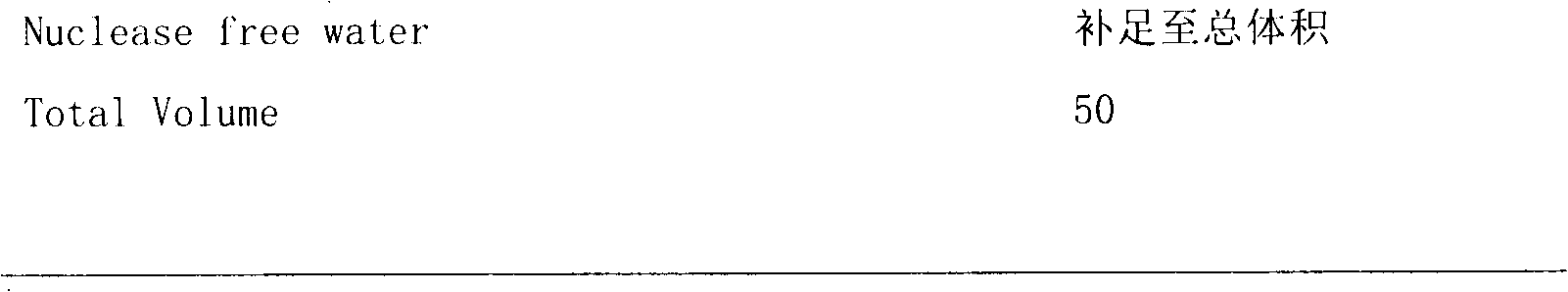
Patents
Literature
38 results about "HRAS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
GTPase HRas also known as transforming protein p21 is an enzyme that in humans is encoded by the HRAS gene. The HRAS gene is located on the short (p) arm of chromosome 11 at position 15.5, from base pair 522,241 to base pair 525,549. HRas is a small G protein in the Ras subfamily of the Ras superfamily of small GTPases. Once bound to Guanosine triphosphate, H-Ras will activate a Raf kinase like c-Raf, the next step in the MAPK/ERK pathway.
Conjugates of cereblon binding compounds and g12c mutant kras, hras or nras protein modulating compounds and methods of use thereof
ActiveUS20180015087A1Inhibiting KRAS-G12C oncogenic activityDecreasing KRAS-G12C protein levelOrganic active ingredientsPharmaceutical non-active ingredientsCereblonDisease
Conjugates of a cereblon-binding compound and compounds having modulatory activity against G12C mutant KRAS, HRAS or NRAS G12C proteins are provided. Methods associated with preparation and use of such conjugates, pharmaceutical compositions comprising such conjugates and methods to modulate the activity of G12C mutant KRAS, HRAS or NRAS G12C proteins for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Kit for testing and identifying genetic cardiac hypertrophy related gene mutation
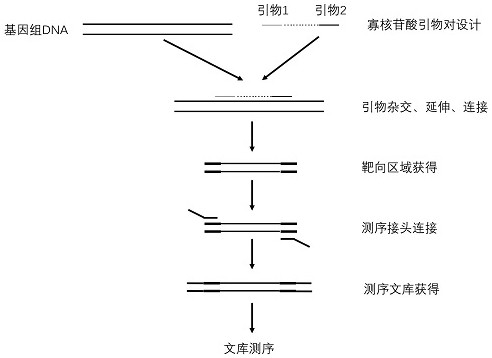
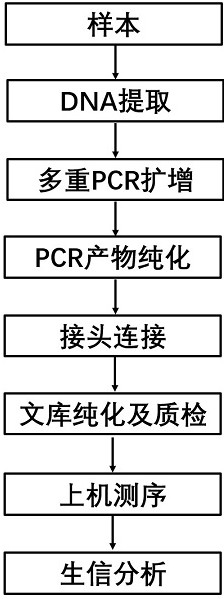
The invention relates to a kit for detecting genetic cardiac hypertrophy related gene mutation samples, particularly to a product for detecting genetic cardiac hypertrophy related genes by a massively parallel sequencing platform technology. The method involved in the kit comprises: a) uniquely designing and preparing a capture probe, which is directed at all exon fragments of genes ACTC1, ACTN2, BRAF, CALR3, CASQ2, CSRP3, GLA, HRAS, JPH2, KRAS, LAMP2, LDB3, MAP2K1, MYBPC3, MYH6, MYH7, MYL2, MYL3, MYLK2, MYOZ2, PRKAG2, RAF1, SOS1, TCAP, TNNC1, TNNI3, TNNT2, TPM1, TTN, TTR, and VCL; b) designing unique joints with tag sequences; c) using universal primers to conduct PCR amplification on probe sequences; and d) designing unique operations for capture of mixed target fragments. The massively parallel sequencing platform prepared by the method and the kit has the advantages of high sample throughput, high efficiency, and easy operation, thus greatly reducing the sequencing testing cost.
Owner:康旭基因技术(北京)有限公司
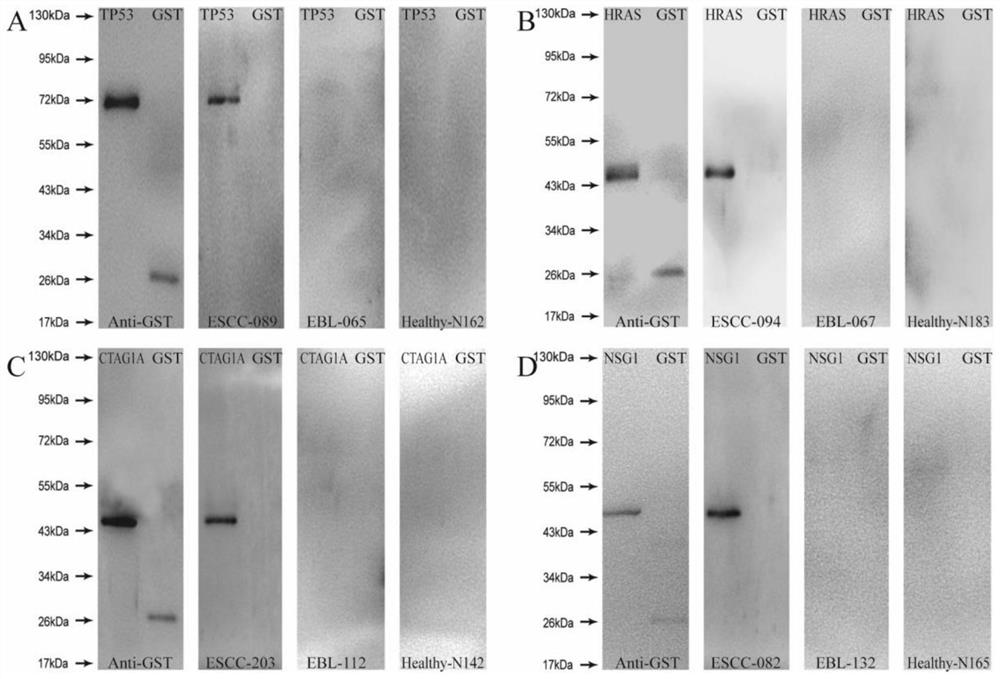
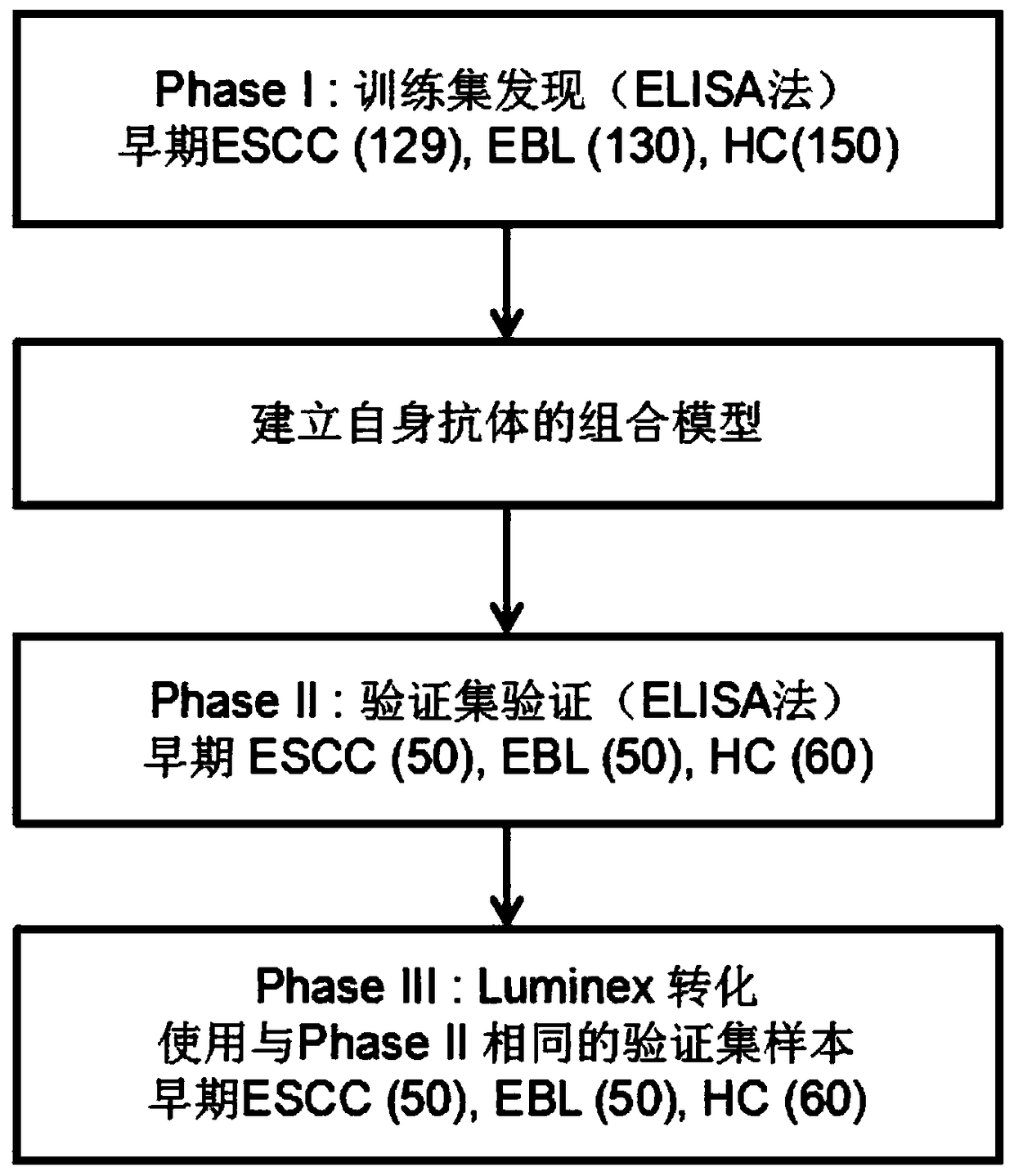
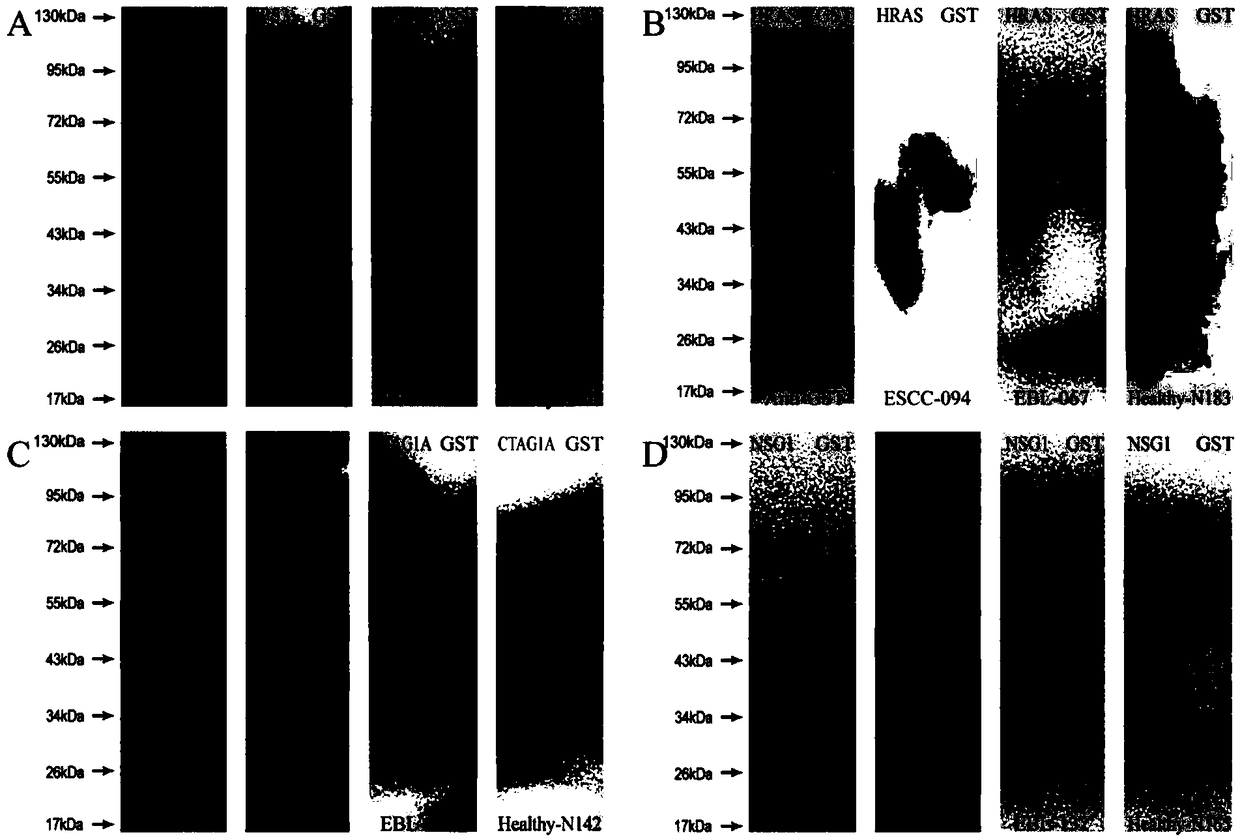
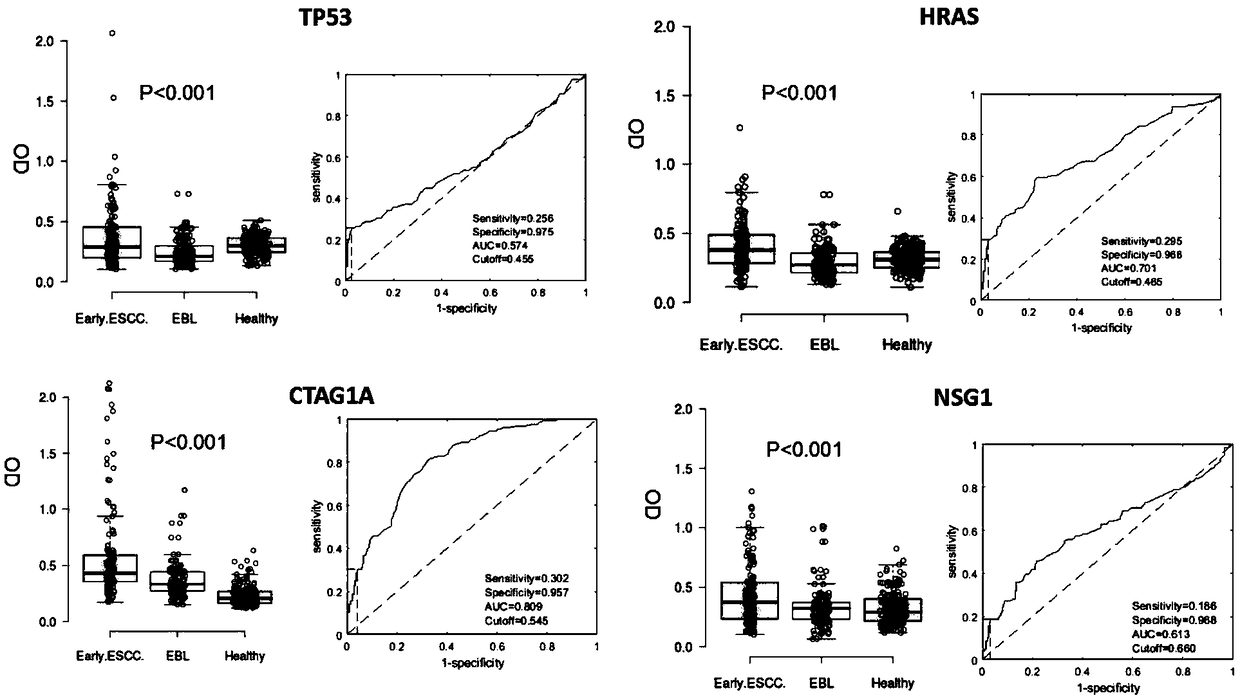
Four-autoantibody combined detection kit for diagnosing early esophageal squamous cancer and application thereof
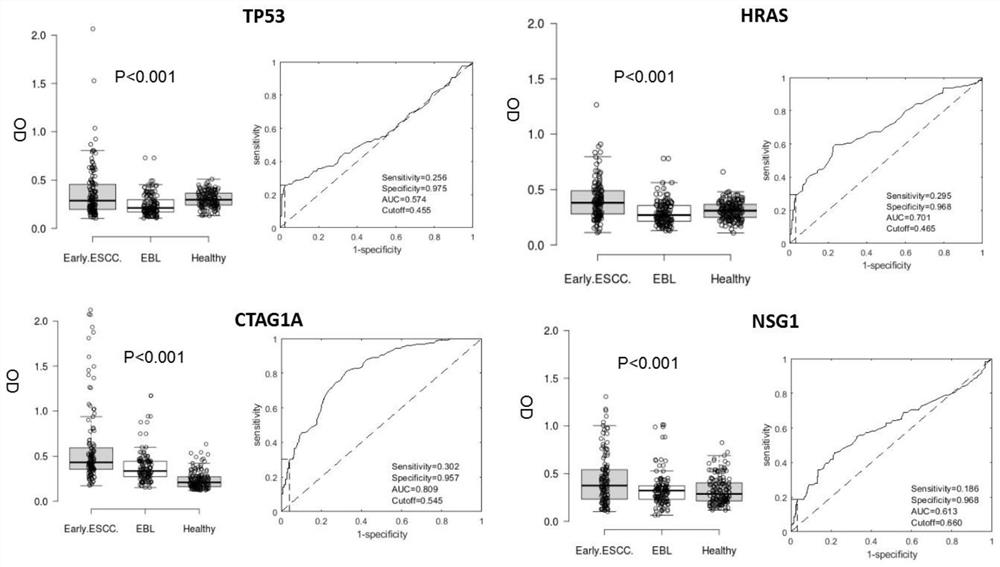
The invention provides a four-autoantibody combined detection kit for diagnosing an early esophageal squamous cancer and application thereof. The four-autoantibody combined detection kit for diagnosing the early esophageal squamous cancer comprises a solid substrate and antigen protein which coats the solid substrate; and the antigen protein comprises TP53, HRAS, CTAG1A and NSG1. The four-autoantibody combined detection kit for diagnosing the early esophageal squamous cancer, provided by the invention, has the advantages that the TP53, the HRAS, the CTAG1A and the NSG1 are firstly adopted as acombination to be used for early diagnosis of ESCC, higher sensibility and higher specificity are realized, the detection kit is conducive to further improvement of serologic screening quality of ESCC early diagnosis, and therefore, the cure rate and survival rate of patients are further promoted.
Owner:FUJIAN PROVINCIAL HOSPITAL
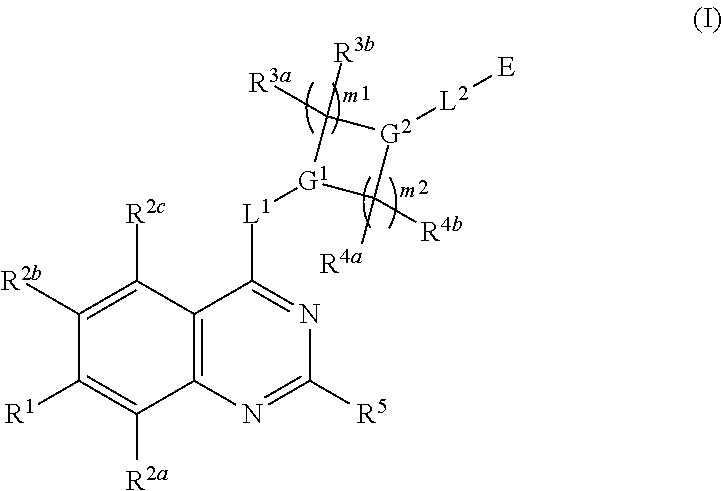
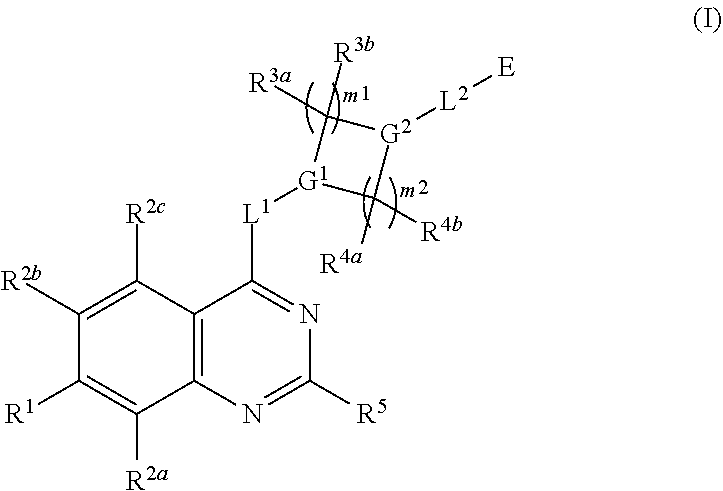
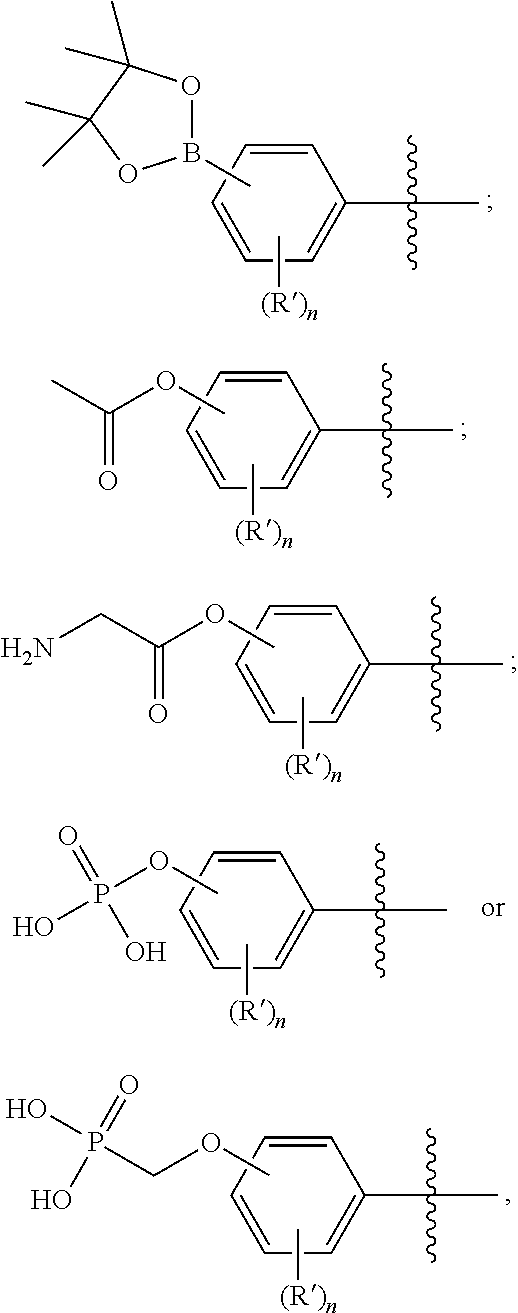
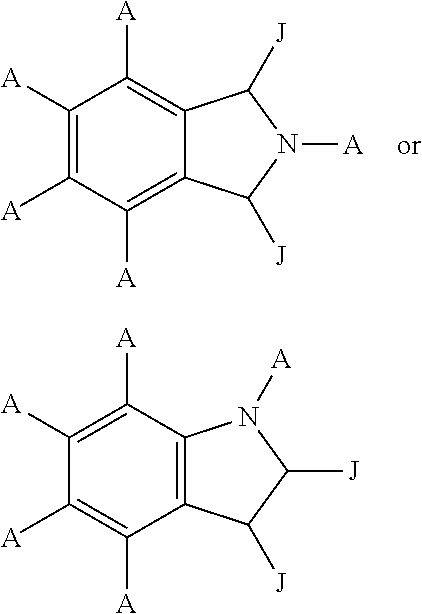
Quinazoline derivatives as modulators of mutant kras, hras or nras
Owner:ARAXES PHARMA LLC
Conjugates of cereblon binding compounds and G12C mutant KRAS, HRAS or NRAS protein modulating compounds and methods of use thereof
Conjugates of a cereblon-binding compound and compounds having modulatory activity against G12C mutant KRAS, HRAS or NRAS G12C proteins are provided. Methods associated with preparation and use of such conjugates, pharmaceutical compositions comprising such conjugates and methods to modulate the activity of G12C mutant KRAS, HRAS or NRAS G12C proteins for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
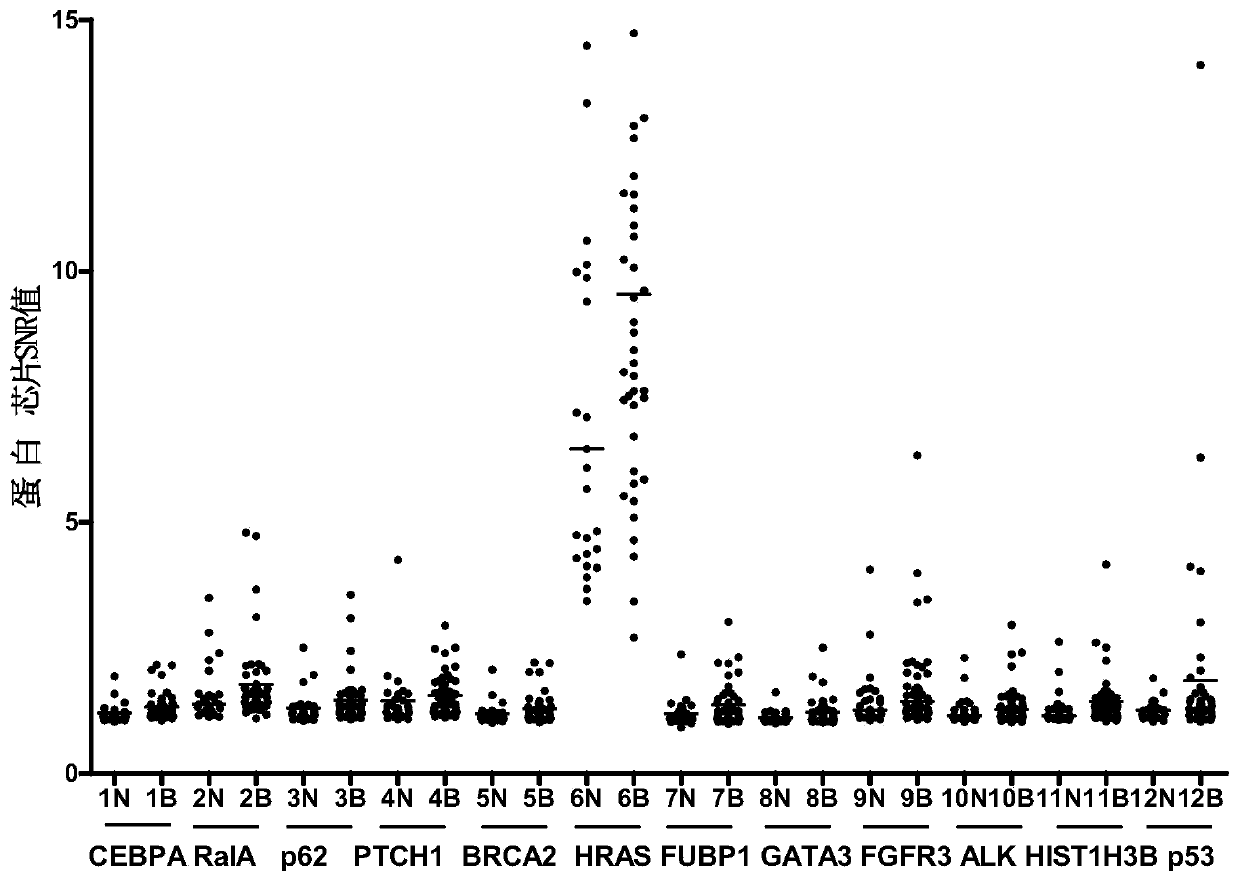
Serum protein marker for early screening and diagnosis of breast cancer, kit and detection method
ActiveCN110716043AGood reference valueHigh sensitivityColor/spectral properties measurementsOncologyGATA3
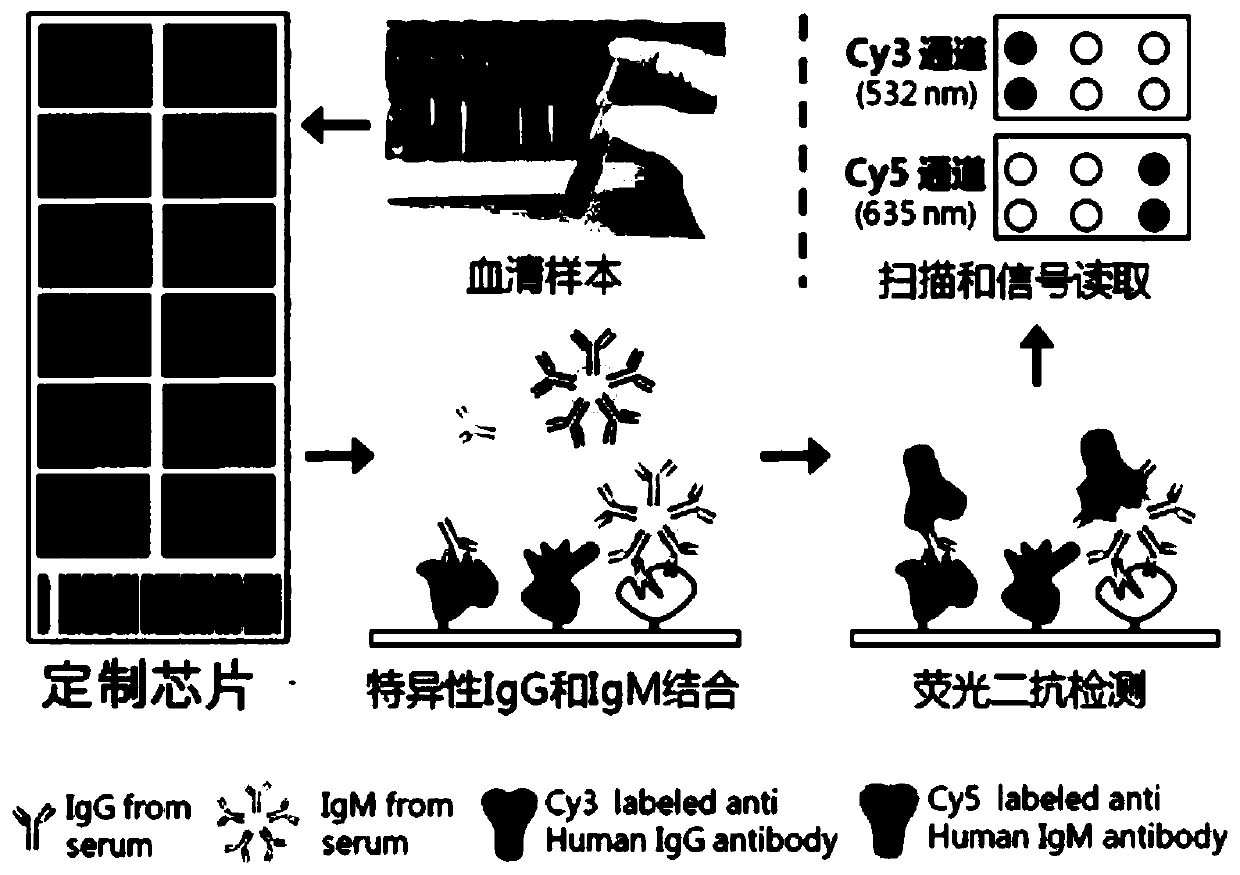
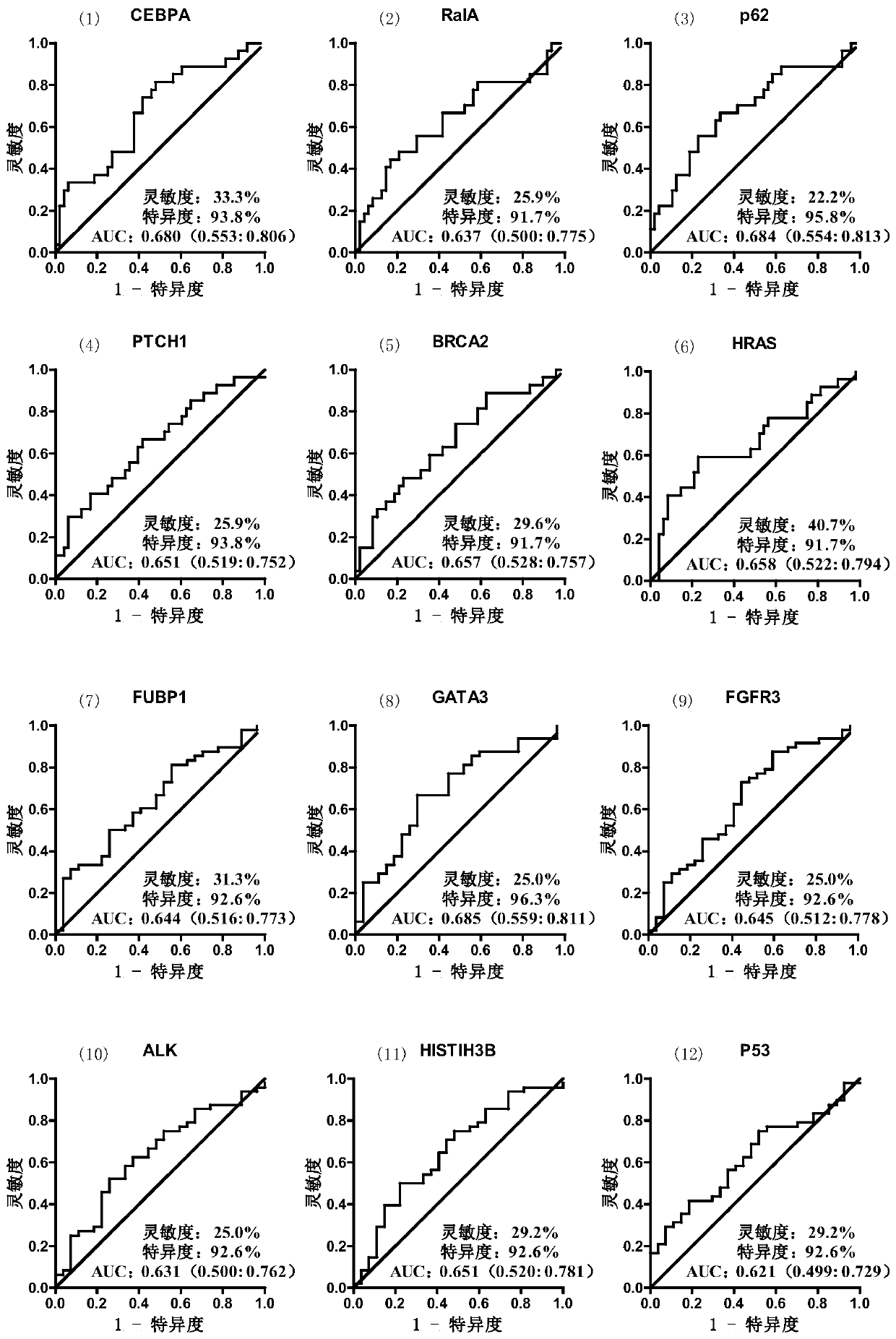
The invention discloses a serum protein marker for early screening and diagnosis of breast cancer, which belongs to the technical field of biomedicine. The serum protein marker for early screening anddiagnosis of the breast cancer is any one or a combination of two or more of the proteins encoded by the CEBPA, RalA, p62, PTCH1, BRCA2, HRAS, FUBP1, GATA3, FGFR3, ALK, HISTIH3B or p53 genes. The invention also discloses a kit and a detection method including the serum protein marker for early screening and diagnosis of the breast cancer. Based on the role played by cancer driver genes in the genesis and development of the tumor, the invention customizes a human protein chip encoded by 138 cancer driver genes, which contains 180 human-derived recombinant proteins. Firstly, an early detectionserum marker of the breast cancer is first screened through the protein chip; then verification is performed by using the ELISA indirect method; and finally, a group of breast cancer serum protein marker that can be used for early screening and diagnosis of the breast cancer is screened out. The area under the ROC curve of the combined diagnosis of breast cancer reaches 0.886; the 95% CI is 0.847to 0.925; and when the specificity is ensured to be 92.2%, the sensitivity is 67.9%, and the consistency rate reaches 80.1%.
Owner:ZHENGZHOU UNIV
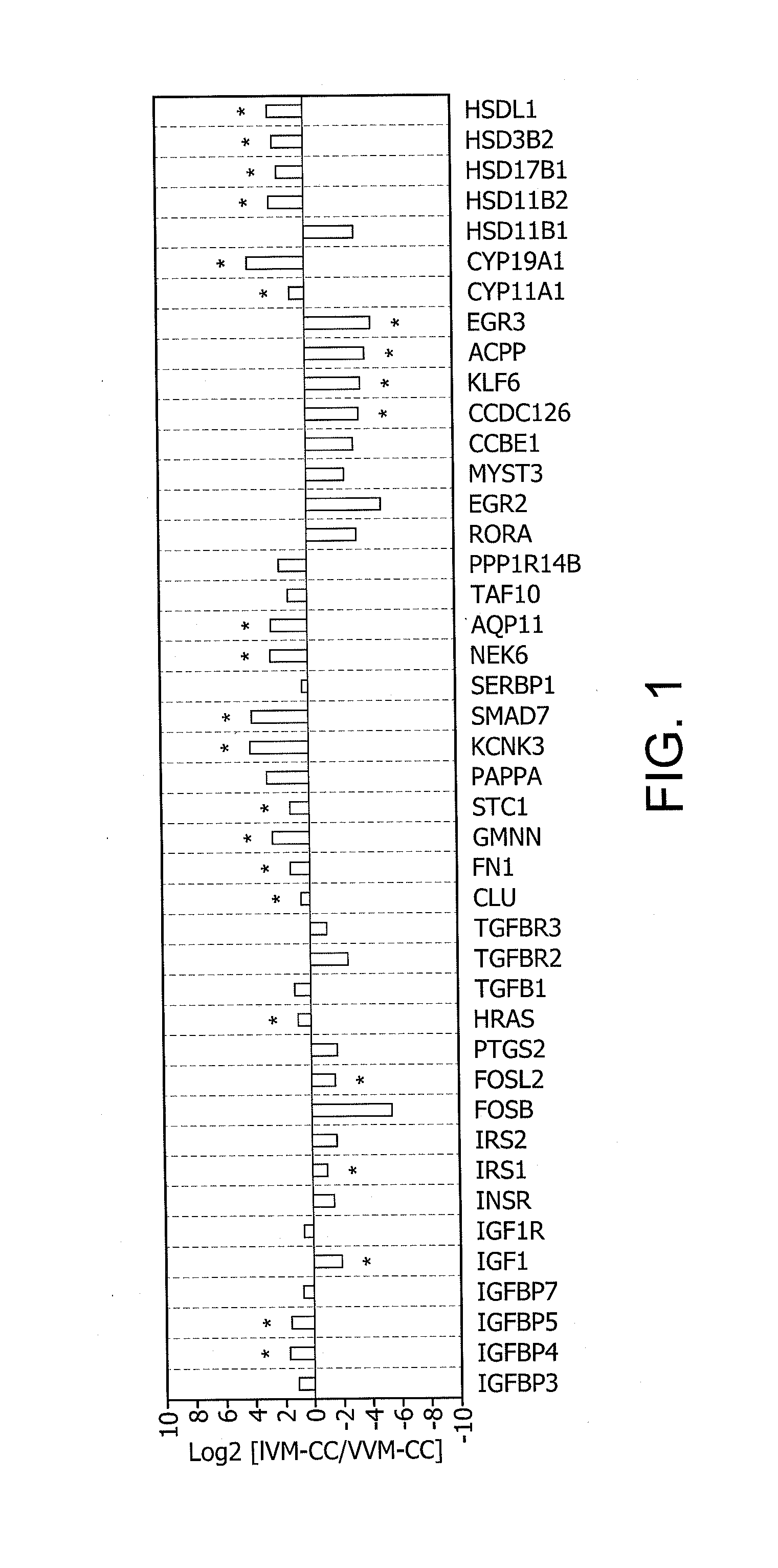
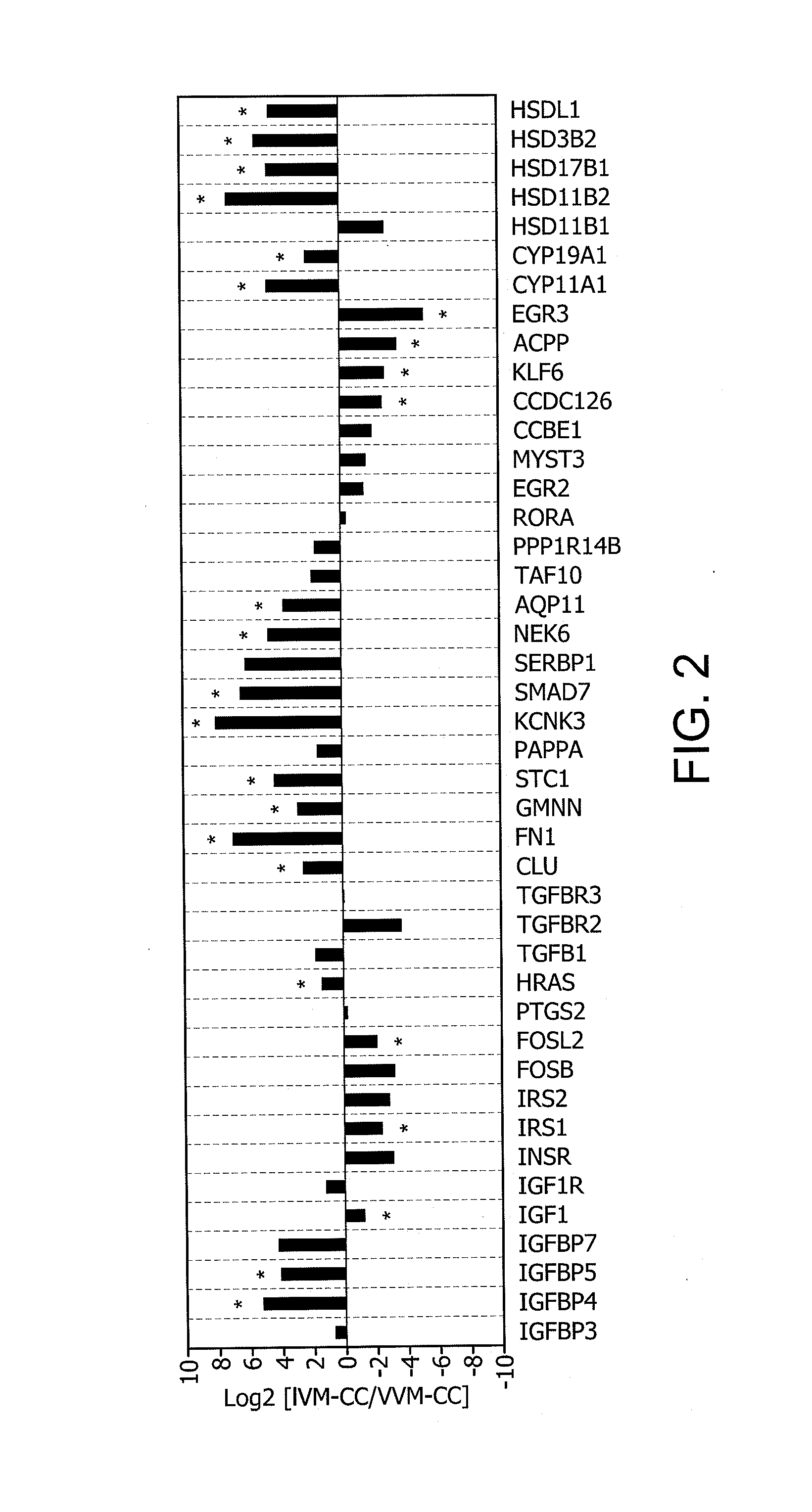
Determination of oocyte quality
A method for evaluating the quality of mammalian oocytes comprises determining the expression level of one or more of the genes ACPP, AQP11, CCDC126, CLU, CYP11 A1, CYP19A1, EGR3, FN1, FOSL2, GMNN, HRAS, HSD3B2, HS-D17B1, HSD11B2, HSDL1, IGF1, IGFBP4, IGFBP5, IRS1, KCNK3, KLF6, NEK6, SMAD7 or STC1 in a test sample derived from a cumulus cell or granulosa cell associated with the oocyte, and comparing the expression level of said at least one marker gene expression in the sample with the expression level in a control. Differential expression of the gene between the sample and the control is indicative of the quality of the oocyte.
Owner:RGT UNIV OF CALIFORNIA +1
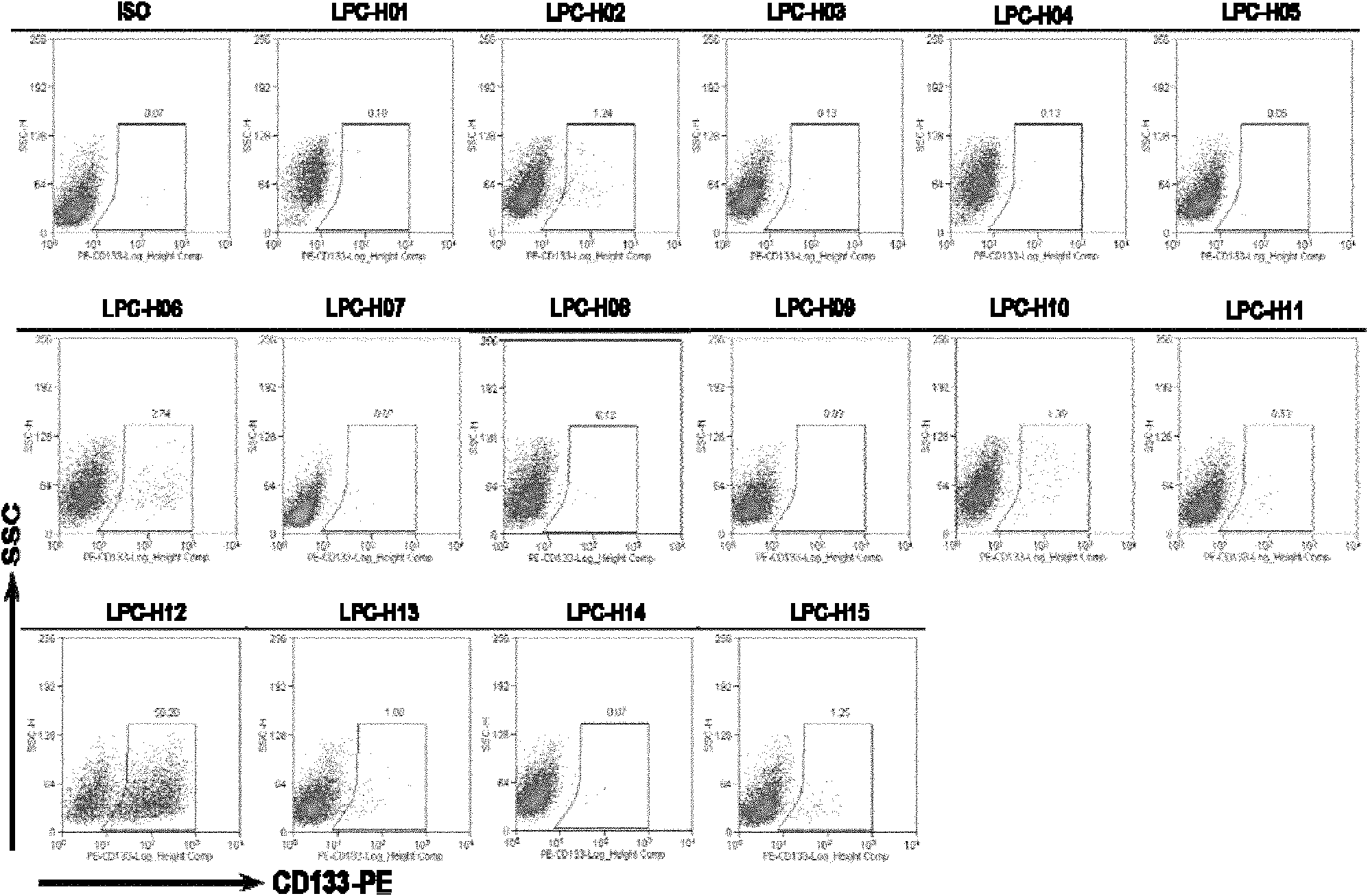
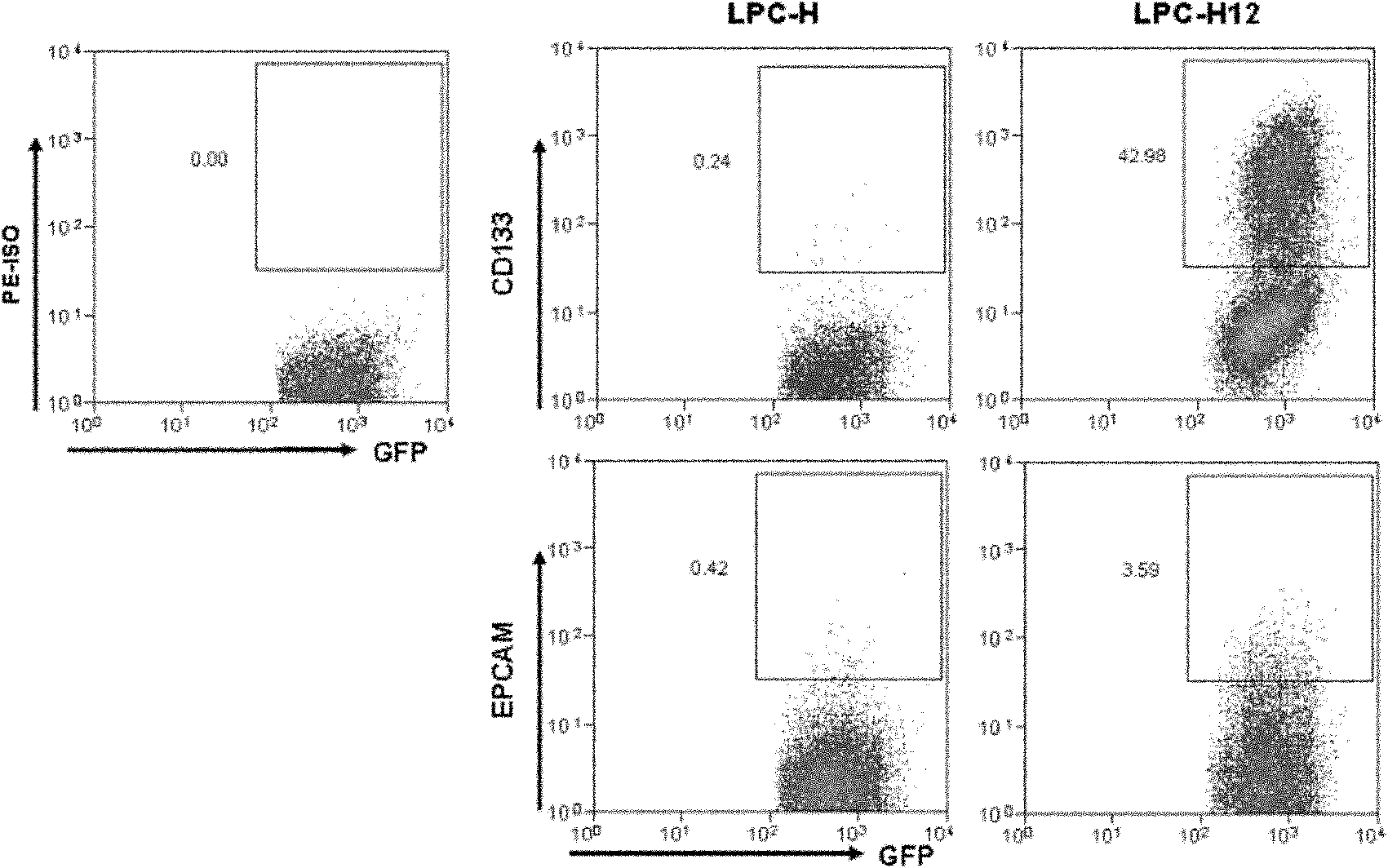
Mouse liver tumor cell line for highly expressing CD133 and preparation method thereof
ActiveCN102181399AHigh expressionHigh in vivo tumorigenicityMicroorganism based processesViruses/bacteriophagesEmbryoCell subpopulations
The invention provides a mouse liver tumor cell line for highly expressing CD133 and a preparation method thereof. The mouse liver tumor cell line with high content of CD133+cell subpopulations is established by the following steps of: importing a cancer gene Hras into p53- / - mouse fetal liver cells, obtaining a single-cell clonal group derived from CD133+cells by utilizing a monoclonal patternmaking technology, and finally screening. The cell line LPC-H12 expresses related genes of various liver stem cells and the stem cells, namely Marker:CD133 and EpCAM, and has high in-vitro balling capacity and in-vitro tumorigenic capacity. The mouse liver tumor cell line provides a powerful tool for researching the action and the mechanism of CD133+liver cancer stem cell subpopulations in the occurrence and development process of liver cancer and screening medicaments used for liver tumor stem cells.
Owner:SHANGHAI INST OF ONCOLOGY
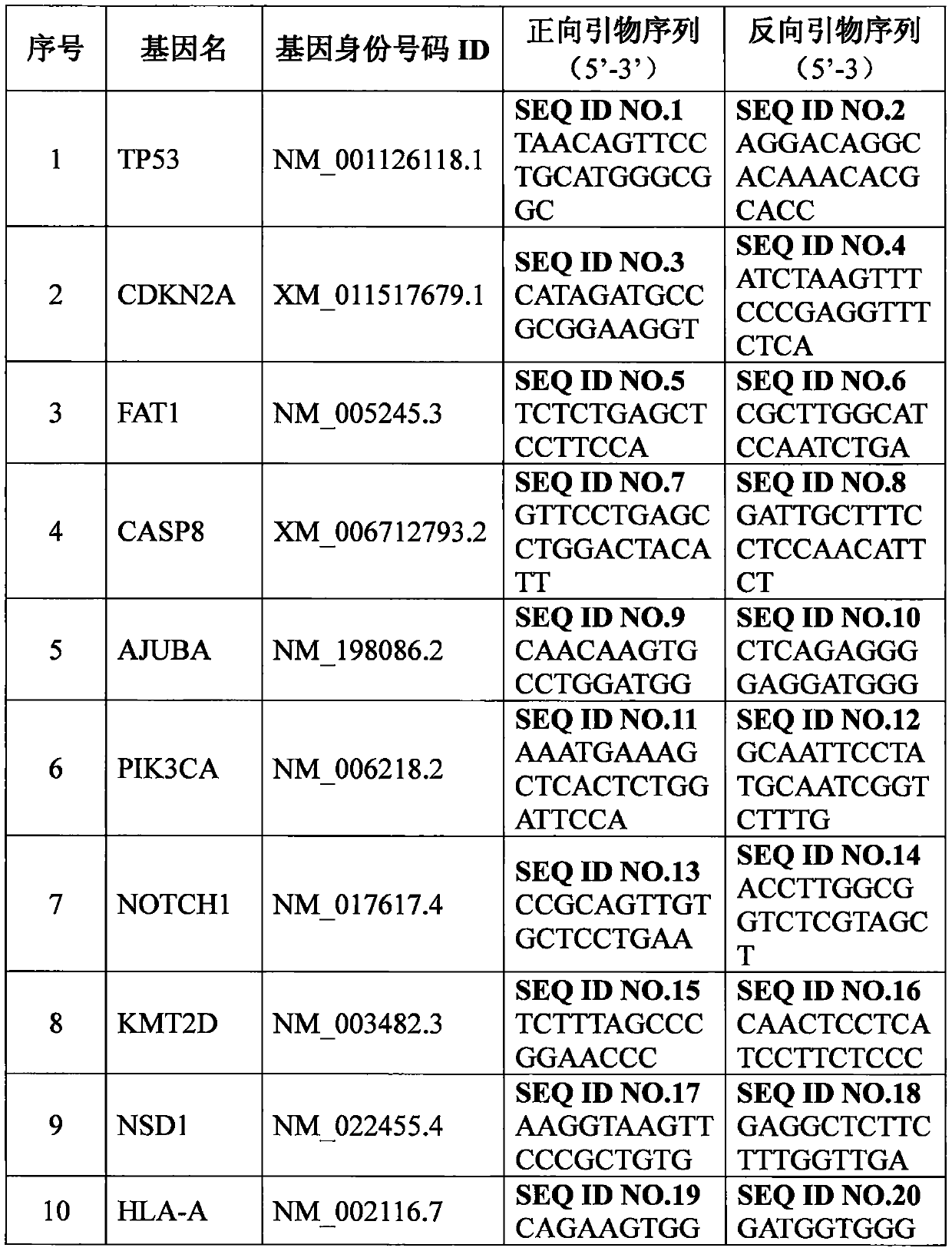
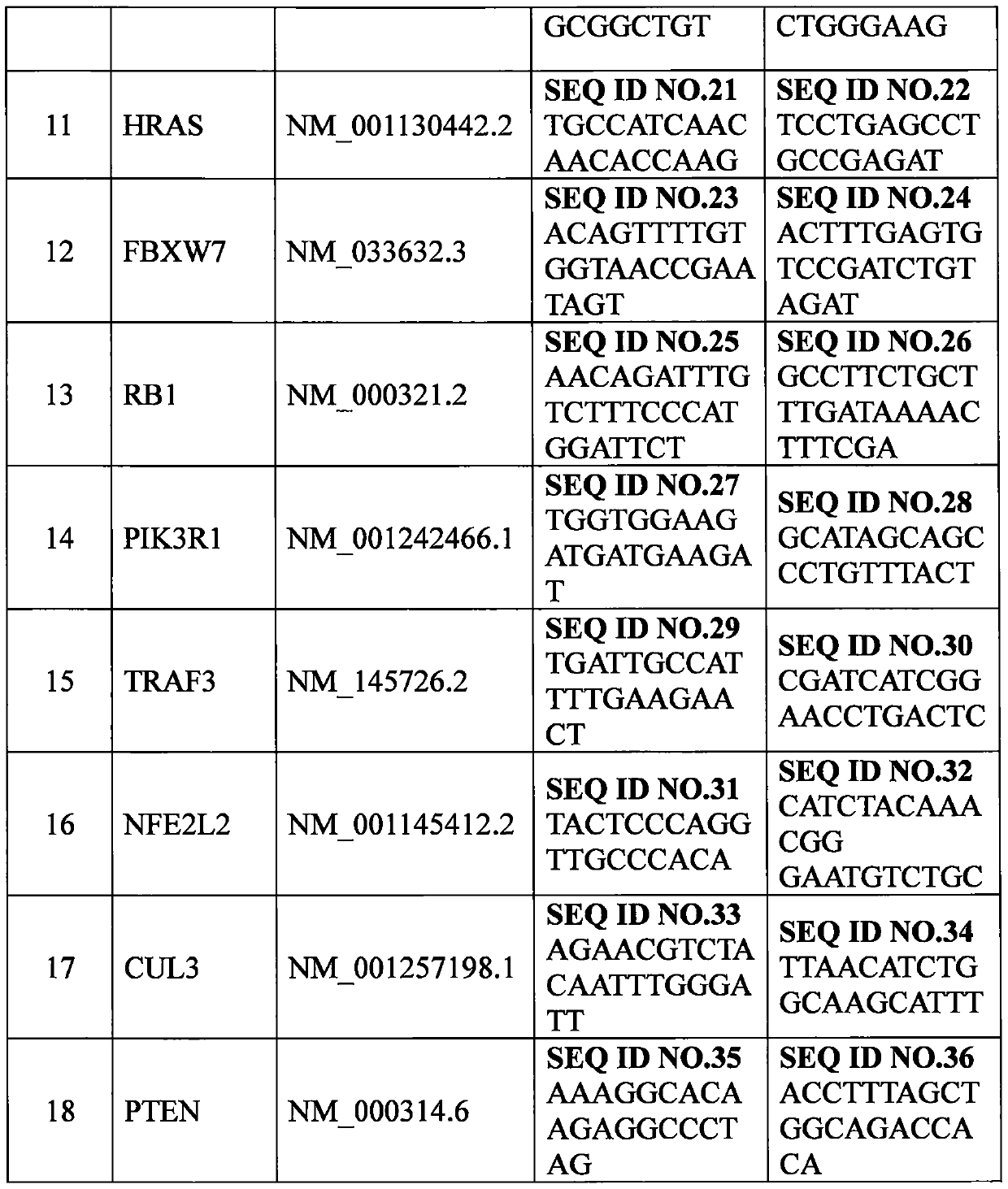
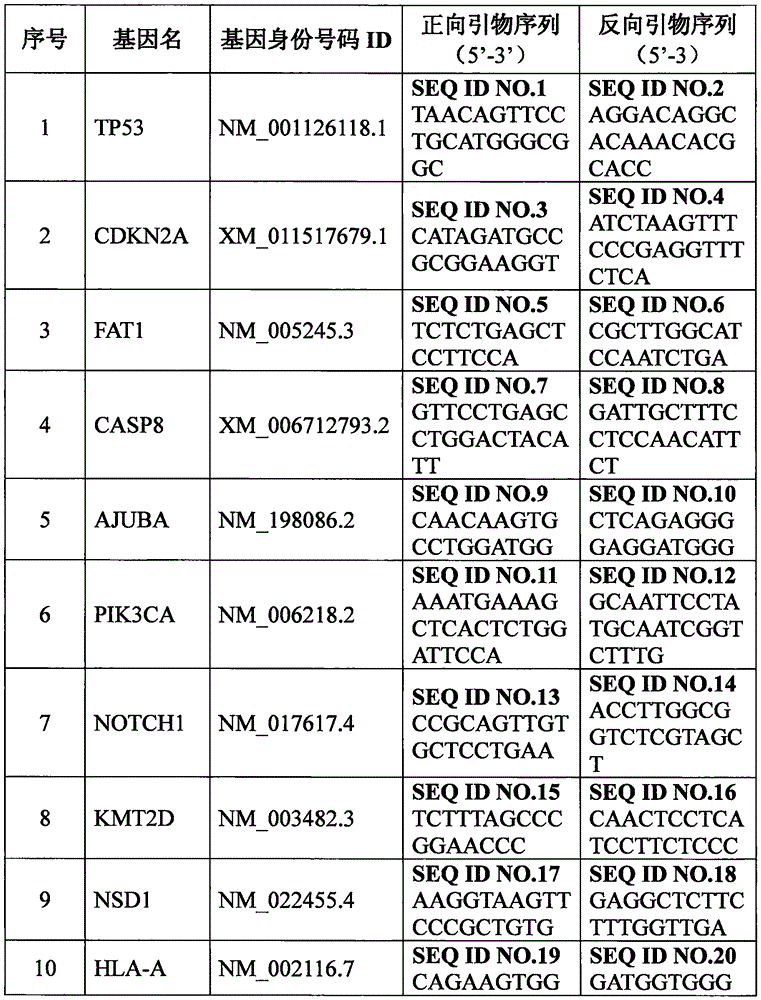
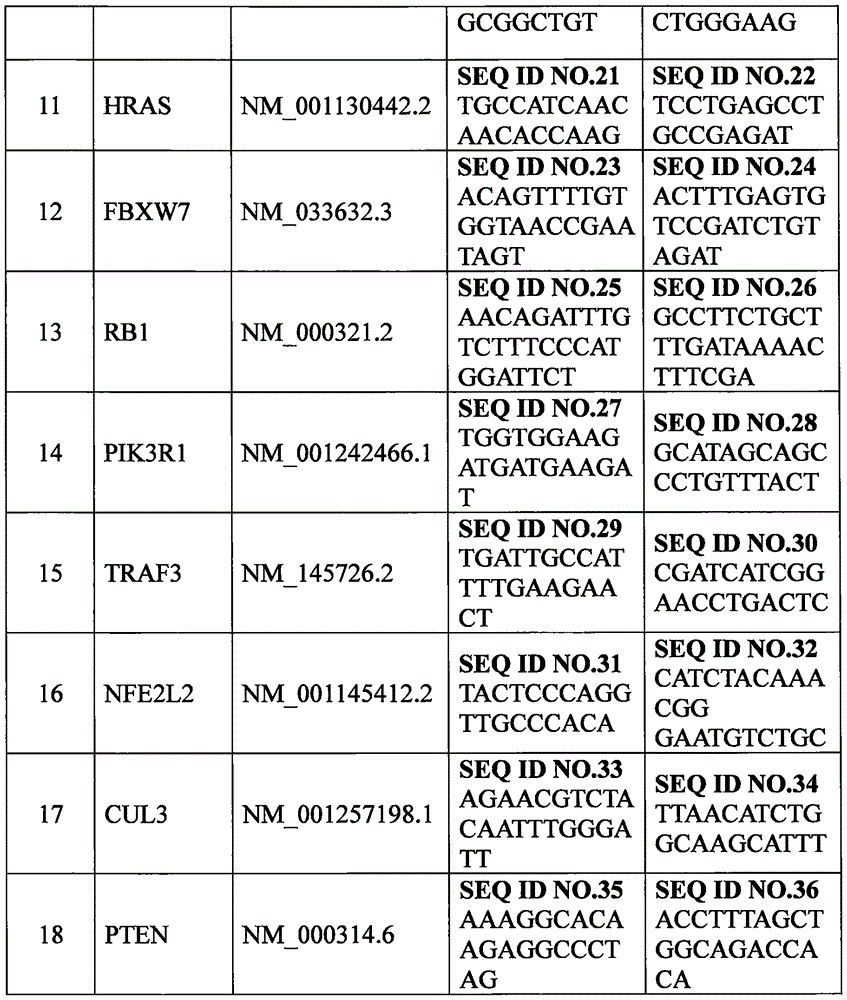
Set of genes for head and neck squamous cell carcinoma (HNSCC) molecular typing and application thereof
ActiveCN105671187AImprove rationalityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationKMT2D geneIndividualized treatment
The invention provides a set of genes for head and neck squamous cell carcinoma (HNSCC) molecular typing. The set of genes comprises TP53 gene, CDKN2A gene, FAT1 gene, CASP8 gene, AJUBA gene, PIK3CA gene, NOTCH1 gene, KMT2D gene, NSD1 gene, HLA-A gene, HRAS gene, FBXW7 gene, RB1 gene, PIK3R1 gene, TRAF3 gene, NFE2L2 gene, CUL3 gene and PTEN gene. The invention also provides application of the genes in preparing a kit and gene chip for HNSCC molecular typing, and a kit and gene chip for HNSCC molecular typing prepared by using the genes. The genes can enhance the HNSCC typing rationality and accuracy, thereby providing key technical supports for implementing early diagnosis, effective interception and individualized treatment on HNSCC.
Owner:SOUTHERN MEDICAL UNIVERSITY
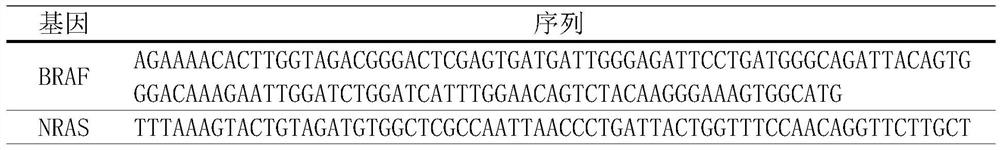
Primer for detecting variation of benign and malignant related genes of thyroid nodules, kit and detection method
ActiveCN110241215AImprove accuracyHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBiologyEIF1AX gene
The invention discloses a primer for detecting variation of benign and malignant related genes of thyroid nodules, a kit and a detection method. According to the primer, the kit and the detection method, variation of 15 loci of the six genes and variation of the three fusion genes can be detected simultaneously, and the BRAF gene, the KRAS gene, the HRAS gene, the NRAS gene, the TERT gene, the EIF1AX gene, RET / PTC1 fusion, RET / PTC3 fusion and PAX8 / PPARgamma fusion are involved. Hotspot mutation and fusion variation of the genes are closely related to the benign and malignant thyroid nodules. Therefore, the primer, the kit and the detection method are used for detecting a sample of a patient and can assist doctors in benign and malignant identification of the thyroid nodules which cannot be clearly diagnosed in the cytology, the accuracy of identifying the benign and malignant thyroid nodules is improved, excessive medical treatment for the patient with the thyroid nodules is reduced, and the primer, the kit and the detection method have important practical significance and high economic benefit for saving medical resources of China.
Owner:润安医学科技(苏州)有限公司
Combination therapy
Reversing resistance to a B-Raf inhibitor for the treatment of a proliferative disease by obtaining a tumor sample from the patient and testing it for genetic alterations in a panel of genes comprising BRAF, CRAF, CCND1, CDK4, HER2, IGF-1R, cMET, FGFR1, FGFR2, FGFR3 EGFR, MAP2K1, MAP2K2, NRAS, KRAS HRAS, PTEN, PIK3CA, and P16 and administering a drug combination therapy comprising the B-Raf inhibitor and a second inhibitor which overcomes resistance to the B-Raf inhibitor, which second inhibitor is selected based on genetic alterations discovered in the tumor sample.
Owner:ARRAY BIOPHARMA
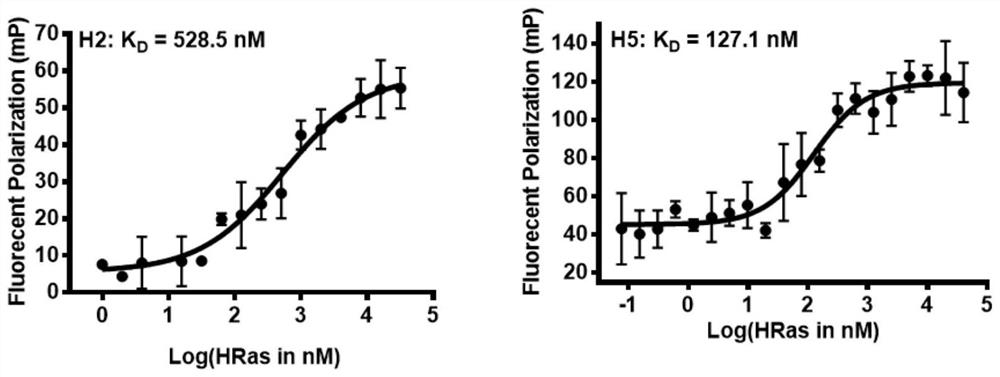
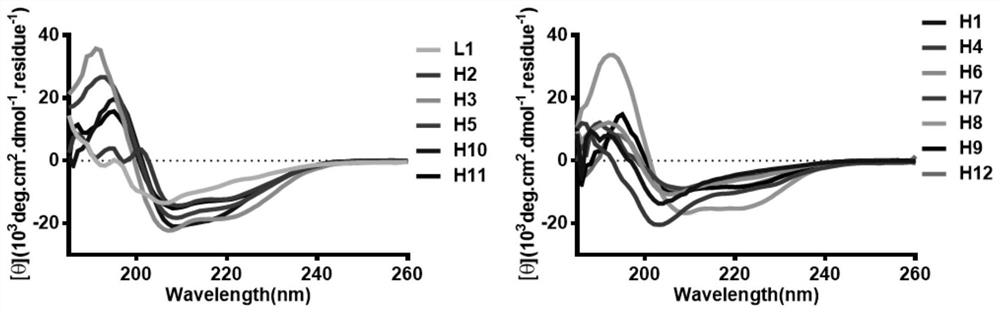
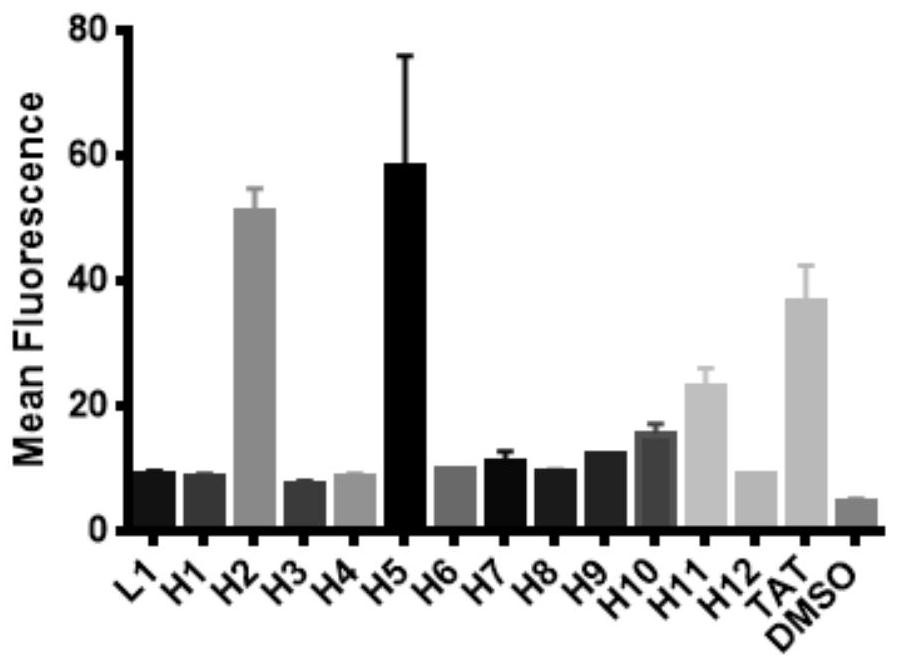
[alpha]-helical peptide inhibitor for targeting HRas protein, and purpose of [alpha]-helical peptide inhibitor
ActiveCN111718398AHigh binding affinityPrevent proliferationTetrapeptide ingredientsPeptidesBiochemistryPharmaceutical Substances
The invention discloses an [alpha]-helical peptide inhibitor for targeting HRas protein. The structural formula of the [alpha]-helical peptide inhibitor is disclosed as follows. The invention also provides a purpose of the above peptide inhibitor for preparing the medicine of the targeting HRas protein. The [alpha]-helical peptide inhibitor for the targeting HRas protein can be applied to a radiotherapy radiation sensitizer.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL +1
HRAS (Health Risk Assessment System) gene detection kit
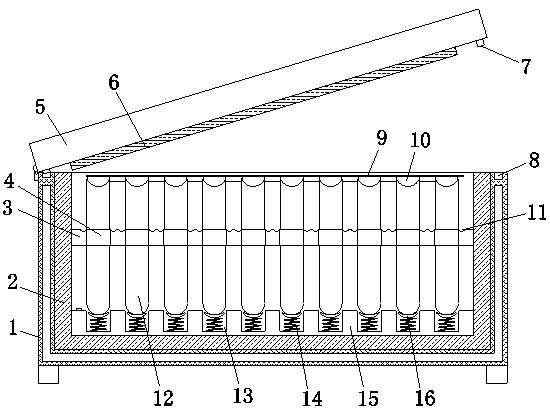
InactiveCN107916214AEasy to fixAvoid damageBioreactor/fermenter combinationsBiological substance pretreatmentsInsulation layerEngineering
The present invention relates to the technical field of medical equipment, in particular to a test kit for HRAS gene detection, which includes a box body, a box cover is installed on the upper side of the box body through a hinge, and the box body is a double-layer structure. The lower part of the bit block is fixedly connected with the upper part of the sealing plug, and the inner bottom of the insulation layer is fixedly provided with a backing plate, and several fixing grooves are equidistantly opened on the backing plate, and springs are fixedly installed in the fixing grooves, The upper part of the spring is fixedly provided with a buffer pad, the side wall of the buffer pad is movably connected with the side wall of the fixing groove, the bottom of the sealing plate is movably connected with the upper part of the limit block, and the bottom of the box cover is close to The outer sides of the sealing plates are provided with clamping strips, and the upper edge of the box body is provided with a clamping groove, and the clamping strips are clamped in the clamping grooves. The structure of the present invention is simple, which effectively improves the safety of transportation, and effectively The sealing effect is improved to ensure the accuracy of the test results.
Owner:CHENGDU YALIAN TECH CO LTD
Thyroid cancer auxiliary molecule diagnosis test kit and use method
InactiveCN111534599AImprove accuracyHigh detection sensitivityMicrobiological testing/measurementBraf genesTert gene
The invention provides a thyroid cancer auxiliary molecule diagnosis test kit. The thyroid cancer auxiliary molecule diagnosis test kit comprises amplification primers and probes for detecting BRAF, HRAS, KRAS, NRAS and TERT genes, internal control gene ACTB primers and probes, and external control gene BRAF primers and probes; the primers are specifically used for detecting a V600E site of the BRAF gene, a Q61R site of the HRAS gene, a G12C site of the KRAS gene, a G12D site of the KRAS gene, a G13R site of the KRAS gene, a G13D site of the KRAS gene, a Q61K site of the NRAS gene, a Q61P siteof the NRAS gene, a Q61H1 site of the NRAS gene, a Q61H2 site of the NRAS gene, a C228T site of the TERT gene and a C250T site of the TERT gene. The invention also provides a use method of the thyroid cancer auxiliary molecule diagnosis test kit. The problems of sensitivity, cost, operation convenience and the like of an existing thyroid cancer auxiliary molecule diagnosis test kit are solved.
Owner:重庆浦洛通基因医学研究院有限公司
A kind of primer and kit for detecting benign and malignant thyroid nodule related gene variation
ActiveCN110241215BImprove accuracyHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationOncologyMalignancy
The invention discloses a primer for detecting variation of benign and malignant related genes of thyroid nodules, a kit and a detection method. According to the primer, the kit and the detection method, variation of 15 loci of the six genes and variation of the three fusion genes can be detected simultaneously, and the BRAF gene, the KRAS gene, the HRAS gene, the NRAS gene, the TERT gene, the EIF1AX gene, RET / PTC1 fusion, RET / PTC3 fusion and PAX8 / PPARgamma fusion are involved. Hotspot mutation and fusion variation of the genes are closely related to the benign and malignant thyroid nodules. Therefore, the primer, the kit and the detection method are used for detecting a sample of a patient and can assist doctors in benign and malignant identification of the thyroid nodules which cannot be clearly diagnosed in the cytology, the accuracy of identifying the benign and malignant thyroid nodules is improved, excessive medical treatment for the patient with the thyroid nodules is reduced, and the primer, the kit and the detection method have important practical significance and high economic benefit for saving medical resources of China.
Owner:润安医学科技(苏州)有限公司
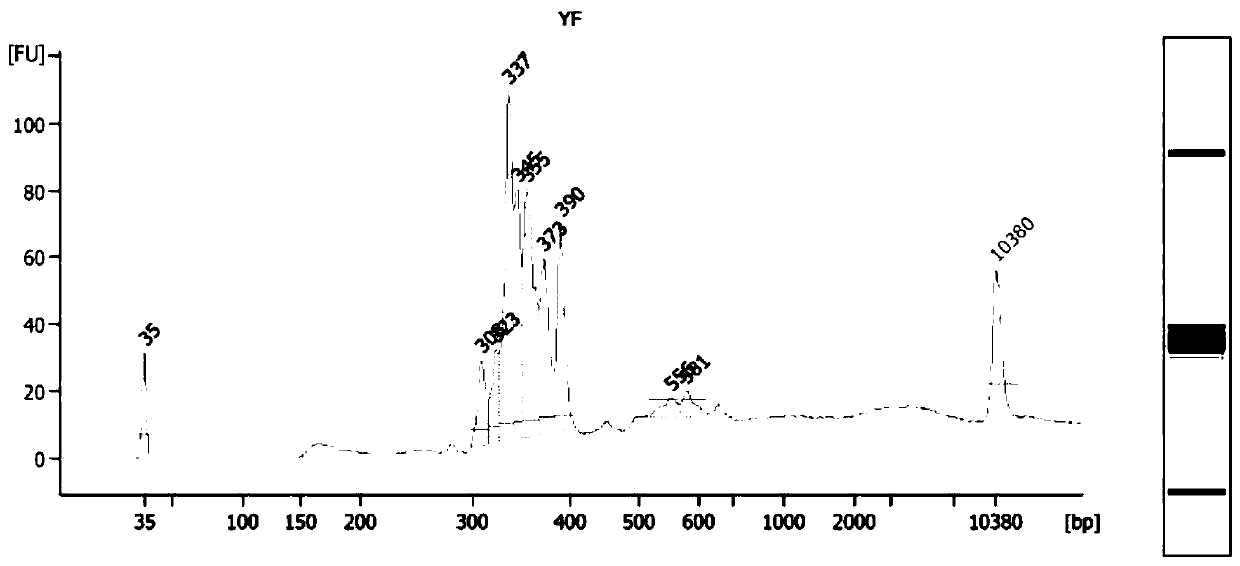
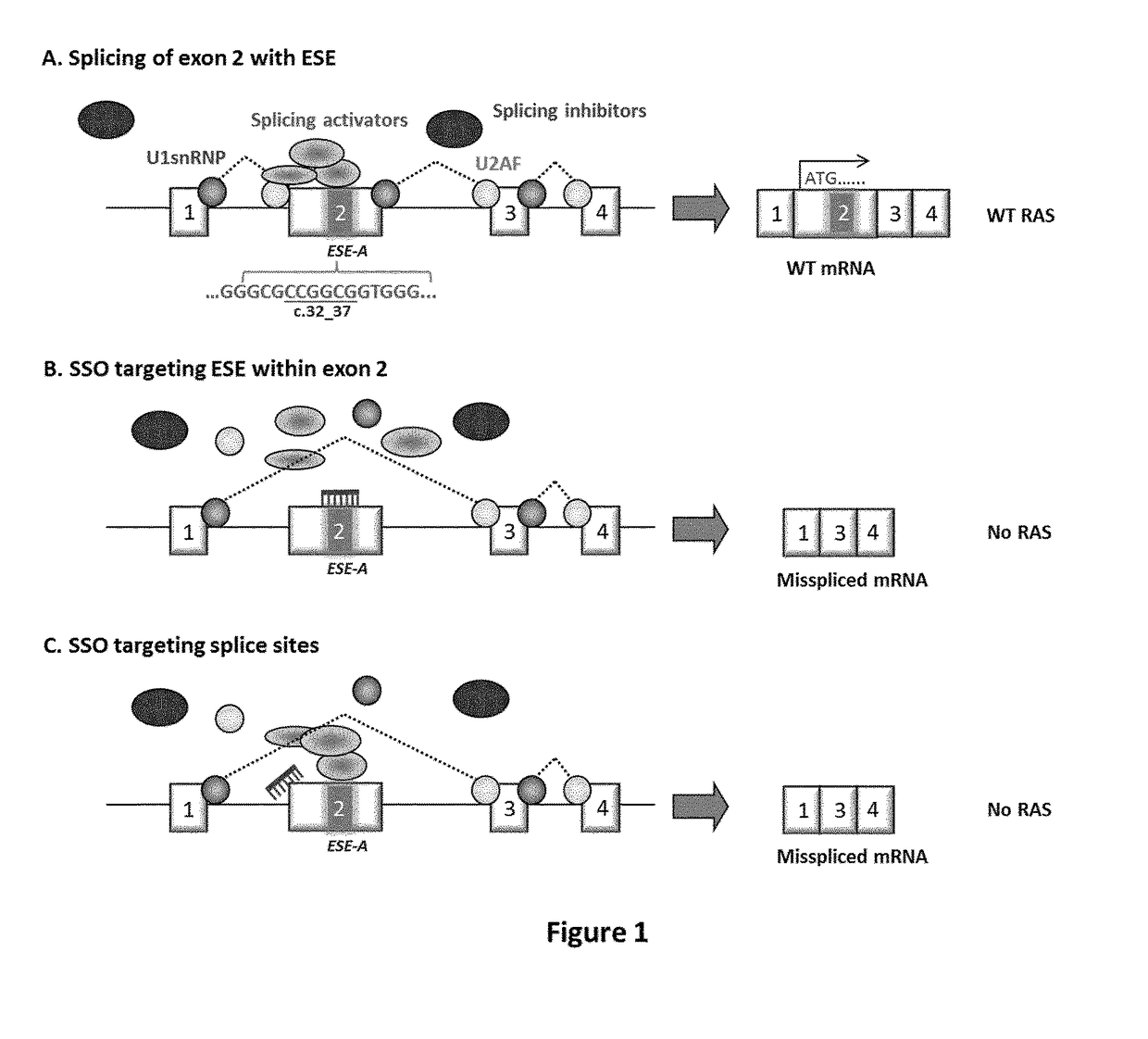
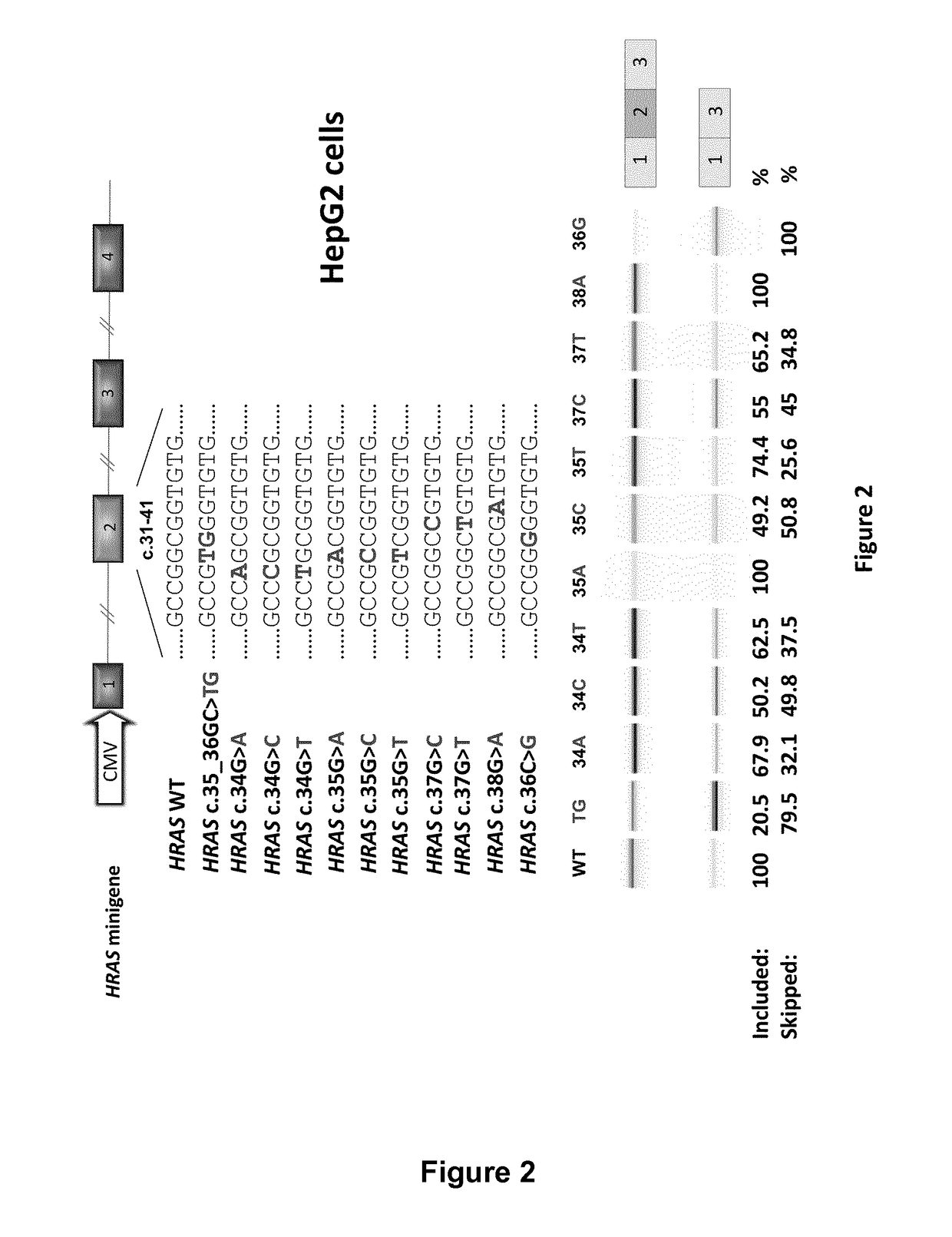
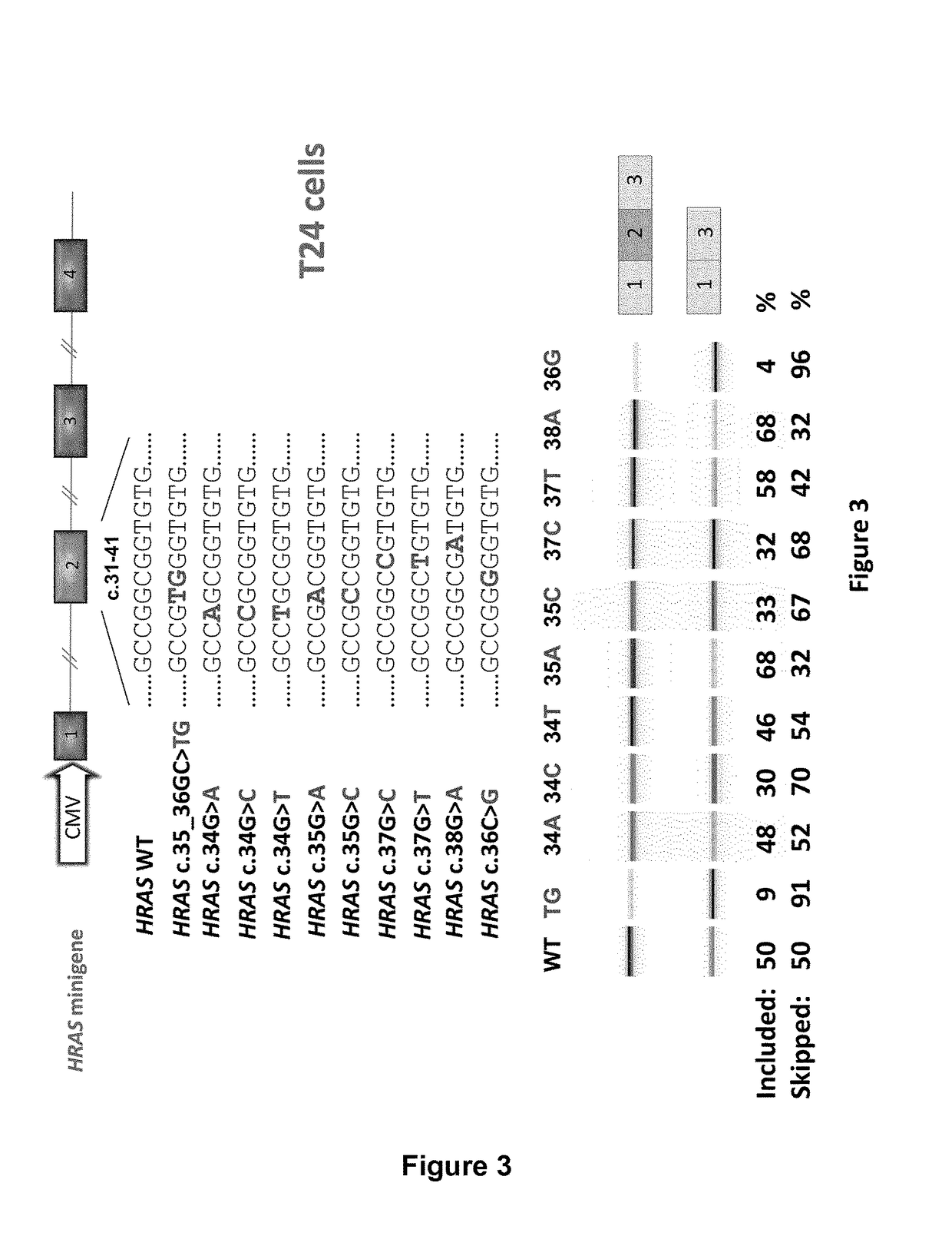
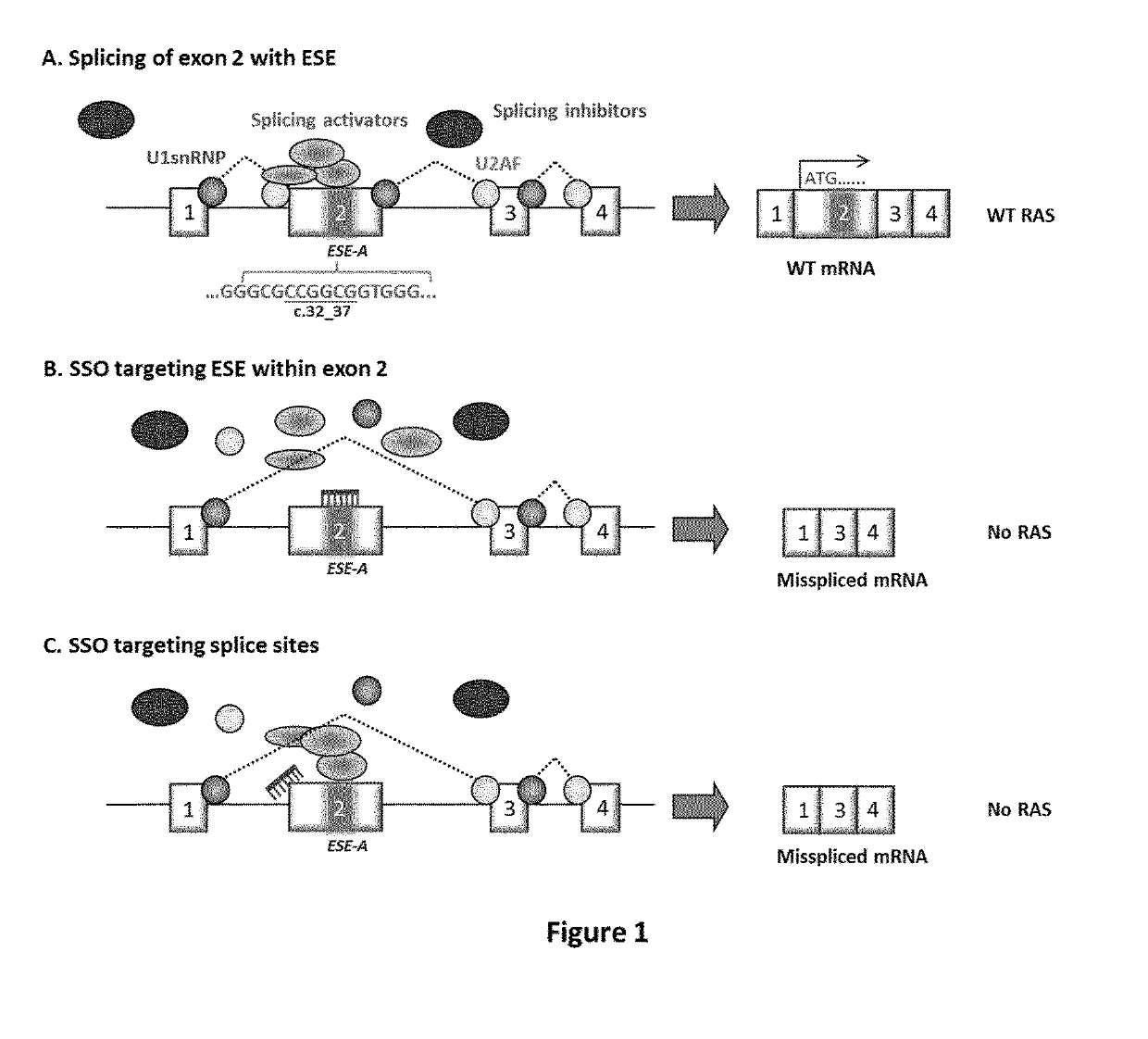
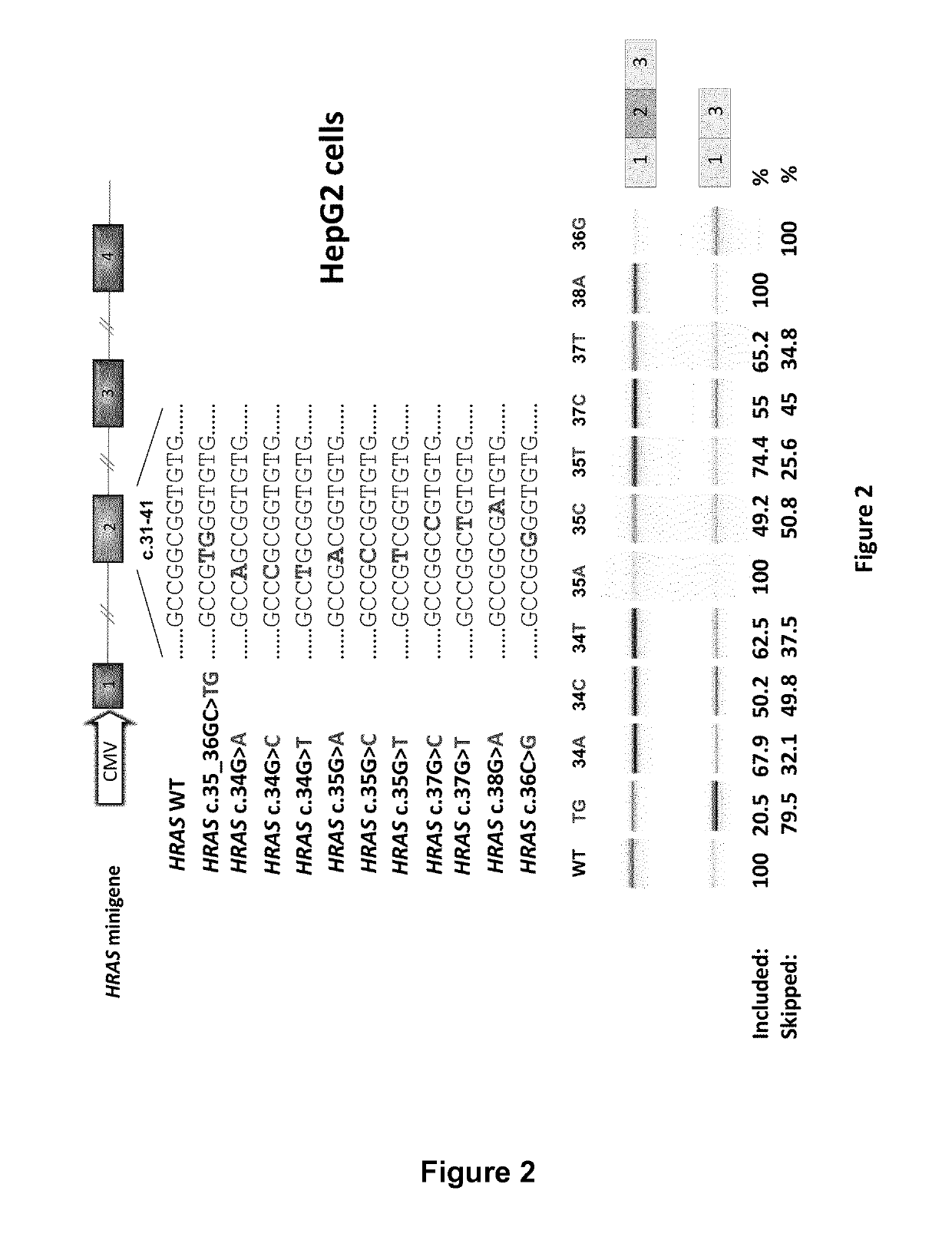
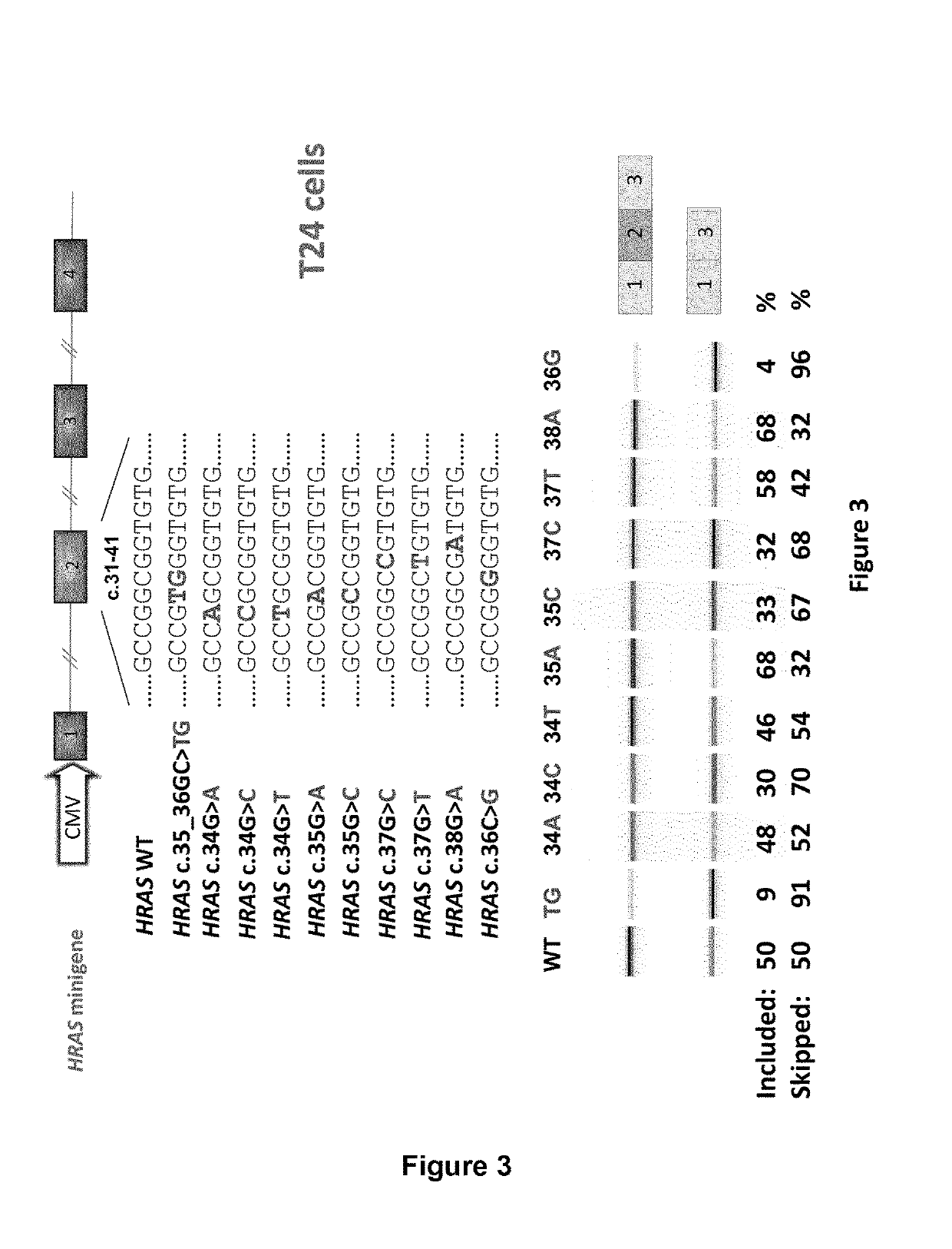
Ras exon 2 skipping for cancer treatment
ActiveUS20170240900A1Improves exon inclusionReduced growth and proliferation and deathSplicing alterationDNA/RNA fragmentationHRASCancer research
There is provided SSOs targeting the region of HRAS, KRAS, and HRAS exon 2 that harbors the activating mutations and which harbors ESE activity. Moreover, there is provided SSOs targeting the 3′- and 5′-splice sites. The SSOs targeting the 5′ splice site sequence of HRAS exon 2, the 3′ splice site sequence of KRAS exon 2 and the 3′ splice site sequence of NRAS exon 2, as well as SSOs that targets ESEs in a conserved part of exon 2 in the HRAS, KRAS and NRAS exon 2 sequences can induce complete or nearly complete exon 2 skipping in cancer cell lines. This results in growth and proliferation inhibition and concomitantly in death of cancer cells. Therefore this invention is directed towards treatment of cancerous diseases and other conditions where RAS signaling is involved.
Owner:SYDDANSK UNIV
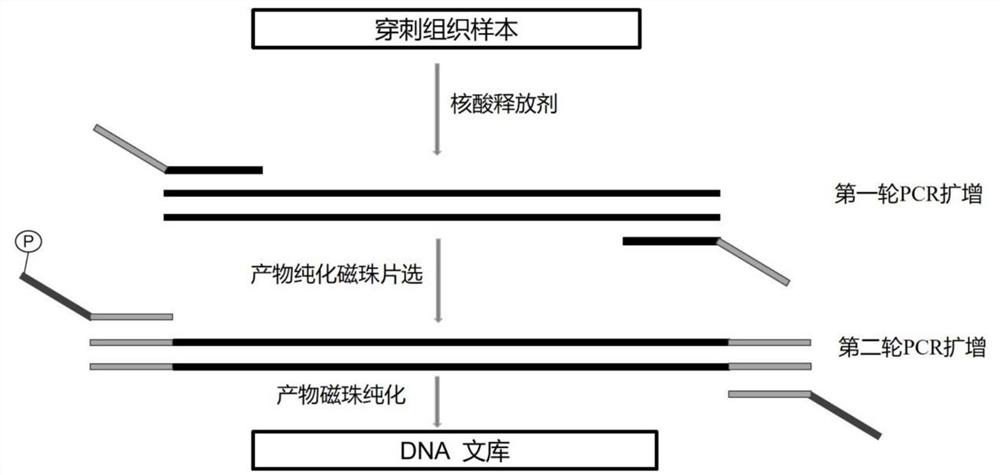
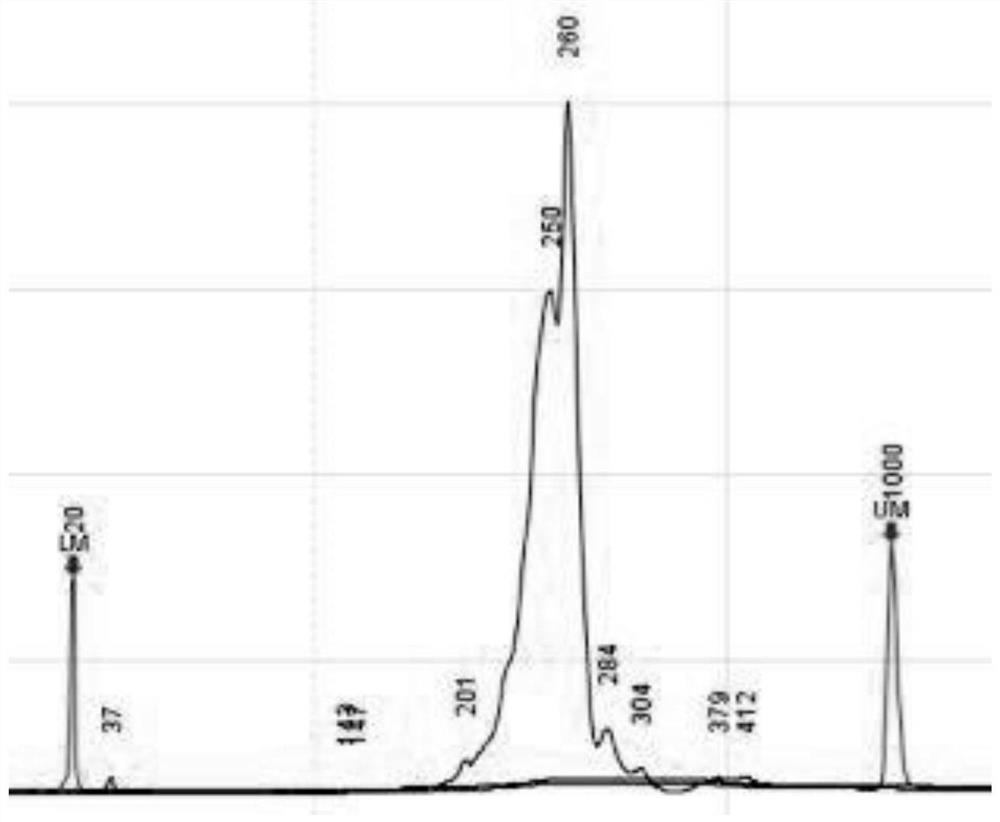
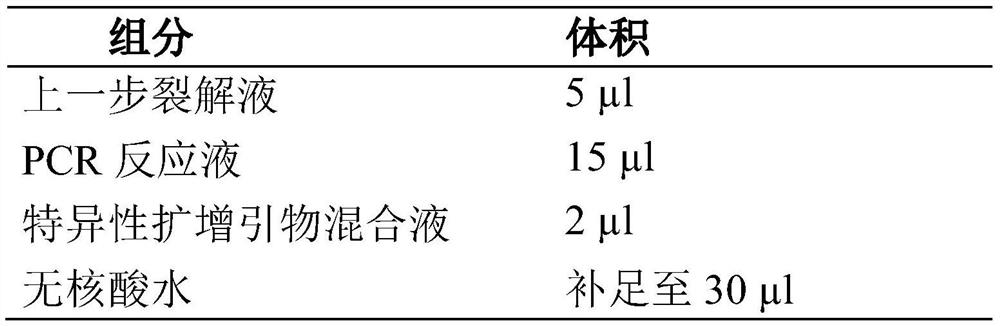
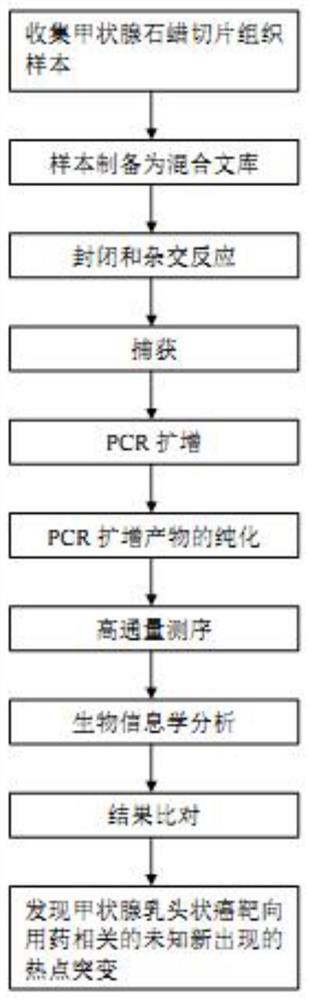
Extraction-free detection kit and detection method for oncogene mutation in human thyroid glands
InactiveCN111979327ARapid expansionReduce distractionsMicrobiological testing/measurementMultiplexGenes mutation
The invention discloses an extraction-free detection kit and detection method for oncogene mutation in human thyroid glands, belonging to the technical field of molecular biology. According to the kit, a specific nucleic acid releasing agent and PCR reaction liquid matched with the specific nucleic acid releasing agent are adopted, a multiplex PCR capture technology and an NGS sequencing technology are cooperatively used, and the kit is used for qualitatively detecting various variations of BRAF, TERT, RET, TP53, NRAS, HRAS, KRAS and PIK3CA genes in a fresh tissue sample with uncertainty according to results of thyroid nodule needle biopsy cytology; and through detection of multiple genes and multiple sites and consideration of clinical pathological analysis results, assistance is providedfor auxiliary diagnosis and treatment of thyroid cancer.
Owner:上海睿璟生物科技有限公司
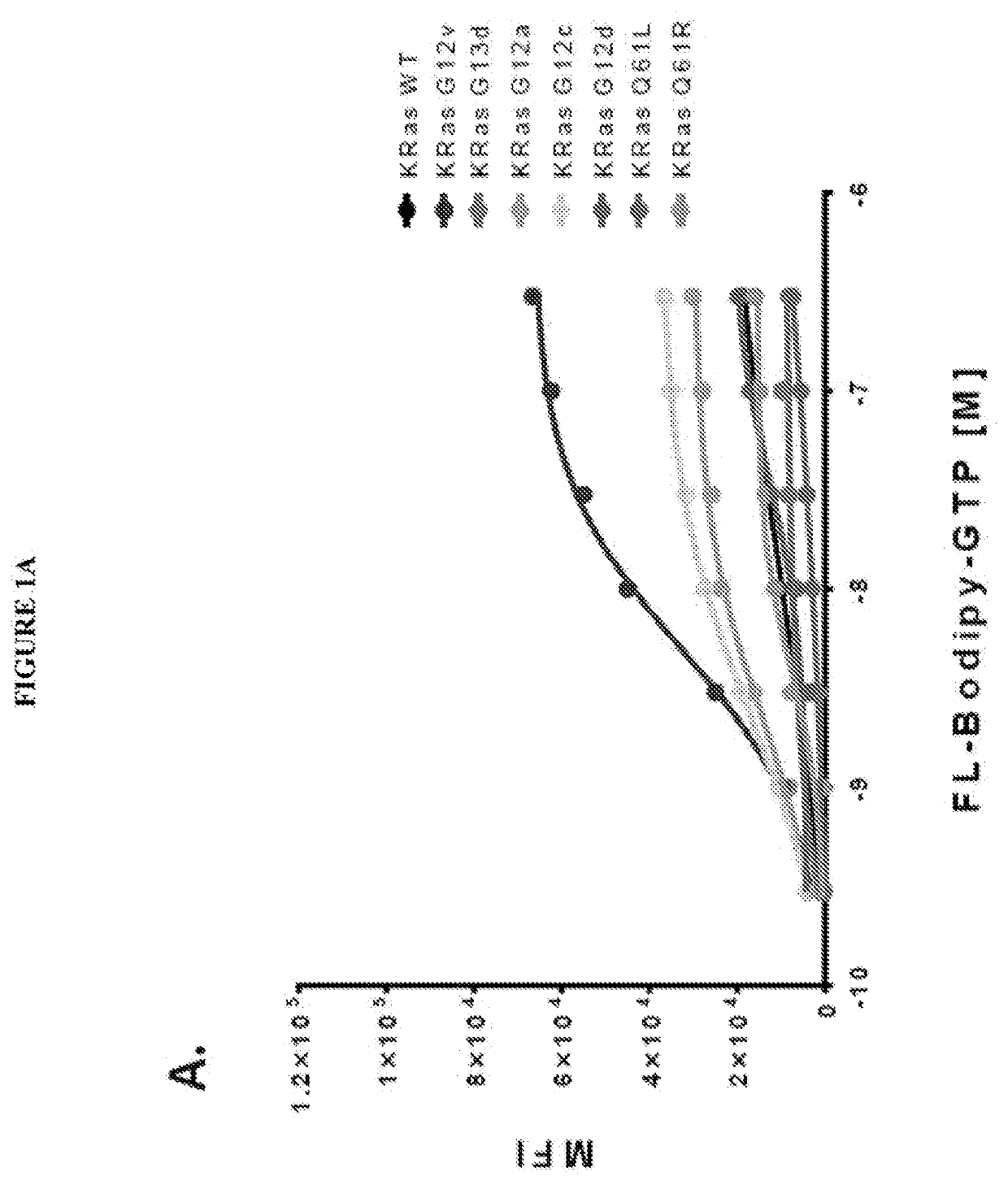
DISCOVERY OF NOVEL MOLECULES AND REPURPOSED DRUGS FOR RAS FAMILY GTPases
InactiveUS20210041441A1Improve understandingGreat tractionCompound screeningOrganic active ingredientsMultiplexPharmaceutical drug
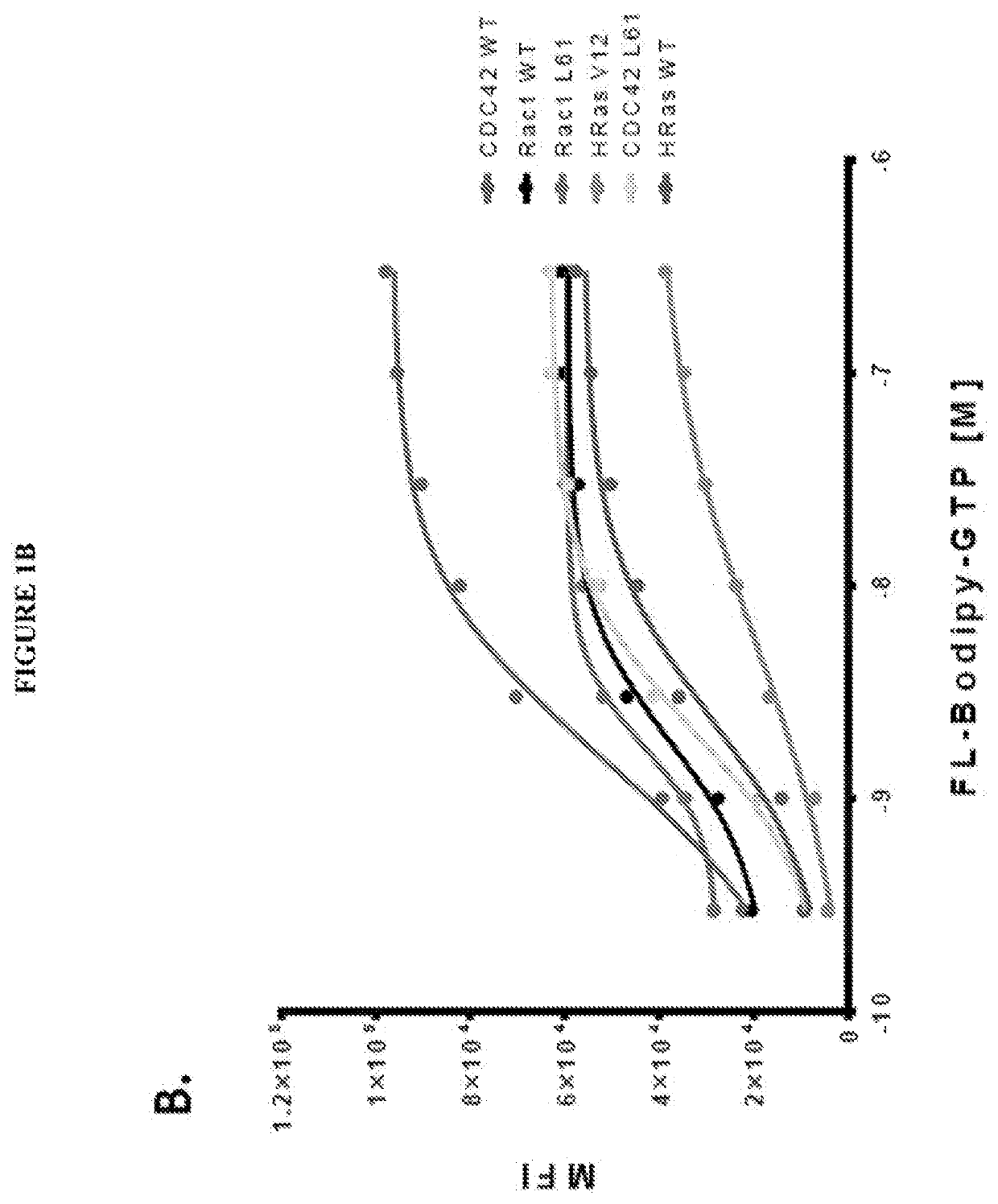
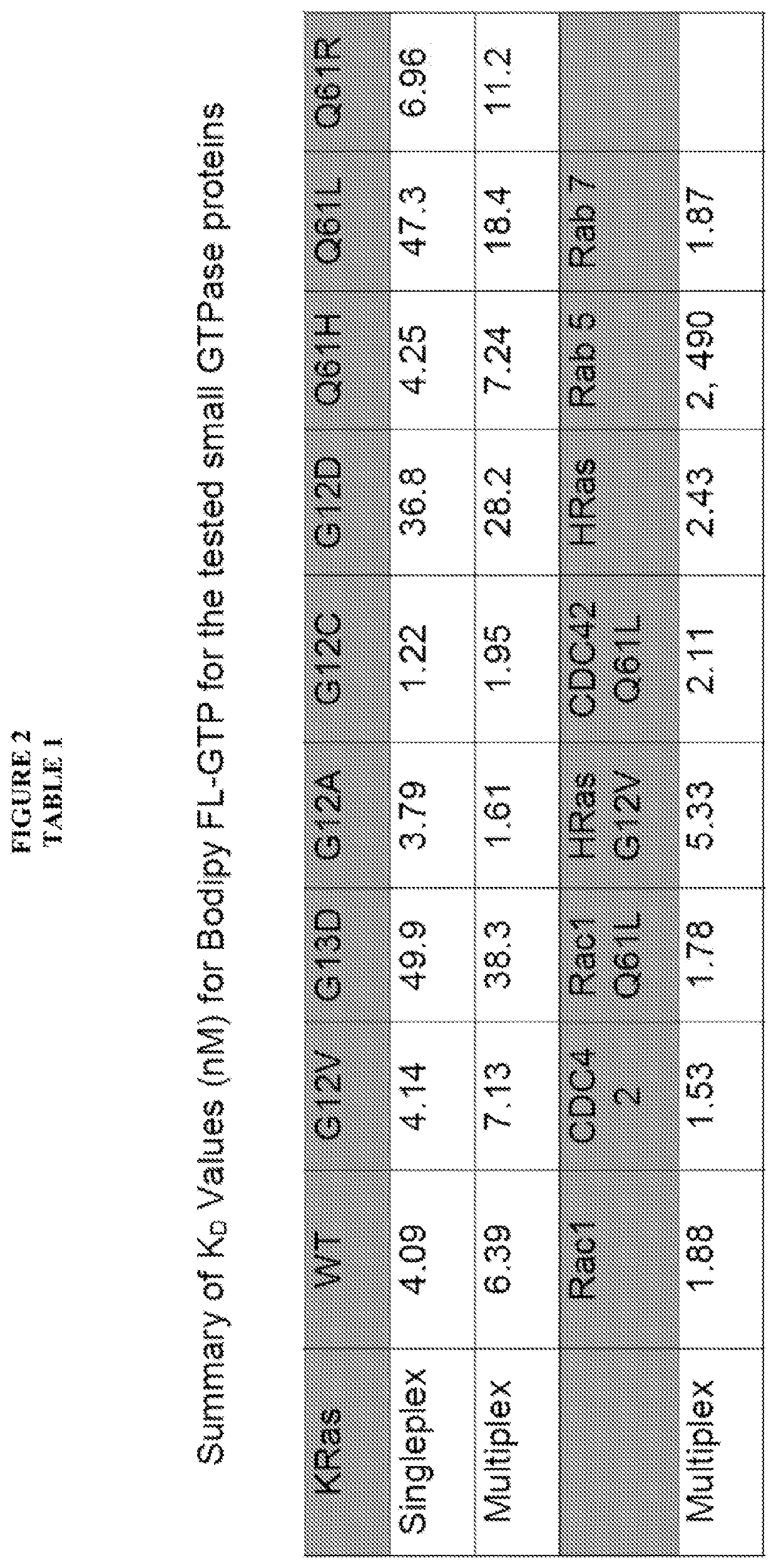
The present invention is directed to compounds, compositions and methods for modulating RAS family GTPases, in particular KRas, HRas and NRas GTPases. These GTPases are upregulated in cancer and in other tissue and represent appropriate targets for therapy. Methods for identifying the activity of compounds with respect to these and other GTPases in multiplex flow cytometry systems represents another aspect of this invention.
Owner:UNM RAINFOREST INNOVATIONS +1
RAS exon 2 skipping for cancer treatment
ActiveUS10266828B2Reduced growth and proliferation and deathReduced growth and proliferationSplicing alterationSugar derivativesDiseaseActivating mutation
There is provided SSOs targeting the region of HRAS, KRAS, and HRAS exon 2 that harbors the activating mutations and which harbors ESE activity. Moreover, there is provided SSOs targeting the 3′- and 5′-splice sites. The SSOs targeting the 5′ splice site sequence of HRAS exon 2, the 3′ splice site sequence of KRAS exon 2 and the 3′ splice site sequence of NRAS exon 2, as well as SSOs that targets ESEs in a conserved part of exon 2 in the HRAS, KRAS and NRAS exon 2 sequences can induce complete or nearly complete exon 2 skipping in cancer cell lines. This results in growth and proliferation inhibition and concomitantly in death of cancer cells. Therefore this invention is directed towards treatment of cancerous diseases and other conditions where RAS signaling is involved.
Owner:SYDDANSK UNIV
A panel of genes and their application for molecular typing of squamous cell carcinoma of the head and neck
ActiveCN105671187BImprove rationalityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationKMT2D geneIndividualized treatment
The invention provides a set of genes for head and neck squamous cell carcinoma (HNSCC) molecular typing. The set of genes comprises TP53 gene, CDKN2A gene, FAT1 gene, CASP8 gene, AJUBA gene, PIK3CA gene, NOTCH1 gene, KMT2D gene, NSD1 gene, HLA-A gene, HRAS gene, FBXW7 gene, RB1 gene, PIK3R1 gene, TRAF3 gene, NFE2L2 gene, CUL3 gene and PTEN gene. The invention also provides application of the genes in preparing a kit and gene chip for HNSCC molecular typing, and a kit and gene chip for HNSCC molecular typing prepared by using the genes. The genes can enhance the HNSCC typing rationality and accuracy, thereby providing key technical supports for implementing early diagnosis, effective interception and individualized treatment on HNSCC.
Owner:SOUTHERN MEDICAL UNIVERSITY
Biomarker for thyroid nodule benign and malignant discrimination, multi-gene joint detection kit and application
PendingCN114807350AHigh sensitivitySimple and fast operationMicrobiological testing/measurementDNA/RNA fragmentationBase JBiomarker (medicine)
The invention belongs to the field of biological medicine, and discloses a biomarker for judging benign and malignant thyroid nodules, a multi-gene joint detection kit and application. The biomarker group comprises at least one of eight DNA single base mutations and four gene fusions; the eight kinds of DNA single base mutations are selected from a BRAF gene V600E, a TERT gene C228T / C250T, a KRAS gene G12C / G12V / Q61R, an NRAS gene Q61R and an HRAS gene Q61R, and the four kinds of gene fusion are selected from CCDC6-RET (Exon1-Exon12), NCOA4-RET (Exon8-Exon12), PAX8-PPARG (Exon10-Exon2) and ETV6-NTRK3 (Exon4-Exon14). The method and the kit are high in detection sensitivity, high in specificity and wide in application scene.
Owner:上海睿璟生物科技有限公司
A thyroid multi-gene combined detection kit
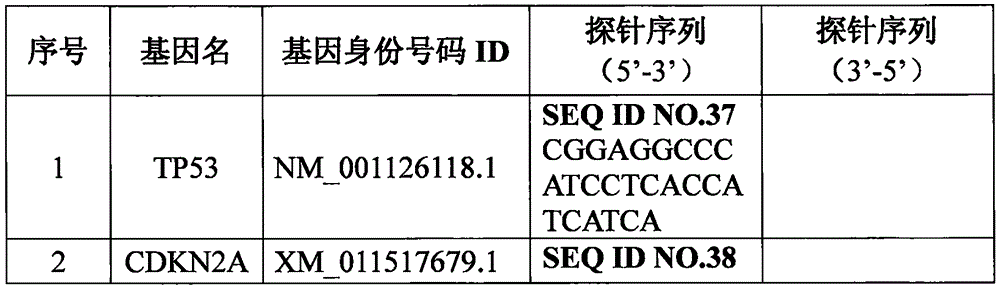
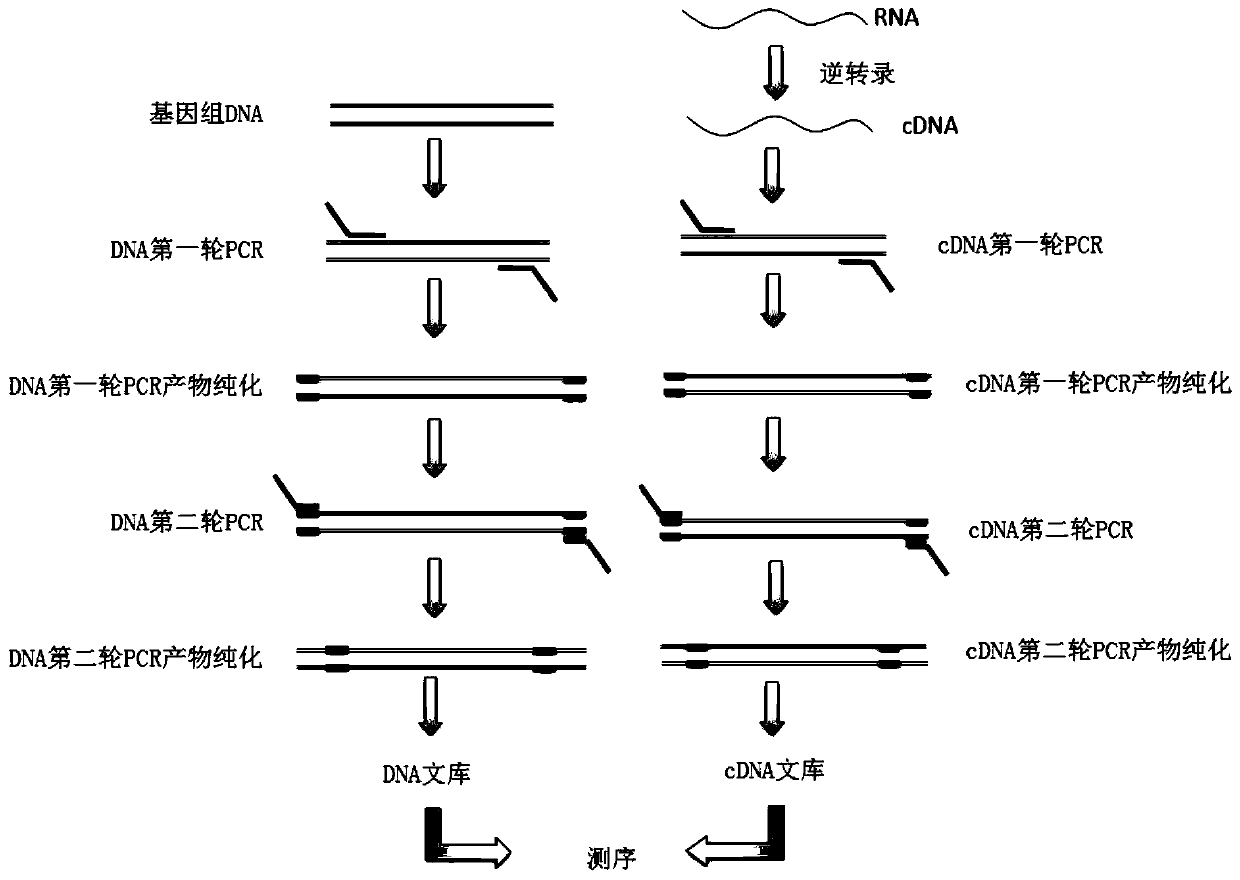
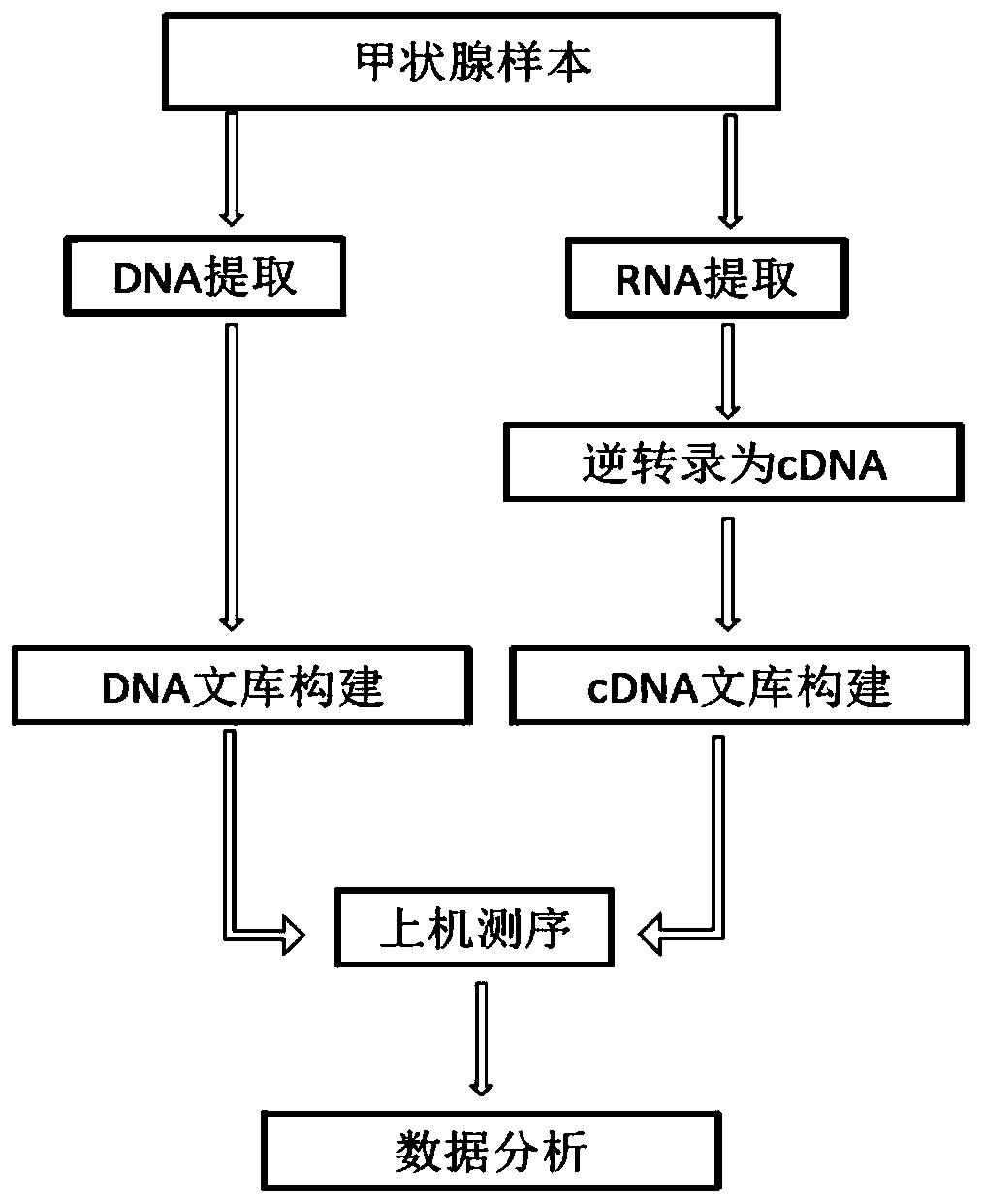
ActiveCN112280864BAchieving Simultaneous DetectionReduce sensitivityMicrobiological testing/measurementDNA/RNA fragmentationA-DNAMolecular Biology Technique
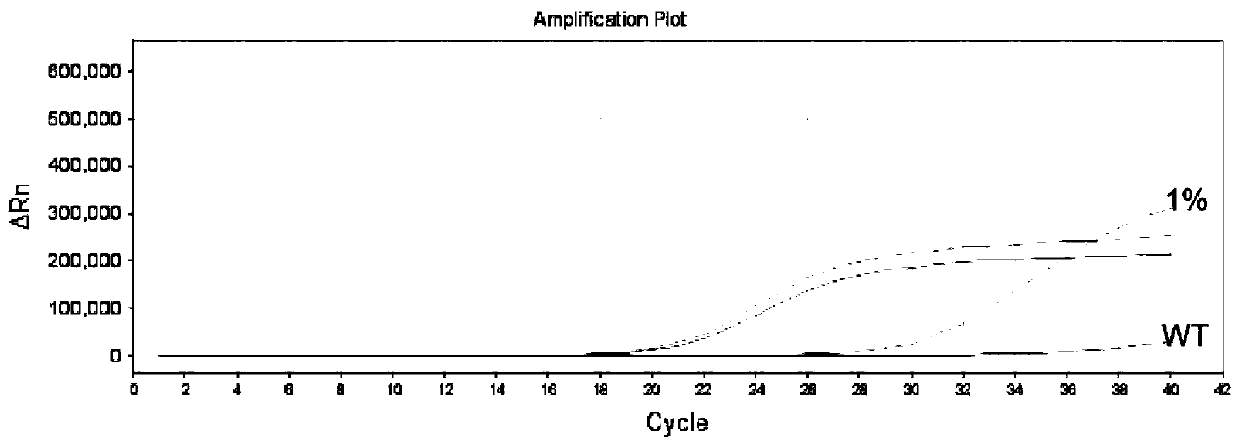
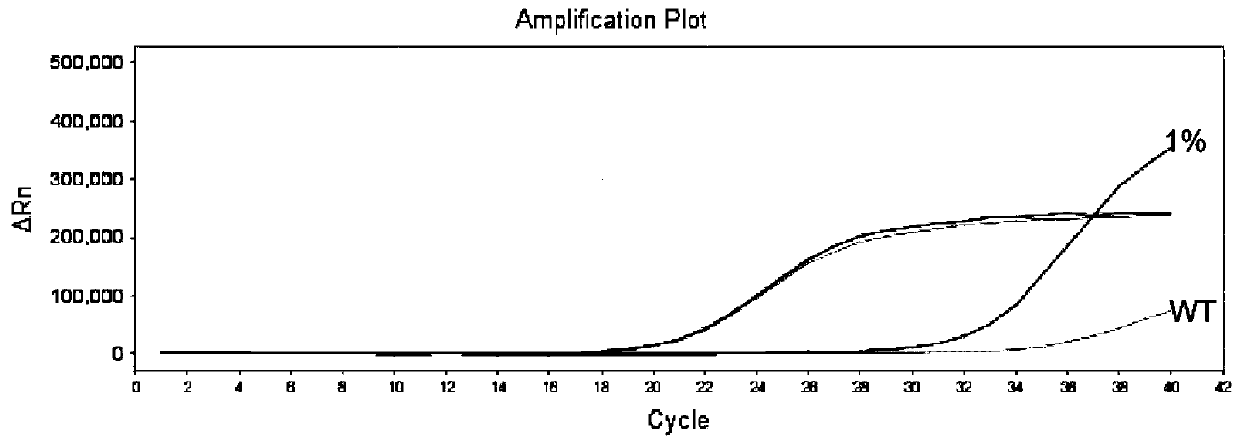
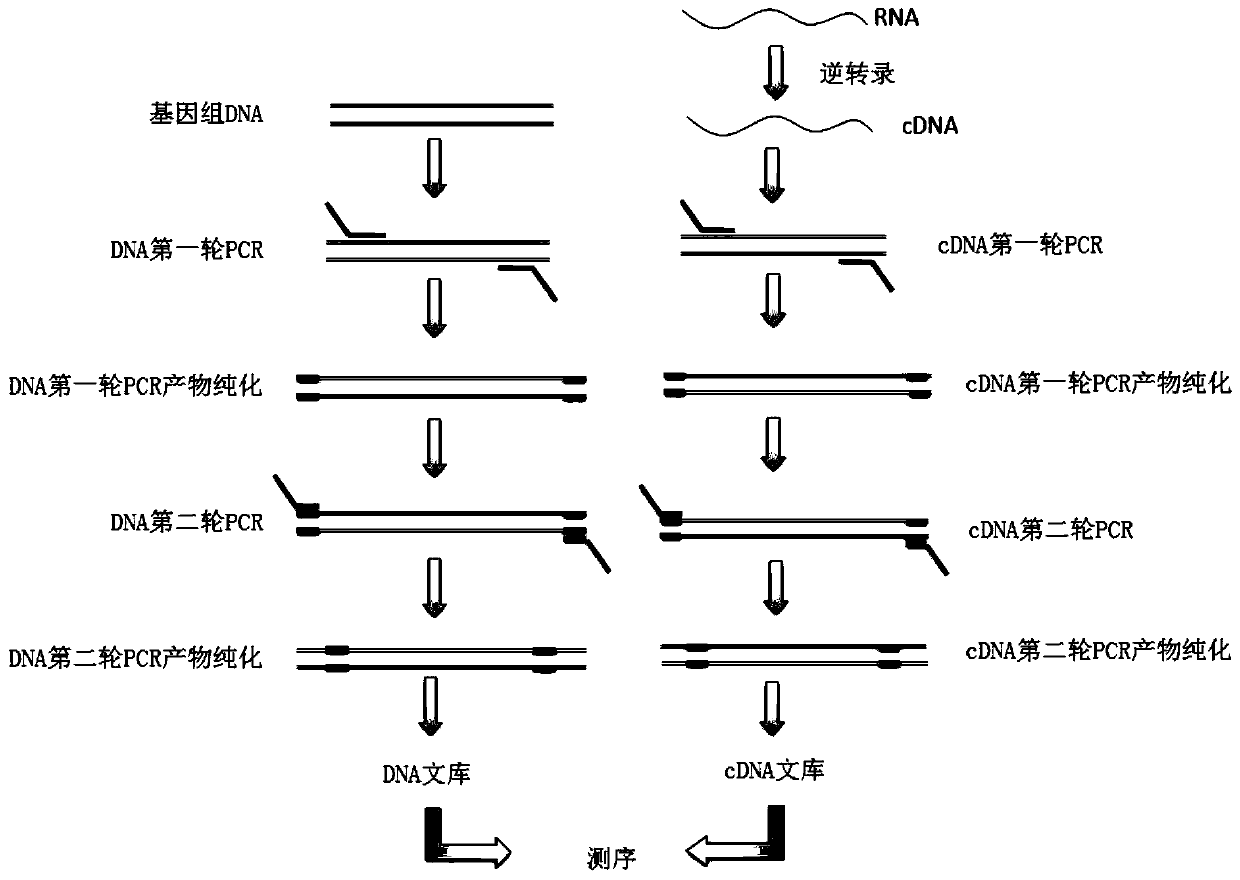
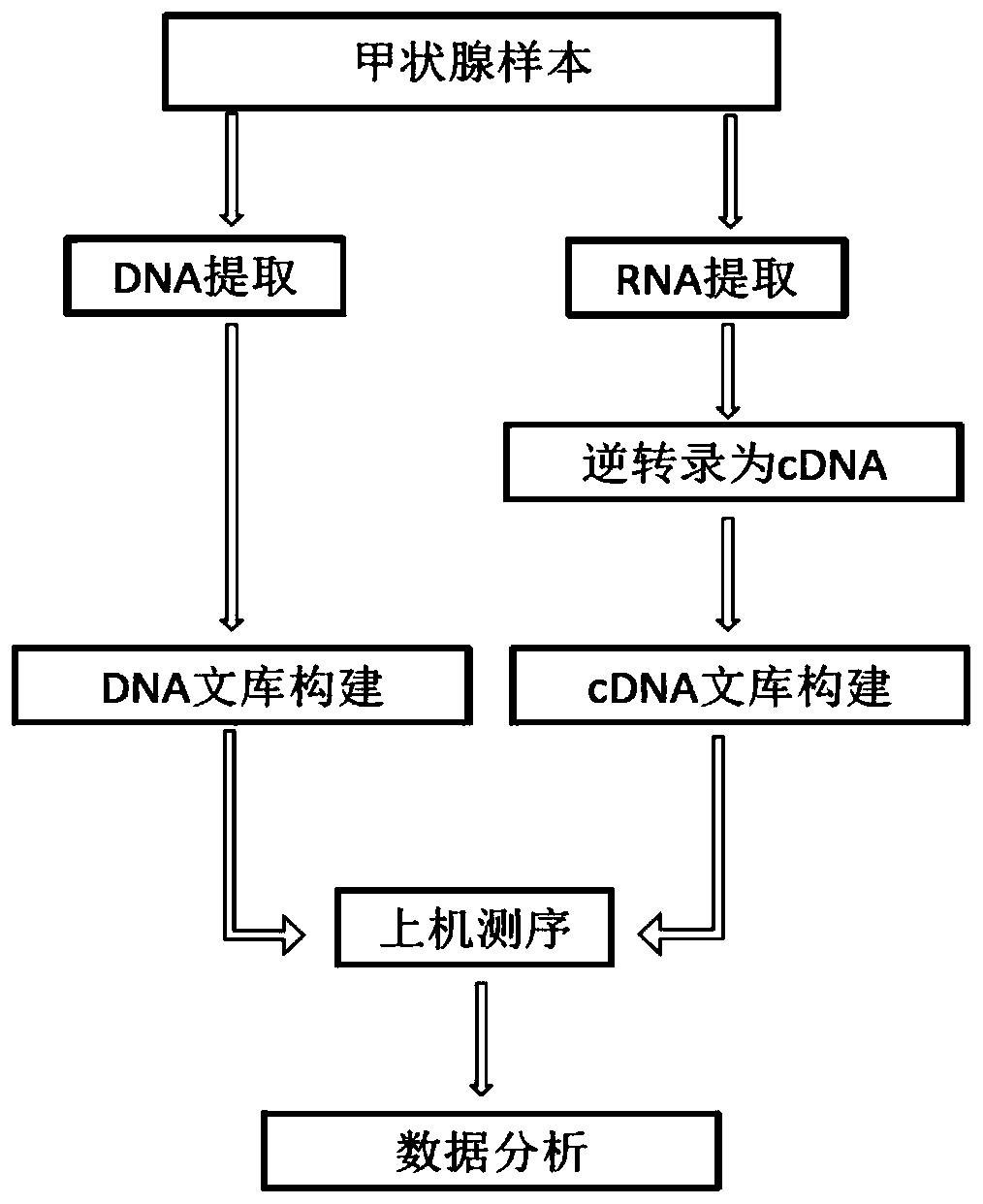
The invention belongs to the technical field of molecular biology, and in particular relates to a thyroid multi-gene combined detection kit. The kit of the invention comprises: a special adapter for DNA library construction; a specific composite primer for detecting fusion genes and point mutations; and a library amplification composite primer. The kit of the present invention jointly extracts the DNA and RNA of the samples submitted for inspection, reverse transcribes the RNA into cDNA, constructs a high-throughput sequencing library together with the DNA, and performs high-throughput sequencing, which can detect CCDC6- RET / NCOA4-RET fusion and PAX8 / PPARγ fusion, BRAF(V600E), HRAS(61), NRAS(61), KRAS(12,13), KRAS(61), TERTp and other point mutations, the operation is simplified and time-consuming Short, the sensitivity can reach 0.1%.
Owner:苏州科贝生物技术有限公司
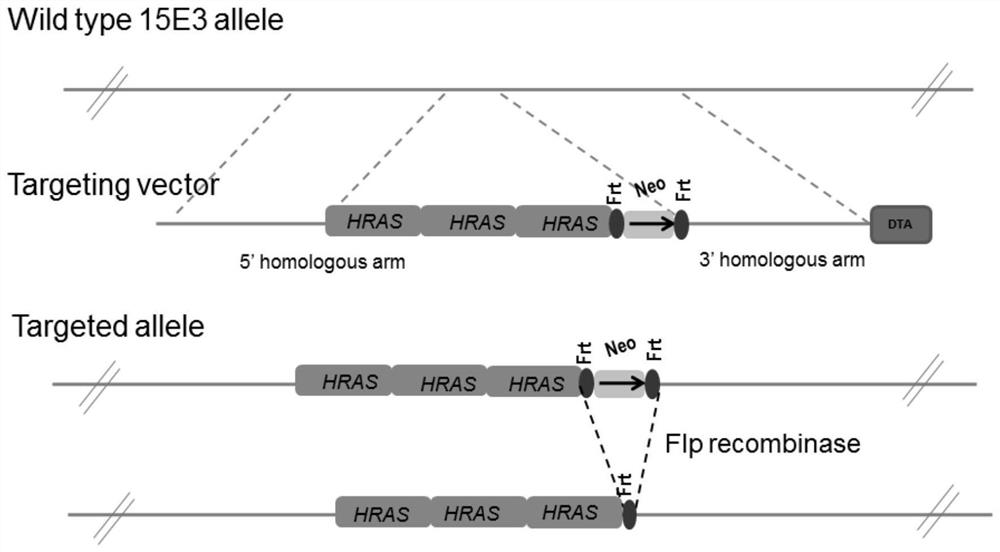
Cancer-causing mouse model as well as establishment method and application thereof
ActiveCN114134178ASmall inter-individual differencesImprove consistencyCompounds screening/testingAnimal reproductionOncogeneMurine model
The invention relates to a carcinogenic mouse model and an establishment method and application thereof, and belongs to the technical field of animal models. The mouse model is obtained by transferring a vector containing a human protooncogene c-Ha-ras (HRAS) gene sequence fragment into C57BL / 6 mouse embryonic stem cells, performing blastocyst injection, transplanting into a receptor mouse and performing screening. According to the mouse model, a stem cell targeting technology is adopted, a transgenic vector for highly expressing human proto-oncogene is established and subjected to fixed-point insertion, the mouse model is obtained through construction, C57BL / 6 stem cells are directly used as a mouse background, a fixed-point insertion model with the pure C57BL / 6 background can be directly obtained without long backcross, and the obtained mouse model is more uniform and better in result consistency.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Application of gene mutation site and mutation site detection method
PendingCN113584171AReduce workloadShorten detection timeMicrobiological testing/measurementAntineoplastic agentsRet geneAdjuvant therapy
The invention belongs to the technical field of medicine, and relates to application of a gene mutation site and a mutation site detection method. The invention mainly discloses application of a PTEN-p.P248L gene locus, a PTEN-p.T319fs gene locus, a BRAF gene V600E locus, an NRAS gene Q61R locus, an RET gene M918T locus, an NRAS gene Q61K locus, an HRAS gene Q61R locus and the like in preparation of thyroid cancer prevention and treatment drugs and / or preparation of thyroid cancer detection reagents. The mutant PTEN gene at least comprises one of the following site mutation: PTEN-p.P248L site mutation, and the base change is c.743C > T; the PTEN-p.T319fs site is mutated, and the basic group is changed into c.955_956insAA. The invention further discloses a capture method in high-throughput sequencing. The invention provides the mutant gene which is related to papillary thyroid carcinoma targeted medication and has a high proportion, so that the sites can be purposefully detected and can be better used for adjuvant therapy, the workload of detection and data analysis is effectively reduced, and the detection time and cost are reduced.
Owner:湖北省中医院
Kit for evaluating effect of 131I on treating differentiated thyroid cancer
InactiveCN112646894AStrong combination specificityAvoid mutual interferenceMicrobiological testing/measurementDNA/RNA fragmentationTreatment effectBraf genes
The invention discloses a primer and kit for evaluating the effect of 131I on treating differentiated thyroid cancer. The kit can be used for simultaneously detecting the variation of nine gene loci of a BRAF gene, a TERT gene, an HRAS gene and an NRAS gene, and the variation of the gene loci is closely related to the treatment effect of 131I on differentiated thyroid cancer. Therefore, when the kit is used for detecting a sample of a patient, a doctor can be assisted in scientifically evaluating the clinical effect of treating a differentiated thyroid cancer patient by using 131I, a basis is provided for making a precise treatment scheme of the patient, and the kit undoubtedly has very important clinical use value.
Owner:润安医学科技(苏州)有限公司
A kind of α-helical polypeptide inhibitor targeting hras protein and its use
ActiveCN111718398BImprove bindingGrowth inhibitionTetrapeptide ingredientsPeptidesPharmaceutical drugBiochemistry
The present invention is an α-helical polypeptide inhibitor targeting HRas protein, and its structural formula is as follows: The present invention also provides the use of the above-mentioned polypeptide inhibitor in the preparation of drugs for targeting HRas protein. The α-helical polypeptide inhibitor targeting HRas protein of the present invention can be applied to radiation sensitizers for radiotherapy.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL +1
A combined detection kit and application of four autoantibodies for diagnosing early esophageal squamous cell carcinoma
ActiveCN109142755BIncreased sensitivityImprove featuresBiological testingStage I Esophageal Squamous Cell CarcinomaSerology
The invention provides a multiple autoantibody combined detection kit and application for diagnosing early esophageal squamous cell carcinoma. The kit comprises a solid-phase substrate and antigenic proteins coated on the solid-phase substrate, and the antigenic proteins are composed of TP53, HRAS, CTAG1A and NSG1. The present invention uses TP53, HRAS, CTAG1A and NSG1 as a combination for the early diagnosis of ESCC for the first time, which has high sensitivity and specificity, and helps to further improve the quality of serological screening for early diagnosis of ESCC, thereby promoting the cure of patients rate increase and survival rate prolongation.
Owner:FUJIAN PROVINCIAL HOSPITAL
Non-invasive detection method and device for arteriovenous malformation and related diseases
PendingCN113981082AAvoid complicationsOperational securityMicrobiological testing/measurementProteomicsDiseaseTissue biopsy
The invention provides a non-invasive detection method for arteriovenous malformation and related diseases. The non-invasive detection method at least comprises the following steps: providing sequencing data of arteriovenous malformation related genes in free DNA (Deoxyribose Nucleic Acid) of plasma of a detected object, wherein the arteriovenous malformation related genes are selected from one or more of RASA1, EPHB4, PTEN, NRAS, HRAS, KRAS, BRAF, MAP2K1, MAPK1, MAPK3 and MAP3K3; and comparing the sequencing data with a reference sequence to obtain mutation related to arteriovenous malformation and related diseases. According to the invention, free DNA liquid biopsy is used for replacing tissue biopsy, a series of complications caused by tissue biopsy are avoided, non-invasion is achieved, operation is safe, materials are convenient to take, side effects are small, and materials can be taken repeatedly; and the method can be widely applied to mutation detection of people suffering from arteriovenous malformation and related diseases, a perfect arteriovenous malformation biological sample library is constructed, and original data support is provided for clinical and biological research.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
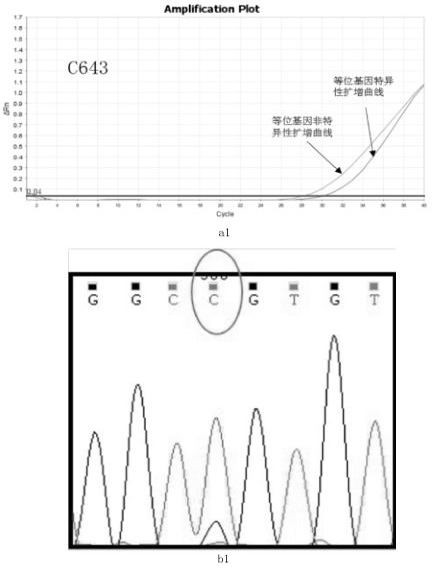
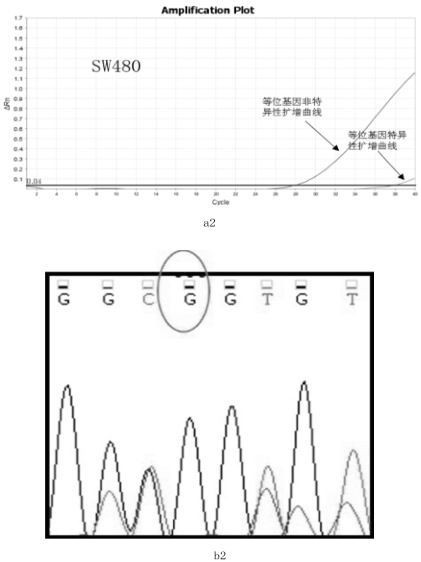
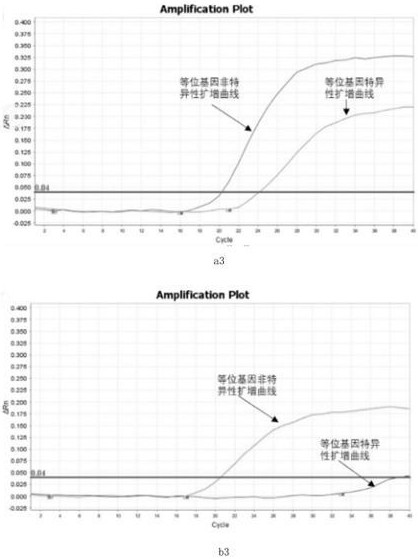
Primer, probe and kit for HRAS G13R mutation detection
PendingCN112725422ADetect mutationImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationMutation detectionGenetics
The invention relates to the technical field of biology, particularly to a primer, a probe and a kit for HRAS G13R mutation detection. The kit comprises allele specific primers, probes, allele non-specific primers, allele non-specific probes and PCR technology common reagents, wherein the PCR common reagents, the primers and the probes form a PCR reaction solution. The kit can effectively and accurately detect the mutation of HRAS G13R in a sample, and has high specificity and sensitivity.
Owner:山东康华生物医疗科技股份有限公司
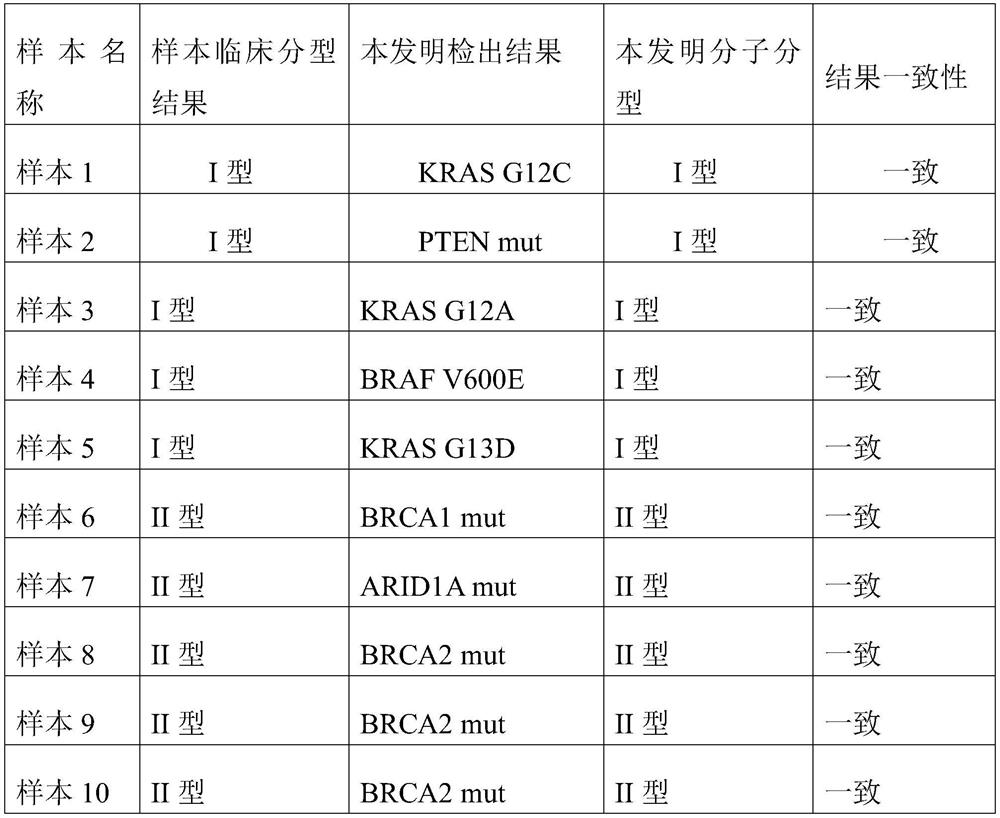
Probe pool for detecting ovarian cancer molecular subtypes, and preparation, application and use method thereof
ActiveCN114410779AImprove throughputHigh precisionMicrobiological testing/measurementDNA/RNA fragmentationOncologyMolecular typing
The invention discloses a probe pool for detecting ovarian cancer molecular subtypes as well as preparation, application and a use method thereof. The preparation method comprises the following steps: 1) designing a probe for I-type ovarian cancer; (2) designing a probe for the type II ovarian cancer; the design of the probe for I-type ovarian cancer typing comprises a combination of a plurality of regions of BRAF, KRAS, NRAS, ARAF, HRAS, PTEN, POLE and POLD genes; the design of the probe for type II ovarian cancer typing comprises combination of a plurality of regions of genes TP53, BRCA1, BRCA2, ATM, ATR, CCND1, CCNE1, ARID1A, CDK4, CDK6, CDK12 and PIK3CA, and the probe has the advantages that two common ovarian cancer molecular typing can be detected at one time by combining and using a targeted next-generation sequencing method, the flux is high, the precision is high, the cost is low, the timeliness is better, and the probe is suitable for clinical application. And a technical guarantee is provided for ovarian cancer molecular typing diagnosis, auxiliary medication guidance and prognosis prediction monitoring.
Owner:SUZHOU FANGKE BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![[alpha]-helical peptide inhibitor for targeting HRas protein, and purpose of [alpha]-helical peptide inhibitor [alpha]-helical peptide inhibitor for targeting HRas protein, and purpose of [alpha]-helical peptide inhibitor](https://images-eureka.patsnap.com/patent_img/3e732361-e49c-46f5-b472-2509a901a850/HDA0002575402620000011.png)
![[alpha]-helical peptide inhibitor for targeting HRas protein, and purpose of [alpha]-helical peptide inhibitor [alpha]-helical peptide inhibitor for targeting HRas protein, and purpose of [alpha]-helical peptide inhibitor](https://images-eureka.patsnap.com/patent_img/3e732361-e49c-46f5-b472-2509a901a850/HDA0002575402620000012.png)
![[alpha]-helical peptide inhibitor for targeting HRas protein, and purpose of [alpha]-helical peptide inhibitor [alpha]-helical peptide inhibitor for targeting HRas protein, and purpose of [alpha]-helical peptide inhibitor](https://images-eureka.patsnap.com/patent_img/3e732361-e49c-46f5-b472-2509a901a850/HDA0002575402620000013.png)