Kit for evaluating effect of 131I on treating differentiated thyroid cancer
A thyroid cancer and kit technology, which is applied to the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., can solve the problems of decreased iodine absorption capacity, no clear determination, and poor radiotherapy, etc. Achieve the effect of less non-specific amplification, overcoming mutual interference, and high uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: A method for evaluating 131 The combination of primers for the effect of I in treating differentiated thyroid cancer
[0045] This embodiment involves with 131 The gene mutation sites related to the effect of I treatment of differentiated thyroid cancer were selected from the COSMIC (Catalogue of Somatic Mutations in Cancer) database, and primers were designed according to the relevant gene sequences. A total of 16 pairs of specific primers were designed, as shown in Table 2. Each pair of specific primers amplifies the target region with a size of 180-200bp, and the size of the amplified product is 220-280bp. It has the advantages of wide coverage, many detection sites, balanced GC content, stable product structure, and less dimer structure. It exhibits good specificity, stability and uniformity in multiple PCR amplification, and can ensure the amplification efficiency while ensuring the specificity of PCR amplification.
[0046] Primers designed in this ex...
Embodiment 2
[0048] Example 2: A method for evaluating 131 Kit for the effect of I treatment of differentiated thyroid cancer
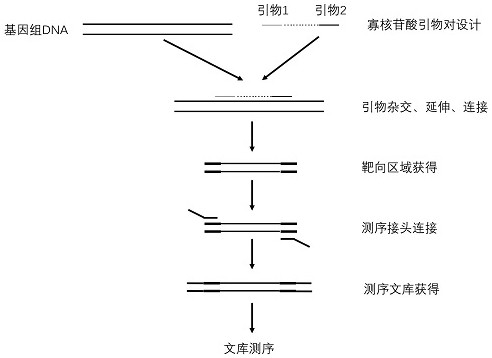
[0049] The one described in this example is used to evaluate 131 I The kit for treating differentiated thyroid cancer effect, its design principle is as follows figure 1 As shown, it mainly includes:
[0050] (1) PCR-specific primers: used to amplify multiple target regions on the target gene of the sample to be tested. The amplification range covers at least the hotspot mutation region of the target gene. The sequences are as shown in Table 2. SEQ ID NO.1 to SEQ ID NO .12. Preferably, multiple amplification primer pairs are mixed together to form a primer pool;
[0051] (2) Universal primers: used to re-amplify the amplification products of the target region amplified by specific primers during the library construction process, to mark the sequencing libraries of different samples to be tested, and then to distinguish different samples. The sequence is shown...
Embodiment 3
[0055] Example 3: A method for evaluating 131 Detection process of the kit for the effect of I treatment of differentiated thyroid cancer
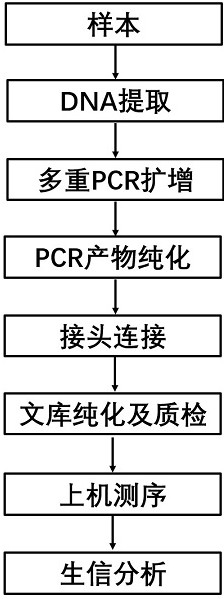
[0056] The detection process of this embodiment is as follows figure 2 shown, including the following steps:
[0057] 1) Sample DNA extraction
[0058] Use the QIAamp DNA Mini Kit (51304) kit to extract fresh tissue DNA. The specific steps are operated according to the kit instructions. After the DNA is extracted, use a spectrophotometer for quality control analysis. The value of A260 / A280 is between 1.9-2.1, using Qubit Concentration determination was performed with a fluorescence quantitator; the extracted DNA was diluted to 10-50 ng / μL using nuclease-free water as a template.
[0059] 2) The first round of ligation PCR
[0060] components volume High Fidelity PCR Enzyme Mix 12.5μL DNA-specific primer mix pool 4μL DNA template 2μL nuclease free water Make up to 25 μL
[0061] DNA multiple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com