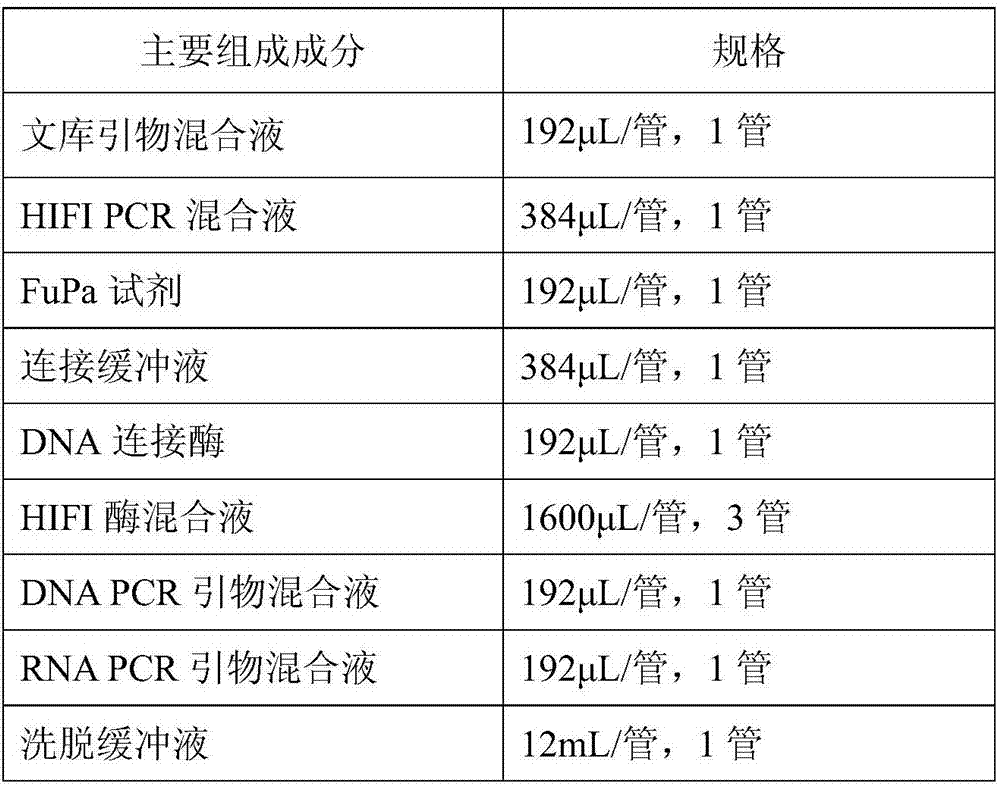
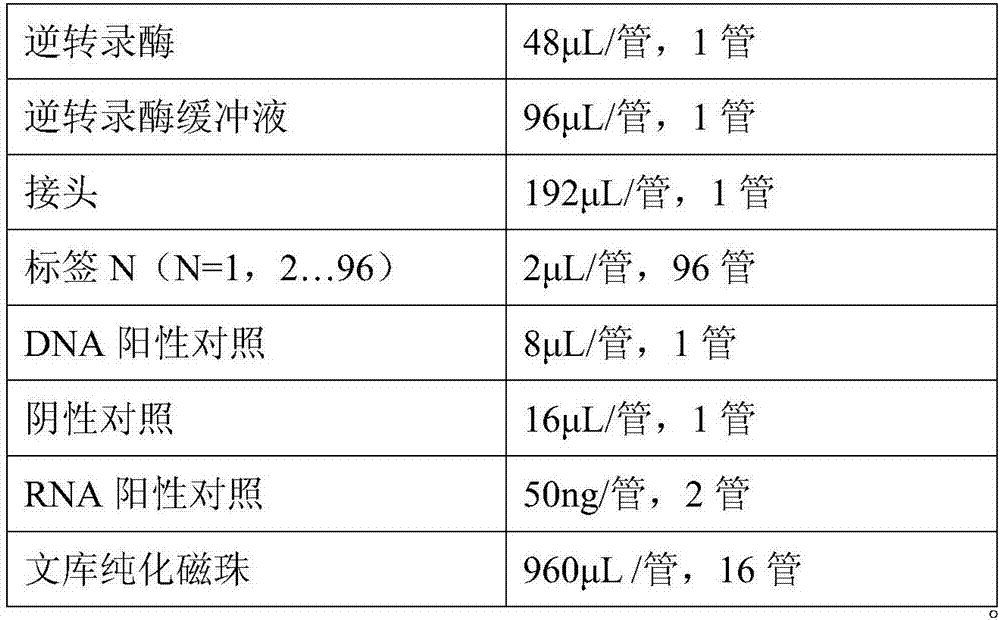
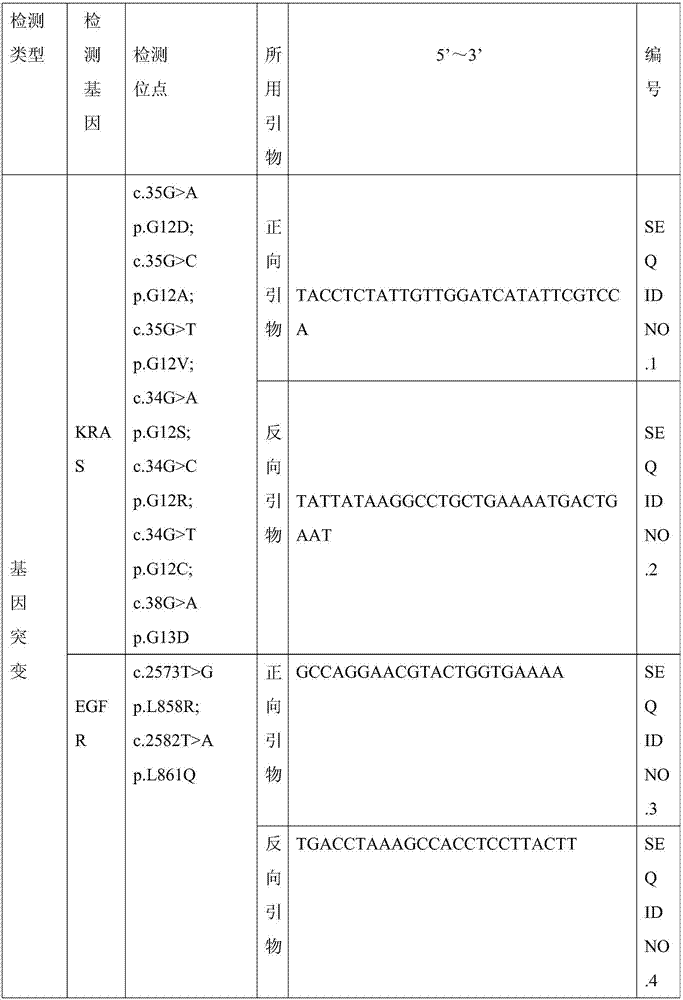
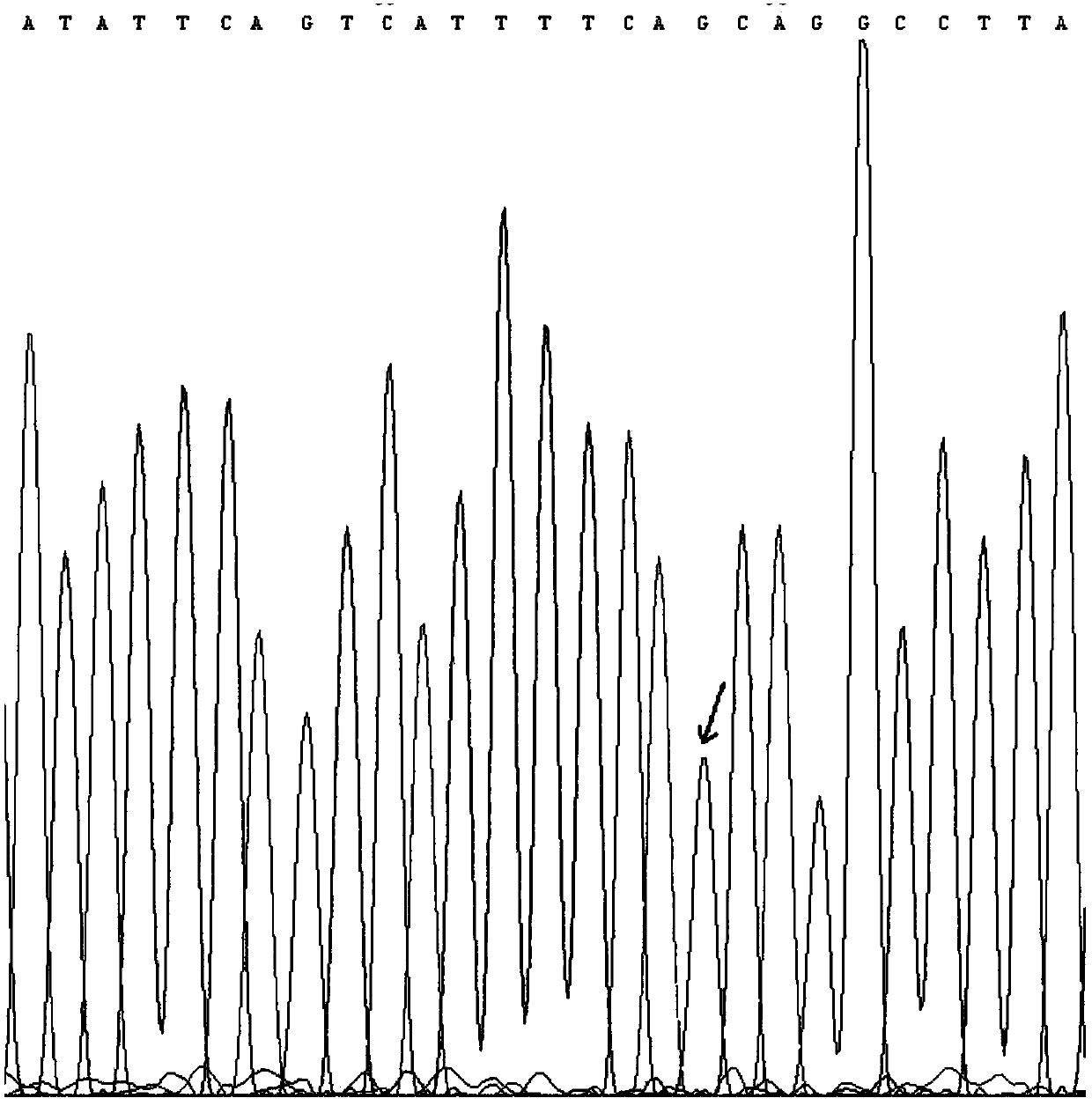
Patents
Literature
91 results about "KRAS Gene Mutation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A change in the nucleotide sequence of the KRAS gene.
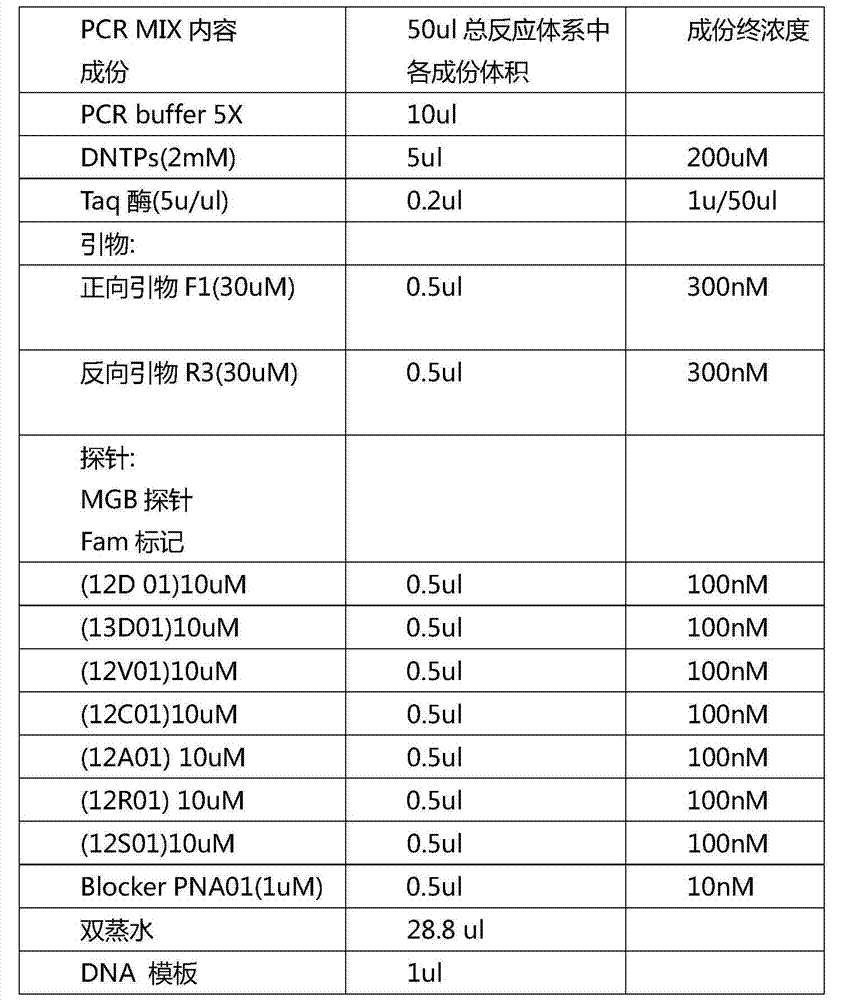
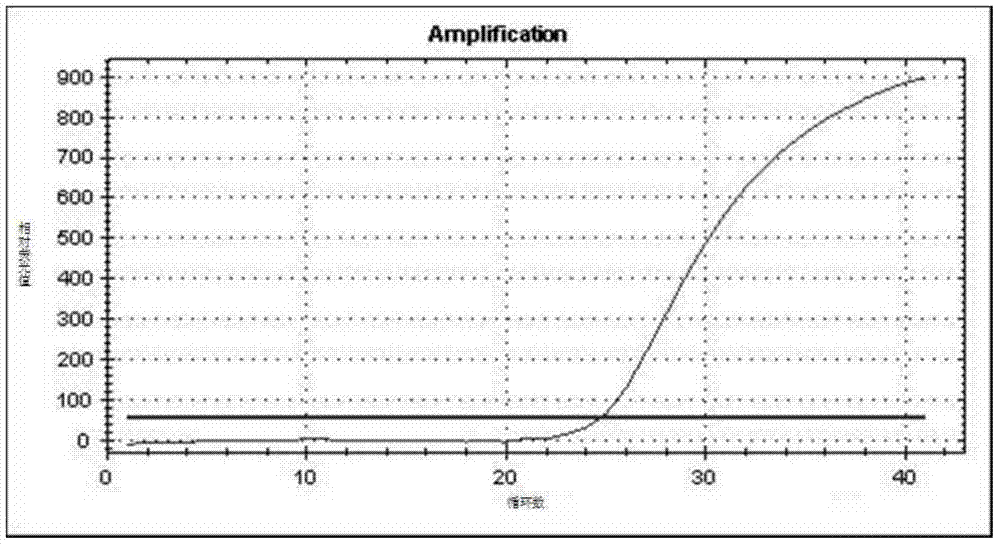
ARMS-qPCR (Allele Refractory Mutation System-quantitative Polymerase Chain Reaction) detection kit for KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) gene mutation subtype and detection method
InactiveCN102367478AIncreased sensitivityQuick checkMicrobiological testing/measurementViral OncogenePositive control
The invention relates to the field of molecular biology and aims to provide an ARMS-qPCR (Allele Refractory Mutation System-quantitative Polymerase Chain Reaction) detection kit for KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) gene mutation subtype and a detection method. The kit comprises a qPCR hybrid reaction solution, a locked nucleic acid retardant probe, a reference primer, an ARMS primer and a positive control sample, wherein the qPCR hybrid reaction solution comprises a PCR buffer solution, dNTPs (Deoxynucleotide Triphosphates), MgCl2, GoldStarbest Taq enzyme, a universal PCR reverse primer and a universal TaqMan probe. The kit provided by the invention can be used for rapidly and accurately detecting specific locus mutation of KRAS genes in various cancer tissues with high sensitivity, has high sensitivity, and can be used for detecting genome DNA with various tissue origins, specially free DNA segments adopting cell-free systems, such as blood serum and blood plasma, orother body fluid origins, wherein the genome DNA is derived from cell systems. Compared with direct sequencing and other mutation detection technologies, the kit and the detection method thereof havethe advantages of strong specificity, high sensitivity, simplicity and rapidness in operation, high throughput, safety, definiteness and objectivity in result identification and the like for detecting the KRAS gene mutation.
Owner:ZHEJIANG UNIV
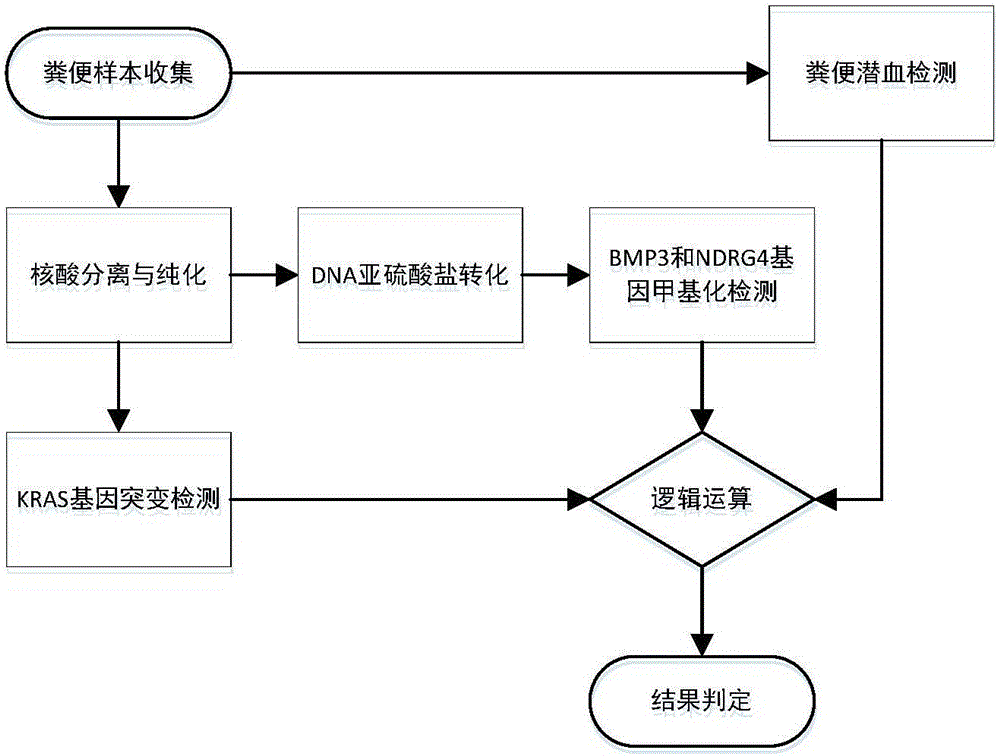
Kit for early stage colorectal cancer auxiliary diagnosis and use method and detection system thereof
InactiveCN106399570ANo detection blind zoneHigh test acceptanceMicrobiological testing/measurementBiological material analysisViral OncogeneGenes mutation
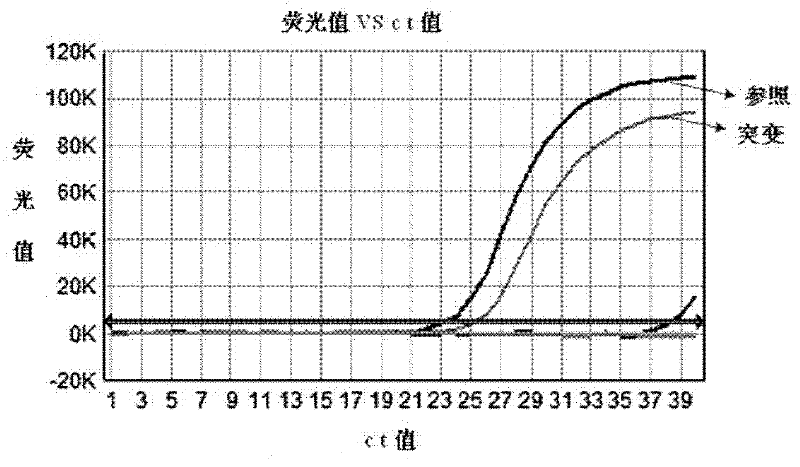
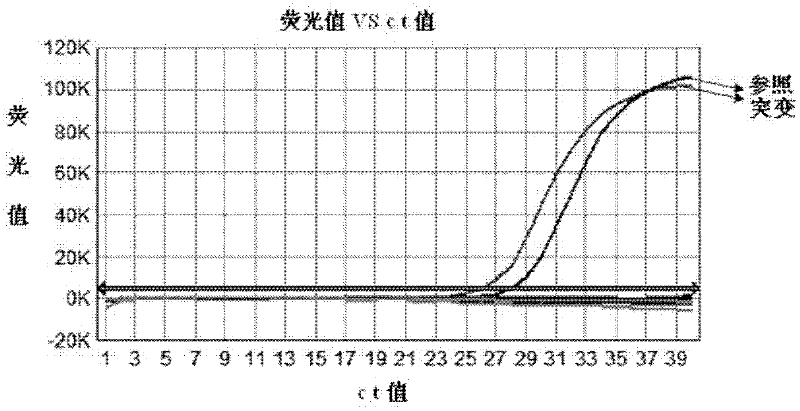
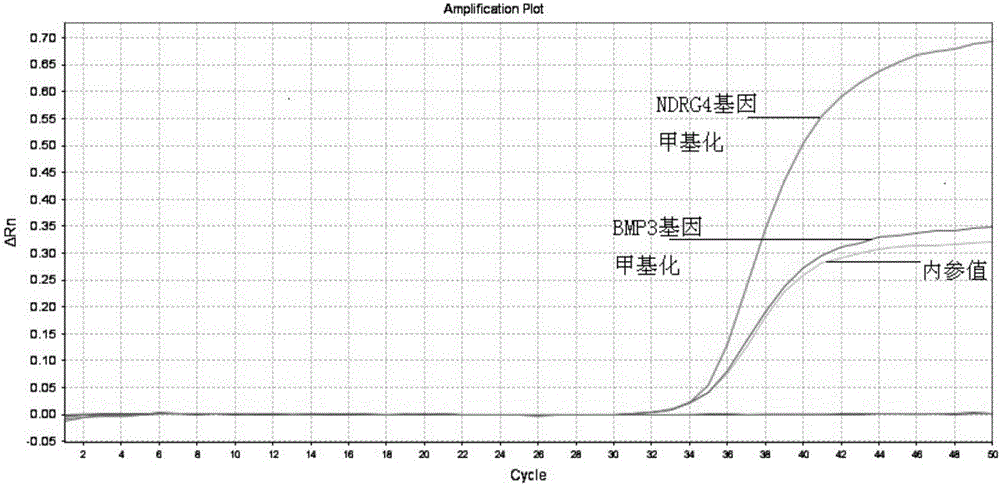
The invention provides a kit for early stage colorectal cancer auxiliary diagnosis and a use method and a detection system thereof. The kit comprises a nucleic acid isolation and purification reagent, a DNA (deoxyribonucleic acid) sulfite conversion reagent, a KRAS (kirsten rat sarcoma viral oncogene homolog) gene mutation detection reagent, a BMP3 (bone morphogenetic protein 3) and NDRG4 (N-myc downsteam regulated gene 4) gene methylation detection reagent and a fecal occult blood detection reagent, wherein the nucleic acid isolation and purification reagent is used for separating and purifying the human DNA in a faeces sample; the DNA sulfite conversion reagent is used for performing sulfite conversion on the purified partial human DNA, and is used for subsequent BMP3 and NDRG4 gene methylation detection. Through detecting two kinds of indexes of DNA and fecal occult blood in the faeces sample in a combined way, the kit provided by the invention is used for the early stage screening of the colorectal cancer. Compared with an FOBT (fecal occult blood test) detection method, the kit provided by the method can realize higher sensitivity on the detection of colorectal cancer, particularly the developing period tumor.
Owner:HANGZHOU NEW HORIZON HEALTH TECH CO LTD
Primers, probes, detection system and kit for one time detection of lung cancer multiple genes
ActiveCN104818320AWill not interfere with each otherLow costMicrobiological testing/measurementDNA/RNA fragmentationTumor targetBRAF Gene Mutation
The present invention discloses primers, probes, a detection system and a kit for one time detection of lung cancer multiple genes, wherein the primers, the probes, and the distribution way for detecting 25 EGFR gene mutations, 7 KRAS gene mutations, 6 BRAF gene mutations, 9 NRAS gene mutations, 5 HER2 gene mutations, 2 PIK3CA gene mutations, 5 fusion genes of ALK5, 13 fusion genes of ROS1, and 6 fusion genes of RET are provided. According to the present invention, the detection kit adopts the 12 linking PCR reaction strip design, each 12 linking PCR strip detects multiple genes of a sample, and the corresponding fusion detection reagents and the internal control reagents are filled in the pipes 1-4 of the 12 linking PCR strip; and with the primers, the probes, the detection system and the kit, the one-time detection of the 24 fusions and the 54 mutations of the lung cancer can be achieved, such that the detection time is substantially shortened, the sensitivity is high, the specificity is strong, the operation is simple and rapid, and the reference for selection of tumor targeting drug therapy on lung cancer patients can be provided for clinician.
Owner:AMOY DIAGNOSTICS CO LTD
Spiro-substituted pyrimido-cyclic compound, and preparation method and medical application thereof
The invention discloses a spiro-substituted pyrimido-cyclic compound with selective inhibition effect on KRAS gene mutation and a pharmaceutically acceptable salt, a stereoisomer, a solvent compound or a prodrug thereof; the spiro-substituted pyrimido-cyclic compound is represented by a formula (I), and the definition of each group or symbol in the formula is shown in the specification in detail.In addition, the invention also discloses a pharmaceutical composition containing the compound and an application of the compound in preparation of cancer drugs.
Owner:GENFLEET THERAPEUTICS (SHANGHAI) INC +1
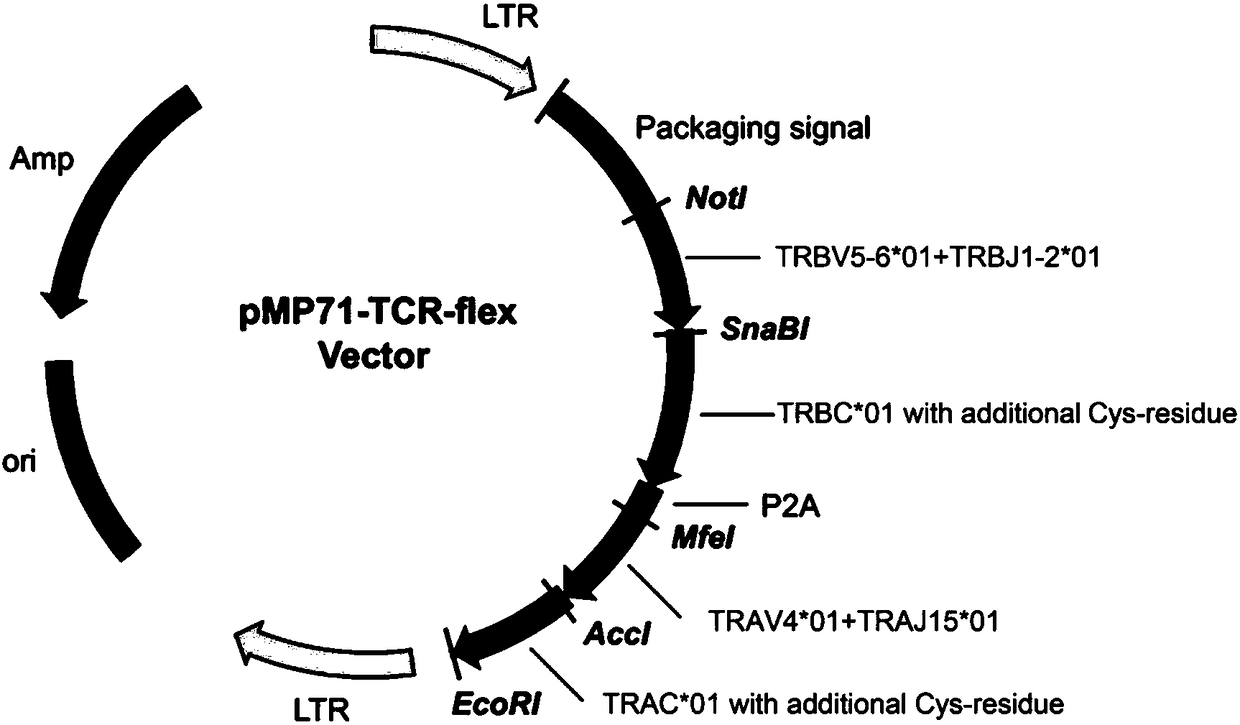
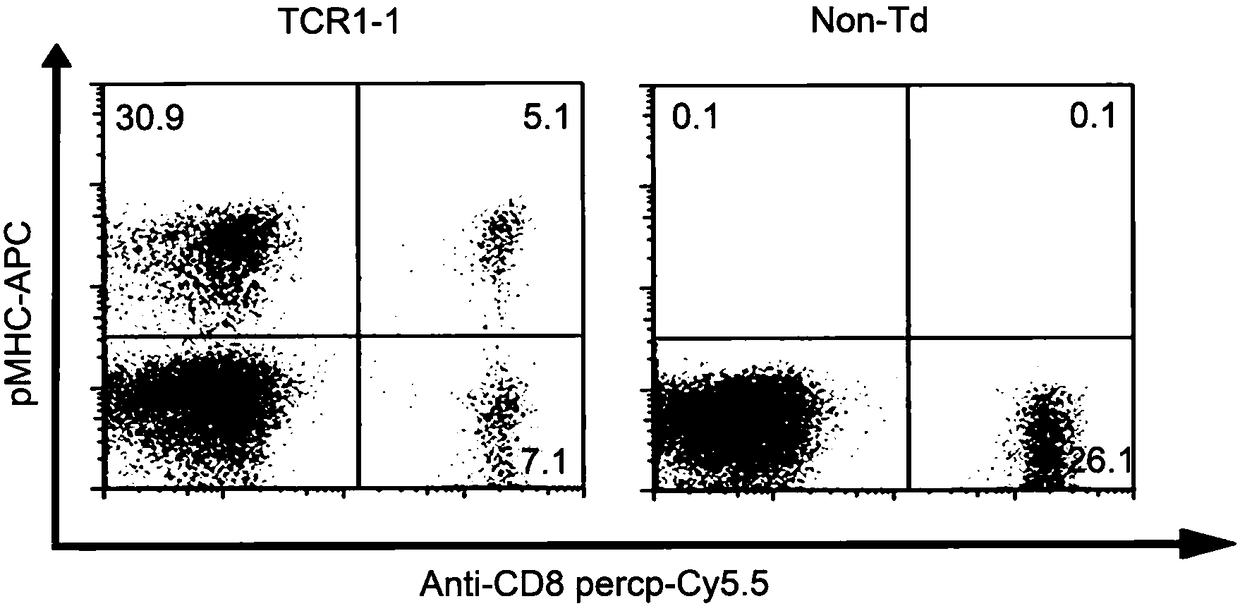
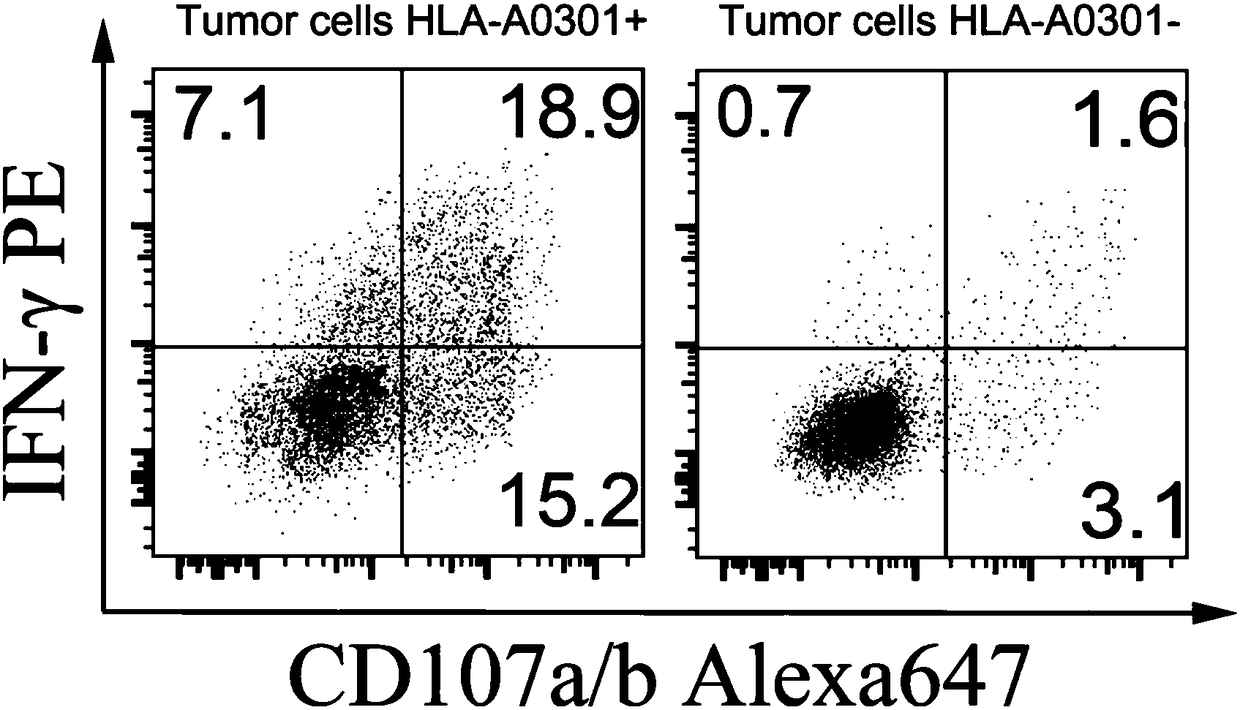
T cell receptor related to KRAS gene mutation
ActiveCN108395479ASignificant precision medical featuresHigh affinityOrganic active ingredientsImmunoglobulin superfamilyDiseaseEphA Receptors
The present invention discloses a T cell receptor, which specifically binds to a KRAS gene codon 12 mutation G12D 26aa peptide, wherein the amino acid sequence of the peptide is represented by SEQ IDNO:1 MTEYKLVVVGADGVGKSALTIQLIQN, the 9-12aa epitope polypeptide with high affinity is represented by SEQ ID NO:48 VVVGADGVGK and SEQ ID NO:49 KLVVVGADGVGK when the T cell receptor can identify the HLAmolecule antigen complex, and the HLA molecule is HLA-A0301. The invention discloses the amino acid sequences of the alpha chain and the beta chain of the T cell receptor in the identification of theantigen complex formed by the KRAS gene G12D mutation peptide and the HLA-A0301, nucleotide sequences encoding the alpha chain and the beta chain of the T cell receptor, a vector containing the nucleotide sequences, cells containing the nucleotide sequences, and applications of the vector or the cells in preparation of drugs for prevention and treatment of diseases related to KRAS gene mutation.
Owner:高军 +2
Rapid detection of KRAS (Kirsten Rat Sarcoma) gene mutation
ActiveCN101875972AHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceNucleotideGenotype
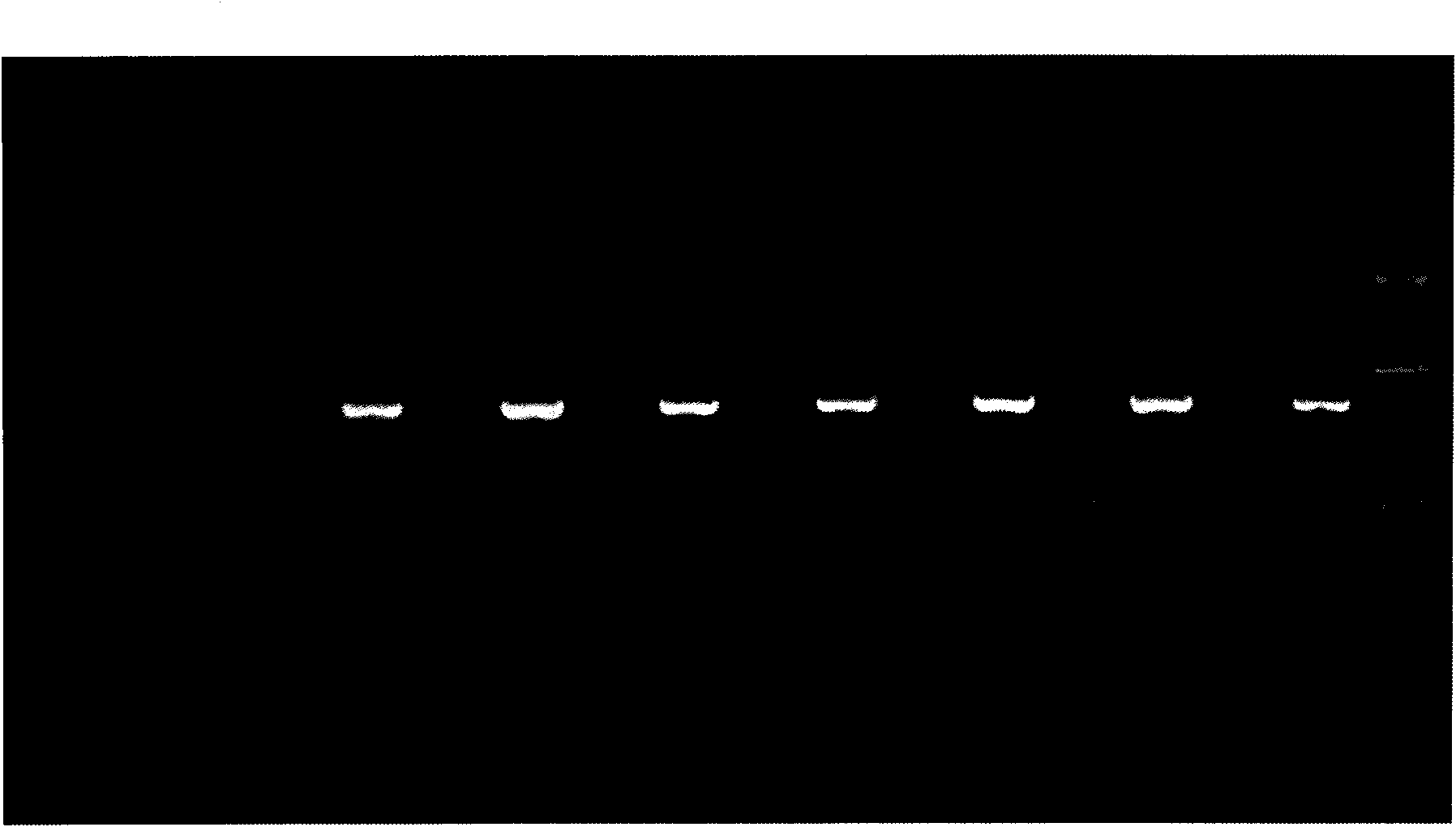
The invention discloses a PCR (Polymerase Chain Reaction) reaction kit for detecting KRAS (Kirsten Rat Sarcoma) gene mutation, which is characterized by comprising a plurality of primer pairs used for specifically amplifying a targeting sequence of the KRAS gene, and each primer pair contains a continuous nucleotide sequence formed by at least 15 continuous nucleotides in a second exon KRAS2 or athird exon KRAS 3 of the KRAS gene, wherein the KRAS2 has the continuous nucleotide sequence of SEQ ID No:1, and the KRAS3 has the continuous nucleotide sequence of the SEQ ID No: 2. The kit completes the judgment on a genotype sample by adopting a saturated probe and a high resolution fusion curve analysis technology so as to provide guidance on medicine selection and diagnosis for various tumors including lung cancers and colorectal cancers.
Owner:JIANGSU MICRODIAG BIOMEDICINE TECH CO LTD
Substituted heteroaromatic ring dihydropyrimidinone derivative as well as preparation method and medical application thereof
ActiveCN112390818AHigh activityGood choiceOrganic active ingredientsOrganic chemistry methodsGenes mutationPharmaceutical medicine
The invention discloses a substituted heteroaromatic ring dihydropyrimidinone derivative with selective inhibition effect on KRAS gene mutation or a pharmaceutically acceptable salt, a stereoisomer, asolvate or a prodrug thereof. The substituted heteroaromatic ring dihydropyrimidinone derivative is shown as a formula (I), and each group in the formula is as defined in the specification. In addition, the invention also discloses a pharmaceutical composition containing the compound and application of the compound in preparation of cancer drugs.
Owner:GENFLEET THERAPEUTICS (SHANGHAI) INC +1
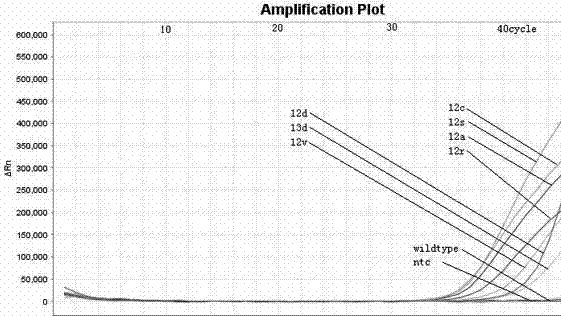
Method and kit for detecting KRAS gene mutations in human colon and rectum cancers
InactiveCN102115792ASignificant progressEasy to detectMicrobiological testing/measurementFluorescenceWild type
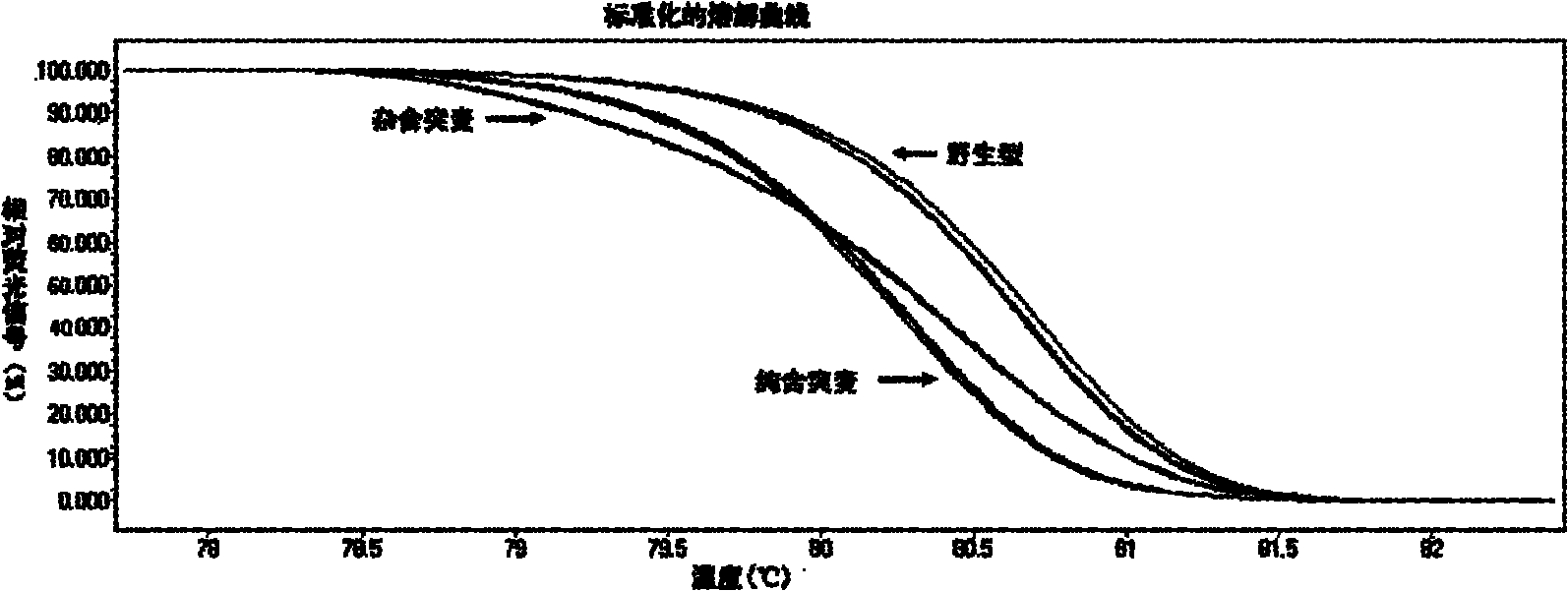
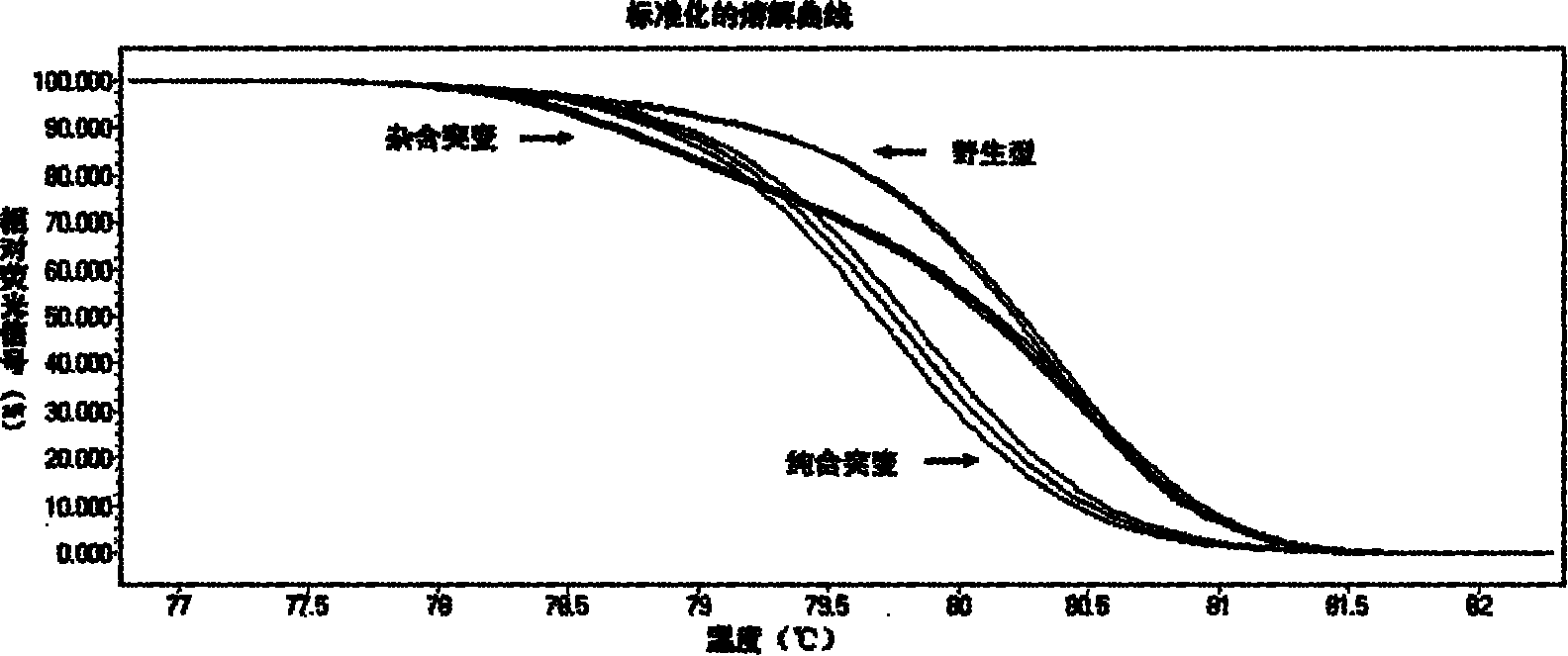
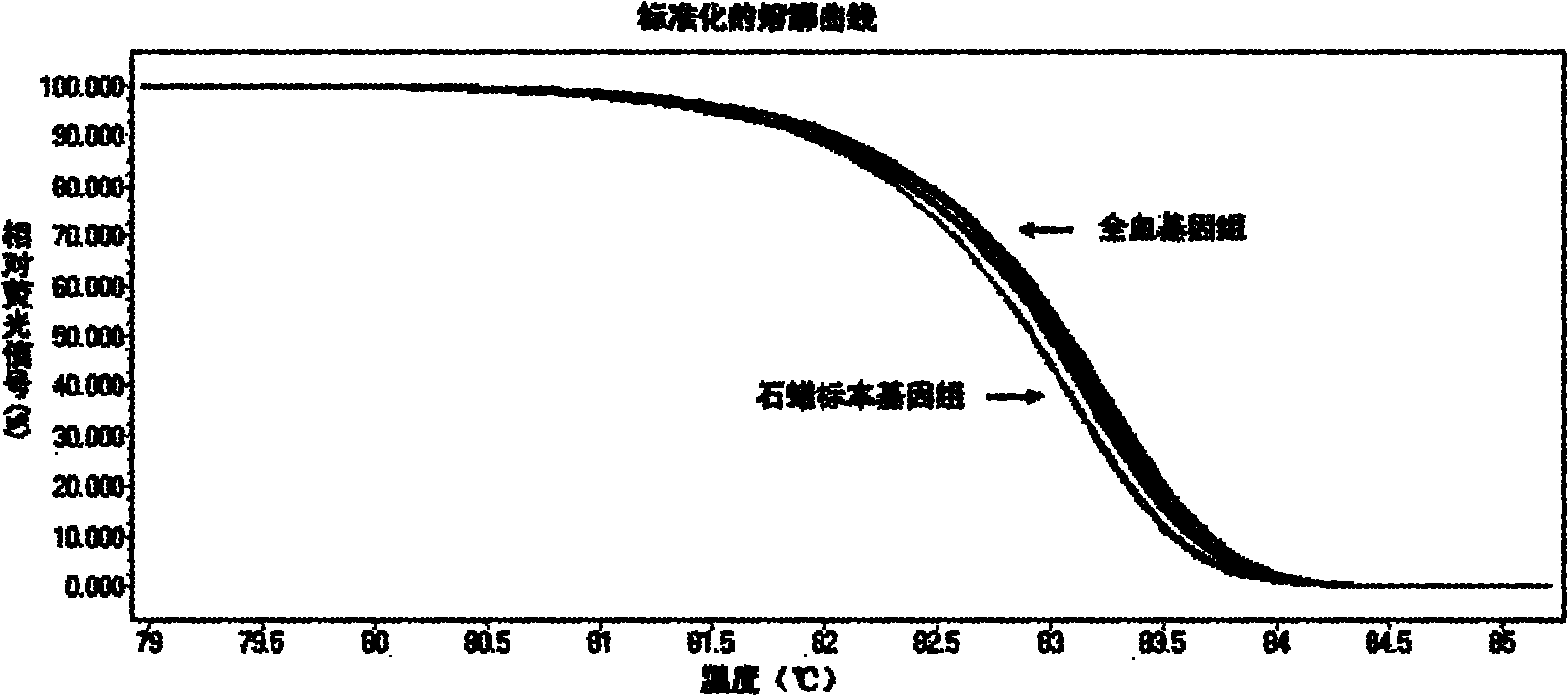
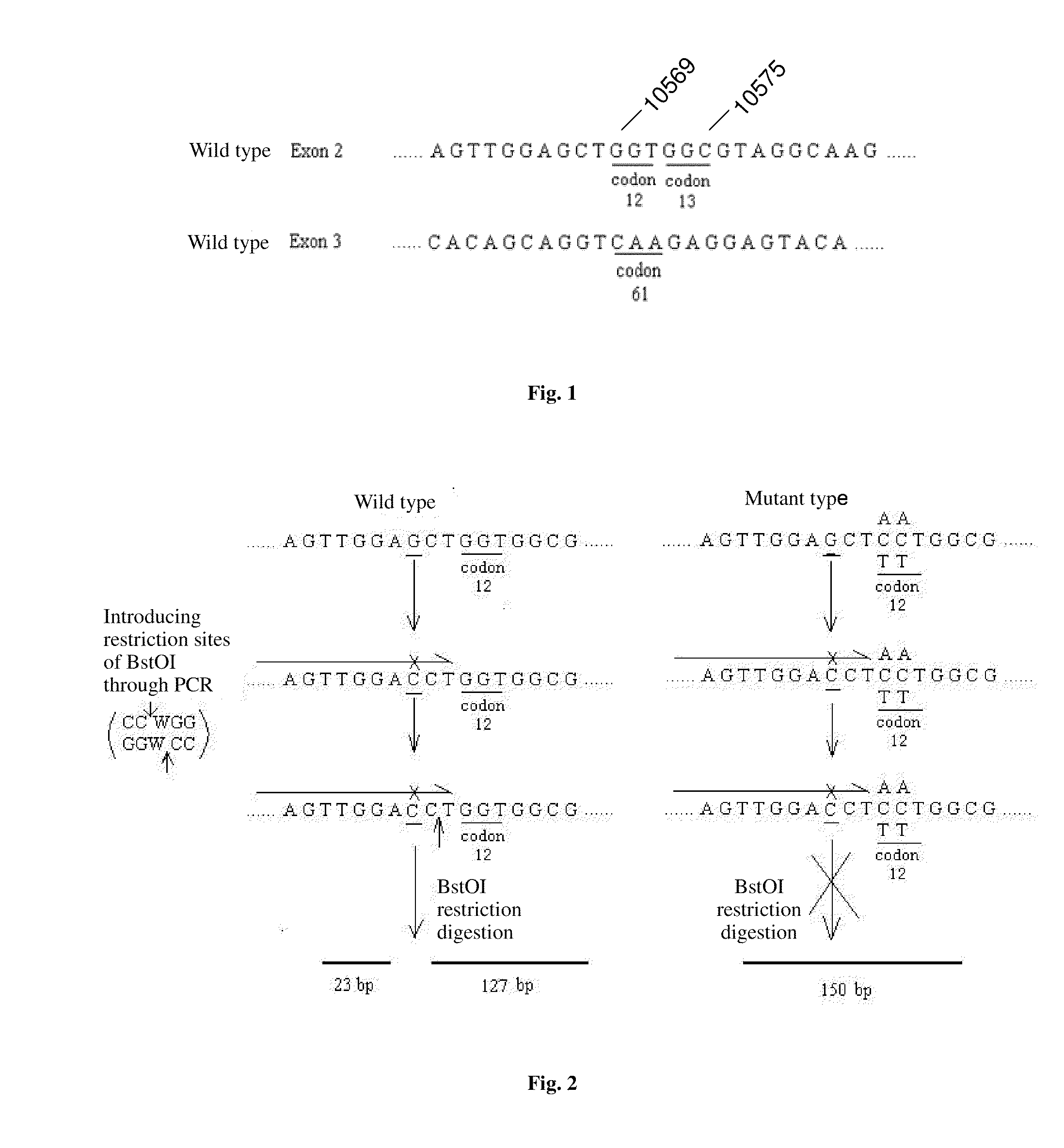
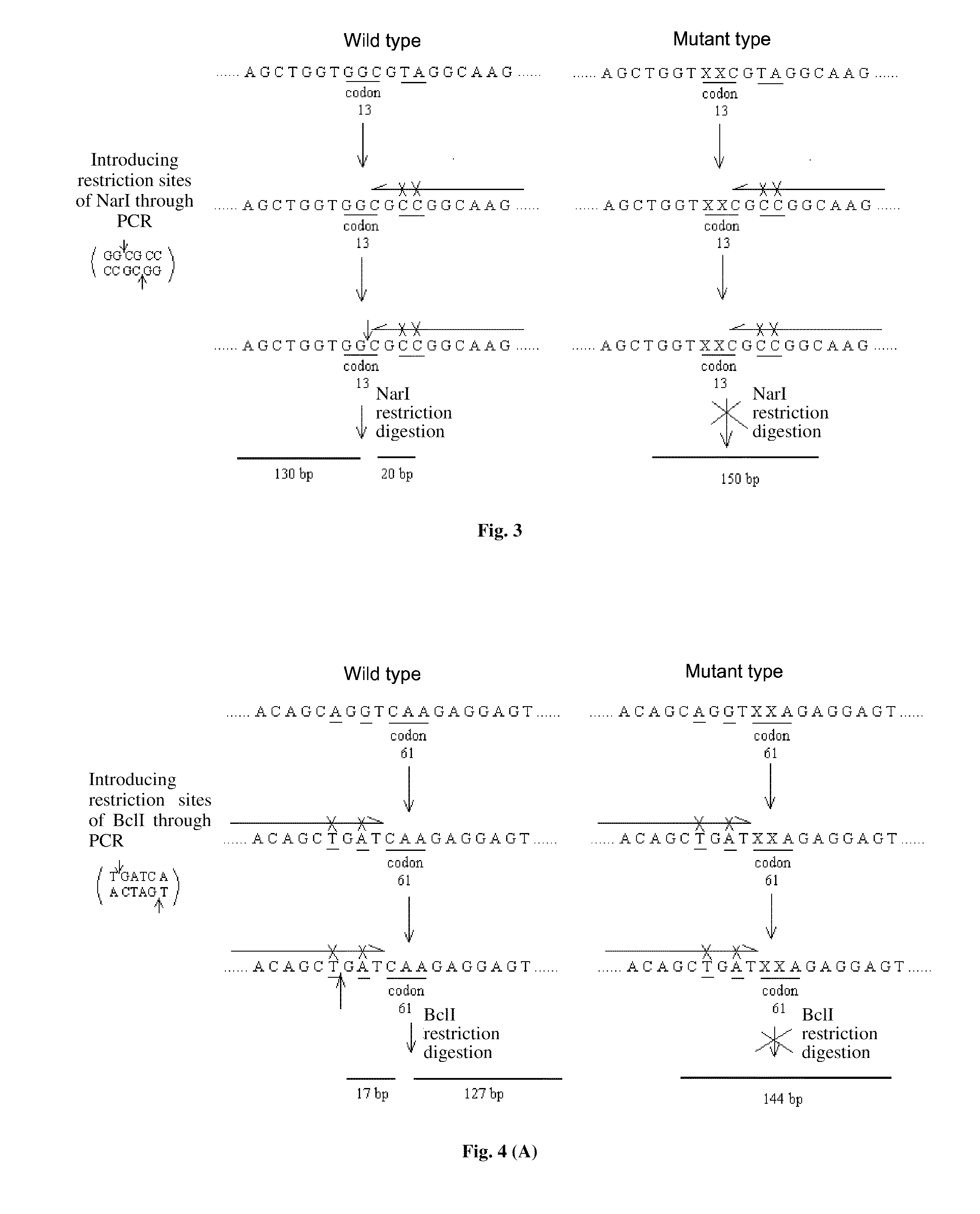
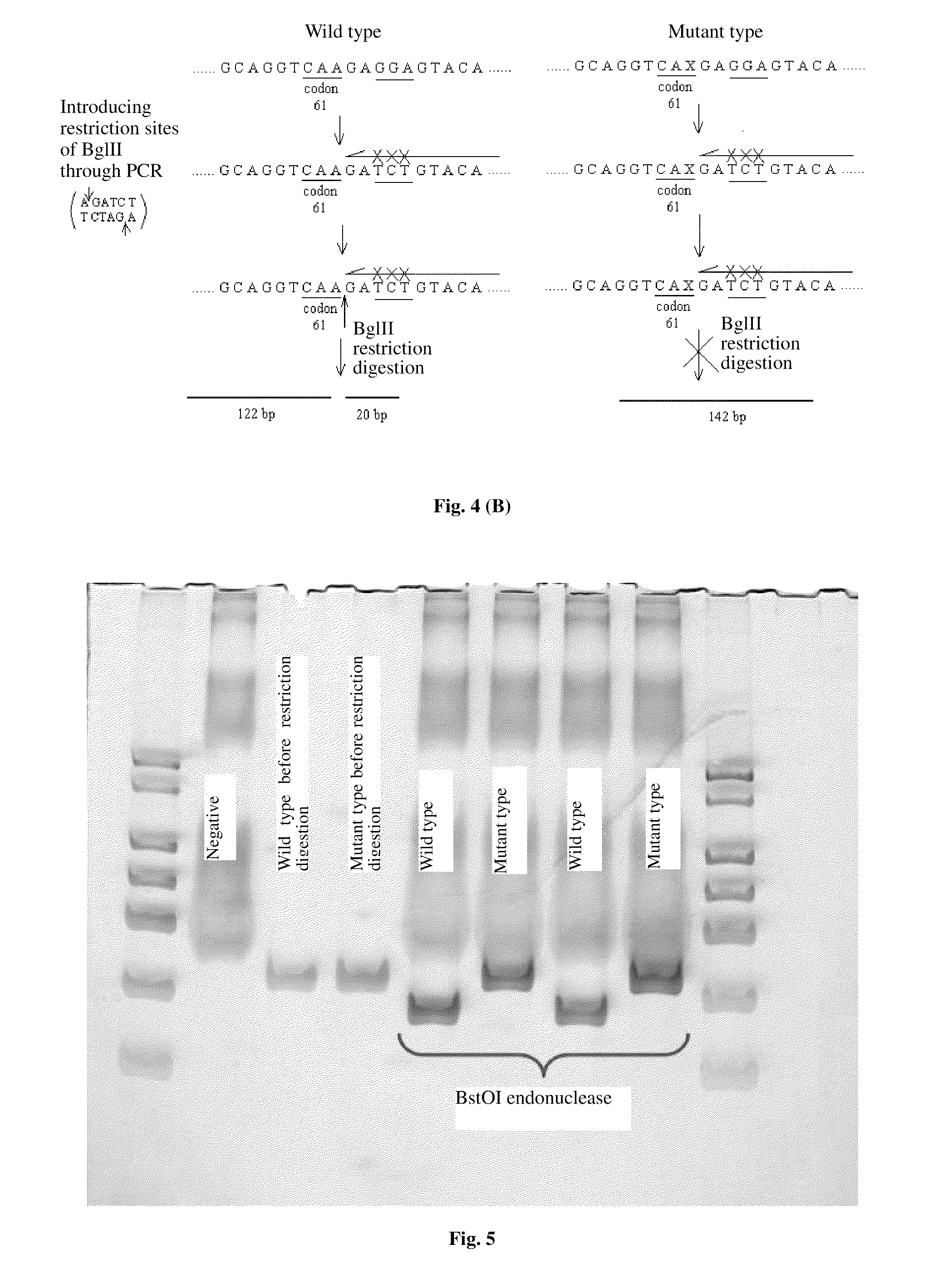
The invention relates to a method and kit for detecting gene mutations, particularly a method and kit for detecting KRAS gene 12, 13 codon mutations. The kit comprises a PCR (Polymerase Chain Reaction) buffer solution, a dNTP (deoxyribonucleotide triphosphate), a DNA (deoxyribonucleic acid) polymerase, a specific primer pair, fluorescent dye, water, a specific probe and a wild-type control. The kit is characterized in that the DNA polymerase is a HotStarTaq DNA polymerase, and the fluorescent dye is SYTO9 fluorescent dye. The method comprises the following steps: (1) acquiring a genome DNA to be analyzed according to a conventional method; (2) carrying out PCR amplification on the genome DNA to obtain a PCR amplified product; after the reaction finishes, carrying out denaturation and renaturation on the PCR product; and (3) carrying out melting curve analysis on the PCR amplified product, and comparing with a melting curve generated by the PCR amplified product of the wild-type genome DNA, wherein the melting curve generated by the PCR amplified product of the mutant genome DNA firstly descends.
Owner:苏州科贝生物技术有限公司
Method for rapidly detecting mutation of KRAS gene
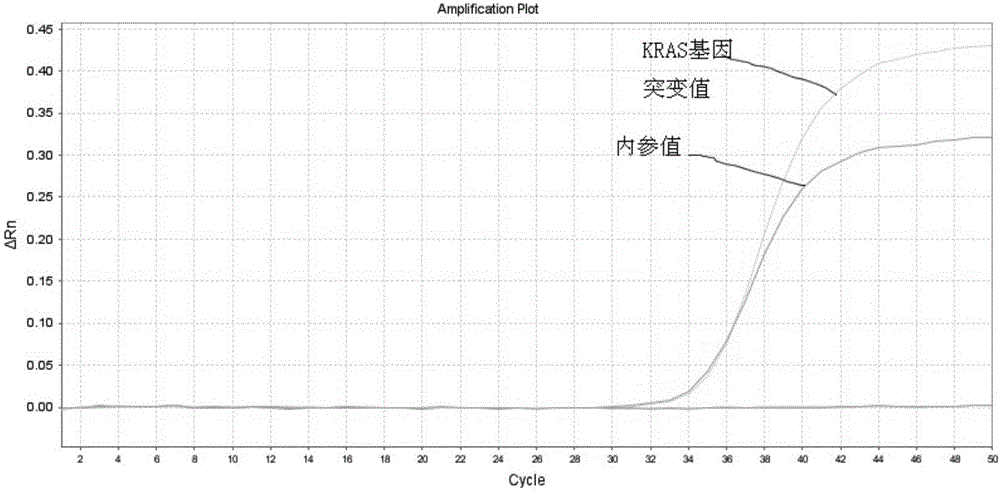
ActiveCN102796817AStrong specificityGuaranteed specific amplificationMicrobiological testing/measurementKras mutationTrue positive rate
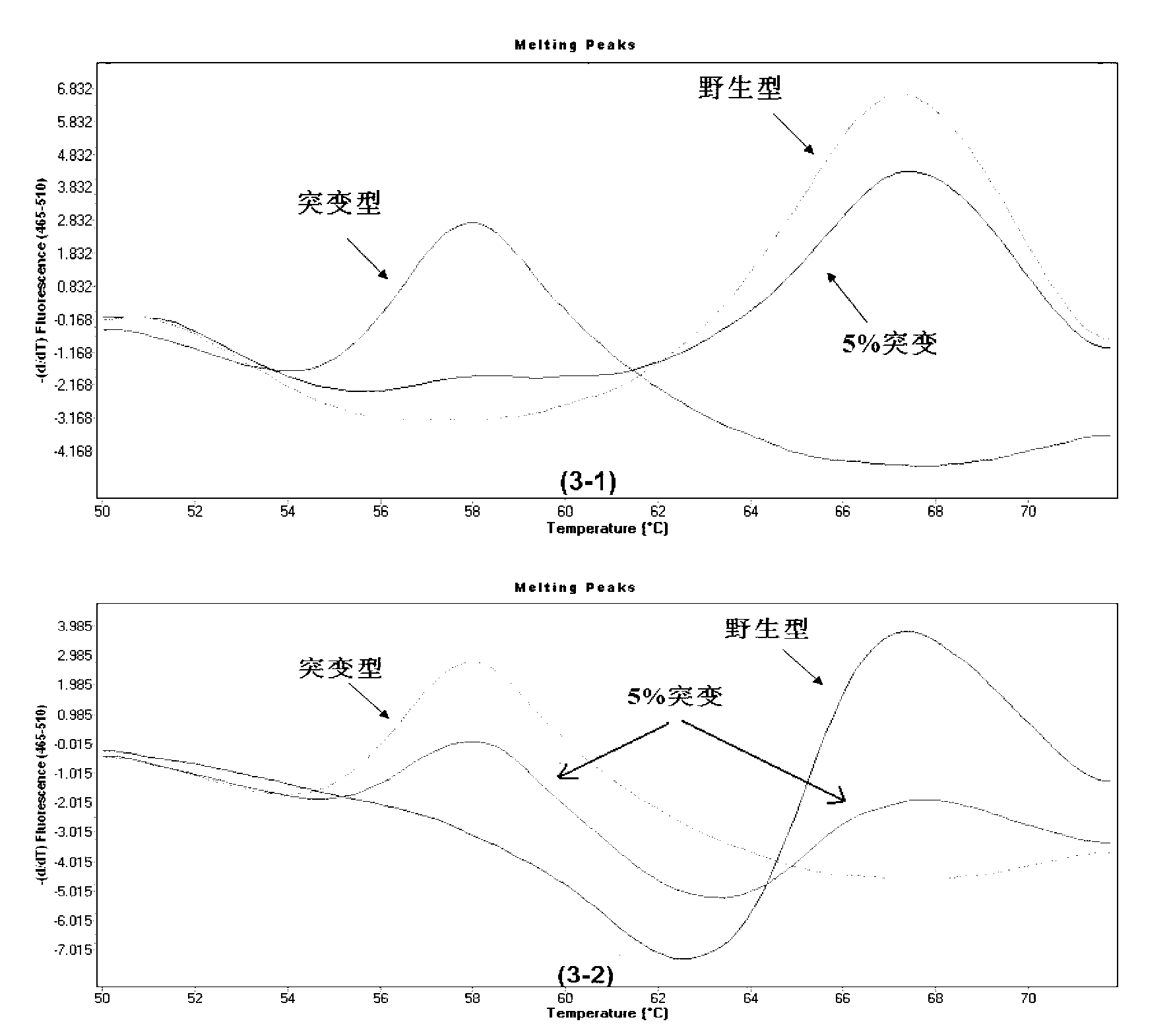
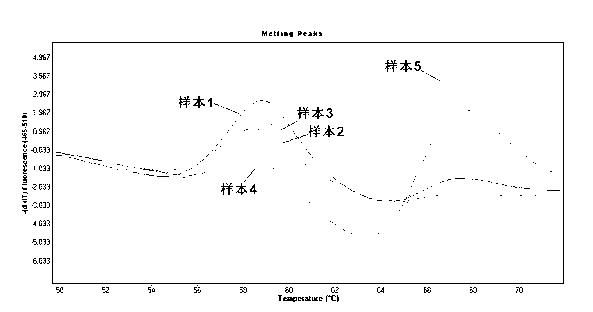
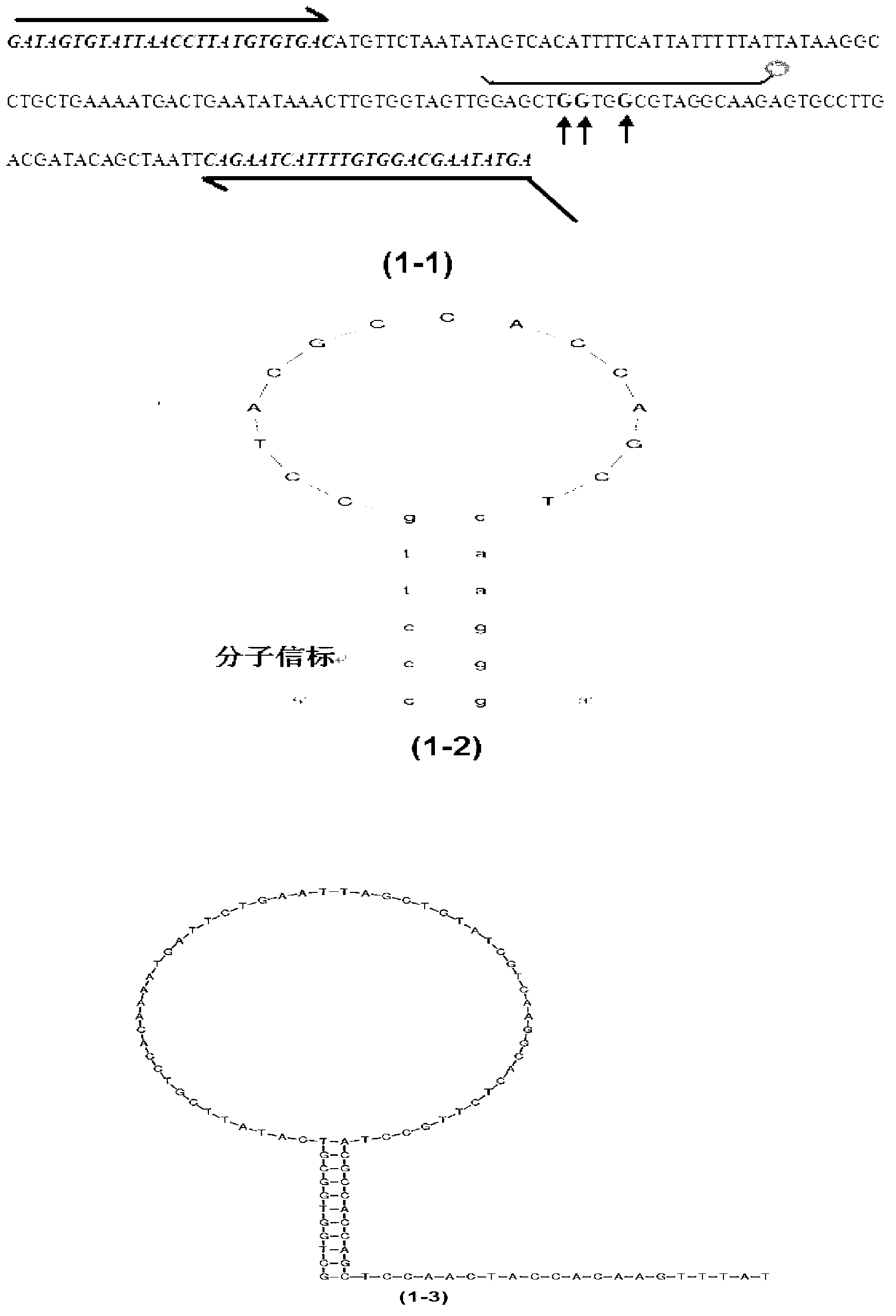
The invention provides a method for rapidly detecting the mutation of KRAS gene, to overcome the disadvantages of low sensitivity and inaccuracy in conventional probe melting curve assay. The method for rapidly detecting the mutation of KRAS gene comprises the following steps: (1) designing and synthesizing molecular beacon probe and template amplification primers; (2) extracting the KRAS genome DNA of a detected sample; (3) preparing a reaction system; (4) performing PCR reaction; performing melting curve analysis after the PCR reaction is finished, and determining if a KRAS mutation occurs in the detected sample through the Tm value of a melting peak. The method combines the molecular beacon probe and extension retardance primers, and uses the DNA polymerase without 5'->3' exonuclease activity to detect KRAS mutation rapidly in vitro, thereby saving consumables simultaneously having the characteristics of high sensitivity and specificity which ensure a more reliable detection result.
Owner:陕西佰美医学检验有限公司
PROBES FOR DETECTING MUTATIONS OF kRas GENE, LIQUICHIP AND DETECTION METHODS THEREOF
InactiveUS20110269640A1Steric hindrance is reducedImprove efficiencySugar derivativesNucleotide librariesMicrosphereMutant allele
Nucleic acid probes for detecting kRas gene mutations, liquid chips and detection methods thereof are provided. The liquid chips for detecting kRas gene mutations comprise: microspheres coupled with wild-type and mutant-type probes, each of which is amino-substituted at 5′-terminal, specific for kRas codons 12, 13 and / or 61, and primers for amplifying the target sequence biotin-labeled at the terminal which are enriched with mutant alleles of kRas codons 12, 13 and / or 61 to be detected. The detection methods are rapid and accurate, and the processes of the methods are easy to perform. The liquid chip can be used to detect mutations of kRas as assistance to early diagnosis of pancreatic cancer, and can be used to prognose efficiency of the molecular targeted therapy to choose right medicine accurately clinically and avoiding economic loss and time loss caused by unnecessary treatment.
Owner:SUREXAM BIO TECH
Test method of KRAS gene mutation for screening and assessing therapeutic effects of molecular targeted agents
InactiveCN101654702AHigh puritySmall amount of sampleMicrobiological testing/measurementMaterial analysis by electric/magnetic meansEGFR Tyrosine Kinase InhibitorsGenomic DNA
The invention relates to a test method of KRAS gene mutation for screening and assessing therapeutic effects of molecular targeted agents, which is characterized by the following specific steps: acquiring serum or tumor tissue samples of testees, extracting genomic DNA, testing a No.2 exon of the KRAS gene, designing corresponding site-specific primers and adopting experimental schemes, reagents and report generation systems for sequence determination and assessing the effectiveness of EGFR tyrosine kinase inhibitors used for treating cancer individuals by simultaneously testing and analyzingwhether mutation exists on the No.2 exon of the individual KRAS gene. The method has the advantage of being used for application of the EGFR tyrosine kinase inhibitors used for treating cancer individuals.
Owner:上海中优医药高科技股份有限公司
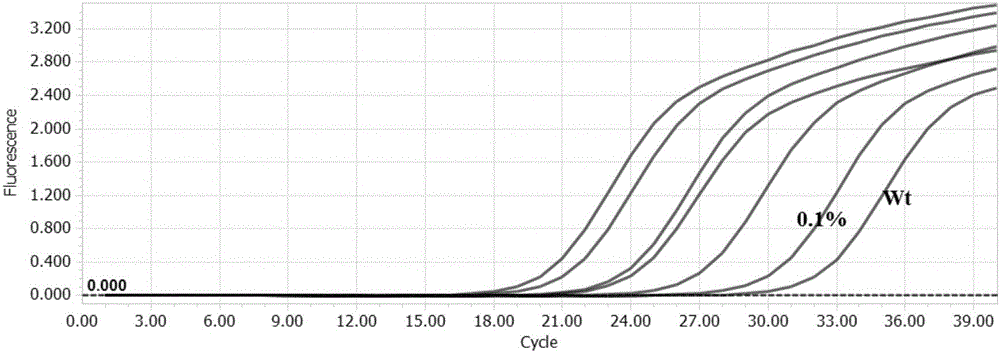
KRAS gene mutation detection kit and application thereof
ActiveCN106148498ADoes not generate non-specific signalStrong specificityMicrobiological testing/measurementFluorescenceBlood plasma
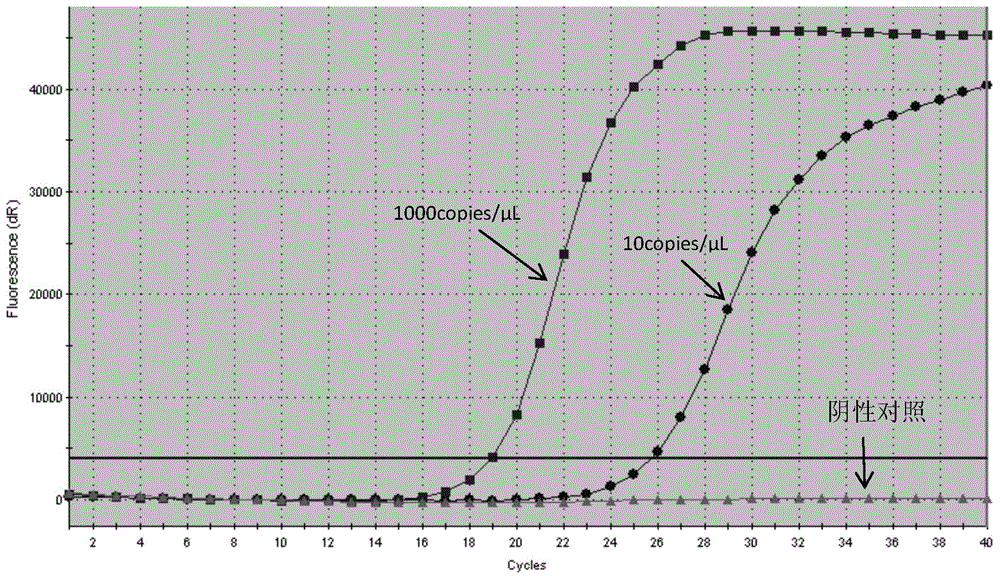
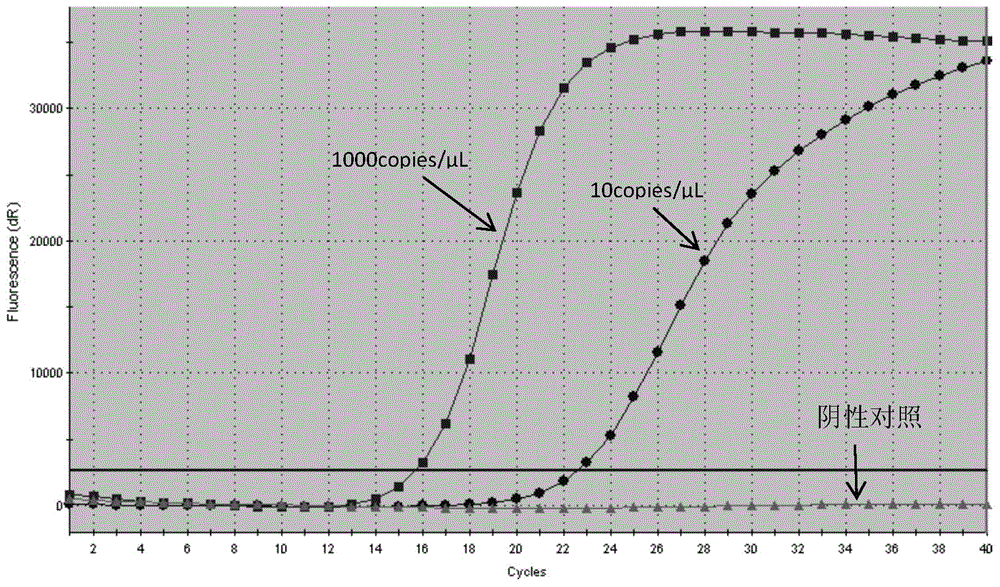
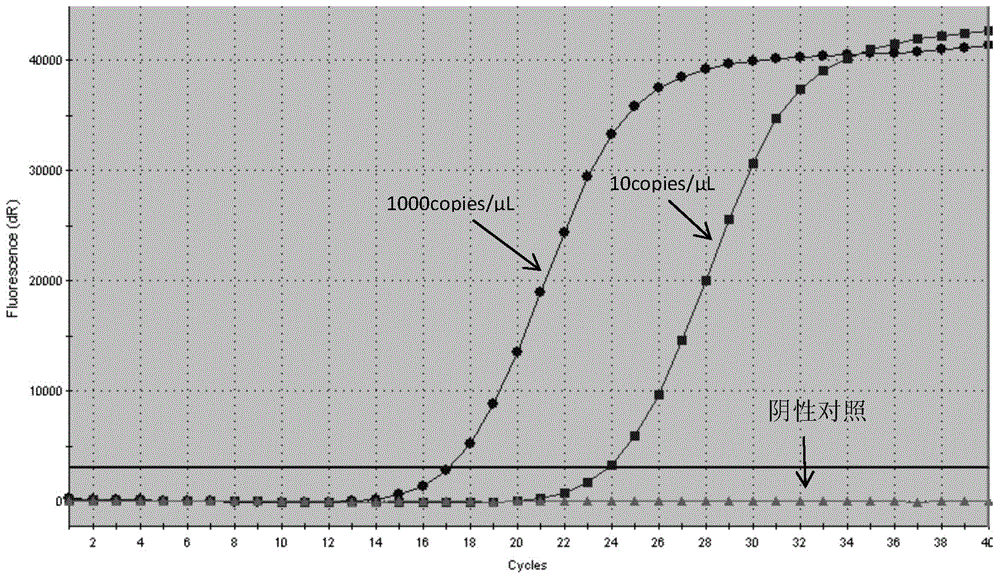
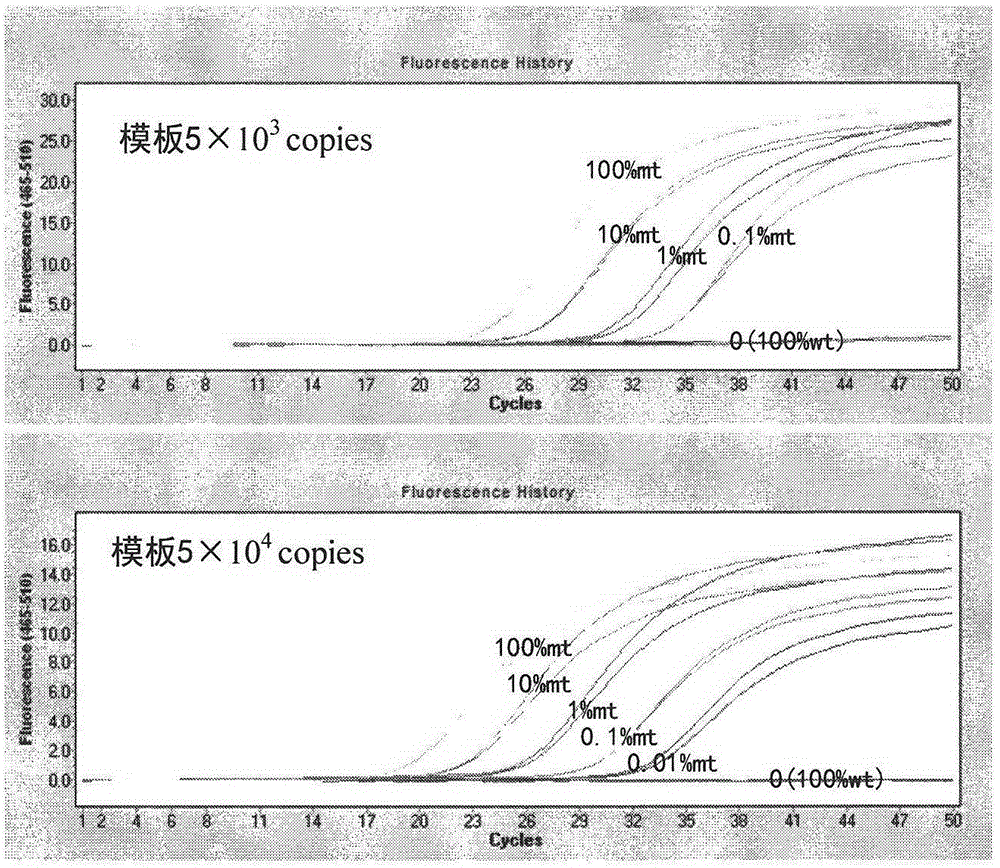
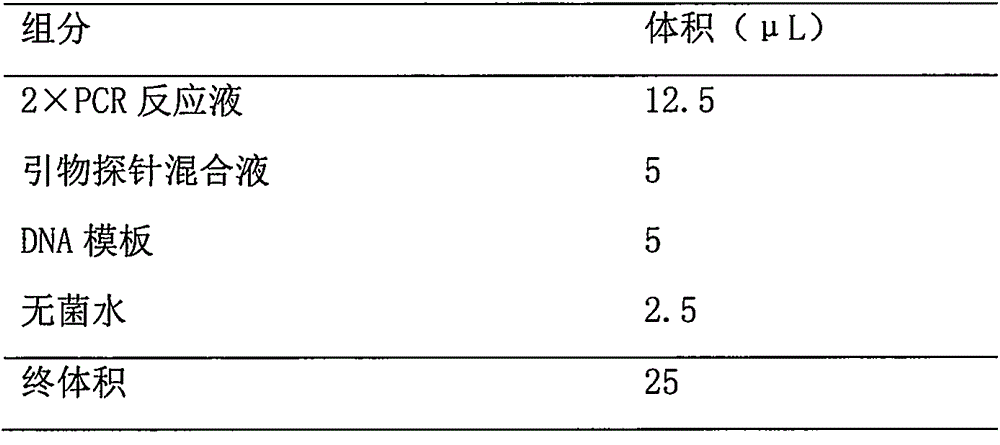
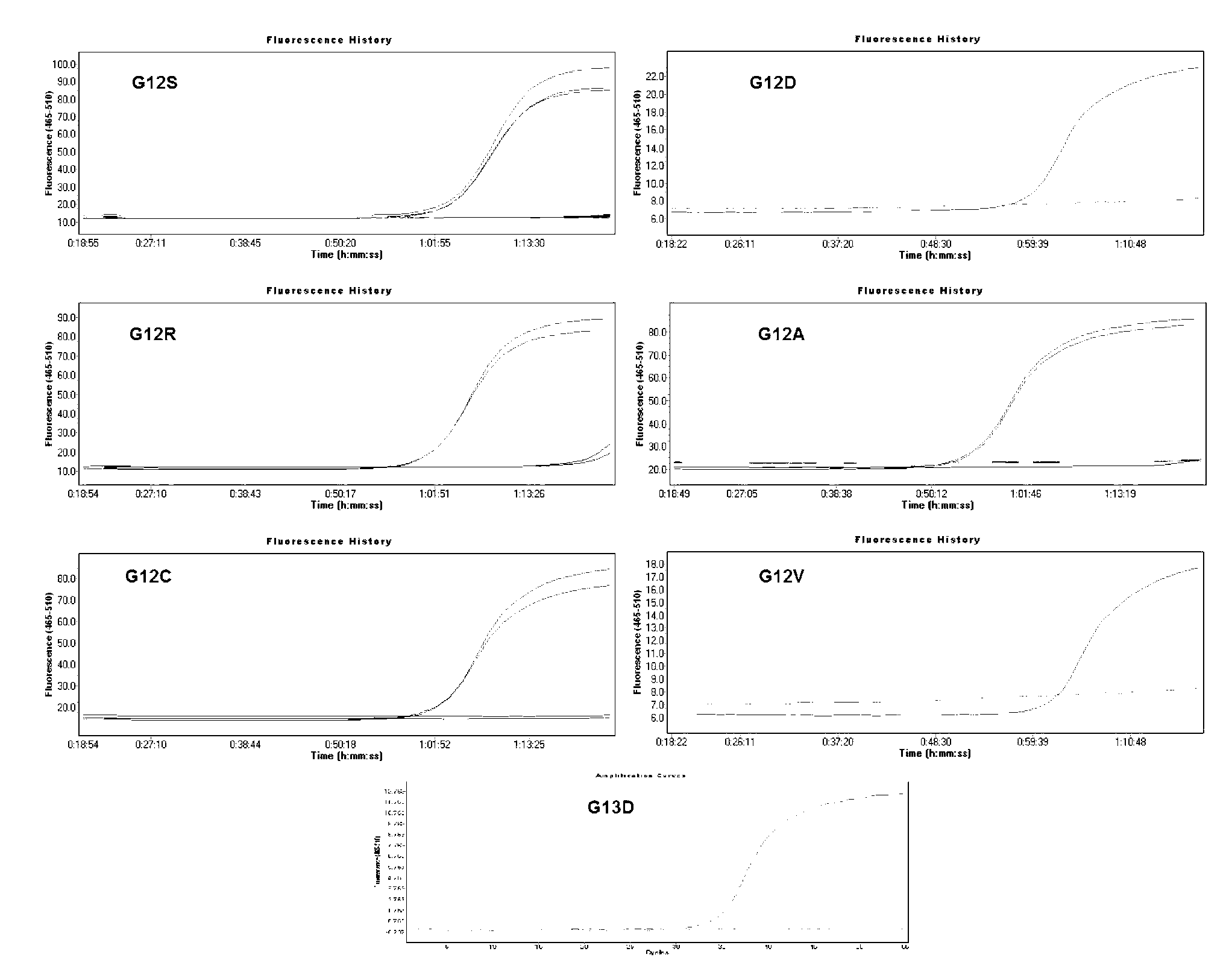
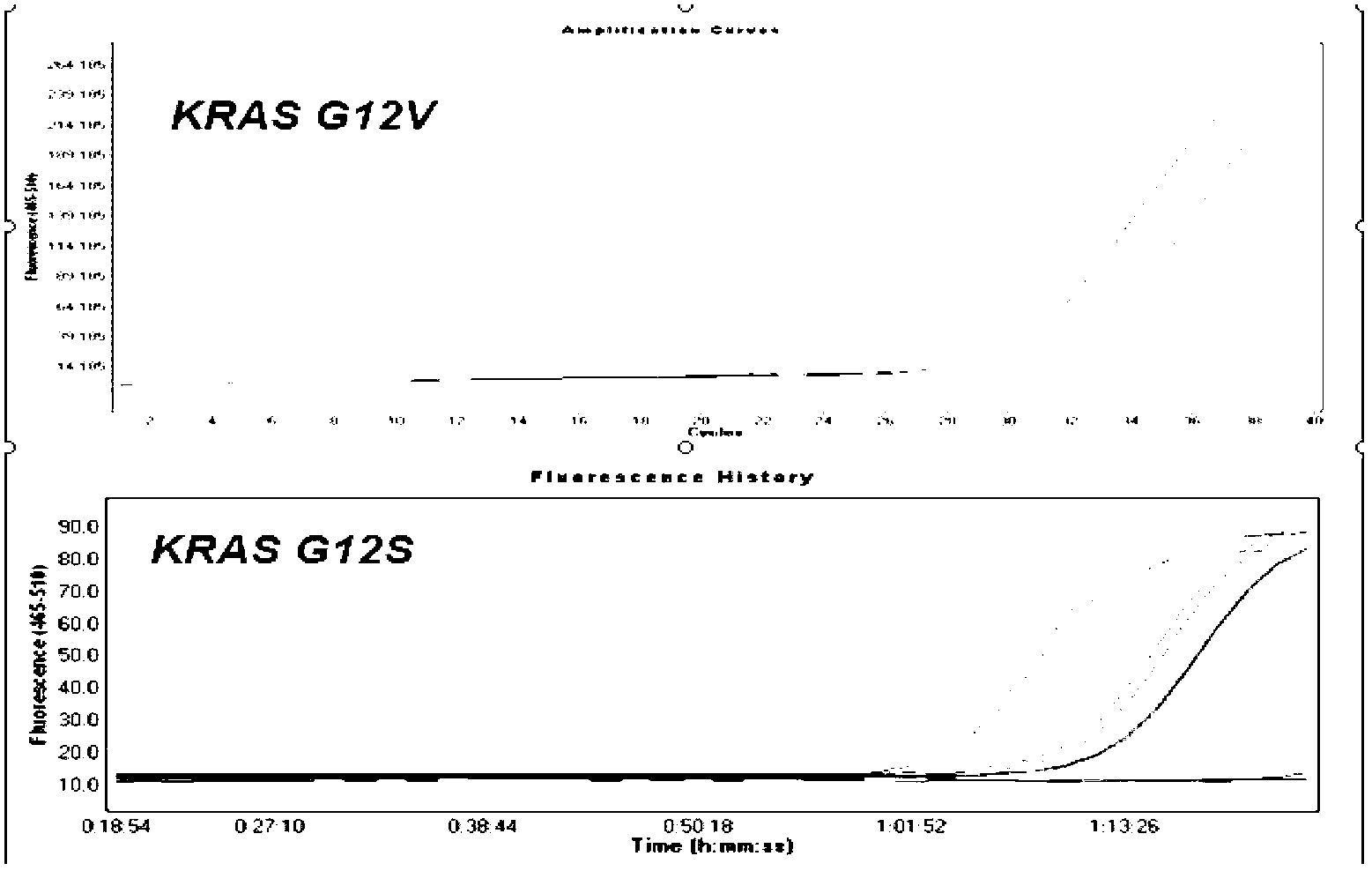
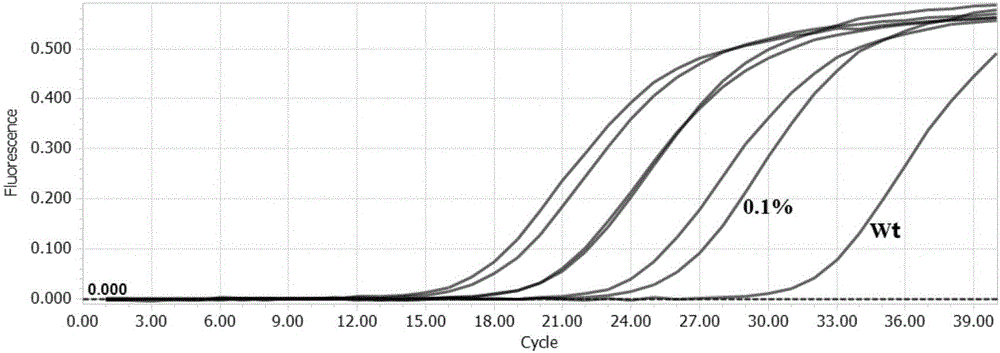
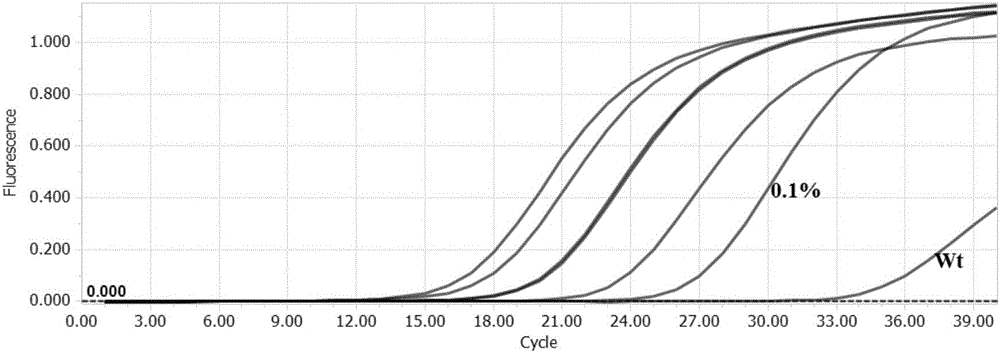
The invention discloses a detection kit and detection method for KRAS gene mutations, and belongs to the technical field of B2D10 currently first developed high-tech industrialization important field guide / biology / novel medical precise diagnosis and treatment equipment. The detection kit is characterized by comprising eight pairs of specifically amplified primers for KRAS gene mutation loci, two efficient blocking probes of a wild sequence and two KRAS gene specific TaqMan fluorescent probes. The kit can detects specimens of which the number of the mutant copies is as low as 5 to 10, and the mutation content is as low as 0.1%. The detection kit can detect five gene mutations of a KRAS gene simultaneously, the sensitivity is high, operation is easy, detection is low in price, the clinical application range is wide, samples can adopt fresh pathological tissue or paraffin-embedded tissue or a pleural fluid or serum or plasma, the detection speed is high, and only 90 minutes are needed for completing the detection process.
Owner:上海济远生物科技有限公司
Method for performing in-vitro accurate test on KRAS gene mutation
ActiveCN102796816AStrong specificityGuaranteed specificityMicrobiological testing/measurementForward primer3-deoxyribose
The invention provides a method for performing in-vitro accurate test on KRAS gene mutation, and the method is used for overcoming the defects that the conventional allele-specific polymerase chain reaction (PCR) method has insufficient specificity and false positive phenomenon. The method for performing the in-vitro accurate test on the KRAS gene mutation comprises the following steps of: (1) designing and synthetizing allele specific primers which comprise the allele specific primers espectively designed for 7 types of mutation of a typical KRAS gene exon 2 and are utilized as forward primers, wherein extension retardant primers are utilized as common downstream primers; (2) extracting the KRAS genomic deoxyribose nucleic acid (DNA) of a test sample, and additionally preparing the wild type genomic DNA (as wild type template); and (3) carrying out the real-time fluorescent PCR reaction. The test method has the advantages of strong specificity, high sensitivity, material conservation and time-saving property.
Owner:陕西佰美医学检验有限公司
LNA probe for detecting KRAS gene mutation and detection method
PendingCN106319074AStrong specificityRealize detectionMicrobiological testing/measurementDNA/RNA fragmentationHybrid materialLocked nucleic acid
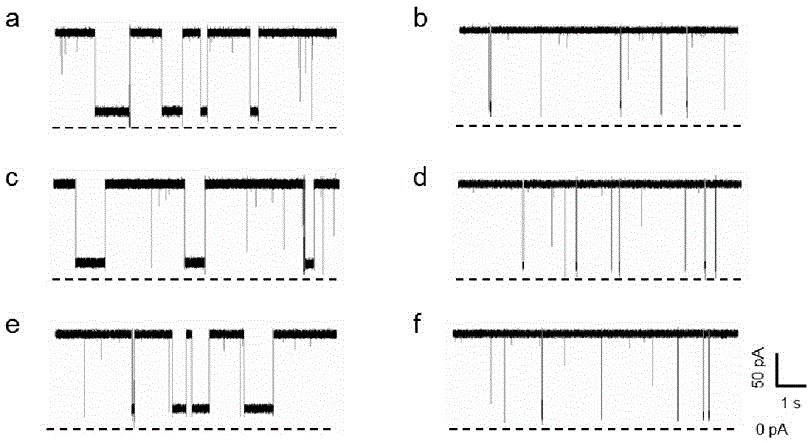
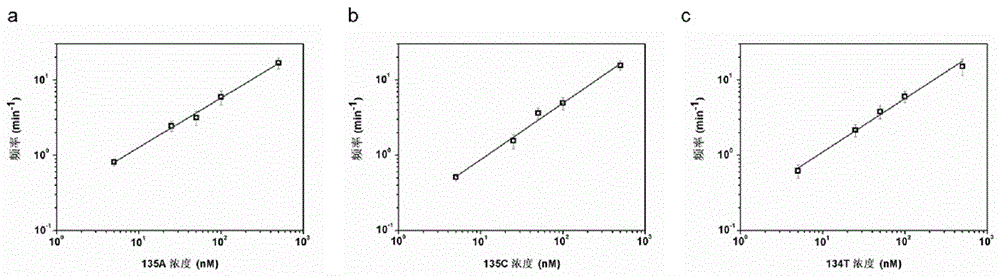
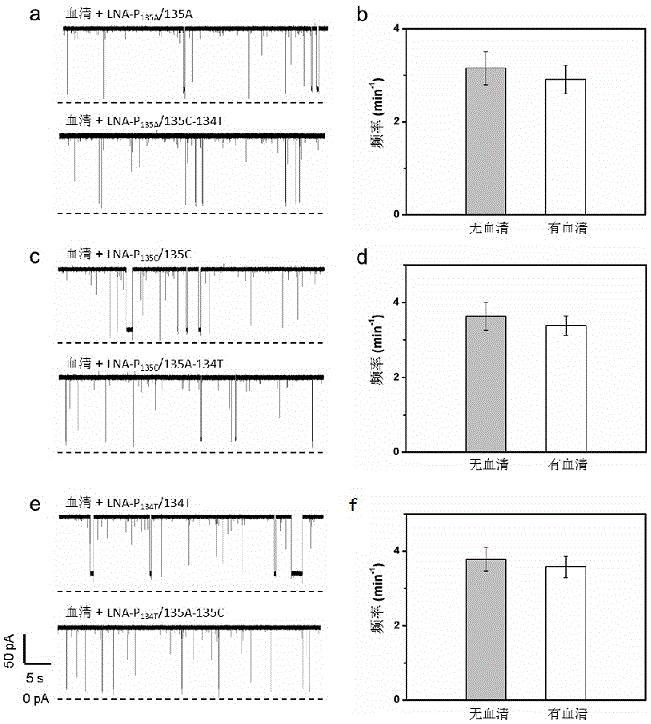
The invention relates to the technical field of gene detection, in particular to an LNA (Locked Nucleic Acid) probe, which is represented by sequences 1, 2, and 3 in a sequence table, for detecting KRAS gene mutation. A detection method for the KRAS gene mutation comprises the following steps: constructing a nanopore sensor by natural alpha-hemolysin, hybridizing the LNA probe with a gene to be detected to obtain a hybrid material, and generating a blocking signal through a nano channel of the nanopore sensor. By the hybridization of the LNA-modified probe and a KRAS mutant gene and detection of the alpha-hemolysin nanopore sensor, the specificity of target gene detection is greatly improved, and a concentration relation between the frequency of the signal and the KRAS mutant gene can be constructed to quantitatively analyze a target; the LNA probe has the advantages of simplicity, quickness and high sensitivity; DNA amplification is not needed; the operation is simple and specific; the LNA probe is high in applicability.
Owner:LINYI UNIVERSITY
Human multi-gene mutation detection kit based on high-throughput sequencing method
InactiveCN107164513AHigh sensitivityStrong specificityMicrobiological testing/measurementLibrary creationMutation detectionEGFR Gene Mutation
The invention belongs to the technical field of biology and particularly relates to a human multi-gene mutation detection kit based on a high-throughput sequencing method and an application thereof. The kit includes a KRAS gene mutation detection primer pair, an EGFR gene mutation detection primer pair and an ALK gene fusion detection primer pair. The kit can simultaneously detect DNA mutation and RNA fusion, is high in sensitivity and low in detection limit, is high in specificity, has good repeatability, and can reach 100% in both accuracy and positive coincident rate.
Owner:SINGLERA GENOMICS (SHANGHAI) LTD
KRAS gene mutation detection quantification standard product as well as preparation method and valuing method thereof
ActiveCN107663532AEasy to operateImprove efficiencyMicrobiological testing/measurementHuman DNA sequencingWild type
The invention discloses a KRAS gene mutation detection quantification standard product as well as a preparation method and a valuing method thereof. The preparation method comprises the following steps: performing PCR (Polymerase Chain Reaction) amplification by taking seven common mutant cell line DNAs (Deoxyribonucleic Acids) containing KRAS genes as a template to obtain a PCR product, and performing Sanger sequencing verification; mixing the PCR product with wild type human genome DNAs being subjected to ultrasonic fragmentation according to a certain proportion through balance weighing toobtain a quantification standard product for seven mutations of a KRAS gene. The invention provides a preparation method of a quantification standard product. Fragmented wild type human genome DNAs are taken as background DNAs, so that the background of an actual detection sample can be better simulated. A valuing method of the quantification standard product has high precision and high accuracy,and is independent of a DNA standard product; a measurement result can be traced to the international basic unit (mass), so that the reliability and traceability of the measurement result are ensured.The quantification standard product can be applied to method verification and quality control of a KRAS gene mutation detection method.
Owner:NAT INST OF METROLOGY CHINA
KRAS gene mutation detection method and kit
ActiveCN104450943ASimplify the experimental stepsImprove experimental efficiencyMicrobiological testing/measurementGene engineeringReaction system
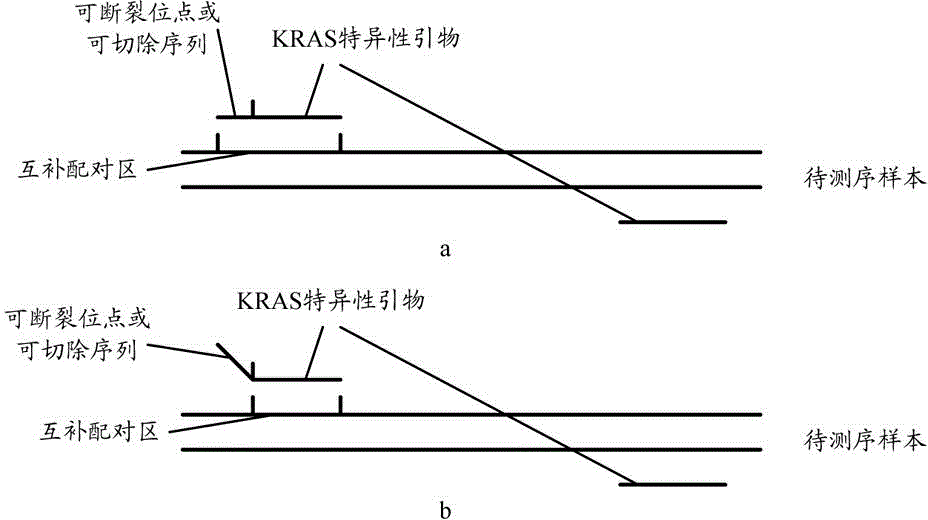
The invention relates to the field of gene engineering and molecular biology, and provides a KRAS gene mutation detection method and a kit. The method comprises the following steps: A, amplifying a target region in a to-be-sequenced sample by using a KRAS specific primer to obtain an amplification product, wherein at least one PCR (Polymerase Chain Reaction) primer in the KRAS specific primer for amplifying each target region comprises a breakable site or a cuttable sequence; B, adding the amplification product into a cutting-connection reaction system to perform cutting and connection so as to connect a joint I and a joint II to the two ends of the amplification products to obtain library molecules; C, performing monomolecular amplification on the library molecules comprising the joint I and the joint II to obtain a monomolecular amplification product; D, performing high-throughput sequencing on the monomolecular amplification product to obtain sequence information on the target region. According to the method and the kit, the experiment steps can be simplified and the detection efficiency is improved.
Owner:SHENGZHEN CHINA GENE TECH COMPANY
Primer probe for detecting mutation of KRAS gene and kit thereof
InactiveCN107022619AStrong specificityTo achieve the purpose of specific bindingMicrobiological testing/measurementDNA/RNA fragmentationTrue positive rateMultiplex pcrs
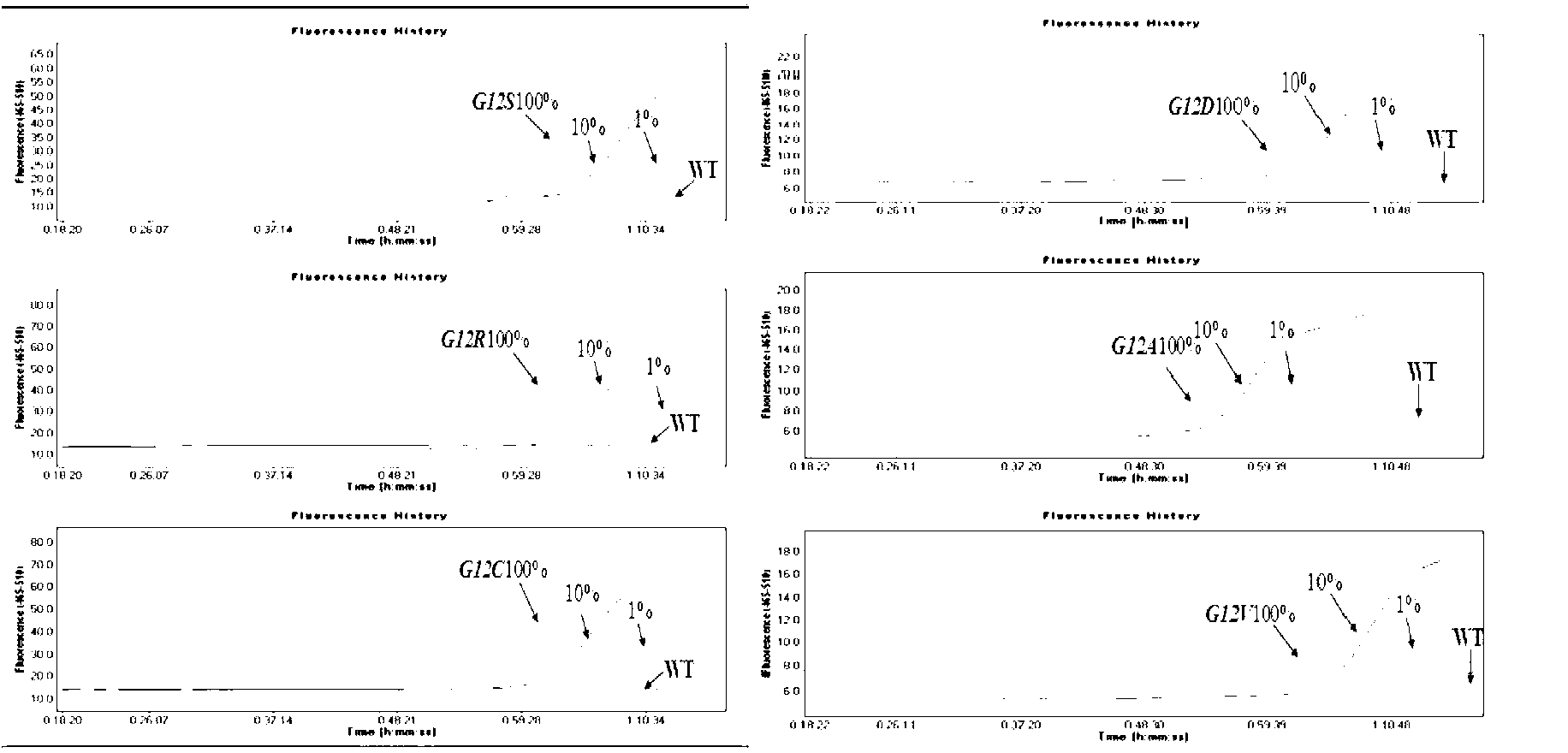
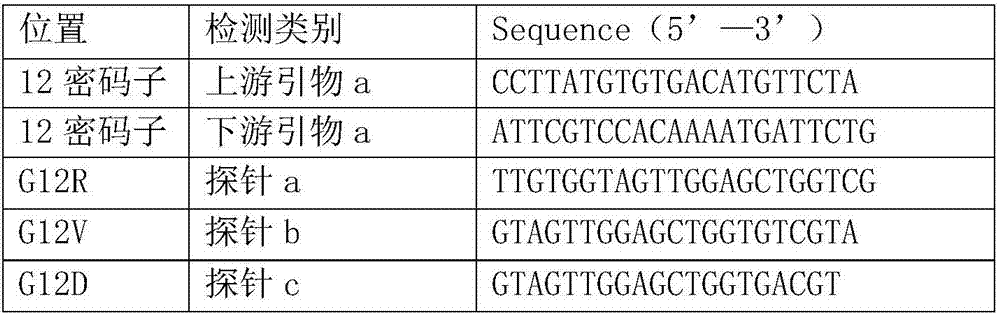
The invention relates to a primer probe for detecting mutation of a KRAS gene and a kit thereof. The kit comprises digital PCR (polymerase chain reaction) premix liquid, a liquid droplet stabilizer and primer probe mixing liquid, wherein the digital PCR premix liquid is used for preparing digital PCR reaction liquid; the primer probe mixing liquid comprises upstream and downstream primers A for detecting a 12th codon, a mutation probe A for a G12R site of the 12th codon, a mutation probe B for a G12V site of the 12th codon, a mutation probe C for a G12D site of the 12th codon, a mutation probe D for a G12A site of the 12th codon, a mutation probe E for a G12S site of the 12th codon, and a mutation probe F for a G12C site of the 12th codon; upstream and downstream primers B for detecting a 13th codon, and a mutation probe G for a G13D site of the 13th codon; upstream and downstream primers C for detecting a 61st codon, and a mutation probe H for a Q61L site of the 61st codon. The kit for detecting the mutation of the KRAS gene has the advantages that the sensitivity and specificity are high, the sample demand amount is small, and multiplex PCR detection can be rapidly and accurately performed.
Owner:上海赛安生物医药科技股份有限公司
Colorectal cancer auxiliary diagnosis kit for fecal nucleic acid detection and use method thereof
ActiveCN110904228AHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationFusobacteriaLogistische regression
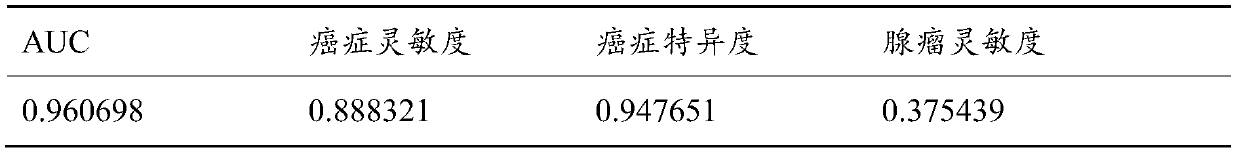
The invention discloses a colorectal cancer auxiliary diagnosis kit for fecal nucleic acid detection and a use method of the colorectal cancer auxiliary diagnosis kit. The kit comprises a KRAS gene mutation detection reagent, a fusobacterium nucleatum detection reagent, a DNA sulfite conversion reagent and a Septin9, SDC2, HOXA11 and NDRG4 gene methylation detection reagent. The use method comprises the following steps: collecting an excrement sample, and then completing DNA purification, KRAS gene mutation detection, fusobacterium nucleatum abundance detection and Septin9, SDC2, HOXA11 and NDRG4 gene methylation detection of the sample by using reagents in the kit; and calculating the obtained data through a logistic regression model, and performing result evaluation and analysis. The colorectal cancer detection sensitivity reaches 89% or above, the specificity reaches 94% or so, high-sensitivity and high-specificity colorectal cancer detection can be achieved, and doctors can be helped to guide follow-up treatment medication of patients.
Owner:GENETALKS BIO TECH CHANGSHA CO LTD
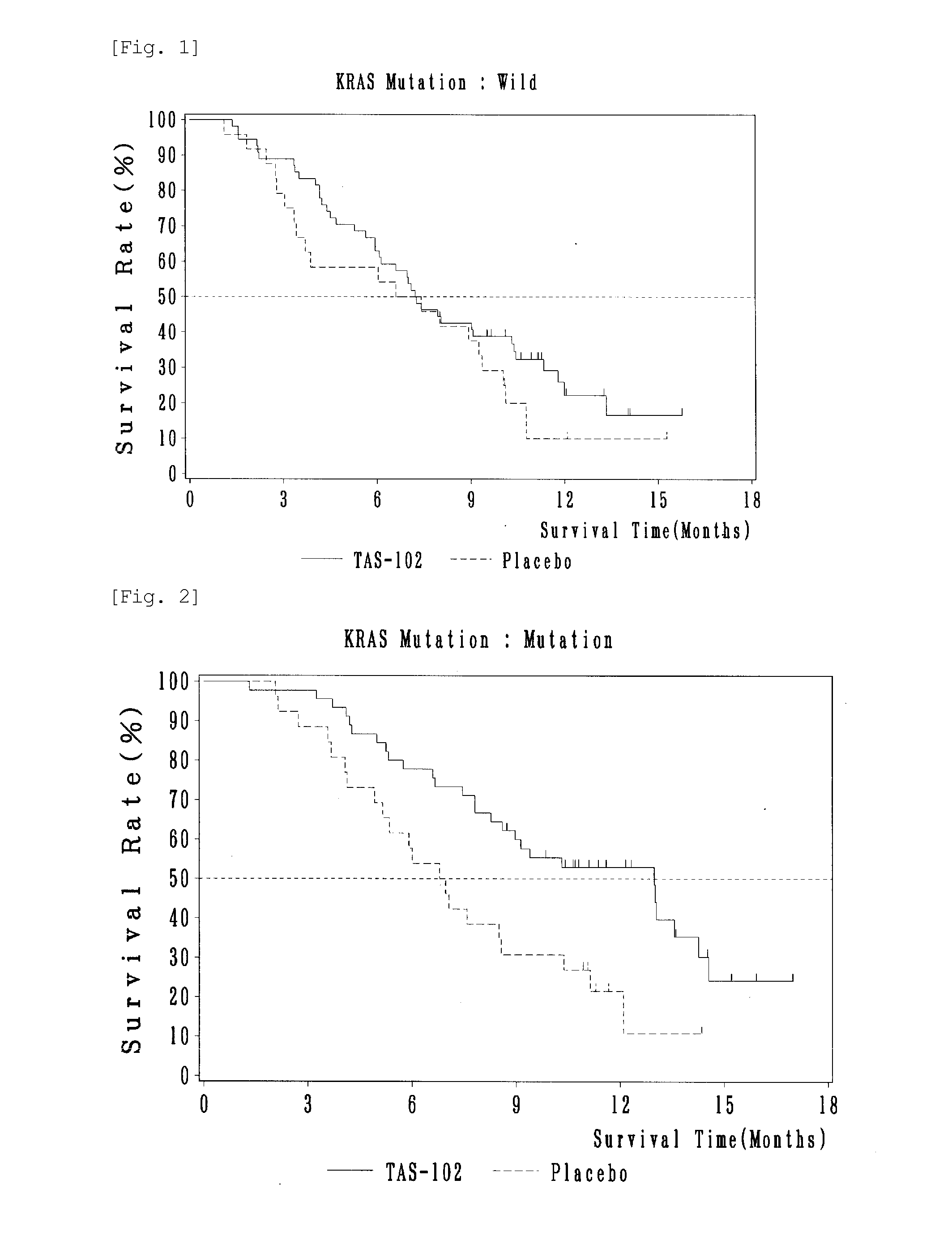
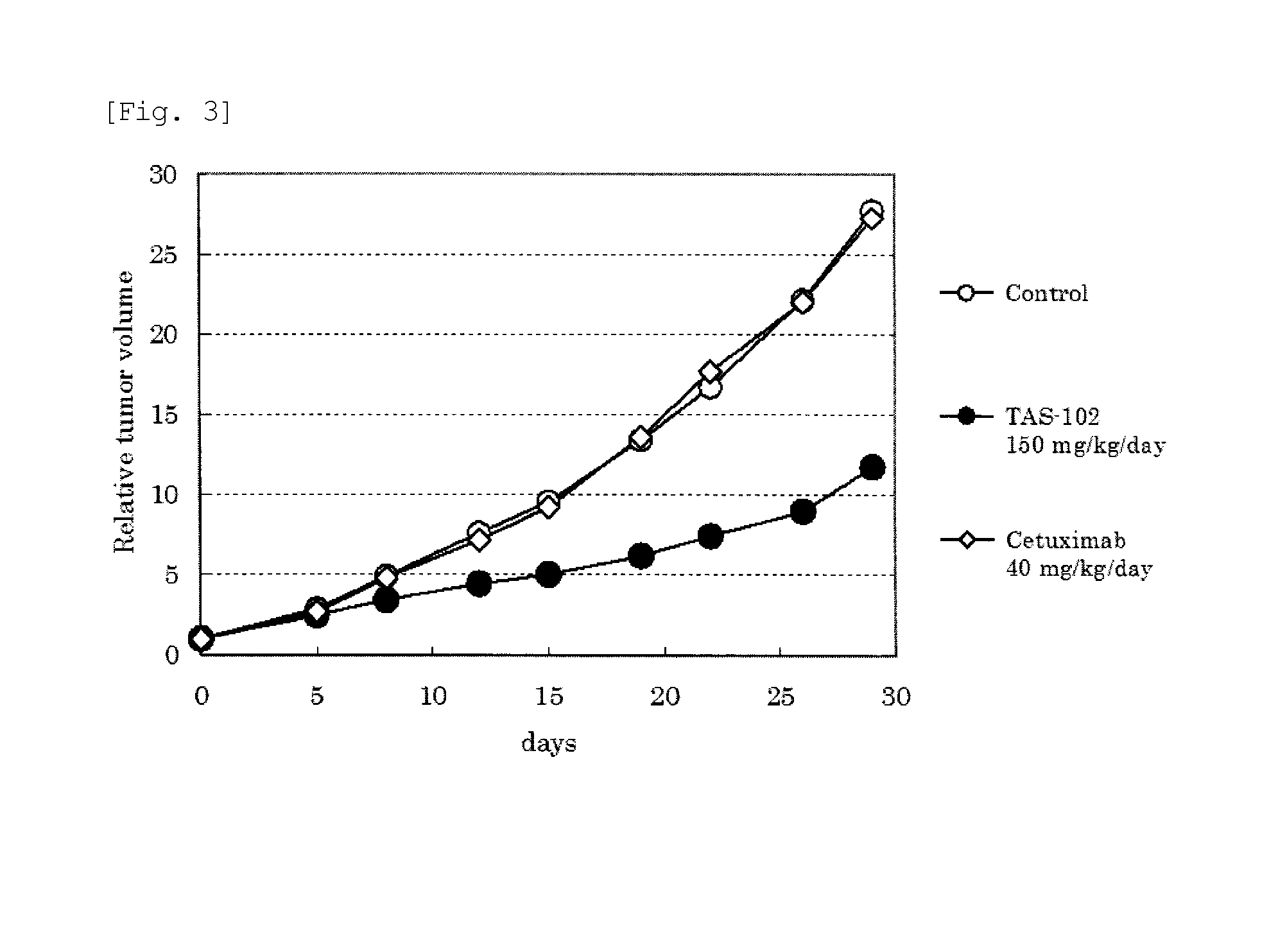
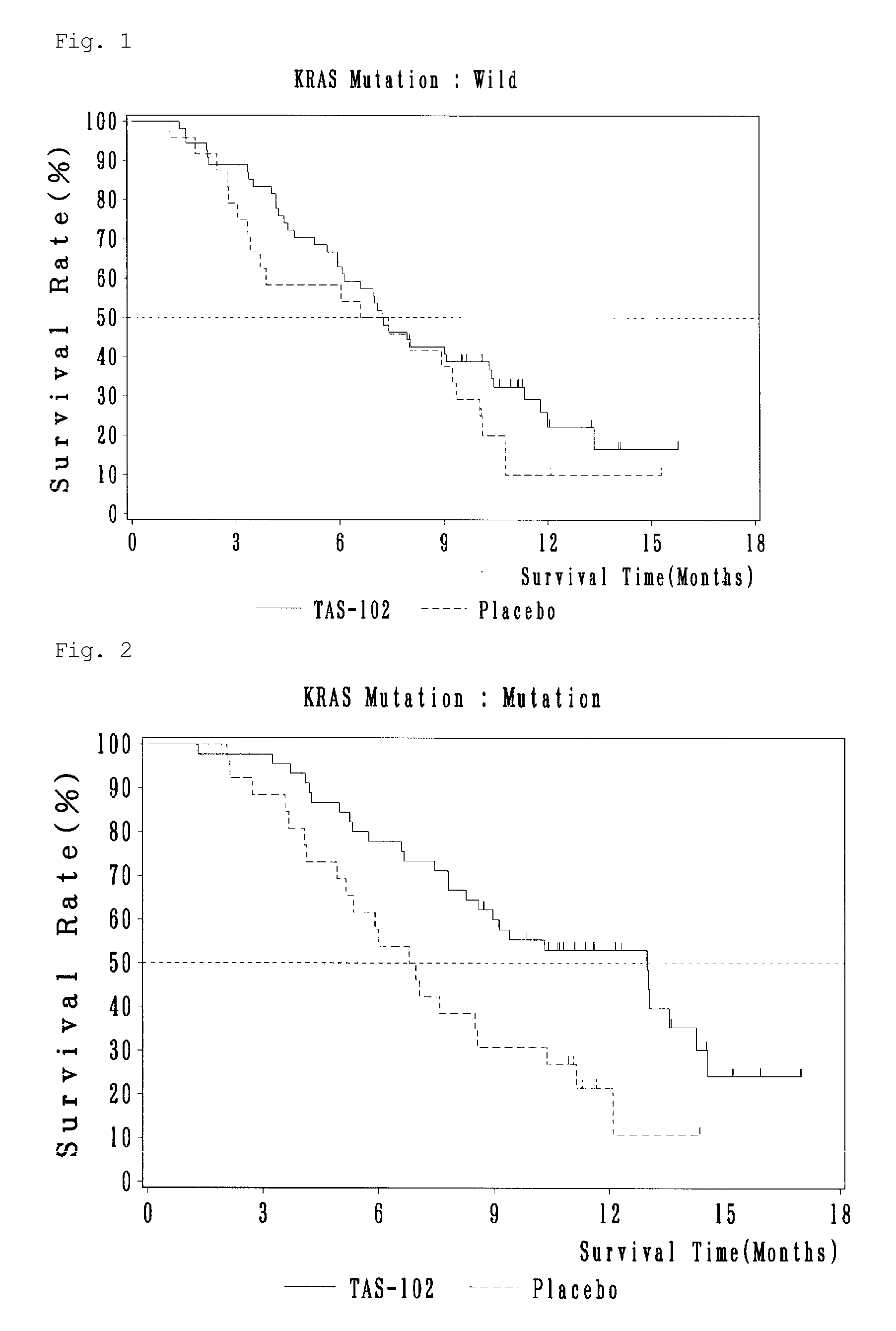
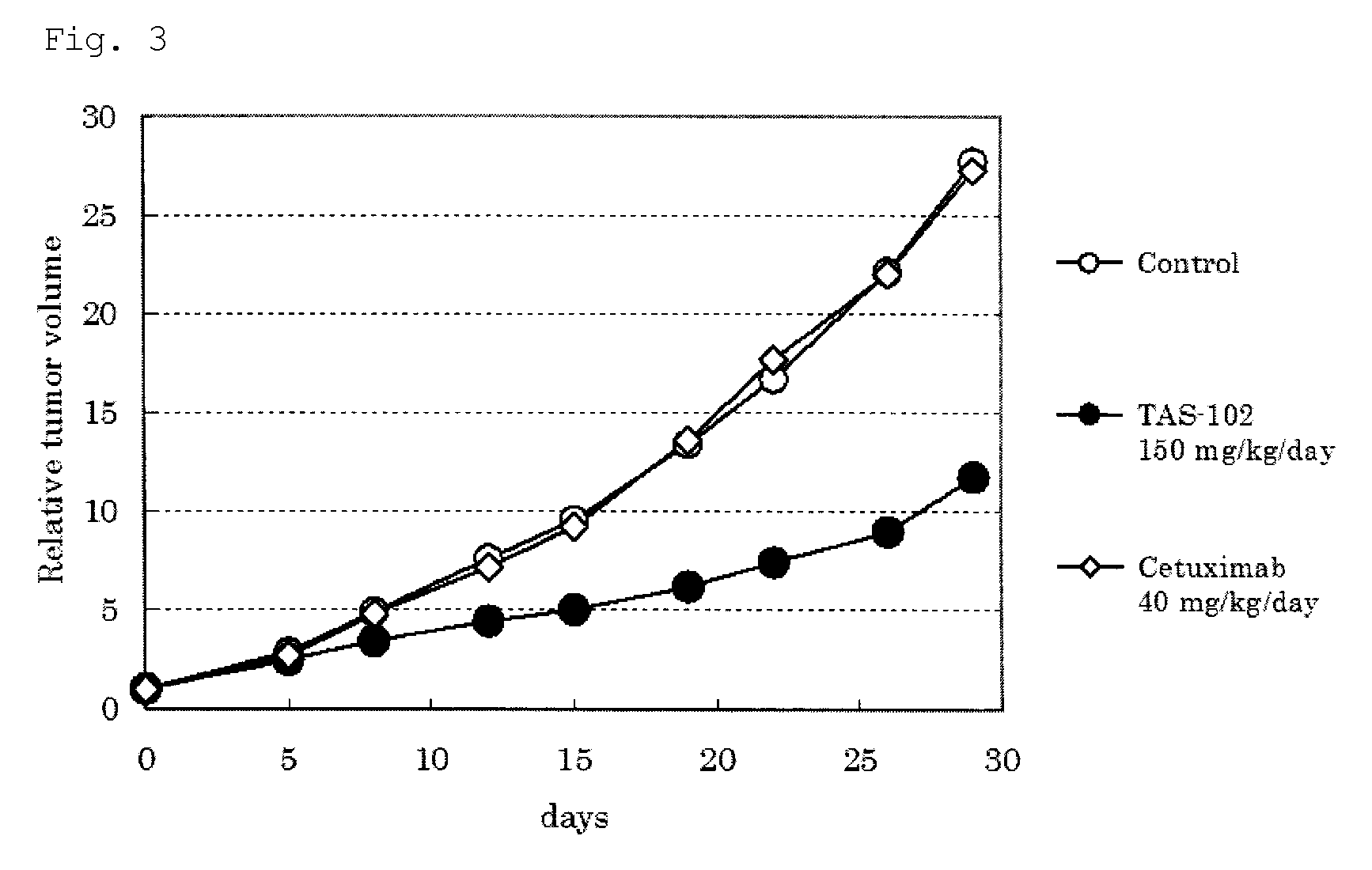
Antitumor agent and therapeutic effect prediction method for patients with kras-mutated colorectal cancer
ActiveUS20140213602A1Remarkable effectImprove survivalOrganic active ingredientsBiocideUracilCurative effect
This invention provides a method for predicting a therapeutic effect of chemotherapy that uses an antitumor agent comprising α,α,α-trifluorothymidine and 5-chloro-6-(1-(2-iminopyrrolidinyl) methyl)uracil hydrochloride at a molar ratio of 1:0.5 on a colorectal cancer patient,the method comprising:(1) detecting the presence or absence of KRAS gene mutation in a biological sample obtained from the patient; and(2) predicting that the patient is likely to sufficiently respond to the chemotherapy, when KRAS gene mutation is detected in Step (1).
Owner:TAIHO PHARMA CO LTD
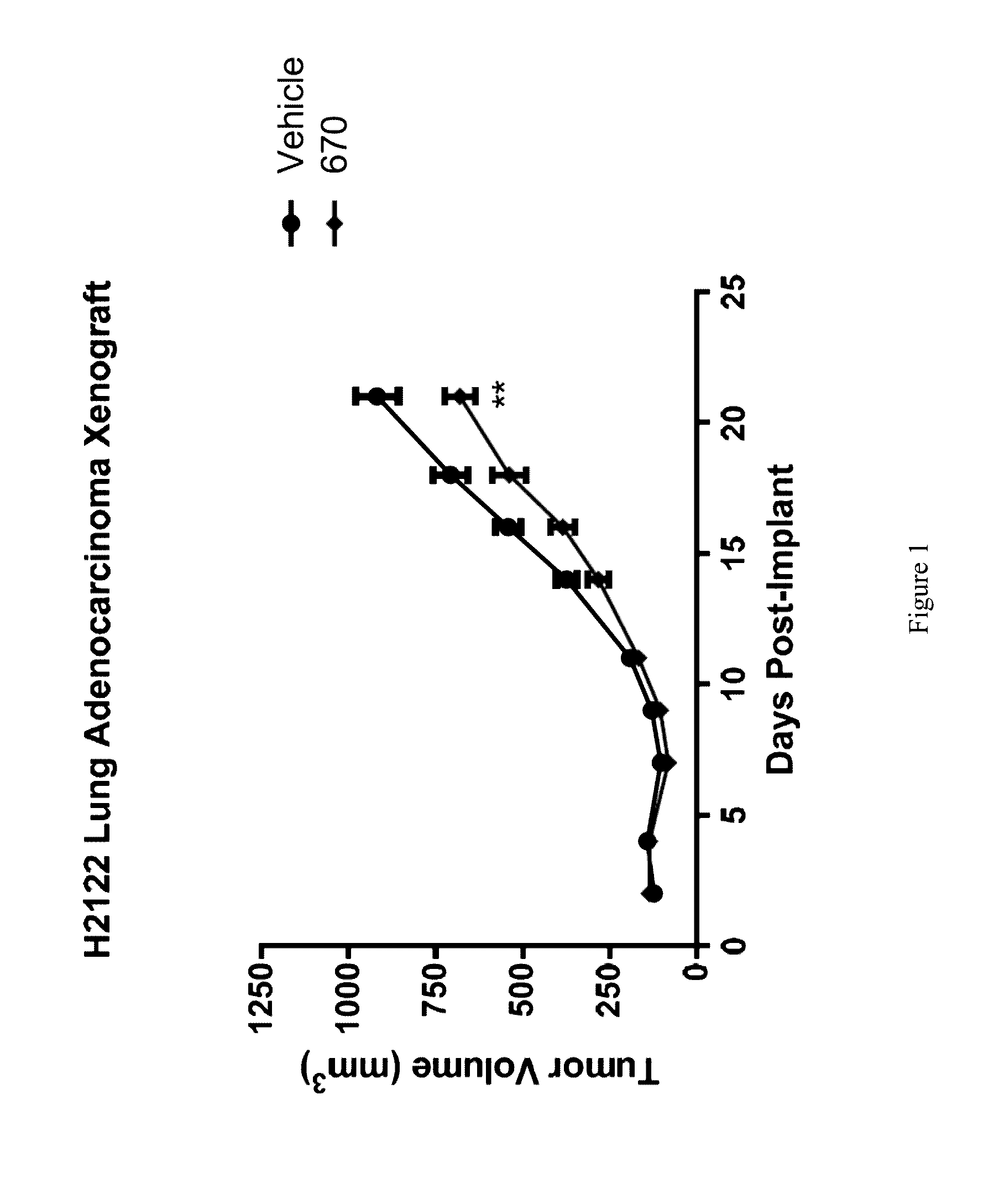
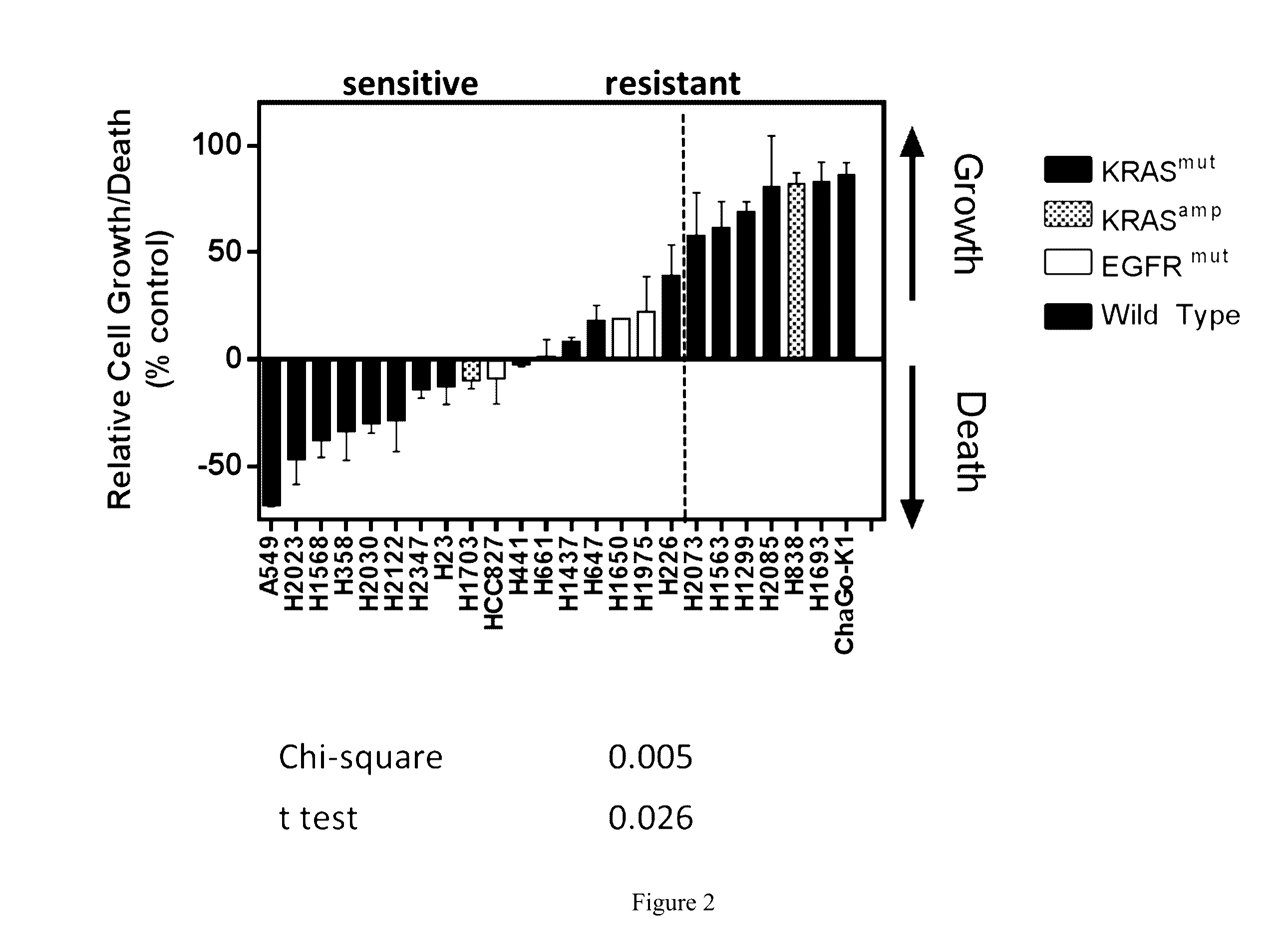
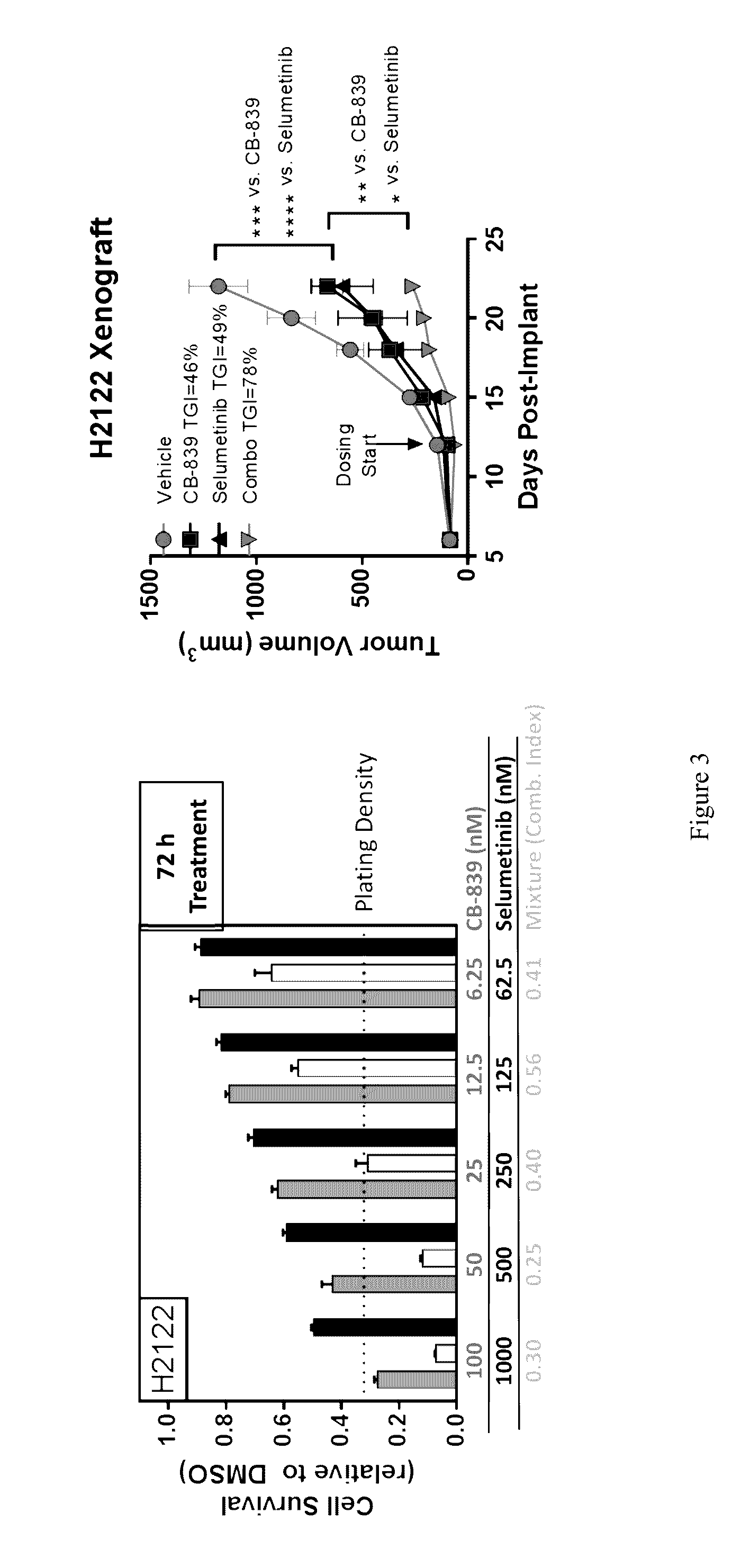
Treatment of Lung Cancer with Inhibitors of Glutaminase
ActiveUS20160287585A1Shrink tumorImprove throughputOrganic active ingredientsMicrobiological testing/measurementKras mutationGlutaminase
The invention relates to methods of treating lung cancer using glutaminase inhibitors. In particular, results demonstrate that lung cancers characterized by an EGFR or KRAS mutation are treated by glutaminase inhibitors.
Owner:CALITHERA BIOSCIENCES INC
Primer and probe for detecting mutant KRAS genes
InactiveCN104498615AGrowth inhibitionInhibitory structureMicrobiological testing/measurementDNA/RNA fragmentationGeneKRAS Gene Mutation
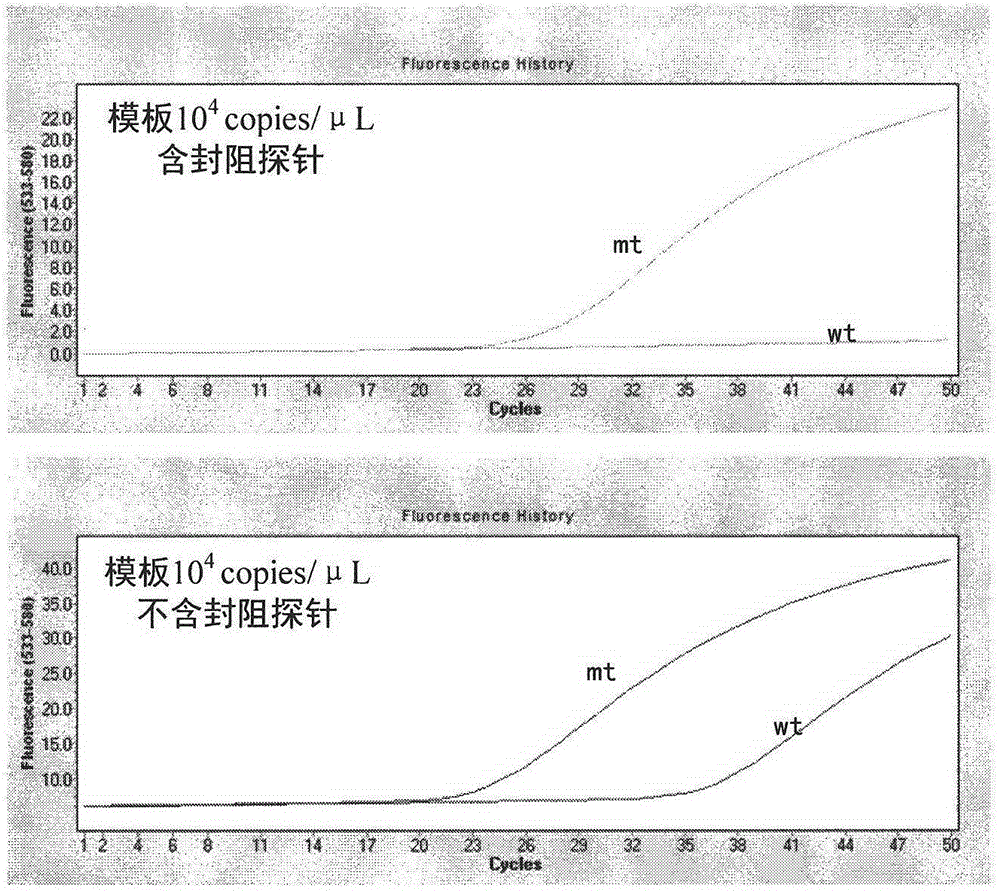
The invention provides a primer and probe for detecting mutant KRAS genes and further provides a method and kit for detecting mutant KRAS genes by using the primer and the probe. By adopting the primer and probe provided by the invention, the amplification of wild KRAS genes can be inhibited, and the combination of the wild KRAS genes with the probe can be also inhibited, thus less than 1% of mutant KRAS genes can be detected.
Owner:HANGZHOU NEW HORIZON HEALTH TECH CO LTD
Kit for detecting hotspot mutation of KRAS gene and detection method thereof
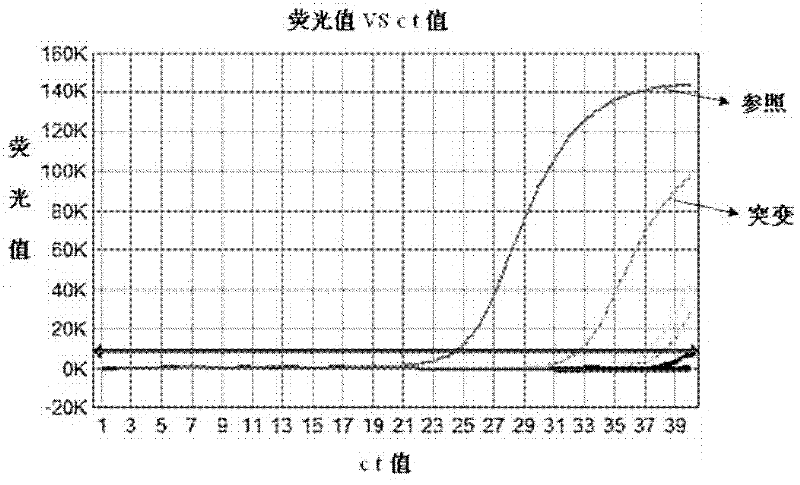
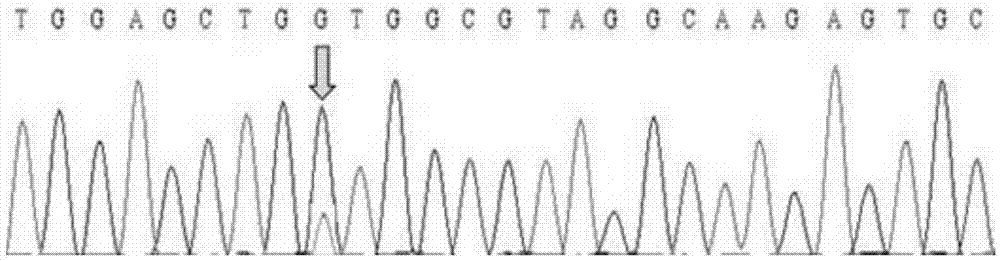
ActiveCN103571947AHigh sensitivityImprove the detection rateMicrobiological testing/measurementWild typeSanger sequencing
The invention belongs to the field of biotechnology and discloses a kit for detecting the hotspot mutation of KRAS gene and a detection method thereof. The kit comprises a PNA (peptide nucleic acid) reagent; and the wild type KRAS gene sequence is closed by a clamp technology so as to improve the sensitivity of a sanger sequencing process and the detection rate of the KRAS gene mutation. The detection method disclosed by the invention is simple and safe to operate and does not need many expensive reagents, thereby being economical and efficient; and meanwhile, the detection method also has the advantage of short operation time, and the whole detection process is finished within 4-6 hours.
Owner:WUHAN BIOTECH GENE ENG
Prime group, probe group and kit for detecting Kras gene mutation
ActiveCN105803088AHigh detection sensitivityFast wayMicrobiological testing/measurementDNA/RNA fragmentationK-ras GenesForward primer
The invention discloses a prime group, probe group and kit for detecting Kras gene mutation. The prime group comprises forward primers, reverse primers and probes which are used for detecting 7 Kras gene mutation types, wherein the forward primers respectively are 34A-Rev-Fp10, 35A-Rev-Fp4, 38A-Rev-Fp13, 34C-Rev-Fp, 34T-Rev-Fp, 35C-Rev-Fp and 35T-Rev-Fp, the reverse primers are Kras-Rev-Rp, and the probes respectively are 34A-Rev-Pb4, 35A-Rev-Pb2, 38A-Rev-Pb3, 34C-Rev-Pb, 34T-Rev-Pb, 35C-Rev-Pb and 35T-Rev-Pb. The prime group, the probe group and the kit are high in detection sensitivity and accuracy of Kras gene mutation, good in specificity and capable of respectively and simultaneously detecting 7 different mutation types.
Owner:CREATIVE BIOSCIENCES (GUANGZHOU) CO LTD
Antitumor agent and therapeutic effect prediction method for patients with KRAS-mutated colorectal cancer
ActiveUS9371380B2Remarkable effectImprove survivalOrganic active ingredientsMicrobiological testing/measurementUracilCurative effect
This invention provides a method for predicting a therapeutic effect of chemotherapy that uses an antitumor agent comprising α,α,α-trifluorothymidine and 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl)uracil hydrochloride at a molar ratio of 1:0.5 on a colorectal cancer patient,the method comprising:(1) detecting the presence or absence of KRAS gene mutation in a biological sample obtained from the patient; and(2) predicting that the patient is likely to sufficiently respond to the chemotherapy, when KRAS gene mutation is detected in Step (1).
Owner:TAIHO PHARMA CO LTD
Detection method of human proto-oncogene KRAS and kit
InactiveCN101736080AHigh degree of standardizationImproving the Meaning of Results AnalysisMicrobiological testing/measurementExonWilms' tumor
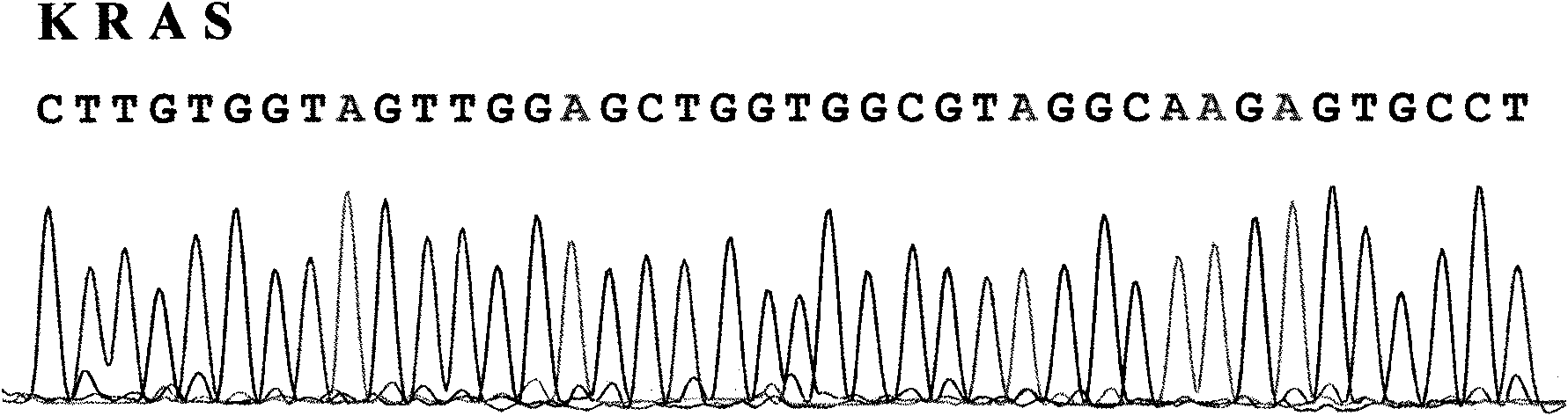
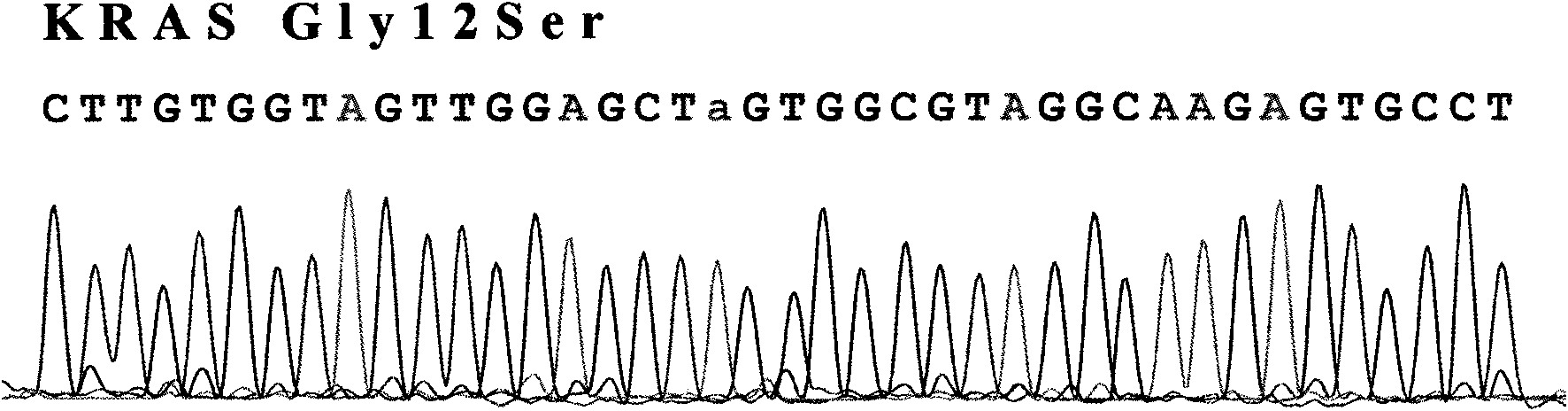
The invention provides a detection method of KRAS gene mutation and a kit. The detection method comprises the following steps of: (a) extracting DNA in the sample, and carrying out PCR amplification by using specific primers under specified conditions; and (b) and sequencing the amplified products, comparing with normal KRAS gene, and determining whether gene mutation exists. The specific primers are 2-4 pairs of primers aiming at different exons of the KRAS gene. By adopting the detection method and the kit of the invention, the detection speed can be improved and the reagent and time are saved. By utilizing the detection result, the administration for clinical chemotherapy of alimentary tract tumors can be guided.
Owner:GENESKY BIOTECH INC
Kit and detection method for detection of human KRAS gene mutations
InactiveCN107164495AHigh sensitivityReduce false negative resultsMicrobiological testing/measurementBlood plasmaFhit gene
The invention discloses a kit for detection of human KRAS gene mutations. The kit comprises specific primer pairs of seven genes and seven probe primers cooperatively used with the specific primers. Mixed liquid of primers and probes includes mutation site genes and internal-control primer probes, quality control genes and internal-control detection primer probes, FAM fluorescein used for labeling mutation and quality control probes and an internal control marker HEX fluorescein; quality control and internal control are taken into consideration in result interpretation; the quality control genes are selected from the conserved segment of the human KRAS gene, and the conserved human beta-actin gene is used as internal control; and a double-control system is employed for monitoring the mass of DNA and PCR reaction process in blood plasma samples. Compared with the prior art, the invention has the following beneficial effects: 1) the method has high sensitivity and can detect 1% trace mutation templates under the background of 100 ng of wild mankind genomes, so false negative results are reduced; 2) a totally-enclosed reaction is carried out to avoid false positive results; and 3) the double-control reaction system is employed, so PCR amplification and nucleic acid extraction are effectively detected via quality control and internal control.
Owner:安徽安龙基因科技有限公司
Tetrahydropyrido[3,4-d]pyrimidine-2-(1H)-ketone compound as well as preparation method and medical application thereof
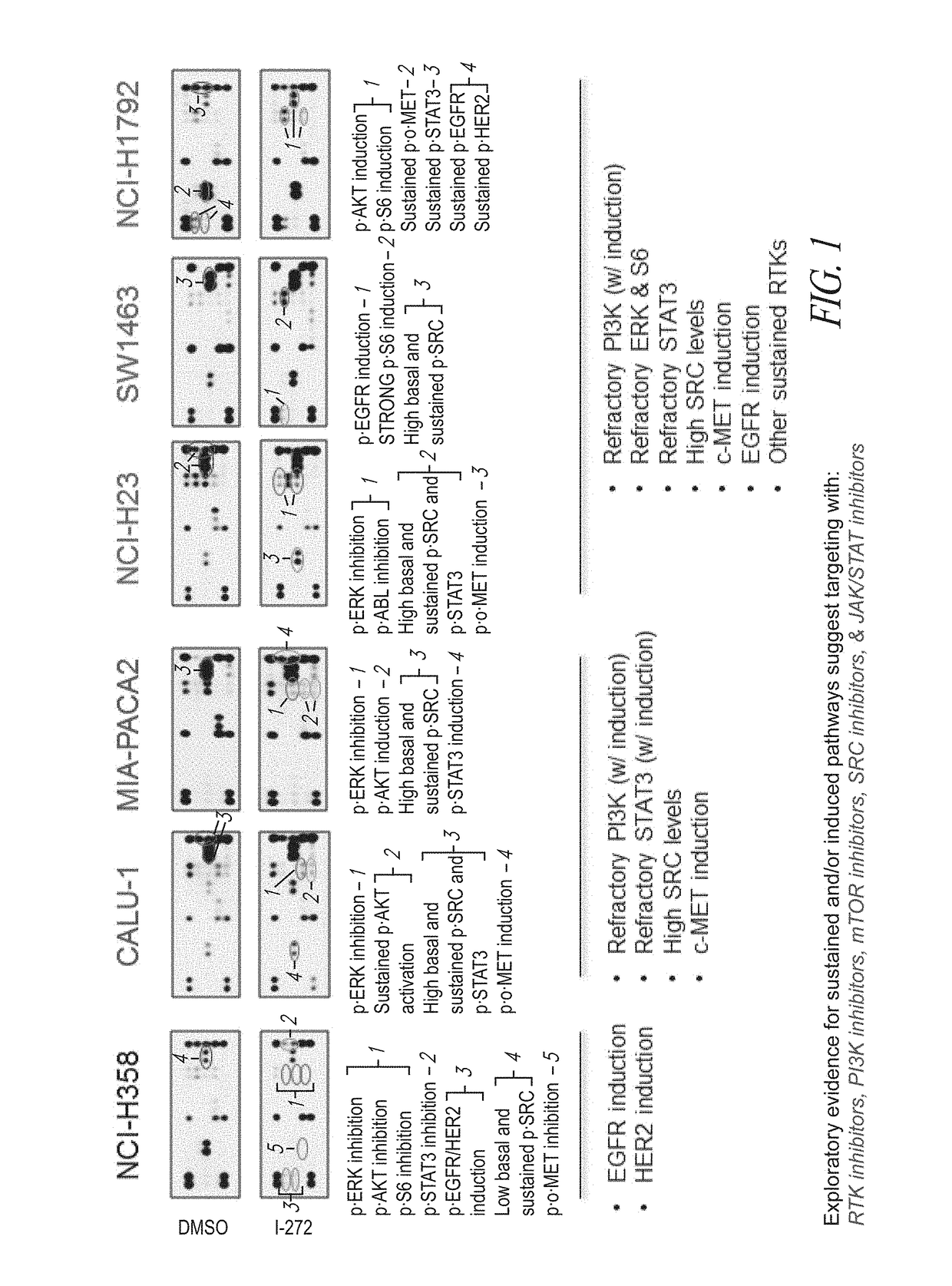
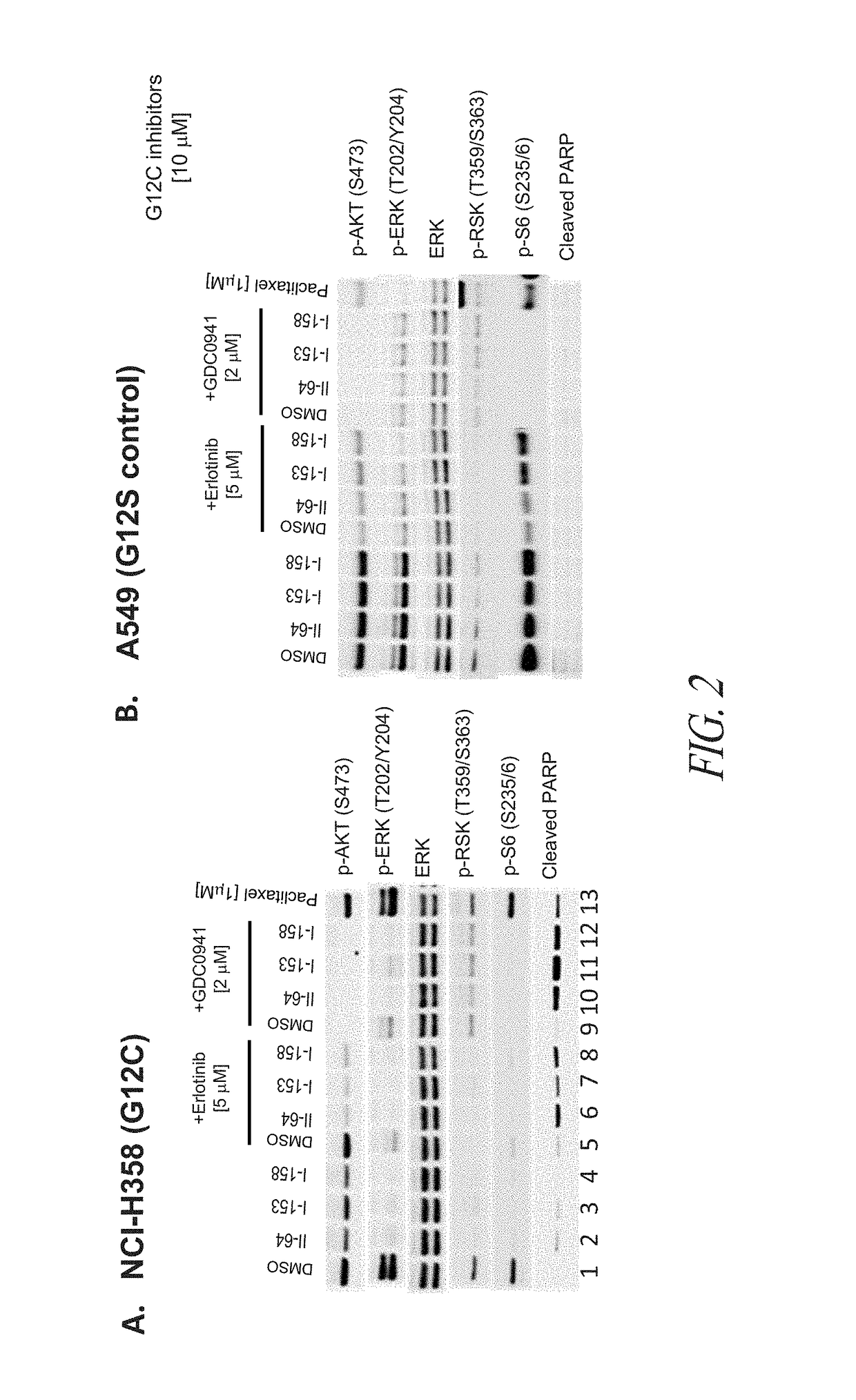
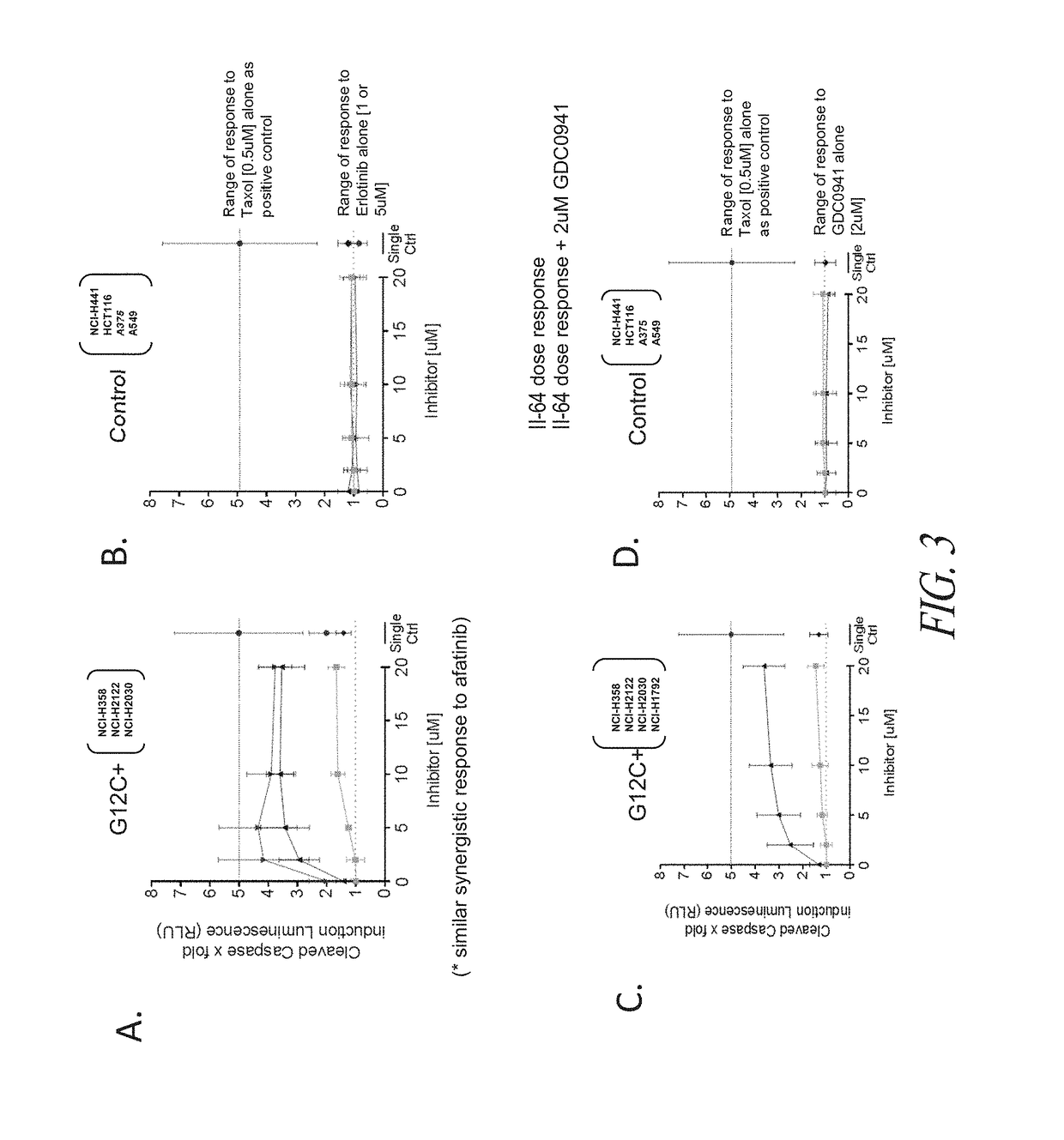
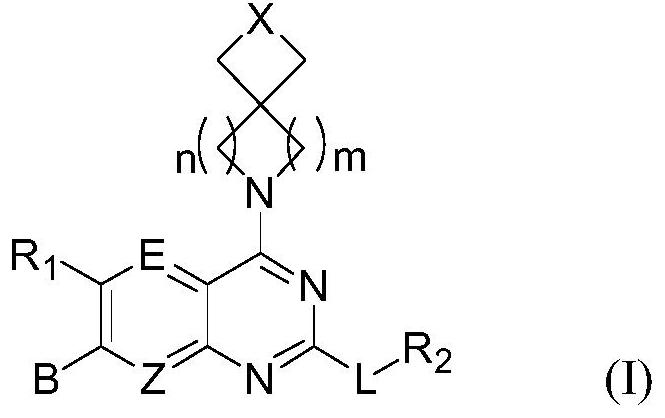
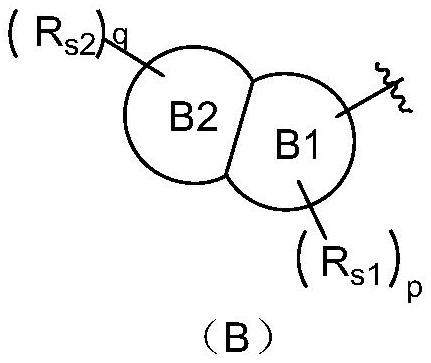
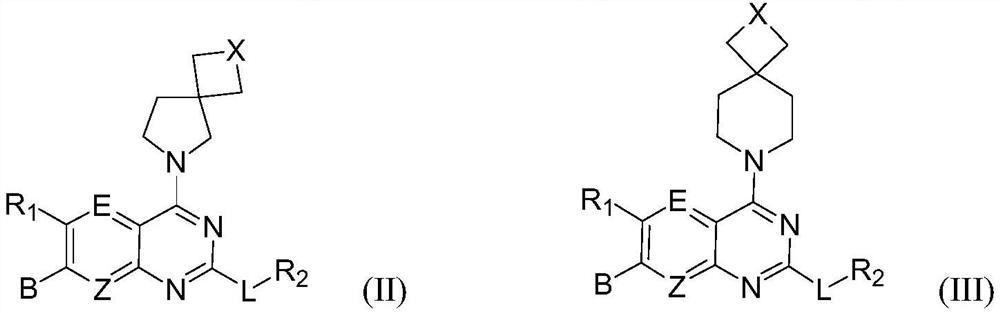
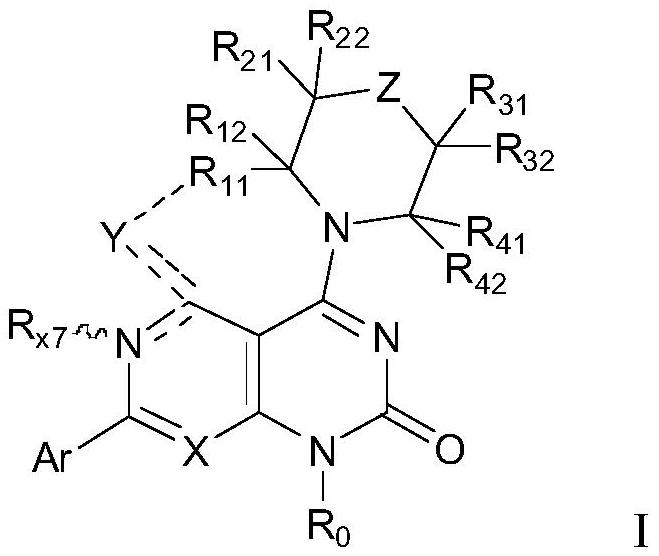
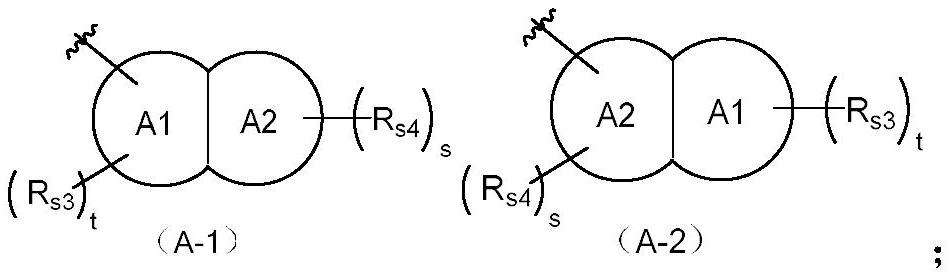
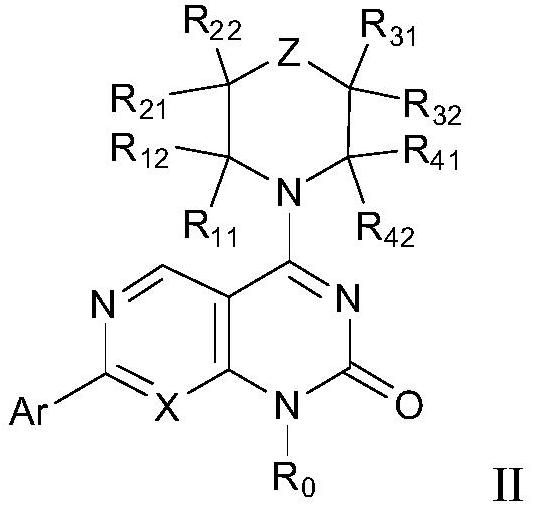
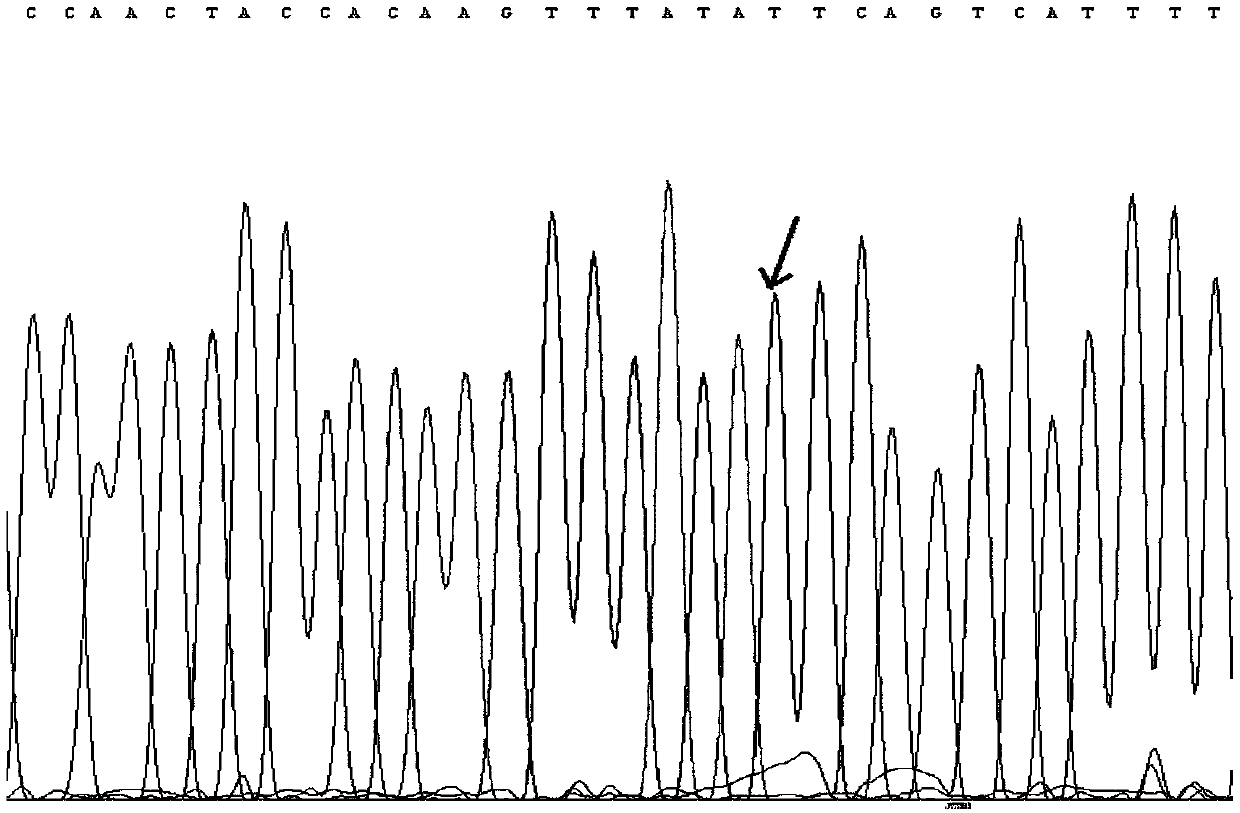
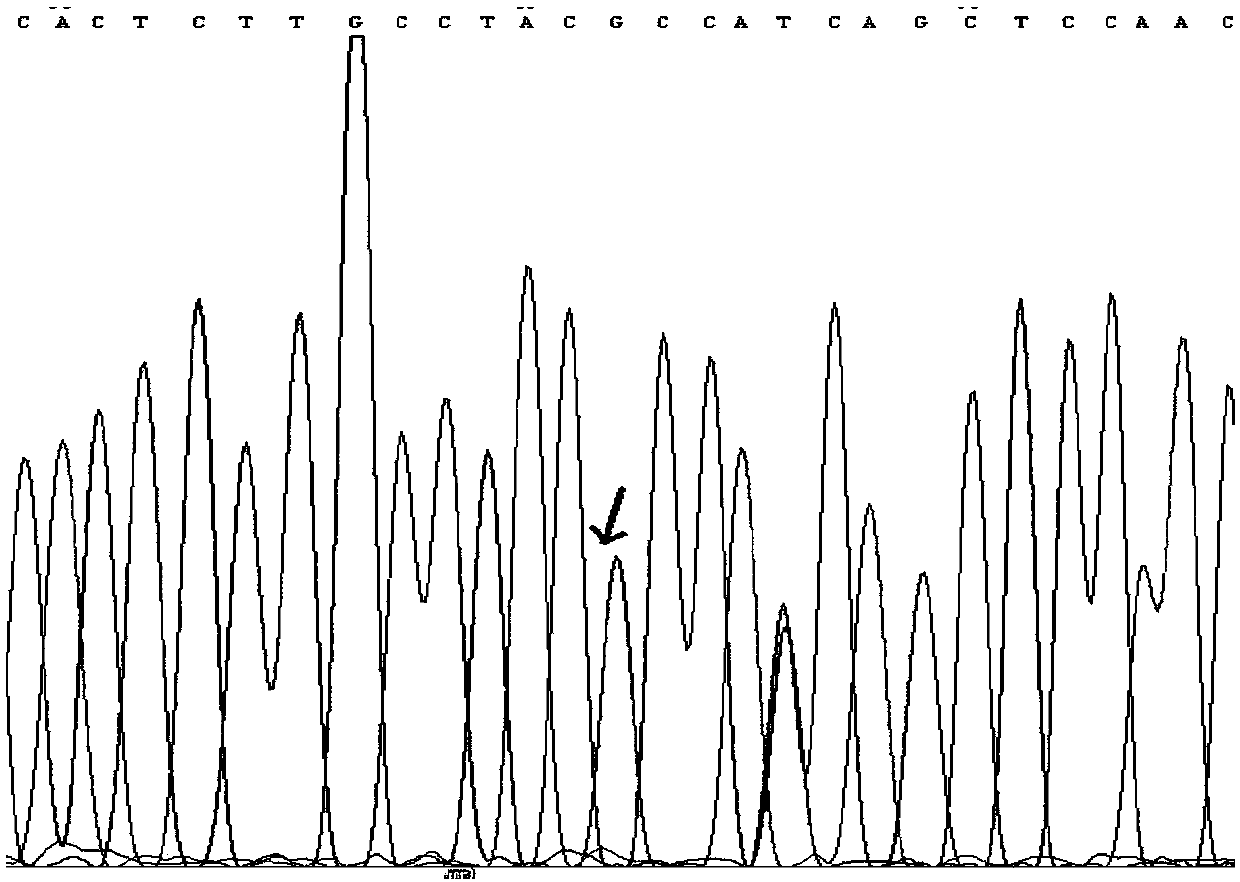
The invention discloses a tetrahydropyrido[3,4-d]pyrimidine-2-(1H)-ketone compound with a selective inhibition effect on KRAS gene mutation and pharmaceutically acceptable salt, stereoisomer, solventcompound or prodrug thereof, the compound is shown as a formula I, and the definition of each group in the formula is shown in the specification in detail. In addition, the invention also discloses apharmaceutical composition containing the compound and application of the compound in preparation of cancer drugs.
Owner:GENFLEET THERAPEUTICS SHANGHAI INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Tetrahydropyrido[3,4-d]pyrimidine-2-(1H)-ketone compound as well as preparation method and medical application thereof Tetrahydropyrido[3,4-d]pyrimidine-2-(1H)-ketone compound as well as preparation method and medical application thereof](https://images-eureka.patsnap.com/patent_img/e2b39f2e-e845-4660-85e0-efe2f6168738/FDA0002525673030000011.png)
![Tetrahydropyrido[3,4-d]pyrimidine-2-(1H)-ketone compound as well as preparation method and medical application thereof Tetrahydropyrido[3,4-d]pyrimidine-2-(1H)-ketone compound as well as preparation method and medical application thereof](https://images-eureka.patsnap.com/patent_img/e2b39f2e-e845-4660-85e0-efe2f6168738/FDA0002525673030000021.png)
![Tetrahydropyrido[3,4-d]pyrimidine-2-(1H)-ketone compound as well as preparation method and medical application thereof Tetrahydropyrido[3,4-d]pyrimidine-2-(1H)-ketone compound as well as preparation method and medical application thereof](https://images-eureka.patsnap.com/patent_img/e2b39f2e-e845-4660-85e0-efe2f6168738/FDA0002525673030000022.png)