KRAS gene mutation detection quantification standard product as well as preparation method and valuing method thereof
A quantitative standard product and genome technology, applied in the direction of biochemical equipment and methods, microbial determination/inspection, etc., to achieve the effect of improving efficiency and reducing the cost of testing quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
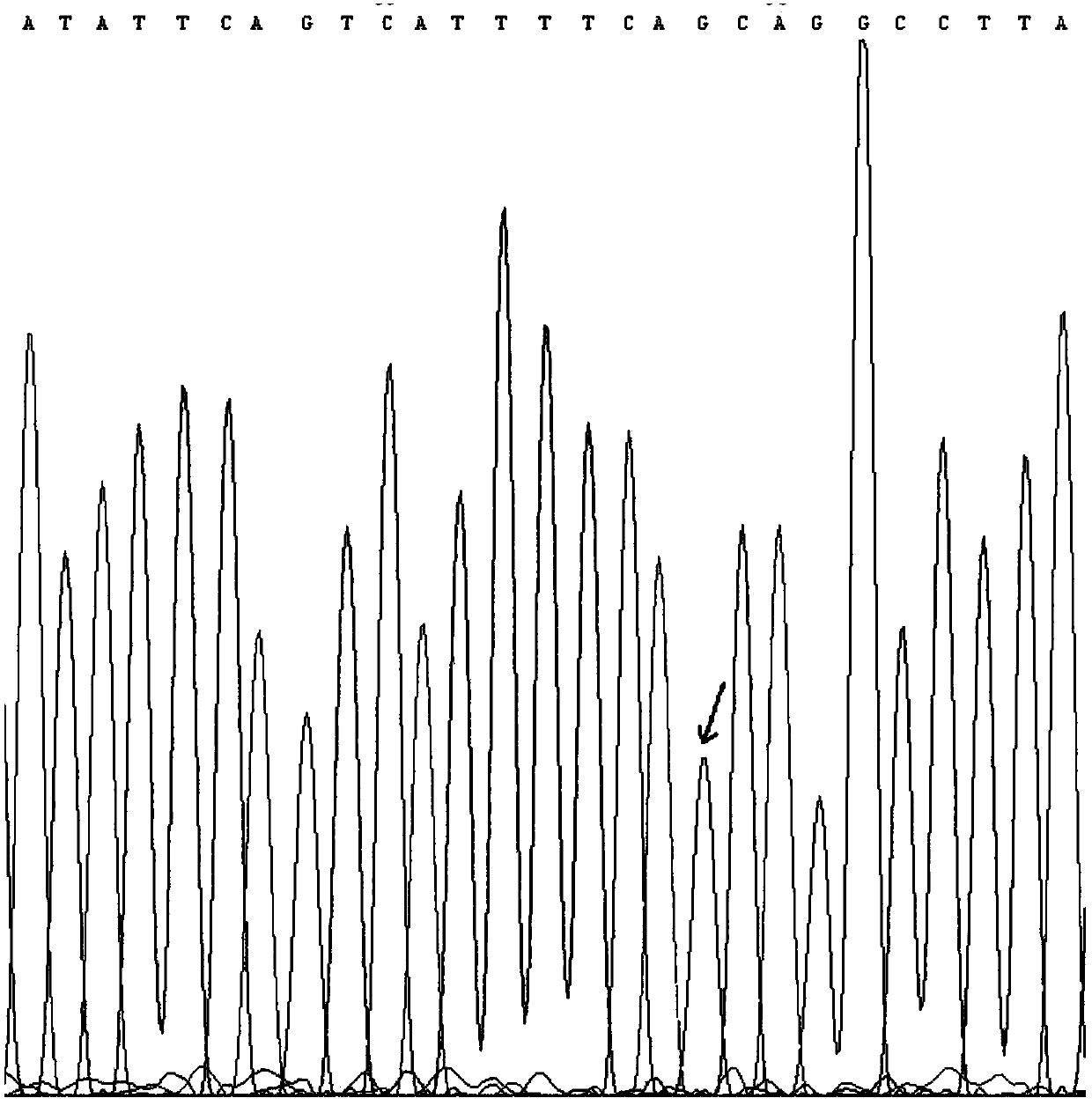
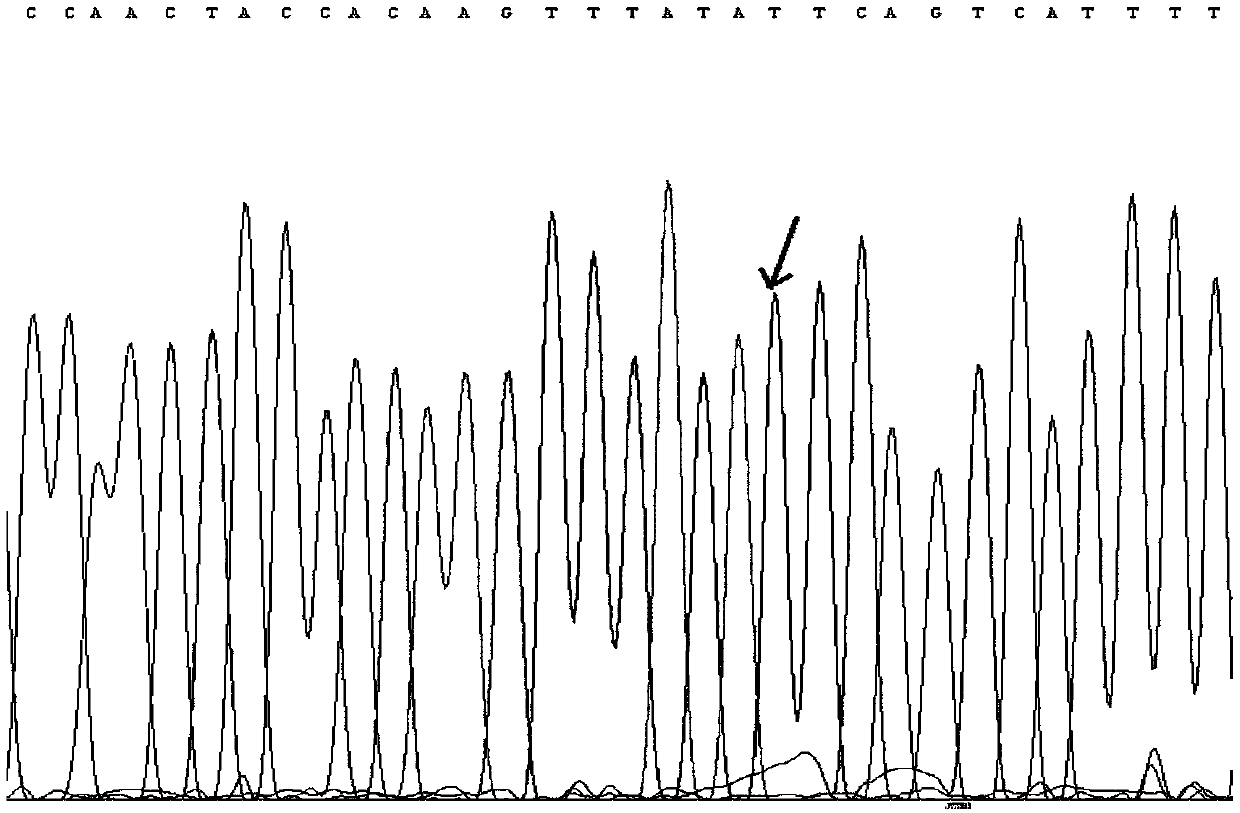
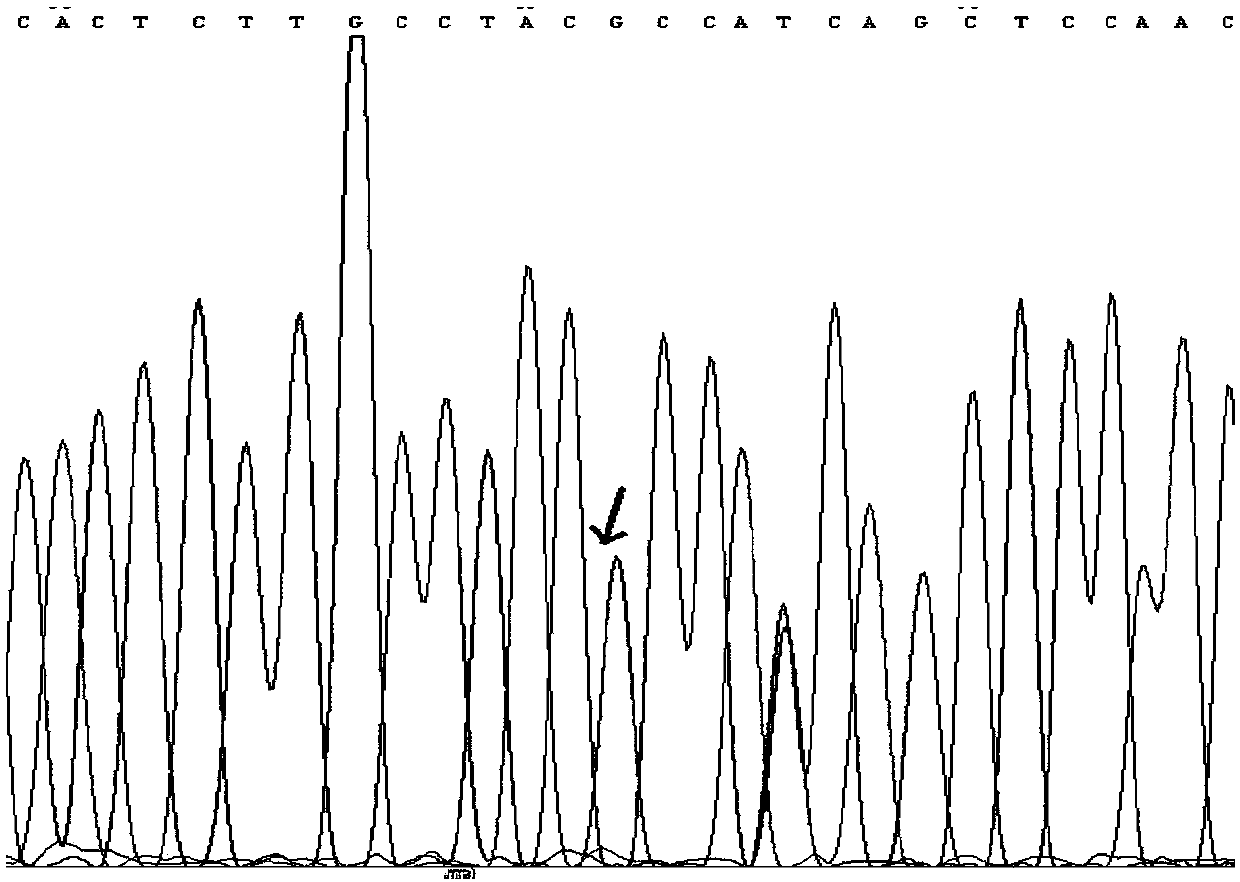
[0100] Preparation and determination of quantitative standards for 7 mutations of KRAS gene
[0101] 1. Materials and methods:
[0102] 1. Cell Lines and Media
[0103] RPMI-8226, SUN-C2B, NCI-H157, SW1573, A549, SW620, HCT-116, PG LCL8, the first 7 cell lines were purchased from ATCC, and the required medium and culture conditions were carried out according to the instructions of ATCC. The eighth cell line is provided by Fudan University, the medium is 1640+10% FBS, 5% CO 2 , cultivated at 37 degrees.
[0104] 2. DNA extraction and PCR amplification
[0105] Genomic DNA of 8 cell lines was extracted using the Genome Purification Kit of Kangwei Century Company, and PCR amplification was performed using the genomic DNA of 7 cell lines containing target mutations as templates to obtain PCR products containing 7 target mutations. 20 μL of PCR amplification system, including 2 μL of upstream and downstream primers with a concentration of 5 uM, 2 μL of DNA template, 10 μL of 2×...
Embodiment 2
[0144] Applicability verification of KRAS gene mutation quantitative standard
[0145] 1. Materials and methods:
[0146] 1. Materials and Reagents
[0147] 7 kinds of mutation detection kits of human KRAS gene from three different manufacturers in the domestic market were selected, and the random numbers were A, B, and C. Quantitative standard substance (code K) and 5% quantitative standard substance (code P) with 7 kinds of mutation levels of human KRAS gene, negative control substance (NC1~NC11), quantitative standard substance and reference substance are all provided by China Metrology Science Institute preparation.
[0148] 2. Instruments and equipment
[0149] Fluorescent quantitative PCR instrument (Roche LC480, Roche LC480II, AB7500), centrifuge.
[0150] 3. Verification indicators
[0151] The following indicators of the kit were verified by real-time fluorescent quantitative PCR method:
[0152]1) Accuracy: 10 parts of 5% quantitative standard were measured, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com