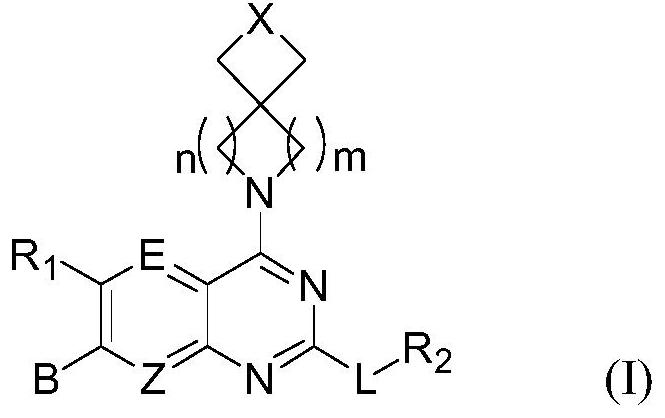
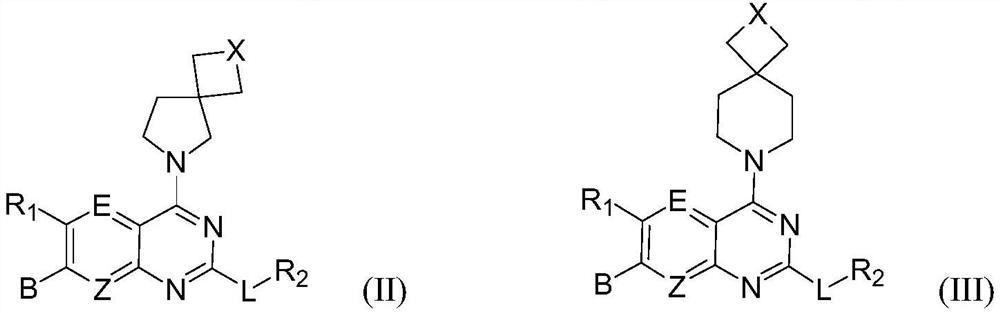
Spiro-substituted pyrimido-cyclic compound, and preparation method and medical application thereof
A compound and solvate technology, applied in the field of medicine, can solve problems such as cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Embodiment 1: Preparation Z1
[0193]
[0194] Step 1: 7-bromo-2,4,6-trichloro-8-fluoroquinazoline (298mg, 0.9mmol) was dissolved in dichloromethane / acetonitrile (3mL / 3mL) and cooled to 0°C. Triethylamine (136 mg, 1.35 mmol) and tert-butyl 2,6-diazaspiro[3.4]octane-2-carboxylate (200 mg, 0.94 mmol) were added thereto, and stirred at room temperature for 3 hours. Dichloromethane (30x 2mL) was extracted, the organic phase was washed with water, washed with brine, dried over anhydrous sodium sulfate, and evaporated to obtain 6-(7-bromo-2,6-dichloro-8-fluoroquinazoline-4- yl)-2,6-diazaspiro[3.4]octane-2-carboxylic acid tert-butyl ester (yellow solid, 620 mg, Y: crude product). ES-API: [M+H]+=506.
[0195] Step 2: tert-butyl 6-(7-bromo-2,6-dichloro-8-fluoroquinazolin-4-yl)-2,6-diazaspiro[3.4]octane-2-carboxylate (620mg, 1.2mmol) was dissolved in N,N-dimethylformamide (10mL), to which was added cesium carbonate (1.17g, 3.6mmol), triethylenediamine (20mg, 0.18mmol) and 1...
Embodiment 2
[0199] Example 2: Preparation of Z2
[0200]
[0201] Step 1: tert-butyl 6-(7-bromo-2,6-dichloro-8-fluoroquinazolin-4-yl)-2,6-diazaspiro[3.4]octane-2-carboxylic acid salt (200mg, 0.395mmol), (S)-(1-methylpyrrolidin-2-yl)methanol (140mg, 1.185mmol), cesium carbonate (687mg, 0.185mmol) and 1,4-diazabicyclo [2.2.2] Octane (10mg, 0.079mmol) was added to dry tetrahydrofuran (5.0mL) and N,N-dimethylformamide (5.0mL), and replaced with argon three to five times. After the reaction solution was stirred at 60°C for 16 hours, it was cooled to room temperature and poured into ice water. Extracted three times with ethyl acetate (10 mL x 3), and combined the organic phases. The organic phase was washed successively with water and saturated brine, and then dried over anhydrous sodium sulfate. Concentration under vacuum afforded crude product (S)-tert-butyl 6-(7-bromo-6-chloro-8-fluoro-2-((1-methylpyrrolidin-2-yl)methoxy) as a yellow solid Quinazolin-4-yl)-2,6-diazaspiro[3.4]octane-2-...
Embodiment 3
[0205] Embodiment 3: Preparation Z3
[0206]
[0207] Step 1: tert-butyl 6-(7-bromo-2,6-dichloro-8-fluoroquinazolin-4-yl)-2,6-diazaspiro[3.4]octane-2-carboxylic acid salt (200mg, 0.395mmol), methylpiperazine (119mg, 1.185mmol), cesium carbonate (687mg, 0.185mmol) and 1,4-diazabicyclo[2.2.2]octane (10mg, 0.079mmol) Add dry tetrahydrofuran (5.0 mL) and N,N-dimethylformamide (5.0 mL), and replace with argon three to five times. After the reaction solution was stirred at 60°C for 16 hours, it was cooled to room temperature and poured into ice water, and extracted three times with ethyl acetate (10.0 mL x 3). The organic phases were combined, washed with water and saturated brine in turn, and dried over anhydrous sodium sulfate. It was then concentrated under vacuum to give the crude product 6-(7-bromo-6-chloro-8-fluoro-2-(4-methylpiperazin-1-yl)quinazolin-4-yl)-2 as a yellow solid, tert-butyl 6-diazaspiro[3.4]octane-2-carboxylate (225 mg, yield: 100%). ES-API: [M+H]+=586.3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com