Fluorescence PCR detection kit for human K-RAS gene mutation
A detection kit and kit technology, applied in the field of fluorescent PCR detection kits for human K-RAS gene mutation, to achieve good specificity and strong anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This embodiment provides a specific human K-RAS gene mutation nucleic acid detection kit, which includes the following components.
[0032] ①Internal standard (positive internal control): The internal standard used in the present invention is the housekeeping gene β-actin in human genomic DNA to prevent false negatives caused by PCR interference substances that may exist in the sample or the sample nucleic acid concentration is too low; The target sequence is as follows:
[0033] 5’-AAGTGCTCGGTGCCTTTAGTGATGGCCTGGCTCACCTGGACAACCTCAAGGGCACCTTTGCCACACTGAGTGAGCTGCACTGTGACAAGCTGCACGTGGATCCTGAGAACTTCAGGGTGAGTCTATGGGAC-3’;
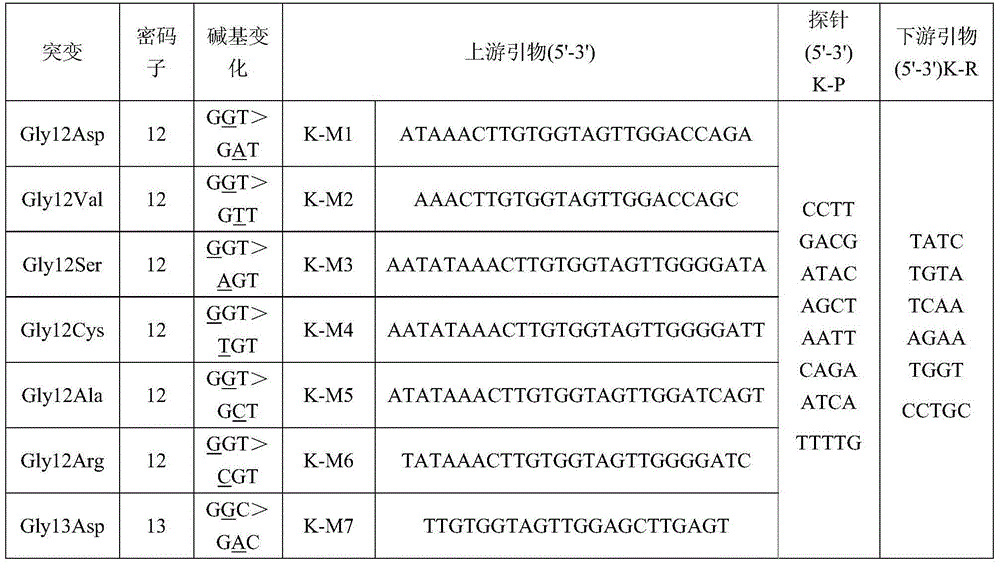
[0034] ②PCR reaction solution: This kit includes 7 PCR reaction solutions to detect Gly12Asp, Gly12Val, Gly12Ser, Gly12Cys, Gly12Ala, Gly12Arg, and Gly13Asp, a total of 7 mutations.
[0035] Gly12Asp mutation detection reaction solution: 10×PCR reaction buffer 5μl, 0.2mmol / L deoxyribonucleoside triphosphate, 0.2μmol / L~0.4μmol / L upstream and downstream primer K-M1 ...
Embodiment 2
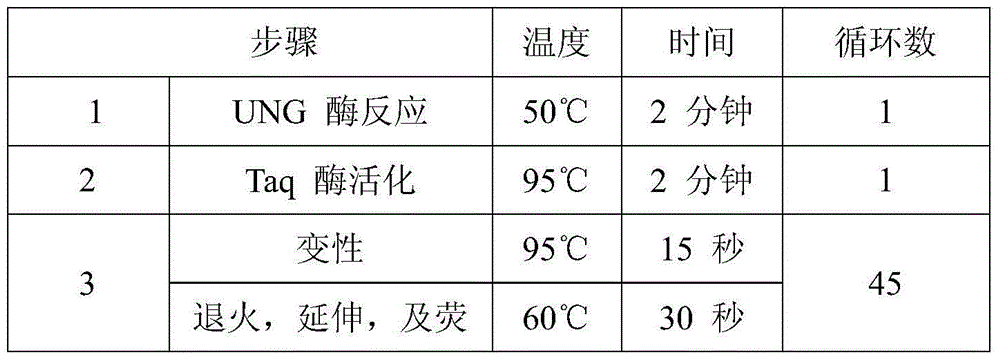
[0053] This embodiment provides an operating method for detecting the mutation of the K-RAS gene in the nucleic acid sample extracted from the paraffin section using the human K-RAS gene mutation nucleic acid detection kit described in Embodiment 1.
[0054] 1. Reagent preparation (in the reagent preparation area)
[0055] Take out the components in the packaging box, place them at room temperature, wait for their temperature to equilibrate to room temperature, mix well and set aside;
[0056] According to the number of samples to be tested, negative control, and positive control, proportionally (Gly12Asp mutation detection reaction solution 43μl / person + enzyme mixture 2μl / person; Gly12Val mutation detection reaction solution 43μl / person + enzyme mixture 2μl / person; Gly12Ser mutation detection reaction solution 43μl / person + enzyme mixture 2μl / person; Gly12Cys mutation detection reaction solution 43μl / person + enzyme mixture 2μl / person; Gly12Ala mutation detection reaction Gly12Arg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com