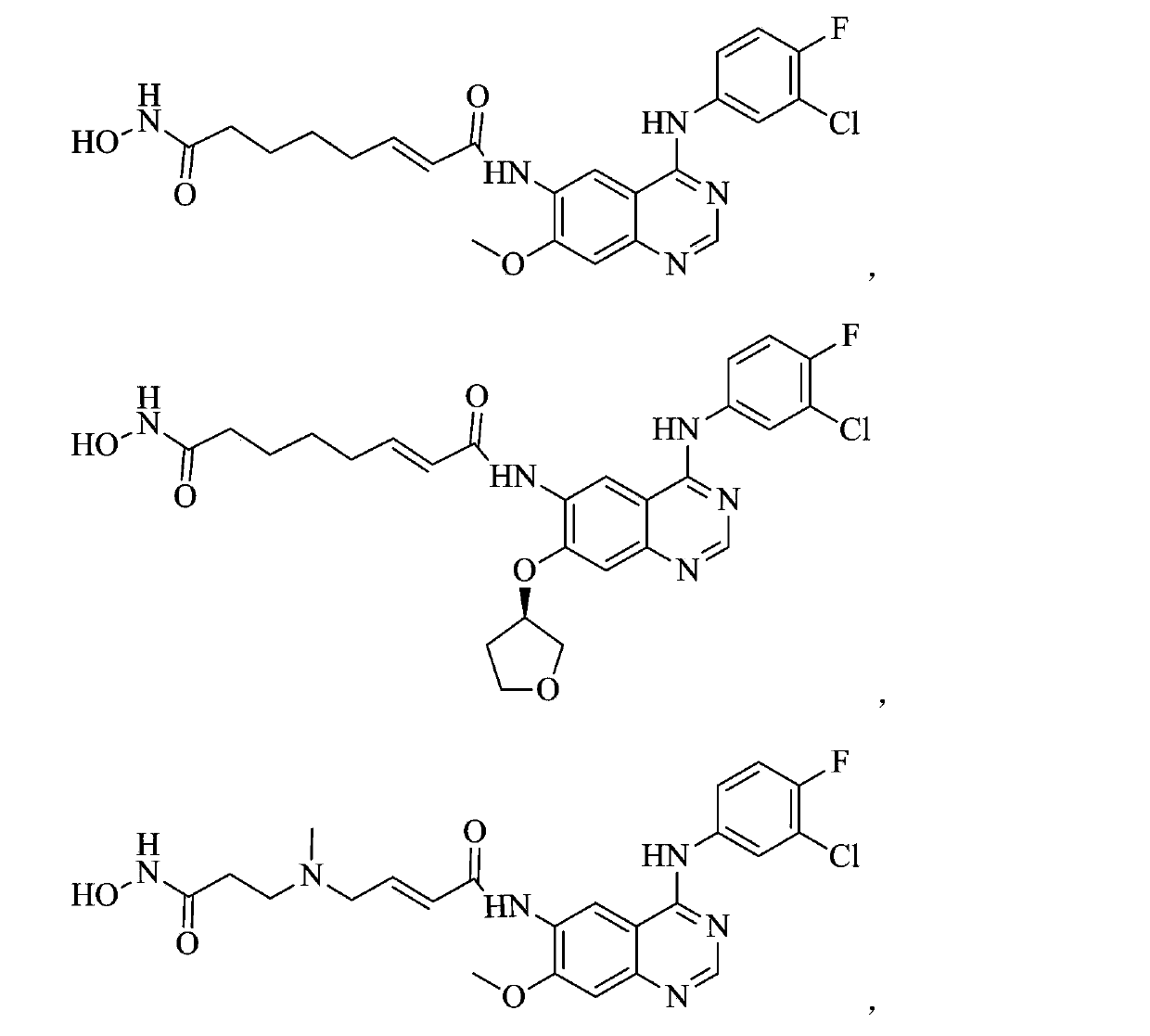
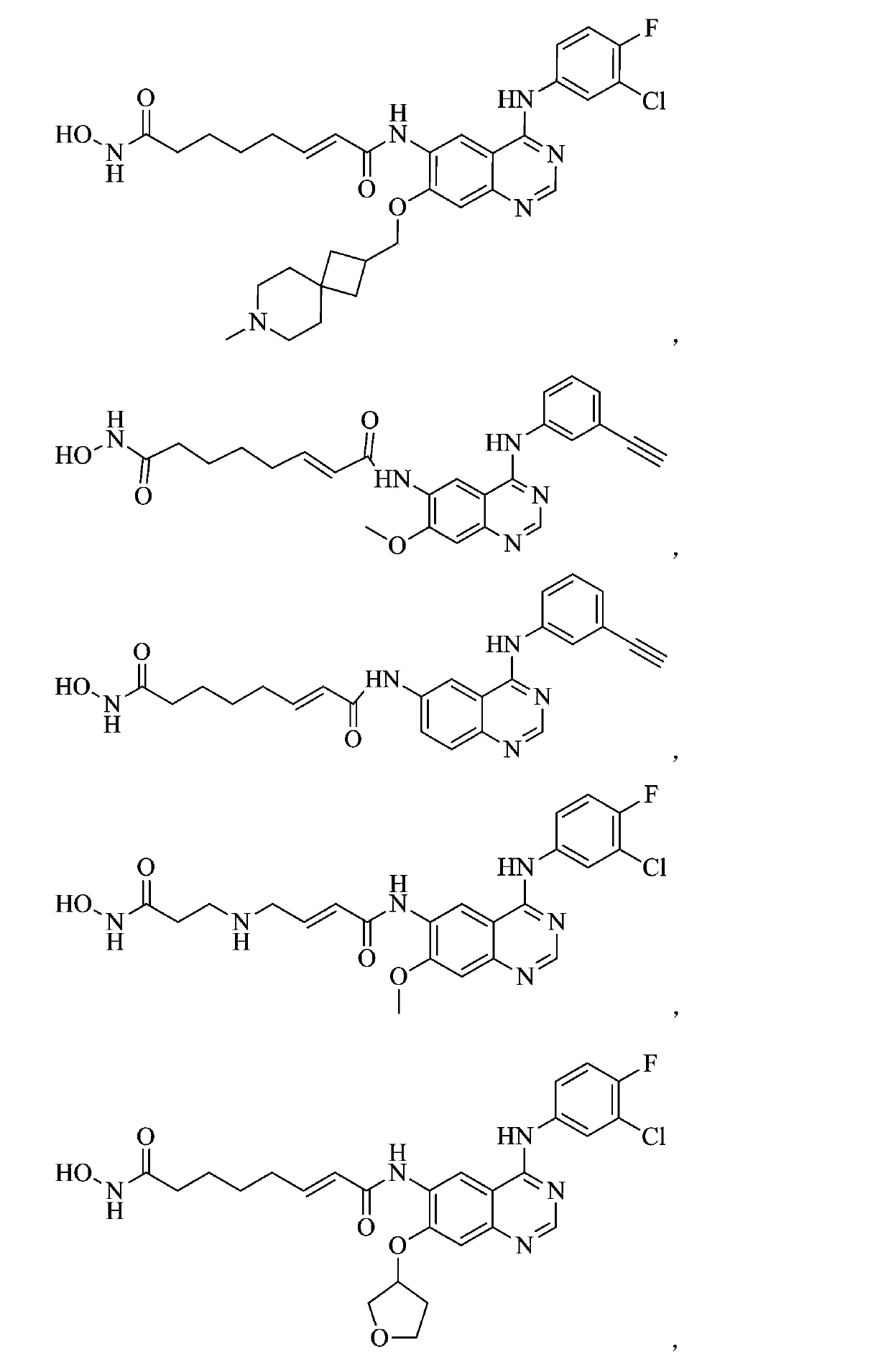
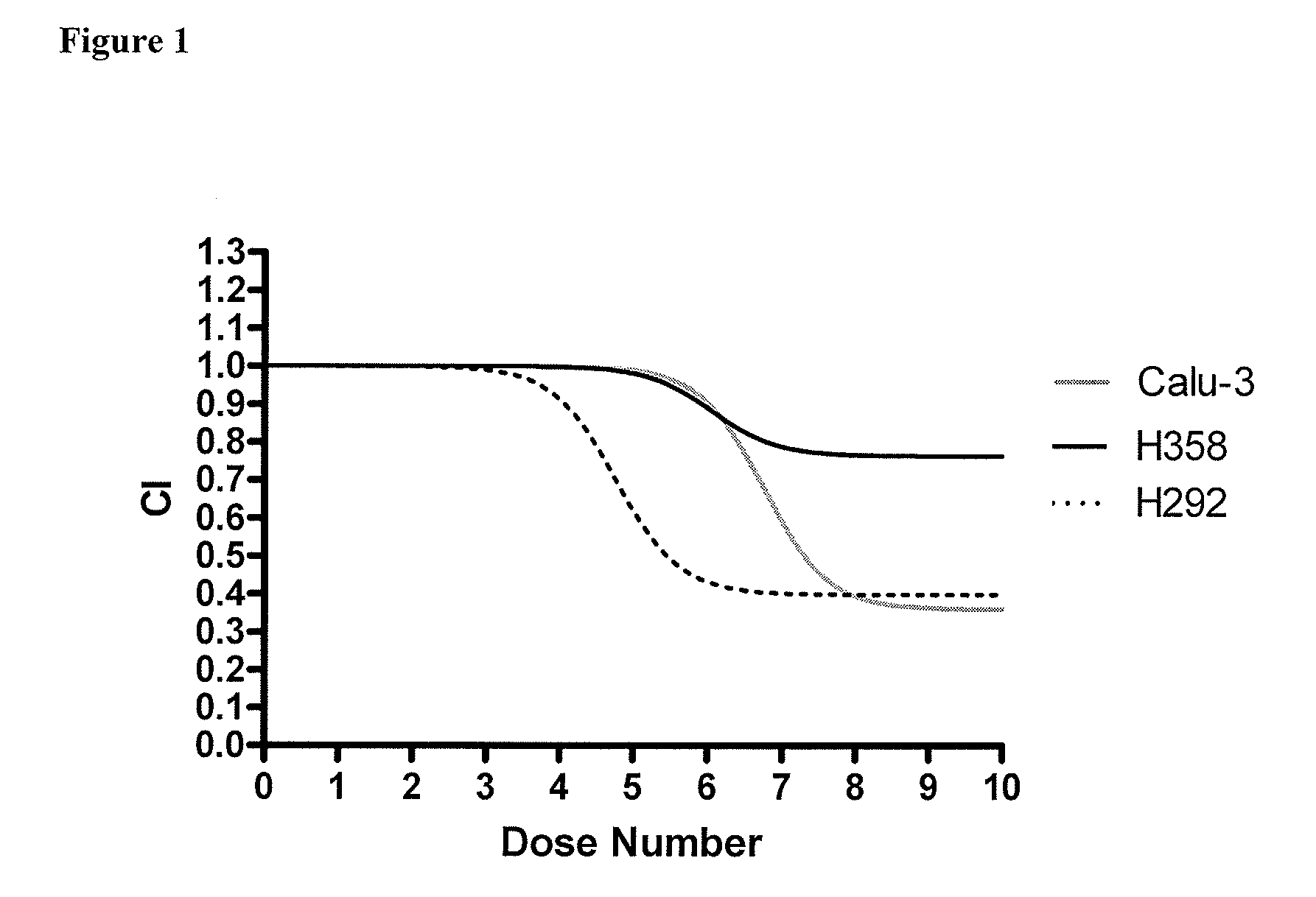
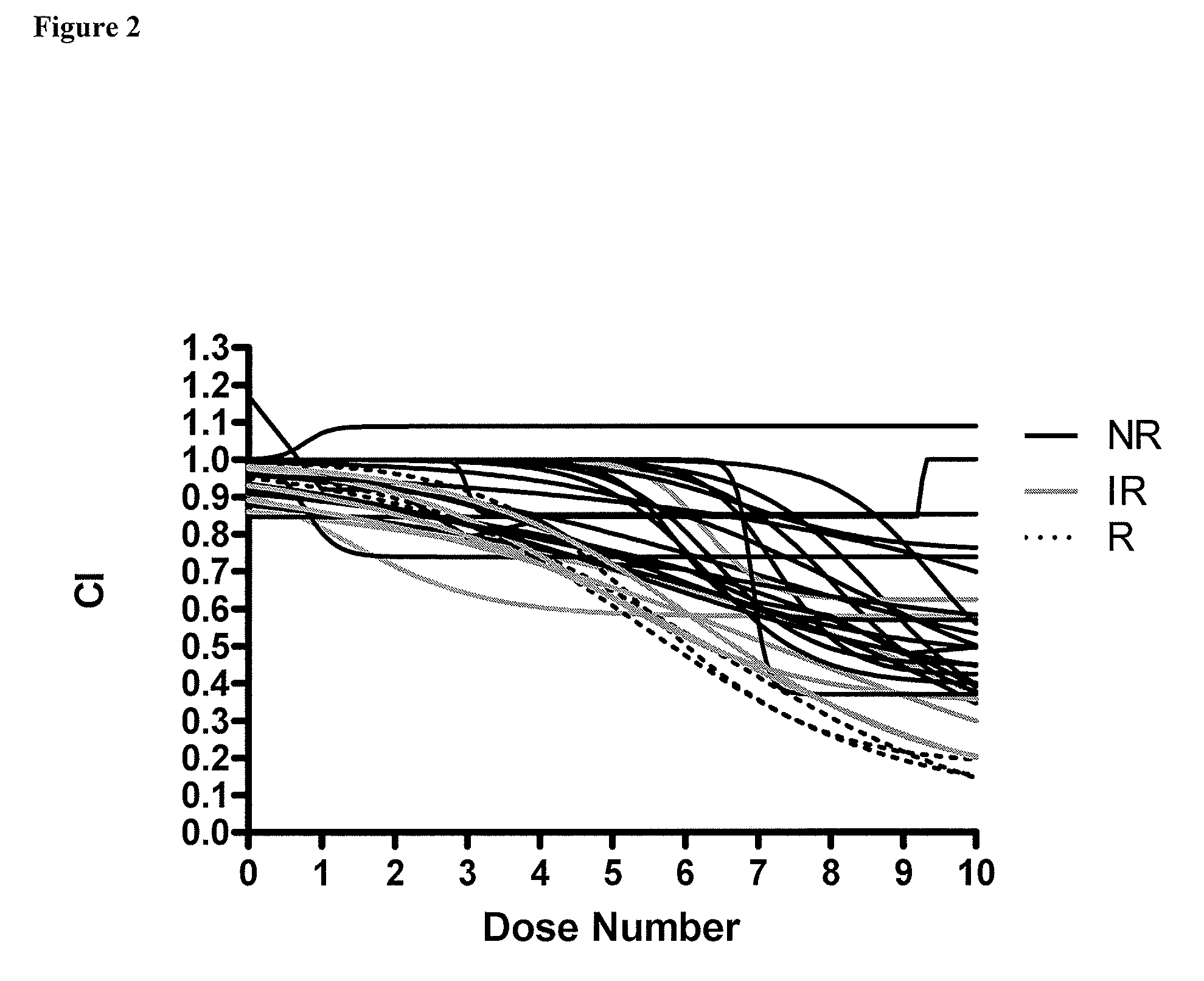
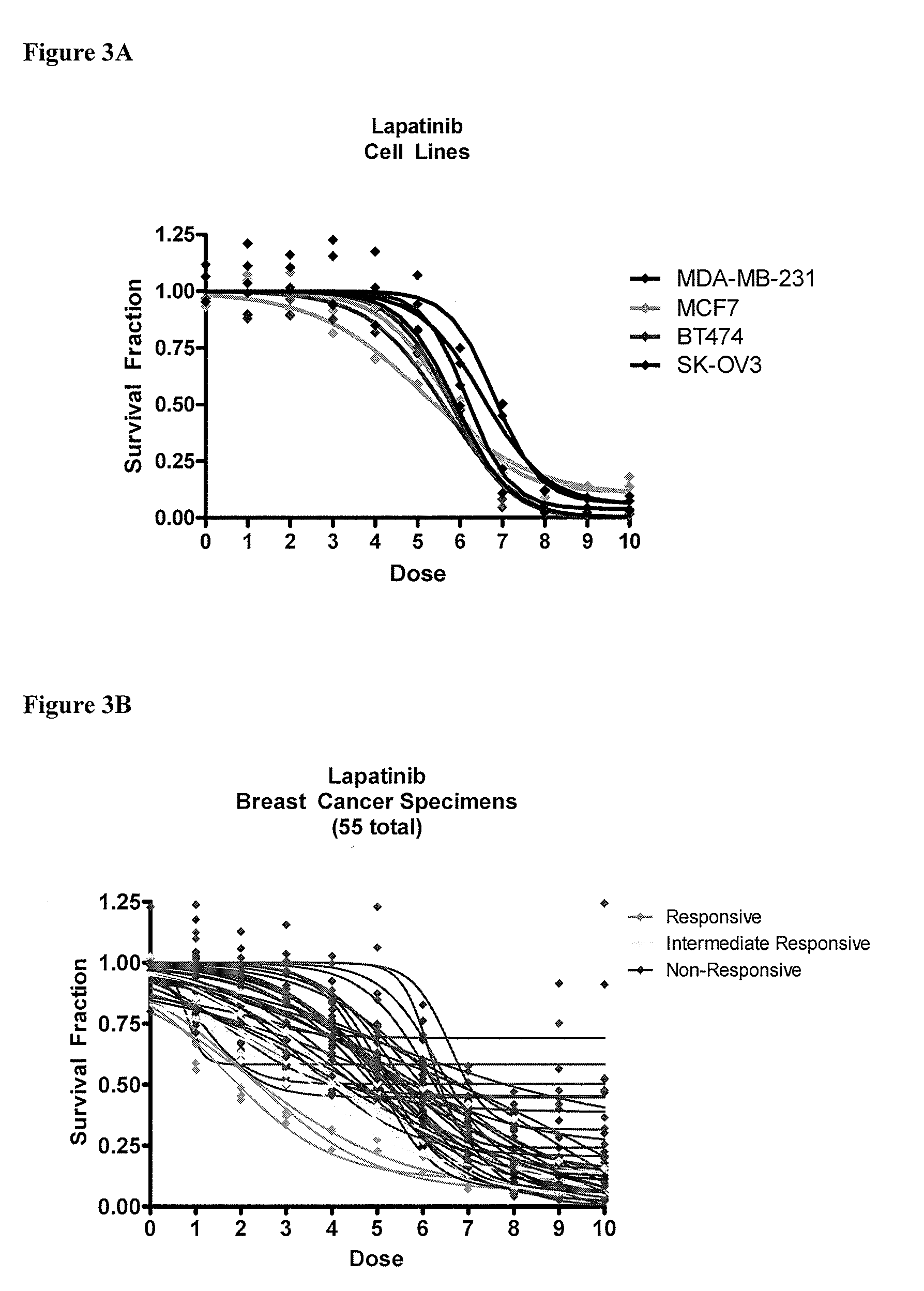
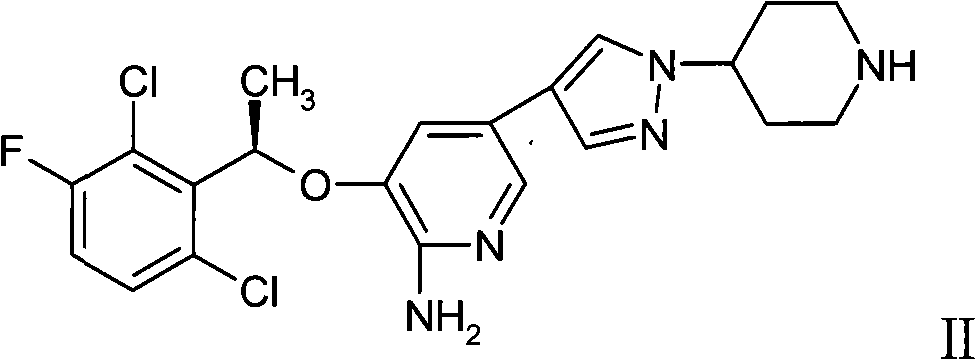
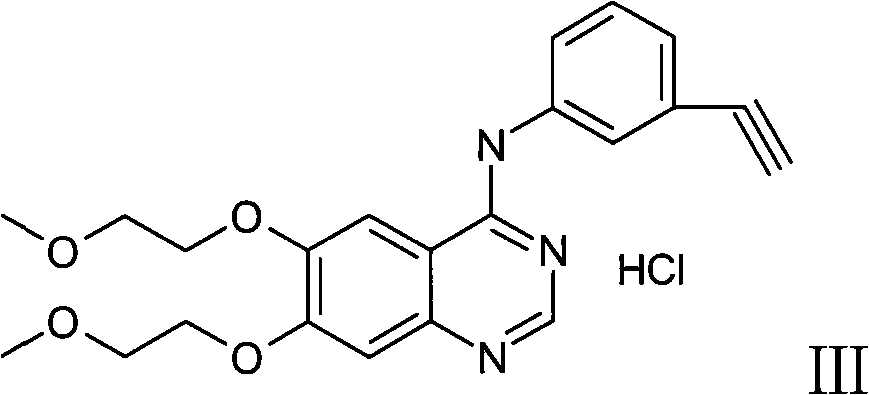
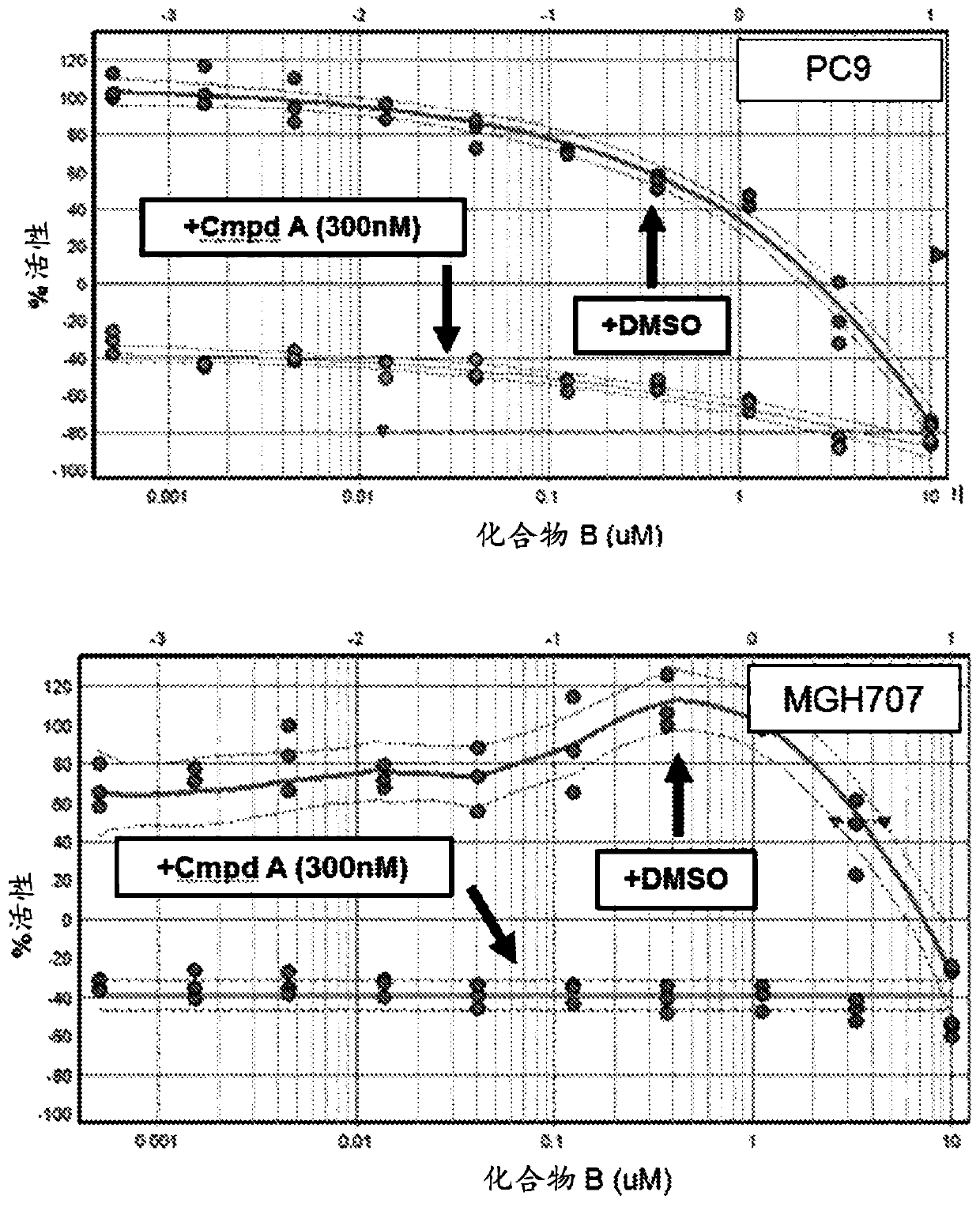
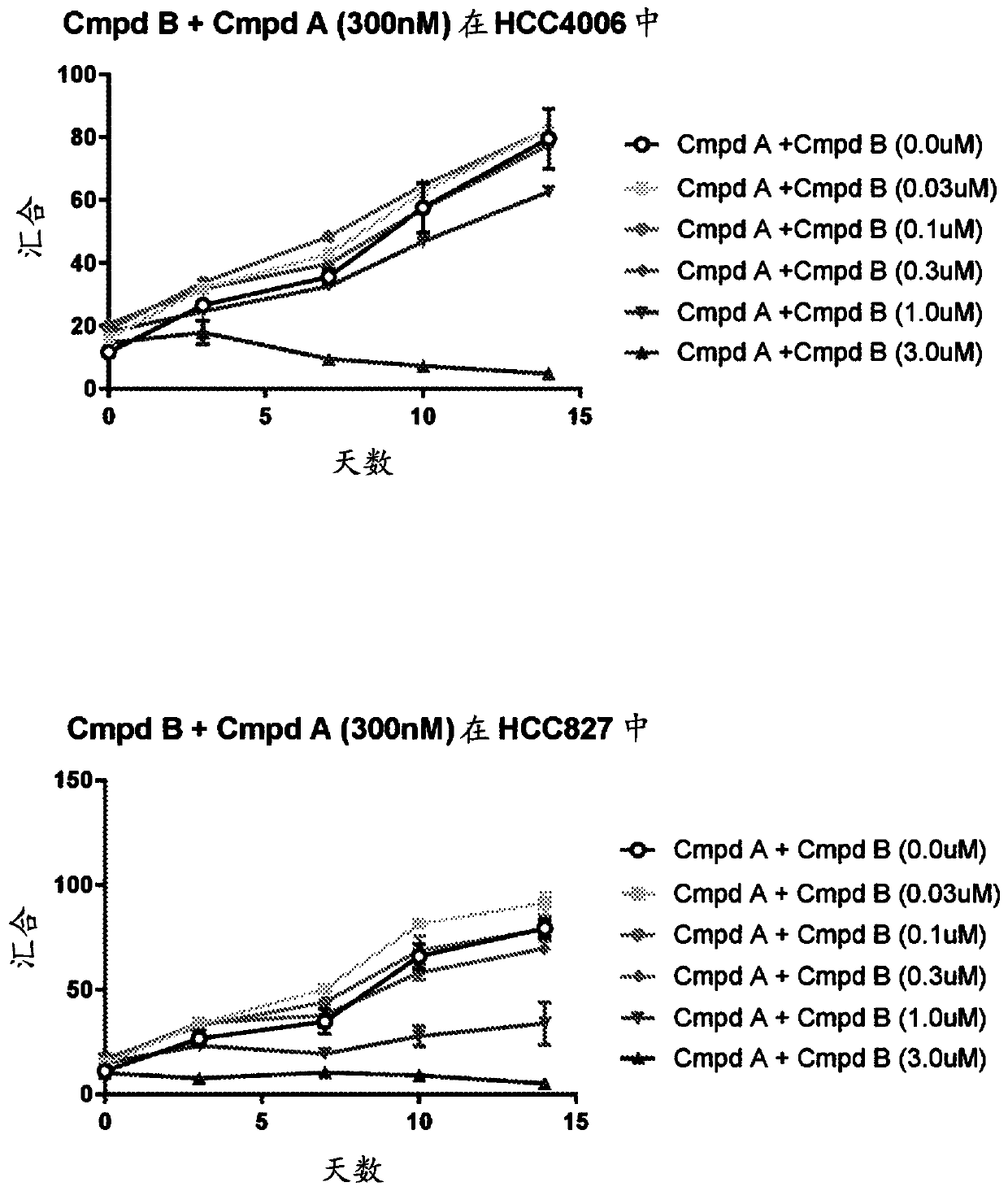
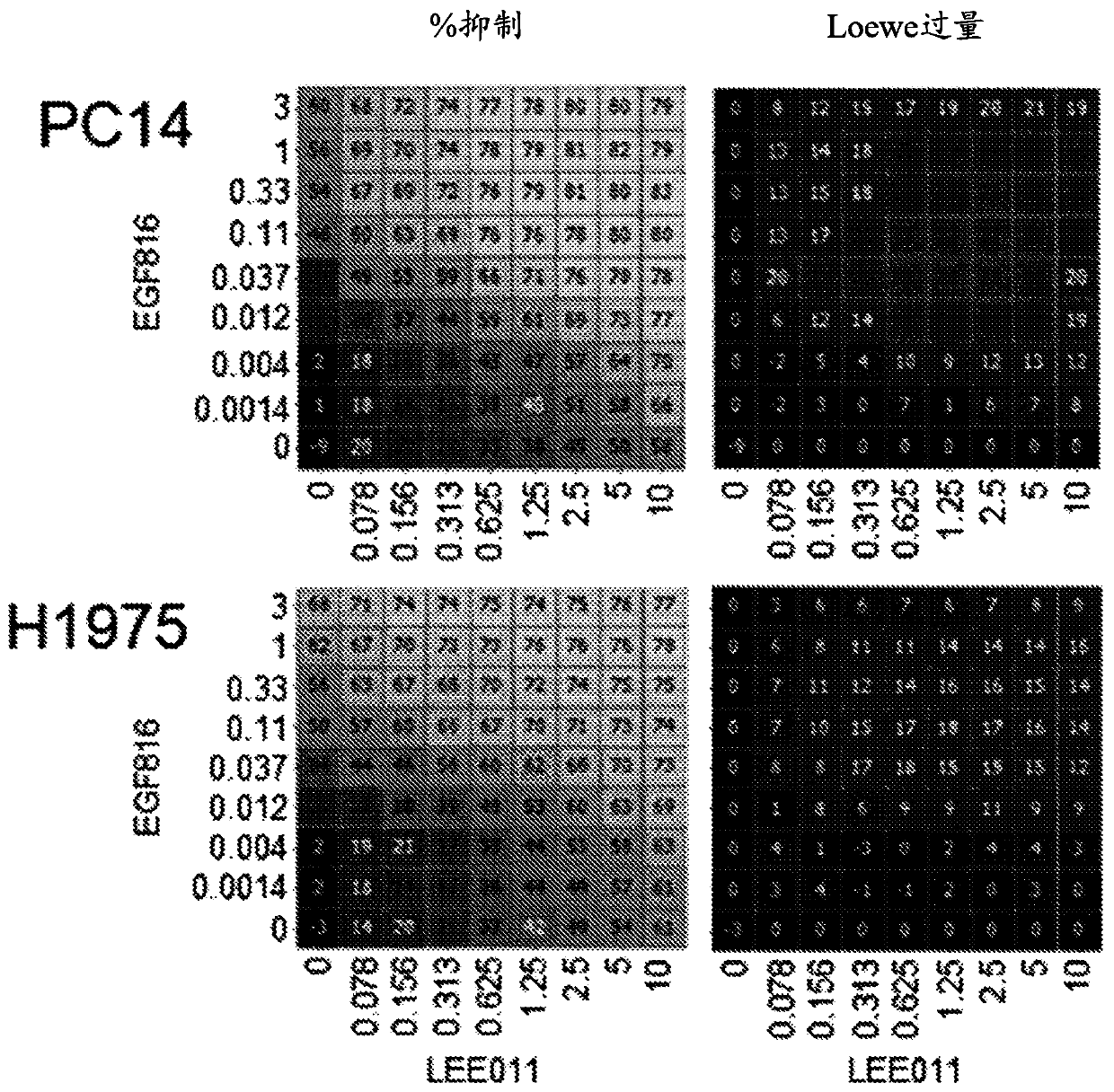
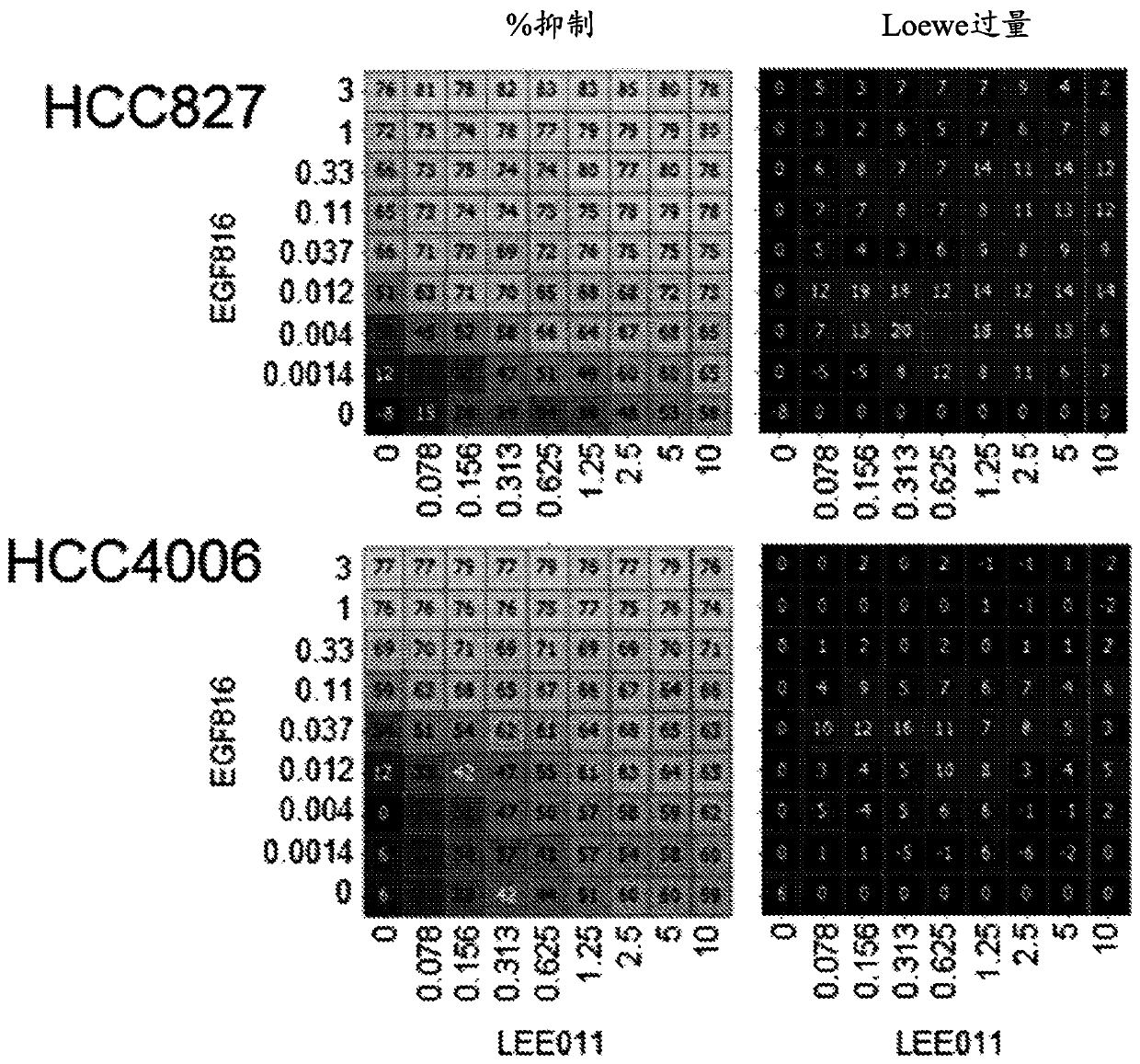
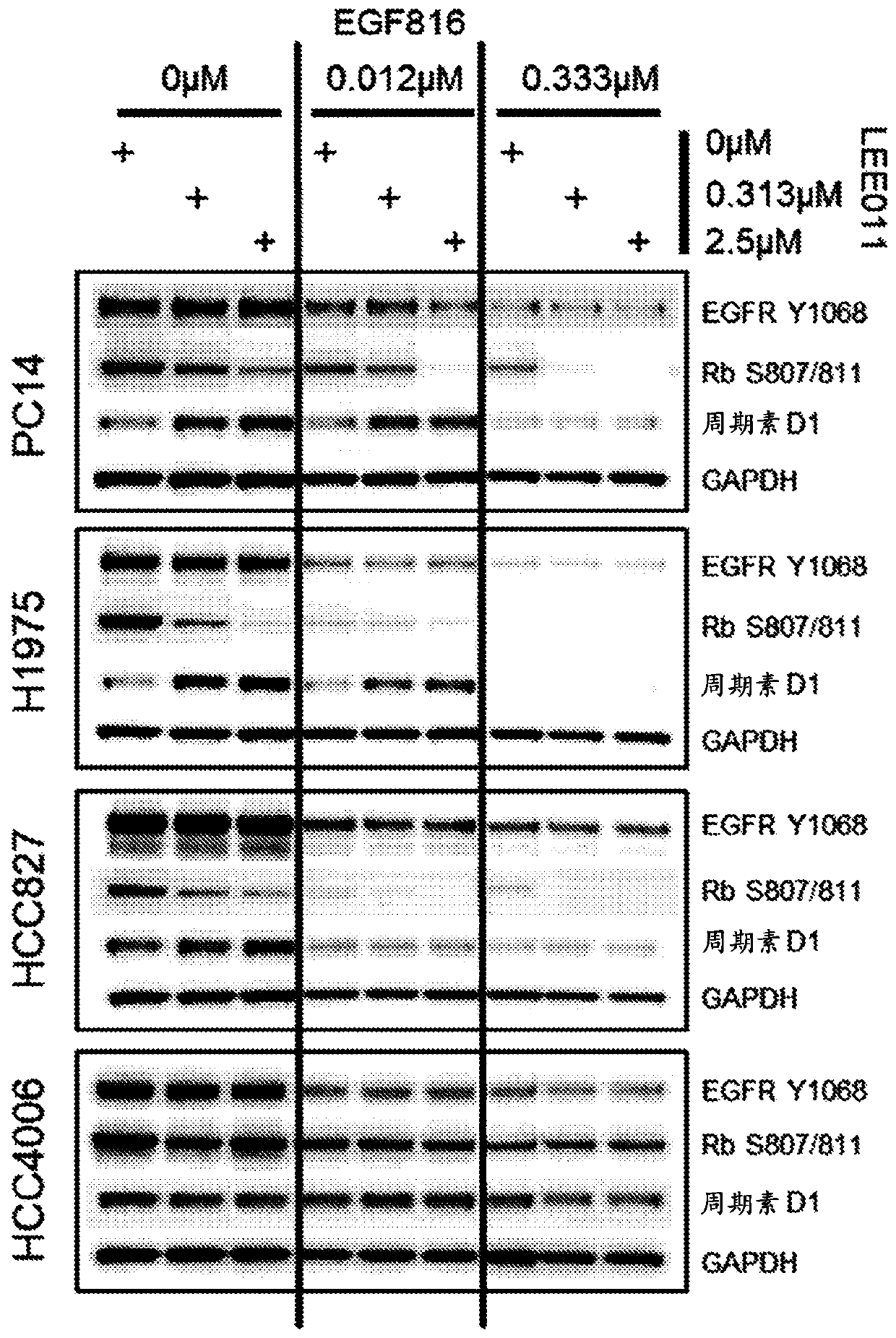
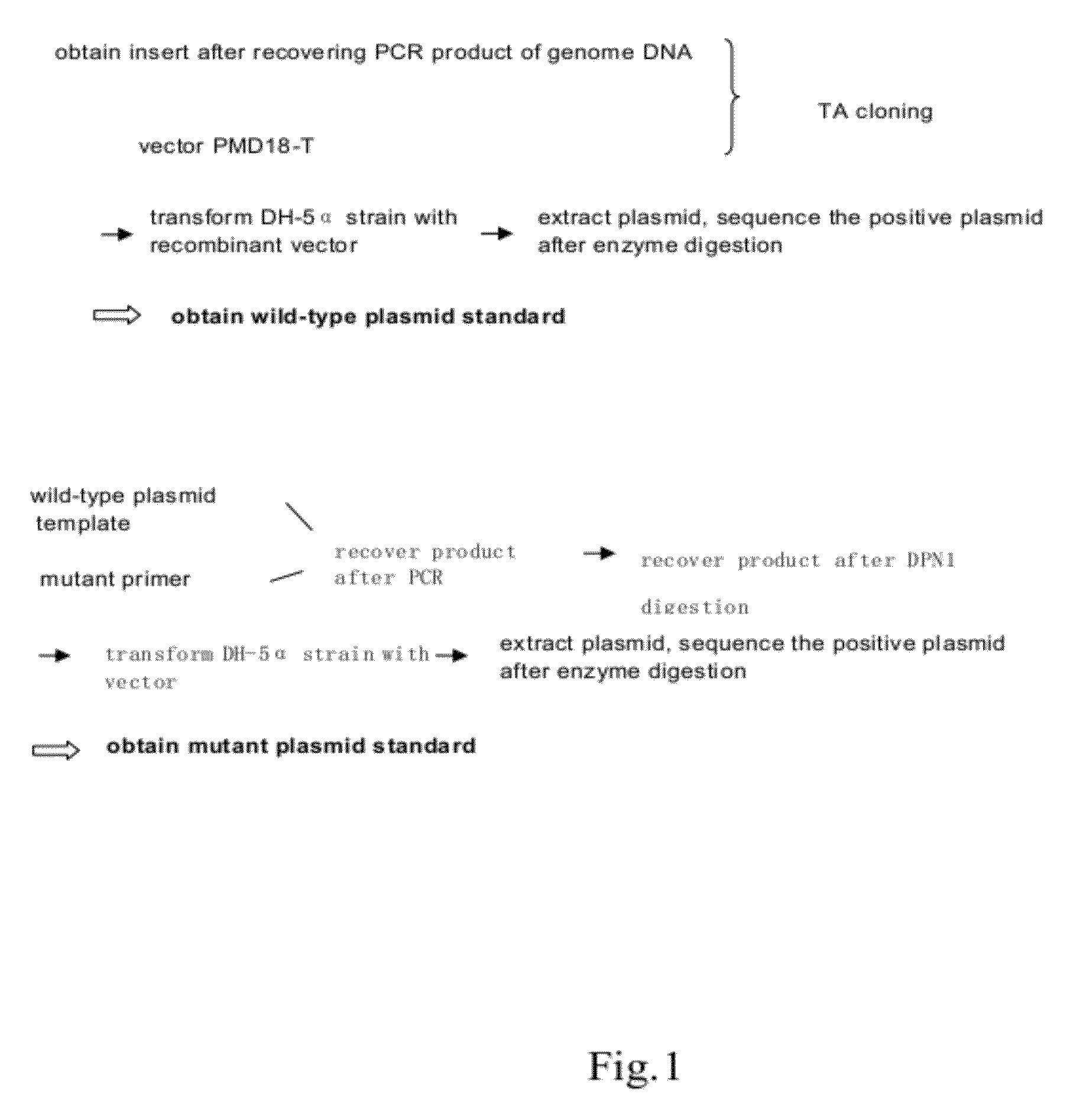
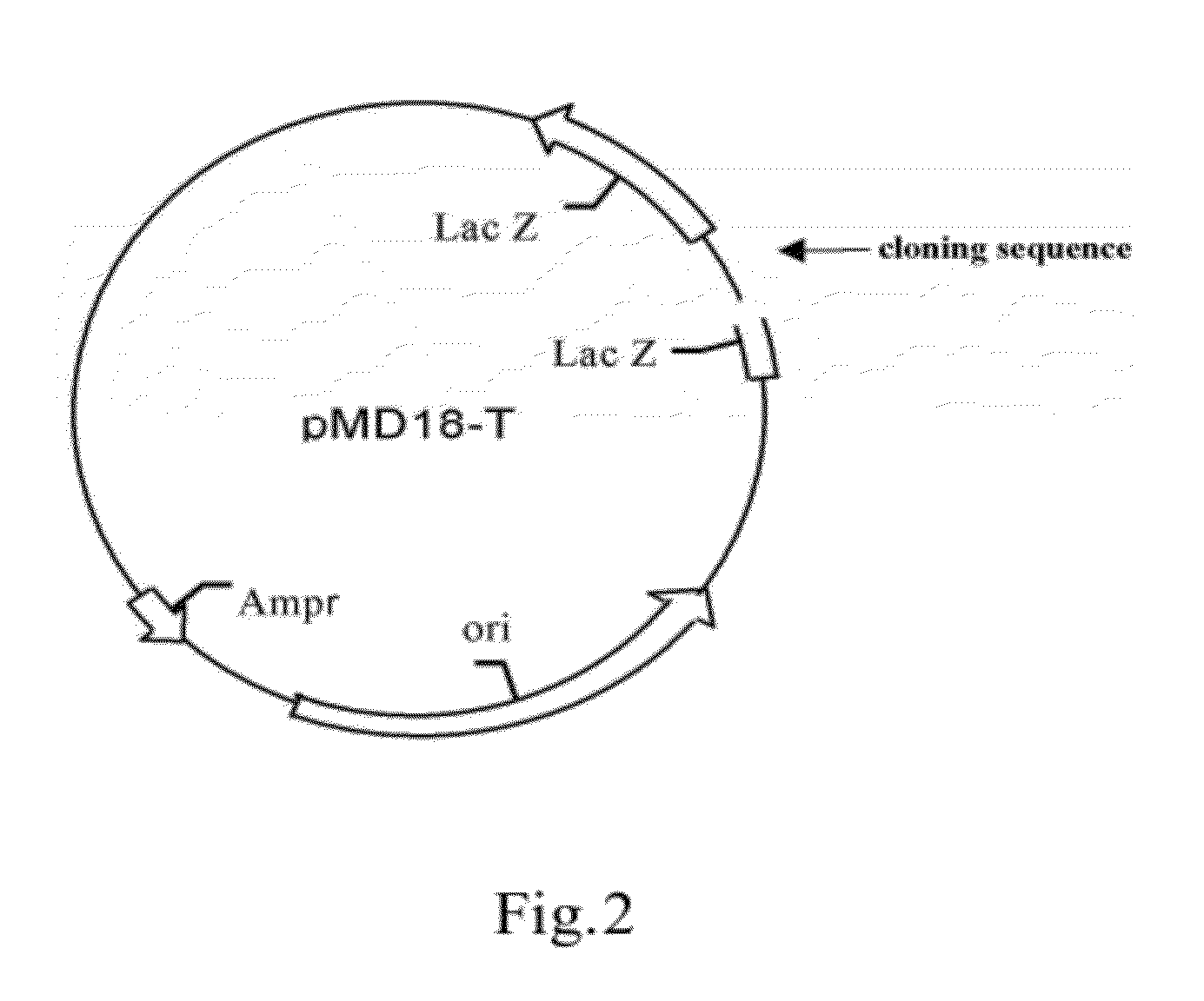
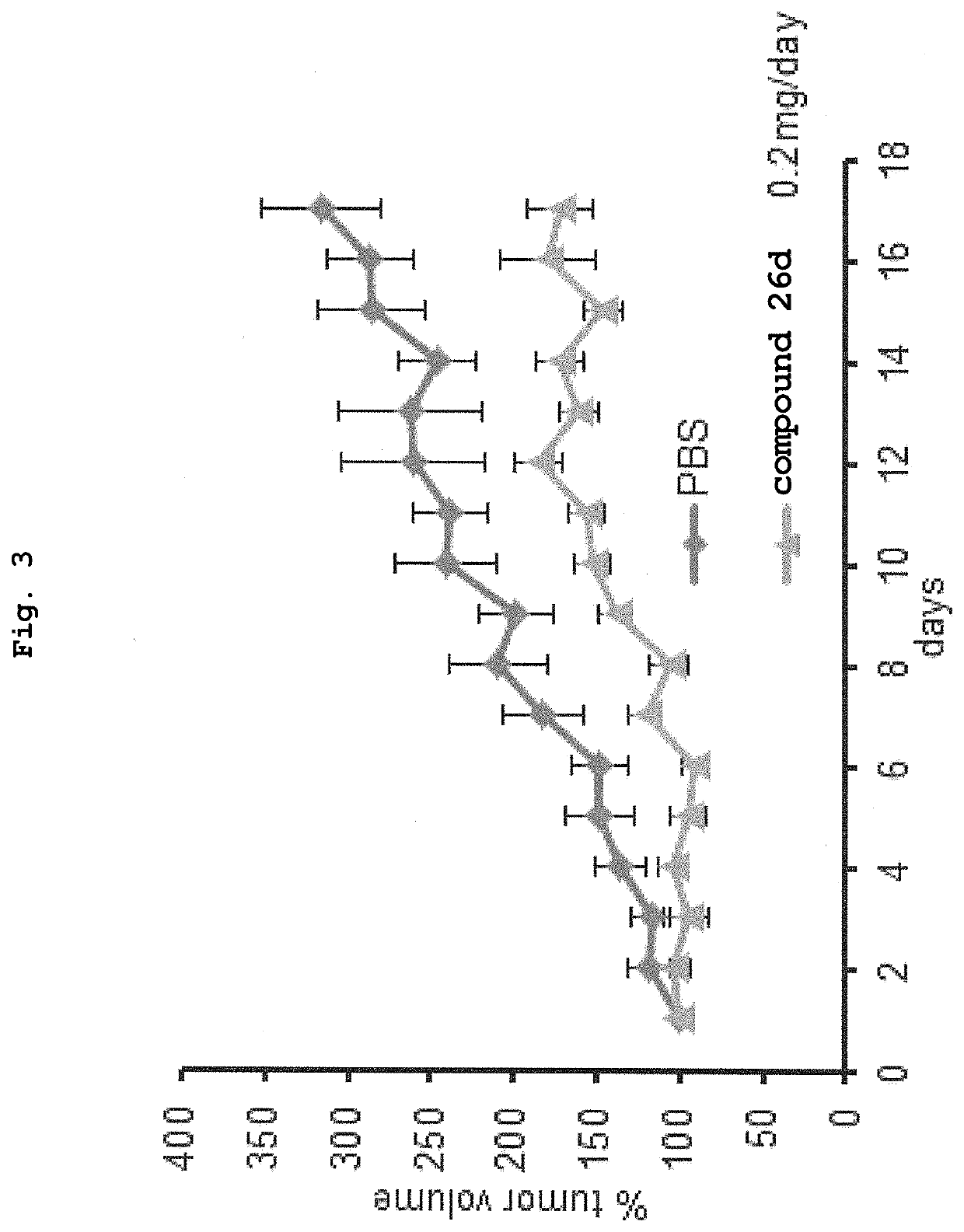
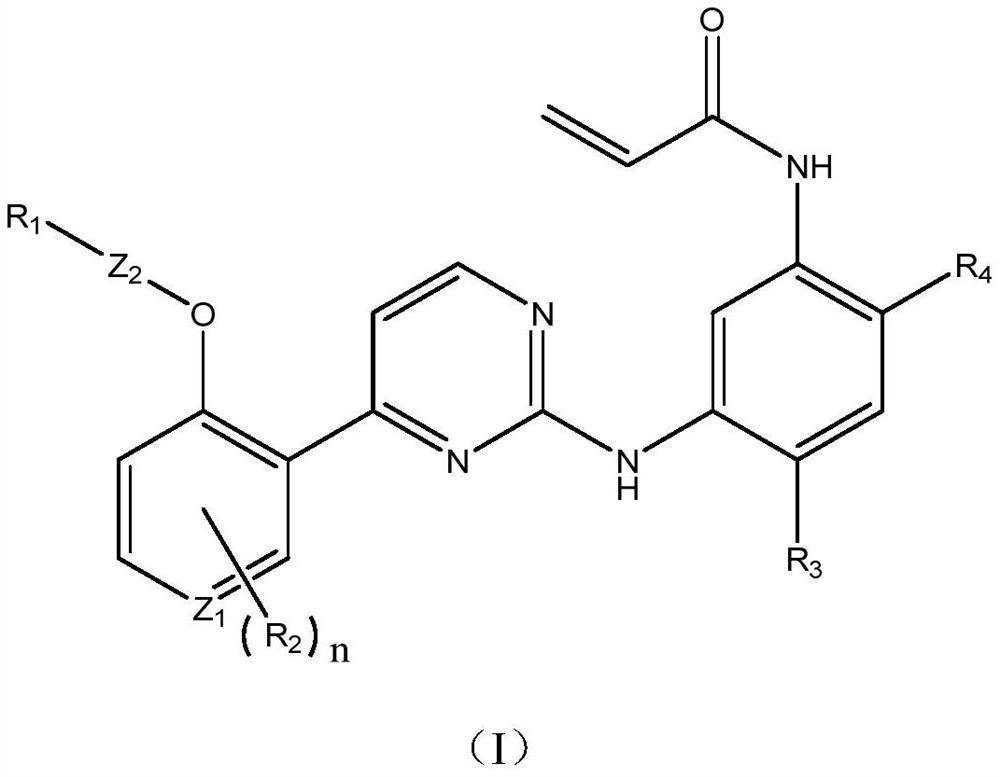
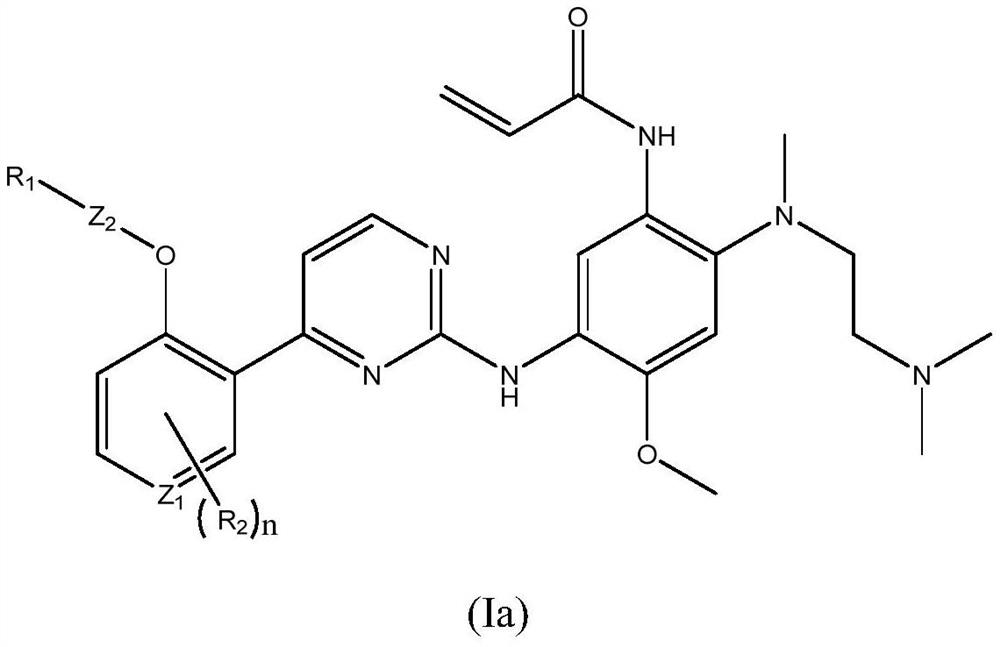
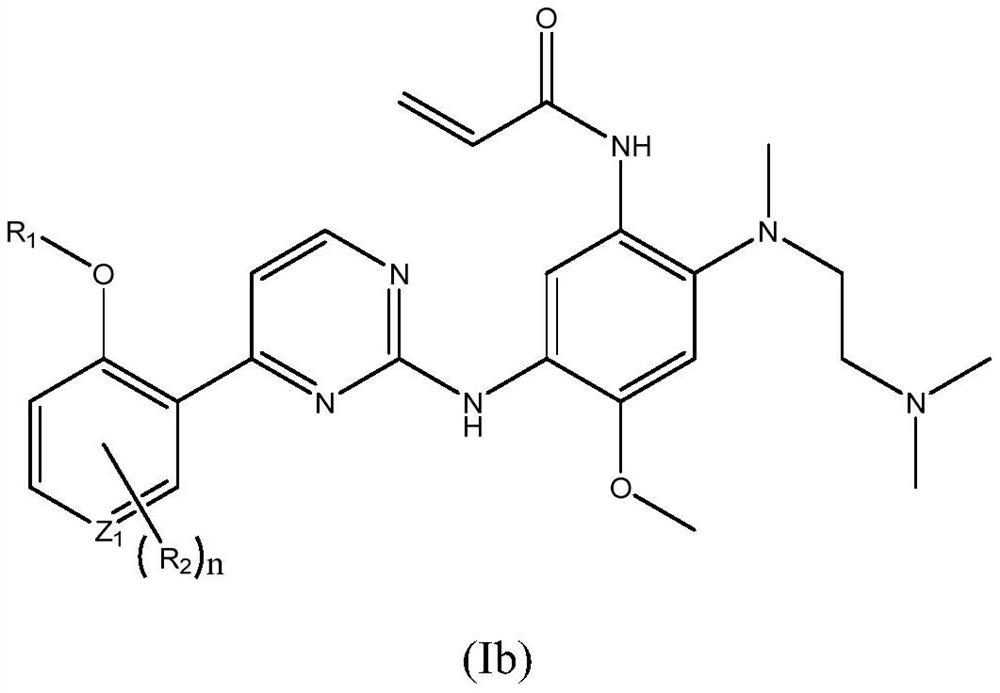
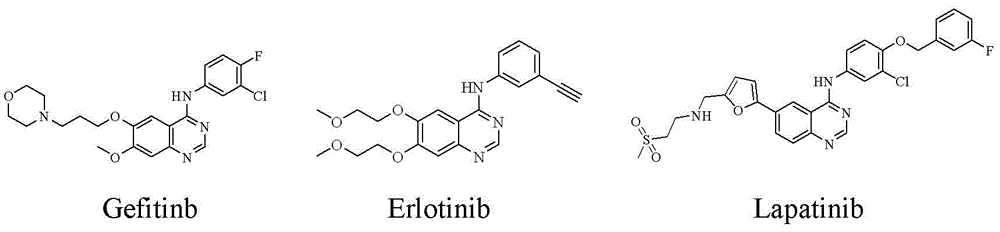
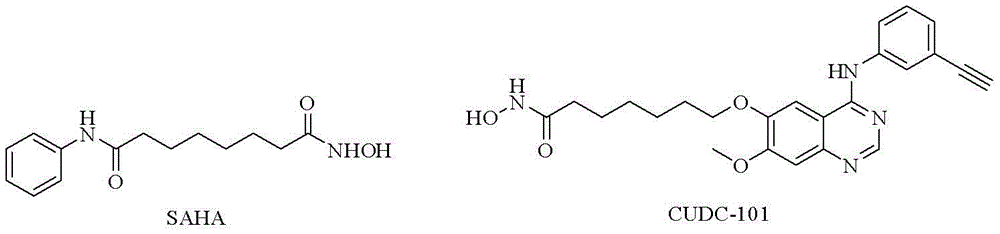
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "EGFR Tyrosine Kinase Inhibitors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
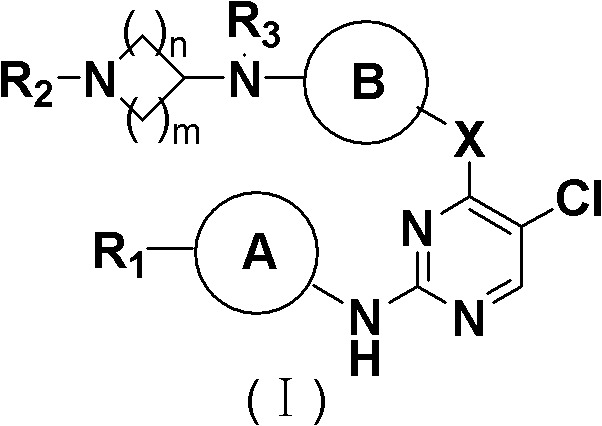
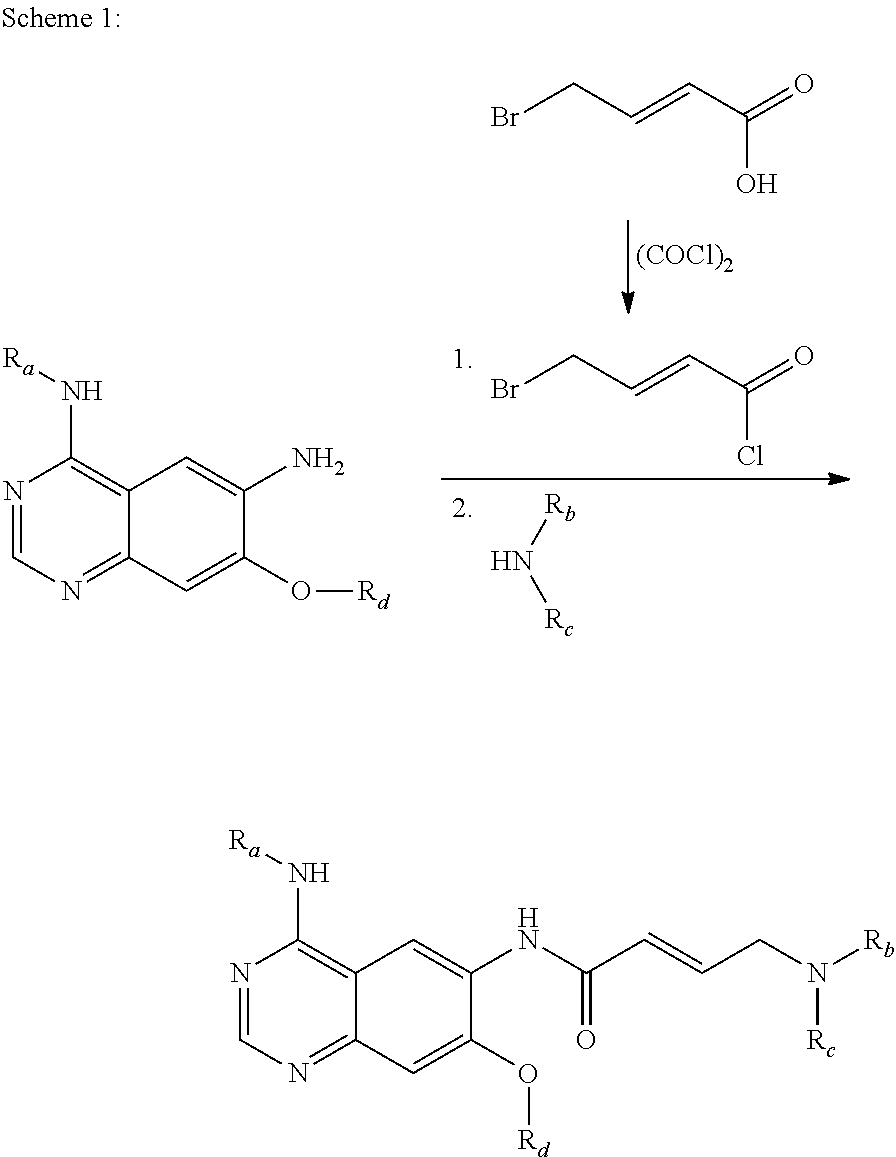
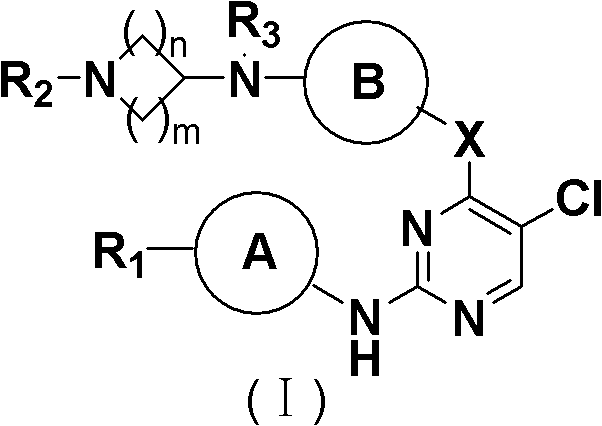
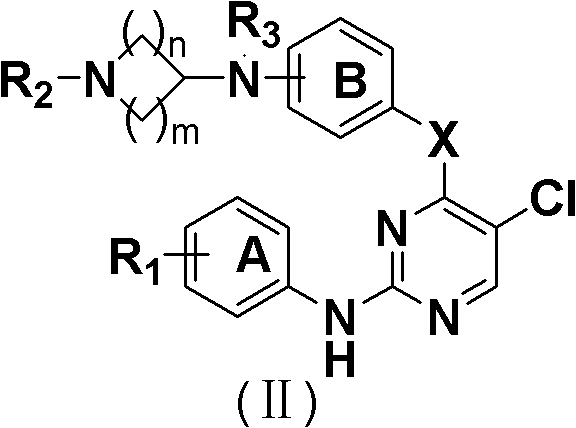
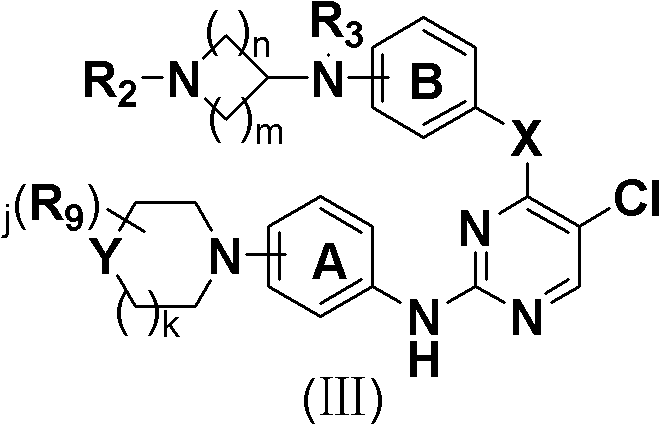
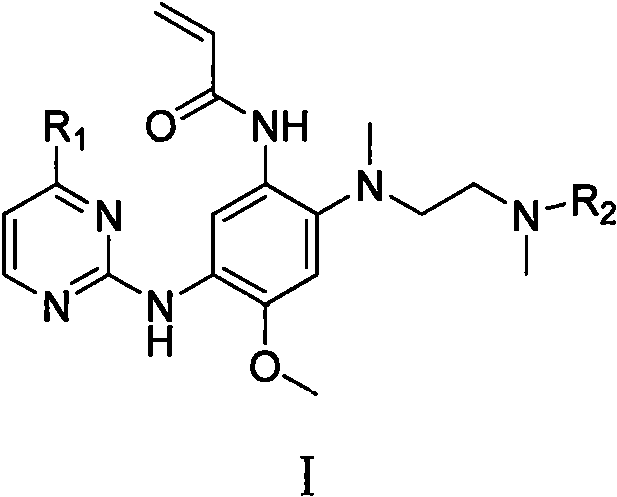
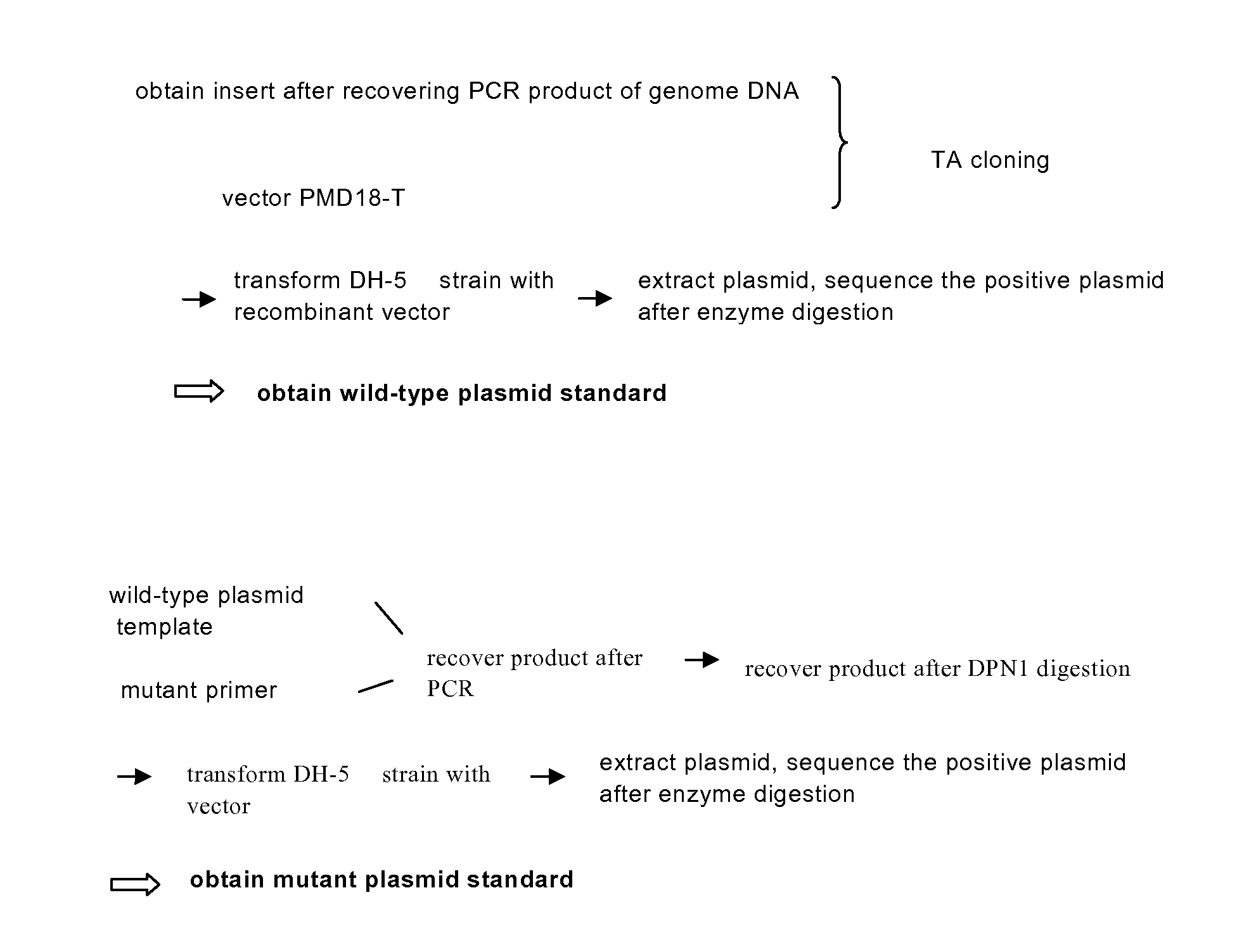
5-Chloropyrimidine compound and application of 5-Chloropyrimidine compound serving as epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor
ActiveCN103159742AHigh inhibitory strengthSmall toxicityNervous disorderOrganic chemistryEGFR Tyrosine Kinase InhibitorsHuman epidermal growth factor receptor
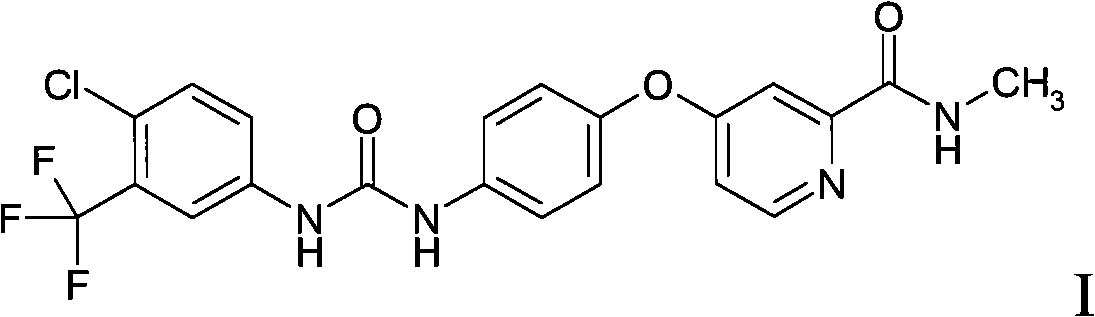
The invention discloses an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, and the structural formula of the inhibitor is shown as in the formula (I). The invention further discloses an application of any compound shown as in the formula (I) and pharmacy-acceptable salt of the compound. The invention further provides a medicine compound for curing, and the medicine compound comprises the compound shown as in the formula (I) and an EGFR modifier.
Owner:BEIJING HANMI PHARMA CO LTD
Diagnostic methods for determining treatment
InactiveUS20070275403A1Prolong survival timeEasy to identifyMicrobiological testing/measurementDisease diagnosisEGFR Tyrosine Kinase InhibitorsTyrosine-kinase inhibitor
The present invention provides methods for identifying cancer patients susceptible to effective treatment with inhibitors of the tyrosine kinase activity of EGFR. The invention is based on the discovery that polysomy of chromosome 7 can be used to selectively identify cancer patients that are likely to be successfully treated with EGFR tyrosine kinase inhibitors or agents that otherwise function similarly to tyrosine kinase inhibitors. The invention is based on the use of nucleic acid technology where nucleic acid probes are allowed to hybridize to cell samples and the number of copies of particular genetic regions quantified. The methods for identifying cancer patients of the invention can be enhanced by determination of expression of pAKT protein in patient samples. The invention also contemplates the treatment of those patients with tyrosine kinase inhibitors.
Owner:RUSH UNIV MEDICAL CENT +1
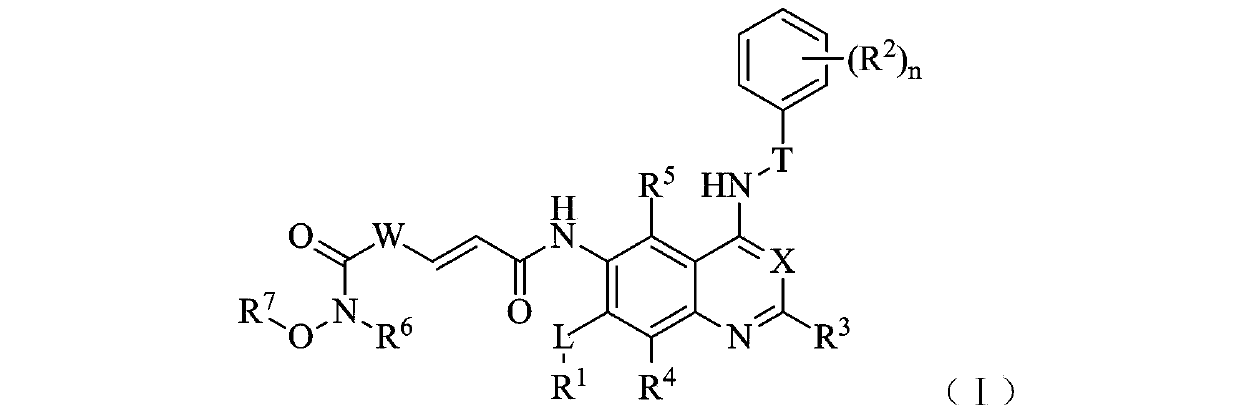
Zinc binding group-containing irreversible EGFR tyrosine kinase inhibitor
ActiveCN103965119AGood antitumor activityDelay drug resistanceOrganic active ingredientsOrganic chemistryEGFR Tyrosine Kinase InhibitorsTyrosine-kinase inhibitor
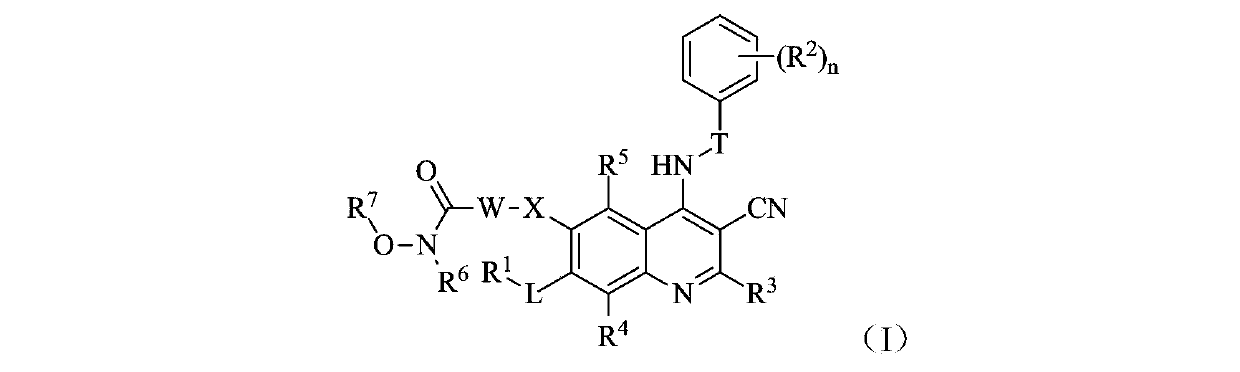
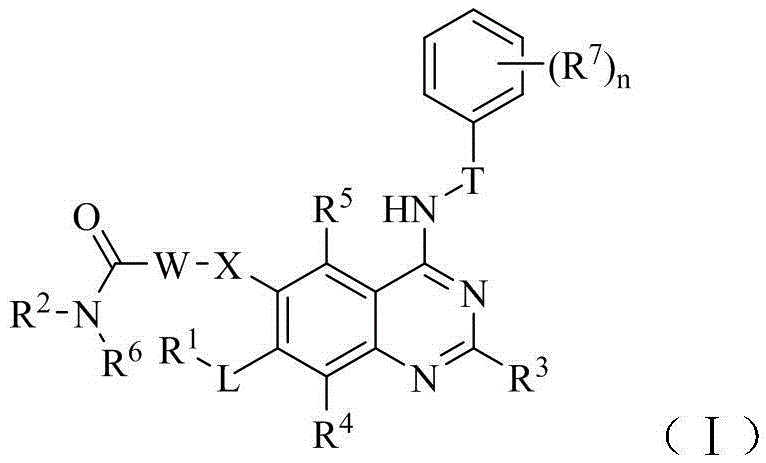
Belonging to the technical field of medicine, the invention in particular relates to a zinc binding group-containing irreversible EGFR (epidermal growth factor receptor) tyrosine kinase inhibitor shown as general formula (I), its deuterated compounds, pharmaceutically acceptable salts or stereoisomers, wherein R1, R2, R3, R4, R5, R6, R7, W, X, L, T, and n are defined as the specification. The invention also relates to a preparation method of the compounds, pharmaceutical preparations containing the compounds, and application of the compounds in preparation of drugs treating and / or preventing tumors. (formula I).
Owner:BEIJING AOHE DRUG RES INST
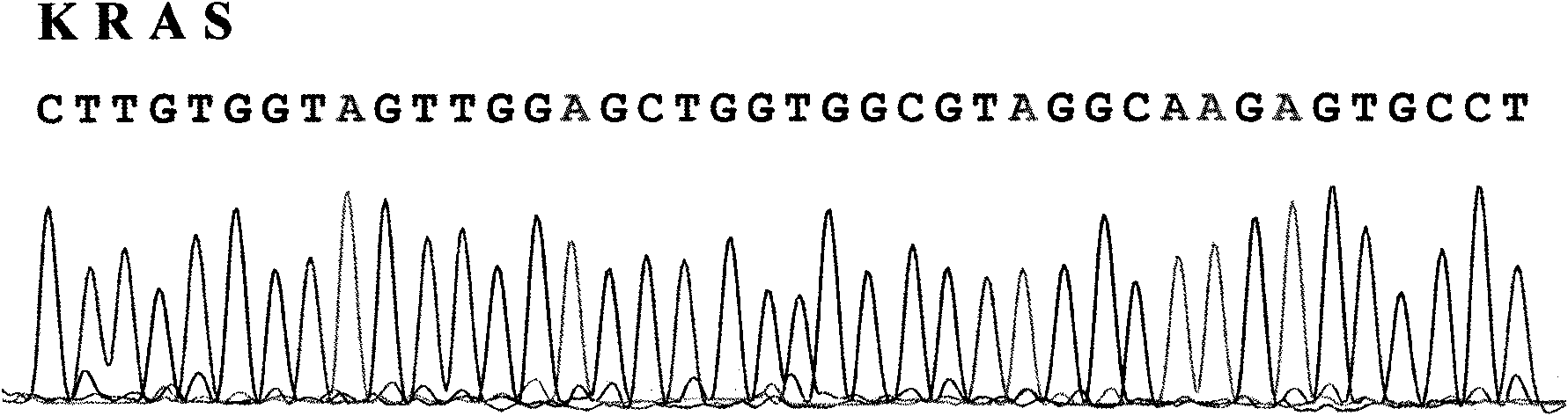
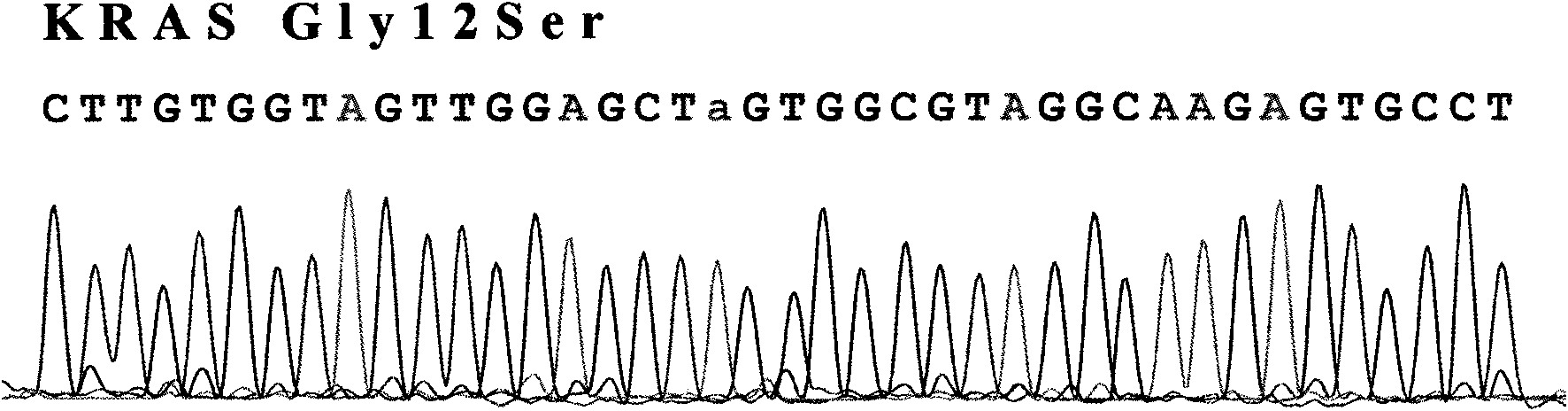
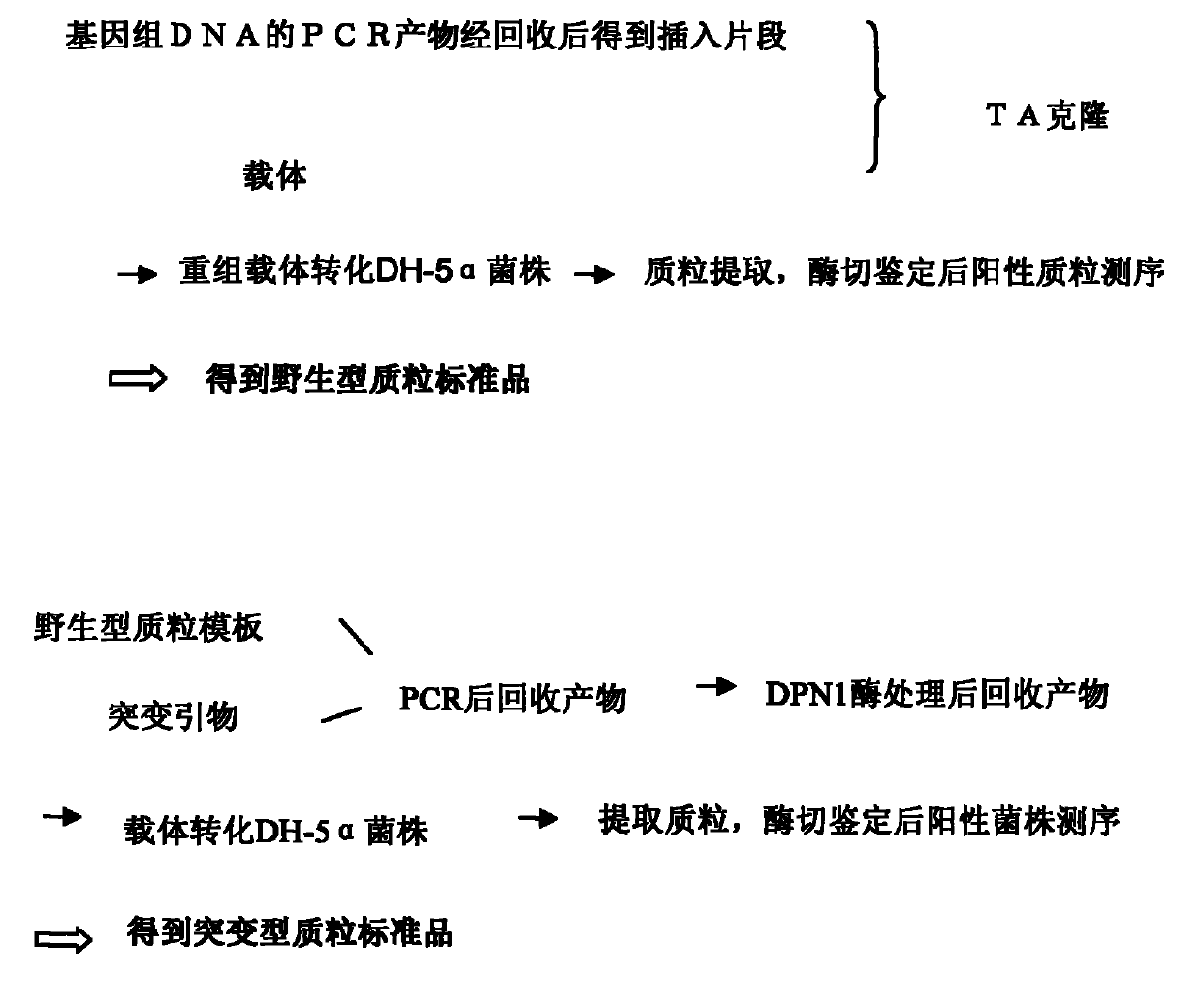
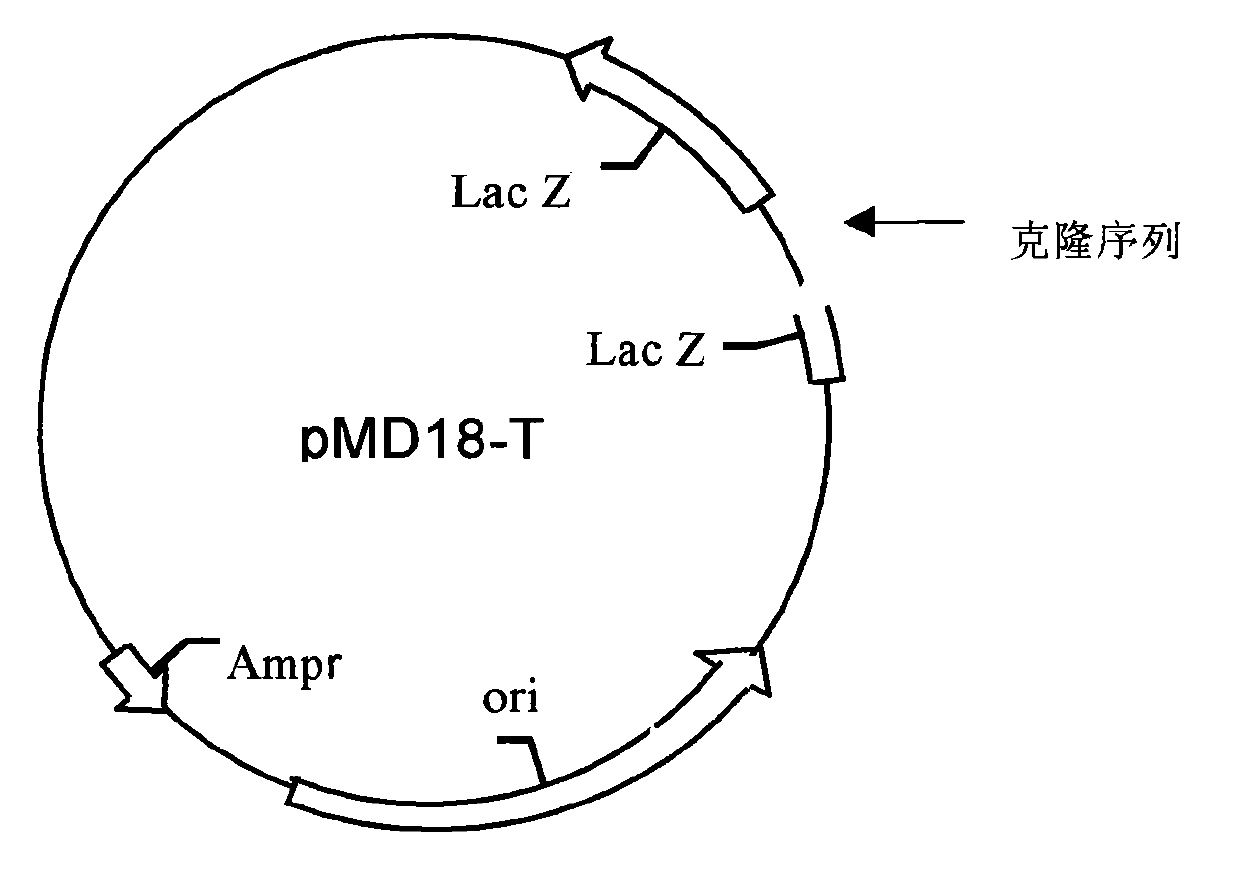
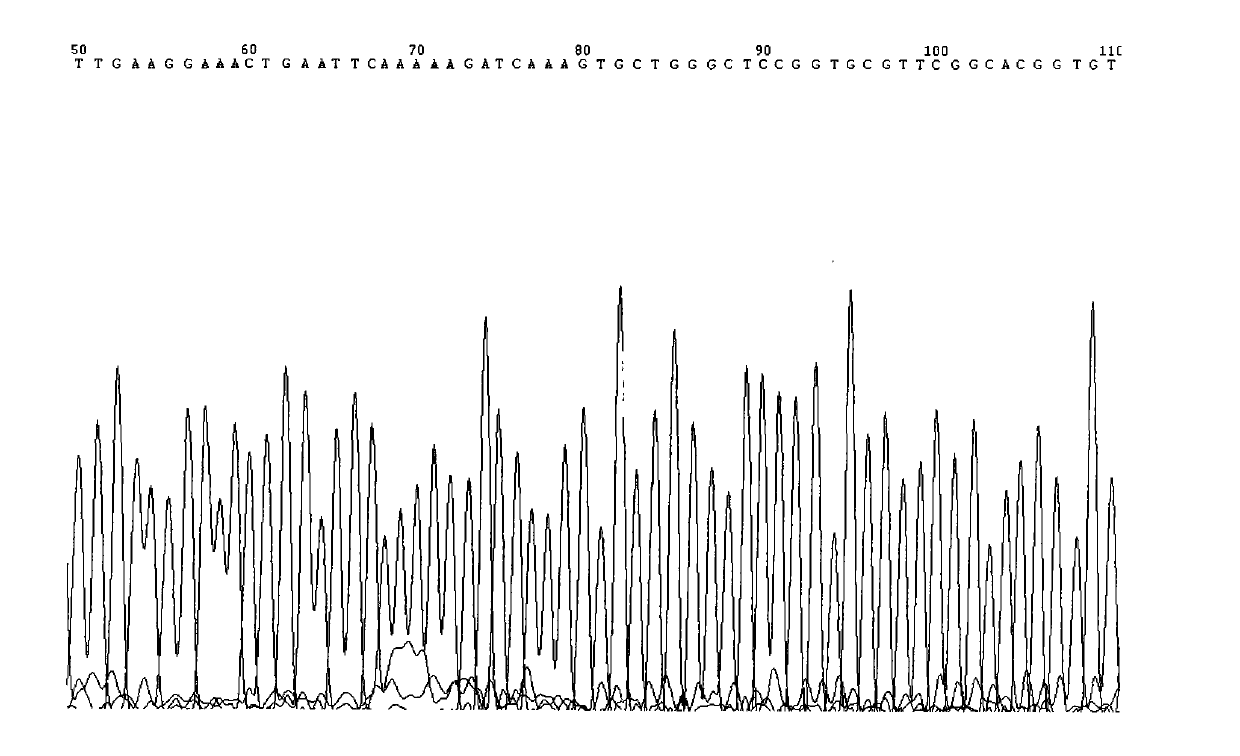
Test method of KRAS gene mutation for screening and assessing therapeutic effects of molecular targeted agents
InactiveCN101654702AHigh puritySmall amount of sampleMicrobiological testing/measurementMaterial analysis by electric/magnetic meansEGFR Tyrosine Kinase InhibitorsGenomic DNA
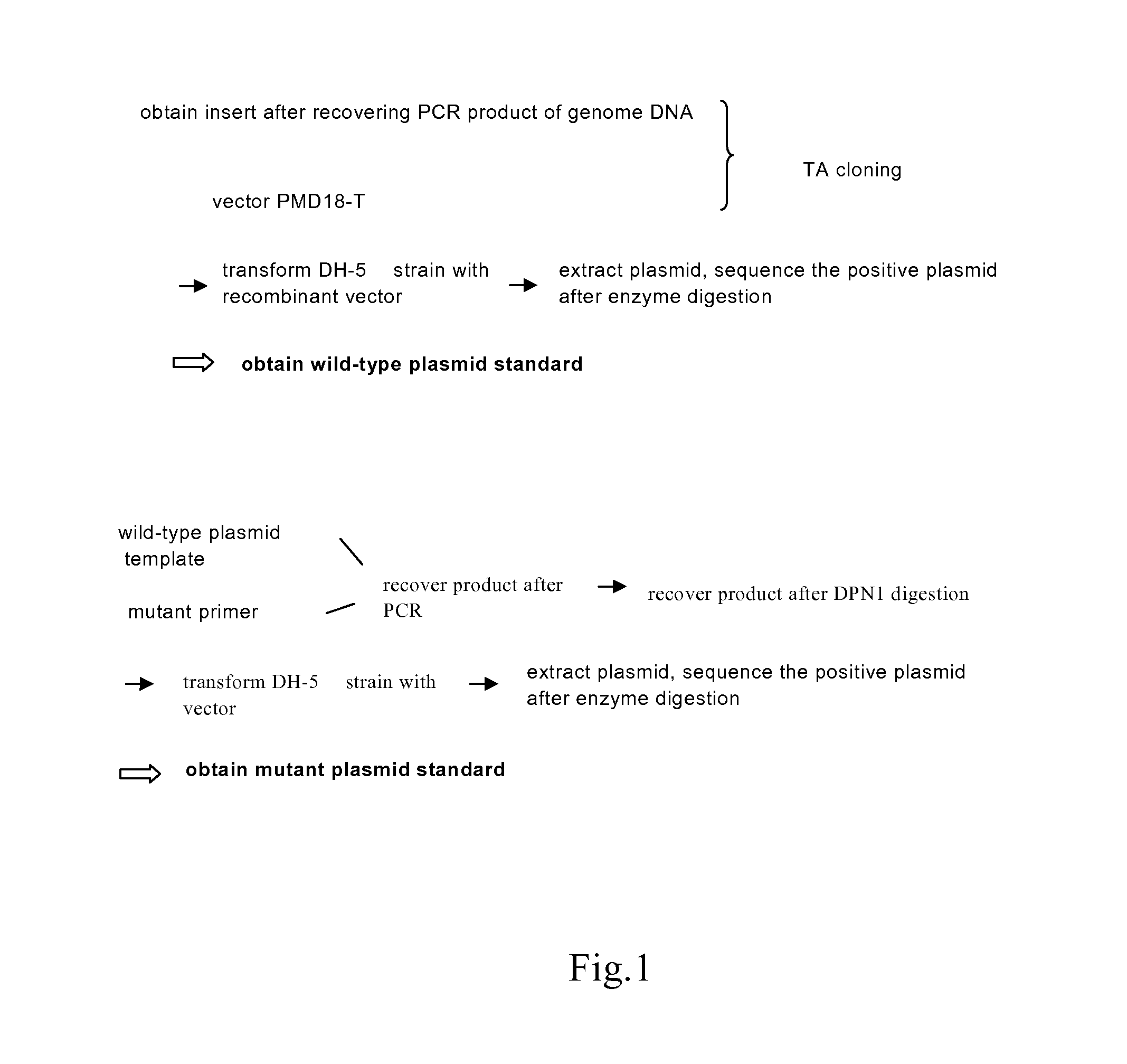
The invention relates to a test method of KRAS gene mutation for screening and assessing therapeutic effects of molecular targeted agents, which is characterized by the following specific steps: acquiring serum or tumor tissue samples of testees, extracting genomic DNA, testing a No.2 exon of the KRAS gene, designing corresponding site-specific primers and adopting experimental schemes, reagents and report generation systems for sequence determination and assessing the effectiveness of EGFR tyrosine kinase inhibitors used for treating cancer individuals by simultaneously testing and analyzingwhether mutation exists on the No.2 exon of the individual KRAS gene. The method has the advantage of being used for application of the EGFR tyrosine kinase inhibitors used for treating cancer individuals.
Owner:上海中优医药高科技股份有限公司
Kit for quantitatively detecting EGFR mutation
InactiveCN102021232AAccurate determination of contentEasy to operateMicrobiological testing/measurementVector-based foreign material introductionEGFR Tyrosine Kinase InhibitorsEGFR Gene Mutation
Owner:BEIJING ACCB BIOTECH
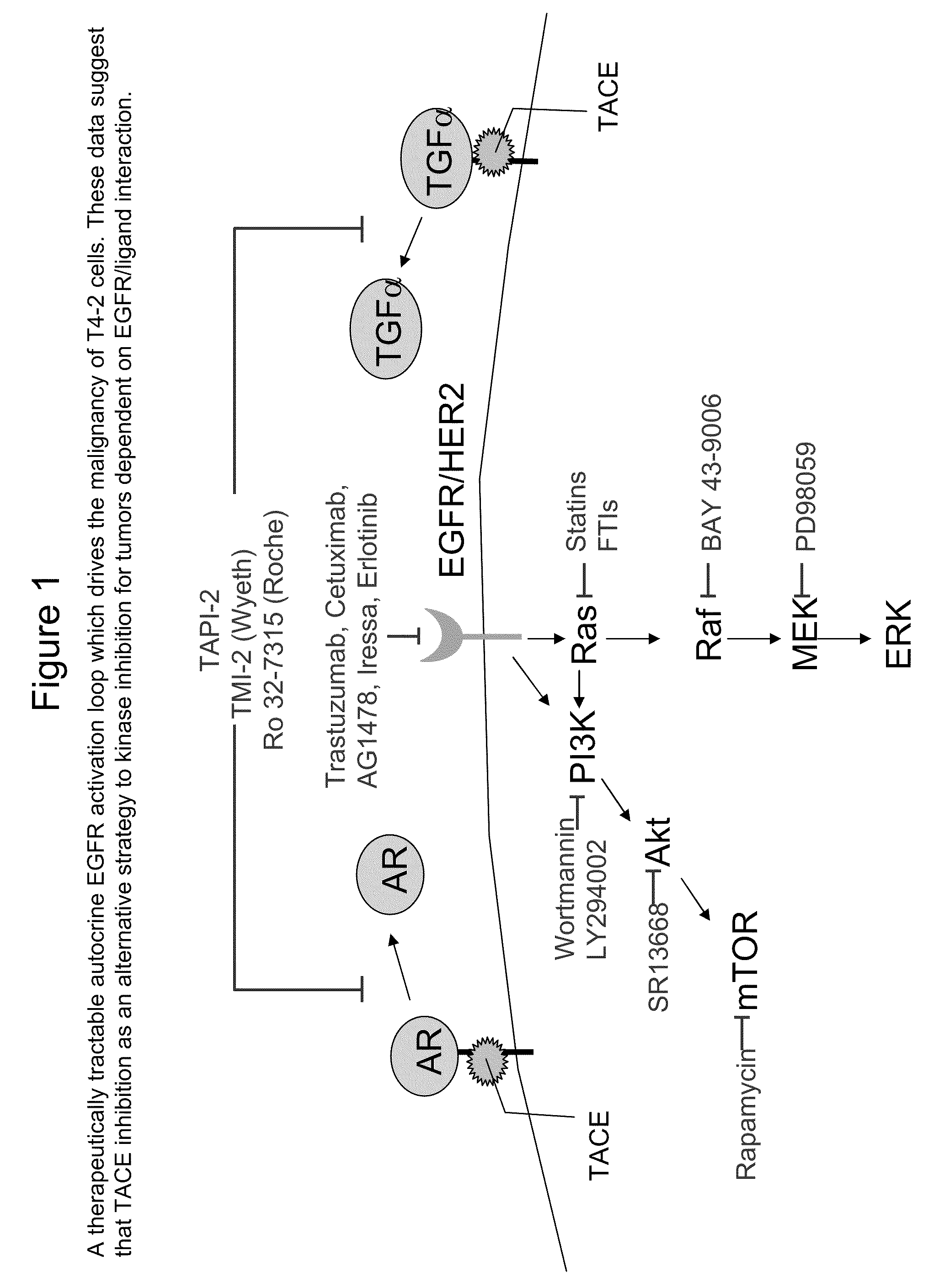
TARGETING TNF-alpha CONVERTING ENZYME(TACE)- DEPENDENT GROWTH FACTOR SHEDDING IN CANCER THERAPY
InactiveUS20090274626A1BiocideHeavy metal active ingredientsTace inhibitorEGFR Tyrosine Kinase Inhibitors
The invention provides methods for modulating tumor cell proliferation by contacting cells (e.g. tumor cells) with a TACE inhibitor and a compound that inhibits EGFR tyrosine kinase, whereby the TACE inhibitor enhances the sensitivity of the cell to the EGFR tyrosine kinase inhibitor. Additionally, methods for treating cancer and methods for identifying TACE inhibitors is also provided.
Owner:RGT UNIV OF CALIFORNIA
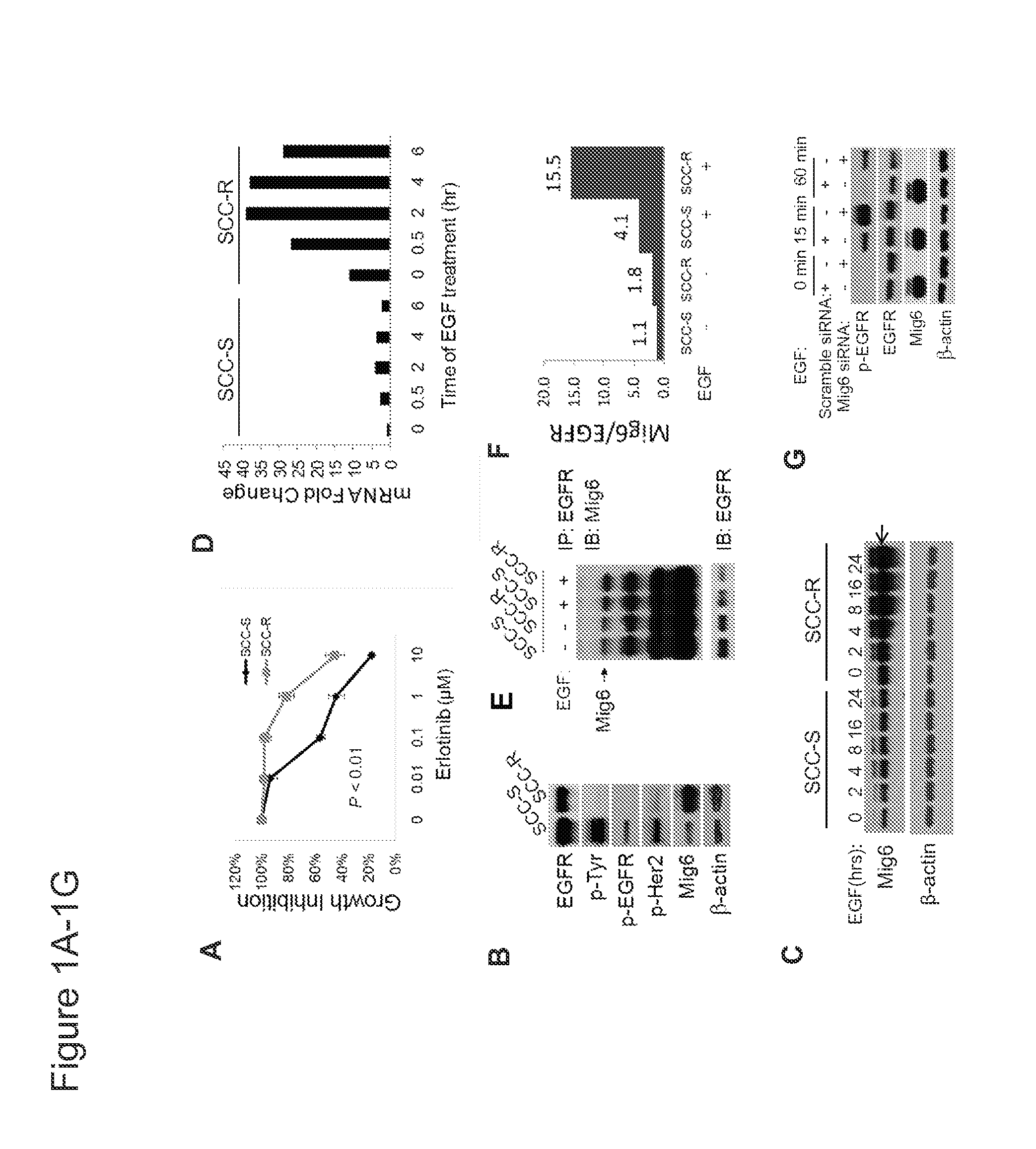
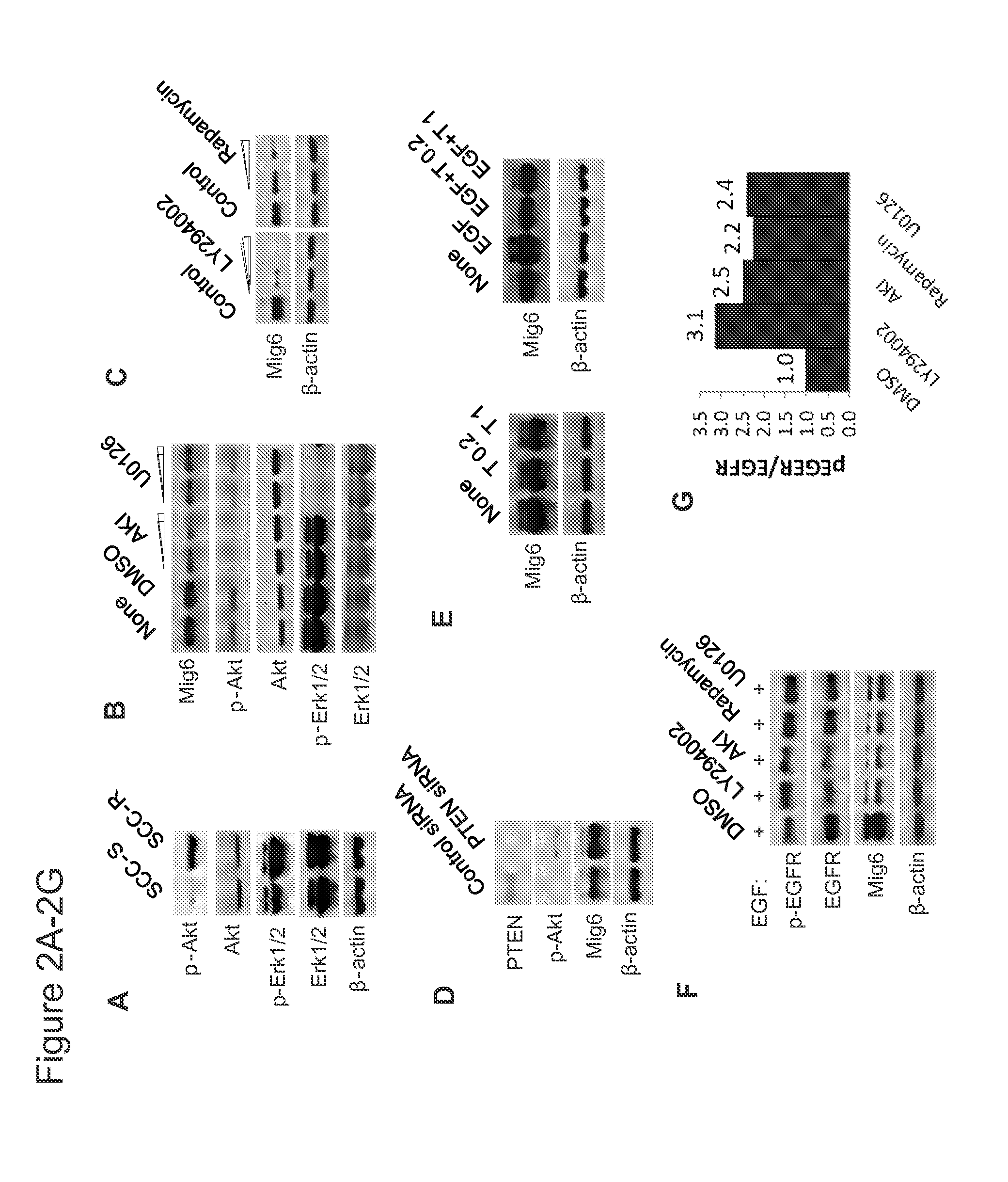
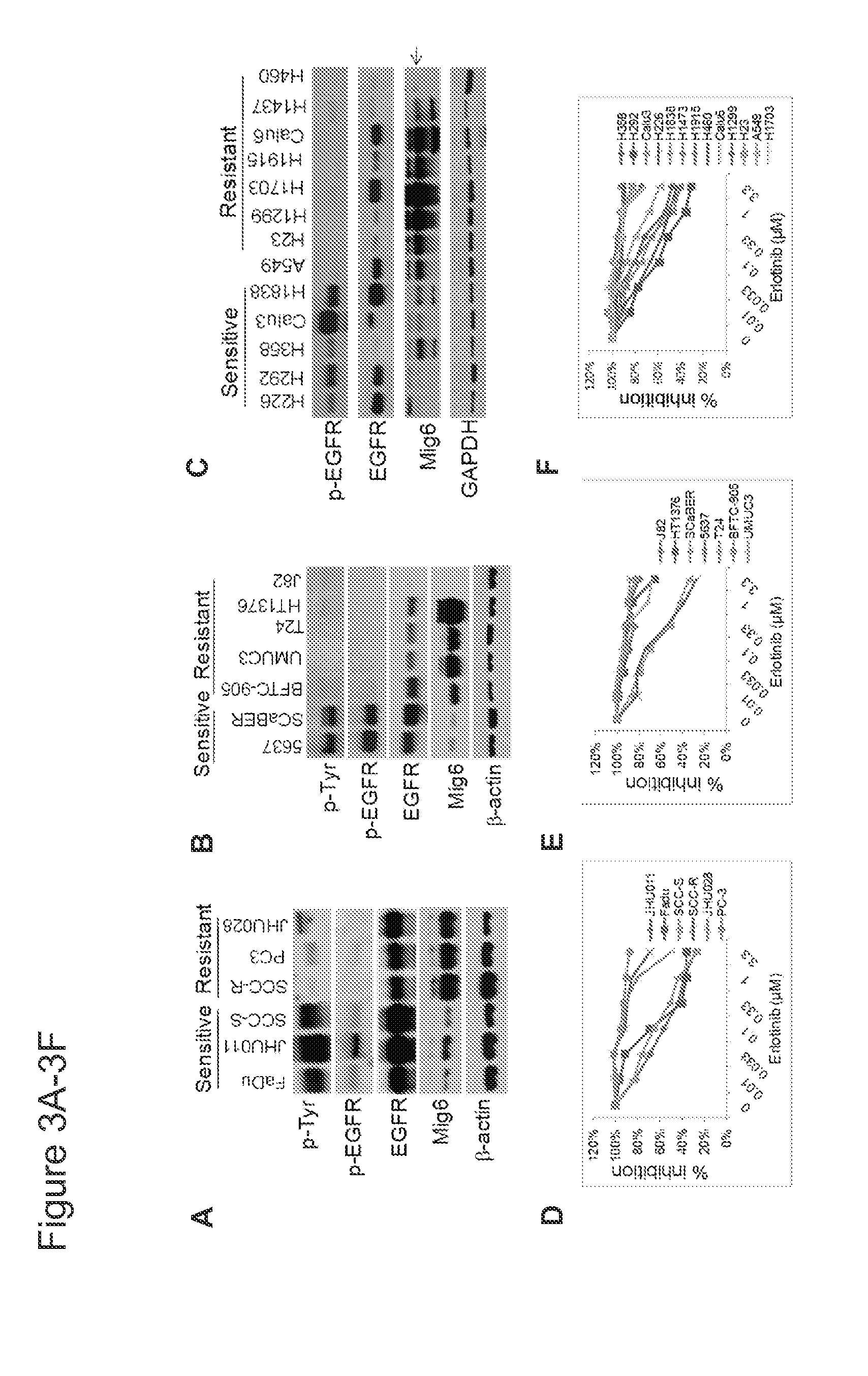
Mig6 and therapeutic efficacy
We identify markers capable of guiding the decision to incorporate epidermal growth factor receptor (EGFR) inhibitors, in particular EGFR tyrosine kinase inhibitors (TKIs), into chemotherapeutic regimens. Mitogen-inducible gene 6 (Mig6), a negative regulator of EGFR, is selectively upregulated during the development of resistance to the EGFR tyrosine kinase inhibitor (TKI) erlotinib, resulting in decreased EGFR phosphorylation. The ratio of Mig6 / EGFR expression highly correlates with erlotinib sensitivity. A low Mig6 / EGFR ratio correlates with a high response rate to gefitinib and a marked increase in progression-free survival for patients. The ratio of Mig6 to EGFR is a major predictor of biologic and clinical responses to EGFR inhibitors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Kit for quantificationally detecting BRAF (Block Repeat Active Flag) mutation
InactiveCN102161990AAccurate determination of contentEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceBRAF Gene MutationEGFR Tyrosine Kinase Inhibitors
The invention relates to a method and kit for detecting BRAF (Block Repeat Active Flag) mutation relevant to the curative effect of a molecular-targeting anti-cancer medicament, in particular relating to a fluorescence quantificational PCR (Polymerase Chain Reaction) detecting method and kit for detecting mutation in BRAF gene mutation hot spot regions and application thereof. According to the invention, mutation at special positions of BRAF genes is detected, the curative effect of the molecular-targeting anti-cancer medicament such as an EGFR (Epidermal Growth Factor Receptor) tyrosine kinase inhibitor, and the like can be forecasted, and furthermore, the individual medicament use schemes of patients with tumors are directed.
Owner:BEIJING ACCB BIOTECH
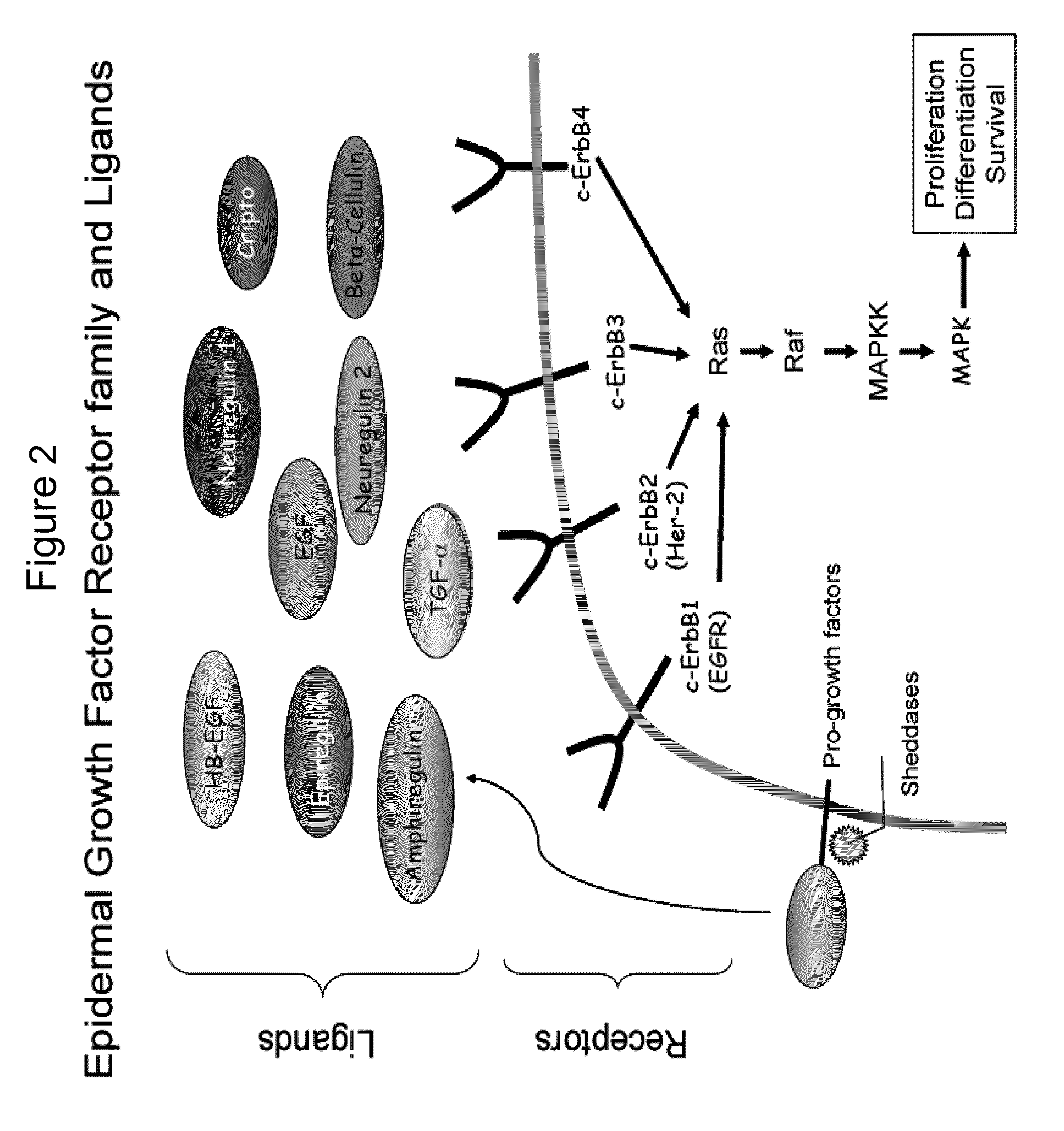
Inhibitors of the EGF-receptor tyrosine kinase and methods for their use
InactiveUS6864286B2Inhibitory activityDesigned for such useBiocideOrganic chemistryEGFR Tyrosine Kinase InhibitorsKinase
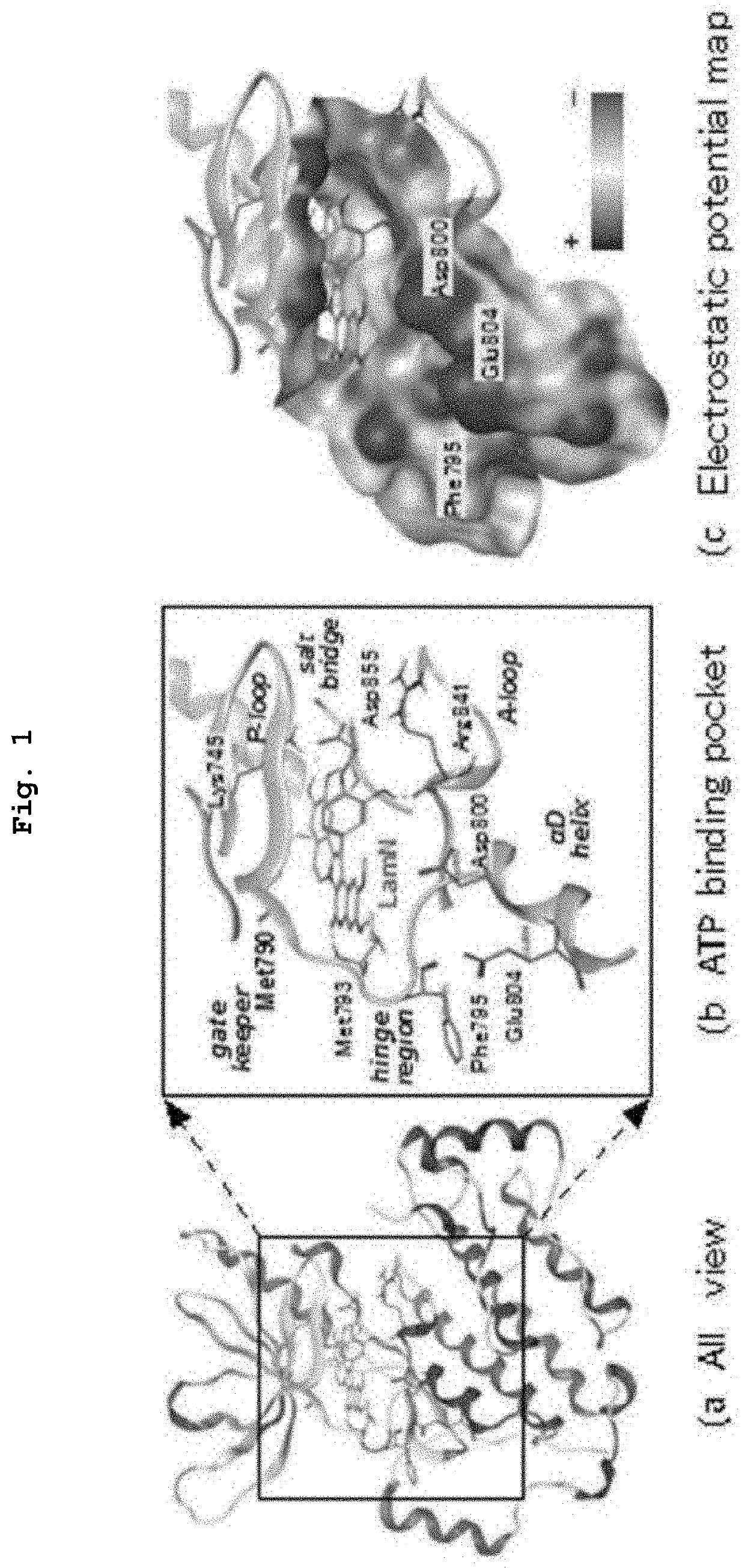
Novel compounds and pharmaceutical compositions useful as EGFR tyrosine kinase inhibitors. Methods of the invention include administration of the EGFR TK inhibitors to treat diseases characterized by enhanced expression of EGF, including cancers, particularly breast cancer. Additionally, a homology model representing the structure of EGFR kinase domain is provided, which model is useful for the rationally design and screening of compounds predicted to bind favorably to EGFR and to inhibit EGFR TK.
Owner:PARKER HUGHES INST
Methods for predicting a patient's response to EGFR inhibitors
InactiveUS20090286276A1Reduce the probability of reactionAvoiding expenseMicrobiological testing/measurementDisease diagnosisRegimenEGFR Tyrosine Kinase Inhibitors
The present invention provides methods for individualizing chemotherapy for cancer treatment, and particularly for evaluating a patient's responsiveness to one or more epidermal growth factor receptor (EGFR) inhibitors prior to treatment with such agents. Particularly, the invention provides an in vitro chemoresponse assay for predicting a patient's response to an EGFR inhibitor, such as an EGFR tyrosine kinase inhibitor or a molecule targeting the extracellular domain of EGFR. The method generally comprises culturing malignant cells from a patient's specimen (e.g., biopsy specimen), contacting the cultured cells with an EGFR inhibitor that is a candidate treatment for the patient, and evaluating the cultured cells for a response to the drug. In certain embodiments, monolayer(s) of malignant cells are cultured from explants prepared by mincing tumor tissue, and the cells of the monolayer are suspended and plated for chemosenstivity testing. The in vitro response to the drug as determined by the method of the invention is correlative with the patient's in vivo response upon receiving the EGFR inhibitor during chemotherapeutic treatment (e.g., in combination with other standardized or individualized chemotherapeutic regimen).
Owner:PRECISION THERAPEUTICS
Medicament composition containing sorafenib, cMet inhibitors and EGFR tyrosine kinase inhibitors and application thereof
InactiveCN101836991AGood curative effectGood synergyAntineoplastic agentsHeterocyclic compound active ingredientsSide effectEGFR Tyrosine Kinase Inhibitors
The invention relates to a medicament composition containing sorafenib, hepatocyte growth factor receptor (cMet) inhibitors and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors and application thereof in preparing medicaments for treating lung cancer, colon cancer, liver cancer, kidney cancer, stomach cancer, encephaloma, sarcoma, pancreatic cancer, ovarian cancer, breast canceror prostatic cancer. The medicament composition has remarkable synergistic effect, improves the curative effect of the medicament, decreases the administration dosage and reduces the occurrence of side effects.
Owner:DINKUM INT INVESTMENT HONG KONG
Gene combination for instructing individualized treatment by medicines such as geftinat and Tarceva
InactiveCN102146434AMicrobiological testing/measurementDNA/RNA fragmentationMedicine allergySide effect
The invention belongs to the technical field of gene techniques and provides a gene combination for instructing the individualized treatment by medicines such as geftinat and Tarceva, which comprises epidermal growth factor receptor (EGFR)-Exon19, EGFR-exon21, Kras-codon12 / 13, and Kras-codon61. In the invention, instruction on medicine allergy is given to a patient to be treated by EGFR-tyrosine kinase inhibitors (TKI) such as geftinat and Tarceva by detecting the mutation condition of the gene loci, namely the EGFR-Exon19, EGFR-exon21, Kras-codon12 / 13, and Kras-codon61, in the tumor tissue of the patient, so that the treatment effect is maximized and the side effect is reduced to the lowest degree.
Owner:SHANGHAI BIOTECAN PHARMA
Methods and Compositions for Detecting a Drug Resistant EGFR Mutant
ActiveUS20120077201A1Microbiological testing/measurementAcquired resistanceGain of function mutation
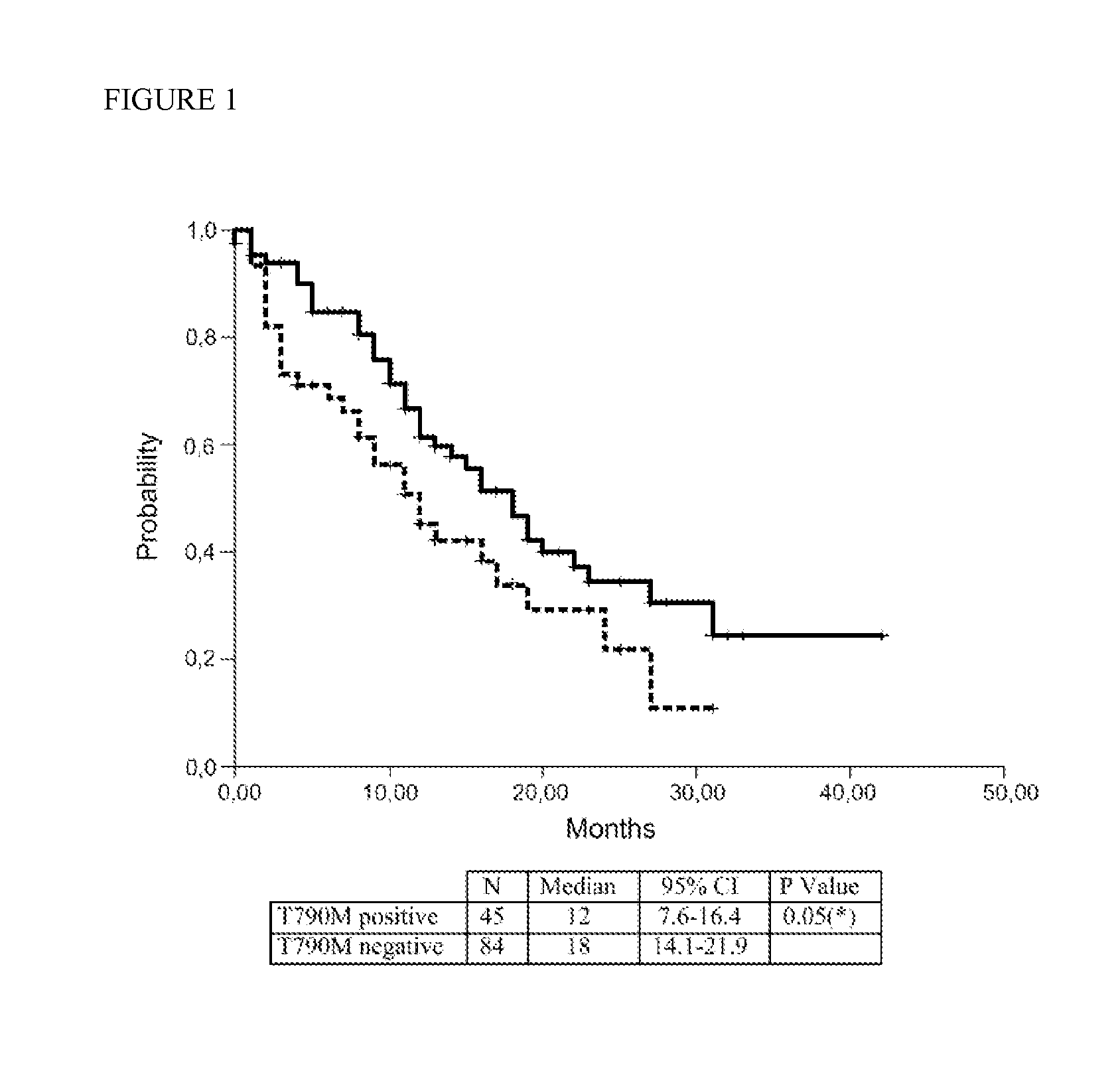
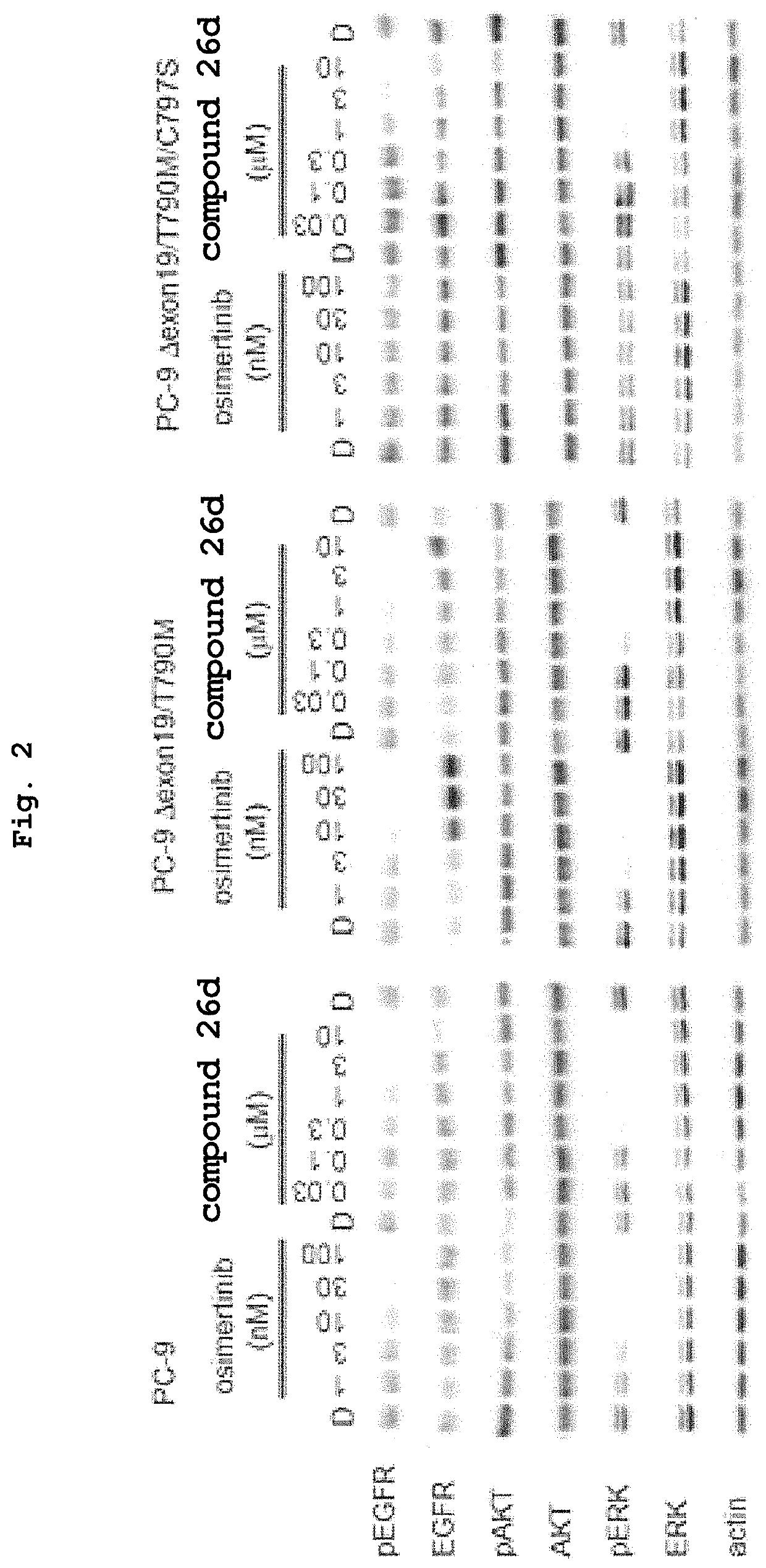
Development of acquired resistance to the therapeutic effects of an epidermal growth factor (EGFR) tyrosine kinase inhibitor in a patient that is suffering from a cancer is predicted by:(a) obtaining a sample from the patient, wherein the cancer harbors a somatic gain-of-function mutation in the tyrosine kinase domain of EGFR that enhances the sensitivity of the cancer to the tyrosine kinase inhibitor, and(b) testing the sample to determine whether the gene encoding EGFR is present in a mutant form that encodes a T790M mutant of EGFR in addition to the somatic gain of function mutation. A finding that the mutant form is present indicates that the cancer has become or will become resistant to the EGFR tyrosine kinase inhibitor.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Kit for quantitative detection of K-ras mutation
ActiveCN102115782AAccurate determination of contentEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesEGFR Tyrosine Kinase Inhibitors
The invention relates to a kit for quantitative detection of K-ras mutation. The invention specifically relates to a detecting method and a detecting kit for K-ras gene mutation relevant with the curative effect of molecular targeted anti-cancer drugs. The invention especially relates to fluorescence quantification PCR detecting method for K-ras gene mutational hotspot region detecting mutational, kit and the application of the fluorescence quantification PCR detecting method. The method of the invention detects the mutation of K-ras gene at a particular locus, predicts the curative effect of molecule targeting anticancer medicament EGFR tyrosine kinases inhibitor, furthermore provides clinic individualized medication scheme for a tumour patient with guidance.
Owner:BEIJING ACCB BIOTECH
Kit for quantitative detection of braf mutation
InactiveUS20130095491A1Accurately and quantitatively ratioEasy to operateMicrobiological testing/measurementChemiluminescene/bioluminescenceBRAF Gene MutationBraf genes
The present invention relates to a method and assay kit for BRAF gene mutations which relates to the effect of molecule-targeting anti-tumor drug. Particularly, the present invention relates to a fluorescent quantitative PCR method and kit for detecting mutations at hotspots of BRAF gene, together with the use thereof. The present invention detects the mutations at specific sites of BRAF gene, and can predict the therapeutic efficacy of anti-EGFR tyrosine kinase inhibitors, an anti-tumor drug. Therefore, the present invention can provide a guidance to individualized treatments for cancer patients.
Owner:BEIJING ACCB BIOTECH
Process for the manufacture of (e)-4-n,n-dialkylamino crotonic acid in HX salt form and use thereof for synthesis of EGFR tyrosine kinase inhibitors
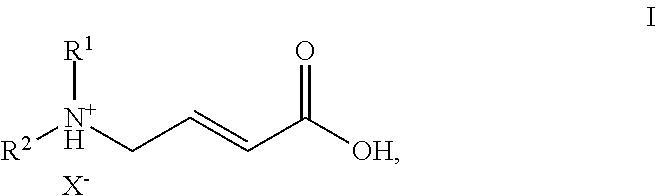
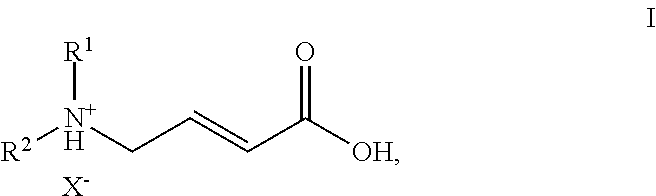
ActiveUS20150183764A1Operational securityOrganic compound preparationAmino-carboxyl compound preparationTrifluoroacetic acidEGFR Tyrosine Kinase Inhibitors
The present invention is directed to an efficient process for the manufacture of (E)-4-N,N-dialkylamino crotonic acid in HX salt form of formula Iwherein R1 and R2 independently denote C1-3-alkyl groups and X− denotes an acid anion, such as the chloride, bromide, tosylate, mesylate or trifluoroacetate anion, with high quality, and a process for synthesis of EGFR tyrosine kinase inhibitors with heterocyclic quinazoline, quinoline or pyrimidopyrimidine core structure, using the acid addition salt I and activated derivatives thereof as intermediates.
Owner:BOEHRINGER INGELHEIM INT GMBH
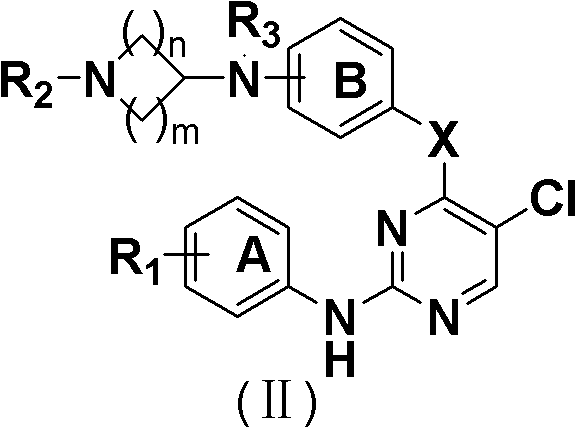
5-Chloropyrimidines and their use as EGFR tyrosine kinase inhibitors
ActiveCN103159742BHas healing propertiesNervous disorderOrganic chemistryEGFR Tyrosine Kinase InhibitorsTyrosine-kinase inhibitor
The invention discloses an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, and the structural formula of the inhibitor is shown as in the formula (I). The invention further discloses an application of any compound shown as in the formula (I) and pharmacy-acceptable salt of the compound. The invention further provides a medicine compound for curing, and the medicine compound comprises the compound shown as in the formula (I) and an EGFR modifier.
Owner:BEIJING HANMI PHARMA CO LTD
Aminopyrimidine derivative and application as EGFR tyrosine kinase inhibitor
ActiveCN113773305AStrong inhibitory activityReduced inhibitory activityOrganic active ingredientsOrganic chemistryEGFR Tyrosine Kinase InhibitorsTyrosine-kinase inhibitor
The invention provides an aminopyrimidine derivative as shown in a formula I, and a pharmaceutically acceptable salt, an isomer, a solvate and a pharmaceutical composition thereof. The invention also provides a preparation method of the compound as shown in the formula I. The compound can be used as an EGFR high-selectivity ligand molecule of EGFRT790M / L858R and EGFRDel19 mutation, and is used for treating related cancers mediated by EGFR kinase.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Therapeutic combination of a third-generation EGFR tyrosine kinase inhibitor and a raf inhibitor
InactiveCN110996960AAntineoplastic agentsHeterocyclic compound active ingredientsEGFR Tyrosine Kinase InhibitorsTyrosine
Owner:NOVARTIS AG
Therapeutic combination of a third generation EGFR tyrosine kinase inhibitor and a cyclin d kinase inhibitor
InactiveCN110996962AAntineoplastic agentsHeterocyclic compound active ingredientsEGFR Tyrosine Kinase InhibitorsTyrosine
This invention relates to a pharmaceutical combination comprising (a) a third generation EGFR tyrosine kinase inhibitor and (b) a cyclin D kinase 4 / 6 (CDK4 / 6) inhibitor, particularly for use in the treatment of a cancer, particularly a lung cancer. This invention also relates to uses of such a combination for the preparation of a medicament for the treatment of a cancer; methods of treating a cancer in a subject in need thereof comprising administering to said subject a jointly therapeutically effective amount of said combination; pharmaceutical compositions comprising such combination and commercial packages thereto.
Owner:NOVARTIS AG
Crystal form of deuterated osimertinib pharmaceutical salt and preparation method thereof
InactiveCN111285852AImprove liquidityImprove solubilityOrganic chemistry methodsSulfonic acids salts preparationEGFR Tyrosine Kinase InhibitorsChemical compound
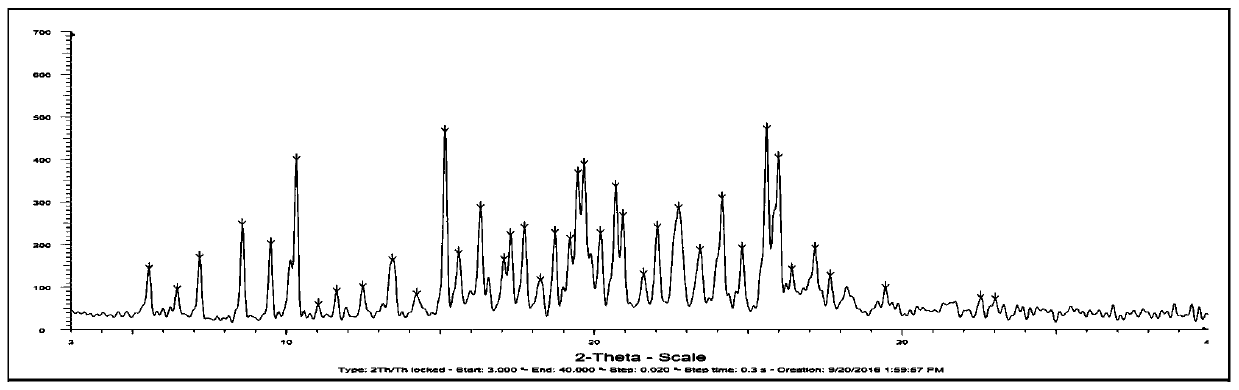
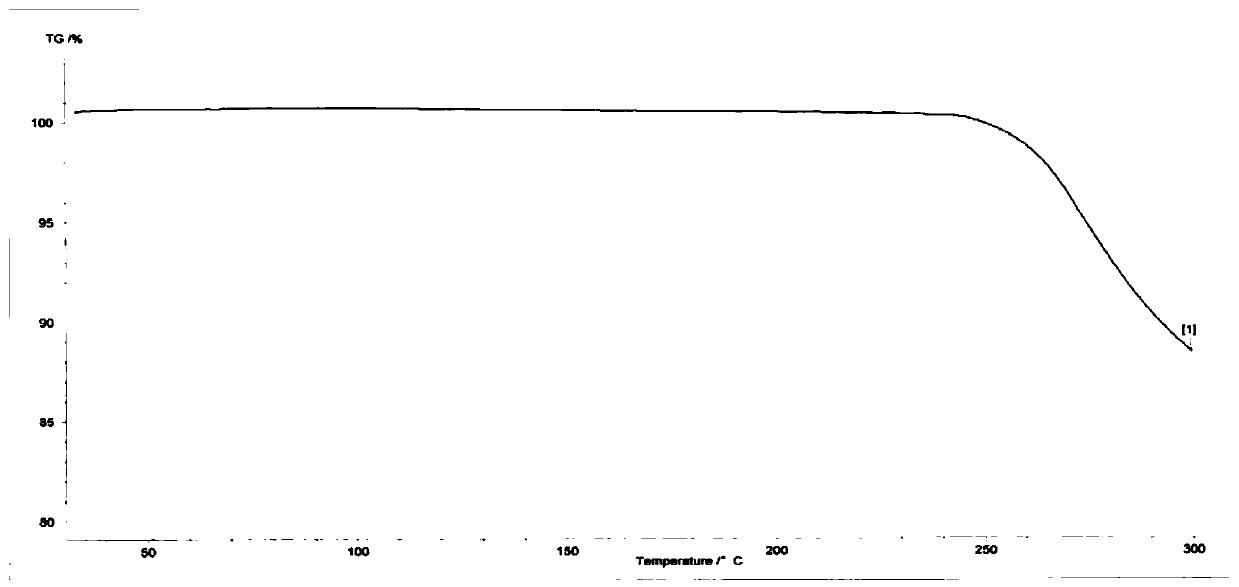
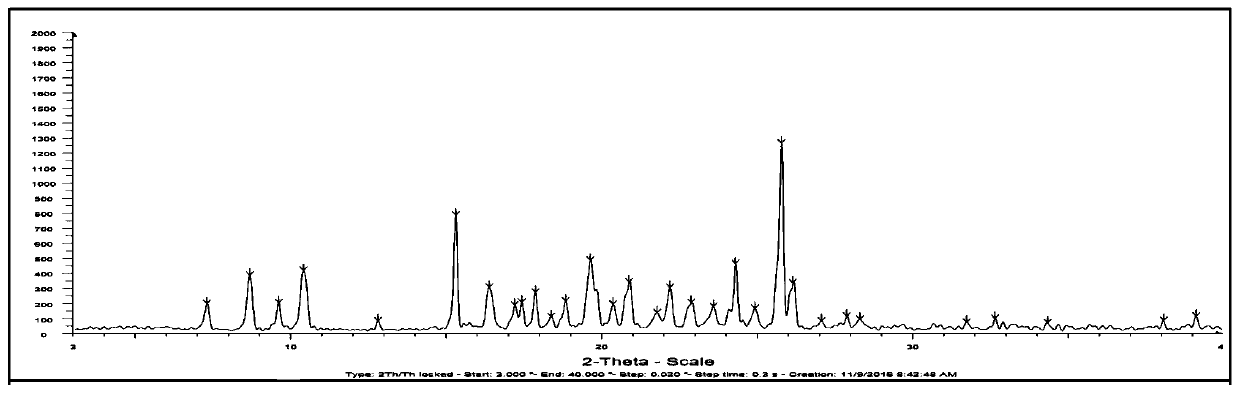
The invention relates to a compound crystal form and a preparation method thereof, in particular to a crystal form I of a deuterated osimertinib pharmaceutical salt as well as a preparation method andapplication thereof. An X-ray powder diffraction pattern of the crystal form represented by a 2theta angle has characteristic diffraction peaks at positions of 5.49 + / -0.2 degrees, 6.42 + / -0.2 degrees, 11.01 + / -0.2 degrees, 11.61 + / -0.2 degrees, 12.46 + / -0.2 degrees, 13.42 + / -0.2 degrees, 14.22 + / -0.2 degrees and 15.58 + / -0.2 degrees. The crystal form is not easy to absorb moisture, has higher solubility, better stability, better fluidity and preparation processability, and has important significance in preparation of EGFR tyrosine kinase inhibitor drugs. The invention also discloses the preparation method of the crystal form I of the deuterated osimertinib pharmaceutical salt, and the crystal form I obtained by the preparation method has higher purity and higher yield.
Owner:GUANGZHOU BOJI MEDICINE SERVICES
Kits for quantitative detection of k-ras mutations
InactiveUS20120288862A1Accurately and quantitatively ratioEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesEGFR Tyrosine Kinase Inhibitors
The present invention relates to an assay kit for quantitatively detecting k-ras gene mutations. Particularly, the present invention relates to detection method and a detection kit for K-ras gene mutations, which relates to the therapeutic efficacy of targeted molecular anti-cancer drugs. More particularly, the present invention relates to a fluorescent quantitative PCR method and kit for detecting mutations at hotspots of K-ras gene, together with the use thereof. The present invention detects the mutations at specific sites of K-ras gene, and can predict the therapeutic efficacy of anti-EGFR tyrosine kinase inhibitors. Therefore, it can provide a guidance to individualized treatments for cancer patients.
Owner:BEIJING ACCB BIOTECH
Molecular biomarkers for predicting response to tyrosine kinase inhibitors in lung cancer
InactiveUS20120316187A1Reduced expression levelOrganic active ingredientsBiocideEGFR Tyrosine Kinase InhibitorsTyrosine-kinase inhibitor
The invention relates to a method for predicting the response to the treatment with an EGFR tyrosine kinase inhibitor of a patient suffering lung cancer an carrying a mutation in the EGFR gene based on the expression levels in a sample of said patient of the BRCA1 gene wherein low BRCA1 expression levels are indicative of a positive response of a patient. This positive response is also observed in patients showing the T790M mutation in the EGFR gene which is usually associated with resistance to EGFR tyrosine kinase inhibitors.
Owner:PANGAEA BIOTECH
New application of total glucosides of paeony as EGFR (epidermal growth factor receptor) tyrosine kinase inhibitor
ActiveCN103550313AImprove securityReduce expressionAntineoplastic agentsPlant ingredientsEGFR Tyrosine Kinase InhibitorsTyrosine-kinase inhibitor
The invention belongs to the field of medicines and relates to a new application of total glucosides of paeony as an EGFR (epidermal growth factor receptor) tyrosine kinase inhibitor. The content of paeoniflorin (C23H28O11) in the total glucosides of paeony is not less than 34.6%. Compared with the traditional EGFR tyrosine kinase inhibitor gefitinib, the total glucosides of paeony have the advantages that the total glucosides of paeony have high safety, can obviously reduce the expression quantity and activation quantity of EGFR tyrosine kinase, are low in price and have abundant resources, thereby providing a new choice for medicine development.
Owner:OCEAN UNIV OF CHINA
Combined preparation and application of combined preparation in preparing non-small-cell lung carcinoma drug
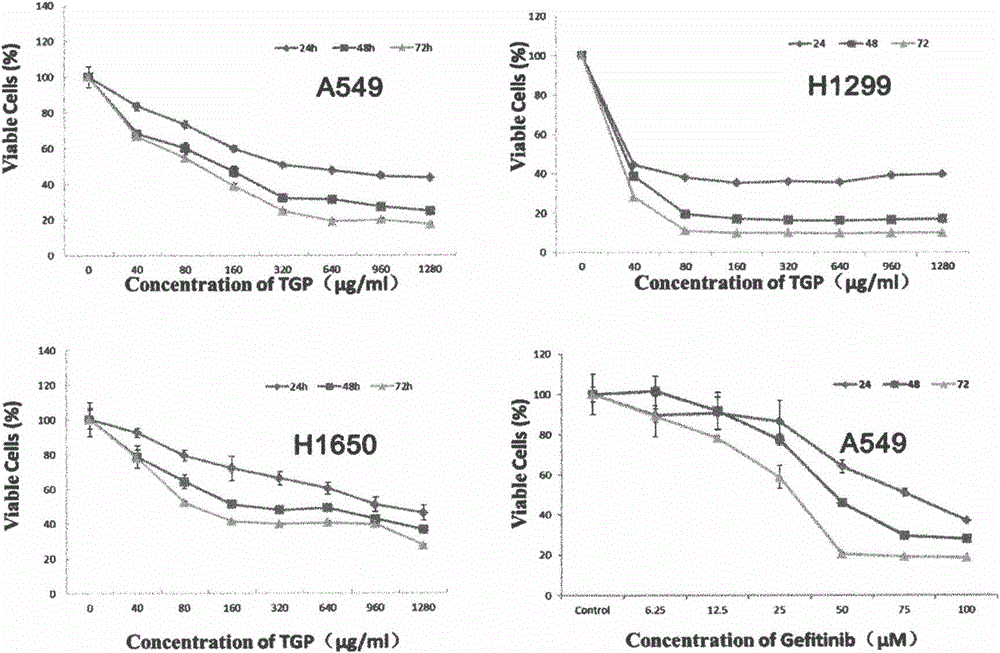
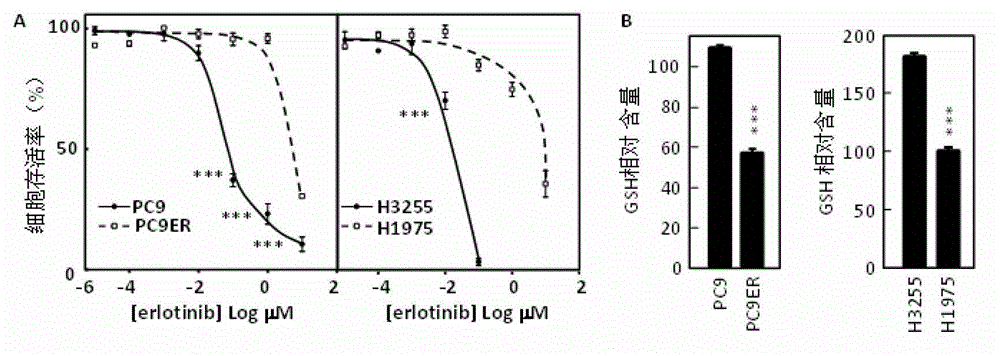
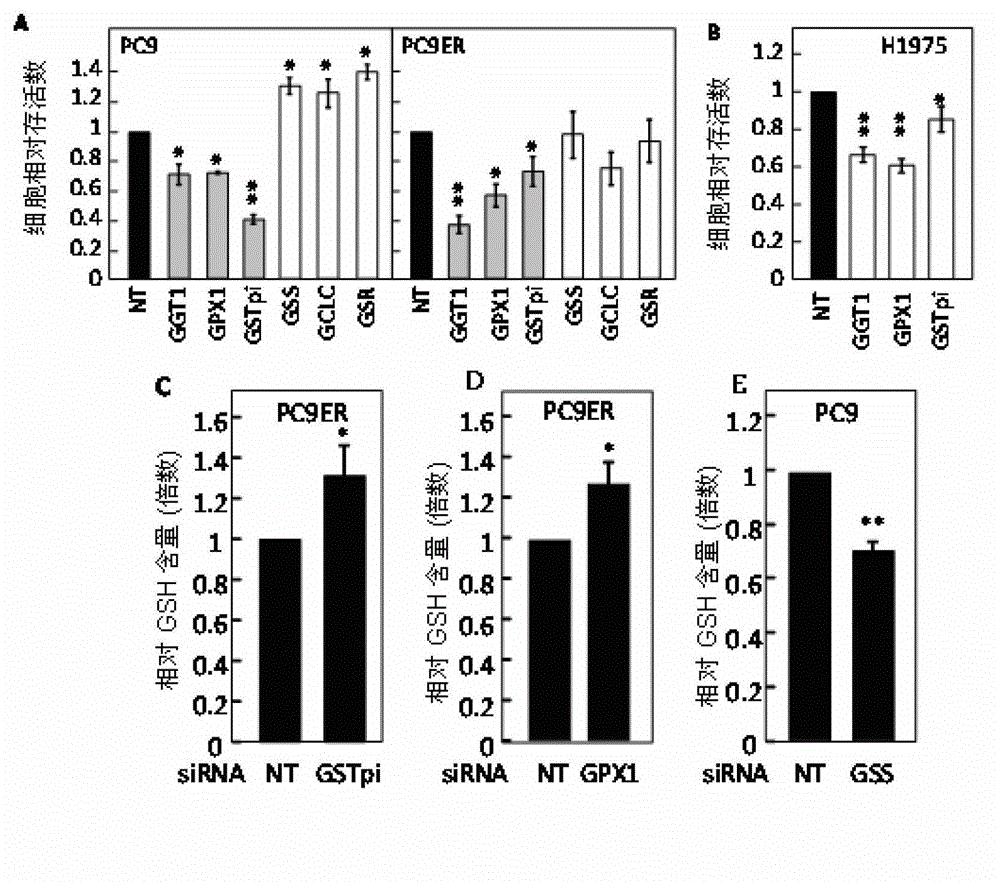
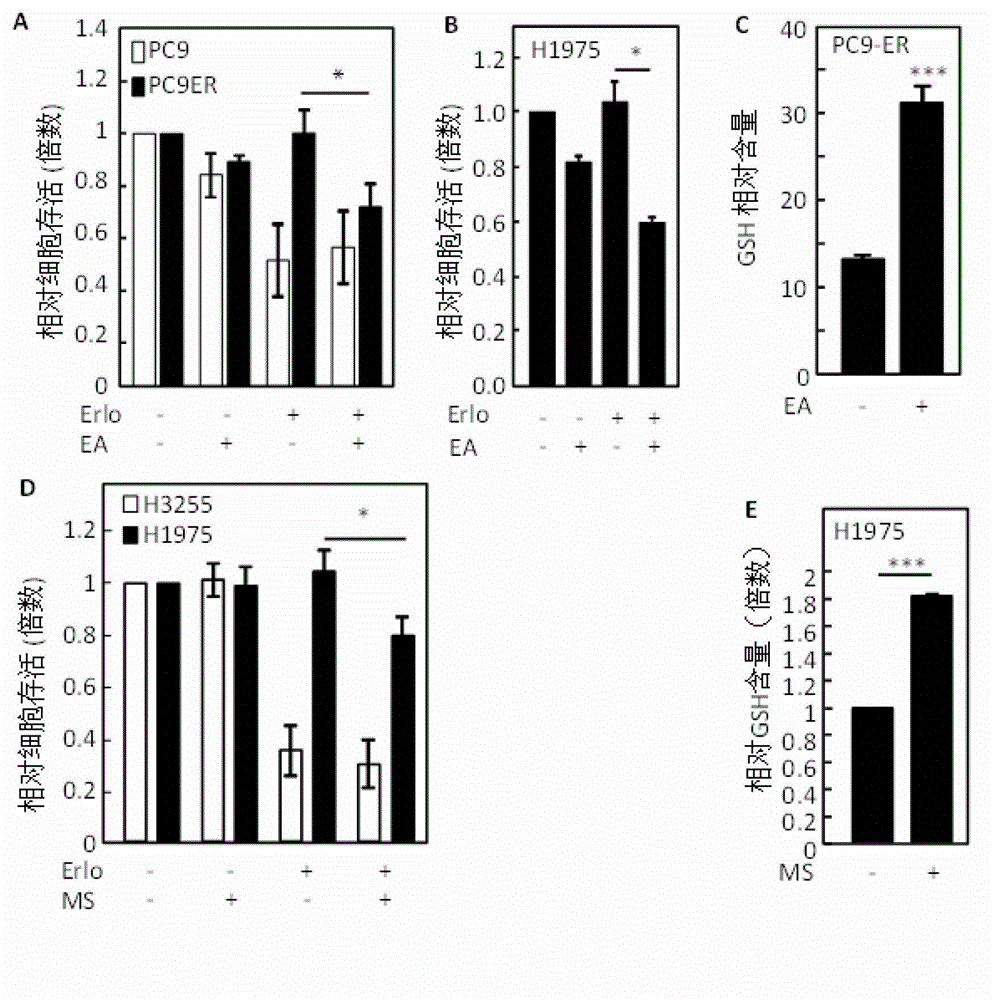
ActiveCN103330940AInhibit killIncrease GSH concentrationOrganic active ingredientsRespiratory disorderEGFR T790MEGFR Tyrosine Kinase Inhibitors
The invention discloses a combined preparation and an application of the combined preparation in preparing a non-small-cell lung carcinoma drug. An EGFR (Epidermal Growth Factor Receptor) tyrosine kinase inhibitor and a preparation for increasing a concentration of glutathione (GSH) in lung carcinoma cells are administered simultaneously or successively. According to the combined preparation and the application, through a systemic research, the GSH plays an important role in resisting the EGFR tyrosine kinase inhibitor to an EGFR T790M mutation non-small-cell lung carcinoma, so that drug resisting cells are sensitive to treatment of the EGFR tyrosine kinase inhibitor again by utilizing a mode of increasing the concentration of the GSH in the lung carcinoma cells; and a cell experiment and an animal experiment prove that the method is safe and effective, can effectively kill the lung carcinoma cells, and can inhibit proliferation of the lung carcinoma cells.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Quinolyl EGFR tyrosine kinase inhibitor containing zinc binding group
InactiveCN103965106AGood antitumor activityDelay drug resistanceOrganic active ingredientsOrganic chemistryEGFR Tyrosine Kinase InhibitorsZinc binding
The invention belongs to the technical field of medicin and specifically relates to a quinolyl EGFR tyrosine kinase inhibitor containing a zinc binding group and a deuterated material, a pharmaceutically acceptable salt or stereoisomer thereof. The inhibitor is represented by a general formula (I) which is described in the specification; and in the general formula (I), R1, R2, R2', R3, R4, R5, R6, R7, W, X, L, T and n are as defined in the specification. The invention further relates to a preparation method for the inhibitor, a medicinal preparation containing the inhibitor and application of the inhibitor in preparation of drugs used for treating and / or preventing tumors.
Owner:TONGHUA SIHUAN PHARM
Reagent kit for quantitatively detecting the mutations of epidermal growth factor receptor(EGFR)
InactiveUS20120237935A1Easy to operateImprove standardizationMicrobiological testing/measurementFluorescence/phosphorescenceEGFR Tyrosine Kinase InhibitorsEGFR Gene Mutation
The present invention relates to a detection method and a detection kit for EGFR gene mutations, which relates to the therapeutic efficacy of molecular-targeted anti-cancer drugs. Particularly, the present invention relates to a fluorescent quantitative PCR method and kit for detecting mutations at hotspots of EGFR gene, together with the use thereof. The present invention detects the mutations at specific sites of EGFR gene, and can predict the therapeutic efficacy of EGFR tyrosine kinase inhibitors. Therefore, it can provide a guidance to individualize treatments for cancer patients.
Owner:BEIJING ACCB BIOTECH
Fourth-generation EGFR tyrosine kinase inhibitor
ActiveUS11286261B2Organic chemistryRespiratory disorderEGFR Tyrosine Kinase InhibitorsTyrosine-kinase inhibitor
Provided is a compound having a tyrosine kinase inhibitory activity specific to C797S resistant mutant EGFR (particularly C797S tertiary-resistant mutant EGFR) and is useful as a C797S resistant mutant EGFR (particularly C797S mutant tertiary-resistant EGFR) specific tyrosine kinase inhibitor, an agent for preventing and / or treating non-small cell lung cancer with resistance mutant EGFR and the like, and the like.
Owner:NAGASAKI UNIVERSITY +2
EGFR tyrosine kinase inhibitor and application thereof
PendingCN113493419APrevent proliferationInhibition of proliferative abilityOrganic active ingredientsOrganic chemistryEGFR Tyrosine Kinase InhibitorsTyrosine-kinase inhibitor
The invention provides a compound as shown in formula (I), a stereoisomer or pharmaceutically acceptable salt thereof, and an application of the compound in preparation of a medicine for preventing or treating related diseases caused by EGFR mutation.
Owner:PHARMABLOCK SCIENCES (NANJING) INC
Quinazoline-based egfr tyrosine kinase inhibitor containing a zinc-binding group
ActiveCN103965174BStrong inhibitory activityOrganic active ingredientsOrganic chemistryEGFR Tyrosine Kinase InhibitorsHuman epidermal growth factor receptor
Belonging to the technical field of medicine, the invention in particular relates to a zinc binding group-containing quinazolinyl EGFR (epidermal growth factor receptor) tyrosine kinase inhibitor shown as general formula (I), its deuterated compounds, pharmaceutically acceptable salts or stereoisomers, wherein R1, R2, R3, R4, R5, R6, R7, W, X, L, and T are defined as the specification. The invention also relates to a preparation method of the compounds, pharmaceutical preparations containing the compounds, and application of the compounds in preparation of drugs treating and / or preventing tumors. (formula I).
Owner:BEIJING AOHE DRUG RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com