Method for detecting tumor mutant gene in blood
A technology for mutated genes and tumors, applied in the field of capillary electrophoresis detection, to achieve the effect of ensuring new reproduction, ensuring accuracy, and less sample consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
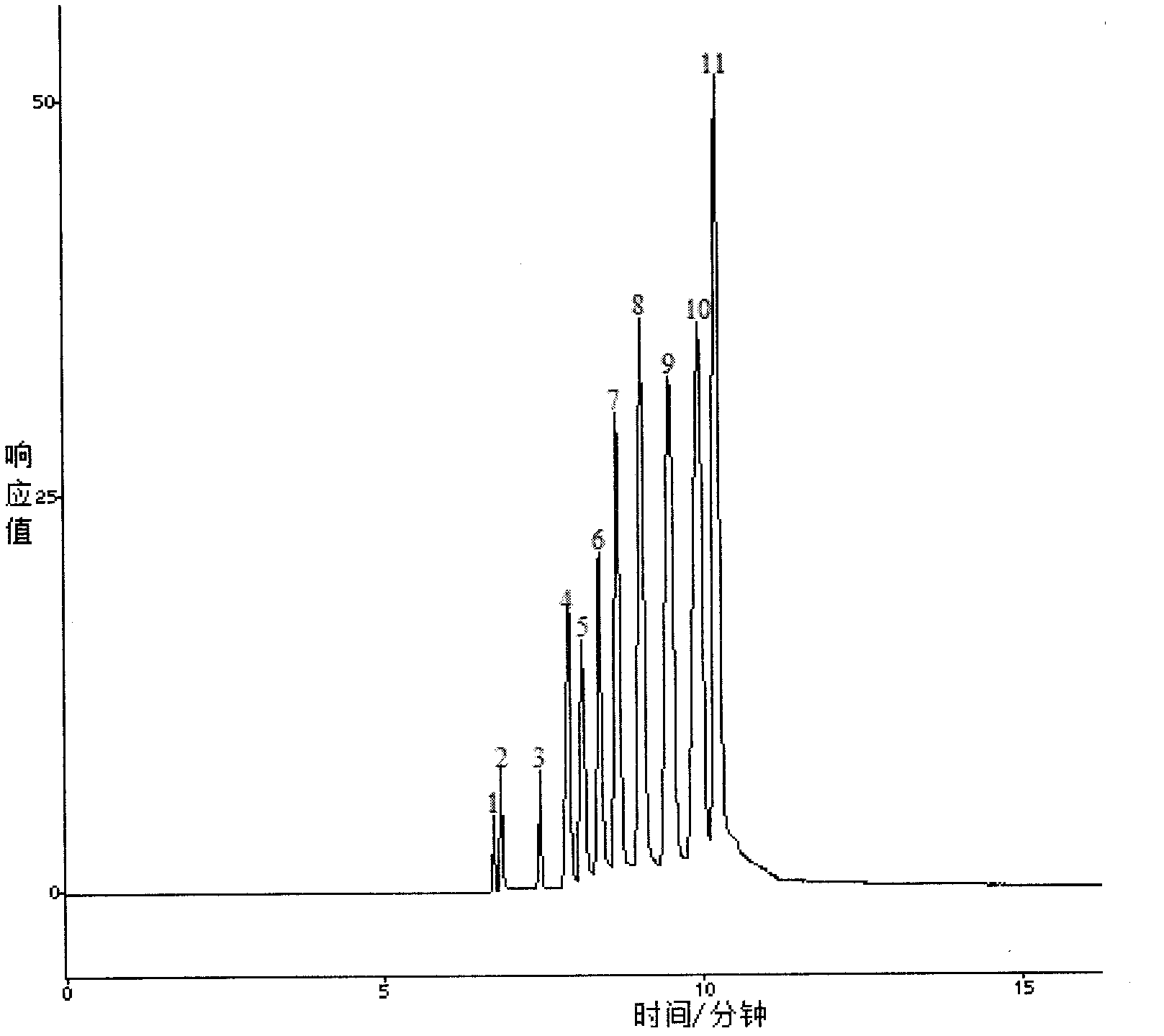
[0043] Example 1 A method for detecting tumor mutation genes in blood samples, which is used for the isolation of pUC19 DNA / MspI / HpaII DNA Marker.
[0044] (1) Preparation of polyethylene oxide sieving medium containing TBE:
[0045] Add polyethylene oxide into 1×TBE buffer solution to make the final solution concentration 3.0% and pH value 8.3, and use a DF-101S magnetic stirrer produced by Yuhua Instrument Co., Ltd., Gongyi City, Henan Province at 400 rpm Stir at a speed of 1 / min until the solution becomes clear, and the clear solution is filtered with a 0.45um water-based microporous membrane to obtain a polyethylene oxide sieving medium.
[0046] Among them: 1 × TBE buffer refers to adding 10.8 grams of Tris (Tris) produced by Shanghai Bioengineering Company, 5.5 grams of boric acid produced by Shanghai Shenggong Company, After stirring and dissolving 0.74 g of ethylenediaminetetraacetic acid disodium salt (EDTA) produced, add deionized water to dilute the solution to 1 l...
Embodiment 2
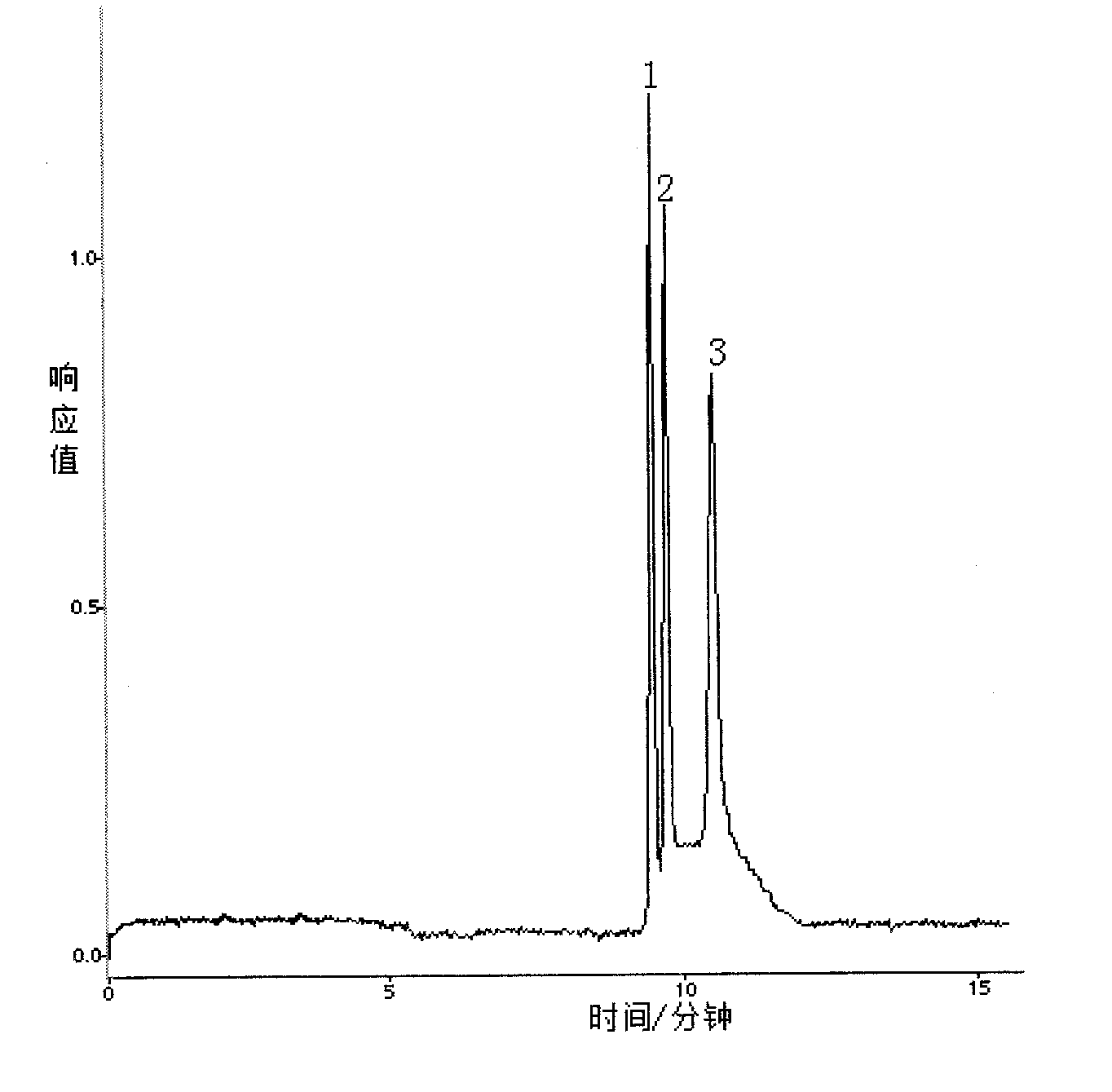
[0053] Embodiment 2 A method for detecting tumor mutation genes in blood, comprising the following steps:
[0054] (1) Preparation of polyethylene oxide sieving medium containing TBE:
[0055] Add polyethylene oxide into 1×TBE buffer solution to make the final solution concentration 2.5% and pH value 8.0, stir until the solution is clear, and filter the clear solution with a 0.22um water-based microporous membrane to obtain Polyethylene oxide sieving media.
[0056] Wherein the 1× TBE buffer solution is the same as in Example 1.
[0057] (2) Genomic DNA extraction from blood samples:
[0058] After adding Tris buffer Ⅰ to the blood sample to lyse the red blood cells, discard the supernatant, add Tris buffer Ⅱ to the pellet to suspend the cell mass, add proteinase K, and incubate in a water bath at 55°C for 4 hours; after the incubation, add phenol-chloroform to precipitate the protein , absorb the supernatant and add absolute ethanol to the supernatant, ice-bath for 1 h, an...
Embodiment 3
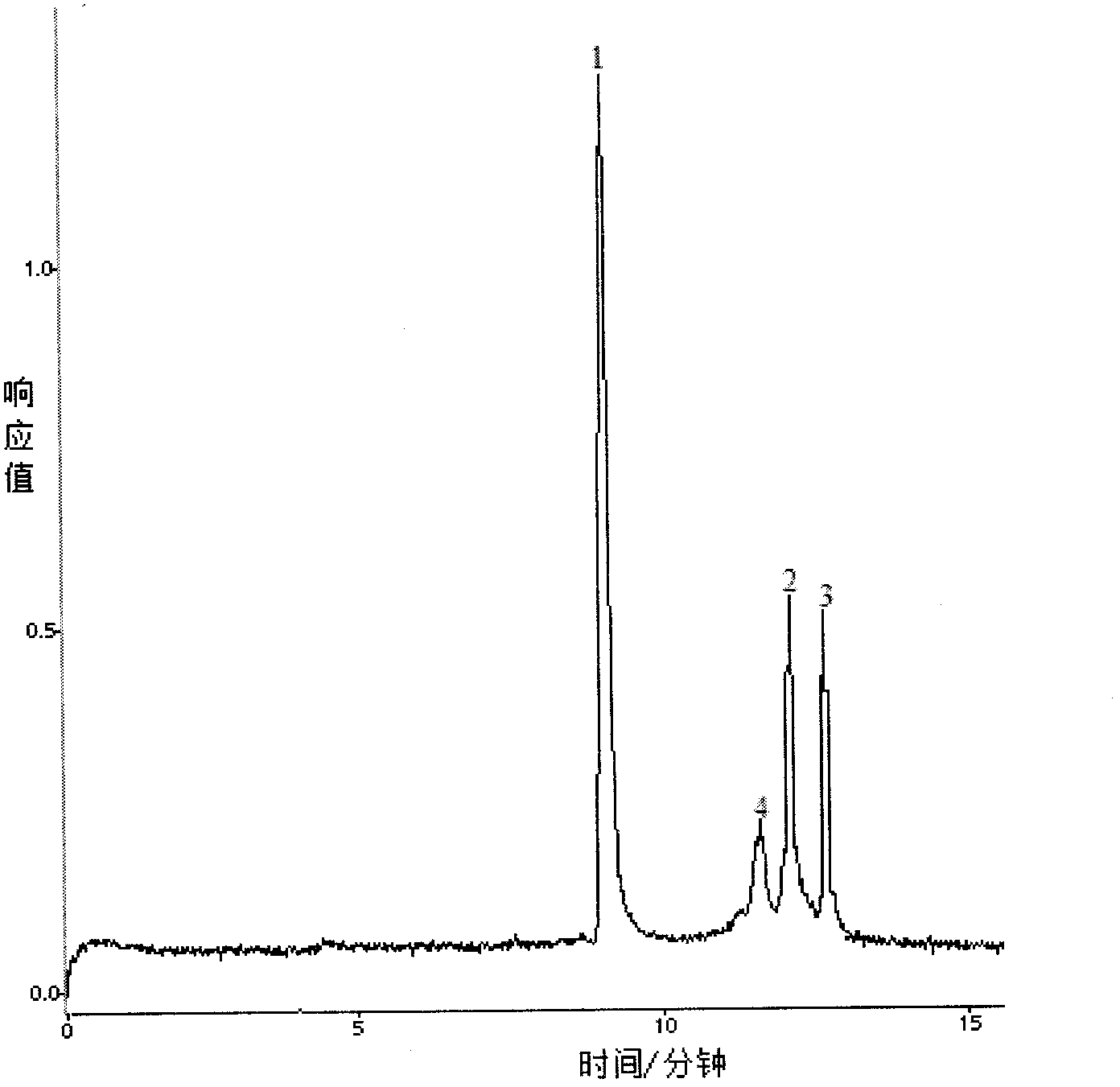
[0070] Embodiment 3 A method for detecting tumor mutation genes in blood, comprising the following steps:
[0071] (1) Preparation of polyethylene oxide sieving medium containing TBE:
[0072] Add polyethylene oxide into 1×TBE buffer solution to make the final solution concentration 2.5% and pH value 7.5, stir until the solution is clear, and filter the clear solution with a 0.22um water-based microporous membrane to obtain Polyethylene oxide sieving media.
[0073] Wherein the 1× TBE buffer solution is the same as in Example 1.
[0074] (2) Genomic DNA extraction from blood samples:
[0075] Add Tris buffer Ⅰ to the blood sample to lyse the red blood cells, discard the supernatant, add Tris buffer Ⅱ to the pellet to suspend the cell mass, add proteinase K, and incubate in a water bath at 56°C for 2 hours; after the incubation, add phenol-chloroform to precipitate the protein , absorb the supernatant and add absolute ethanol to the supernatant, ice-bath for 1 h, and centrif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com