Kit and detection method for detection of human KRAS gene mutations
A kit and human technology, applied in the fields of biotechnology and medicine, can solve the problems of high detection cost, limited detection ability, high specificity, etc., and achieve the effects of avoiding false positive results, high sensitivity, and reducing false negative results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
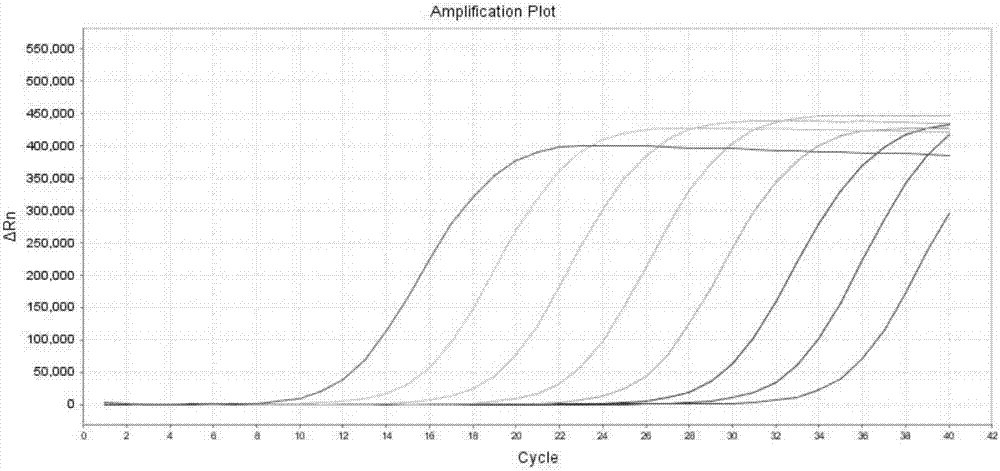
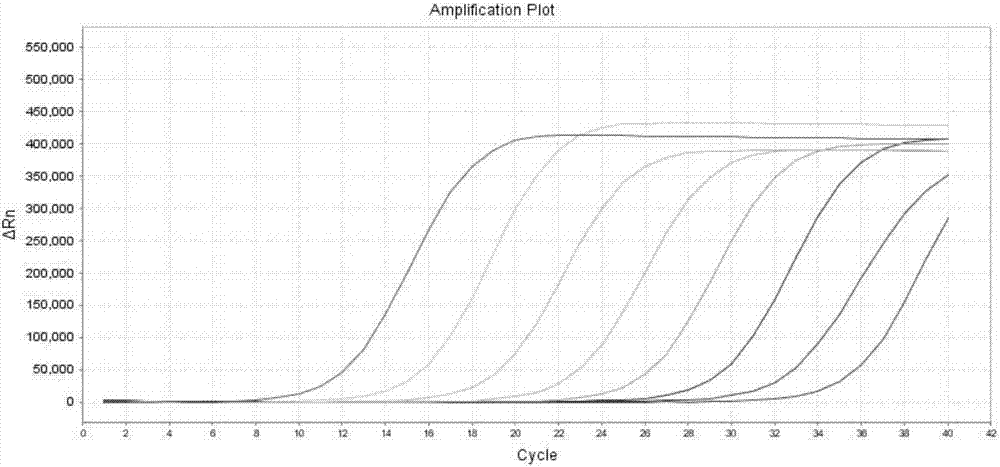
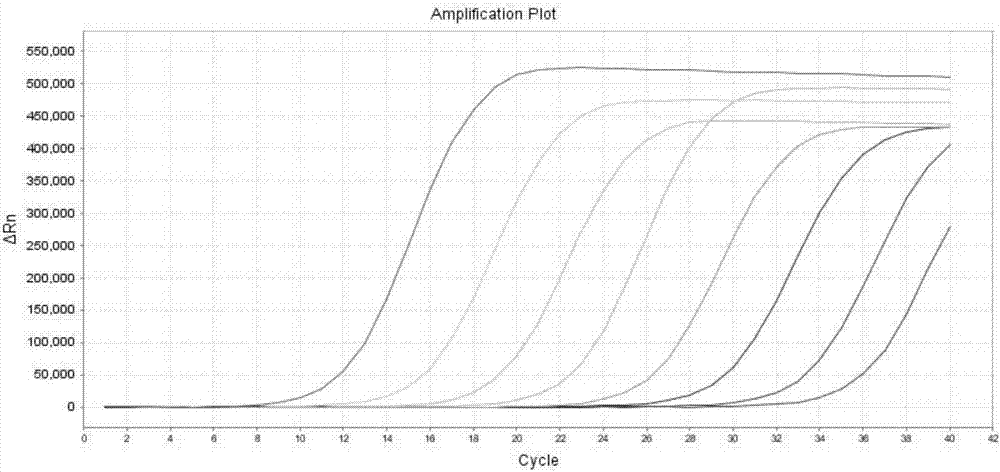
[0051] The method for detecting KRAS 7 gene mutations by real-time fluorescent PCR of the present invention comprises the following steps:
[0052] 1. Nucleic acid extraction
[0053] Taking paraffin-embedded tissue as an example, extract genomic DNA from paraffin-embedded tissue according to the recommended QIAamp DNA FFPE Tissue Kit instructions.
[0054] 2. Preparation of PCR reaction system (25ul)
[0055] Reagent name
Amount added
PCR reaction solution
16ul
Enzyme 1
2ul
Primer Probe Mix
2ul
template DNA
5ul
[0056] The template DNA includes sample DNA and negative and positive control DNA.
[0057] 3. PCR amplification
[0058] Real-time PCR amplification conditions are: 50°C for 2min, 1 cycle; 95°C for 5min; 95°C for 10s, 55°C for 15s, 72°C for 30s, 5 cycles; 95°C for 10s, 60°C for 45s (collect FAM and HEX fluorescence signals) , 40 cycles.
[0059] 4. Result judgment
[0060] 1) In addition to the negative c...
Embodiment 2
[0068] Using the present invention to detect 10 cases of paraffin-embedded tissue samples of KRAS gene mutation positive patients, and compared with the sequencing results, the results: 4 cases of 12VAL positive, 5 cases of 12ASP positive, 1 case of 13ASP positive were detected, and the coincidence rate with the sequencing method was 100%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com