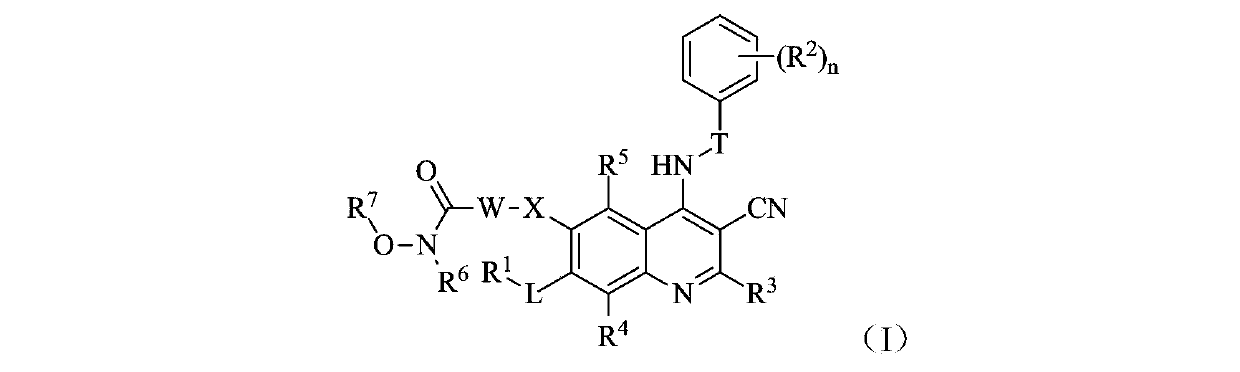
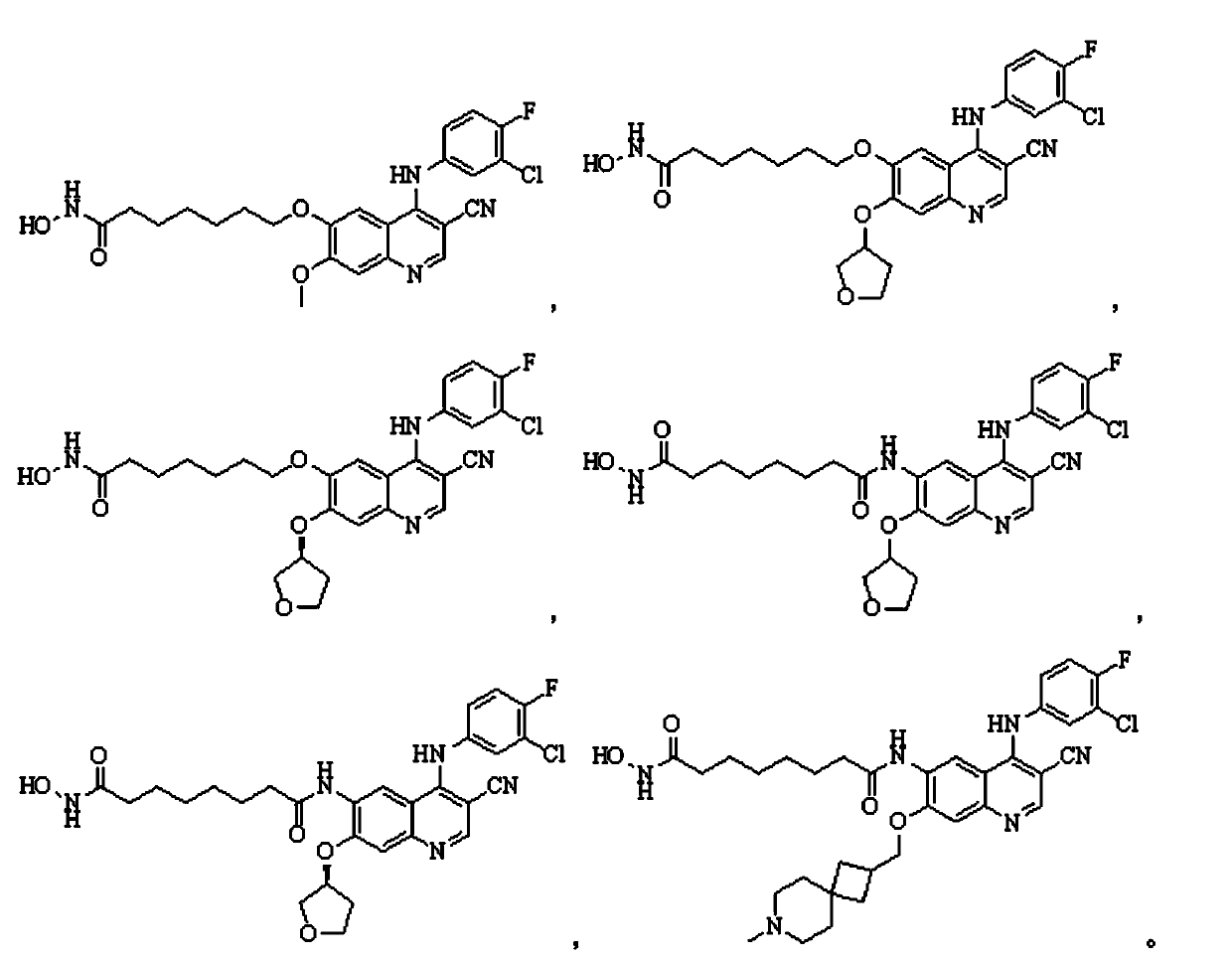
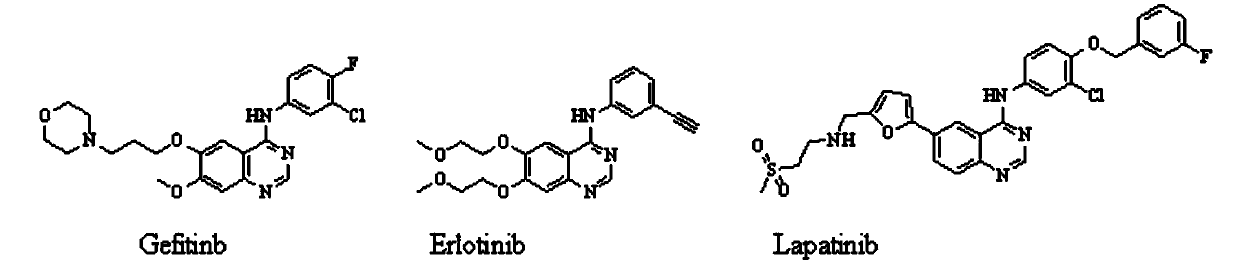
Quinolyl EGFR tyrosine kinase inhibitor containing zinc binding group
A technology of alkylamino and alkyl, which is applied in the field of medicine and can solve the problems of drug resistance of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Example 1 The preparation of 7-(4-(3-chloro-4-fluorophenylamino)-3-cyano-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide ( Compound 1)
[0131]
[0132] (1) Preparation of ethyl 2-cyano-3-ethoxyacrylate
[0133]
[0134] Dissolve triethoxymethane (16.65 mL, 0.1 mol) and ethyl dicyanoacetate (10.65 mL, 0.1 mol) in acetic anhydride (80 mL) solution, react at 150-160 °C for 5 hours, and cool to room temperature , concentrated under reduced pressure to remove the solvent to obtain the product (13.5 g, yield 80%).
[0135] (2) Preparation of ethyl 7-(4-amino-2-methoxyphenoxy)heptanoate
[0136]
[0137] 7-(2-Methoxy-4-nitrophenoxy)methyl heptanoate (17 g, 0.05 mol) was dissolved in THF / H 2 O (volume ratio 1:1, 200 mL) mixed solution was added palladium carbon (8 g), stirred under hydrogen atmosphere for 20 hours, filtered, and the filtrate was concentrated under reduced pressure to obtain the product (14 g, yield 94.8%).
[0138] (3) (Z)-Ethyl 7-(4-(2-cyano-3-et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com