A kind of α-helical polypeptide inhibitor targeting hras protein and its use
A polypeptide inhibitor and α-helix technology, which is applied in the field of α-helix polypeptide inhibitors, can solve the problems of weak cell membrane penetration and low binding affinity, and achieve the effects of increasing radiation sensitivity, solving resistance, and broadening the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation and separation and purification steps of the polypeptide of embodiment 1:
[0043] Using solid-phase synthesis to synthesize peptides, the core steps for preparing the above-mentioned stable peptides are as follows (taking H5 peptide as an example):
[0044]
[0045] The specific operation steps are:
[0046] (1) Polypeptide solid-phase synthesis: Weigh Rink amide MBHA resin in the peptide tube and use morpholine / N,N-dimethylformamide with a reagent volume ratio of 50% (v / v) in the peptide tube (DMF) removes the Fmoc protecting group on the resin, in N 2 React under blowing, 30min / time, a total of two times, so that the resin exposes free amino groups. Then use DMF solvent / dichloromethane (DCM) / N-methylpyrrolidone (NMP) and other solvents to wash alternately 6-9 times respectively. Then add 5eq Fmoc-protected amino acid but carboxyl exposed amino acid, 5eq HCTU, 10eq DIPEA, in N 2 React under agitation, 1h / time, react 2 times. Then alternately wash 6...
Embodiment 2
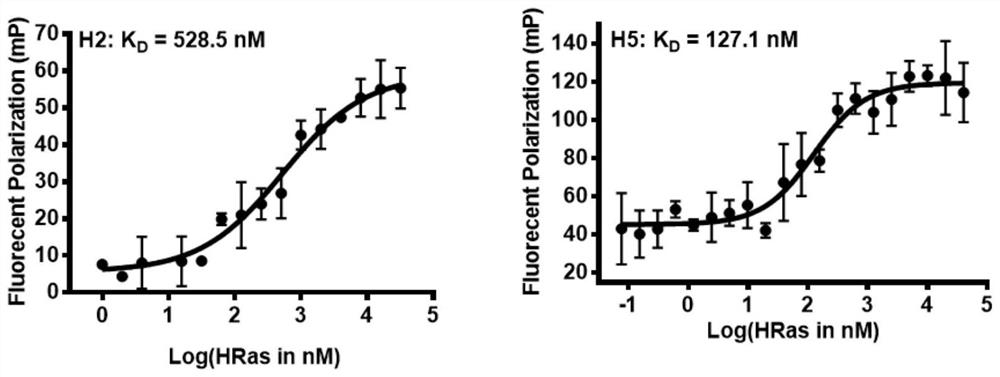
[0048] Example 2 Fluorescence Polarization (FP) Experimental Screening of Polypeptides Combined with HRas
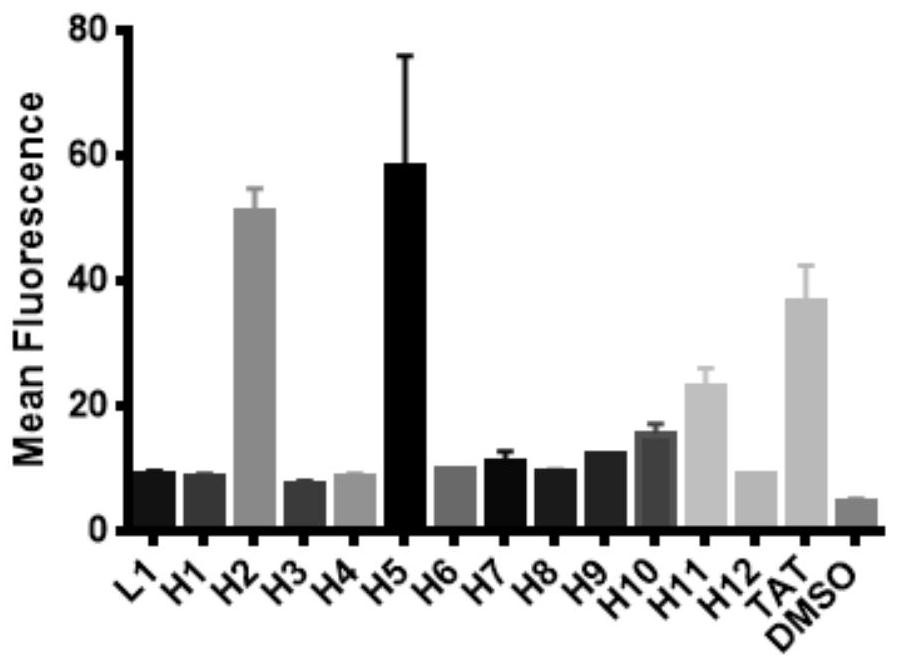
[0049] In the present invention, fluorescence polarization (FP) experiment is used to measure the binding affinity between FITC-labeled polypeptide and HRas protein, and the experimental results are summarized in Table 1. According to the results of the FP experiment, the linear polypeptide L1 only shows a weak interaction with the HRas protein, indicating that the affinity between the linear polypeptide L1 and HRas is weak, and as the original sequence based on the key fragment of the Sos protein, it can be used as a follow-up experiment the control peptide.
[0050] The peptides H3 and H4 designed based on the peptide sequence (FEGIYRLELLKAEEAN) have a certain interaction with HRas, but the binding affinity is above the micromolar level, and the binding affinity is weak, indicating that the inhibitor designed based on the peptide sequence needs further research. Only ...
Embodiment 3
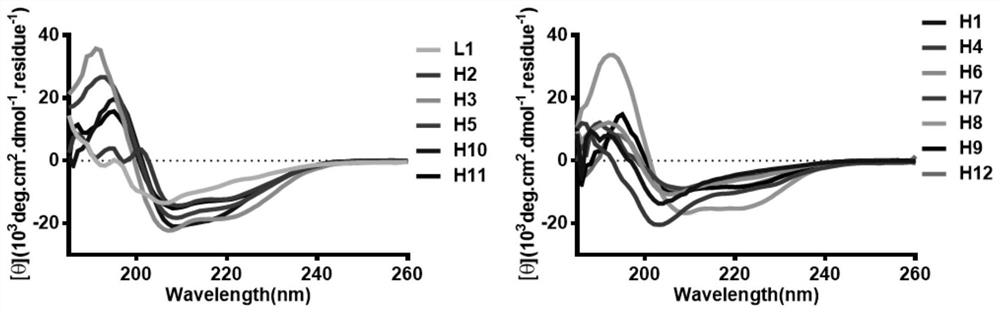
[0053] The circular dichroism spectrum result of embodiment 3Ras polypeptide
[0054] In order to determine the secondary conformation of the polypeptide in aqueous solution, the present invention uses a circular dichroism spectrometer to measure the circular dichroism (CD) spectrum of the polypeptide. According to the characteristic negative absorption peaks near 208nm and 222nm and the characteristic positive absorption peak near 190nm in the circular dichroism spectrum, it can be judged that the polypeptide has a typical α-helical conformation. The results of CD experiments showed that peptides H2, H3, H5, H8, H10, and H11, which were ring-closed with terminal aspartic acid nucleating templates, displayed typical α-helical conformations in their circular dichroism (CD) assays ( figure 2 ), while the unclosed linear polypeptide L1 showed a random coil conformation, which confirmed that the TD-terminal nucleation template can stabilize the polypeptide in the α-helical confor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com