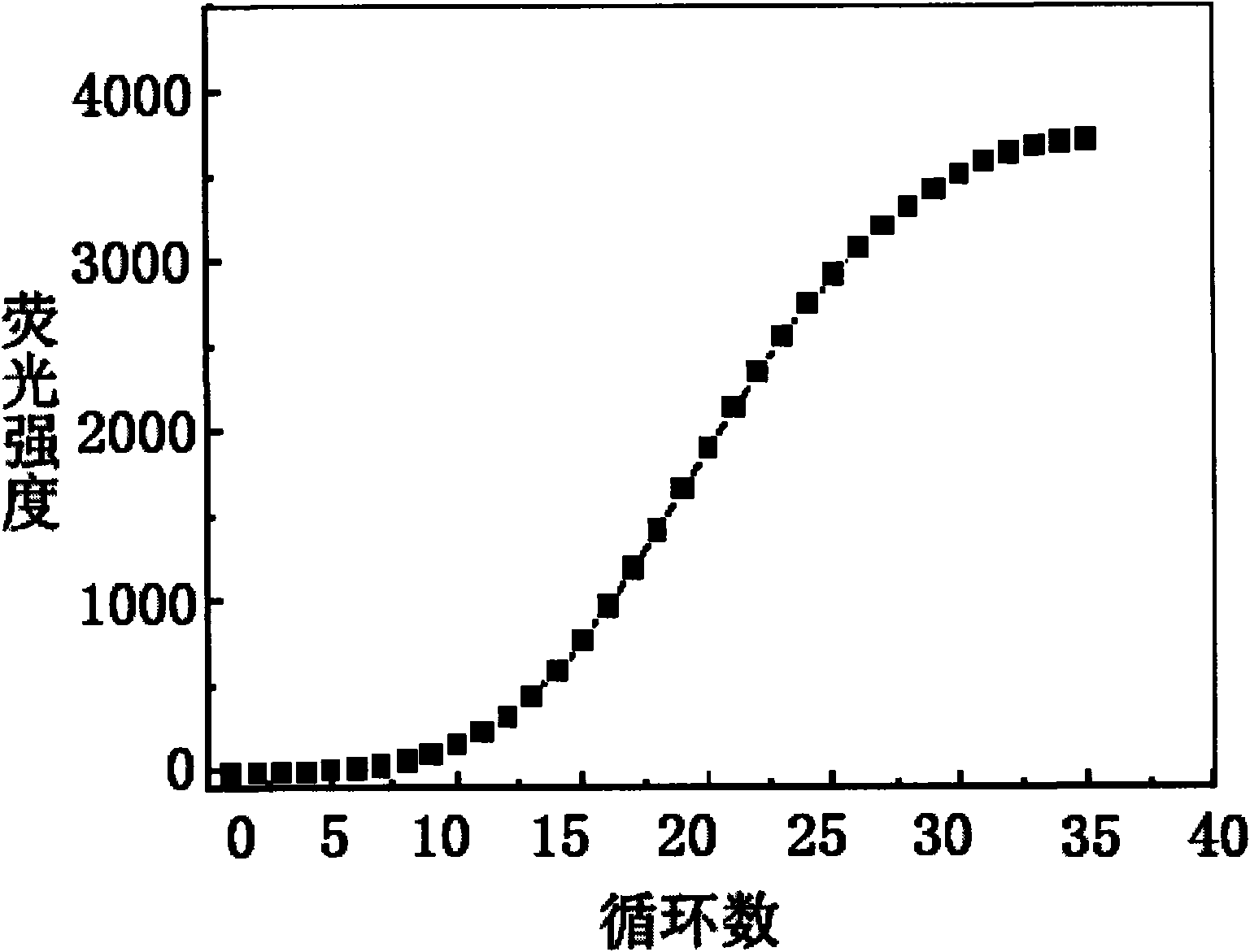
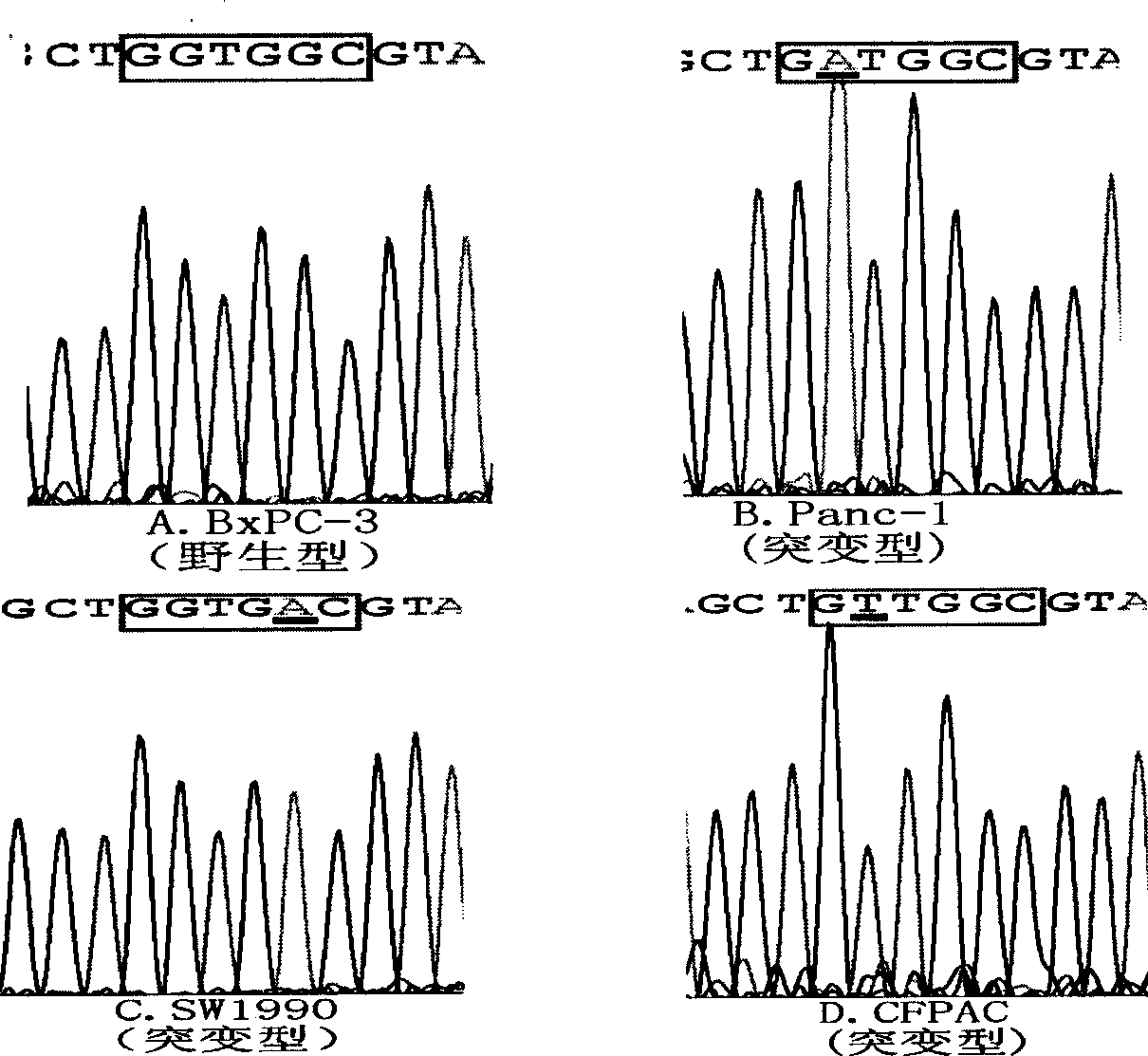
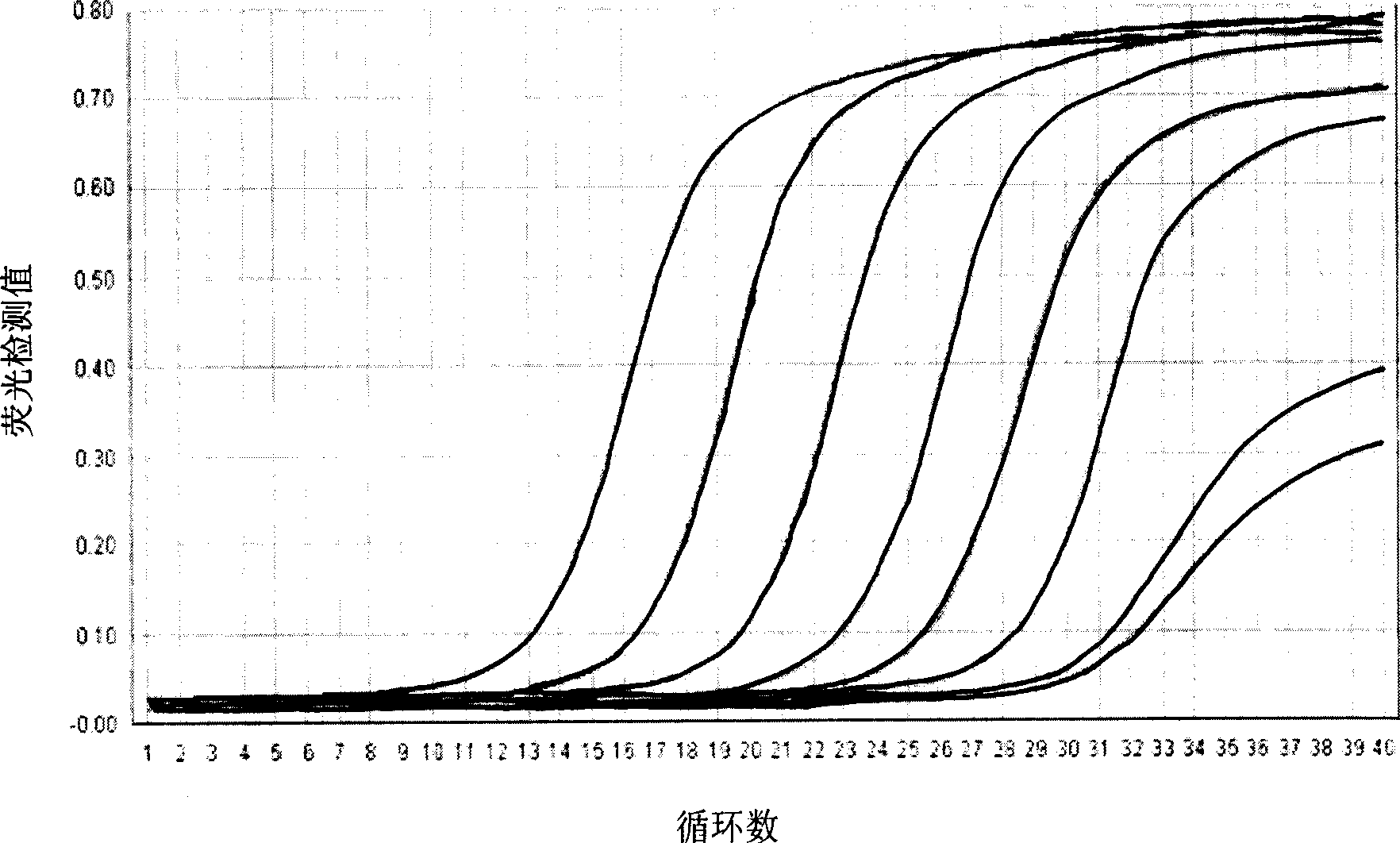
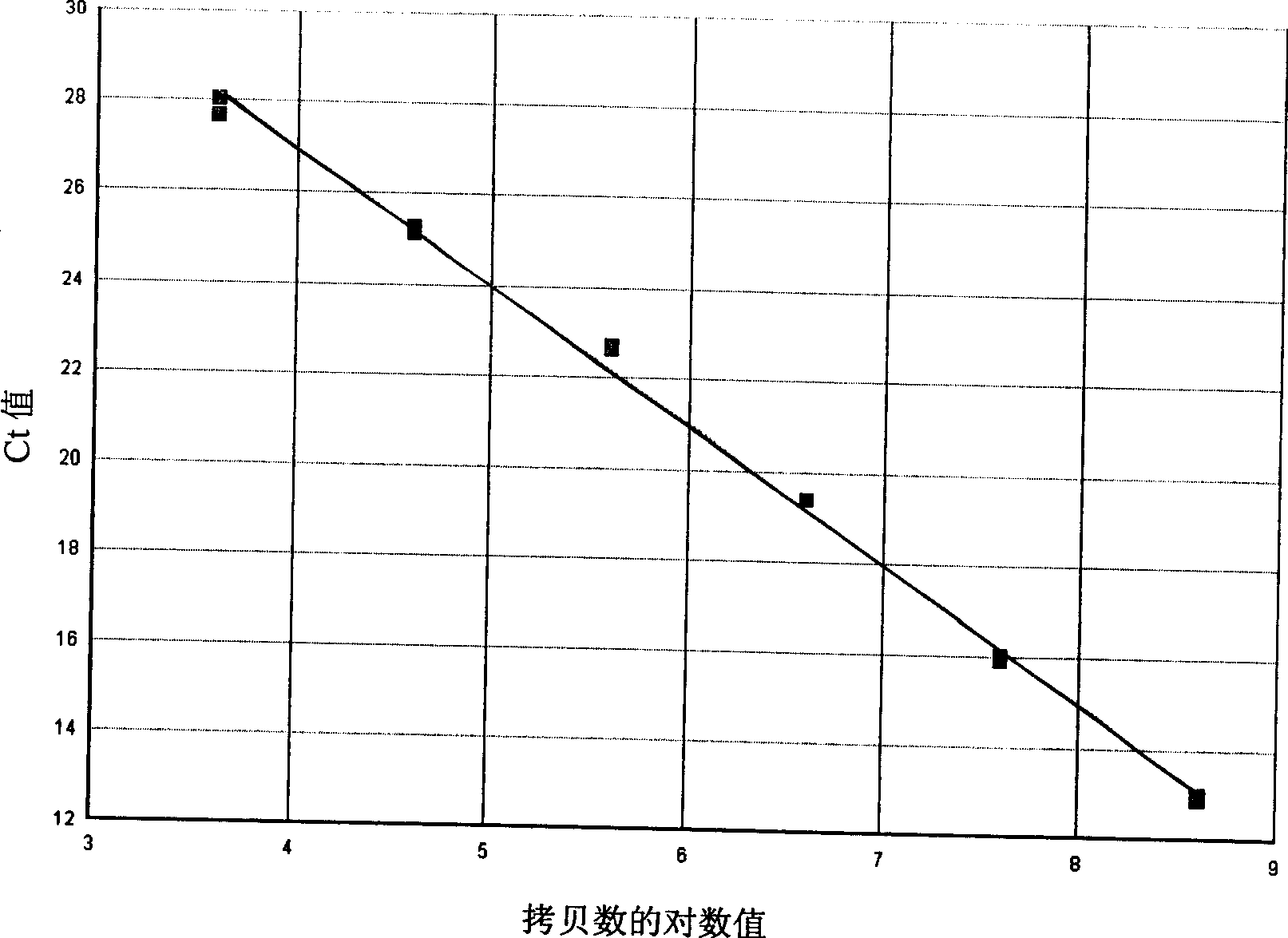
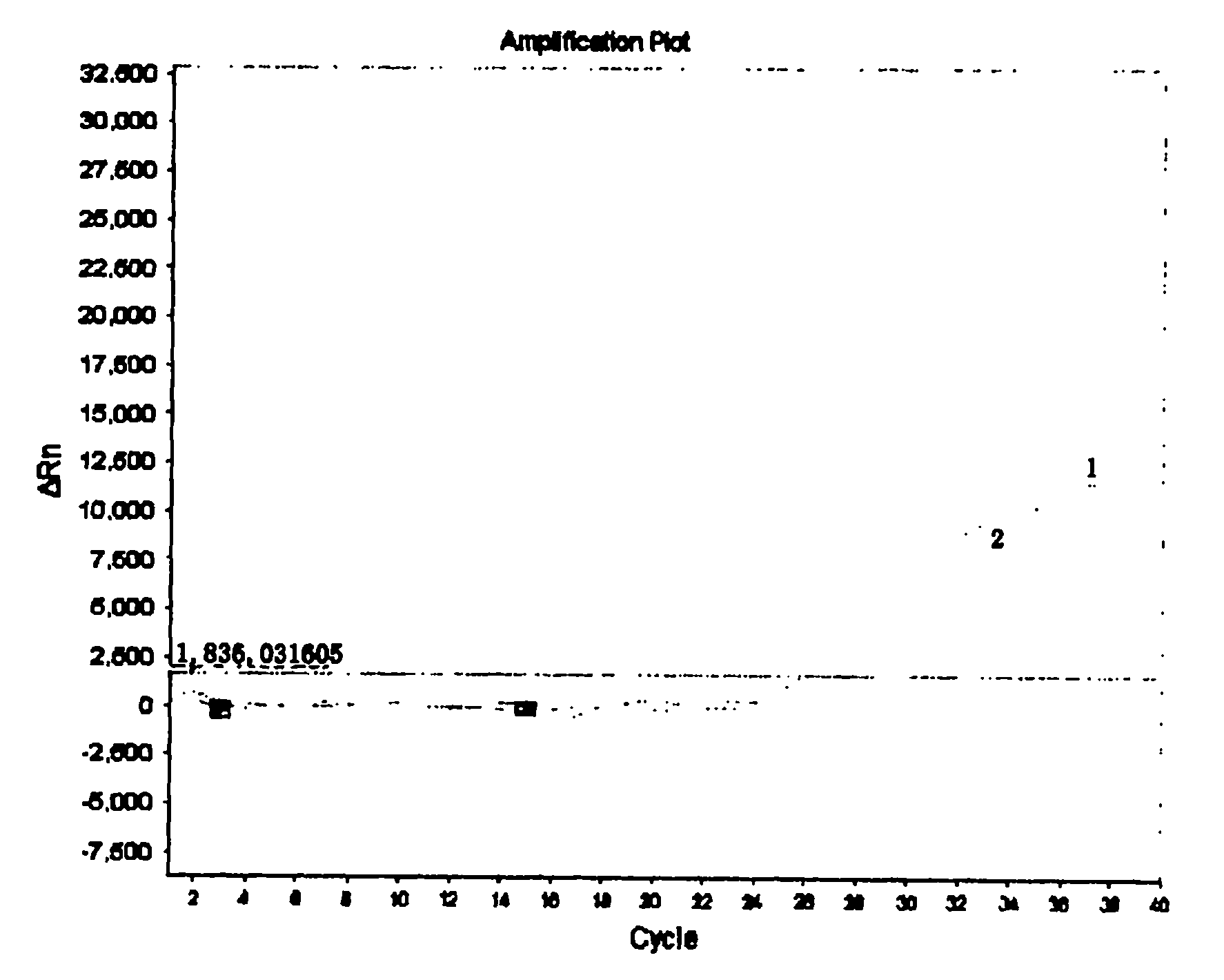
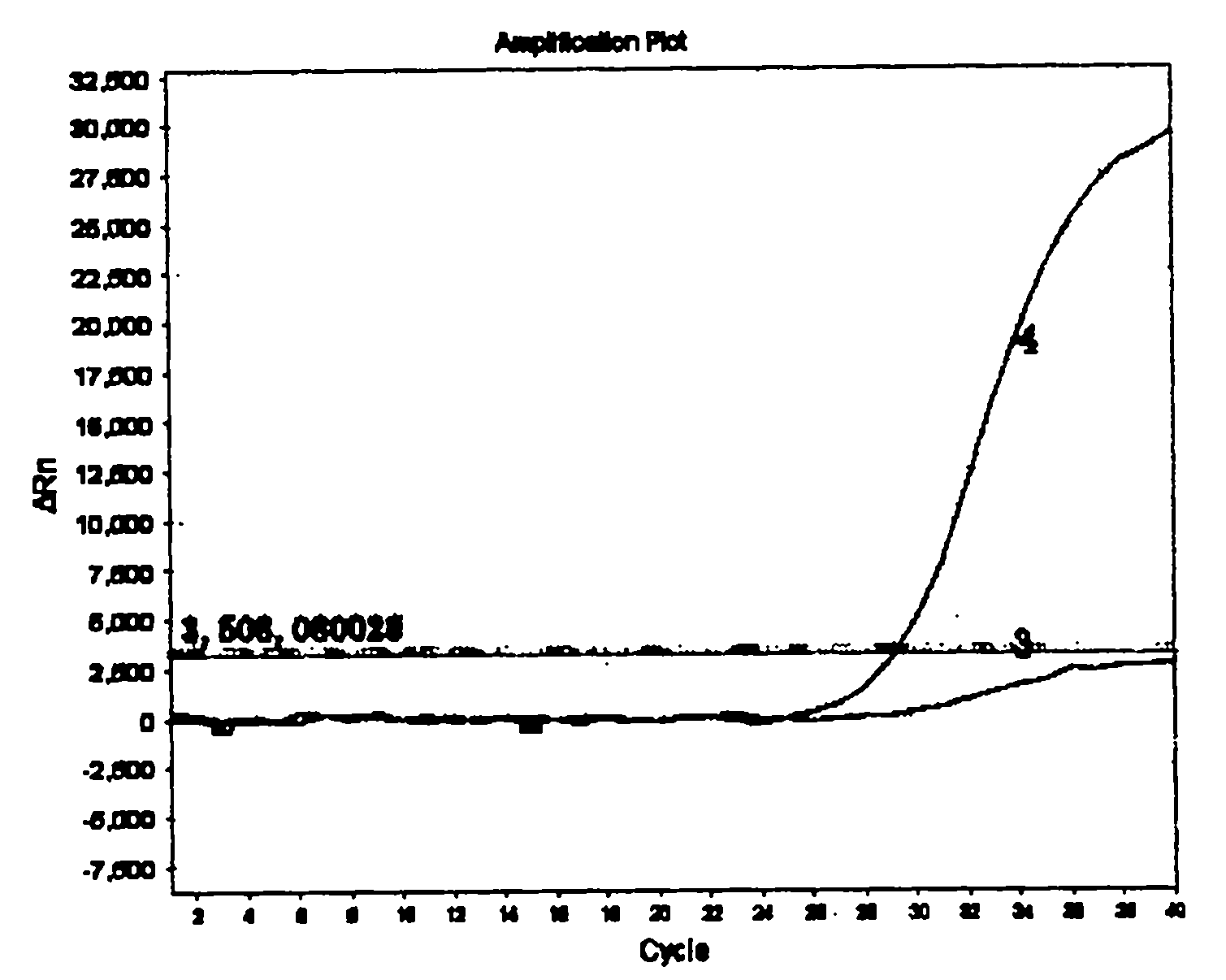
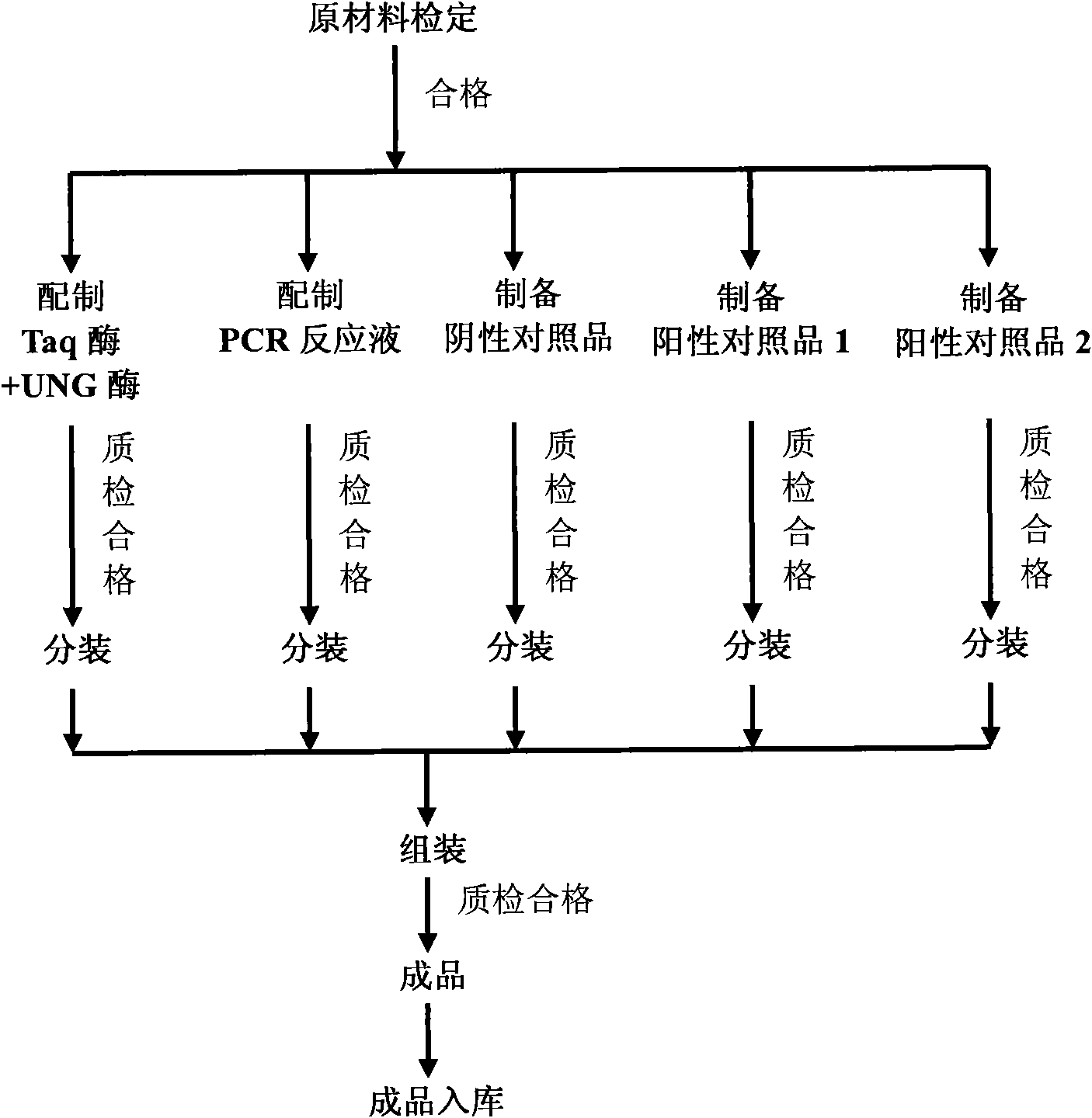
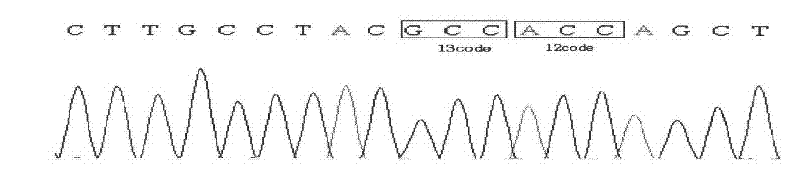
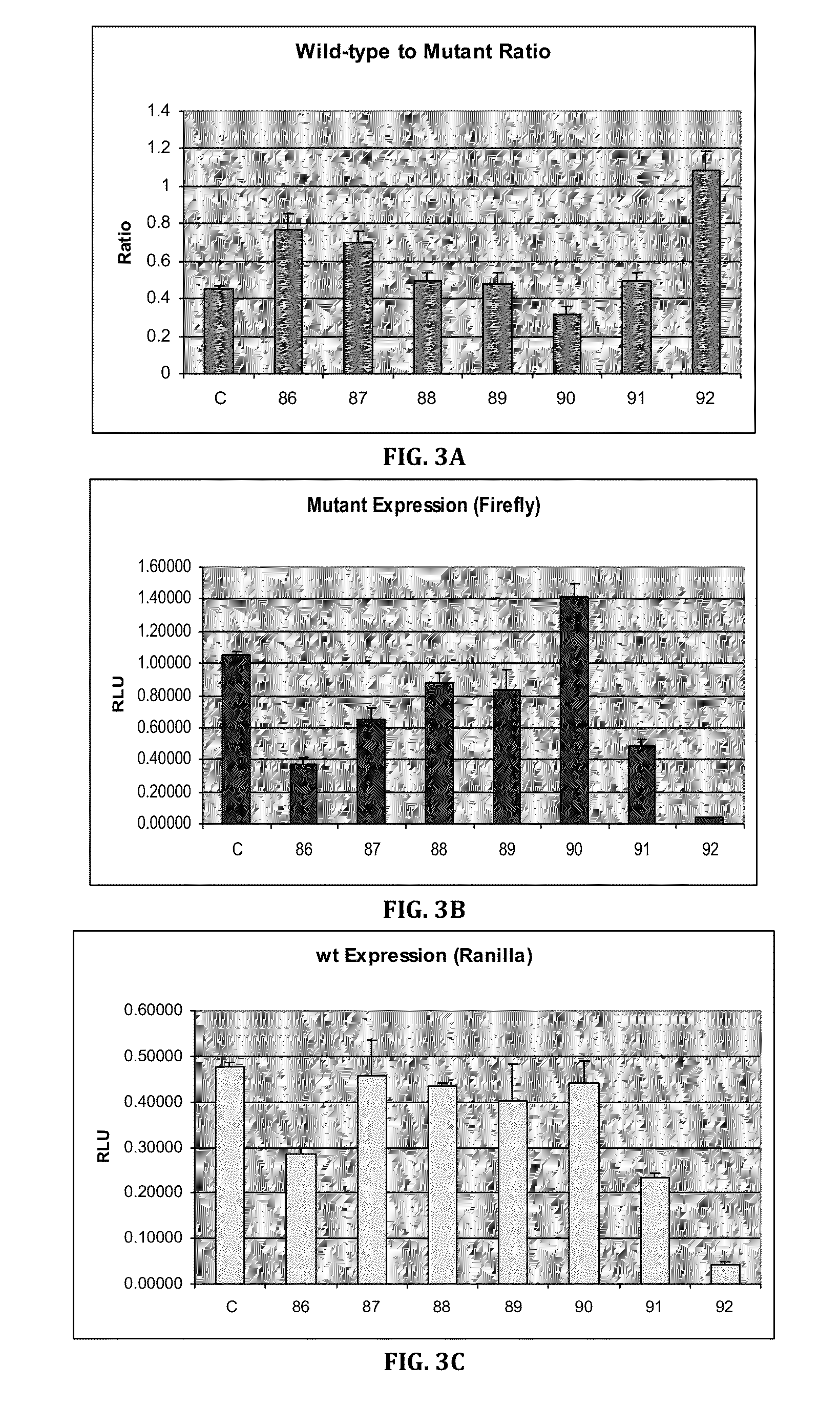
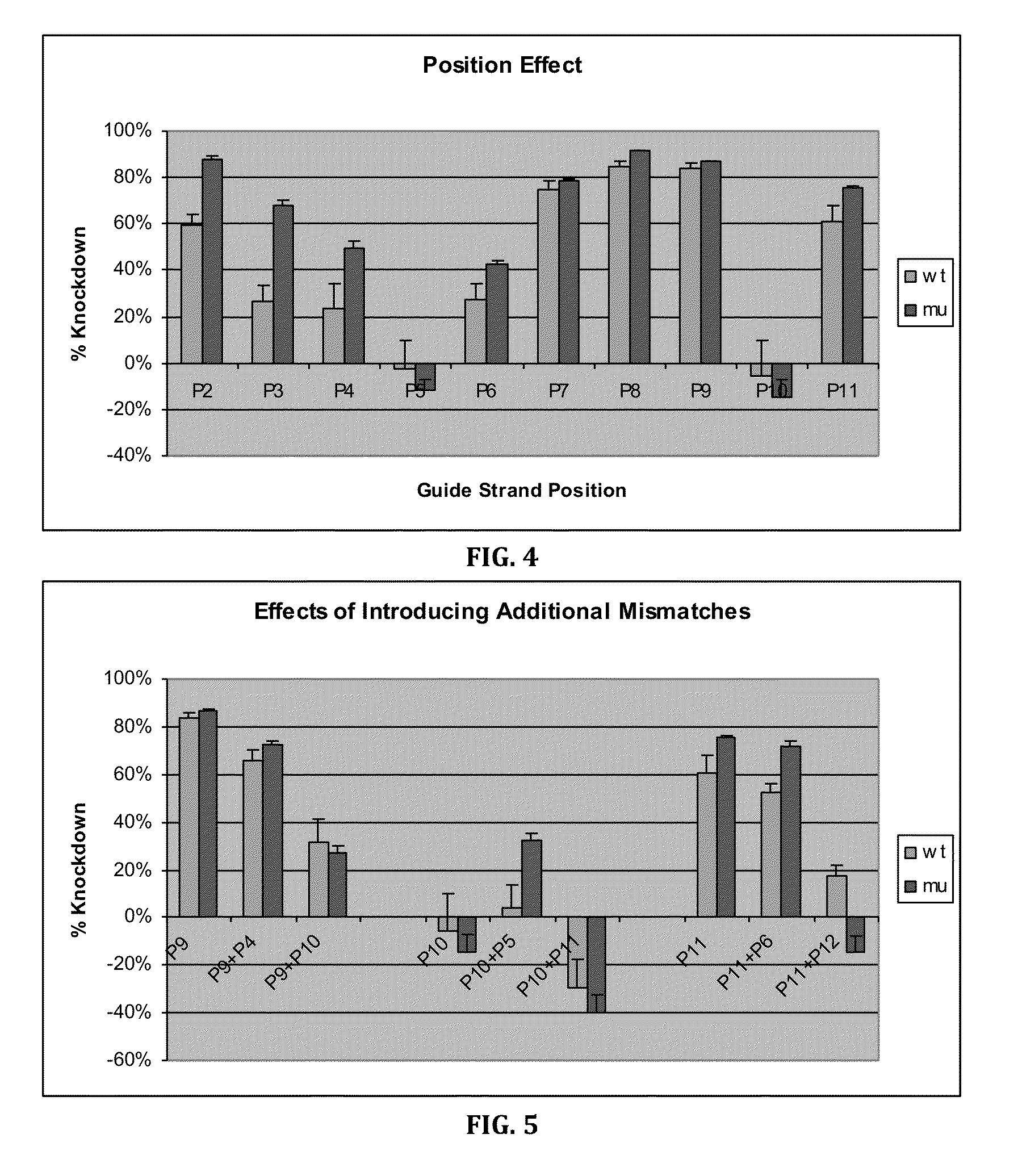
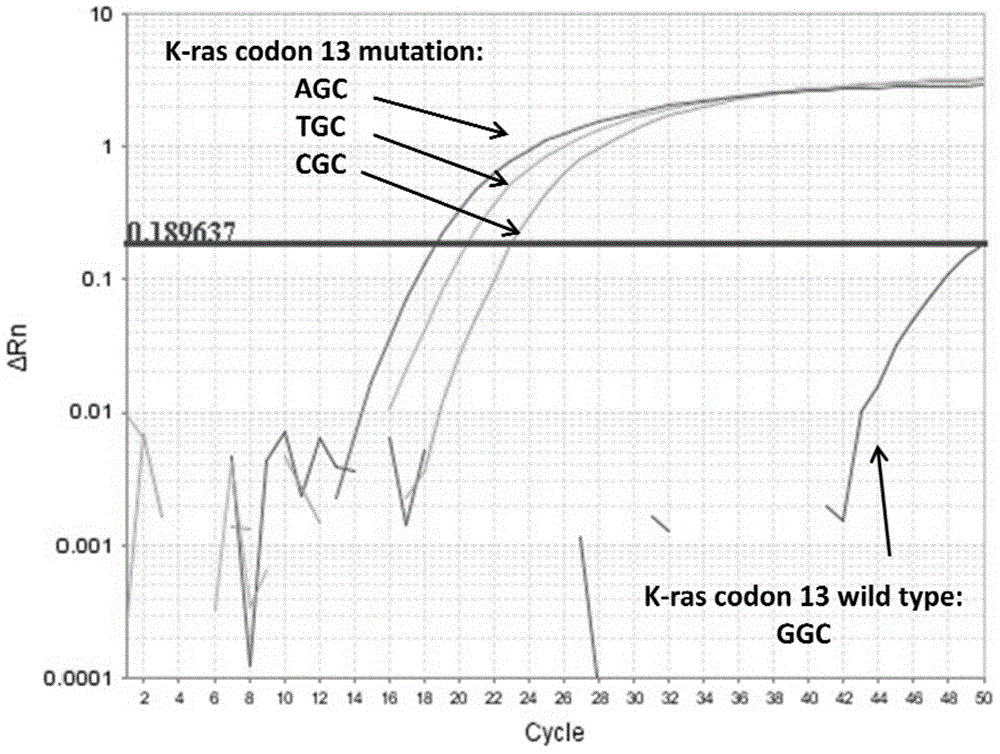
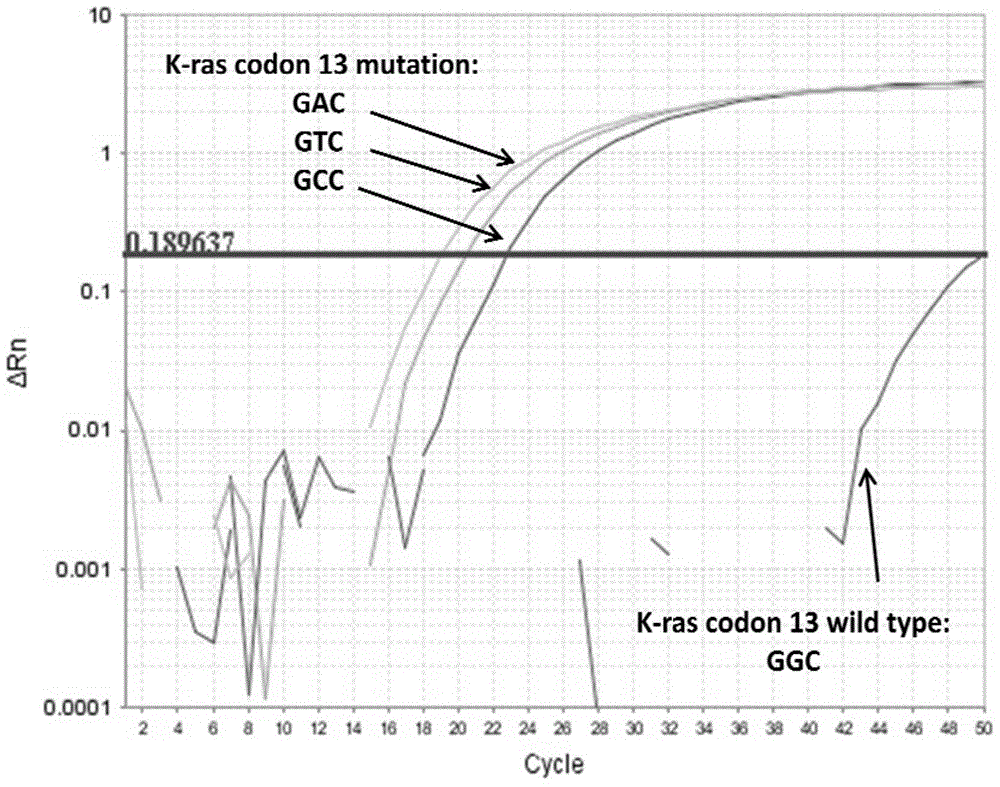
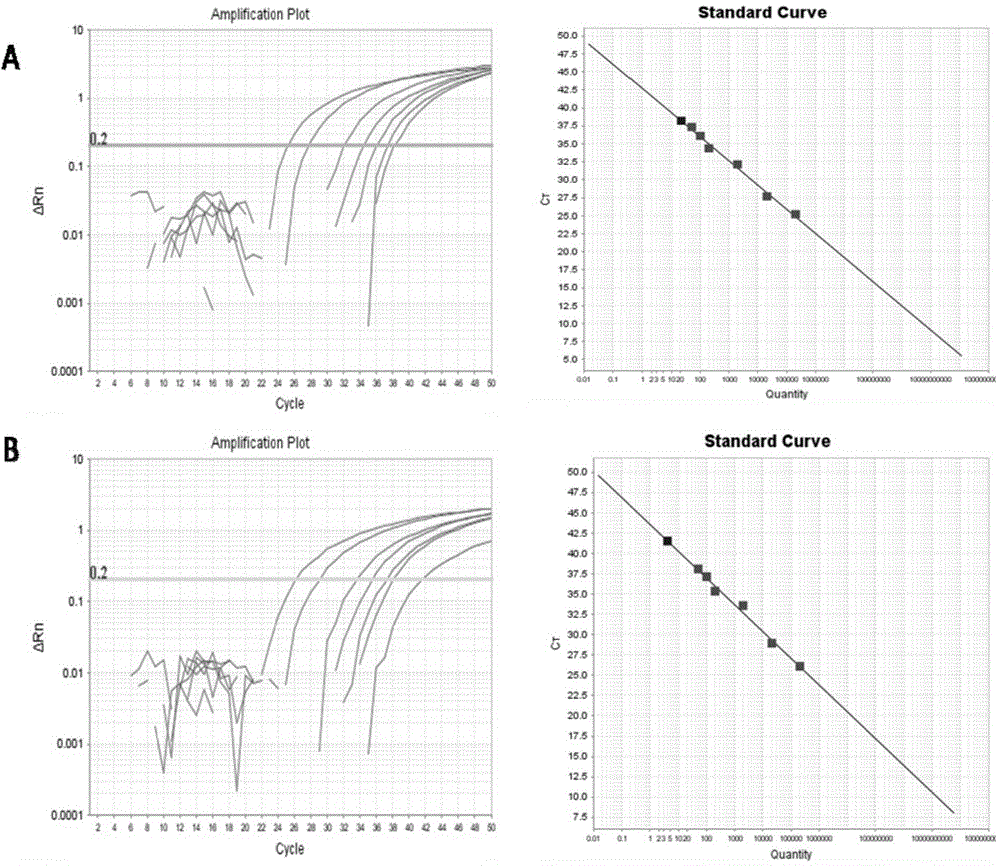
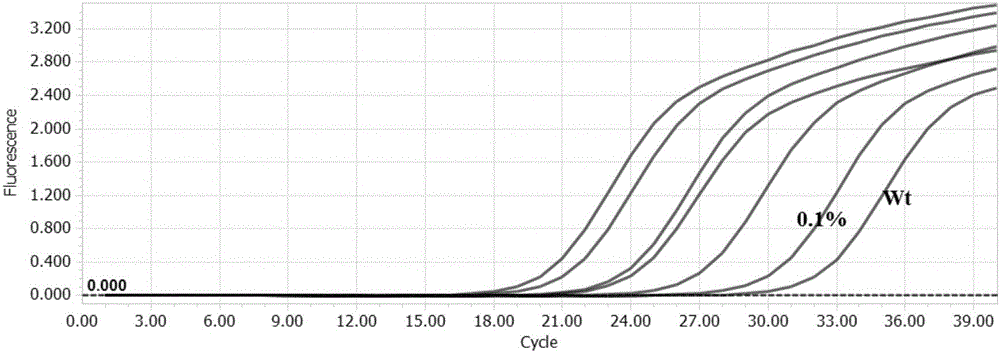
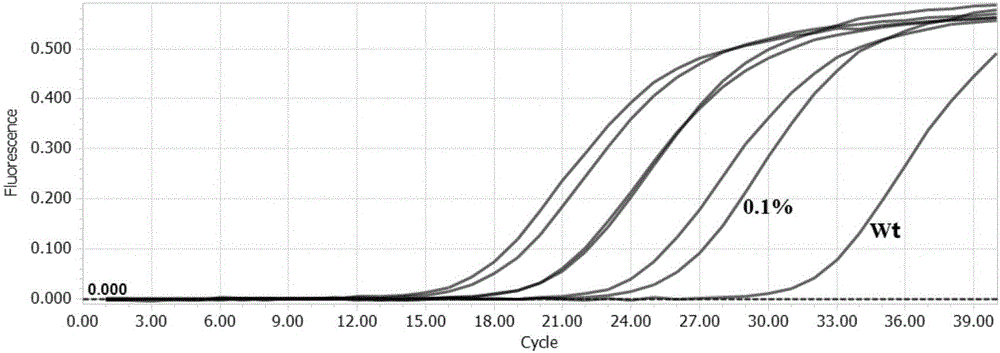
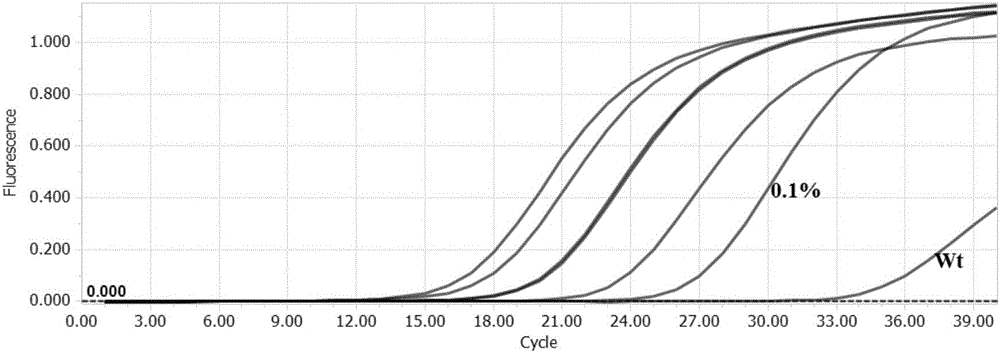
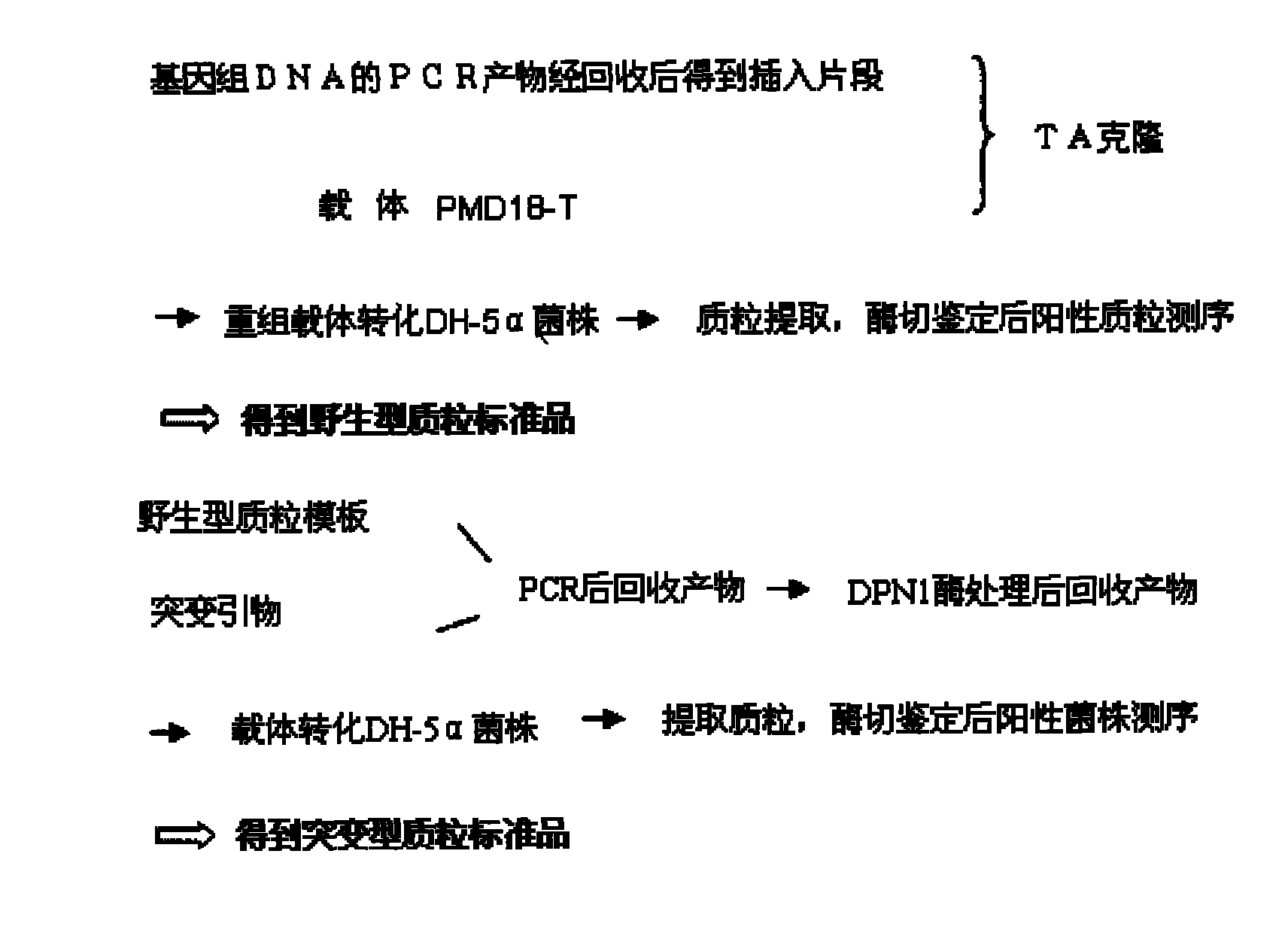
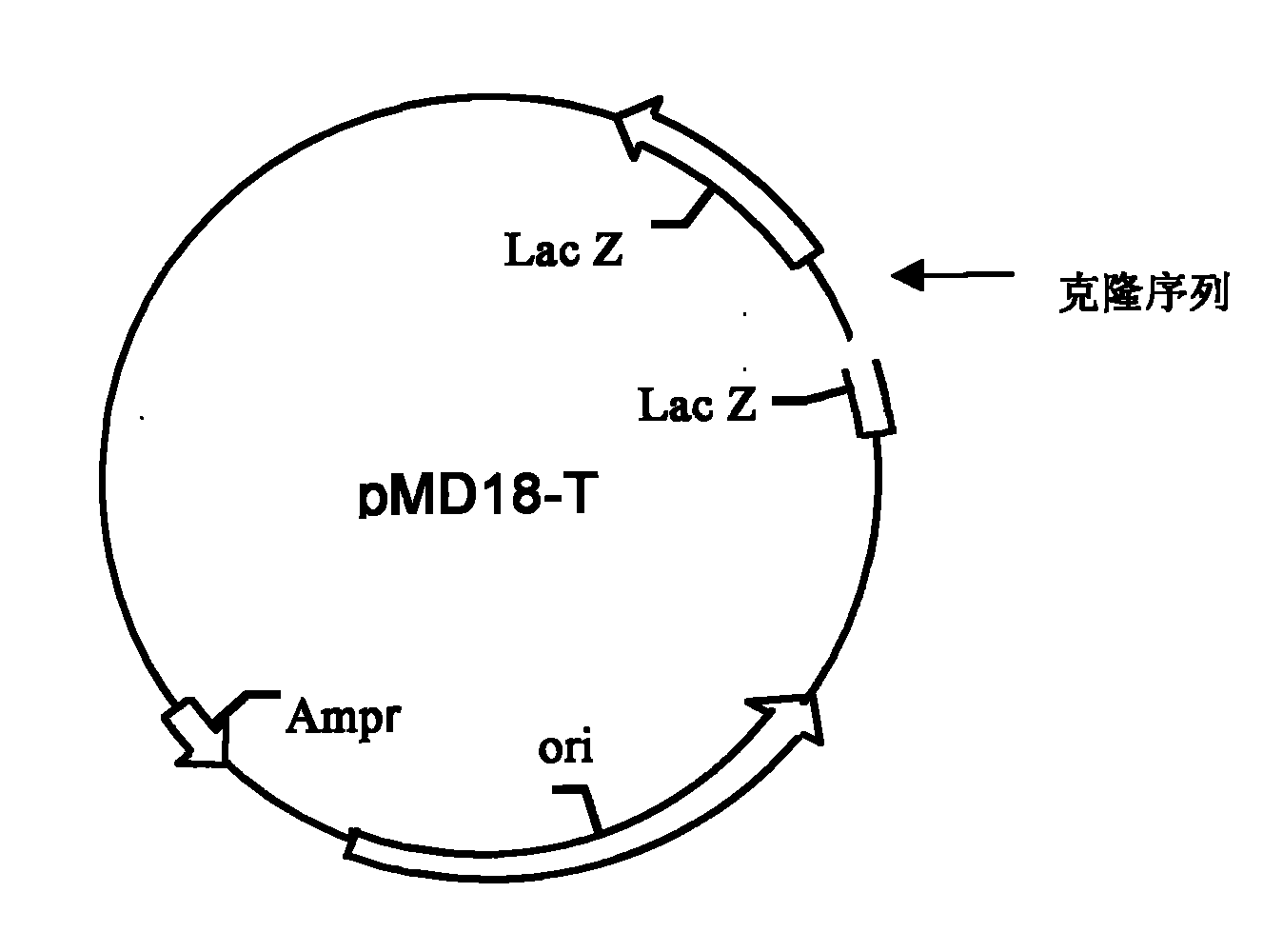
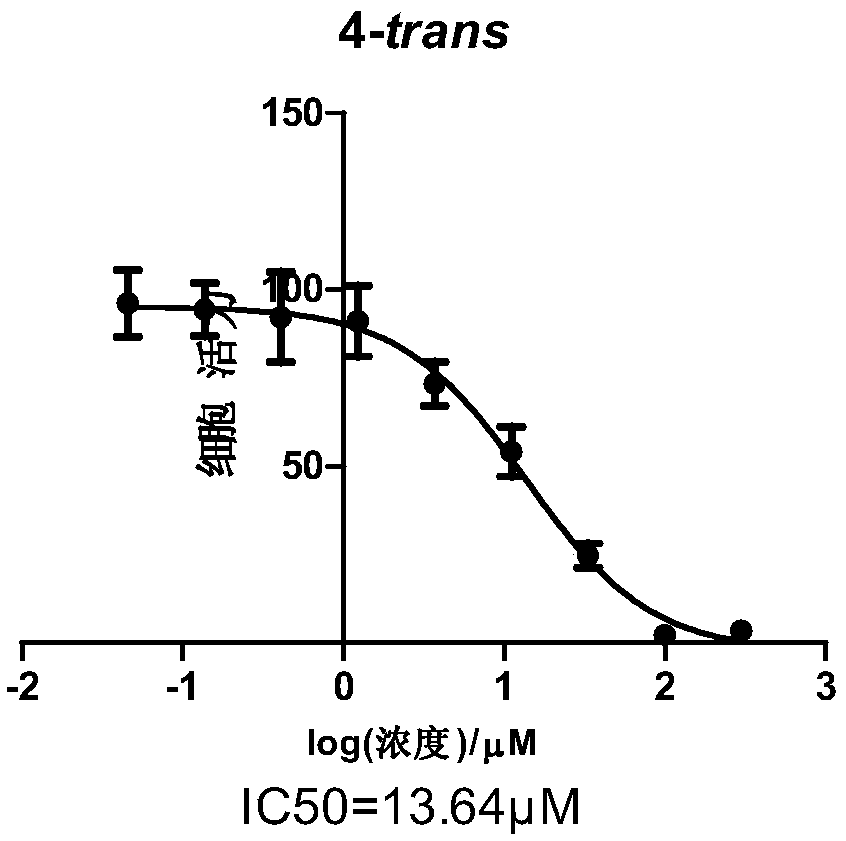
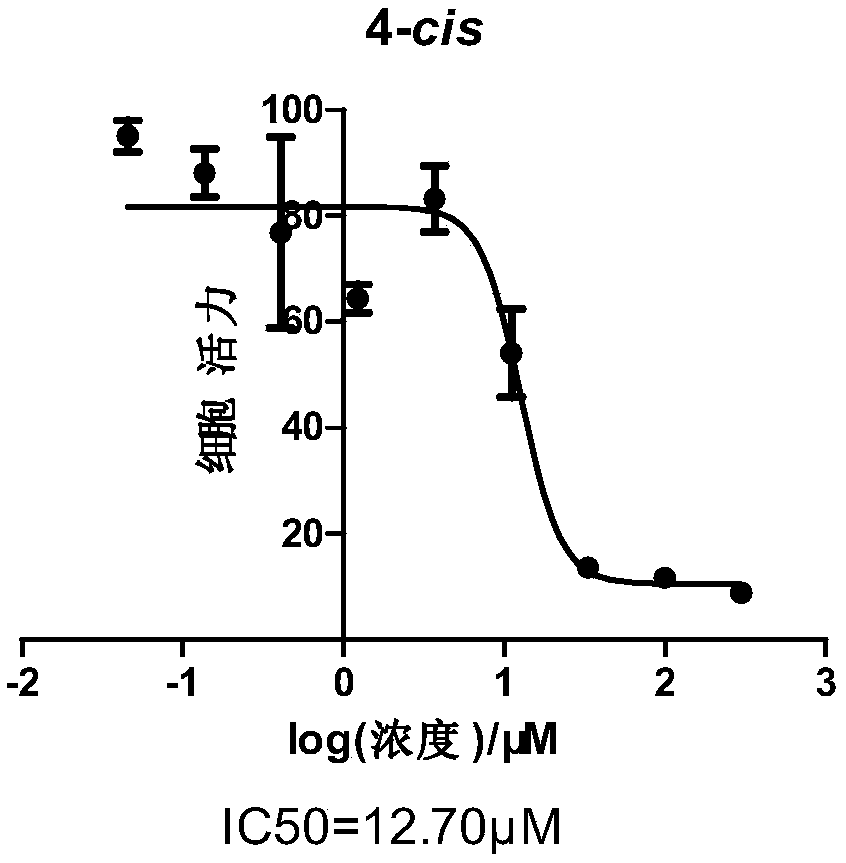
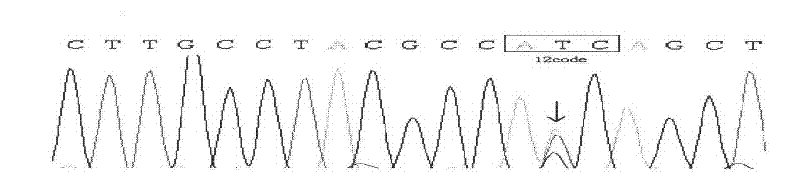Patents
Literature
50 results about "K-ras Genes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Primer, probe and kit for detecting EGFR and/or K-ras genetic mutation
ActiveCN105624309AEasy to optimizeMeet the requirements of rapid detectionMicrobiological testing/measurementDNA/RNA fragmentationK-ras GenesFluorescence
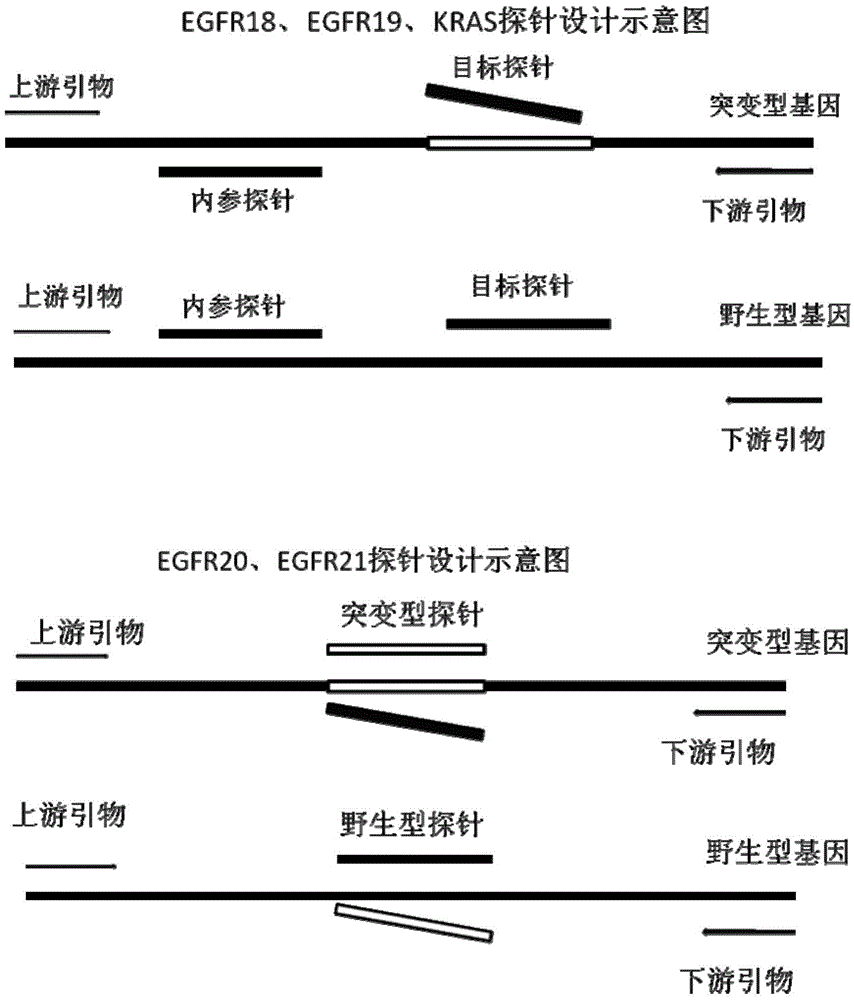
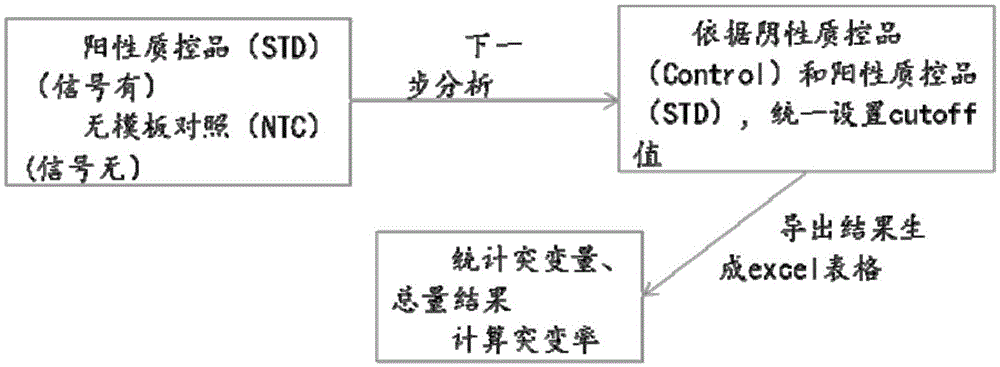
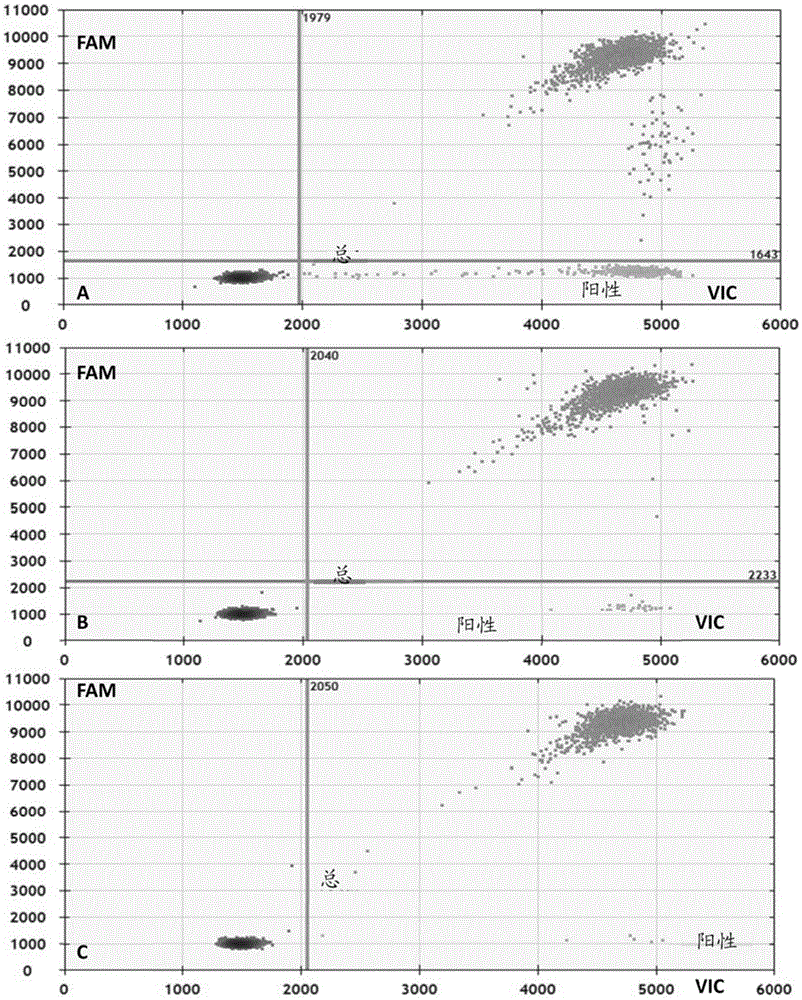
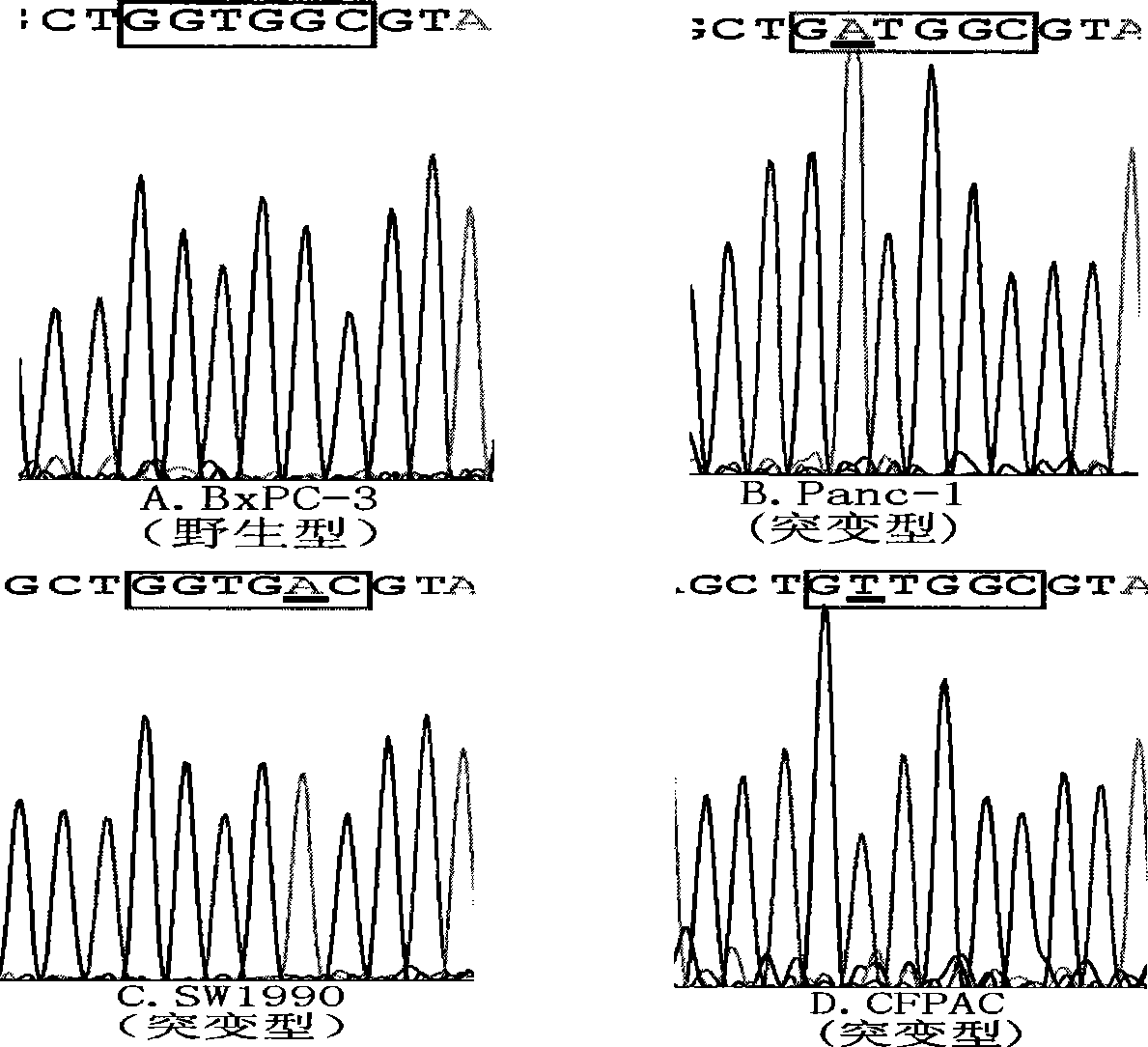
The invention discloses a primer and probe for detecting a human epidermal growth factor receptor (EGFR) gene and / or a K-ras gene, a kit containing the primer and the probe and a device for detecting genetic mutation on the basis of a digital PCR platform.The method for detecting the genetic mutation by means of the primer and the probe comprises the steps that the prime and the probe are provided; DNA of a sample to be detected is extracted; a fluorescent PCR reaction system capable of amplifying a mutant gene sequence is prepared; a target probe and an internal reference probe are utilized to be hybridized with amplified products respectively, and fluorescent signals of corresponding fluorescent groups are detected; existence of the genetic mutation is judged and / or the mutation rate is calculated according to the strength and proportion of the fluorescent signals of the target probe and the internal reference probe.According to the method for detecting the genetic mutation, the needed primers and probes are small in number, the optimization procedure is simple, related mutation of EGFR and / or K-ras gene can be qualitatively or quantitatively detected, and the detection sensitivity is high; a DNA sample with low initial amount can also be detected stably.
Owner:SHENZHEN HUADA GENE INST
PNA, probe, primer and method for detecting K-ras gene mutation
InactiveCN101423866ASimple and fast operationIncreased sensitivityMicrobiological testing/measurementK-ras GenesDisease
The invention discloses PNA, probe and primer for testing K-ras genetic mutation, and a method for quantitatively testing the K-ras genetic mutation in real time by the PNA, the probe and the primer. The PNA is hybridized with the 12th codon and / or the 13th codon in the first exon of wild K-ras gene; and the PNA is combined with real-time quantitative PCR to quantitatively test the mutation amount of the K-ras gene in real time. The method has the advantages of simple and convenient operation, high sensitivity and high specificity, and can be applied to early auxiliary diagnosis for pancreatic cancer and other diseases.
Owner:SHANGHAI CHANGHAI HOSPITAL
Combinatorial methods for inducing cancer cell death
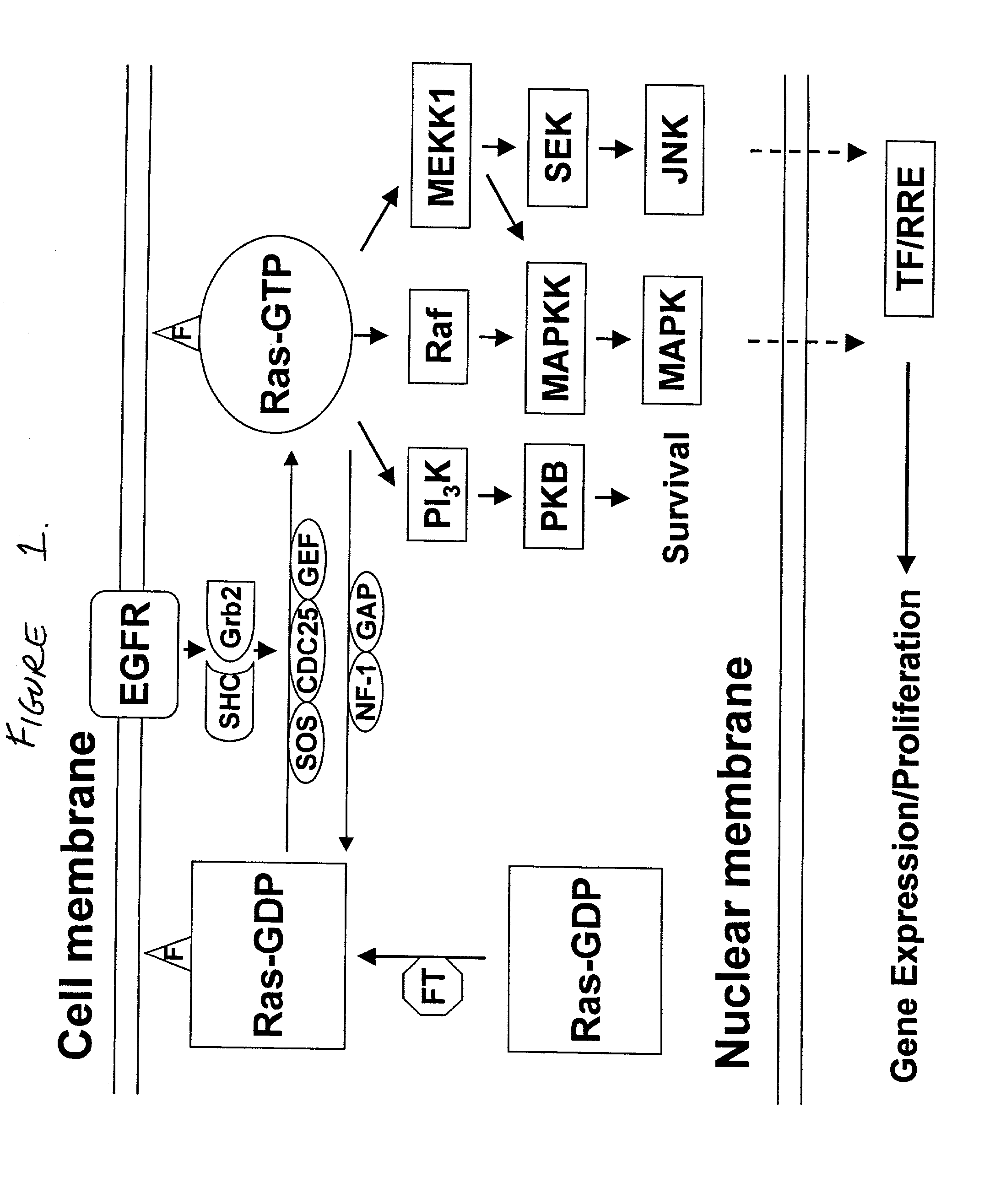
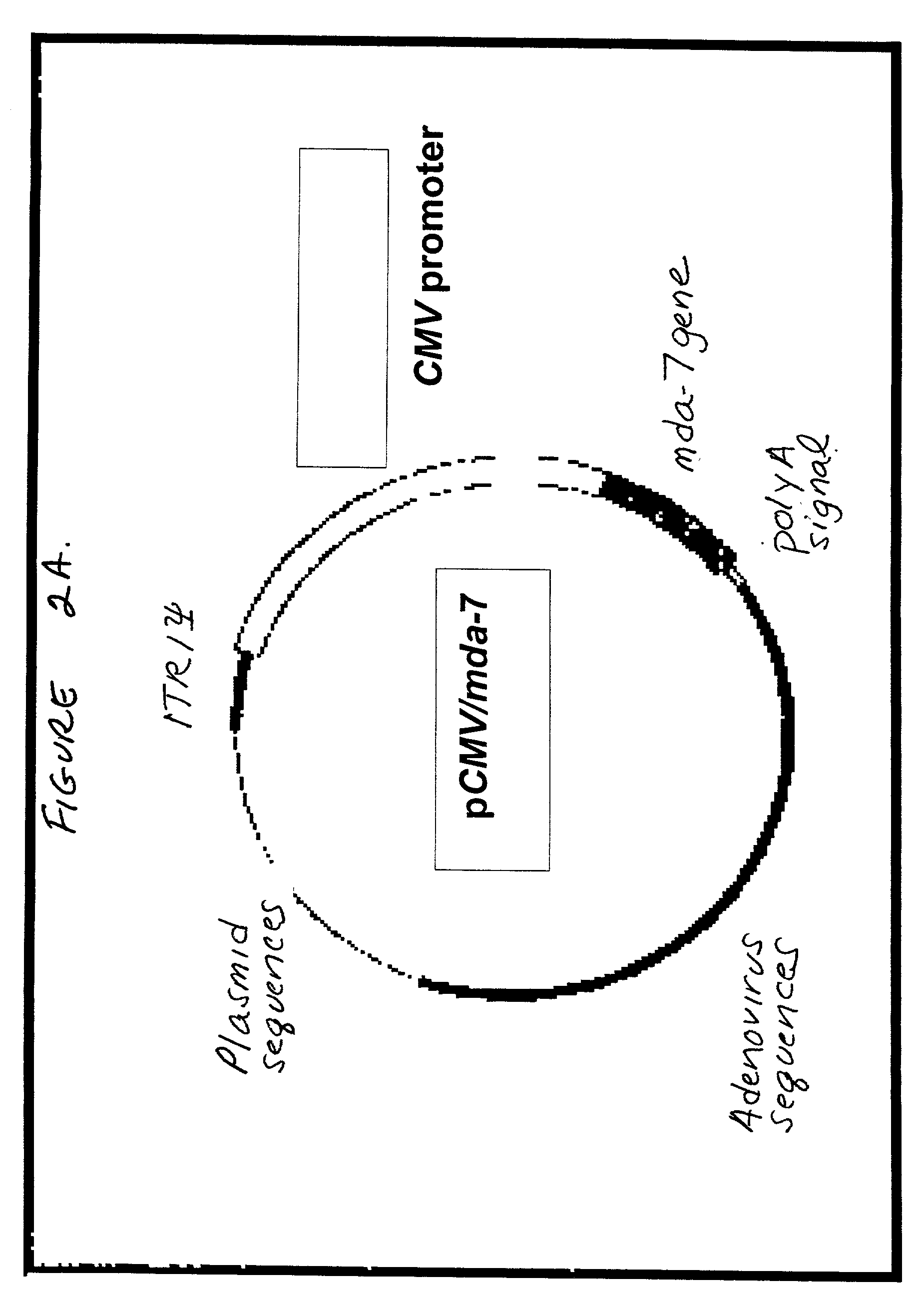
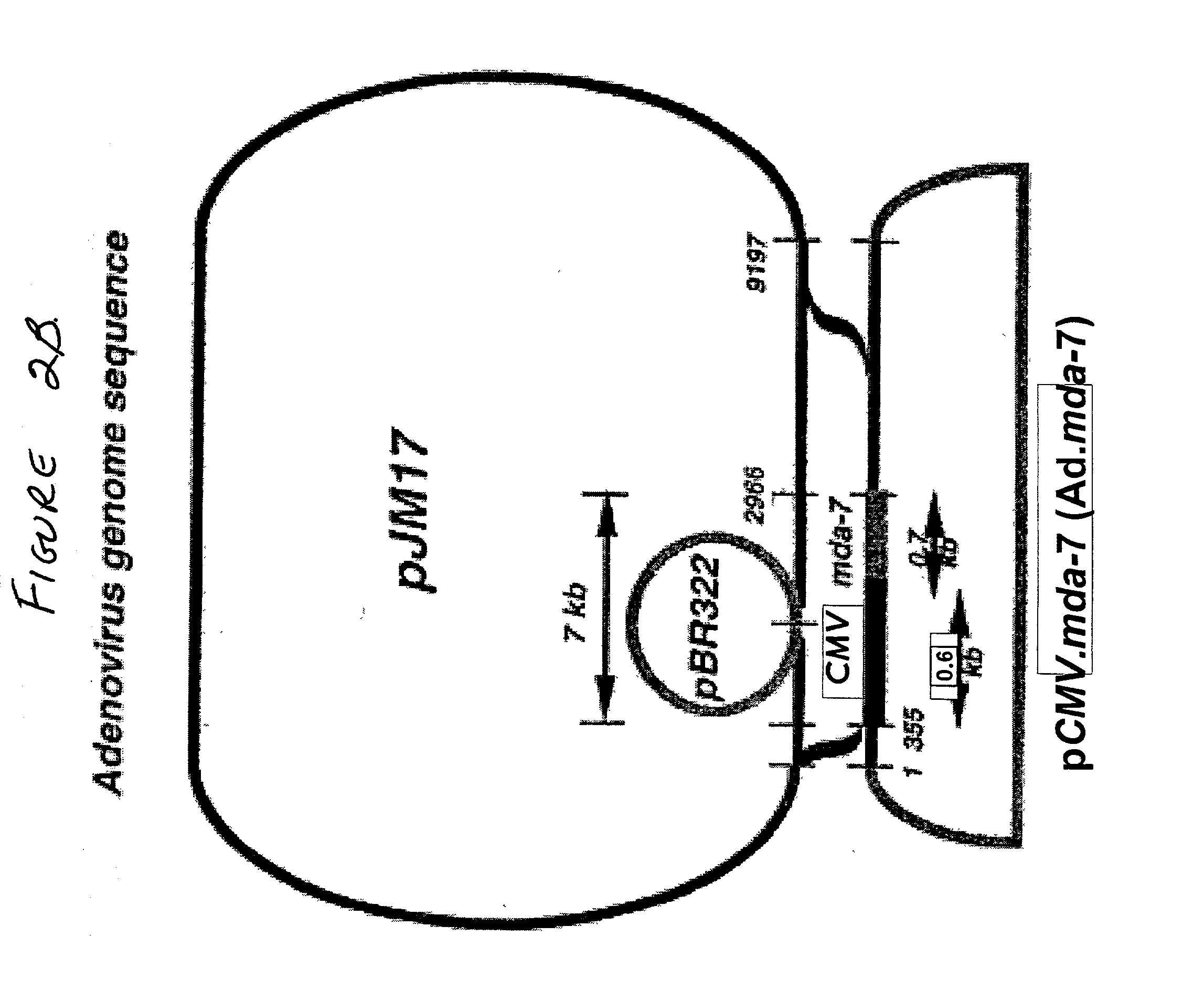
The present invention relates to methods and compositions for inhibiting proliferation and inducing cell death in a population of cancer cells by (i) increasing the amount of the differentiation associated protein MDA-7, and (ii) decreasing RAS activity within the population. It is based, at least in part, on the discovery that decreasing expression of a mutated, activated K-ras gene, together with introducing an expressible mda-7 gene, in pancreatic cancer cells had a synergistic growth-inhibitory and anti-survival effect, and abolished tumorigenicity of the cells in athymic nude mice. The methods of the invention may be directed to the therapy of pancreatic cancer and other malignancies.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Human K-ras (K-rat sarcoma) gene mutation parting type fluorescent quantitation PCR (polymerase chain reaction) detecting reagent kit and detecting method
InactiveCN102747158AGrowth inhibitionAdvantages and Notable ImprovementsMicrobiological testing/measurementFluorescence/phosphorescenceWild typeMinor groove
The invention discloses a human K-ras (K-rat sarcoma) gene mutation parting type fluorescent quantitation PCR (polymerase chain reaction) detecting reagent kit and a detecting method. The detecting reagent kit comprises PCR mixed reaction liquid, a peptide nucleic acid probe, a taqman-MGB (minor groove binder) probe, an inner control forward and reverse primer and probe, an external control forward and reverse primer and probe and a positive reference article, wherein the inner control forward and reverse primer and probe is used for K-ras gene mutation detection, and the external control forward and reverse primer and probe is used for the K-ras gene mutation detection. The PNA (peptide nucleic acid) technology and the taqman-MGB technology are combined, relevant ARMS (amplification refractory mutation system) primers are designed, PNA can be stably combined with wild K-ras genes 12 or 13 codon sequences, only mutation specimens can be amplified, and the K-ras gene mutation can be detected. The method has the advantages that the speed is high, simplicity and convenience are realized, the specificity is good, the sensitivity is high, and the method can be used for the clinical K-ras gene mutation screening.
Owner:武汉海吉力生物科技有限公司
Fluorescent genotyping detection kit and detection method for eight K-ras gene mutations
InactiveCN103695555AReduce the number of reaction tubesFew samplesMicrobiological testing/measurementK-ras GenesFluorescence
The invention belongs to the field of biotechnology and clinical molecular diagnosis and in particular relates to a fluorescent genotyping detection kit and a detection method for eight human K-ras gene mutations. According to the method, an MGB (minor groove binder) probe technology is combined with a mismatch ARMS (amplification refractory mutation system) technology, an MGB probe complementary with a DNA (deoxyribonucleic acid) plus strand and an ARMS downstream primer complementary with a DNA minus strand are designed at allelic gene loci, a mismatch locus is introduced in the ARMS downstream primer, the MGB probe is overlapped with the ARMS primer by not more than 5 bp, and the eight human K-ras gene mutations are subjected to genotyping detection by four reaction pipes based on multiple fluorescent PCR (polymerase chain reaction). The method has the advantages of good specificity, high sensitivity, simplicity and quickness for operation, accuracy for genotyping, simplicity for result interpretation and the like, and can be used for detecting the clinical K-ras gene mutations and helping doctors screen the crowed with effective anti-EGFR (epidermal growth factor receptor) treatment.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Primers and probes for detecting human K-ras gene mutation as well as use method thereof
ActiveCN101608241AHigh sensitivityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesFluorescence
The invention relates to primers and probes for detecting human K-ras gene mutation as well as use method thereof, relating to detection of gene mutation. In the invention, the fluorescent quantitative PCR technology and the fluorescence dual ring probes technology are combined to develop a multiple real-time fluorescent PCR method capable of fast detecting gene mutation and design the relevant primers. The method is used for detecting mutation of the twelfth and the thirteenth codons of the K-ras gene, mutant type primers designed specific to different mutation types are respectively adopted to detect samples to be detected, only the mutation type samples can be smoothly amplified to obtain double-strand DNA products which can be combined with DNA probes to send out fluorescence signals to be detected. The invention can detect mutate type genes with content being 1 / 1000 in the samples to be detected, has the advantages of high speed, convenience and simplicity, safety, high sensitivity, high throughput and low cost and can be applied to K-ras gene mutation screening of numerous clinical samples.
Owner:AMOY DIAGNOSTICS CO LTD
Quantitative determination method for K-ras gene mutation
InactiveCN101434985AChange in melting temperatureSimple and fast operationMicrobiological testing/measurementHybrid typeK-ras Genes
The invention discloses a quantitative detection method of K-ras gene mutation, comprising the following steps: a PNA which is hybridized with a twelfth and / or thirteenth codon in a first exon of a wild-typed K-ras gene is designed; SYBR-RT-PCR quantitative detection is carried out to the mutant-typed and wild-typed standard products with known copy numbers respectively with the PNA for obtainingstandard and melting curves; SYBR-RT-PCR quantitative detection is carried out to the sample nucleic acid to be detected with the PNA; and after the sample nucleic acid to be detected is judged to bewild type or mutant type or mutant / wild hybrid type according to the melting curve, the K-ras gene mutation quantity of the sample nucleic acid to be detected is obtained according to the standard curve. The method of the invention has simple operation, high sensitivity and high specificity, and can be applied to early auxiliary diagnosis on diseases such as pancreatic cancer, etc.
Owner:SHANGHAI CHANGHAI HOSPITAL
Nucleotide sequence, method and kit for detecting exons 12, 13 mutation of human K-ras gene
ActiveCN102010894AMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesFluorescence
The invention relates to a nucleotide sequence, a method and a kit for detecting exons 12, 13 mutation of a human K-ras gene. The nucleotide sequence for detecting the exons 12, 13 mutation of the human K-ras gene comprises any one nucleotide sequence shown from SEQ ID NO:1 to SEQ ID NO:10, wherein two ends of the nucleotide sequence shown in the SEQ ID NO:3 are labeled with JUP (Junction Plakoglobin) fluorescence groups, and two ends of the nucleotide sequences shown in the SEQ ID NO:4 to the SEQ ID NO:10 are labeled with MAR fluorescence groups. The kit of the invention can be used for instructing the clinical medication of Erbitux and other EGFR (Epithelial Growth Factor Receptor) antibody medicines.
Owner:上海源奇生物医药科技有限公司 +1
Double fluorescent marker probe real-time quantitative detection method for K-ras gene 12 codon mutation and application
InactiveCN102041313ASimple and fast operationIncreased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceWild typeTarget peptide
The invention belongs to the technical field of biology. K-ras gene mutation plays an important role in occurrence and development of pancreatic cancer, but the current universal K-ras gene mutation detection methods comprise a limited fragment length polymorphism analysis method and an amplification blocked mutation system method, and the two methods have low specificity and cannot qualitativelyand quantitatively detect the mutation of K-ras genes at the same time. The invention aims to provide a double fluorescent marker probe real-time quantitative detection method for K-ras gene 12 codonmutation with operation convenience, high sensitivity and high specificity. The method comprises the following steps of: designing wild K-ras gene 12 codon-targeted peptide nucleic acid (PNA) and corresponding mutation detection probes, namely a K-ras-FAM Tagman MGB probe and a K-ras-VIC Tagman MGB probe; performing real-time quantitative polymerase chain reaction (PCR) detection on a plasmid standard substance of known mutation quantity by using the PNA and the probes to acquire a standard curve and a fluorescent type; and extracting and purifying sample DNA, measuring the concentration, performing real-time quantitative PCR detection, and judging K-ras gene mutation quantity and mutation type according to the standard curve and the fluorescent type.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Methods for the Detection of Colorectal Cancer
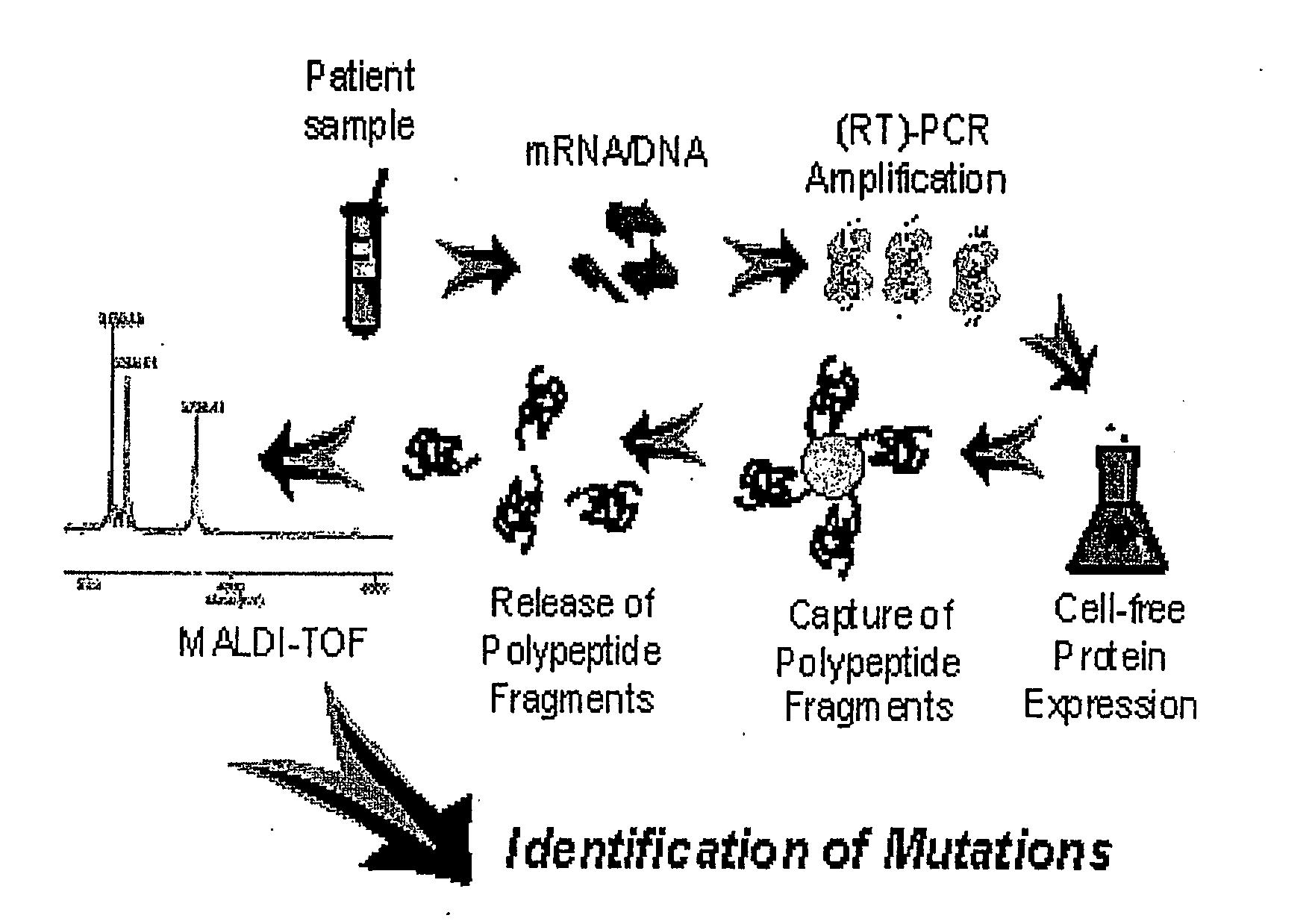
InactiveUS20110045991A1Microbiological testing/measurementLibrary screeningCell-free protein synthesisAmino acid
This invention relates to an approach for detection of chain truncating mutations based on the utilization of existing sample collection methods such as FOBT platforms, together with advanced methods for cell-free protein expression. “When further combined with mass spectrometry, the invention provides the ability to simultaneously detect changes in the amino acid sequence of multiple peptides. In some embodiments, DNA is isolated from a patient fecal sample and specific regions of a gene (i.e., for example, a K-ras gene or an APC gene) are PCR amplified using specifically designed primers that allow translation of encoded peptide fragments in a cell-free protein synthesis system. Nascent proteins are affinity purified and their mass is detected by MALDI-TOF which allows identifying low levels of mutations.
Owner:AMBERGEN INC
Preparation method for nucleic acid mass spectrum for detecting mutation of colorectal cancer K-ras gene and product for detecting mutation of colorectal cancer K-ras gene
ActiveCN103667490ARealize detectionConvenient Medication JudgmentMicrobiological testing/measurementFhit geneMass spectrometric
Owner:BIOYONG TECH
Fluorescence PCR detection kit for human K-RAS gene mutation
InactiveCN104531881AStrong specificityImprove anti-interference abilityMicrobiological testing/measurementForward primerK-ras Genes
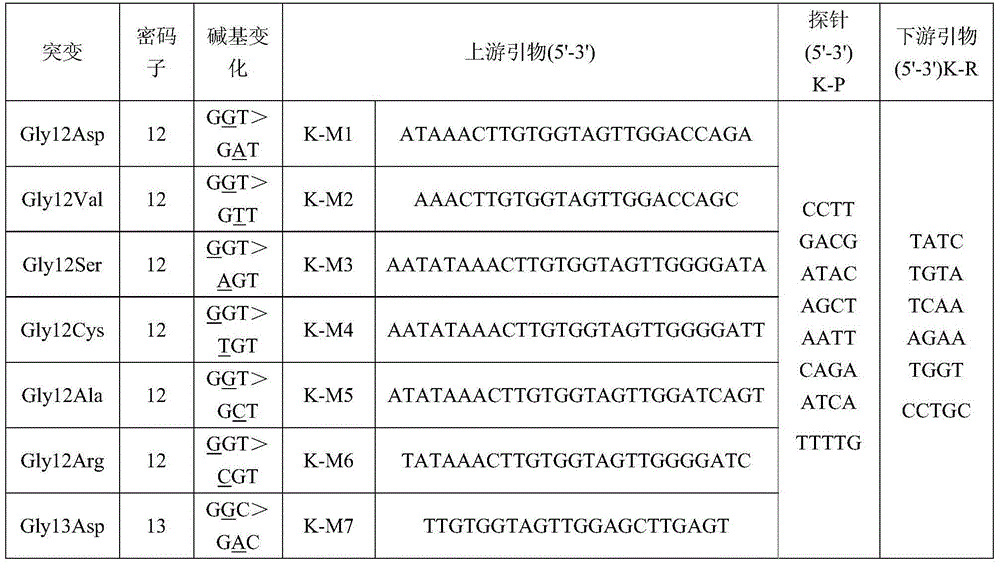
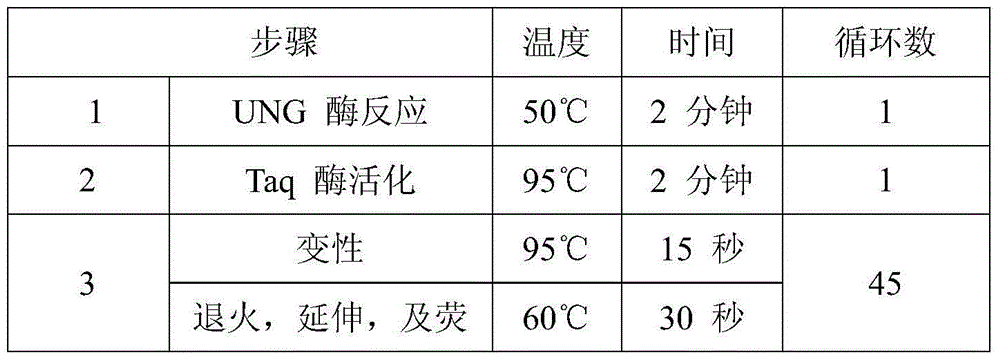
The invention provides a fluorescence PCR detection kit for human K-RAS gene mutation. The kit comprises a forward primer sequence K-M1 PCR reaction solution, a forward primer sequence K-M2 PCR reaction solution, a forward primer sequence K-M3 PCR reaction solution, a forward primer sequence K-M4 PCR reaction solution, a forward primer sequence K-M5 PCR reaction solution, a forward primer sequence K-M6 PCR reaction solution and a forward primer sequence K-M7 PCR reaction solution which are used for detecting Gly12Asp mutation, Gly12Val mutation, Gly12Ser mutation, Gly12Cys mutation, Gly12Ala mutation, Gly12Arg mutation and Gly13Asp mutation on a codon 12 and codon 13. According to the kit, under the 100ng / reaction wild-type human DNA background, amplification signals and the false positive condition are avoided, and it shows that the kit is good in specificity and high in antijamming capability. By the adoption of the kit, human K-RAS gene mutation in samples such as clinical paraffin sections can be detected quickly, and a reliable experiment basis is provided for diagnosing related cancer therapies.
Owner:SANSURE BIOTECH INC
Use of ursolic acid as colon tumor resistant medicament
InactiveCN101991579ABlock EGFR signaling pathwayOrganic active ingredientsAntineoplastic agentsSide effectApoptosis
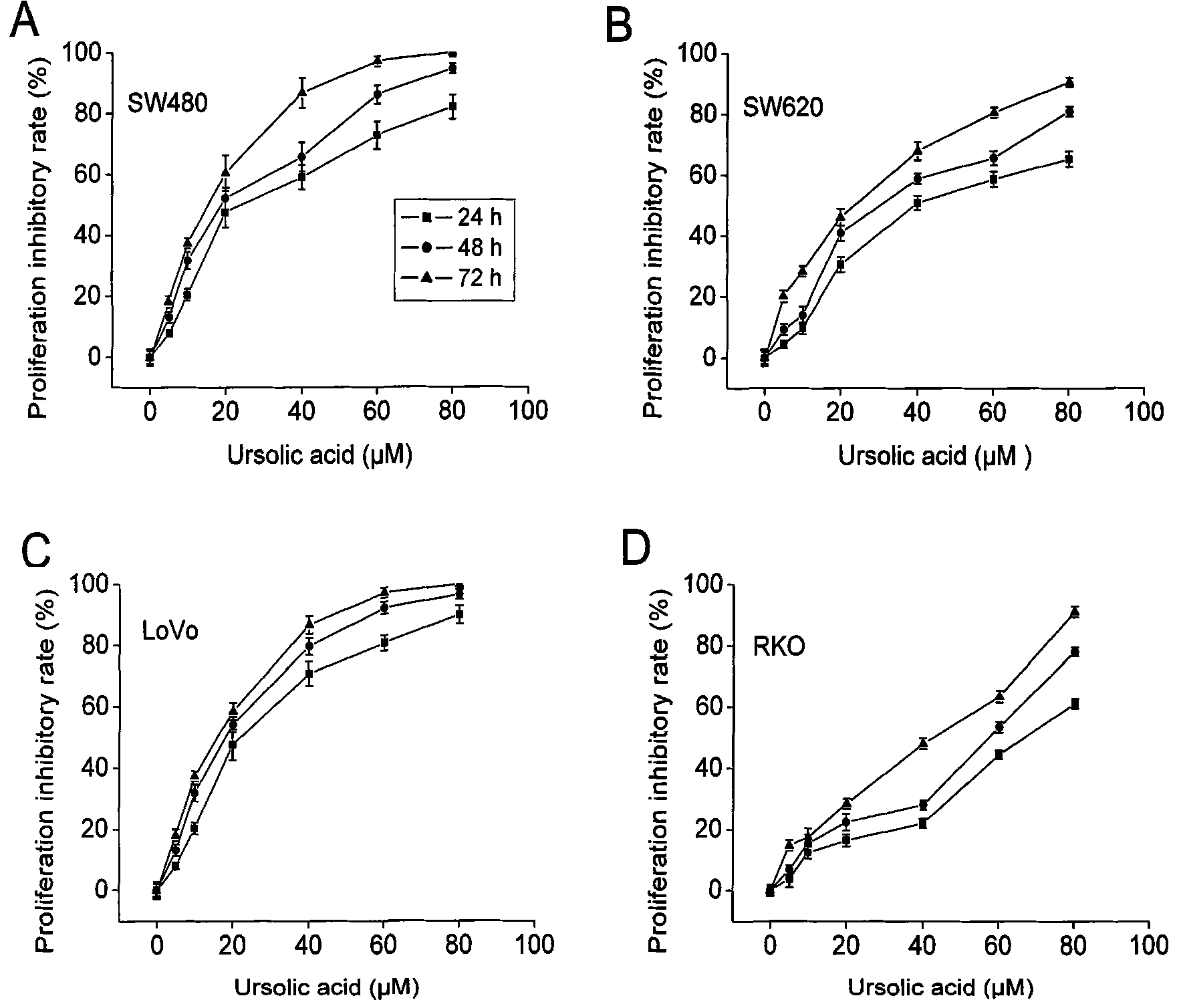
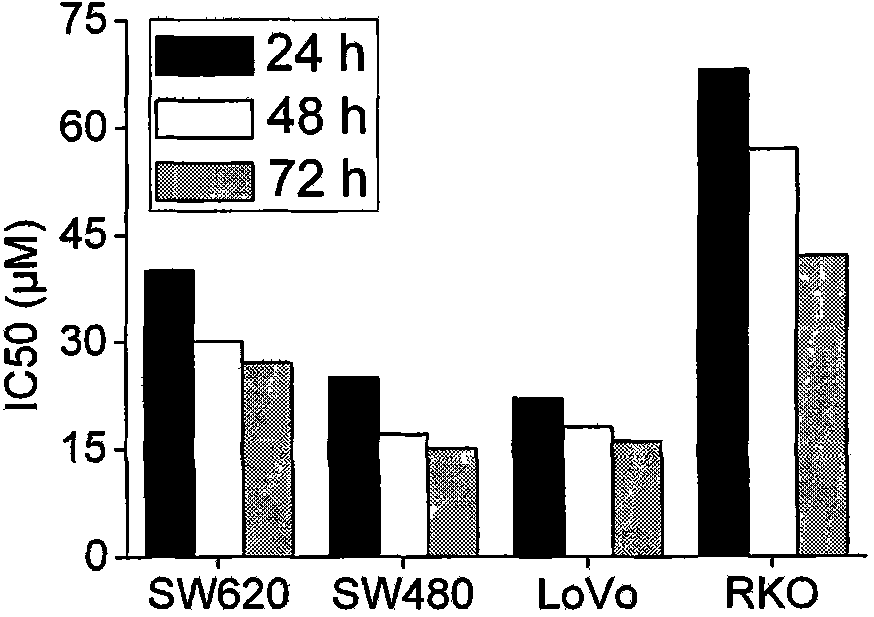
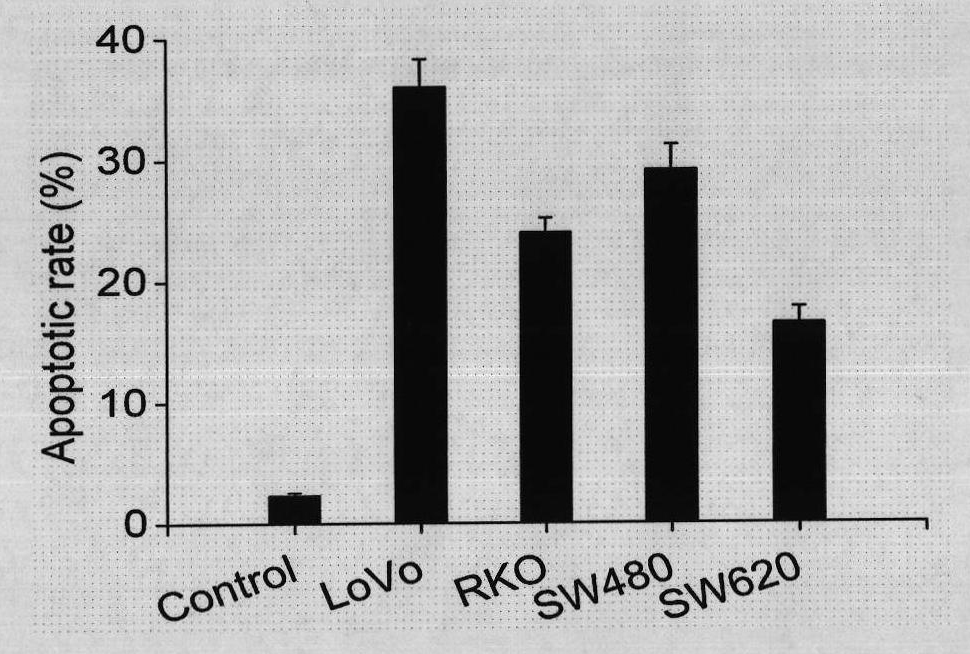
The invention relates to use of ursolic acid as a colon tumor resistant medicament. The ursolic acid has the effects of inhibiting colon cancer / tumor cell proliferation and inducing apoptosis. In naked mouse in vivo experiments, the ursolic acid can effectively inhibit the proliferation of colon cancer transplanted tumor and inhibit the generation of tumor blood vessels, and has no obvious toxic or side effect on mice. The ursolic acid has the effect of inhibiting EGFR / MAPK signal channel phosphorylation of colon cancer cells, and the key target of the ursolic acid is to inhibit ERK1 / 2 protein phosphorylation. The ursolic acid which is synergetic with oxaliplatin serving as a clinical first-line chemotherapy medicament of the colon cancer has better curative effect. The ursolic acid has better proliferation inhibiting effect on the colon cancer cells of K-RAS gene mutation. The use of the ursolic acid as the colon tumor resistant medicament provides a new thought for treating the colon tumor.
Owner:ZHEJIANG UNIV
Kit for detecting K-ras gene mutation and detection method with kit
ActiveCN103114146AReduce the risk of contaminationHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceWild typeMinor groove
The invention discloses a kit for detecting K-ras gene mutation and a detection method with the kit. The kit comprises an amplification primer pair, a TaqMan-MGB (minor groove binder) probe and PNA (pentose nucleic acid), wherein the sequences of amplification primers are shown as SEQ ID NO:1 and SEQ ID NO:2; and the sequence of the TaqMan-MGB probe is shown as SEQ ID NO:3; and the sequence of PNA is shown as SEQ ID NO:4. The detection method comprises the following steps of: extracting DNA (deoxyribonucleic acid) of a sample to be detected; preparing K-ras wild and mutation plasmid standard products; and detecting the K-ras gene mutation, performing qualitative judgment on the K-ras gene mutation, drawing a standard curve, performing quantitative analysis on the mutation, and the like. The kit has the characteristics of quickness, simplicity, convenience, high sensitivity and high flux, and can be used for performing qualitative or quantitative detection on the K-ras gene mutation of clinical samples of tissue, urea, serum or plasma.
Owner:CHANGSHA 3G BIOTECH
Method and kit for detecting mutation of K-ras gene
ActiveCN102399872ASimple methodLow costMicrobiological testing/measurementDNA/RNA fragmentationK-ras GenesForward primer
The invention discloses a method and kit for detecting the mutation of a K-ras gene by using PCR (Polymerase Chain Reaction) amplification, belonging to the field of molecular biology. The kit comprises a conventional PCR assembly and a specific primer, and the specific primer comprises three forward primers and one reverse primer, wherein the sequences of the three forward primers respectively have nucleotide sequences represented by SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3; and the reverse primer has a nucleotide sequence represented by SEQ ID No.4. The existence of a target product band of PCR amplification through electrophoretic separation is used for judging whether the K-ras gene has at least one of three hot spot mutations including GGT-GAT and GGT-GTT of No.12 codon and GGC-GAC of No.13 codon. The kit and the method for detecting the mutation of a K-ras gene provided by the invention have the advantages of quickness, accuracy, simple method and low cost, in favor of clinic application and popularization.
Owner:苏州科贝生物技术有限公司
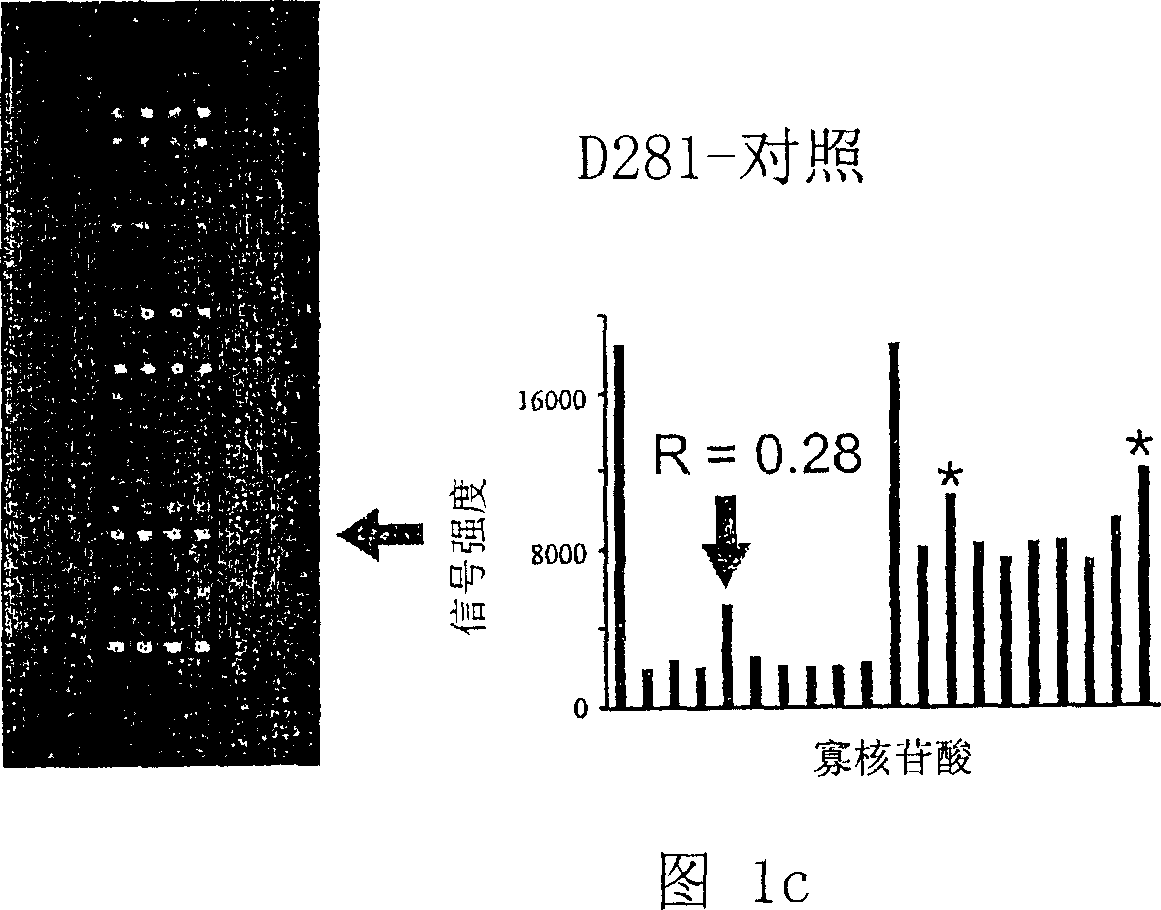
K-ras oligonucleotide microarray and method for detecting K-ras mutations employing the same
Owner:NAT CANCER CENT
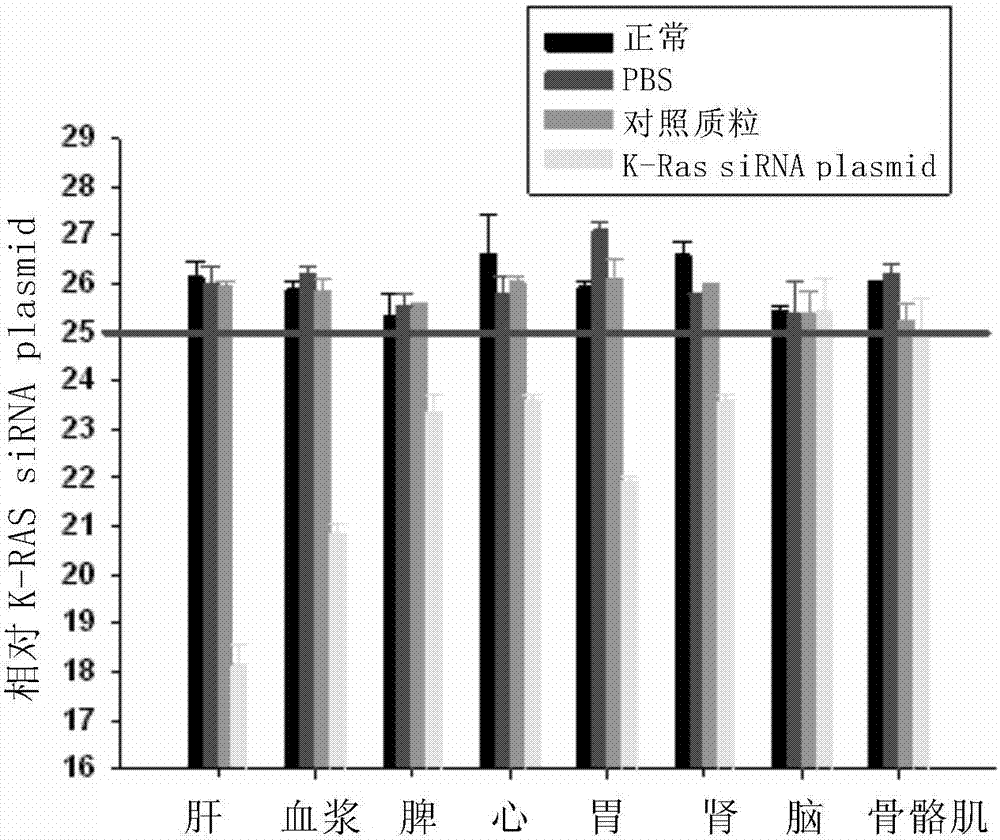
siRNA for inhibiting expression of K-RAS genes as well as precursor and application thereof
InactiveCN107345230AAvoid interferenceOrganic active ingredientsAntineoplastic agentsK-ras GenesWilms' tumor
Owner:JIANGSU MICROMEDMARK BIOTECH
Method for detecting tumor mutant gene in blood
InactiveCN102251046AGuaranteed accuracyGood temperature control systemMicrobiological testing/measurementMaterial analysis by electric/magnetic meansAbnormal tissue growthSingle-strand conformation polymorphism
The invention relates to a method for detecting a tumor mutant gene in blood. The method comprises the following steps of: (1) preparing a polyethylene oxide sieving medium containing TBE (Tetrabromoethane); (2) extracting a genome DNA (Deoxyribonucleic Acid) in a blood sample; (3) amplifying a gene target fragment needing to be detected: performing conventional PCR (Polymerase Chain Reaction) amplification by taking the genome DNA as an amplifying template to obtain a p53 gene PCR amplification sample and a k-ras gene PCR amplification sample; (4) treating the samples: performing denaturation or enzyme digestion on the p53 gene PCR amplification sample and the k-ras gene PCR amplification sample to obtain a denatured sample solution and an enzyme digestion solution respectively; and (5) detecting the samples: adding a 10*SYBR Green I fluorescence dye and the genome DNA into the denatured sample solution and the enzyme digestion solution respectively, uniformly mixing, adding deionized water till a system is 30-50 mu l, automatically filling the polyethylene oxide sieving medium by using a capillary electrophoresis apparatus pressure system and performing CE-SSCP (Capillary Electrophoresis-Single Strand Conformation Polymorphism) detection or CE-RFLP (Capillary Electrophoresis-Restriction Fragment Length Polymorphism) detection respectively. The method is easy to operate, and has high repeatability.
Owner:王荣
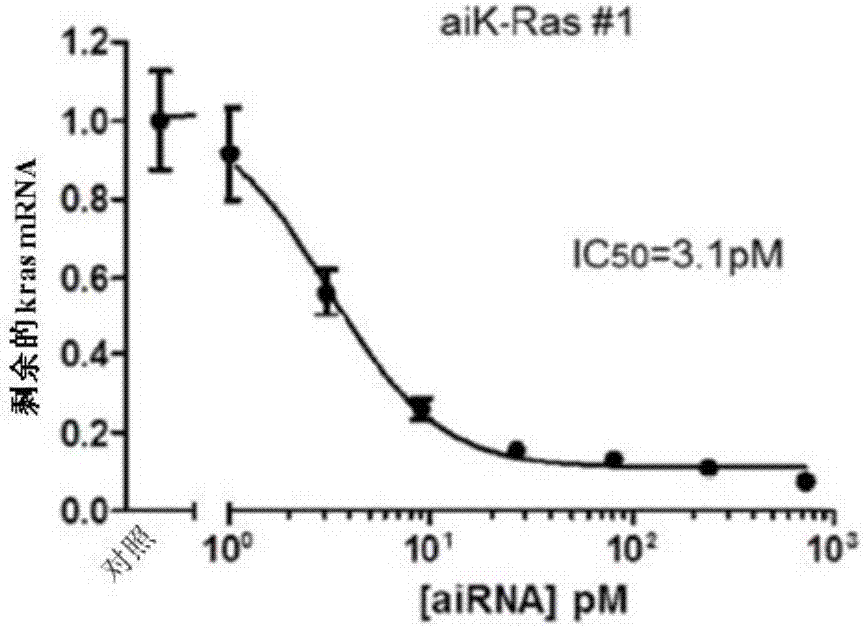
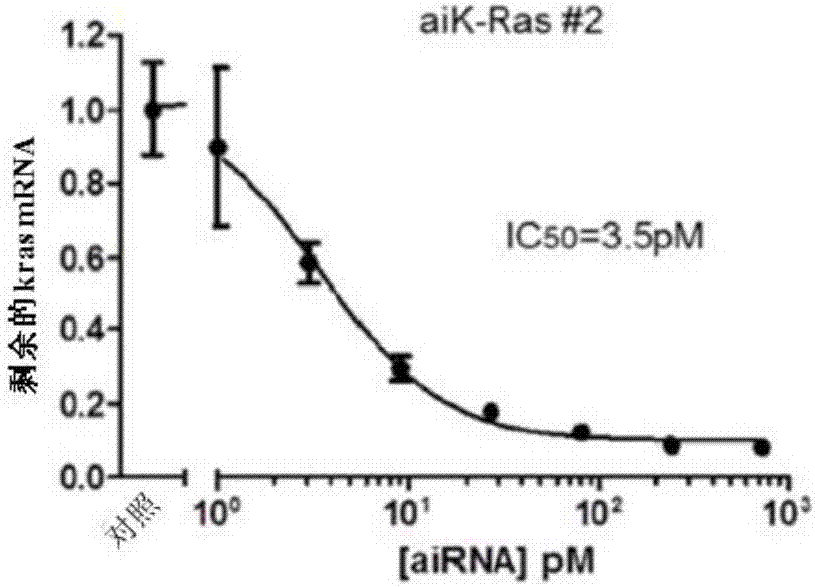
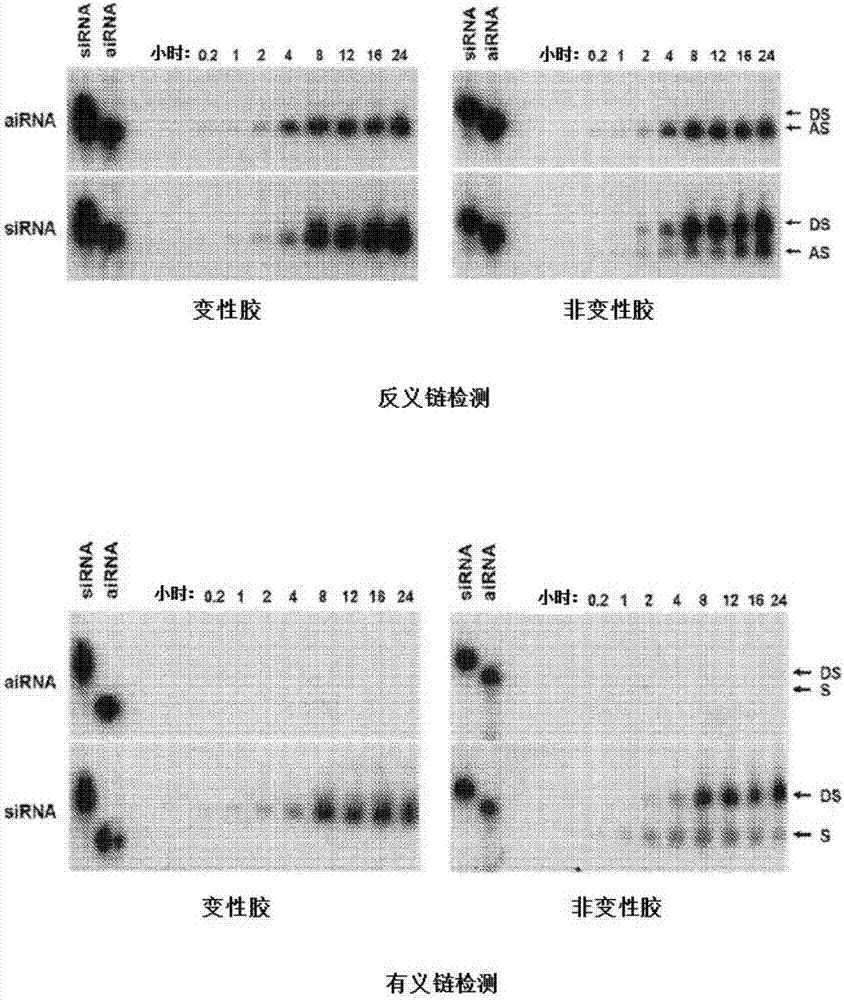
Bi-functional short-hairpin RNA (bi-shRNA) specific for single-nucleotide KRAS mutations
ActiveUS9353373B2Reduced expression levelInhibit expressionOrganic active ingredientsSpecial deliveryK-ras GenesKRAS
The present invention includes compositions and methods for making and using a bifunctional shRNAs capable of reducing an expression of a K-ras gene, e.g., a mutated K-ras gene, wherein at least one target site sequence of the bifunctional RNA molecule is located within the K-ras gene and wherein the bifunctional RNA molecule is capable of activating a cleavage-dependent and a cleavage-independent RNA-induced silencing complex for reducing the expression level of K-ras.
Owner:GRADALIS
Probe for detecting K-ras gene mutation, primer, kit and method
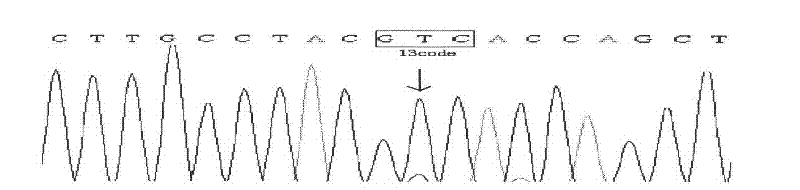
InactiveCN104694622ASimple and fast operationHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationK-ras GenesNucleotide
The invention discloses a probe for detecting K-ras gene mutation. The probe is Taqman MGB fluorescence probe in specific binding with a mutant type sequence of the 13th code of K-ras gene. The invention further discloses a primer and a kit for detecting K-ras gene mutation, the kit comprises the Taqman MGB fluorescent probe in specific binding with the mutant type sequence of the 13th code of the K-ras gene, a primer pair for amplifying the nucleotide sequence of the 13th code containing the K-ras gene; and PNA designed aiming at the 13th code GCC of the wild type K-ras gene. The invention further discloses a dual-fluorescent mark probe real-time quantitative detection method in K-ras gene mutation. The kit and the detection disclosed by the invention are capable of qualitatively and quantitatively detecting the mutation of the 13th code of K-ras gene at the same time, the sensitivity is high, the operation is simple and convenient, and wide application prospect is realized.
Owner:SHANGHAI CHANGHAI HOSPITAL
Prime group, probe group and kit for detecting Kras gene mutation
ActiveCN105803088AHigh detection sensitivityFast wayMicrobiological testing/measurementDNA/RNA fragmentationK-ras GenesForward primer
The invention discloses a prime group, probe group and kit for detecting Kras gene mutation. The prime group comprises forward primers, reverse primers and probes which are used for detecting 7 Kras gene mutation types, wherein the forward primers respectively are 34A-Rev-Fp10, 35A-Rev-Fp4, 38A-Rev-Fp13, 34C-Rev-Fp, 34T-Rev-Fp, 35C-Rev-Fp and 35T-Rev-Fp, the reverse primers are Kras-Rev-Rp, and the probes respectively are 34A-Rev-Pb4, 35A-Rev-Pb2, 38A-Rev-Pb3, 34C-Rev-Pb, 34T-Rev-Pb, 35C-Rev-Pb and 35T-Rev-Pb. The prime group, the probe group and the kit are high in detection sensitivity and accuracy of Kras gene mutation, good in specificity and capable of respectively and simultaneously detecting 7 different mutation types.
Owner:CREATIVE BIOSCIENCES (GUANGZHOU) CO LTD
Kit for quantitative detection of K-ras mutation
ActiveCN102115782AAccurate determination of contentEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesEGFR Tyrosine Kinase Inhibitors
The invention relates to a kit for quantitative detection of K-ras mutation. The invention specifically relates to a detecting method and a detecting kit for K-ras gene mutation relevant with the curative effect of molecular targeted anti-cancer drugs. The invention especially relates to fluorescence quantification PCR detecting method for K-ras gene mutational hotspot region detecting mutational, kit and the application of the fluorescence quantification PCR detecting method. The method of the invention detects the mutation of K-ras gene at a particular locus, predicts the curative effect of molecule targeting anticancer medicament EGFR tyrosine kinases inhibitor, furthermore provides clinic individualized medication scheme for a tumour patient with guidance.
Owner:BEIJING ACCB BIOTECH
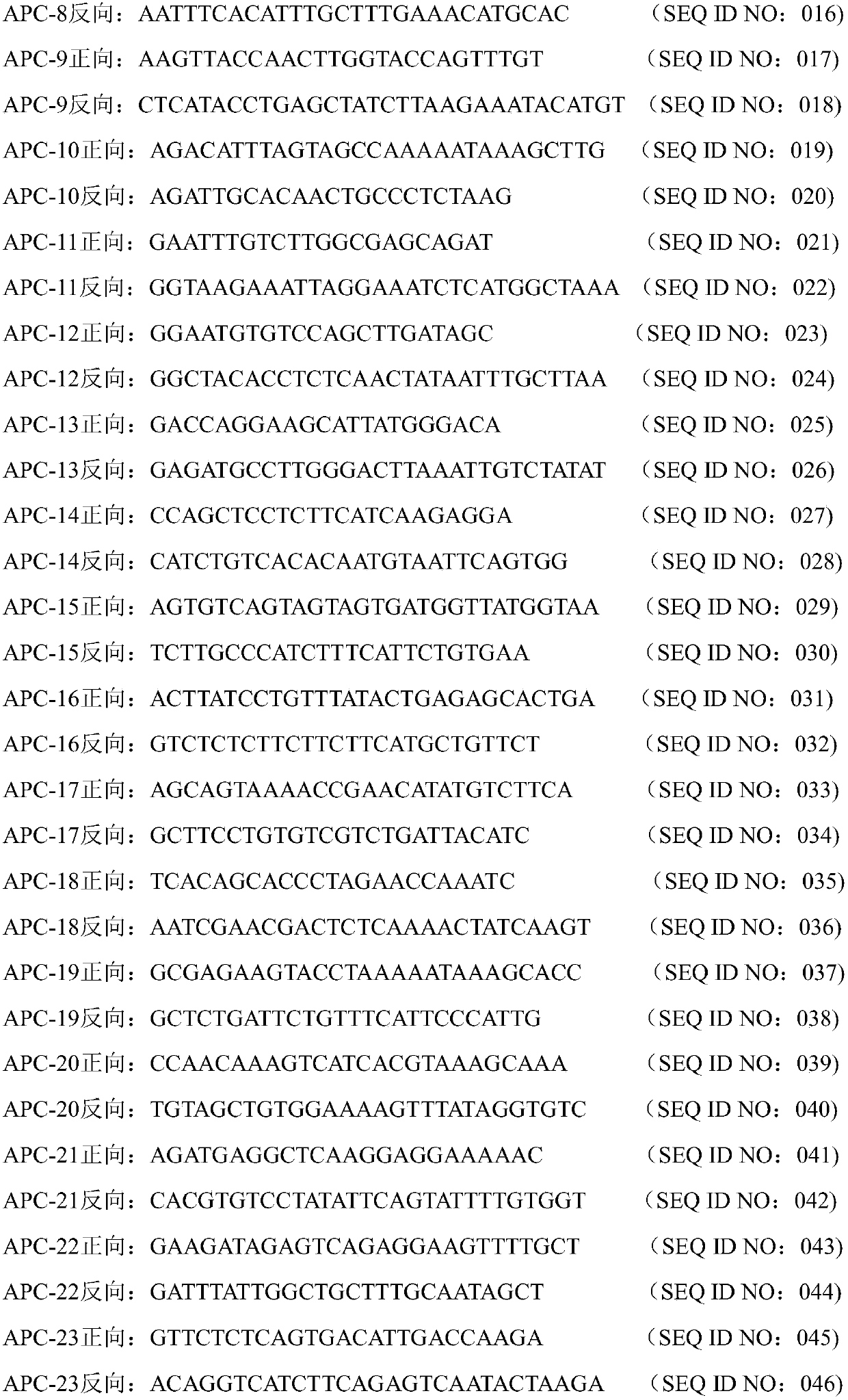
Colorectal cancer early screening primer group based on four genes and kit
ActiveCN108018353AReduce the ratioImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationK-ras GenesCTNNB1 gene
The invention provides a colorectal cancer early screening primer group based on four genes. The primer group specifically comprises the following primers including 58 pairs of forward and reverse primer groups, 20 pairs of forward and reverse primer groups, 27 pairs of forward and reverse primer groups and 7 pairs of pairs of forward and reverse primer groups, wherein the 58 pairs of forward andreverse primer groups are used for amplifying, covering and detecting whole exon sequence mutation sites of the APC gene, and the nucleotide sequences of the primer groups are as shown in SEQ ID NO:001-116; the 20 pairs of forward and reverse primer groups are used for amplifying, covering and detecting whole exon sequence mutation sites of the CTNNB1 gene, and the nucleotide sequences of the primer groups are as shown in SEQ ID NO:117-156; the 27 pairs of forward and reverse primer groups are used for amplifying, covering and detecting whole exon sequence mutation sites of the B-raf gene, andthe nucleotide sequences of the primer groups are as shown in SEQ ID NO:157-210; the 7 pairs of forward and reverse primer groups are used for amplifying, covering and detecting whole exon sequence mutation sites of the K-ras gene, the nucleotide sequences of the primer groups are as shown in SEQ ID NO:211-224. Exons of the four genes can be completely amplified through the method, all the mutation sites to be detected can be covered, and the accuracy of screening detection is ensured.
Owner:达瑞医学检验(广州)有限公司
K-Ras Oligonucleotide Microarray and Method for Detecting K-Ras Mutations Employing the Same
InactiveUS20070298419A1Fast and reliableBioreactor/fermenter combinationsBiological substance pretreatmentsK-ras GenesBiology
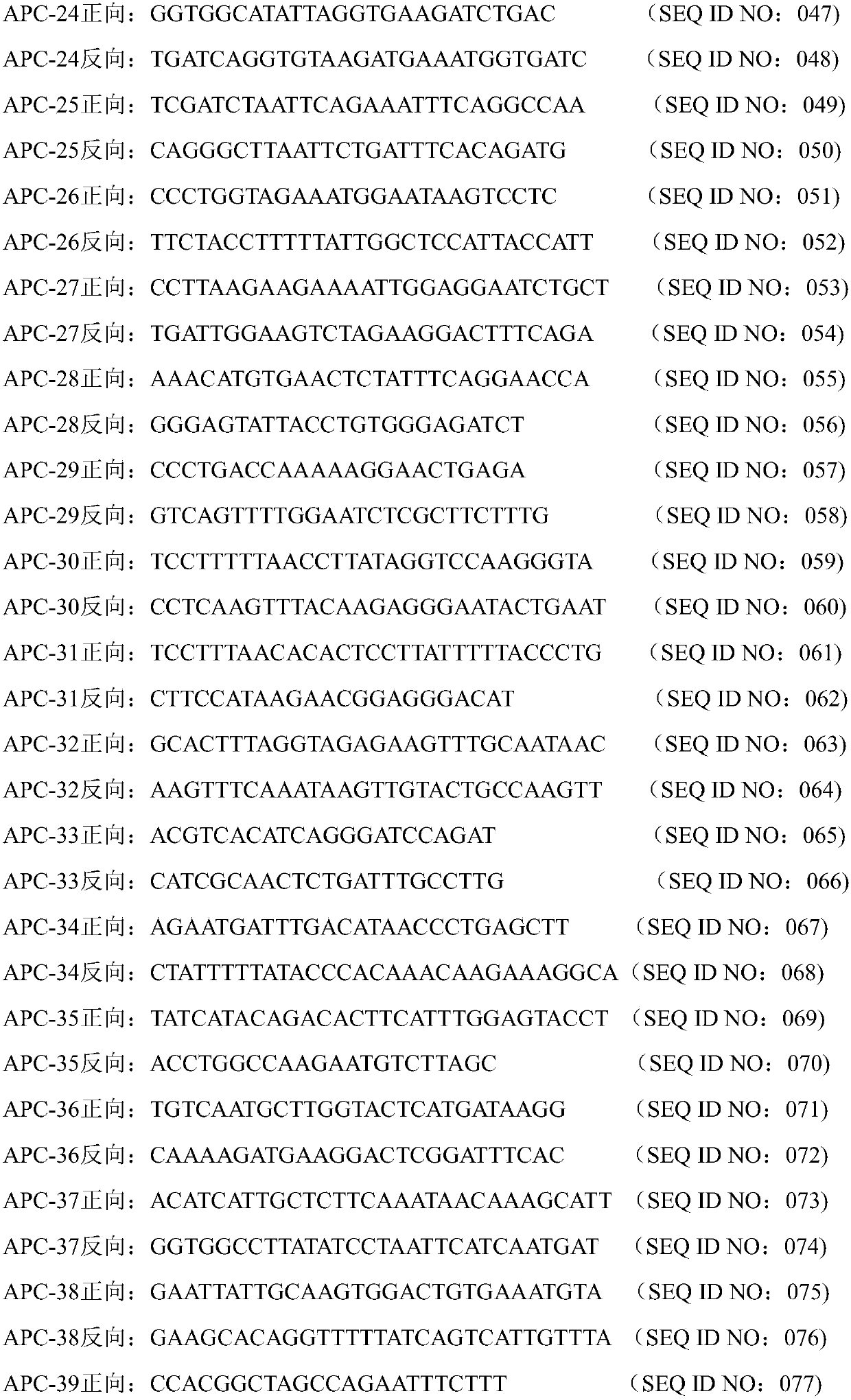
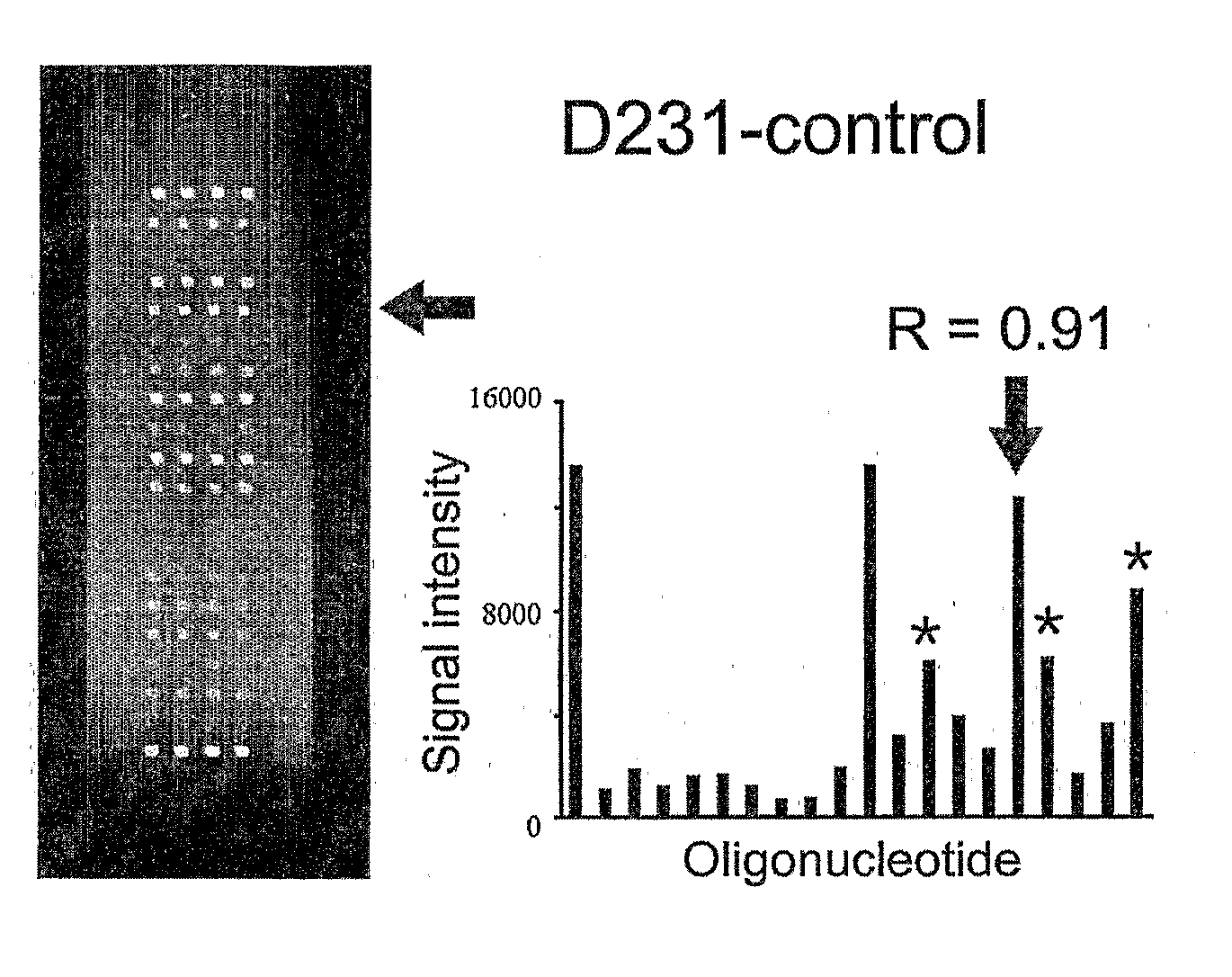
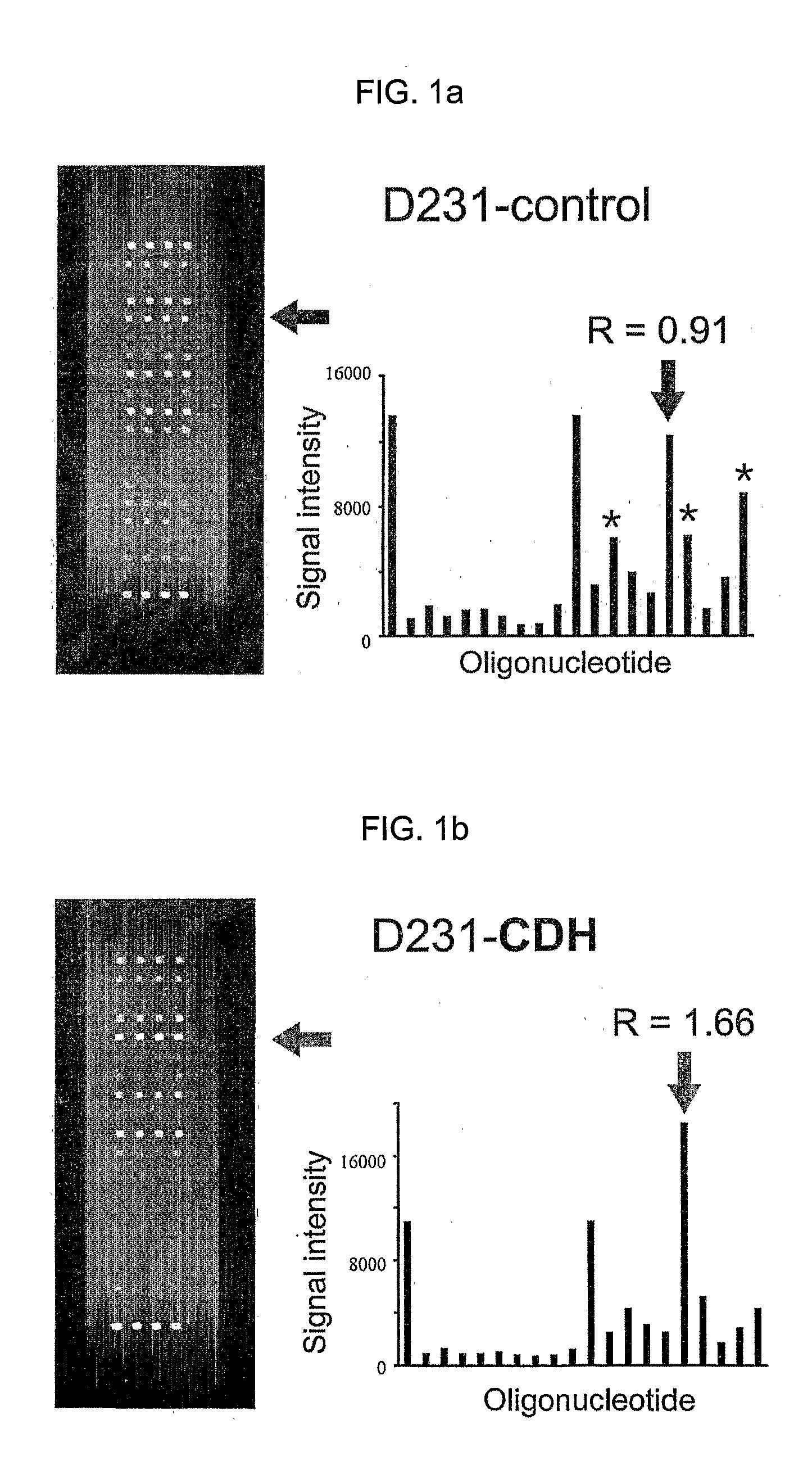
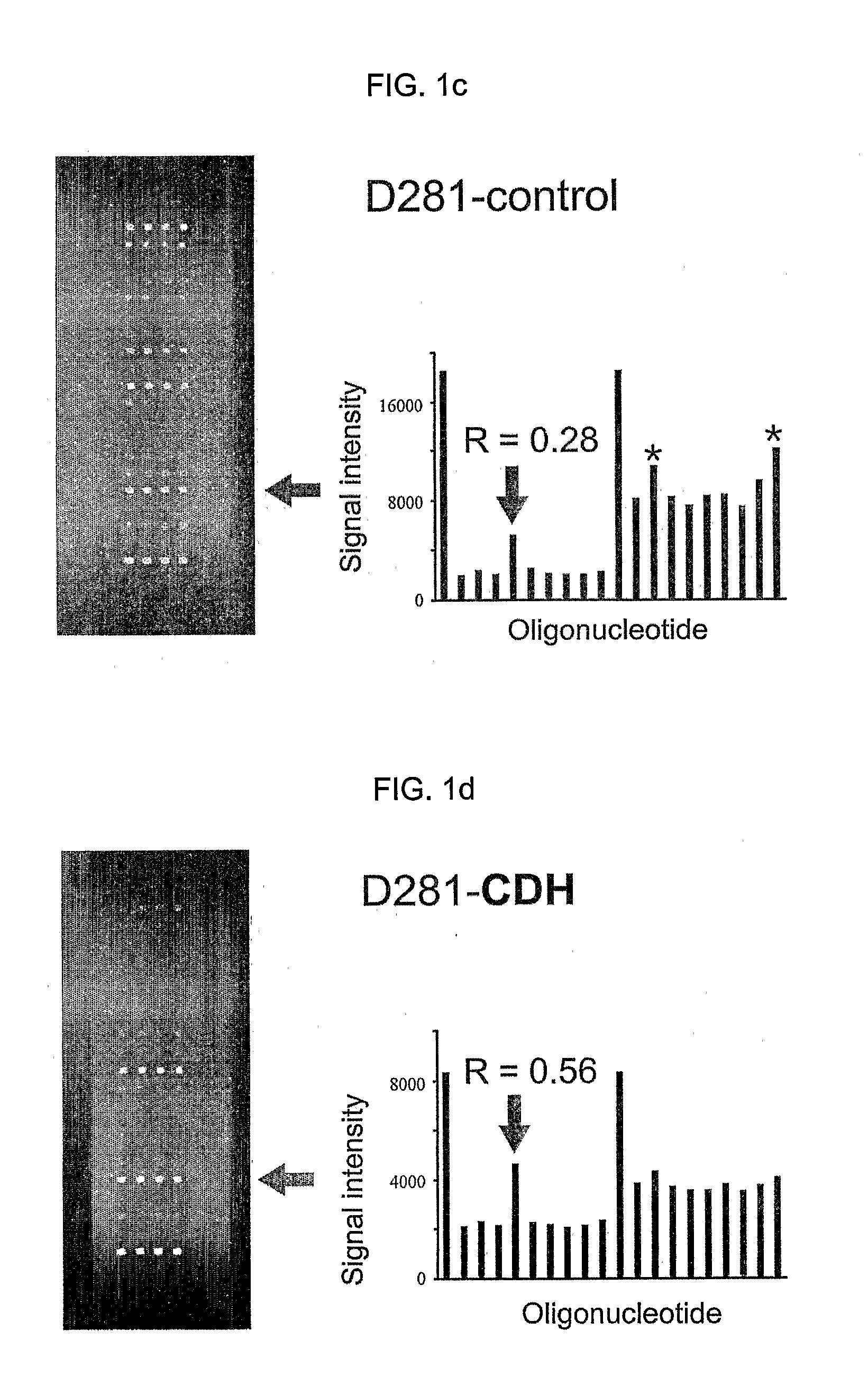
Since the K-ras oligonucleotide microarray of the present invention can detect K-ras mutations by applying a competitive DNA hybridization method to the oligonucleotides spotted on a solid matrix different from the previously reported method for detecting a mutation, it makes the more precise analysis and can reduce experimental cost and time. Accordingly, the K-ras oligonucleotide microarray of the present invention can be used in studies to detect K-ras mutations and unravel the signal transduction mechanism and tumorigenesis related to K-ras gene. Further, since the microarray of the present invention can be applied to other genes having mutational hot spot regions such as K-ras, it has wide applicable range.
Owner:NAT CANCER CENT
Primer for detecting K-ras genic mutation and application thereof
ActiveCN102094078AHigh reference significanceStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationK-ras GenesErlotinib
The invention relates to the field of molecular tests guiding oncotherapy and particularly discloses a primer for detecting the mutation condition of K-ras gene codons 12 / 13 and 61 and application of the primer to the identification of cancer patient sensibility to medicines such as geftinat, erlotinib and the like. DNA extraction is performed on tumor tissues of a patient, a target segment is amplified by using the primer designed by the invention, and mutational sites of the K-ras codons 12 / 13 and 61 are detected by sequencing the target segment, so that the treatment on the tumor patients can be guided.
Owner:SHANGHAI BIOTECAN PHARMA
Acetamino azobenzene derivative and preparation and application thereof
ActiveCN109574871AEasy to prepareEasy to separate and purifyOrganic chemistryAntineoplastic agentsK-ras GenesStructural formula
The invention relates to an acetamino azobenzene derivative and preparation and application thereof. The derivative has a structure in a general formula as shown in the specification. The acetamino azobenzene derivative has characteristics of structural changes and activity changes under illumination conditions, is in a structure of a structural formula I in a dark environment and is in a structure of a structural formula II after ultraviolet irradiation, wherein the structure II has K-Ras(G12C) protein inhibiting activity, and the structure I is weak in activity. The compound can be used forpreparation of selective medicines for treatment of K-Ras gene mutation related tumors such as colorectal cancer, lung cancer and prostatic cancer.
Owner:SHANGHAI JIAO TONG UNIV
Kits for quantitative detection of k-ras mutations
InactiveUS20120288862A1Accurately and quantitatively ratioEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceK-ras GenesEGFR Tyrosine Kinase Inhibitors
The present invention relates to an assay kit for quantitatively detecting k-ras gene mutations. Particularly, the present invention relates to detection method and a detection kit for K-ras gene mutations, which relates to the therapeutic efficacy of targeted molecular anti-cancer drugs. More particularly, the present invention relates to a fluorescent quantitative PCR method and kit for detecting mutations at hotspots of K-ras gene, together with the use thereof. The present invention detects the mutations at specific sites of K-ras gene, and can predict the therapeutic efficacy of anti-EGFR tyrosine kinase inhibitors. Therefore, it can provide a guidance to individualized treatments for cancer patients.
Owner:BEIJING ACCB BIOTECH
Rapid detection kit for K-ras gene mutation
InactiveCN110004224AThe detection process is fastMicrobiological testing/measurementK-ras GenesFluorescence
The invention relates to a rapid detection kit for K-ras gene mutation, and belongs to the technical field of molecular biology. The kit consists of an ARMS upstream primer, a downstream primer, a trigger primer and a fluorescent probe for detection of 10 sensitive mutation sites. The K-ras gene detection kit can detect 10 major mutations simultaneously under a single-tube condition. With design of the trigger primer, a reaction system generates fluorescence when any kind of mutation exists, and the effect of rapid detection and determination of K-Ras mutation can be achieved. The kit has theadvantages of fast detection speed and the sensitivity degree reaching 0.001%.
Owner:邓定平
Asymmetric interfering RNA compositions that silence K-RAS and methods of uses thereof
InactiveCN107428794ADelay gastric cancer progressionImprove gastric cancer symptomsOrganic active ingredientsSugar derivativesK-ras GenesRelated disorder
The invention provides novel compositions for use in silencing K-Ras gene expression. More particularly, the invention provides novel asymmetrical interfering RNA molecules as inhibitors of K-Ras expression, and to pharmaceutical compositions and uses thereof in the treatment of cancer or a related disorder in a mammal.
Owner:1GLOBE BIOMEDICAL CO LTD
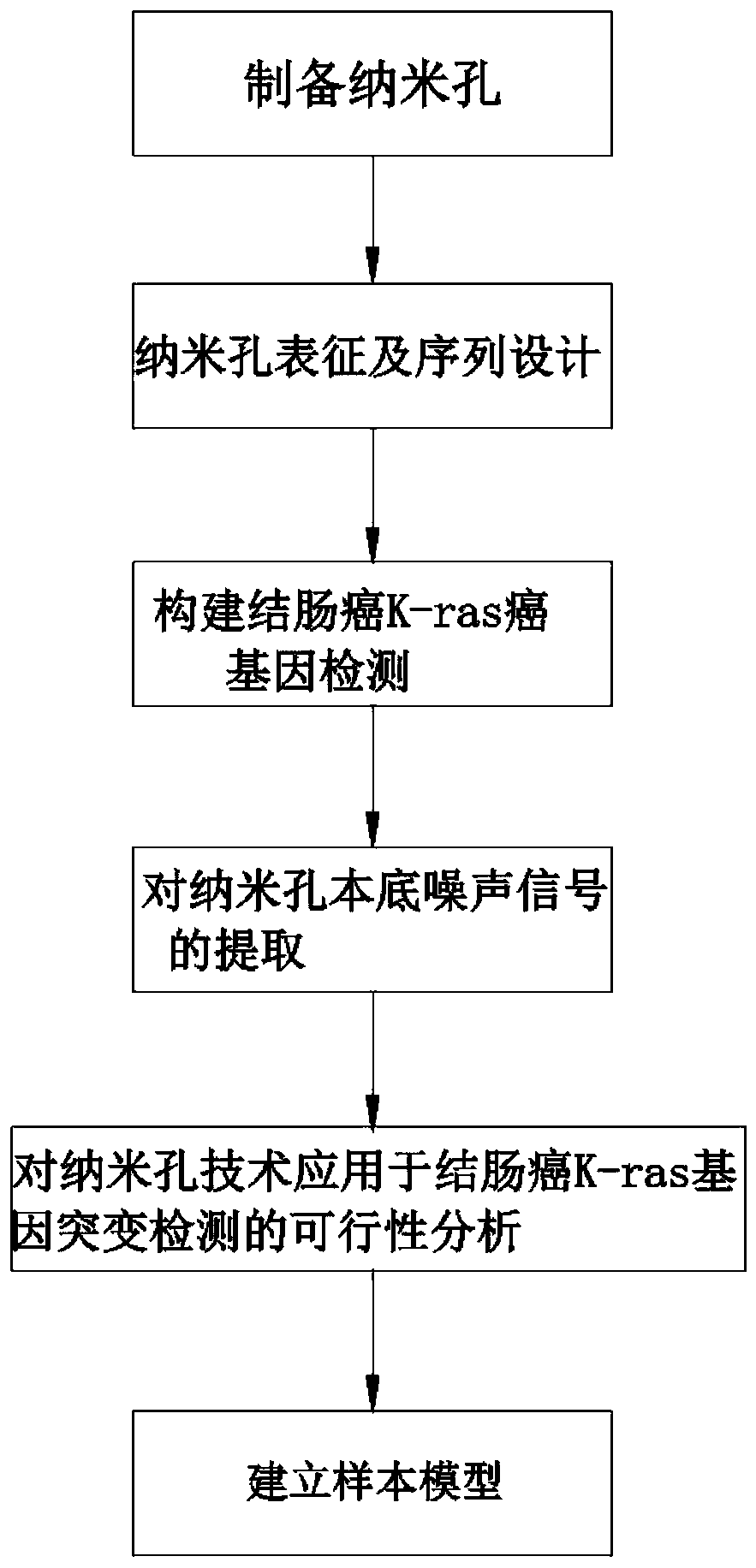
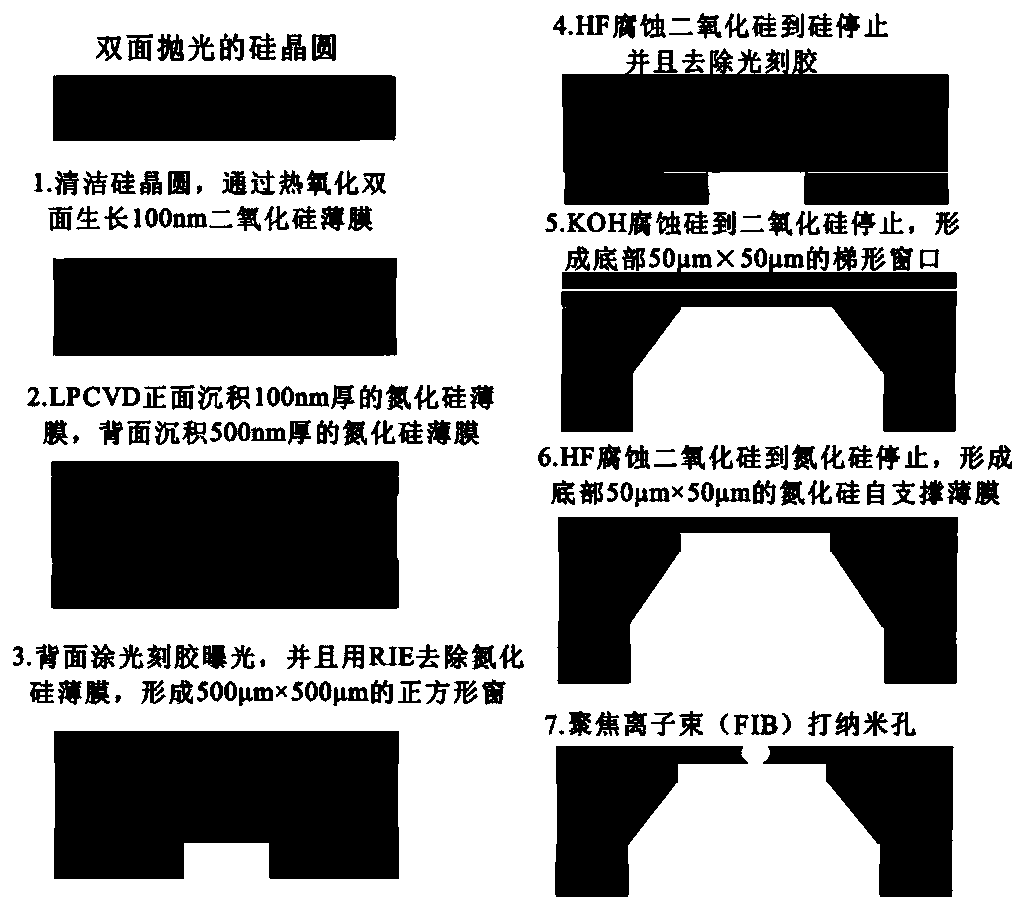
Colon cancer K-ras oncogene mutation detection system
PendingCN110408701AReveal the law of motionExplain the kinetic mechanismMicrobiological testing/measurementEnzyme digestionWild type
The invention discloses a colon cancer K-ras oncogene mutation detection system, wherein the system includes the following steps: A, preparation of nanopores; B, nanopore characterization and sequencedesign; C, construction of colon cancer K-ras oncogene detection; D, extraction of nanopore background noise signals; E, feasibility analysis of a nanopore technology applied to colon cancer K-ras gene mutation detection; and F, establishment of a sample model. The system focuses on the advantages of stable solid-state nanopores, easy design and processing, high throughput and easy physical and chemical modification. An MvaI enzyme digestion site is introduced at a codon 12 of a wild-type K-ras oncogene, and translocation behavior of mutation of the colon cancer K-ras gene after enzyme digestion and the wild type in a nano-scale environment is studied by using a highly sensitive nanopore ion current detection technology, the law of molecular motion is revealed and the dynamic mechanism isexplained.
Owner:苏州丽纳芯生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com