Crystal form of deuterated osimertinib pharmaceutical salt and preparation method thereof
A medicinal salt and deuterated technology, applied in the field of medicine, can solve the problems of undisclosed compound crystal form, hygroscopicity, solubility physicochemical data, other crystal forms and stability data of unreported compounds, etc., to improve bioavailability and The effect of drug safety, improvement of drug production quality, and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Preparation of crystalline form I of deuterated Osimertinib pharmaceutical salt
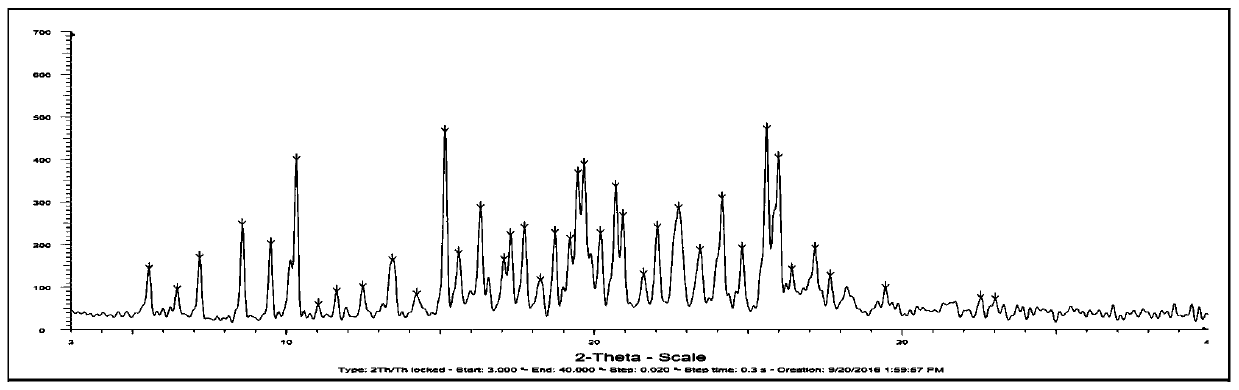
[0039] This embodiment is used to prepare the crystal form I of the deuterated Osimertinib medicinal salt shown in formula VI, comprising the following steps: dissolving 2.00 g of deuterated Osimertinib in a 20ml mixed solvent (volume ratio of ethanol, acetone and isobutyl acetate 1:2:1.5), heated to 45°C, added methanesulfonic acid aqueous solution (0.46g methanesulfonic acid dissolved in 3ml water) to the solution at a flow rate of 0.2ml / min, stirred for 2h after addition, cooled to 5°C, The cooling rate is 10°C per hour. Subsequently, stand for crystallization for 3 h, and then filter, and vacuum-dry the filter cake at room temperature for 10 h to obtain 2.20 g of a yellow solid with a yield of 92%. Its X-ray powder diffraction pattern is as figure 1 shown.
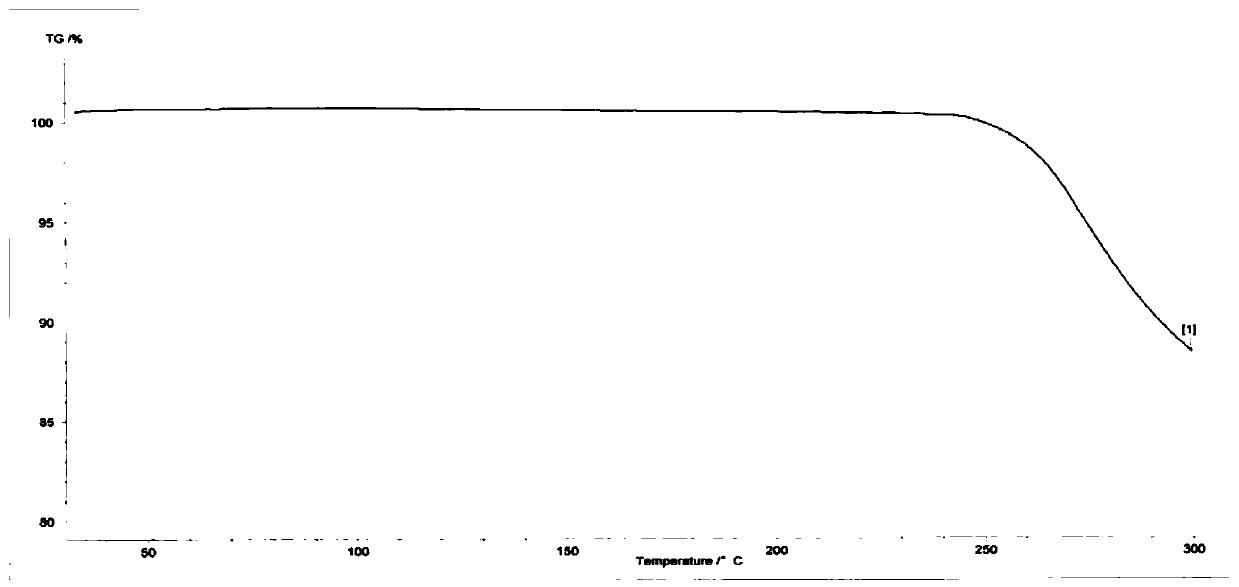
[0040] Thermogravimetric analyzer (model: NETZSCH TG 209) thermal weight loss of crystalline form I of deuterated ...
Embodiment 2
[0041] Embodiment 2: Preparation of crystalline form I of deuterated Osimertinib pharmaceutical salt
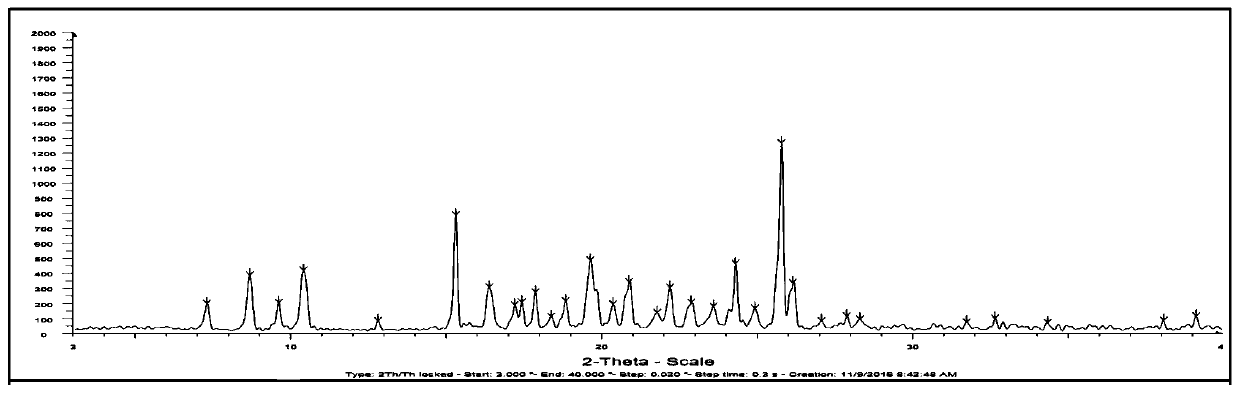
[0042] This embodiment is used to prepare the crystal form I of the deuterated Osimertinib medicinal salt shown in formula VI, comprising the following steps: dissolving 4.00 g of deuterated Osimertinib in a 20ml mixed solvent (volume ratio of ethanol, acetone and isobutyl acetate 1:1:2), heated to 30°C, added methanesulfonic acid aqueous solution (1.14g methanesulfonic acid dissolved in 9ml water) to the solution at a flow rate of 0.1ml / min, stirred for 2h after addition, cooled to 0°C, The cooling rate is 10°C per hour. Subsequently, the crystallization was allowed to stand for 4 hours, and then filtered, and the filter cake was vacuum-dried at room temperature for 14 hours to obtain 4.28 g of a yellow solid with a yield of 90%. The X-ray powder diffraction spectrum obtained by measuring the crystalline form I of the deuterated Osimertinib pharmaceutical salt obtained by u...
Embodiment 3
[0043] Embodiment 3: Preparation of crystalline form I of deuterated Osimertinib pharmaceutical salt
[0044] This embodiment is used to prepare the crystal form I of the deuterated Osimertinib medicinal salt shown in formula VI, comprising the following steps: dissolving 4.00 g of deuterated Osimertinib in a 32ml mixed solvent (volume ratio of ethanol, acetone and isobutyl acetate 1:1.2:1), heated to 50°C, added methanesulfonic acid aqueous solution (0.76g methanesulfonic acid dissolved in 6ml water) into the solution at a flow rate of 0.1ml / min, stirred for 2h after addition, cooled to 10°C, The cooling rate is 10°C per hour. Subsequently, the crystallization was allowed to stand for 2 h, and then filtered, and the filter cake was vacuum-dried at room temperature for 10 h to obtain 4.23 g of a yellow solid with a yield of 89%. The X-ray powder diffraction spectrum obtained by measuring the crystalline form I of the deuterated Osimertinib pharmaceutical salt obtained by usin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com