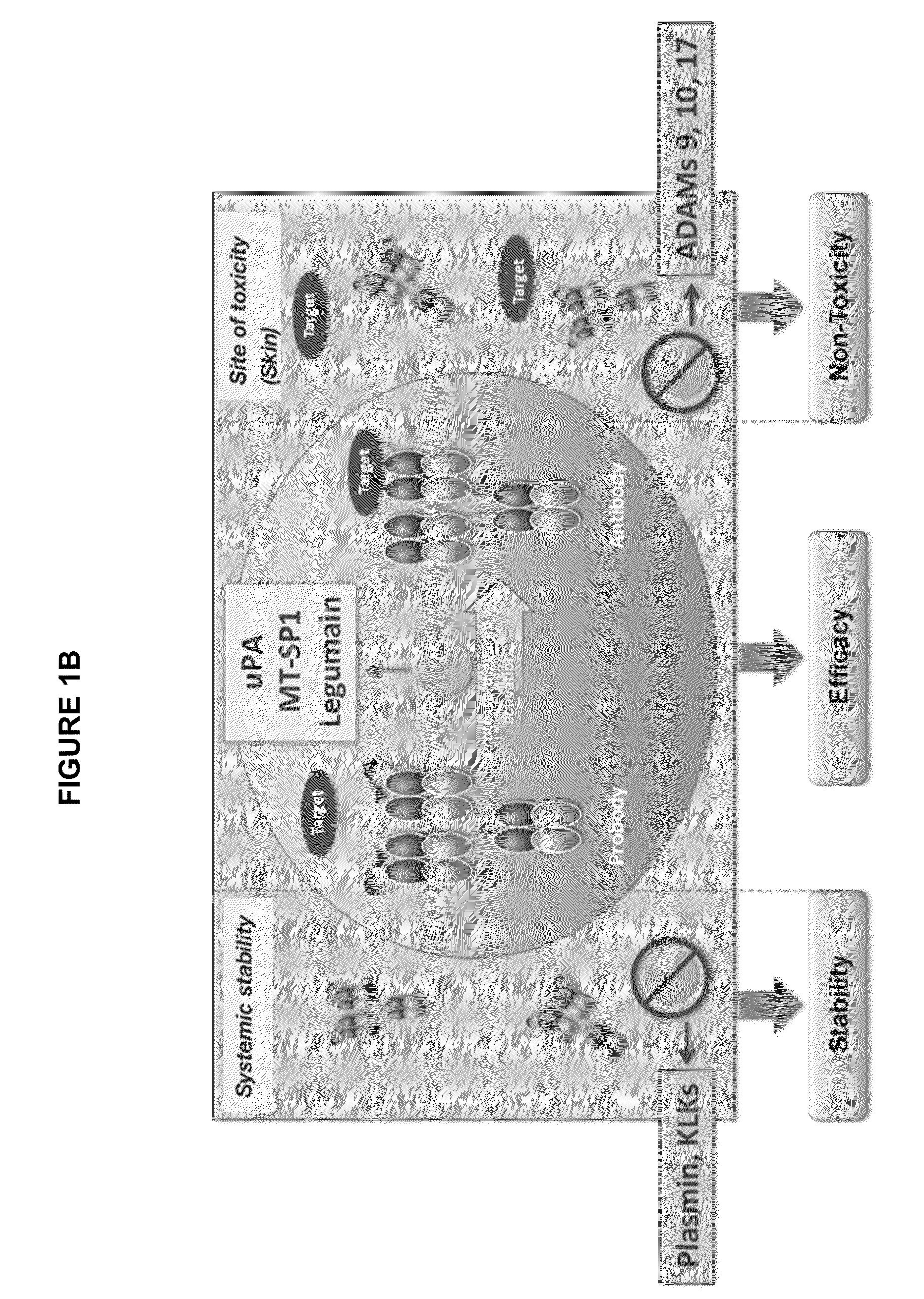
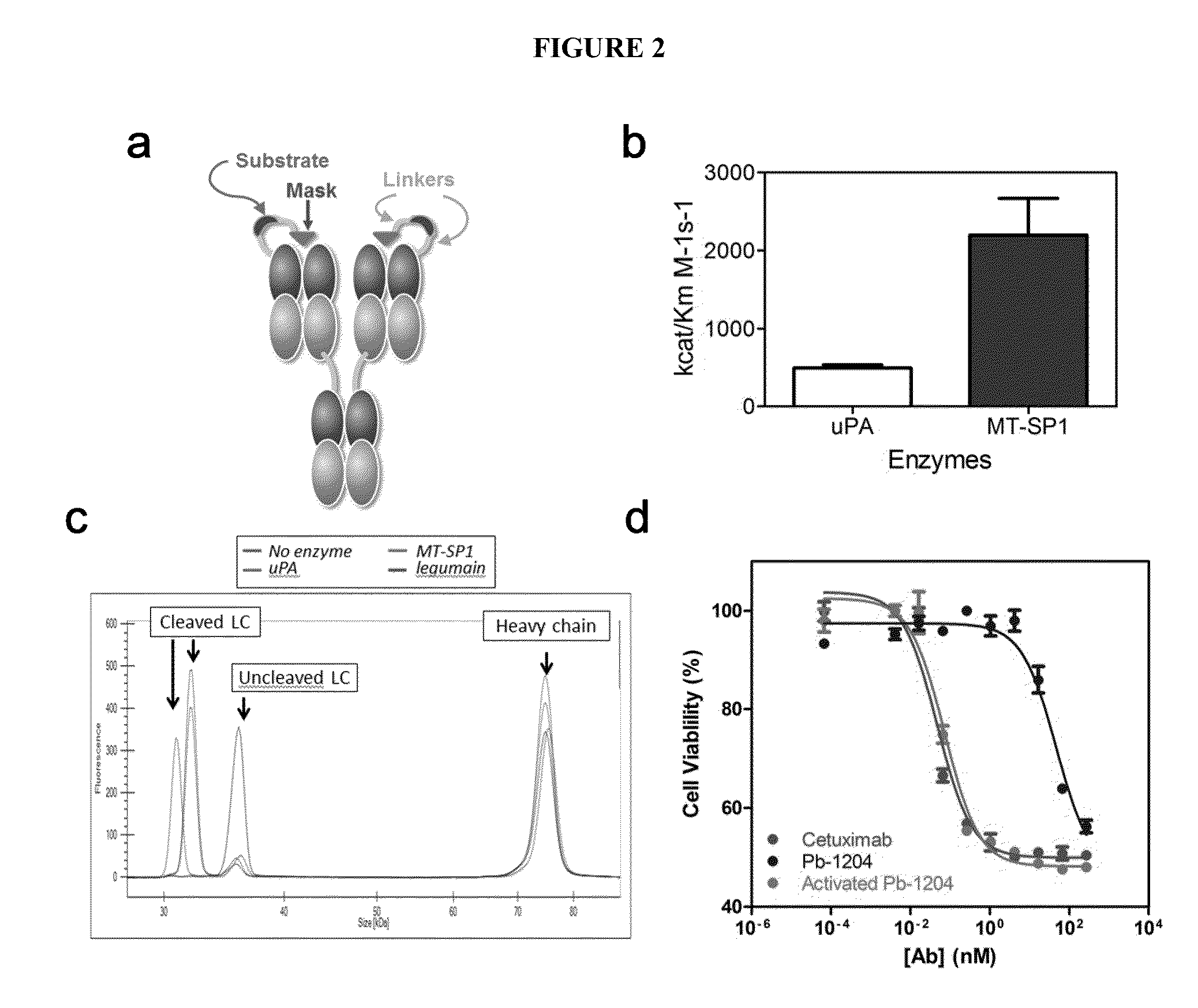
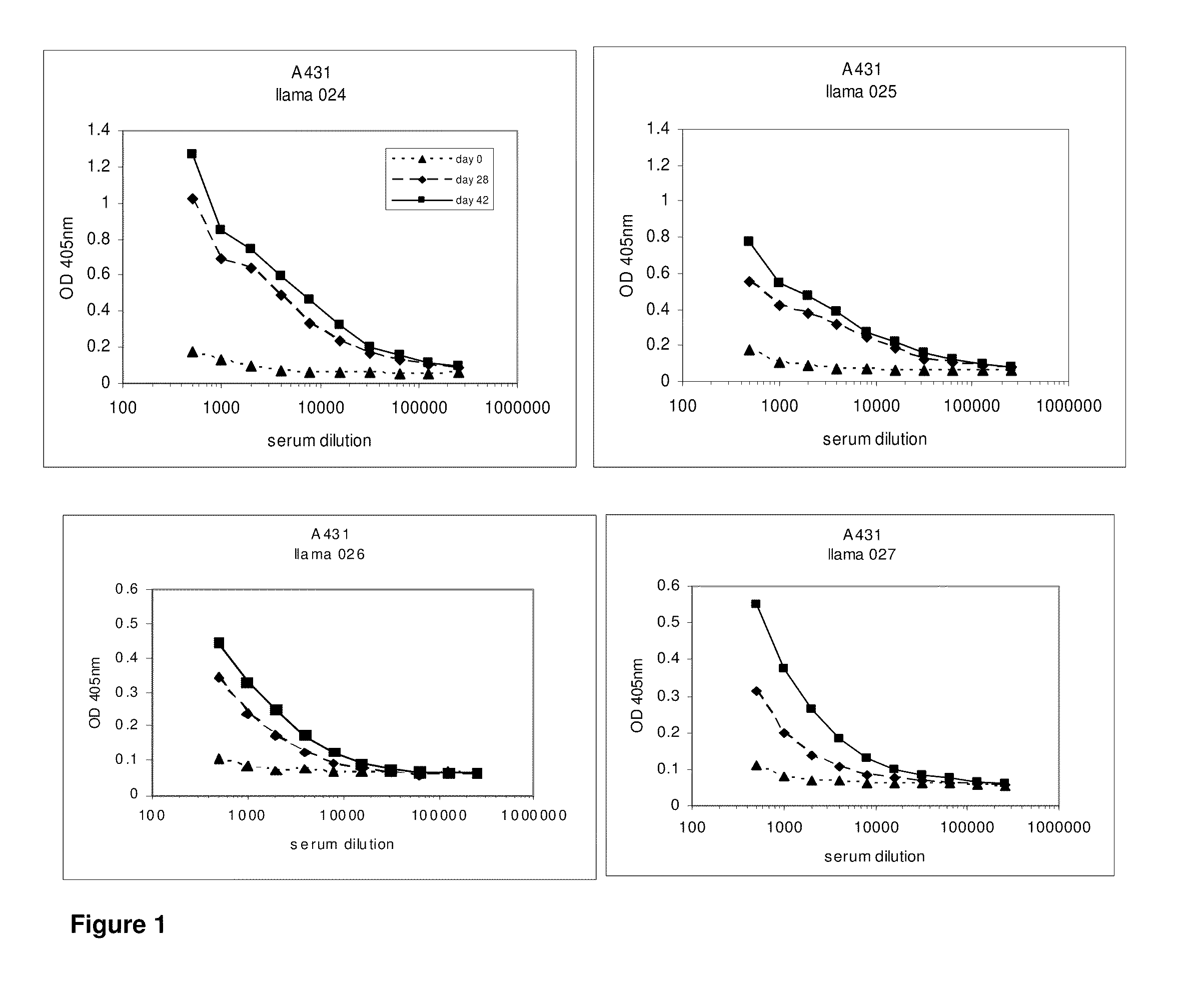
Activatable antibodies that bind epidermal growth factor receptor and methods of use thereof
Owner:CYTOMX THERAPEUTICS INC
Single domain antibodies directed against epidermal growth factor receptor and uses therefor
InactiveUS20110123529A1Preventing and alleviating symptomPeptide/protein ingredientsFermentationInhalationHuman epidermal growth factor receptor
The present invention relates to polypeptides derived from single domain heavy chain antibodies directed to Epidermal Growth Factor Receptor. It further relates to single domain antibodies that are Camelidae VHHs. It further relates to methods of administering said polypeptides orally, sublingually, topically, intravenously, subcutaneously, nasally, vaginally, rectally or by inhalation. It further relates to protocols for screening for agents that modulate the Epidermal Growth Factor Receptor, and the agents resulting from said screening. The invention further a method for delivering therapeutic molecules to the interior of cells.
Owner:ABLYNX NV
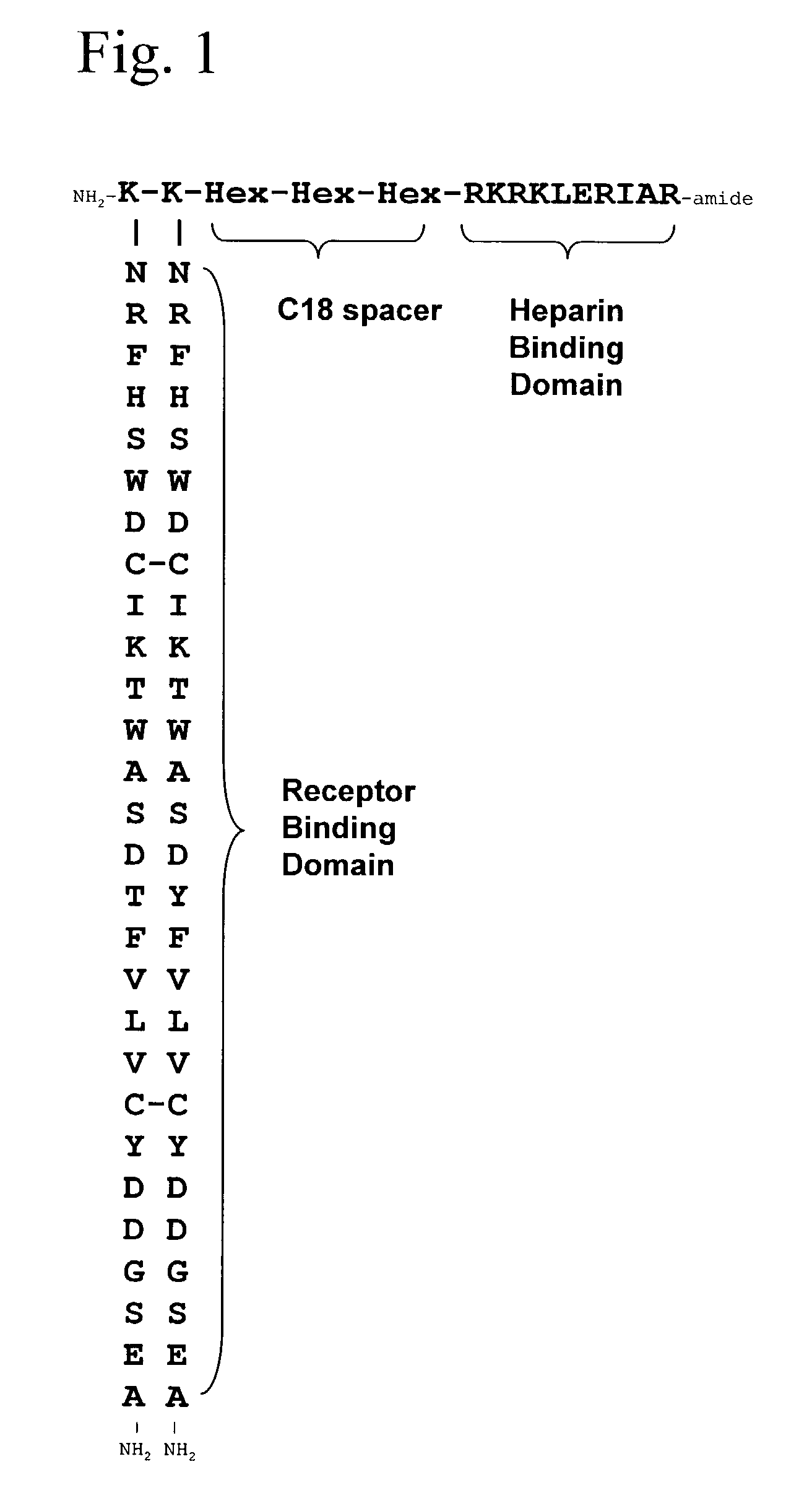
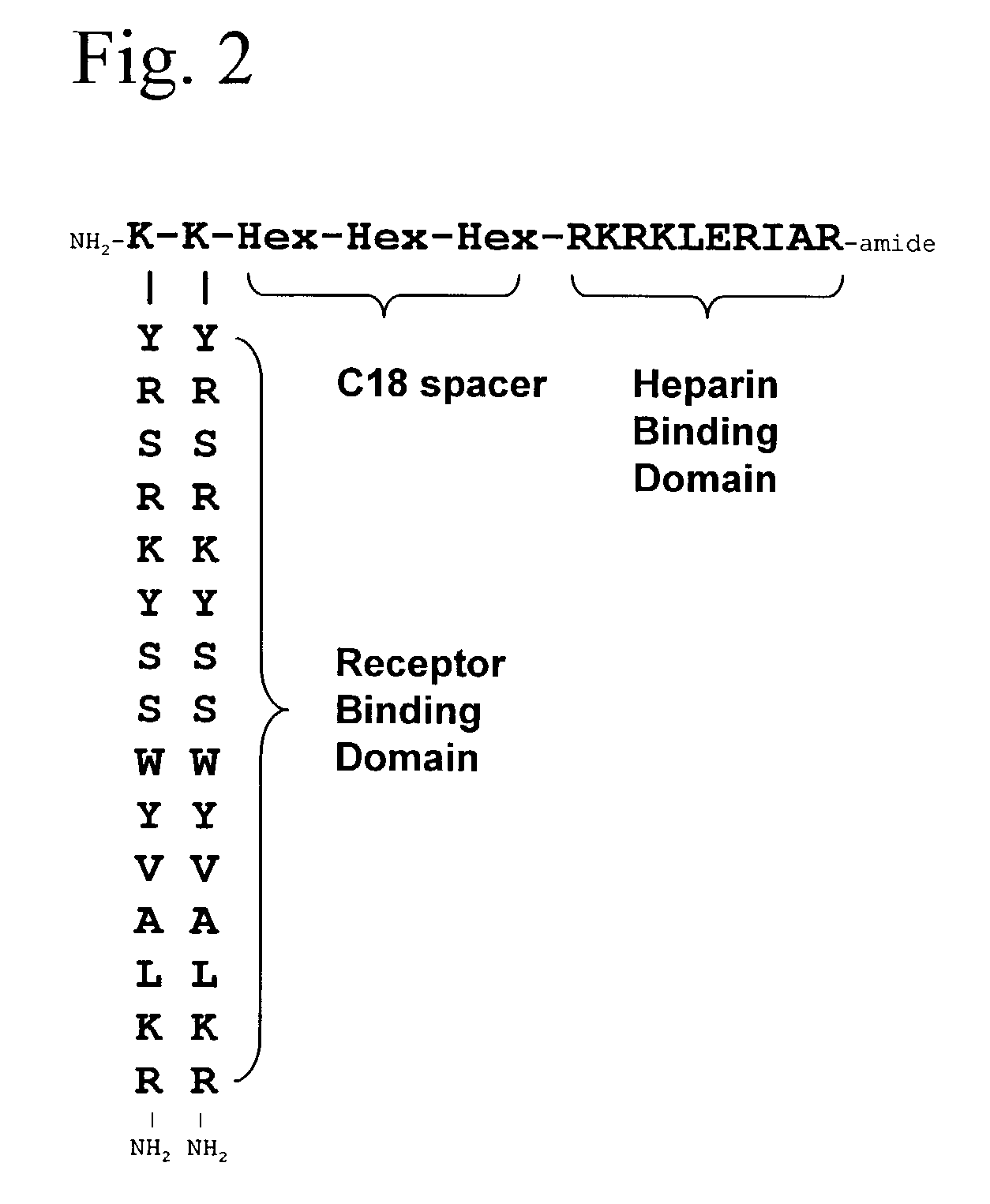
Synthetic heparin-binding growth factor analogs
InactiveUS7166574B2Ameliorate harmful effect of radiationPeptide/protein ingredientsAntibody mimetics/scaffoldsFactor iiBinding domain
The invention provides synthetic heparin-binding growth factor analogs having at least one peptide chain that binds a heparin-binding growth factor receptor, covalently bound to a hydrophobic linker, which is in turn covalently bound to a non-signaling peptide that includes a heparin-binding domain. The synthetic heparin-binding growth factor analogs are useful as soluble biologics or as surface coatings for medical devices.
Owner:BROOKHAVEN SCI ASSOCS +1
Targeted therapeutics based on engineered proteins that bind EGFR
ActiveUS8524244B2Improves one or more pharmacokinetic properties of the polypeptidesIncreased serum half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideADAMTS Proteins
Owner:BRISTOL MYERS SQUIBB CO
Process for preparing aminocrotonylamino-substituted quinazoline derivatives
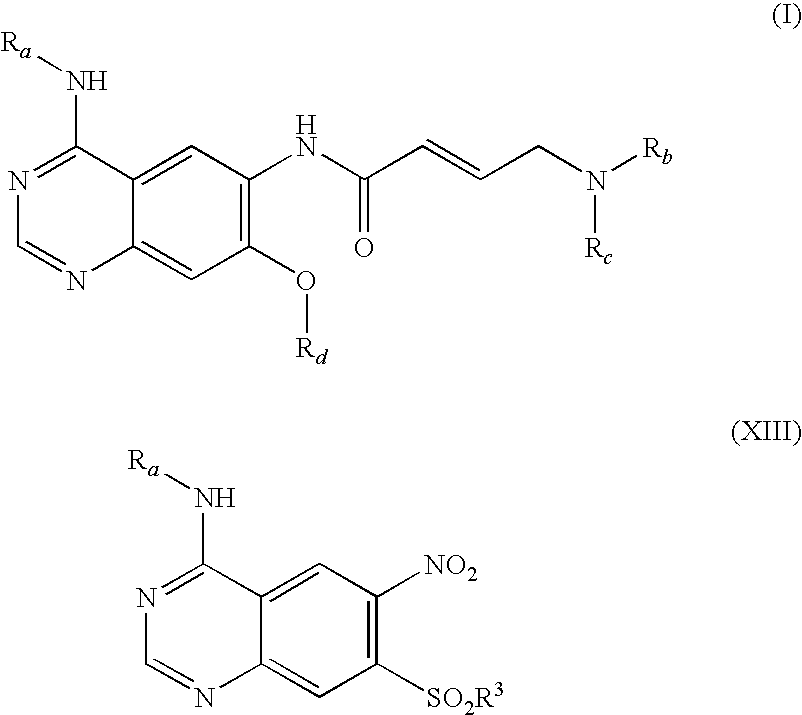
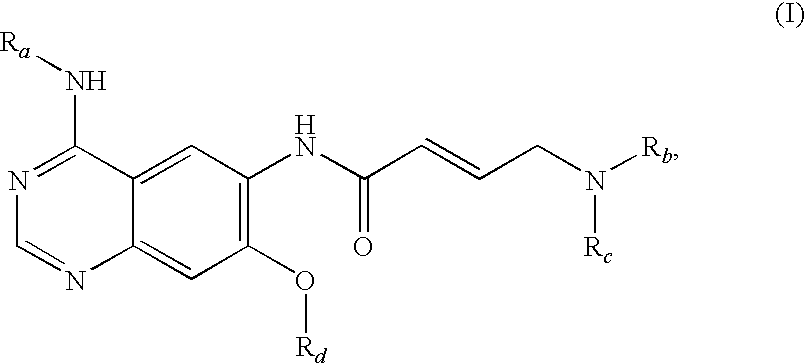
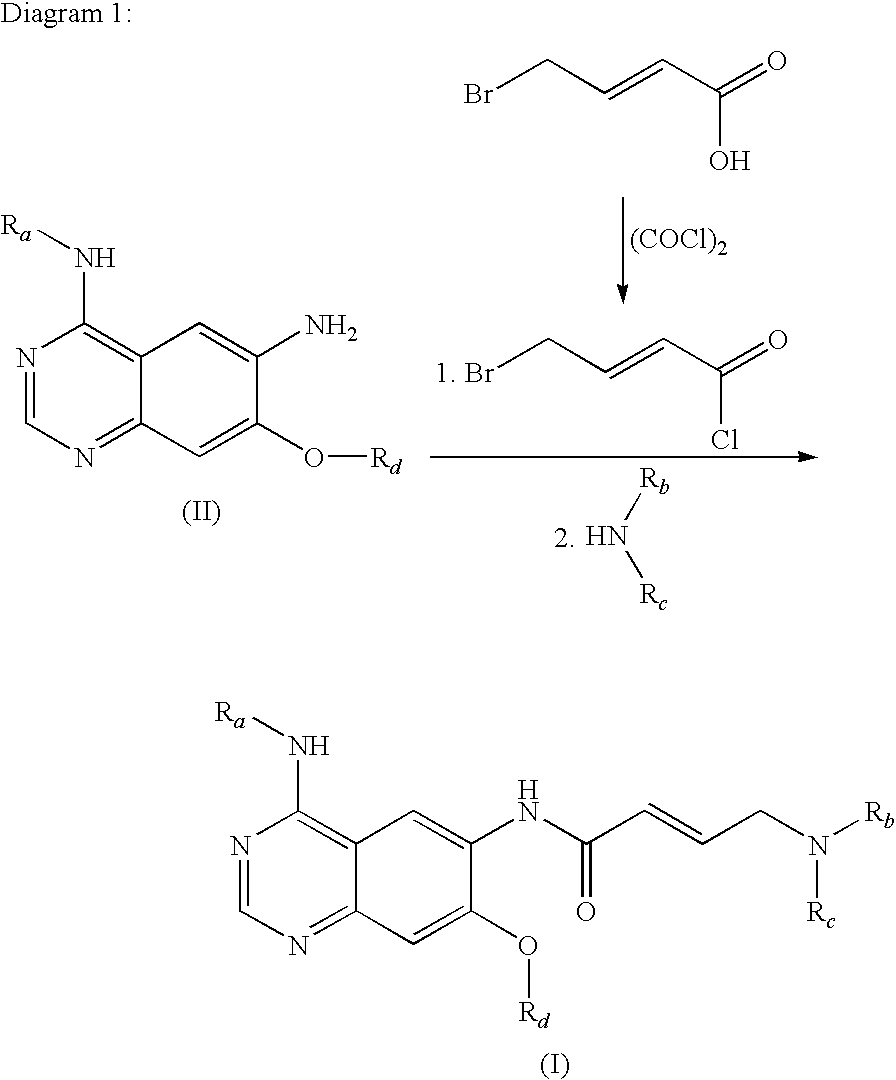
The invention relates to an improved process for preparing aminocrotonylamino-substituted quinazoline derivatives of general formula (I) wherein the groups Ra, Rb, Rc and Rd have the meanings given in the claims, as well as sulphonyl derivatives of formula (XIII) and the use thereof as synthesis components for preparing quinazolines of formula (I). The quinazoline derivatives of formula (I) are inhibitors of signal transduction mediated by tyrosinekinases and by the Epidermal Growth Factor-Receptor (EGF-R) and are therefore particularly suitable for the treatment of tumoral diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Single domain antibodies directed against epidermal growth factor receptor and uses therefor
InactiveUS20100003253A1Improve breathabilityPreventing and alleviating symptomImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsInhalationSingle-domain antibody
The present invention relates to polypeptides derived from single domain heavy chain antibodies directed to Epidermal Growth Factor Receptor. It further relates to single domain antibodies that are Camelidae VHHs. It further relates to methods of administering said polypeptides orally, sublingually, topically, intravenously, subcutaneously, nasally, vaginally, rectally or by inhalation. It further relates to protocols for screening for agents that modulate the Epidermal Growth Factor Receptor, and the agents resulting from said screening. The invention further a method for delivering therapeutic molecules to the interior of cells
Owner:ABLYNX NV
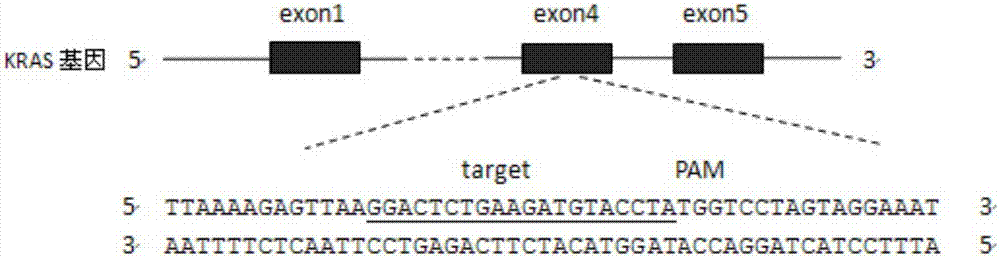
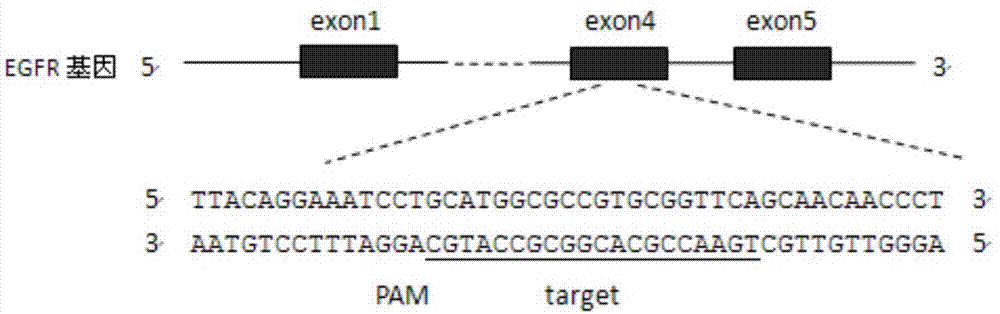
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 system capable of simultaneously knocking out KRAS genes and EGFR (Epidermal Growth Factor Receptor) genes and application thereof
ActiveCN107130000AHigh knockout efficiencyEasy to operateOrganic active ingredientsHydrolasesAbnormal expressionKRAS
The invention discloses a CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 system capable of simultaneously knocking out KRAS genes and EGFR genes. The system comprises sgRNA for specifically targeting KRAS genes and sgRNA for specifically targeting EGFR genes, wherein a corresponding DNA sequence of the sgRNA for specifically targeting KRAS genes is shown as SEQ ID NO.1 or / and SEQ ID NO.2; and a corresponding DNA sequence of the sgRNA for specifically targeting EGFR genes is shown as SEQ ID NO.11 or / and SEQ ID NO.12. The invention further discloses application of the system in preparation of medicines for treating cancers. The CRISPR-Cas9 system disclosed by the invention is capable of simultaneously and efficiently knocking out two cancer driving factors KRAS and EGFR which are highly-expressed in lung cancer. The system is simple in operation and high in knockout efficiency and is expected to be applied to treatment of the lung cancer. The system disclosed by the invention is applicable to multiple cancers with abnormal expressions of the EGFR and KRAS.
Owner:浙江卫未生物医药科技有限公司
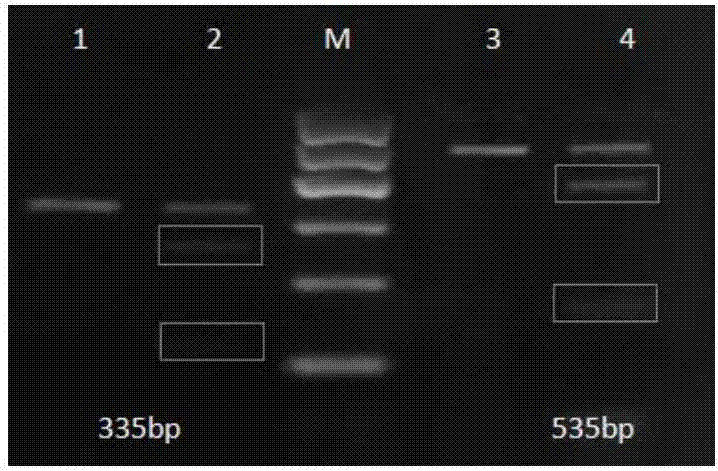
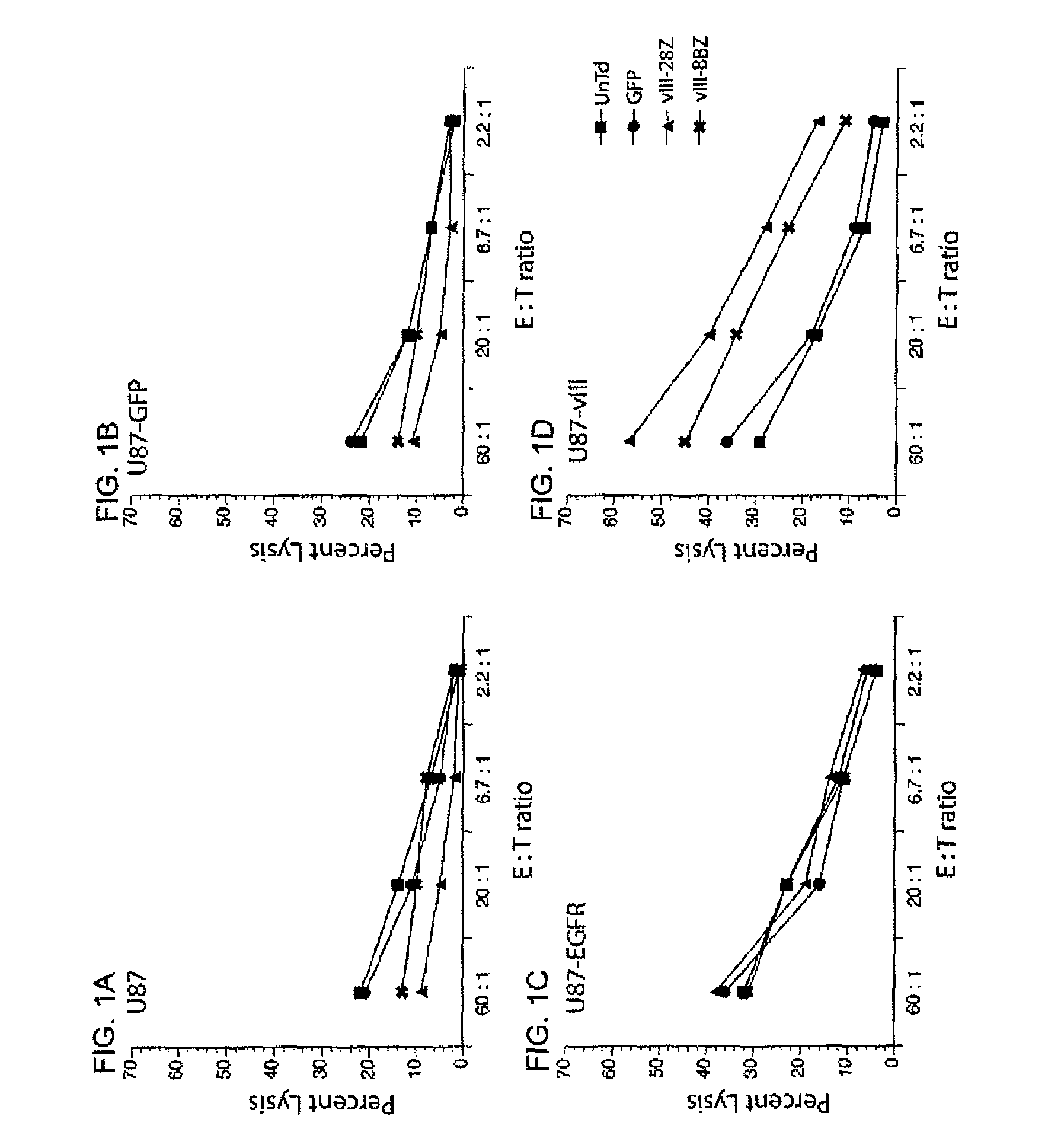
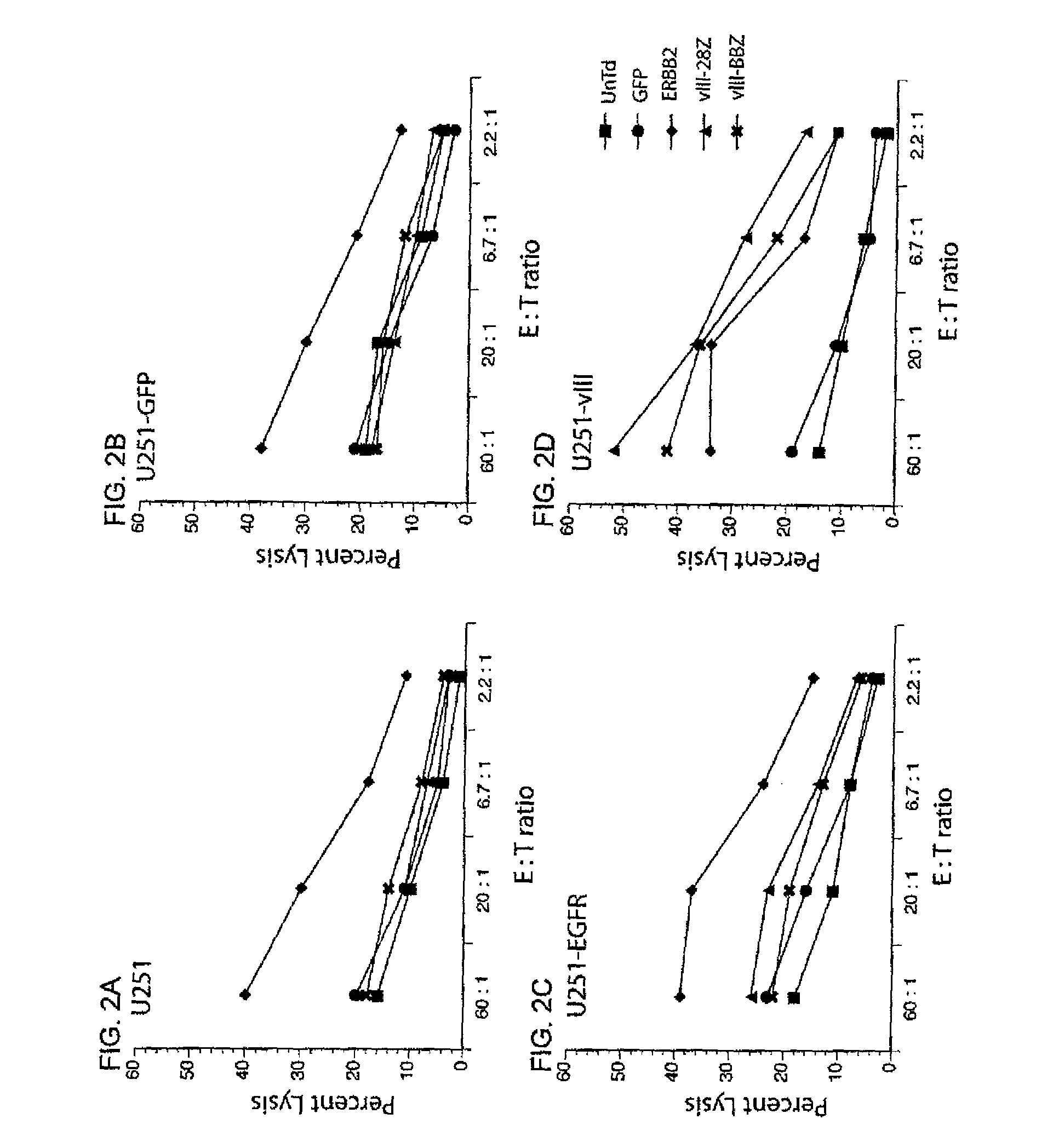
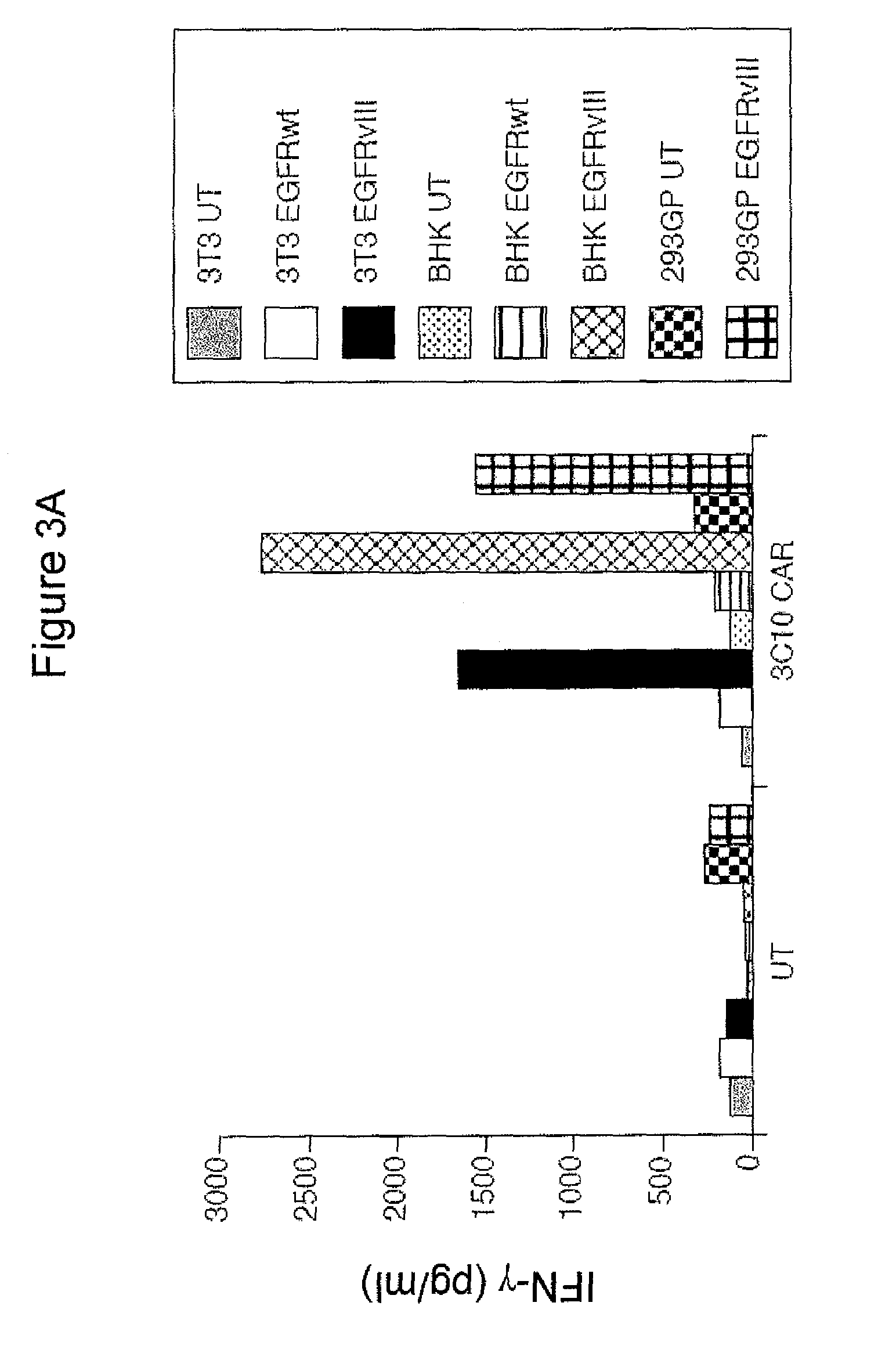
Anti-epidermal growth factor receptor variant III chimeric antigen receptors and use of same for the treatment of cancer
ActiveUS9266960B2Peptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsAntigen binding
The disclosure provides chimeric antigen receptors (CARs) comprising an antigen binding domain of human antibody 139, an extracellular hinge domain, a transmembrane domain, and an intracellular domain T cell receptor signaling domain. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a host and methods of treating or preventing cancer in a host are also disclosed.
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com