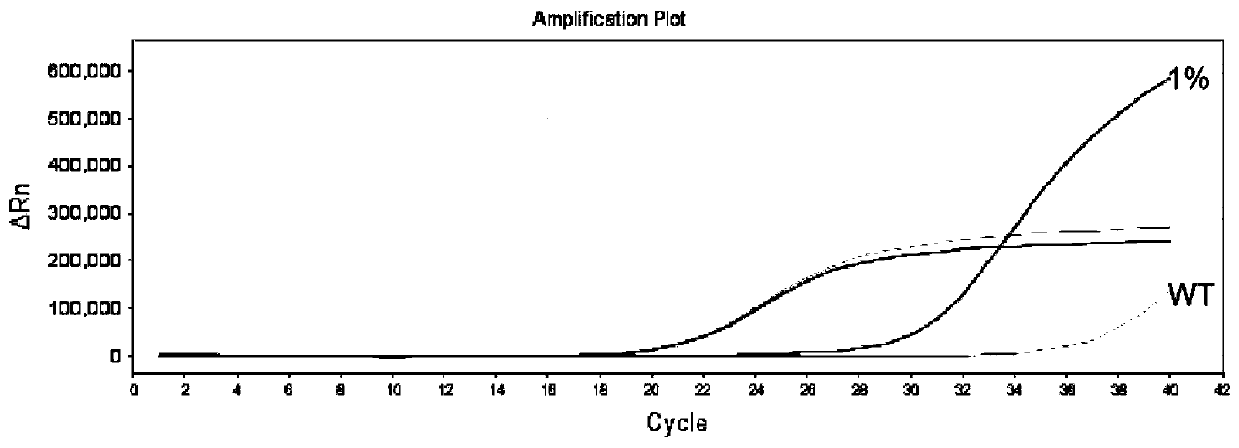
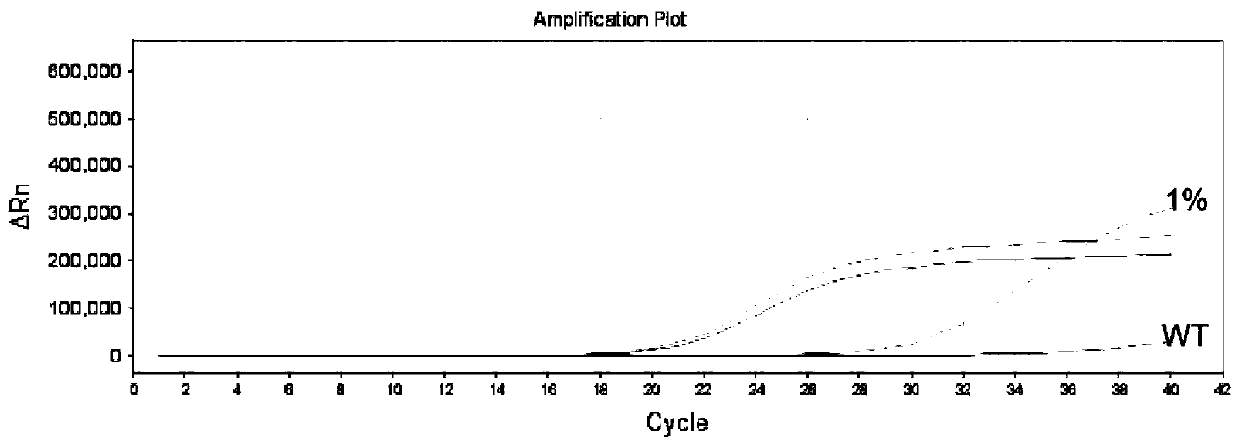
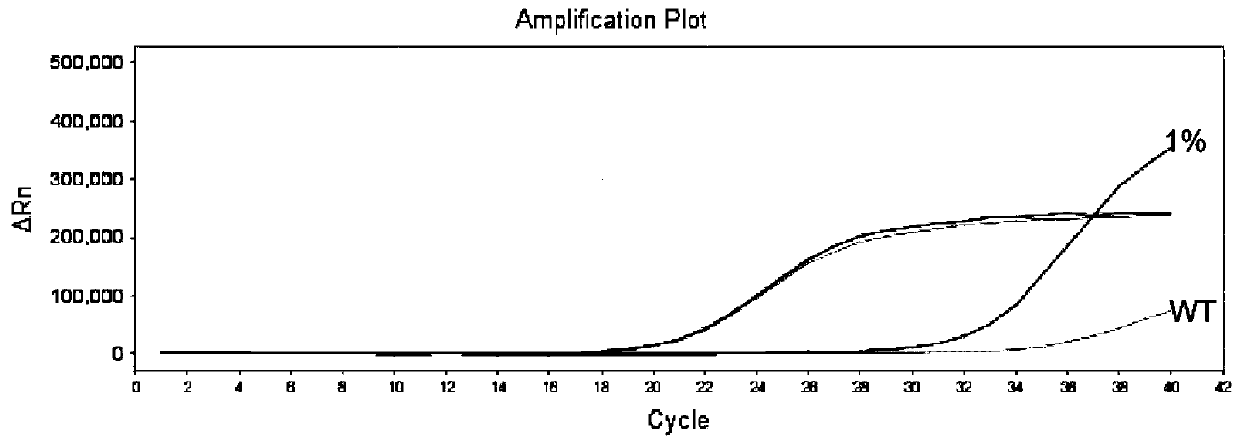
Thyroid cancer auxiliary molecule diagnosis test kit and use method
A molecular diagnosis, thyroid cancer technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve the problems of reduced PCR efficiency, primer extension block, short detection time, etc., to achieve good specificity and improved accuracy. Good reproducibility and repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Primer and probe combination design and use
[0044] The present invention collects BRAF (p.V600E), HRAS (p.Q61R), KRAS (p.G13D), KRAS (p.G13R), KRAS (p.G12D), KRAS (p.G12C), NRAS (p.Q61H ), NRAS(p.Q61P), NRAS(p.Q61K), TERT(C228T) and TERT(C250T) 5 genes sequence information of 12 kinds of mutation sites, after large-scale experimental screening, the optimal primers and The probes are as follows:
[0045] (1) Amplification primer pair and probe for detecting BRAF gene V600E
[0046] BRAF-F: GTGATTTTGGTCCAGCCAGAAA
[0047] BRAF-R: TCTAGTAACTCAGCAGCATCTCAGG
[0048] BRAF-P: FAM-ACTGATGGGACCCACTCCATCGA-TAMRA
[0049] (2) Amplification primer pair and probe for detection of HRAS gene Q61R
[0050] HQ61R-F5:CATCCTGGATACCGCCGTCAG
[0051] HQ61R-R:CTTGGTGTTGTTGATGGCAAAC
[0052] HQ61R-P: FAM-AGGAGTACAGCGCCATGCGGGA-TARMA
[0053] (3) Amplification primer pair and probe for detecting KRAS gene G12C
[0054] KG12C-F3:TATAAACTTGTGGTAGTTGGAGCCT
[0055] K1213-R...
Embodiment 2
[0107] Example 2: Acquisition of Positive Quality Controls
[0108] The method of obtaining positive quality control products is: according to BRAF(p.V600E), HRAS(p.Q61R), KRAS(p.G13D), KRAS(p.G13R), KRAS(p.G12D), KRAS published on NCBI (p.G12C), NRAS(p.Q61H), NRAS(p.Q61P), NRAS(p.Q61K), TERT(C228T), TERT(C250T) and ACTB gene sequence information, artificially synthesized nucleic acid fragments, and then Insert the fragment into the T vector, transform and extract the plasmid with Escherichia coli DH5α strain, measure the concentration and purity with nanodrop 2000, mix the plasmid in equal volumes and use it as the positive quality control product of the kit of the present invention.
[0109] The BRAF (p.V600E) quality control sequence is shown in SEQ ID NO.43, the HRAS (p.Q61R) quality control sequence is shown in SEQ ID NO.44, and the KRAS (p.G13D) quality control sequence is shown in SEQ ID As shown in NO.45, the KRAS (p.G13R) quality control sequence is shown in SEQ ID N...
Embodiment 3
[0111] Embodiment 3: the configuration of PCR reaction system
[0112] In the present invention, the PCR reaction liquid contains substances such as hot start taq enzyme, buffer solution, magnesium ions, dNTPs and the like required by the PCR reaction. Specifically: Tris-HCl 20mM at pH 8.3, KCl 100mM, Mg 2+ 2mM, dNTPs 0.4mM, hot start Taq DNA polymerase 0.2U / μL.
[0113] The kit of the present invention is designed with 12 PCR reaction strips. Tubes 1-11 are composed of gene detection reagents and internal control detection reagents, which respectively indicate 12 mutation sites of 5 genes of BRAF, HRAS, KRAS, NRAS and TERT. The gene detection reagents are composed of FAM signal indication, the internal control detection reagent is used to monitor the quality of sample DNA and its addition, indicated by the VIC signal; tube 12 is composed of external control detection reagents for BRAF non-mutated regions, used to quality control the quality of DNA extraction, and is also ind...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com