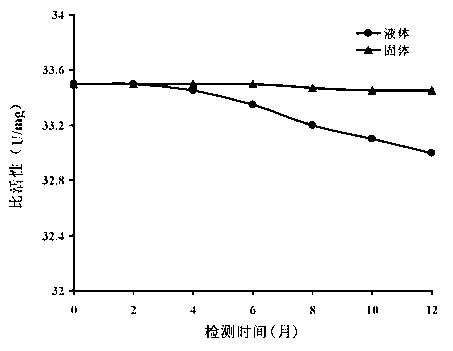
Gene mutation type recombined protease K and industrialized production method thereof
A protease and amino acid technology, which is applied in the field of gene mutant recombinant proteinase K and its industrial production, and can solve problems such as industrial production of undiscovered gene mutant recombinant proteinase K.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0059] Examples of such eukaryotic cells include, but are not limited to, yeast cells. According to a preferred embodiment of the present invention, the eukaryotic cells are yeast cells. According to yet another preferred embodiment of the present invention, the yeast cells are Pichia cells, Candida cells, Hansenula polymorpha cells, Torulopsis cells, Schizosaccharomyces cells and Kluyveromyces cells.
[0060] According to an especially preferred embodiment of the present invention, the yeast cells are Pichia pastoris cells. Due to more in-depth research on the fermentation conditions of Pichia pastoris, and the cost of the Pichia pastoris cell is the lowest in the fermentation preparation, which can meet the technical requirements and the needs of product marketization, the Pichia pastoris cell is considered to be a genetic mutation. The most preferred transformed cells for the production of recombinant proteinase K.
[0061] According to a preferred embodiment of the pres...
Embodiment 1
[0083] Example 1 Construction of Proteinase K Expression Vectors pPIC9K-ProK and pPIC9K-mutProK
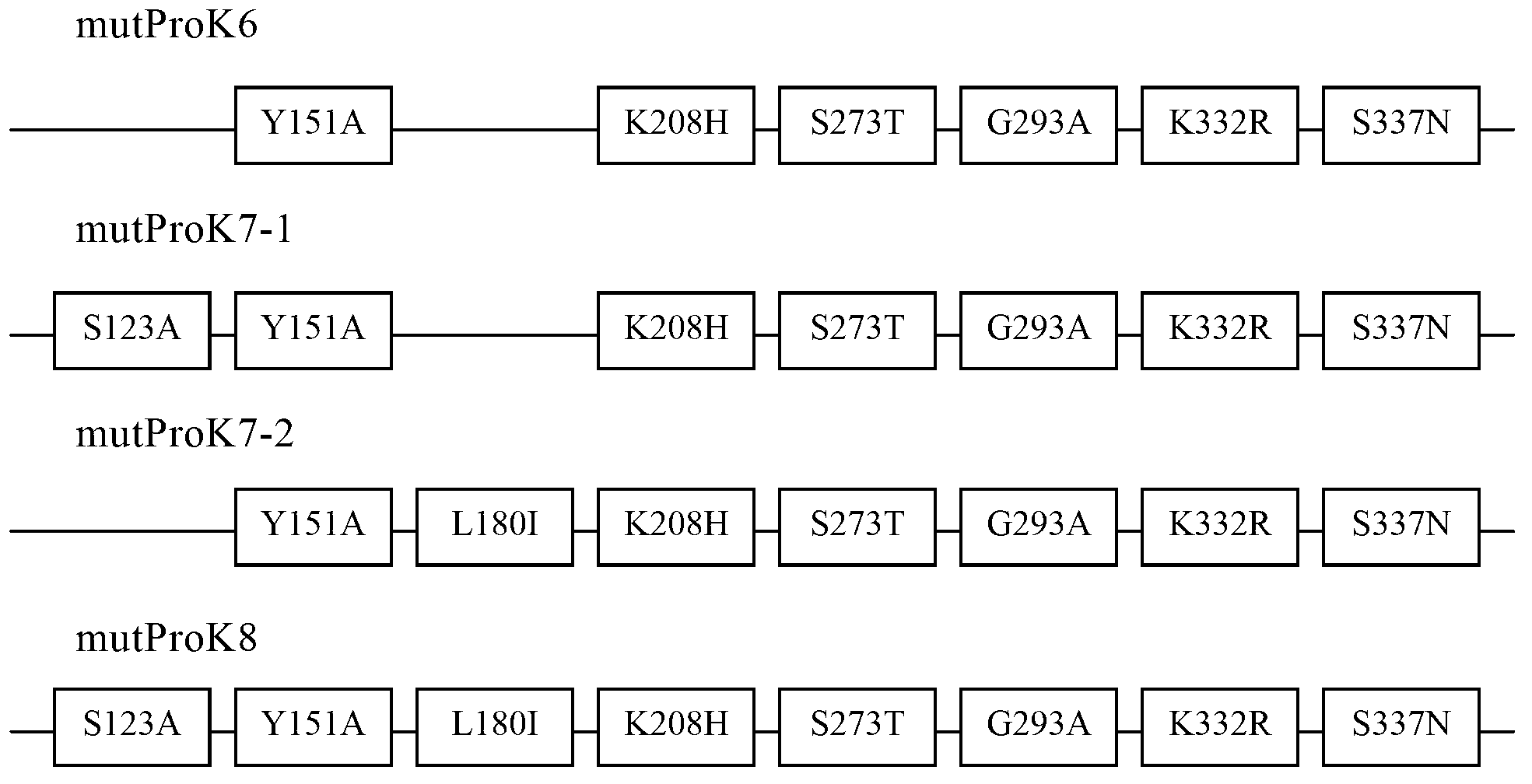
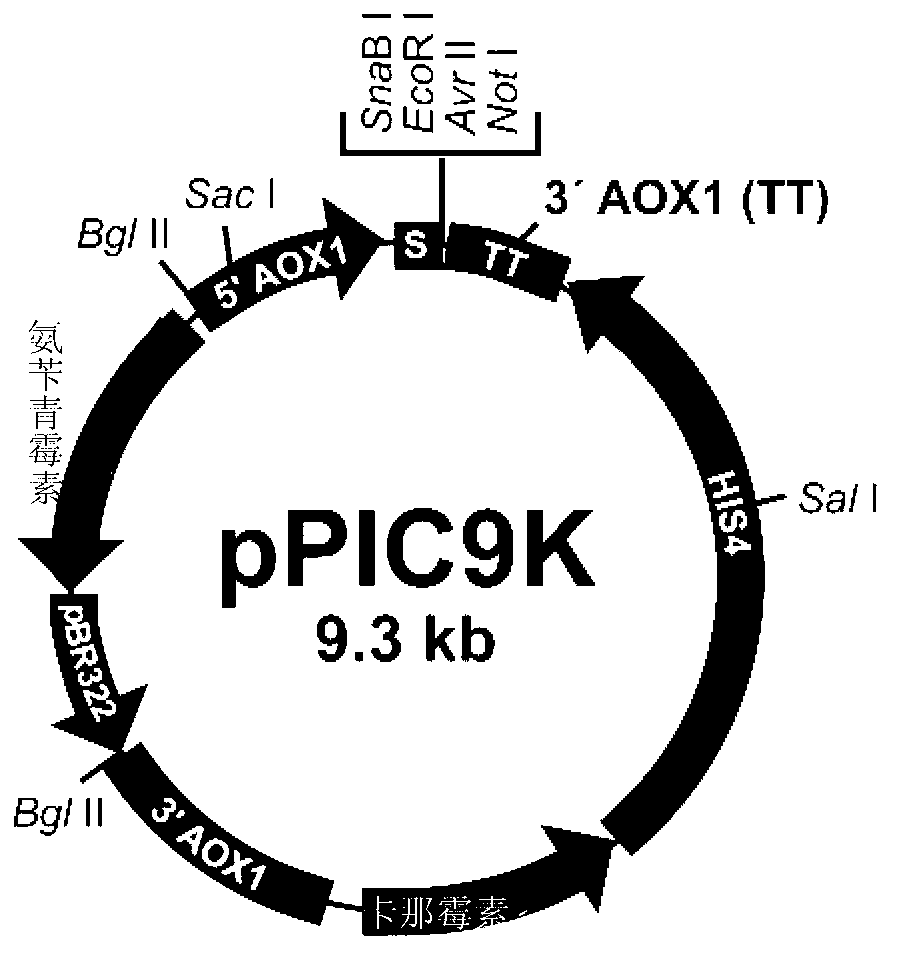
[0084] Replace the coding sequence of wild-type proteinase K (SEQ ID NO.3) with the DNA sequence composed of Pichia pastoris preferred codons, add the SnaB I restriction site TACGTA at the 5' end, and add the hexamerization site in sequence at the 3' end The histidine tag CACCATCACCACCATCAC (SEQ ID NO.4), the stop codon TAA and the NotI restriction site GCGGCCGC were synthesized by chemical methods (SEQ ID NO.5). Replace the SnaB I restriction site TACGTA added at the 5' end with the EcoR I restriction site GAATTC, and further introduce corresponding mutations (such as figure 1 shown), and the obtained sequence (SEQ ID NO.6-9) was synthesized by chemical method (BioBasic Inc., a Canadian gene synthesis technology service company). The above chemically synthesized sequences have been cloned into the pUC57 plasmid (BioBasic Inc., a Canadian gene synthesis technology service company...
Embodiment 2
[0087] Sequencing results showed that the target fragments were correctly inserted into the above expression vectors. Example 2 Transformation of Proteinase K Expression Vectors pPIC9K-ProK and pPIC9K-mutProK
[0088] The recombinant plasmid constructed in Example 1 was digested with the restriction endonuclease Sal I to make it linearized, and then dissolved to a concentration of 1 μg / μL with TE buffer (pH 8.0), and 20 μL of the dissolved plasmid was mixed with GS115 yeast Competent cells were mixed evenly, transferred to an ice-precooled electroporation cuvette (0.1 cm gap between poles), and kept in an ice bath for 5 min. Using the method of electric shock transformation, the above-mentioned expression vector was transformed into GS115 yeast competent cells by electric shock using the built-in Pichia pastoris transformation parameters of the electric transformation instrument.
[0089] After the electric shock, quickly add 1mL of ice-cooled 1M sorbitol solution to the elec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com