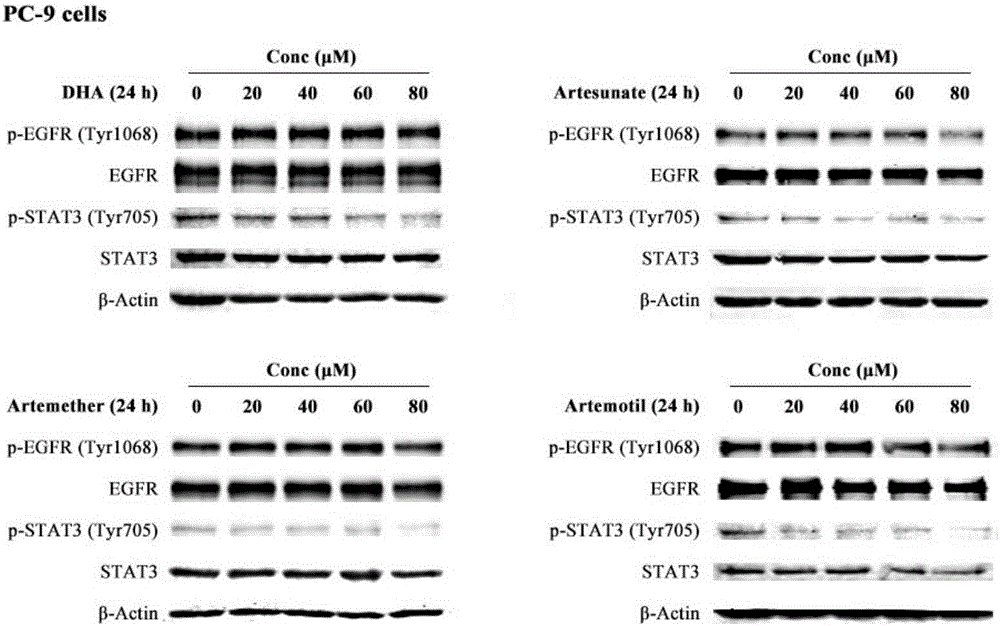
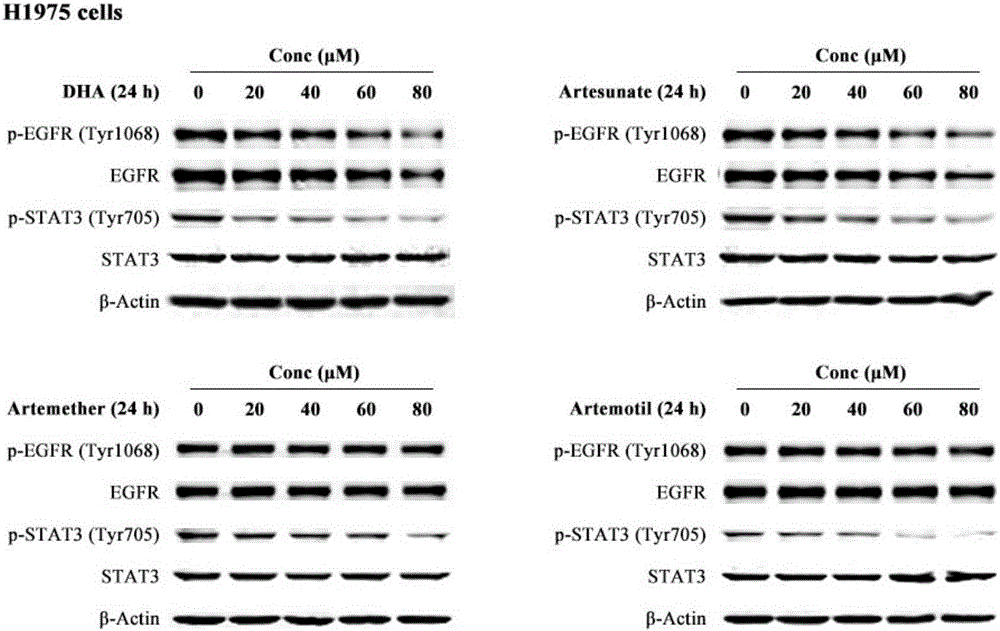
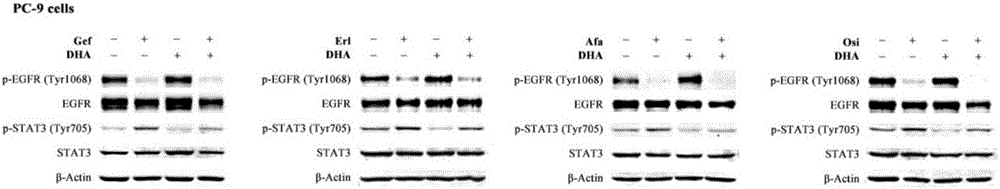
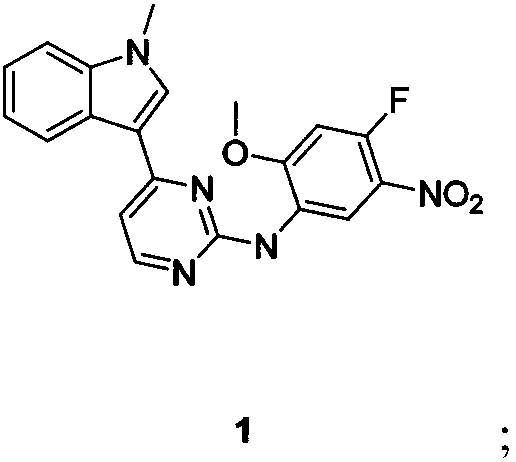
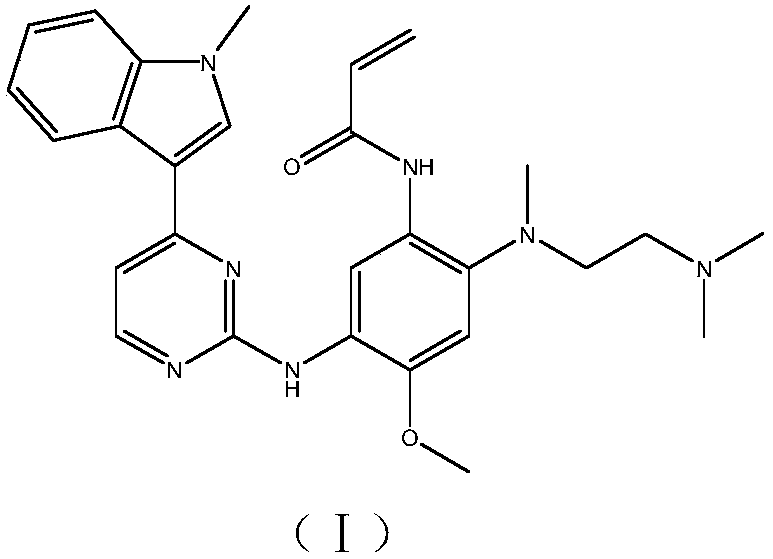
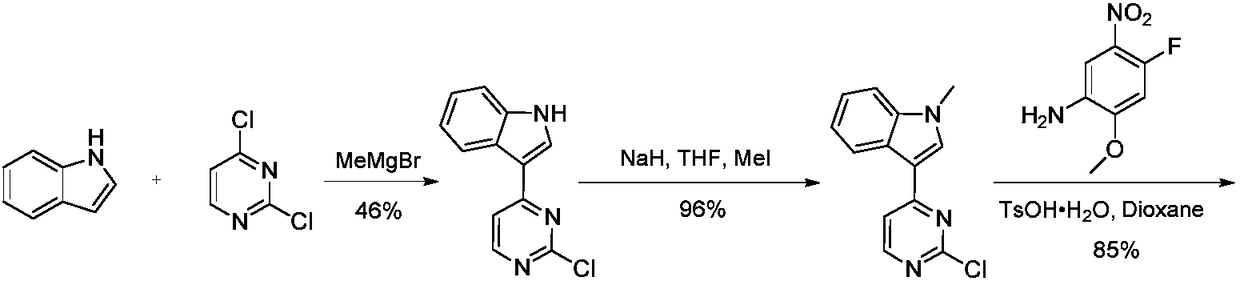
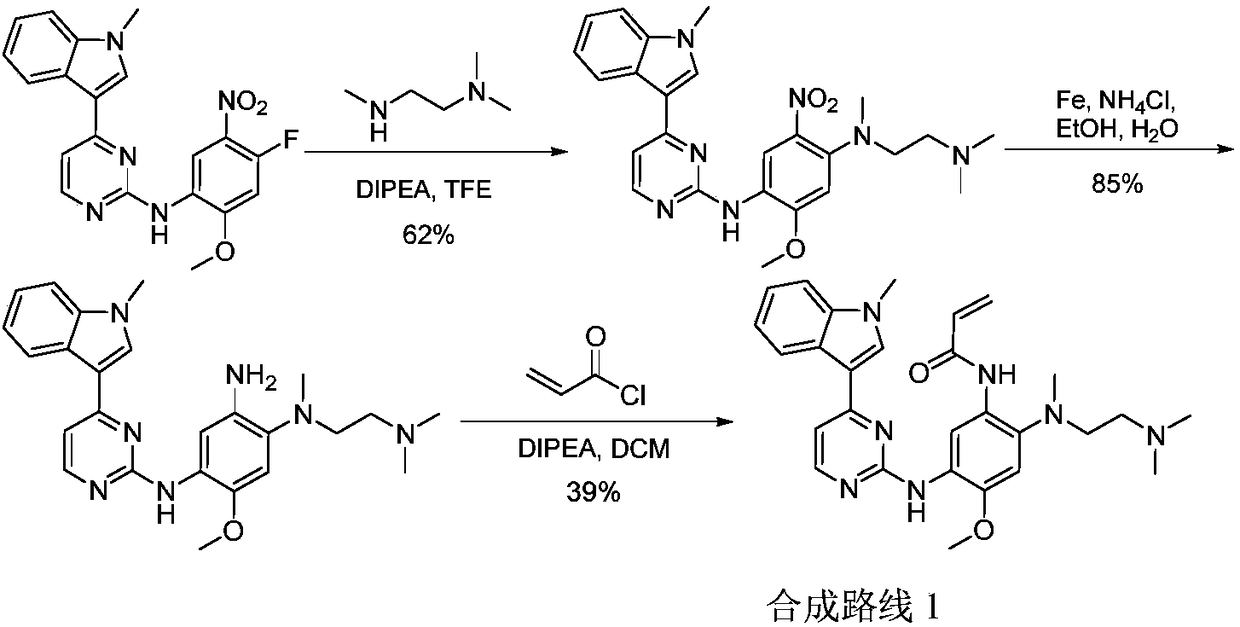
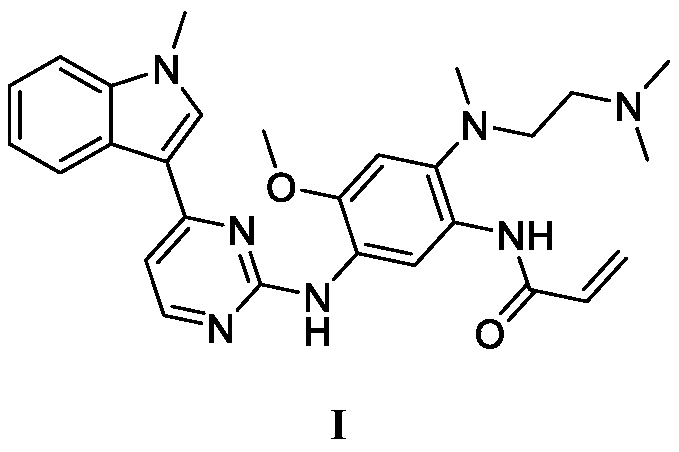
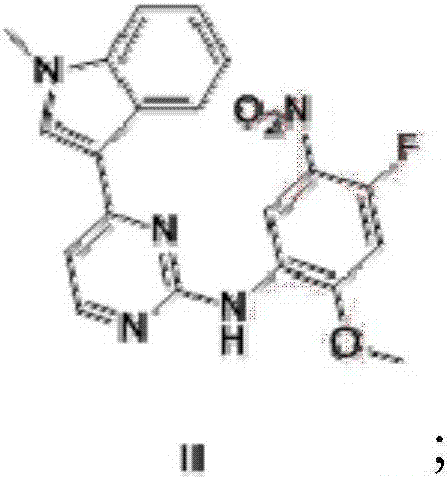
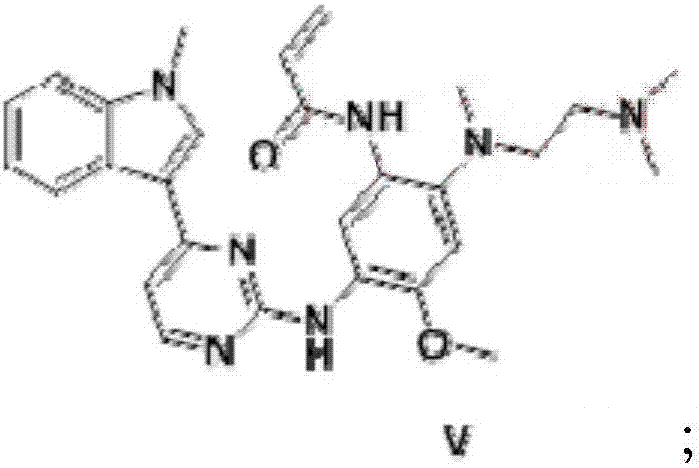
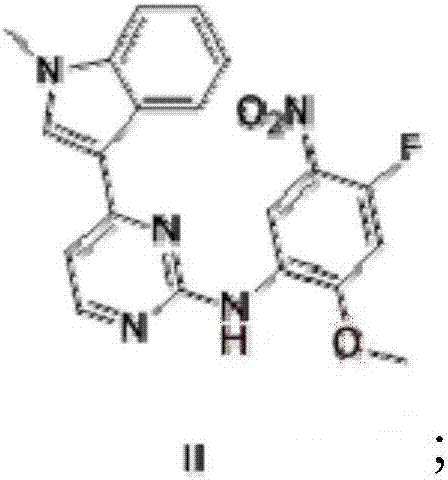
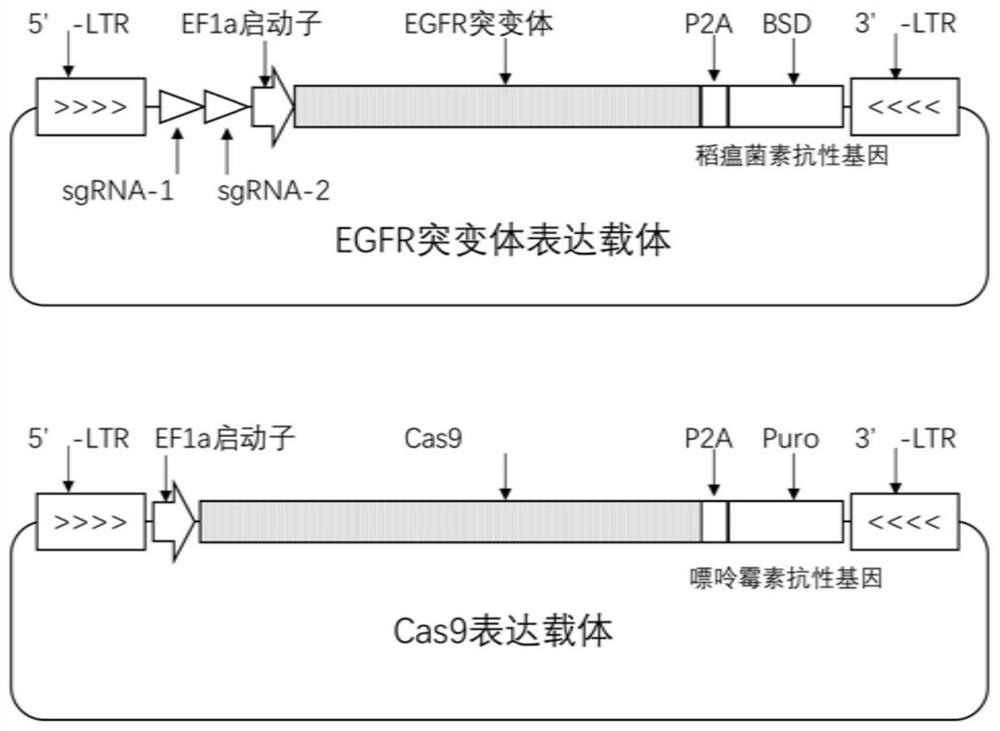
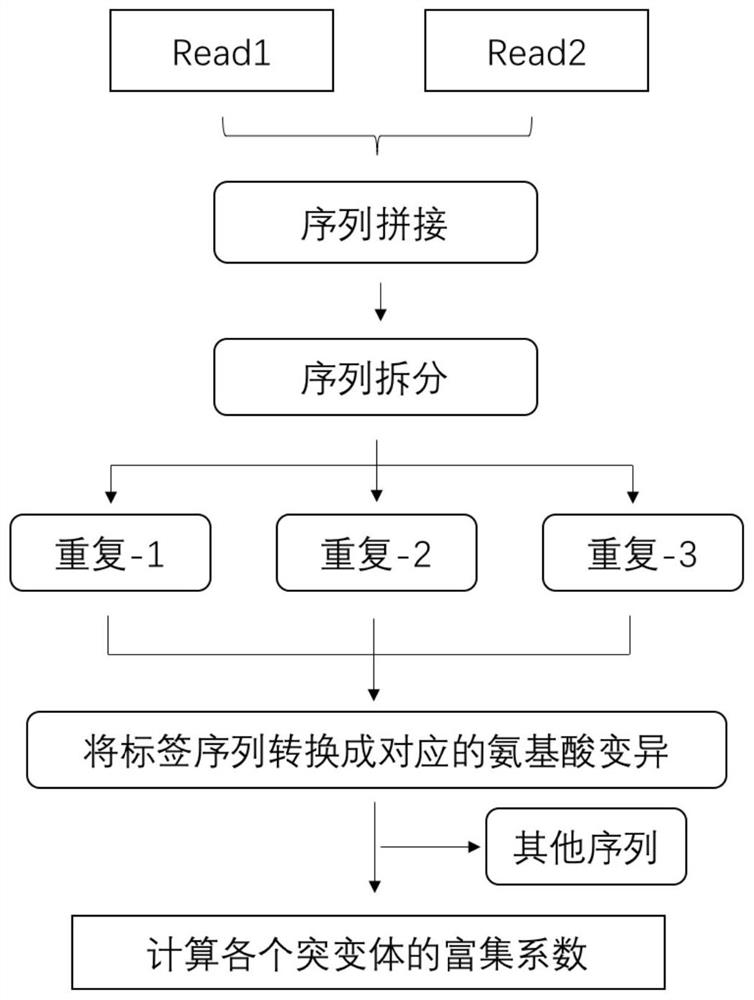
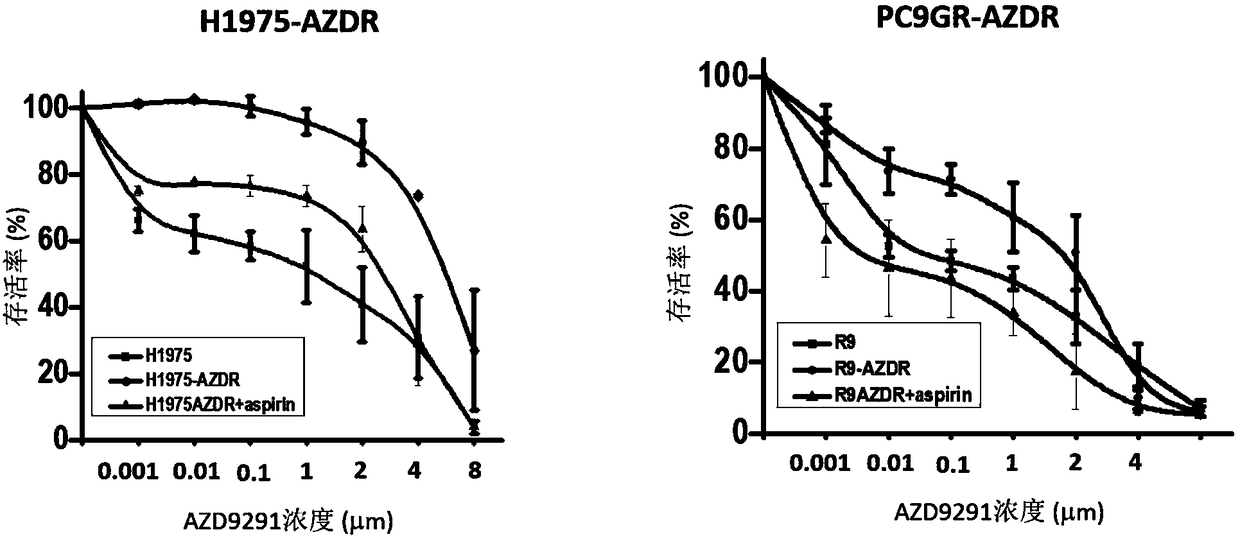
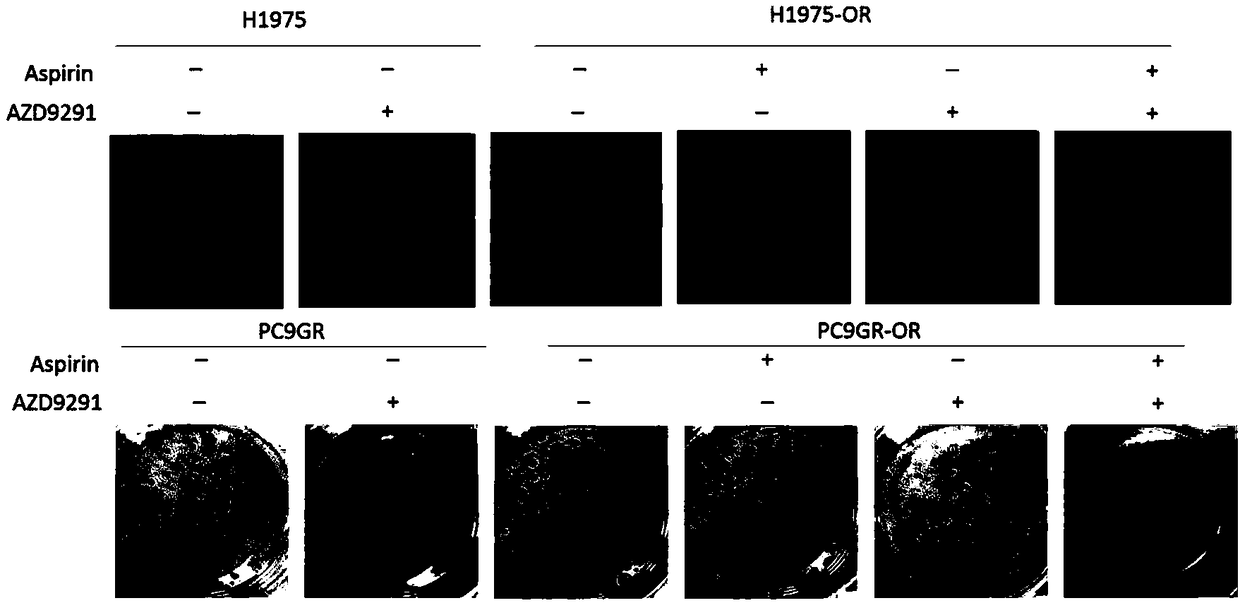
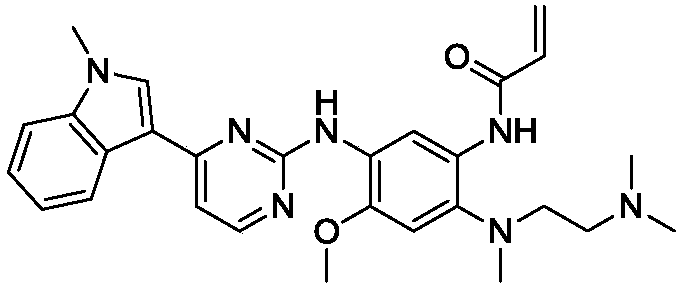
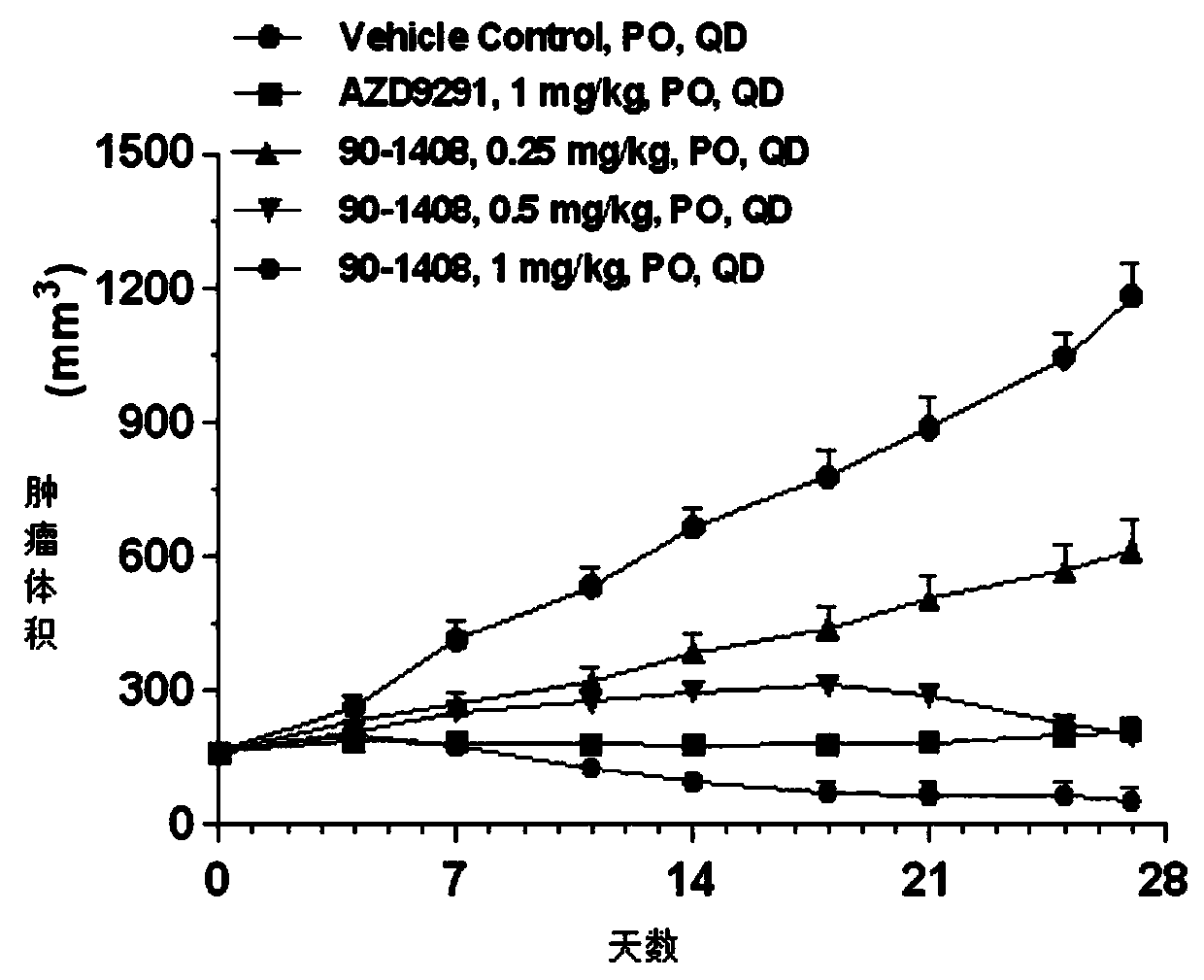
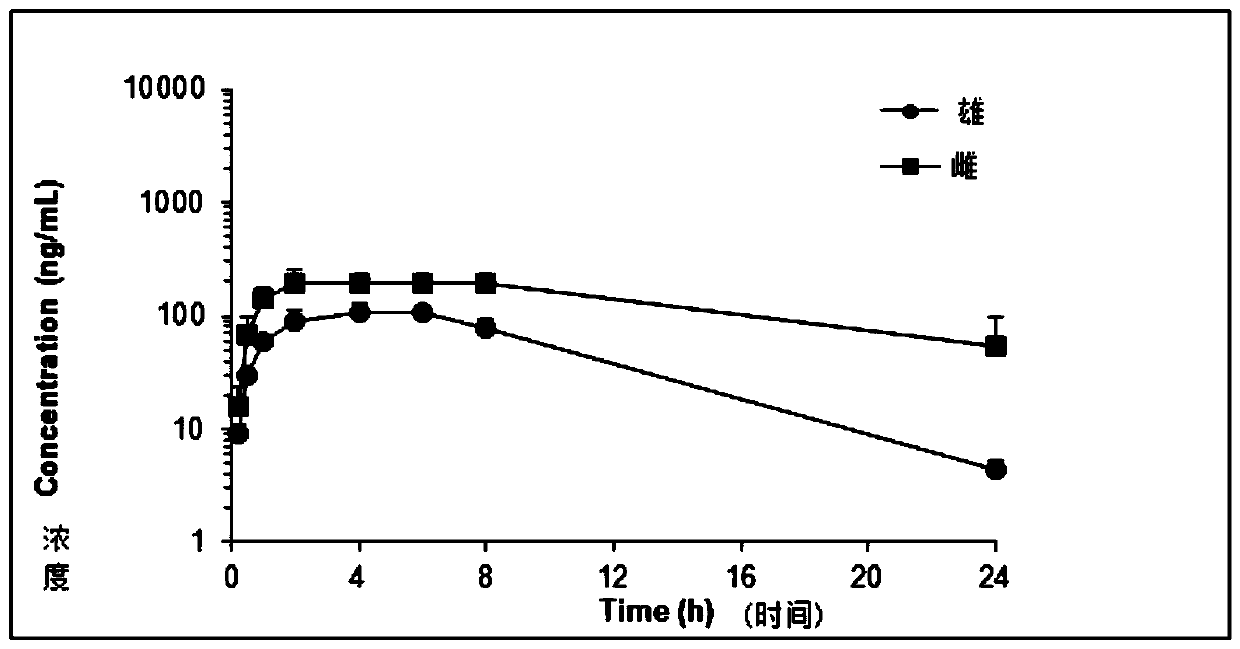
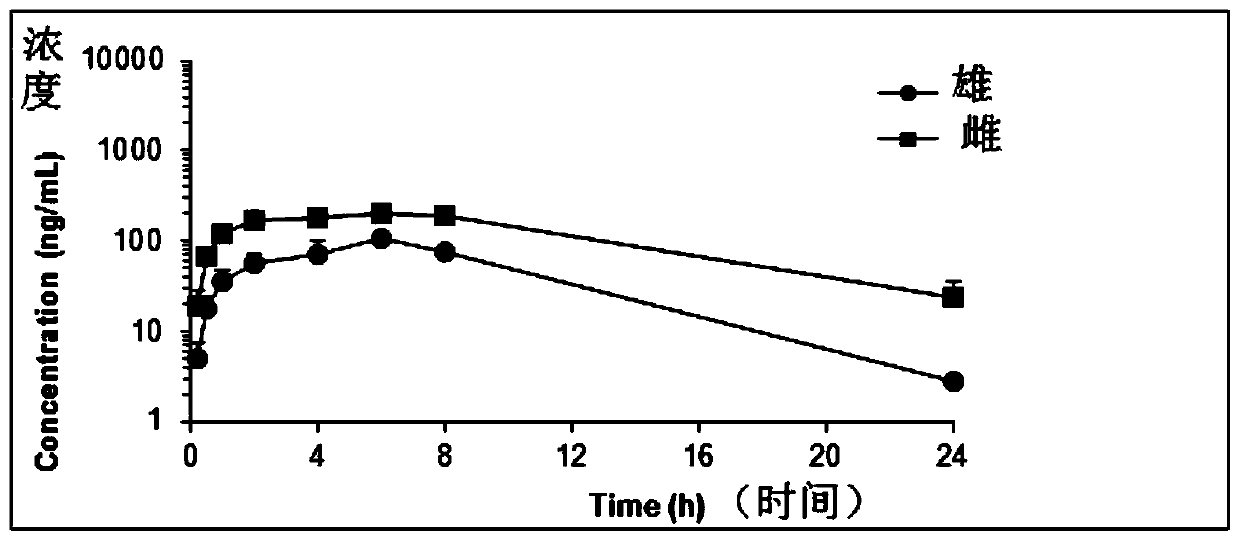
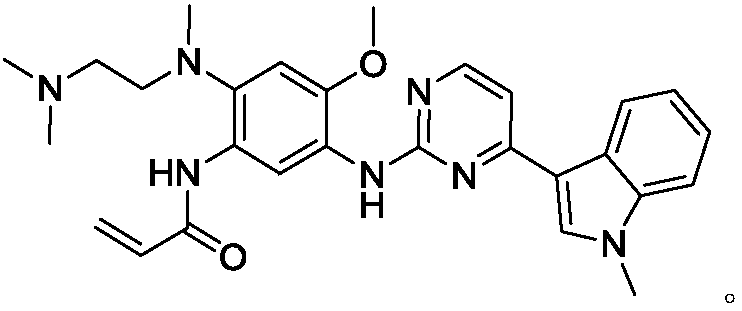
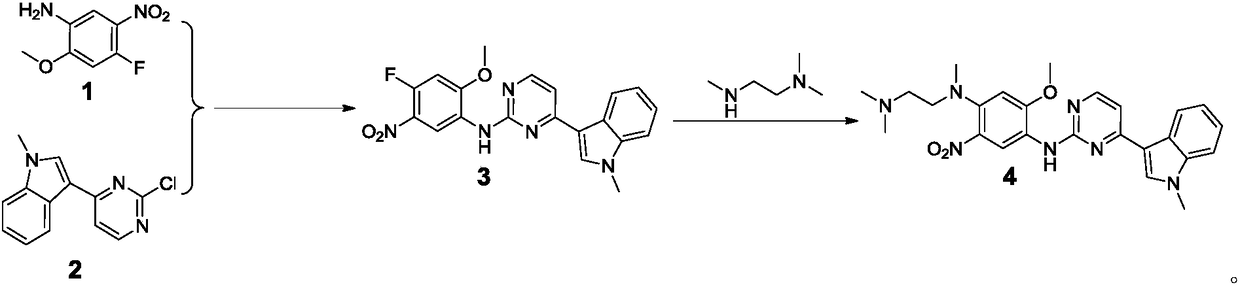
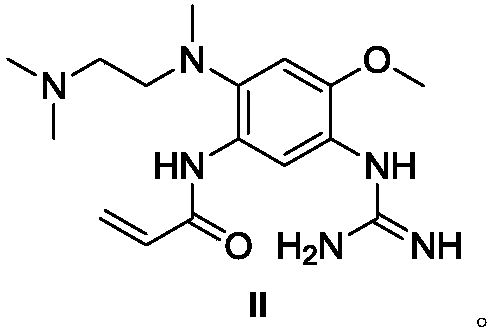
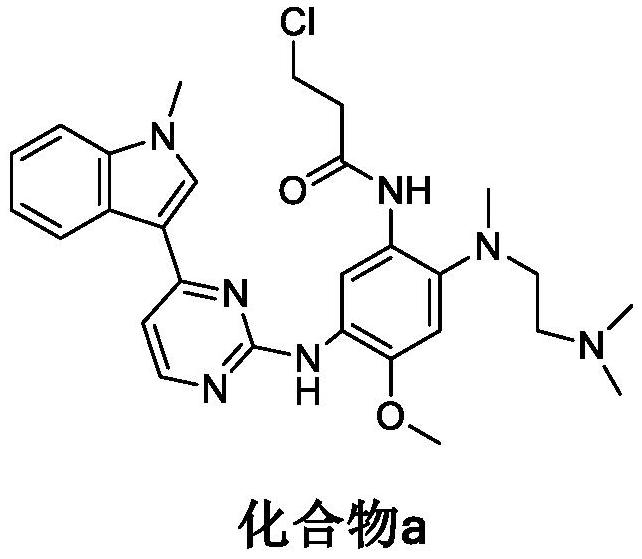
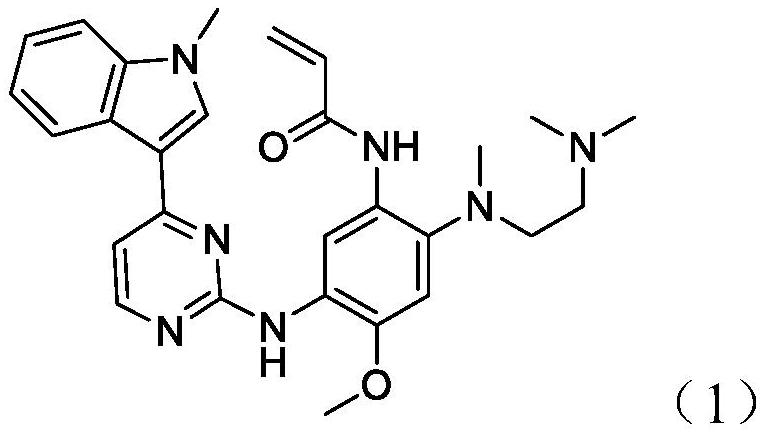
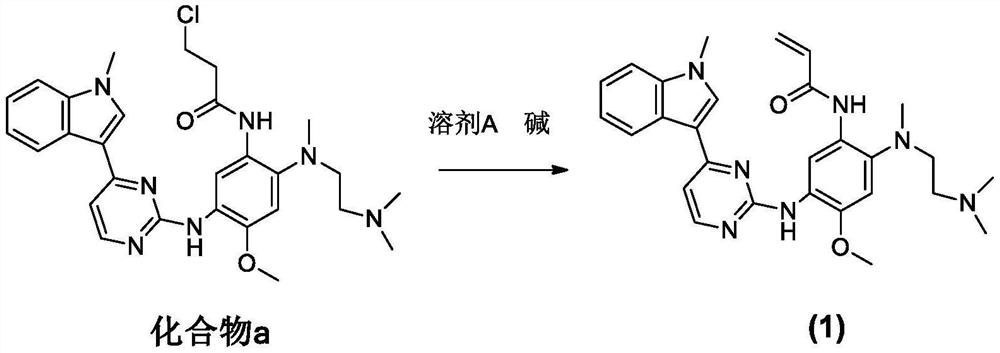
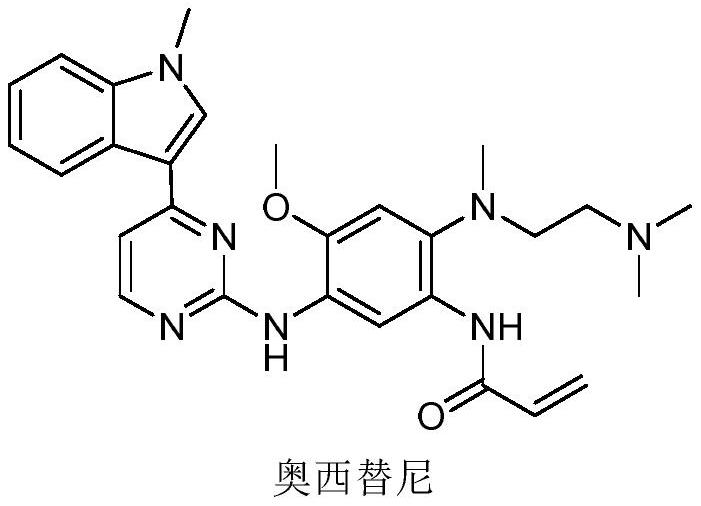
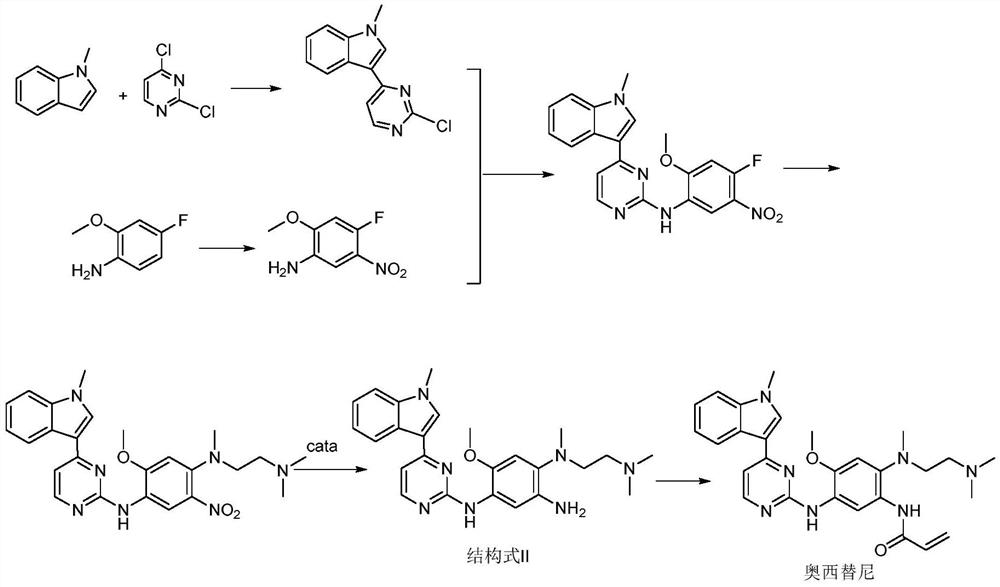
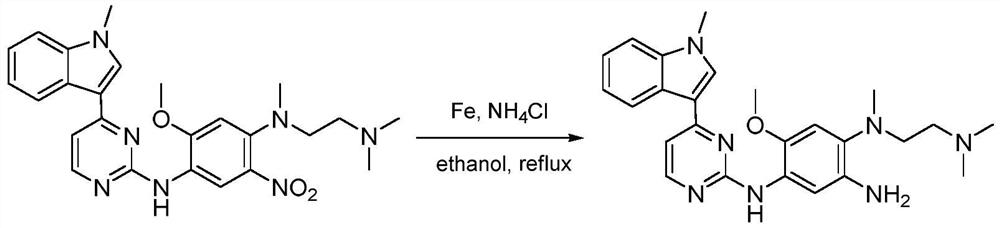
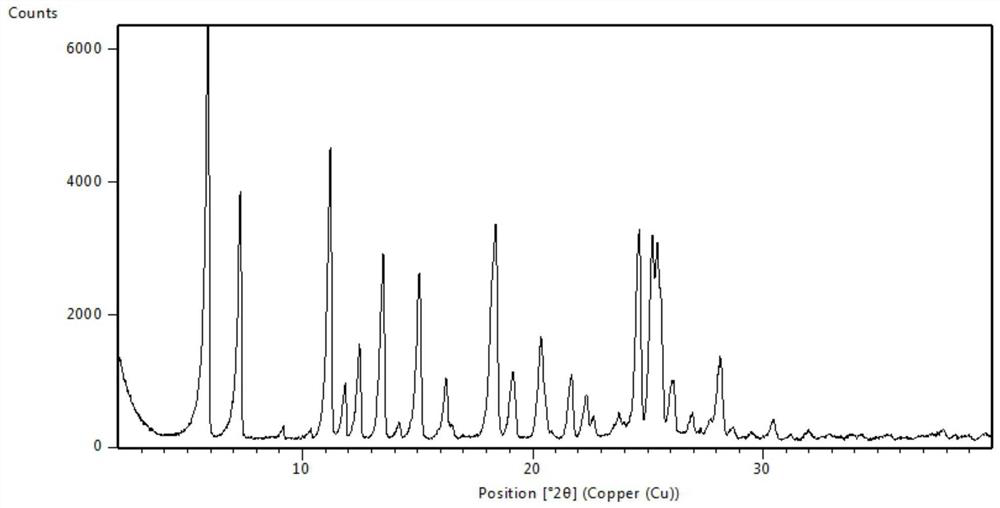
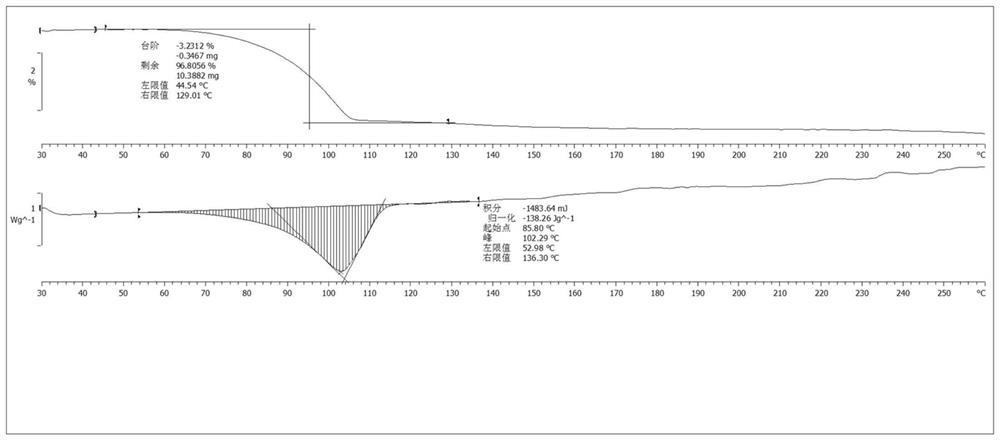
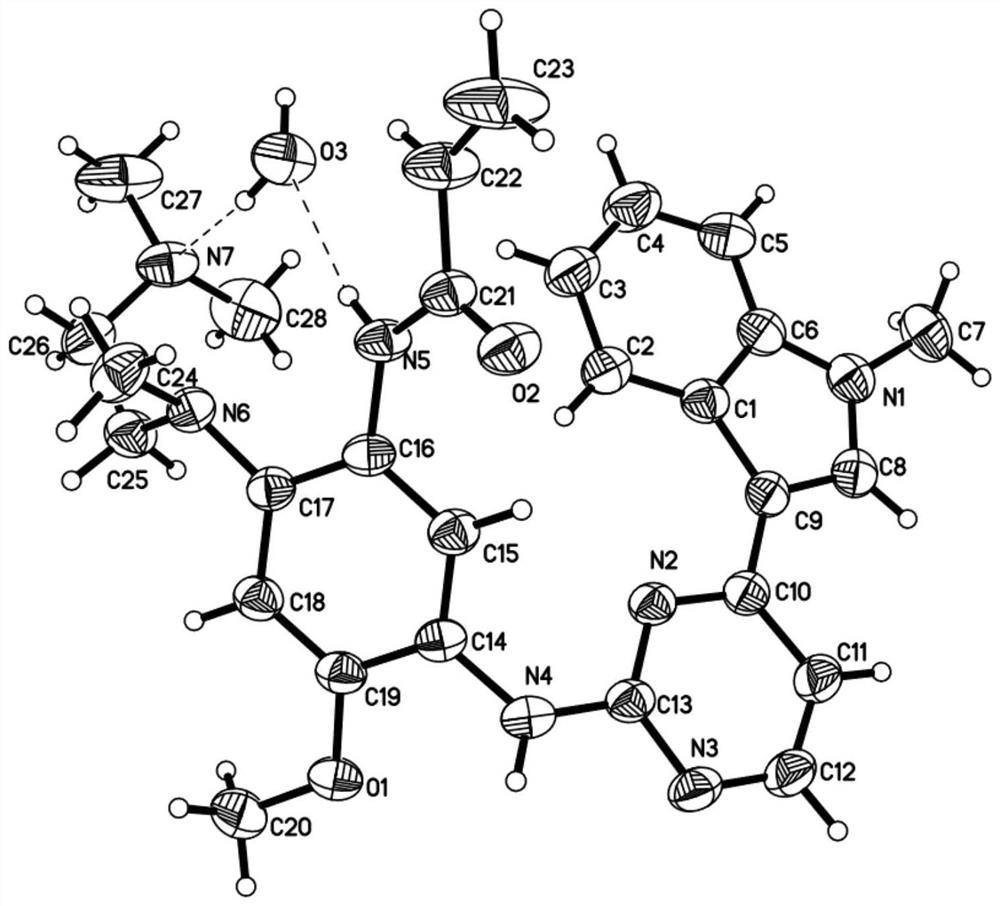
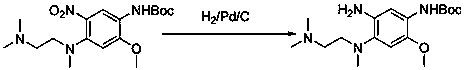Patents
Literature
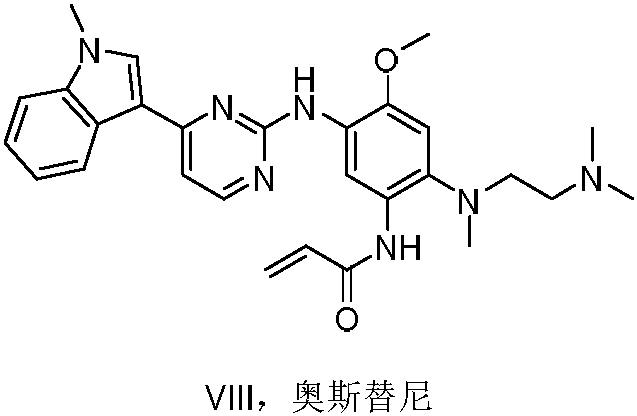
79 results about "Osimertinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
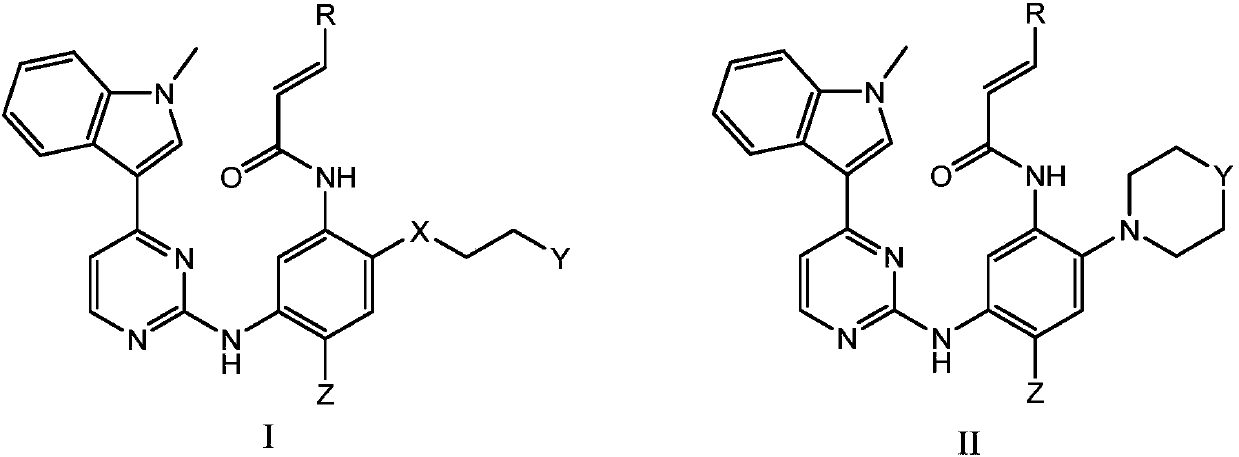
This medication is used to treat lung cancer.
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
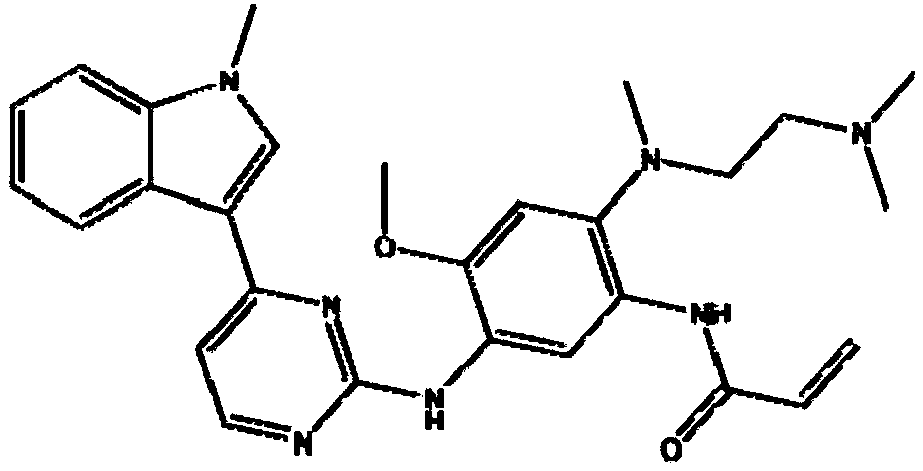
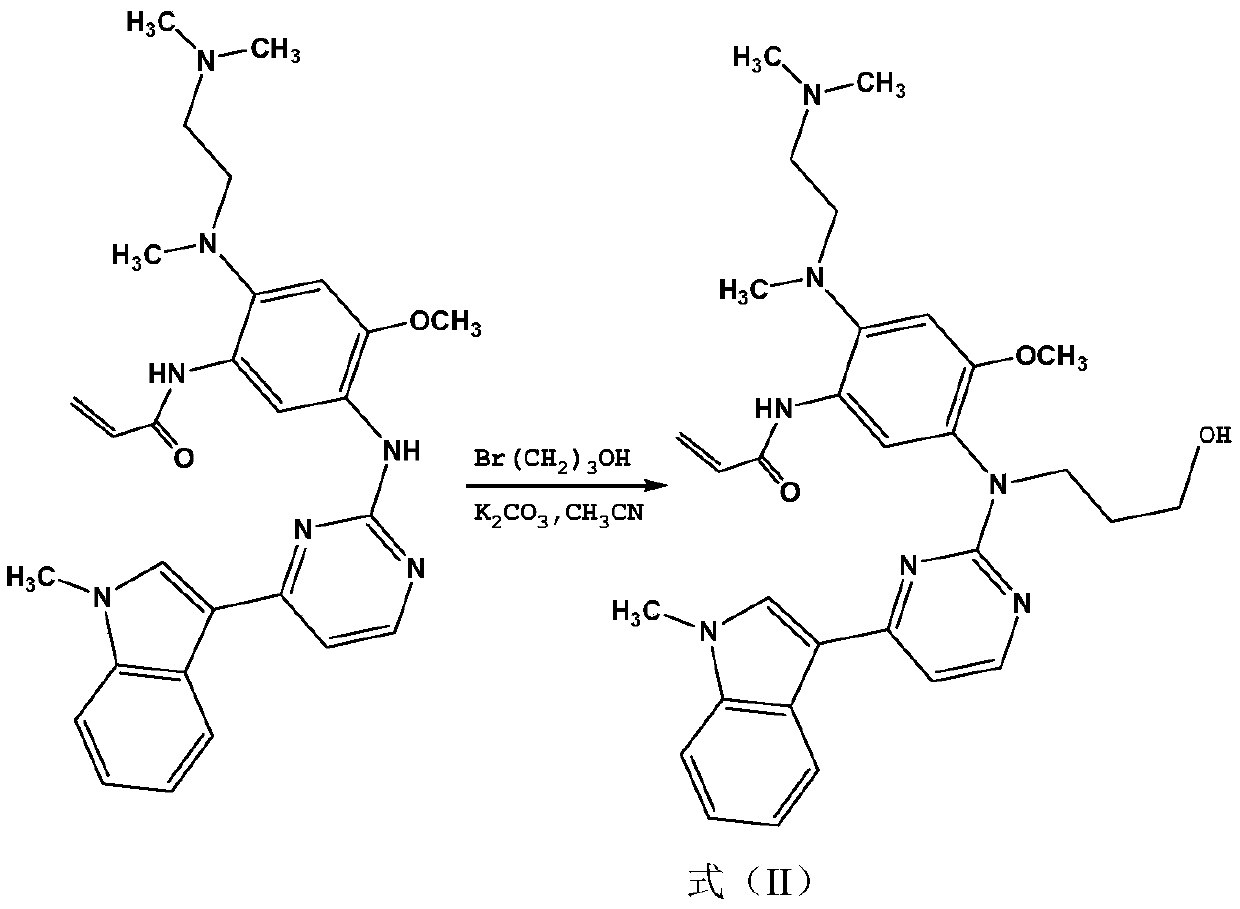
Preparation method of osimertinib
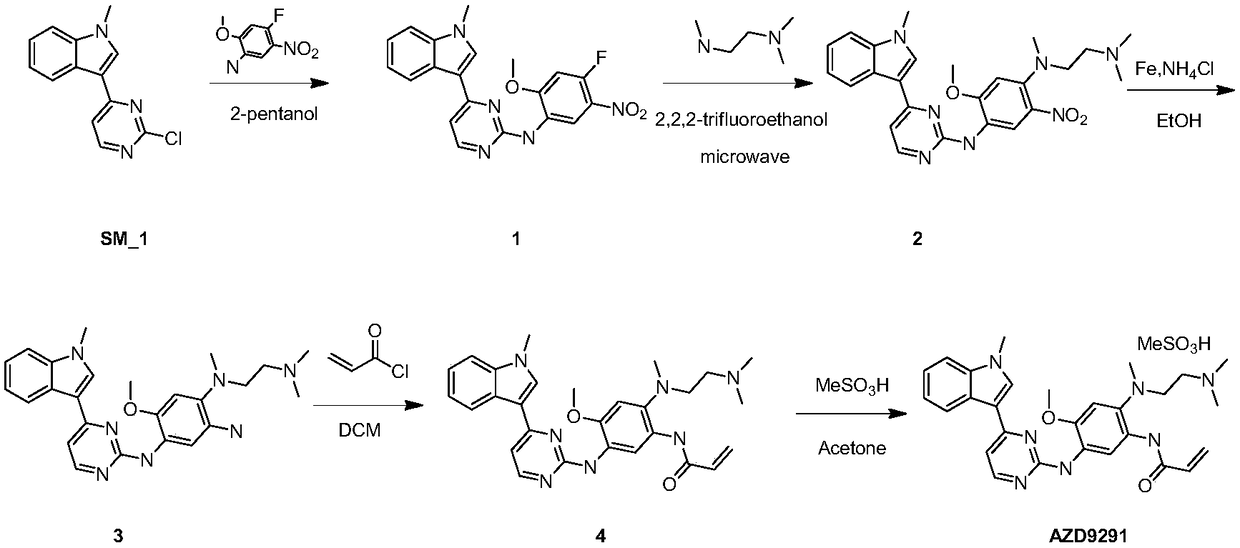
The invention relates to a preparation method of osimertinib. The method comprises the steps: taking 5-fluorine-2-nitroanisole as a starting material, performing aminolysis, reduction, nitration and Lewis acid reaction, carrying out chlorination with N-methyl indol, performing nitryl reduction and amidation to form a product, and finally performing salification. The yield of each step of the technology is higher and reaches above 80%; the method is simple to operate and suitable for amplified production; and the purity is high.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Medicinal composition for resisting non-small cell lung cancer, and application thereof
InactiveCN106668866ASolve the problem of partial drug resistanceInhibition of phosphorylation levelsOrganic active ingredientsAntineoplastic agentsActive componentDihydroartemisinin
The invention discloses a medicinal composition for resisting non-small cell lung cancer. The active components of the medicinal composition comprise an artemisinin derivative and EGFR-TKI (epidermal growth factor receptor-tyrosine kinase inhibitor), wherein the artemisinin derivative is selected from one of dihydroartemisinin, artesunate, artemether and arteether; and the EGFR-TKI is selected from one of gefitinib, erlotinib, afatinib and osimertinib. The invention also discloses application of the medicinal composition to preparation of medicines for treating and resisting the non-small cell lung cancer. When the medicinal composition provided by the invention is used for treating the non-small cell lung cancer, the medicinal effect which is more excellent than that of the singly used EGFR-TKI can be achieved, the sensitizing effect is achieved, the problem of partial medicine resistance of the non-small cell lung cancer EGFR-TKI is solved, and a scientific basis is provided for the development of new medicines.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Drug-resistance cell system for lung cancer and preparation method thereof
InactiveCN108118031AEffectively induce the formation ofManufacturableCulture processTumor/cancer cellsCell systemDrug resistance
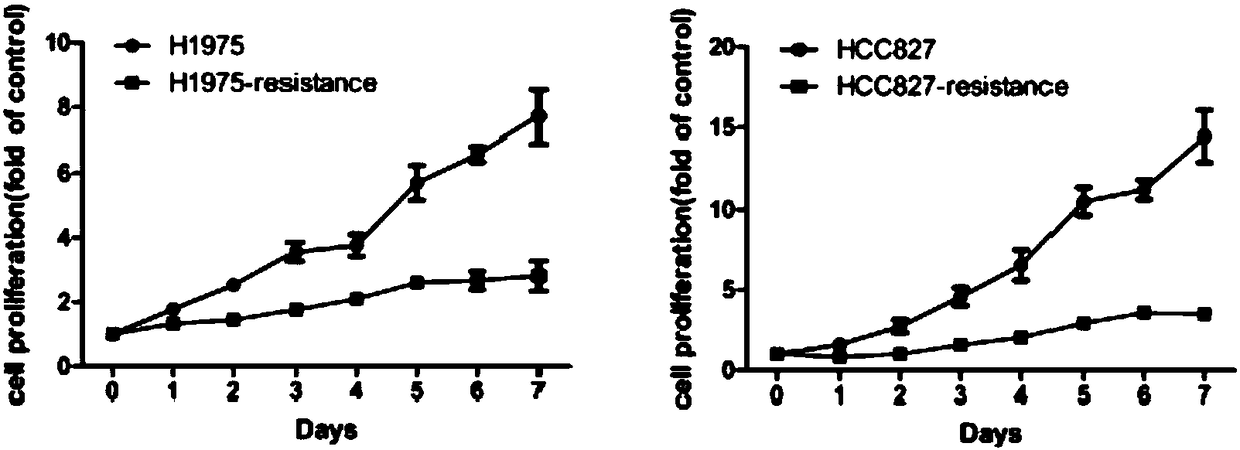
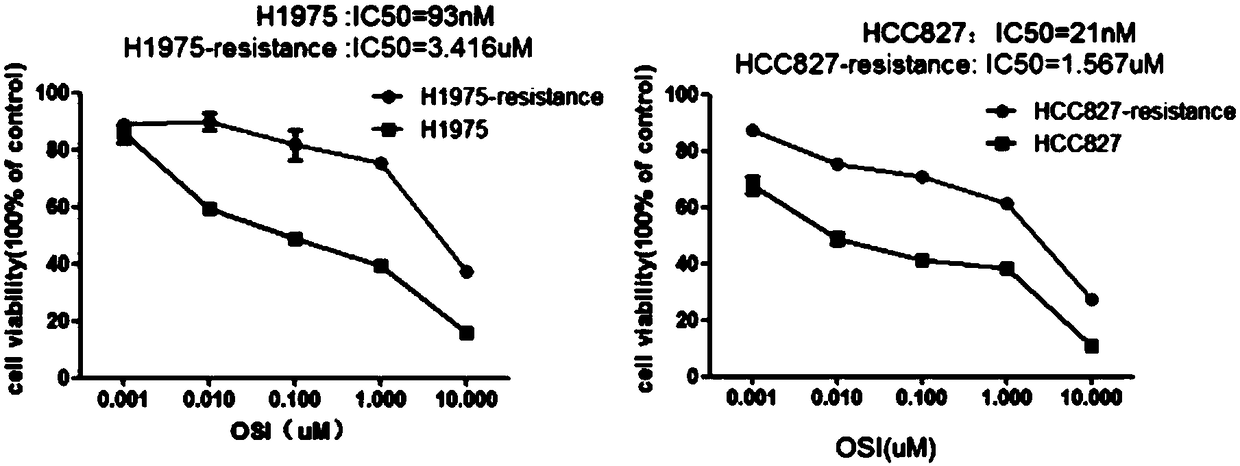
The invention relates to the field of drug-resistance cell systems, and particularly relates to a drug-resistance cell system and a preparation method thereof. The method comprises the following steps: inoculating the lung cancer cells to a culture medium containing a 21-93nM lung cancer targeted drug; culturing until cells can normally live and multiply under the same concentration; improving andculturing at 50nM concentration difference; improving and culturing at concentration difference of every 100nM when the cell multiplying inhibiting concentration is 4 times of IC50; improving and culturing at concentration difference of every 1 microns when the drug sieving concentration is 8 times of IC50; and obtaining the drug-resistance cell systems for the lung cancer when the lung cancer cells can live and multiply in a cell culture system of 10 microns osimertinib. According to the method, the drug-resistance cell system is prepared on the basis of gradually increasing the concentration of drugs in the culture medium; the foundation is provided for later simulation of clinical drug resistance, researching of drug resistance mechanism of lung cancer targeted drugs, and developing ofeffective drug resisting drugs.
Owner:SHANGHAI CHEST HOSPITAL
Osimertinib preparation method
The present invention relates to an osimertinib preparation method, which comprises: (1) carrying out a nucleophilic substitution reaction on 3-(2-chloro-4-pyrimidinyl)-1-methyl-1H-indole and 4-fluoro-2-methoxy-5-nitroaniline to prepare a compound 1; (2) carrying out a substitution reaction on the compound 1 and N,N,N-trimethylethylenediamine in the presence of an organic alkali to prepare a compound 2; (3) reducing the compound 2 in the presence of a reducing agent to prepare a compound 3; (4) carrying out condensation on the compound 3 and 3-chloropropionyl chloride in the presence of an alkali to obtain a compound 4; (5) carrying out a heat elimination reaction on the compound 4 through heating in the presence of an alkali to prepare a compound 5; and (6) carrying out salification on the compound 5 and methanesulfonic acid under a heating condition to obtain osimertinib. According to the present invention, the osimertinib synthesis process has characteristics of stability, controllability, no high-toxicity solvent is used, energy saving and environmental protection.
Owner:上海赛诺克医药科技有限公司
Preparation method of osimertinib intermediate
ActiveCN109485638AEmission reductionImprove protectionOrganic chemistryN dimethylformamideDimethyl acetal
The invention relates to a preparation method of an osimertinib intermediate, comprising: subjecting ortho-nitrotoluene and 3,3-dialkoxypropanenitrile as raw materials to alkali-catalyzed nucleophilicaddition to obtain 1-(2-nitro)phenyl-4,4-dialkoxy-2-butanone, subjecting 1-(2-nitro)phenyl-4,4-dialkoxy-2-butanone and N,N-dimethylformamide dimethyl acetal to thermal condensation to obtain 1-dimethylamino-2-(2-nitro)phenyl-5,5-dialkoxy-3-n-pentanone, subjecting the attained reaction liquid to direct catalytic hydrogenation to obtain 3-(3,3-dialkoxy)propionylindole, subjecting 3-(3,3-dialkoxy)propionylindole to reaction with a methylation reagent under alkaline conditions to generate 3-(3,3-dialkoxy)propionyl-N-methylindole, and subjecting 3-(3,3-dialkoxy)propionyl-N-methylindole and 2-methoxy-4-fluoro-5-nitrophenylguanidine to exocondensation to obtain the osimertinib intermediate. The materials herein are low in price and easy to attain, the route is short, and the preparation method is environmentally friendly and high in yield.
Owner:XINFA PHARMA
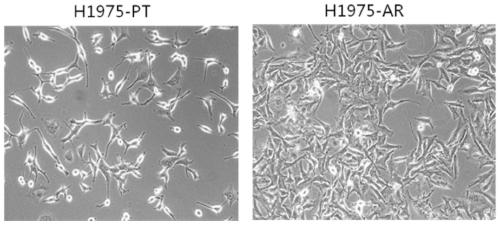
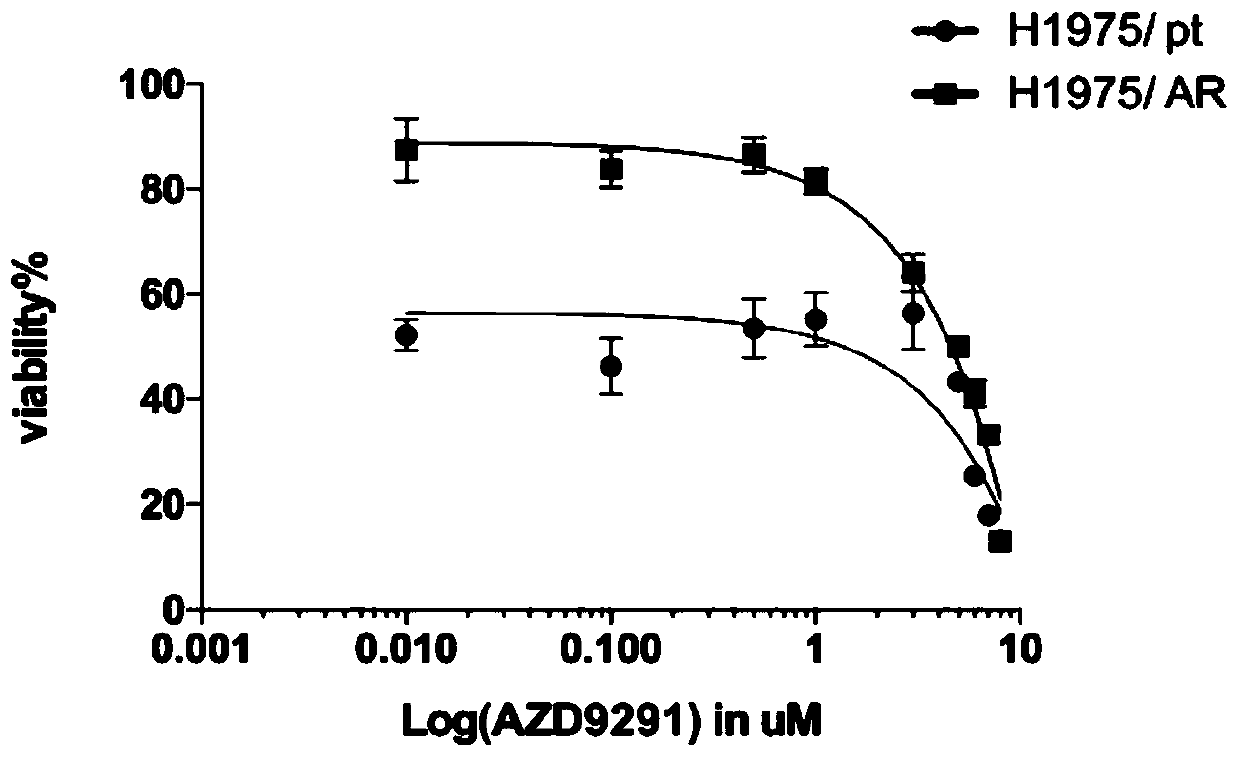
Osimertinib drug-resistant cell line NCI-H1975/AR and application thereof
The invention discloses an osimertinib drug-resistant cell line NCI-H1975 / AR and an application thereof. The drug-resistant cell line is a non-small-cell lung cancer-resistant AZD9291 cell line, namely human lung adenocarcinoma cell NCI-H1975 / AR, the drug-resistant cell line is preserved on June 14, 2019 at China Center for Type Culture Collection, the preservation number is CCTCC NO:C201982. Thedrug resistance index of the drug-resistant line is 10 times or more, and the drug-resistant line can be stably grown and passaged in a culture system with an action concentration of 100 nmol AZD9291,the drug resistance index (RI) to the AZD9291 is 17.7 times, and the drug-resistant cell line provides a drug-resistant cell model for the drug-resistance mechanism of non-small cell lung cancer to atargeted drug, finding of effective treatment methods to overcome drug resistance in the non-small cell lung cancer, and guiding of clinical medication, and has important effects and good applicationprospects.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
Therapeutic agent for lung cancer that has acquired egfr-tki resistance
ActiveUS20190160066A1Good treatment effectGood effectOrganic active ingredientsMicrobiological testing/measurementFirst generationBULK ACTIVE INGREDIENT
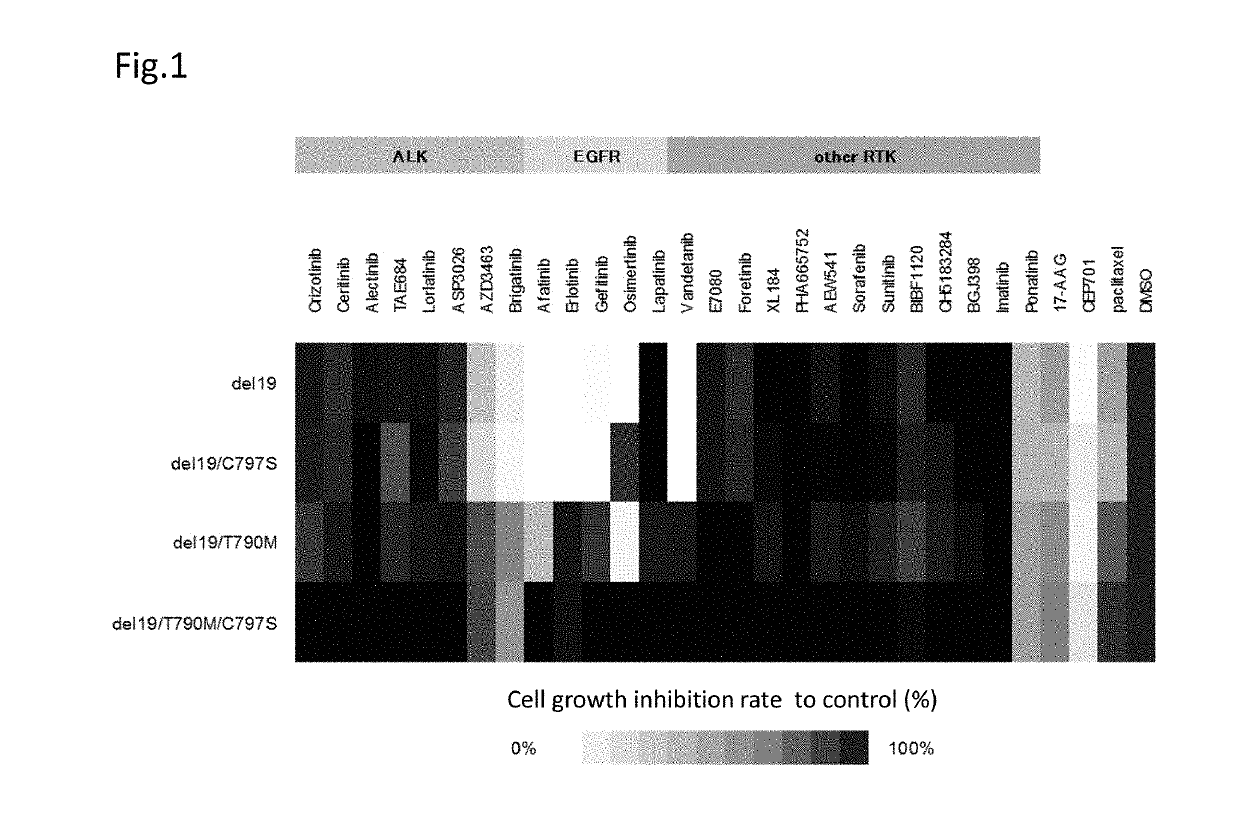
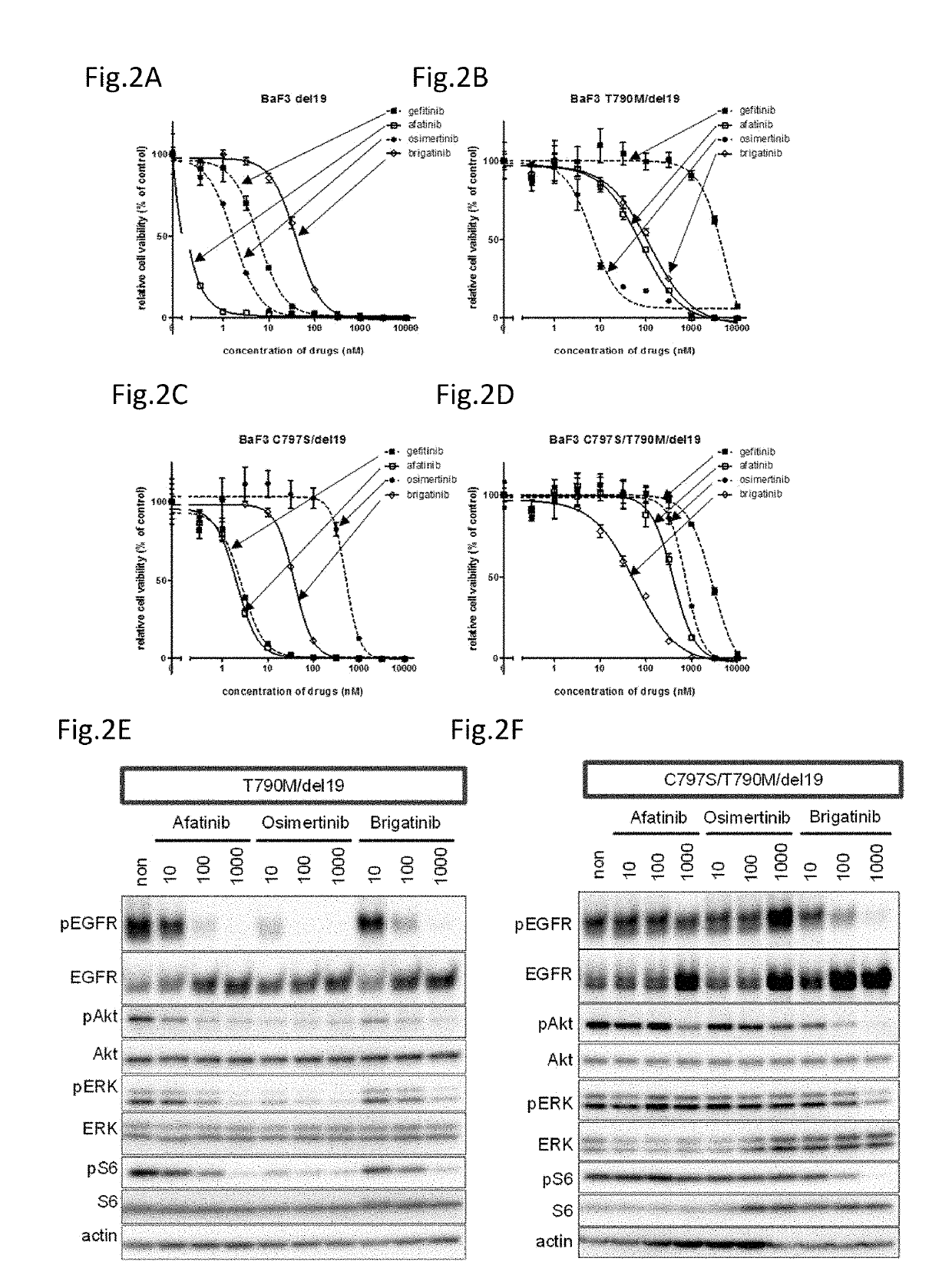
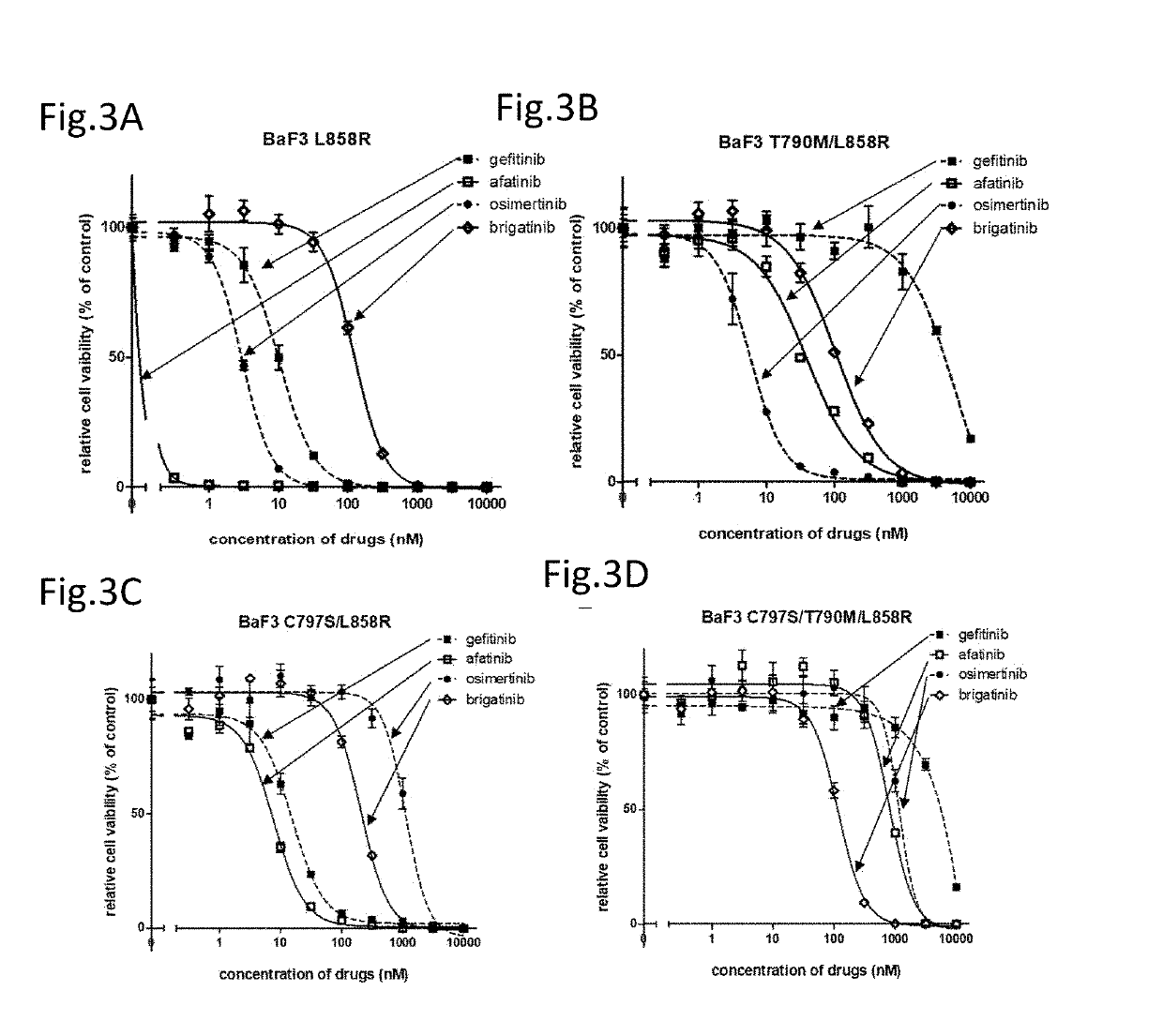
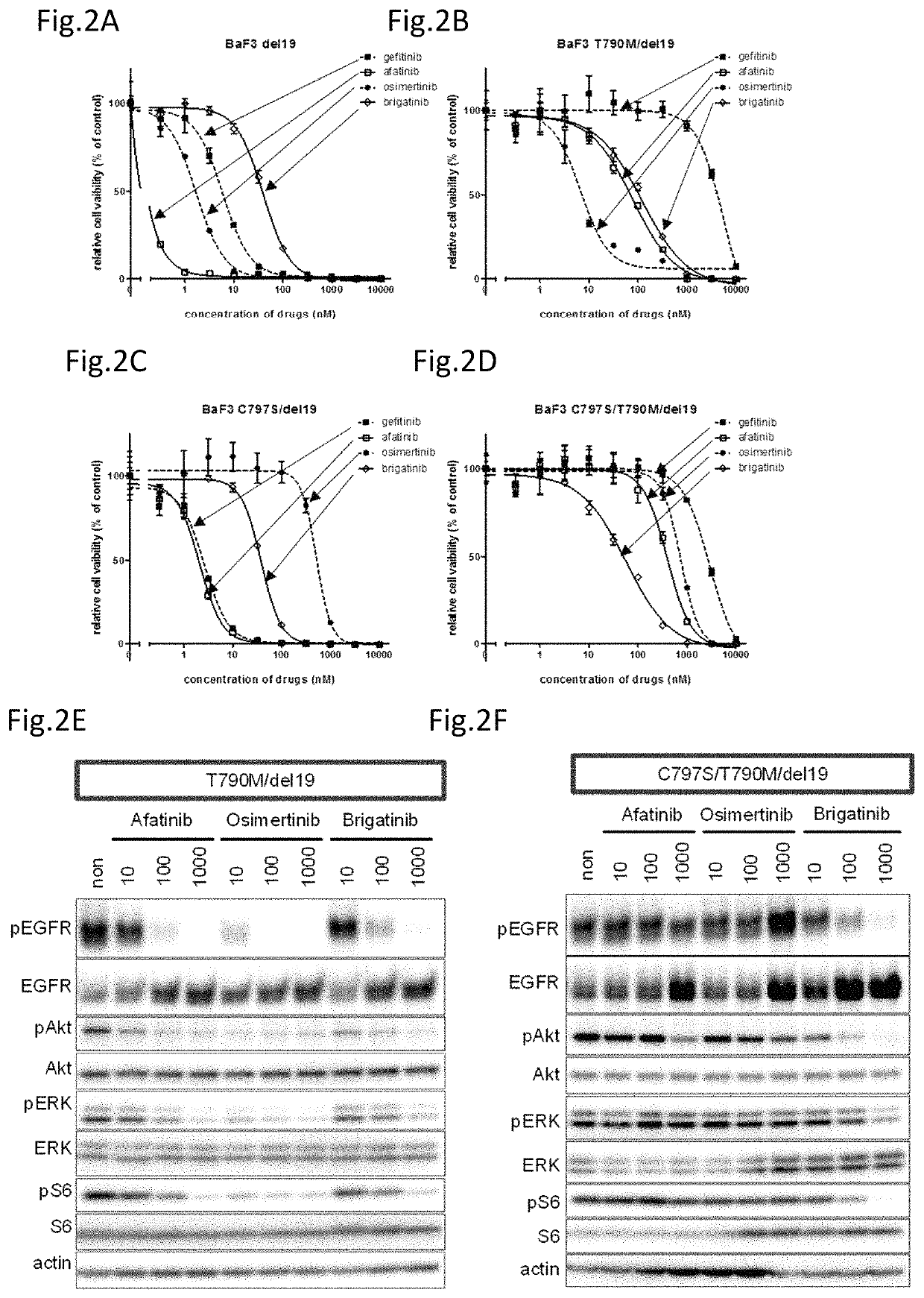
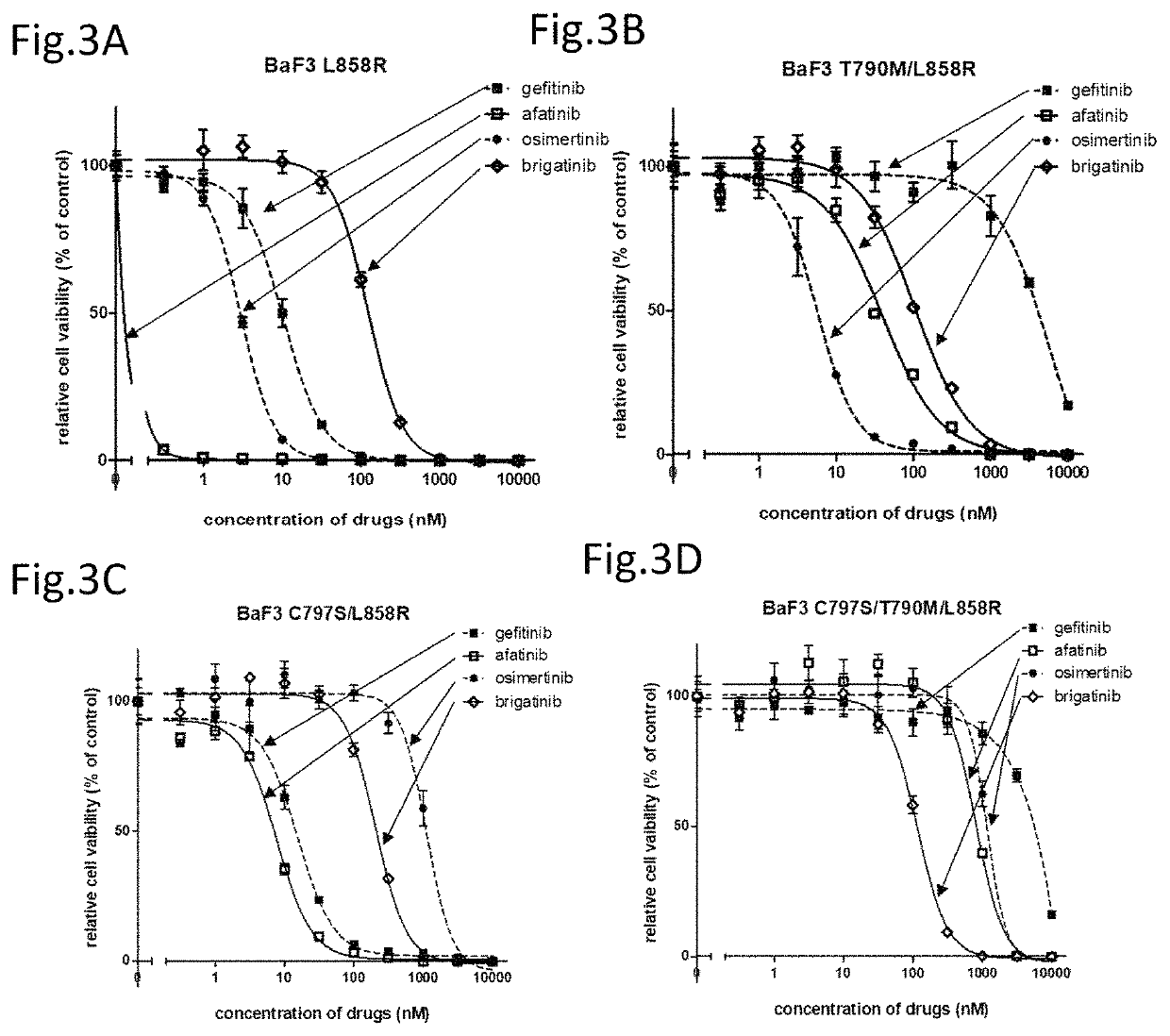
A drug containing, as an active ingredient, a compound represented by ALK inhibitors such as brigatinib, AP26113-analog, and AZD3463 has been found to be effective against a non-small cell lung cancer having a point mutation at C797S in EGFR which has acquired a resistance to chemotherapy agents. Further, the drug used in combination with an anti-EGFR antibody demonstrates a notable suppression effect on the tumor growth. The drug has a potential to be a therapeutic agent effective against a non-small cell lung cancer which is resistant to gefitinib, a first generation therapeutic agent and osimertinib, a third generation therapeutic agent.
Owner:JAPANESE FOUND FOR CANCER RES
Novel N-phenyl-2-pyrimidinaminederivative and application thereof to preparation of anti-tumor medicine
InactiveCN107935995AImprove solubilityImprove bioavailabilityOrganic active ingredientsOrganic chemistrySolubilityHalogen
The invention discloses a novel N-phenyl-2-pyrimidinaminederivative. A side chain of the novel N-phenyl-2-pyrimidinaminederivative contains a sulfoxide SO functional group, a sulphone SO2 functional group, a hydroxyl functional group, a halogen functional group or a methylmercapto functional group, which replaces a NCH3 group and a N(CH3)2 group in osimertinib. As sulfoxide SO, sulphone SO2, hydroxyl, halogen or methylmercapto is introduced into the side chain, favorable activity is achieved on the solubility, the bioavailability and the antitumor activity of a medicine.
Owner:SUN YAT SEN UNIV
Synthesis method of Osimertinib
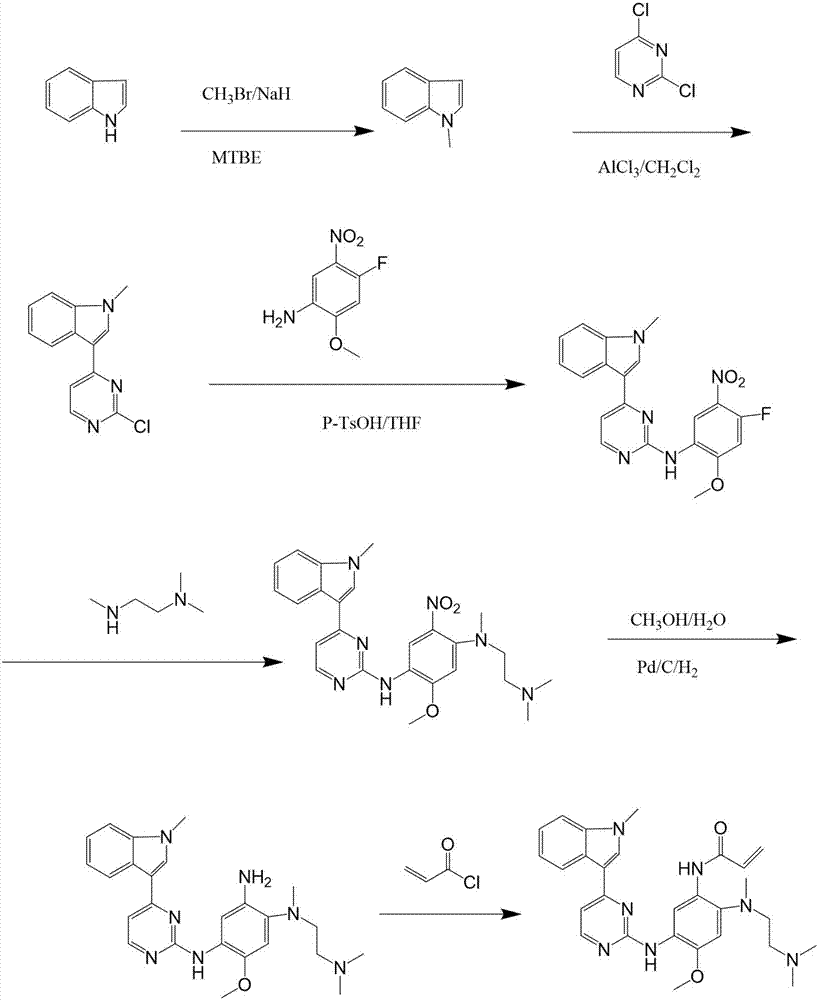
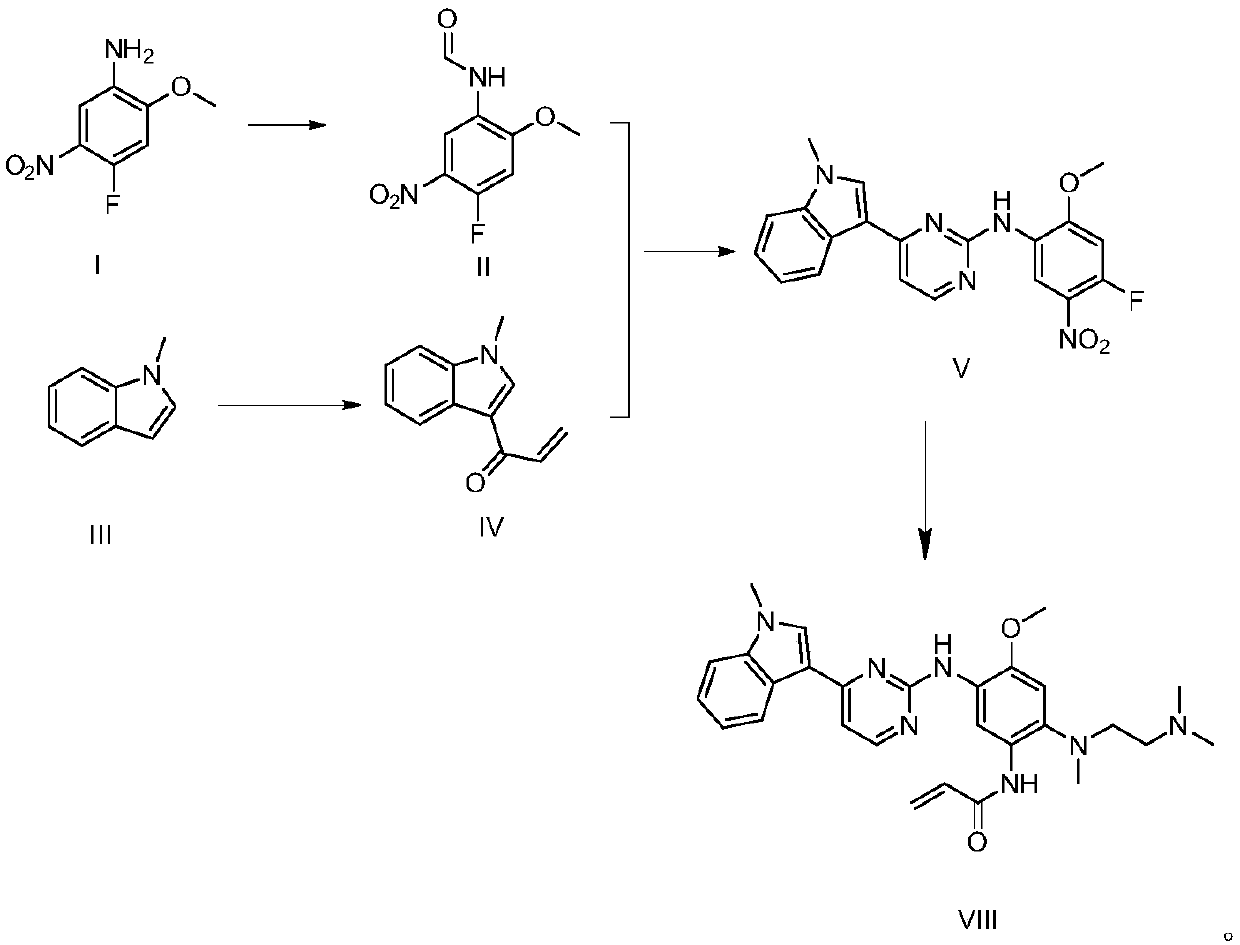
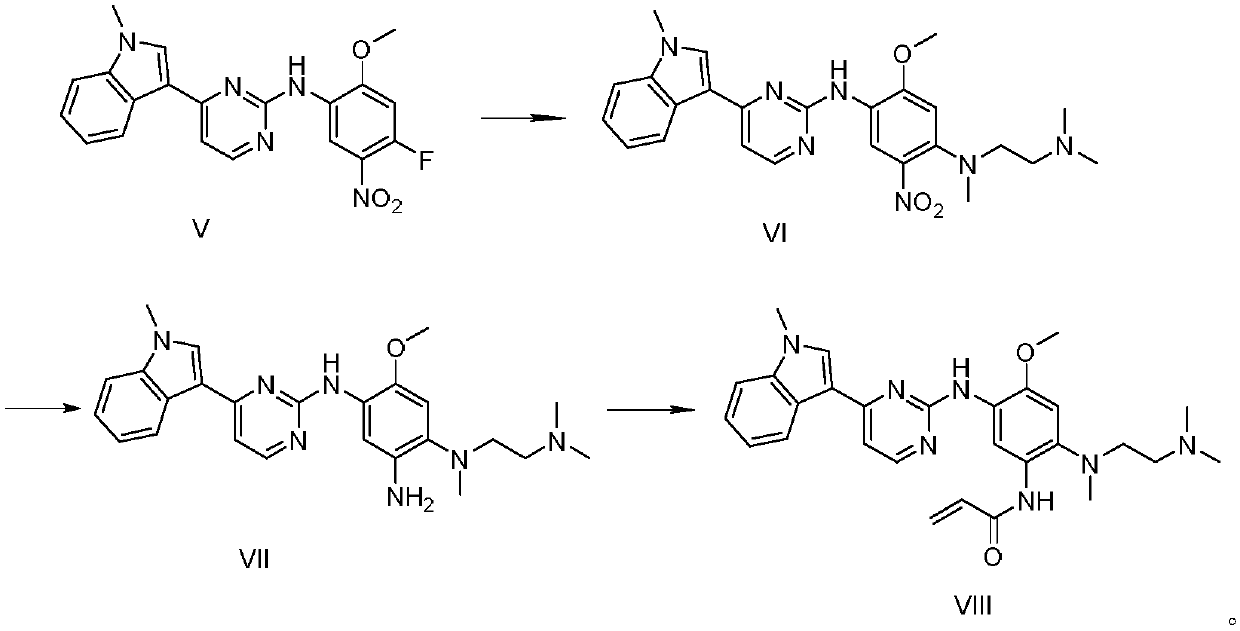
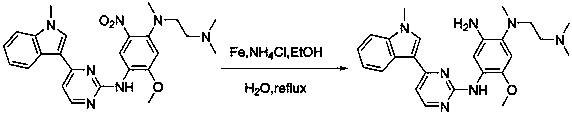
The invention discloses a synthesis method of Osimertinib. The method comprises the following steps that (1) 4-fluoro-2-methoxyl-5-nitroaniline is subjected to formylation to obtain N-(4-fluoro-2-methoxyl-5-nitrophenyl) methanamide; (2) N-methylindole is subjected to acylation reaction to prepare 3-(1-methyl-1H-indole-3-yl)prop-2-en-1-one; (3) products generated in the former two-step reactions are subjected to one-step cyclization to obtain 2-(4-fluoro-2- methoxyl-5- nitroanilino)-4-(1-methyl-1H-indole-3-yl) pyrimidine; (4) the product generated in the step (3) is sequentially subjected to nucleophilic substitution reaction, reduction reaction and condensation amidation reaction to obtain the Osimertinib. The method provided by the invention has the advantages that the raw materials can be easily obtained; the steps are simple; the yield is high; the reaction conditions are mild; the synthesis method is suitable for industrial production.
Owner:安庆奇创药业有限公司
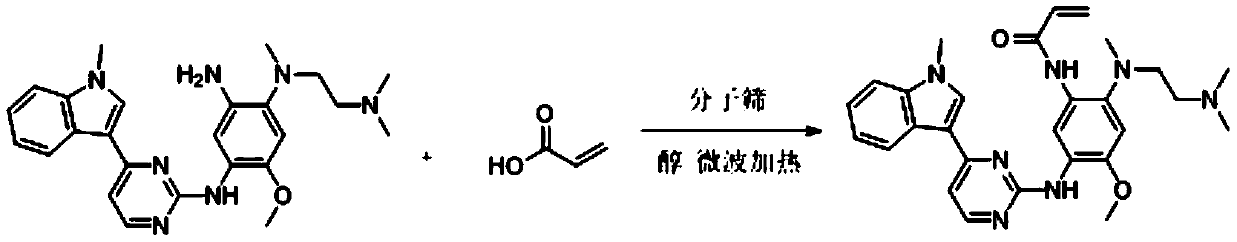
Method for synthesizing osimertinib by molecular sieve catalysis
The invention relates to the field of organic synthesis, in particular to a novel method for synthesizing osimertinib through molecular sieve catalysis. N-1-[2-(dimethylamino) ethyl]-5-methoxy-N1-methyl-N4-[4-(1-methyl-1H-indole-3-yl-2-pyrimidinyl]-1,2,4-triaminobenzene and acrylic acid as raw materials, acrylic acid is added into an alcohol solvent, reaction is carried out through molecular sievecatalysis and microwave heating, and after the reaction is completed, post-treatment is carried out to obtain the osimertinib. According to the method, the reaction conditions are mild, industrial production is facilitated, the raw materials are environmentally friendly in use, used highly toxic raw materials are decreased, the yield is high, and the reaction efficiency is high.
Owner:滨州市鸿源工程有限公司
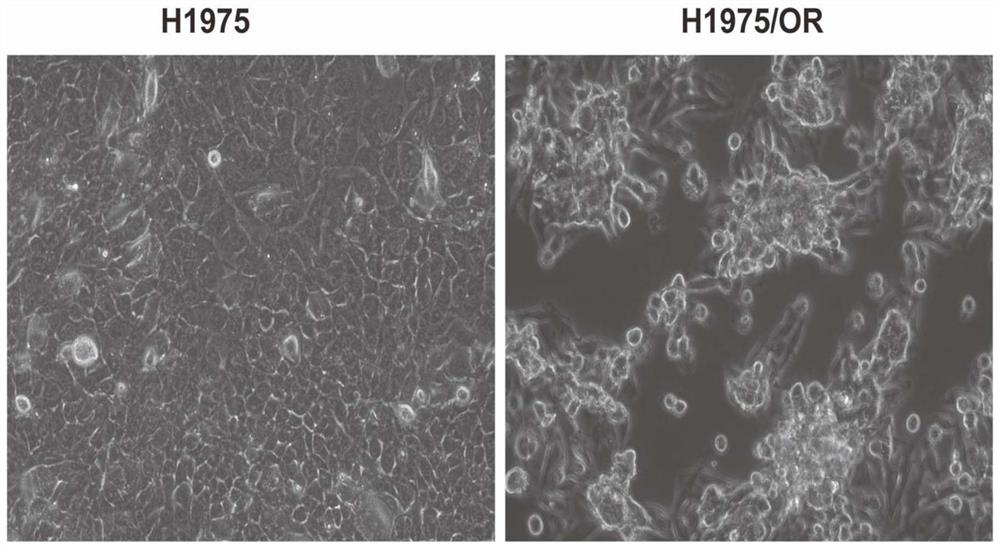
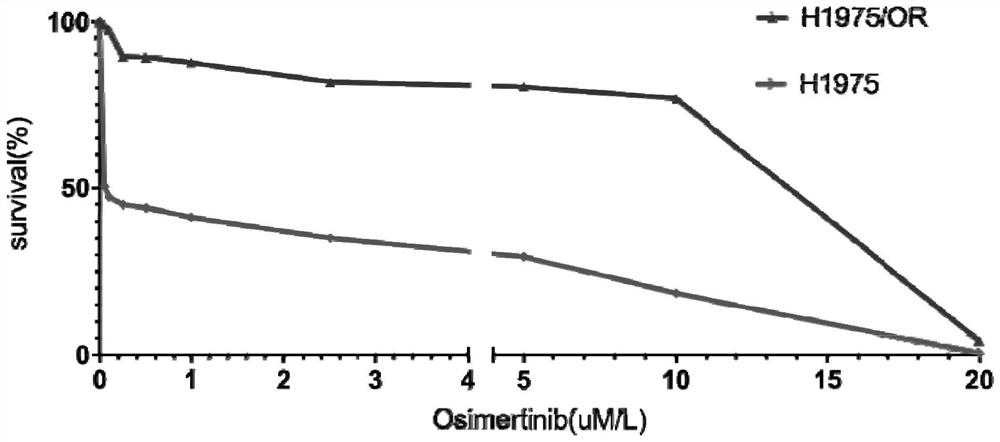
Osimertinib-resistant human non-small cell lung cancer cell strain H1975/OR and application thereof
ActiveCN111793604AIncrease production capacityCompound screeningApoptosis detectionTumor therapyOncology
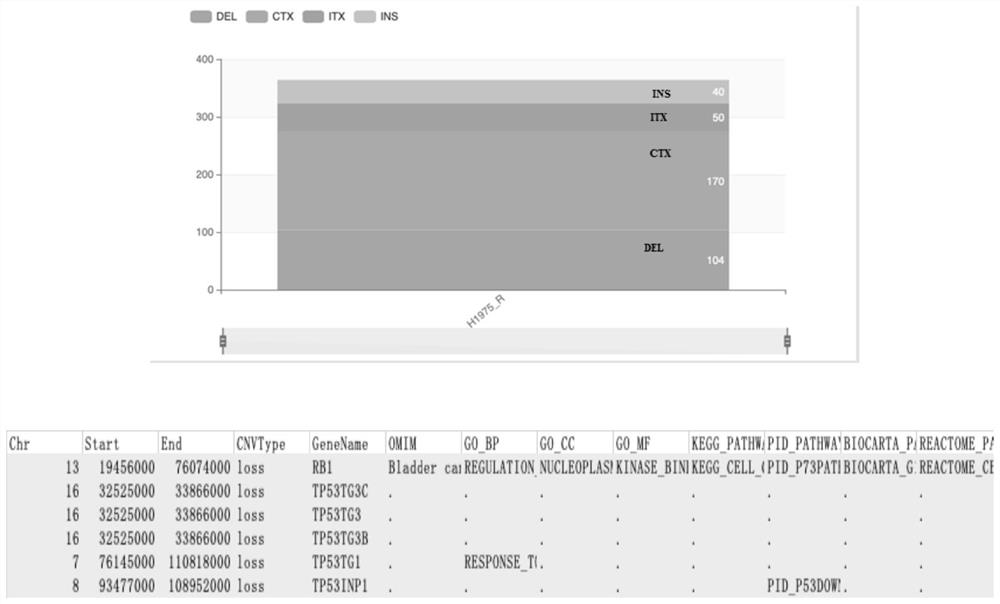
The invention discloses an osimertinib-resistant human non-small cell lung cancer cell strain which is named as osimertinib-resistant human lung cancer cell line H1975 / OR, and has a preservation number of CCTCC NO:C2019300. According to the cell strain, EGFR exon21: c.T2573G: p.L858R mutation and exon20: c.C2369T: p.T790M mutation which are sensitive to osimertinib are reserved, RB1 gene deletionoccurs after drug resistance is acquired, and a small cell lung cancer phenotype is shown. The osimertinib-resistant human non-small cell lung cancer cell strain H1975 / OR disclosed by the invention can be used for researching the morphological and biological characteristics of osimertinib-resistant human non-small cell lung cancer cells, studying a tumor drug resistance mechanism, analyzing the sensitivity of the anti-tumor drug, screening and evaluating the anti-tumor drug, developing a tumor drug resistance reversal drug and studying a more effective tumor treatment method, and can be used for discussion of a small cell lung cancer transformation mechanism after EGFR-TKIs treatment and research of related signal channels, has high scientific research and production application values, and is expected to produce good scientific research, economic and social benefits.
Owner:SUN YAT SEN UNIV CANCER CENT
Osimertinib ketorolac salt crystal form and preparation method thereof
PendingCN110483486AReduce humidityImprove stabilityOrganic active ingredientsAntipyreticKetorolacX-ray
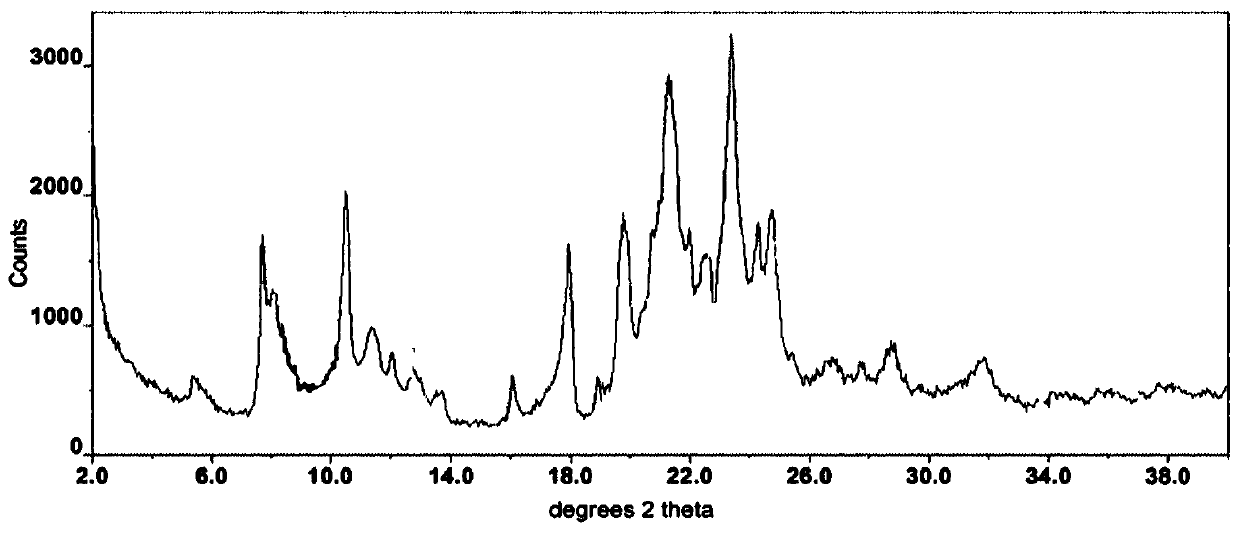
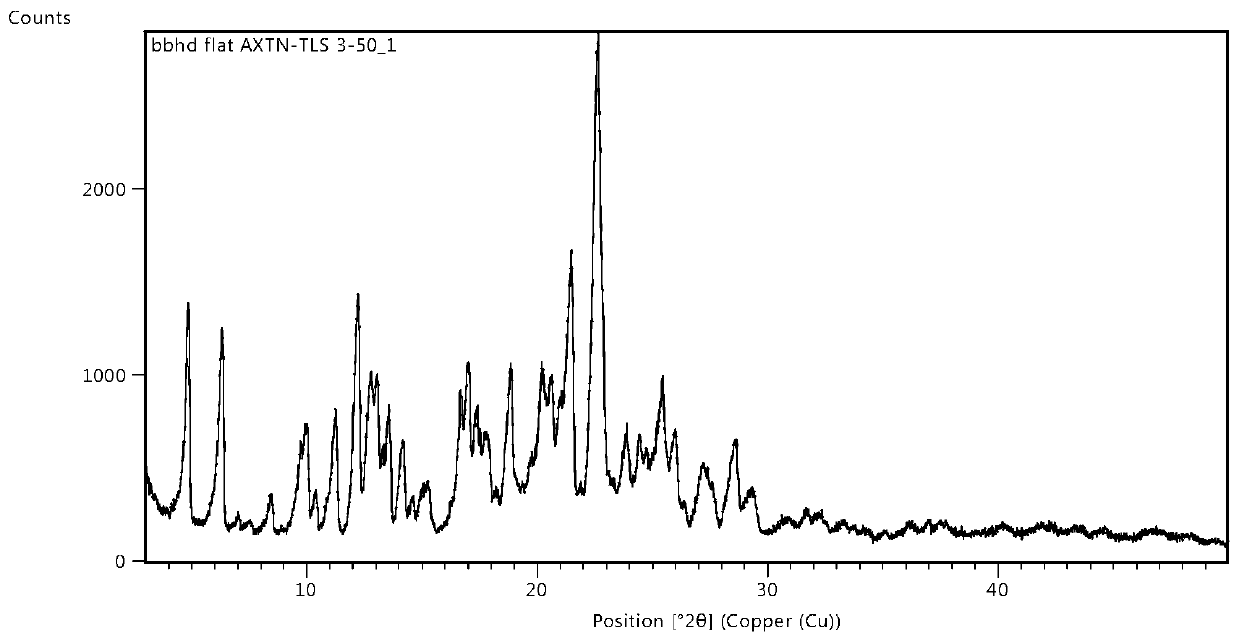
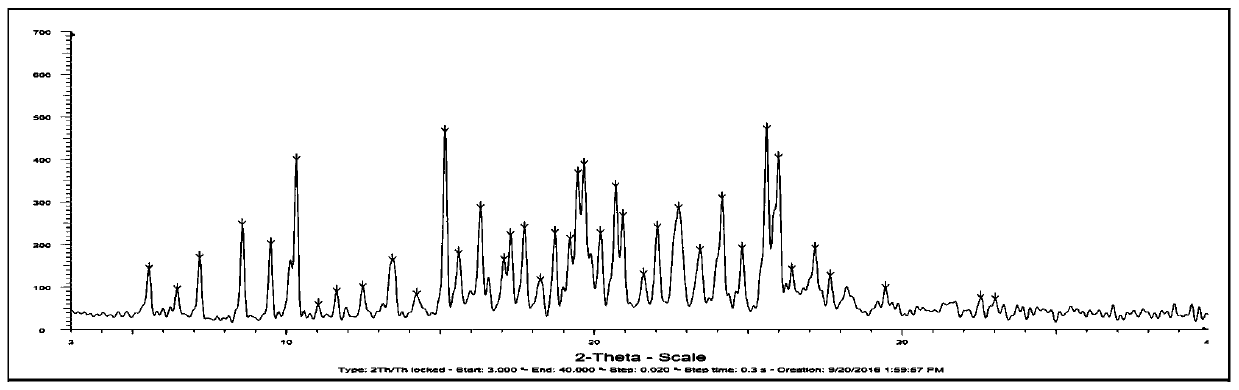
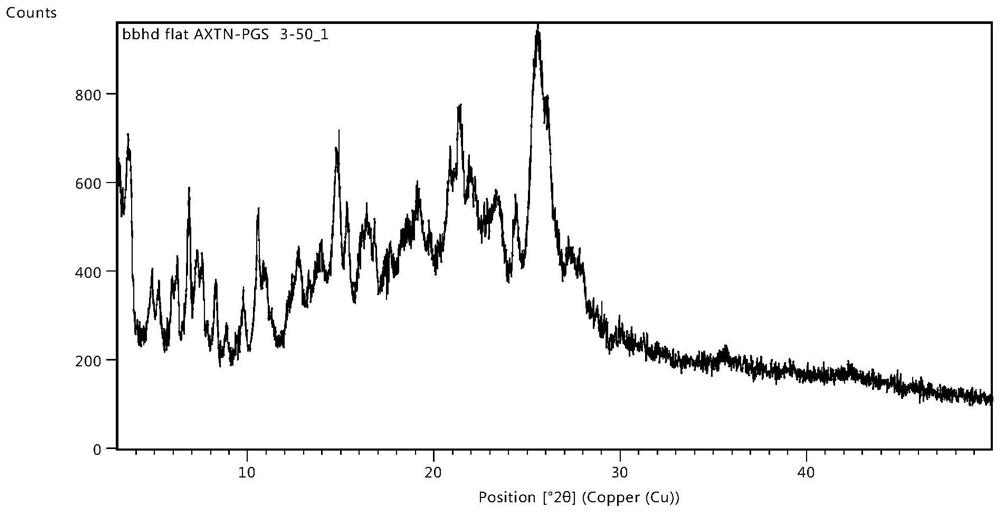
The invention belongs to the technical field of medicines, and particularly relates to an osimertinib ketorolac salt crystal form and a preparation method thereof. An X-ray diffraction pattern expressed by 2theta and utilizing Cu-Kalpha radiation, of the crystal form, has characteristic peaks at 4.9+ / -0.2 degrees, 6.4+ / -0.2 degrees, 12.2+ / -0.2 degrees, 21.+ / -0.2 degrees and 22.6+ / -0.2 degrees. Theosimertinib ketorolac crystal form is low in hygroscopicity, simple in preparation process, stable in property and suitable for large-scale production.
Owner:LUNAN PHARMA GROUP CORPORATION
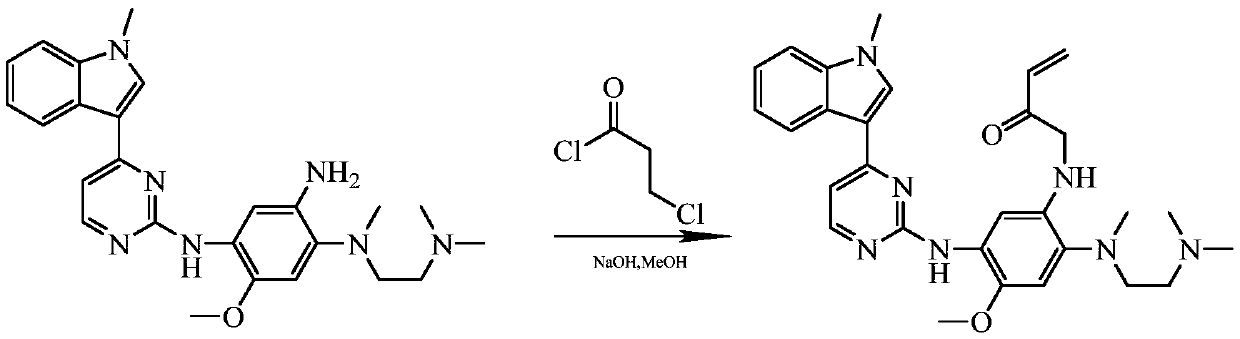
Method for preparing osimertinib mesylate
InactiveCN107188888AImprove protectionLow costSulfonic acids salts preparationEthylenediamineMethyl-1H-indole
The invention discloses a method for preparing Osimertinib mesylate. The method comprises the steps that after 3-(2-chlorine-4-pyrimidinly)-1-methyl-1H-indole is subjected to substitution reaction with 4-fluorine-2-methoxy-5-nitroaniline, 3-(2-chlorine-4-pyrimidinly)-1-methyl-1H-indole is subjected to substitution reaction with N,N,N'-trimethyl-ethylenediamine, nitro is restored after catalytic hydrogenation, then 3-(2-chlorine-4-pyrimidinly)-1-methyl-1H-indole is subjected to coupling reaction with 3-chloropropionyl-chloride, osimertinib is obtained after elimination with sodium hydroxide, and osimertinib reacts with salt mesylate in acetone and water to obtain osimertinib mesylate, wherein a catalyst for restoring nitro by the catalytic hydrogenation is raney nickel, palladium carbon or palladium-carbon hydroxide. The method for preparing osimertinib mesylate is low in cost, high in yield, little in environmental pollution, simple and easy in production process operation and suitable for industrialized production.
Owner:LUOXIN PHARM SHANGHAI CO LTD +1
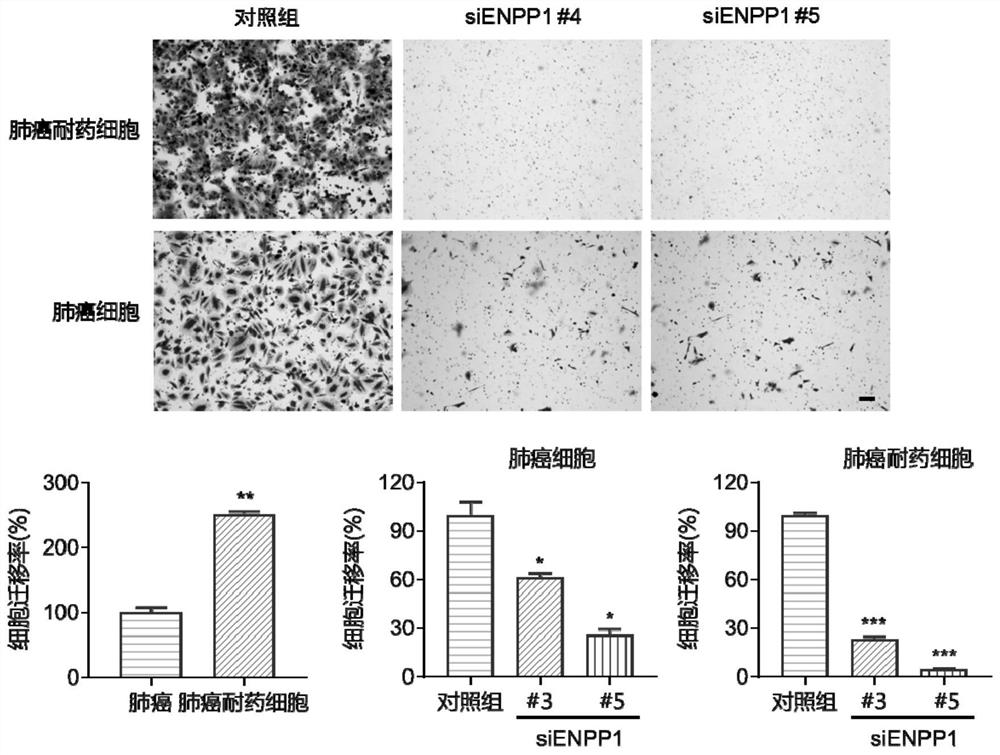
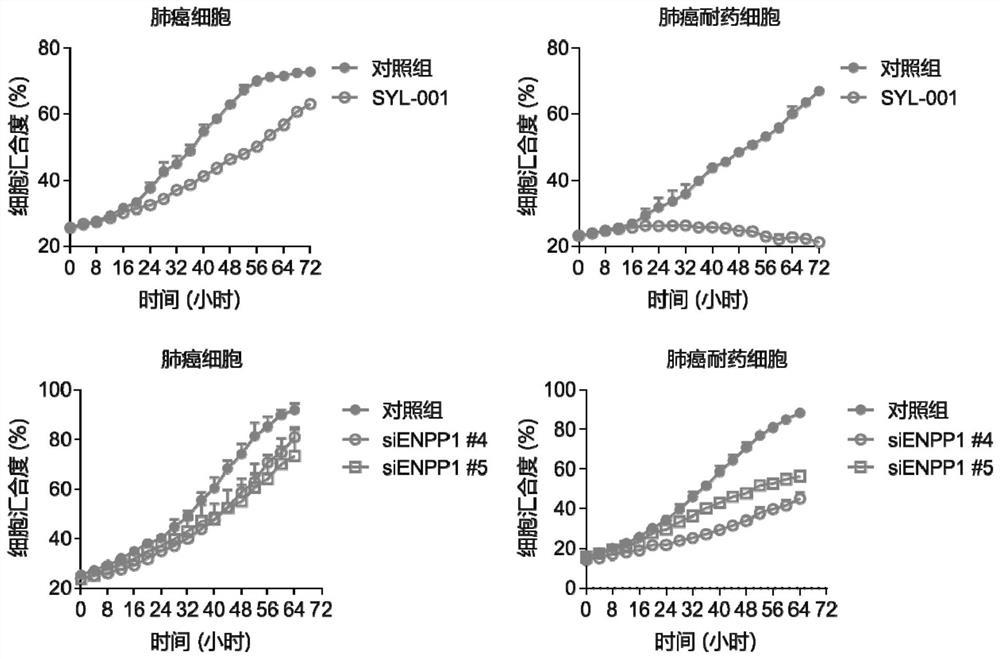
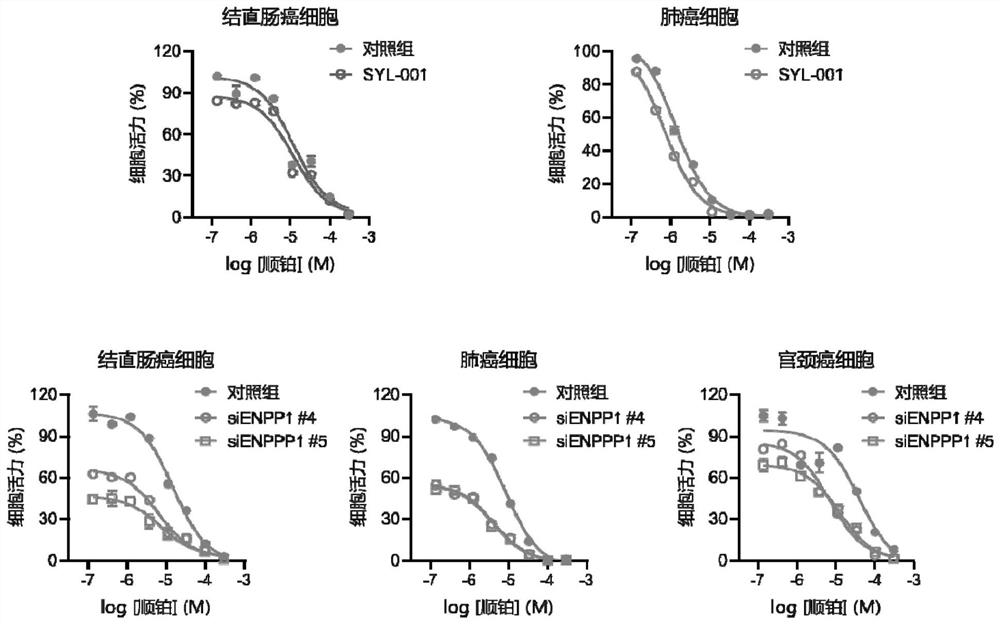
Application of drug combination of ENPP1 inhibitor and anticancer drug to preparation of antitumor drug
ActiveCN111973748AGrowth inhibitionDelay drug resistanceOrganic active ingredientsInorganic active ingredientsOncologyChemo therapy
The invention provides application of a drug combination of an ENPP1 inhibitor and anticancer drug to preparation of antitumor drug. The ENPP1 inhibitor comprises SYL-001 or carrier-delivered siENPP1,shENPP1 and CRISPR-Cas9 preparations; and the anticancer drug comprises cis-platinum, tarceva, gefitinib or osimertinib. According to the invention, the results that the ENPP1 inhibitor and chemotherapeutic drug of cis-platinum and lung cancer targeted drug (tarceva, gefitinib and osimertinib) cooperate to achieve the antitumor effects; the occurrence of phenomena of drug resistance and recurrence are obviously inhibited and delayed; and the cancer includes colorectal cancer, cervical cancer and lung cancer. More importantly, the ENPP1 inhibitor particularly and significantly inhibits drug-resistant recurrent lung cancer cell proliferation, migration and reversing drug resistance. Therefore, the ENPP1 inhibitor can be applied to treatment and prevention of drug resistance and reversing drug resistance, and has efficient clinic application prospects.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
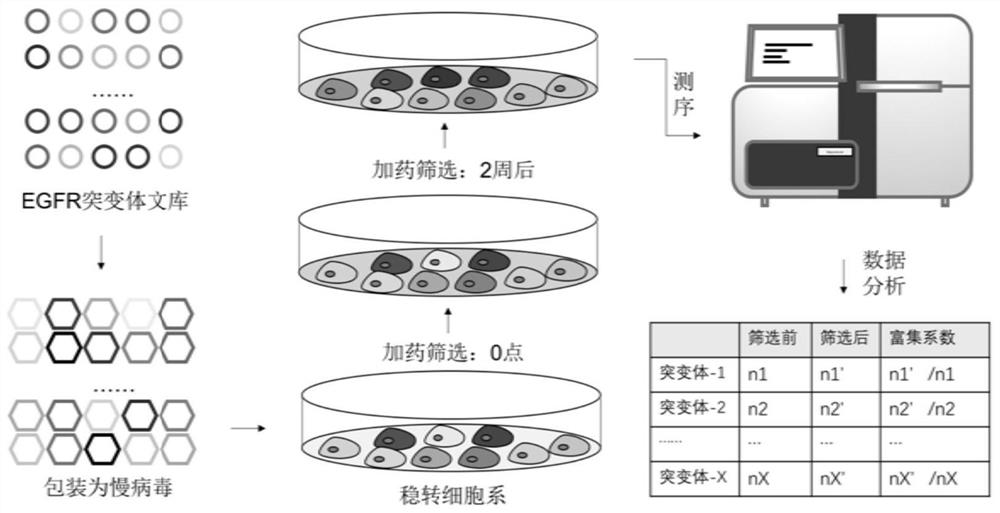
Human EGFR gene missense mutation molecular marker and application thereof in predicting drug resistance of targeted inhibitor
The invention belongs to the field of biomedicine and gene detection, and particularly relates to a human EGFR gene missense mutation molecular marker and application thereof in predicting drug resistance of a targeted inhibitor. The molecular marker provided by the invention comprises 242 types of EGFR missense mutants related to the drug resistance of erlotinib, gefitinib and icotinib, and other 15 types of EGFR missense mutants related to the drug resistance of afatinib and osimertinib, and the mutants can be used for predicting the drug resistance of non-small cell lung cancer patients to targeted inhibitor treatment. According to the invention, a mutant library of EGFR gene tyrosine kinase functional regions is constructed by using a synthetic biology method, and the EGFR mutants are highly enriched in drug screening; the molecular marker can be clinically used as a potential molecular marker for predicting drug resistance of a lung cancer patient after the lung cancer patient is treated by a targeted inhibitor.
Owner:卫国朋
Application of aspirin in preparing medicine for treating NSCLC (non-small cell lung cancer)
InactiveCN108096257AOvercoming acquired drug resistanceIncreased sensitivityOrganic active ingredientsAntineoplastic agentsAspirinOncology drugs
The invention belongs to the field of antitumor drugs and specifically discloses application of aspirin in preparing medicine for treating NSCLC (non-small cell lung cancer). In comparison with the fact that osimertinib is applied alone to treat EGFR(+) NSCLC, the drug combination of the aspirin and the osimertinib can effectively inhibit proliferation of the EGFR(+) NSCLC cells, so as to promoteapoptosis of the EGFR(+) NSCLC cells. The drug combination of the aspirin and the osimertinib enhances sensitivity of the NSCLC patient to EGFR-TKI, overcomes the defect of highly possible drug resistance to the osimertinib, increases the effective treatment time of the osimertinib and can further improve the prognosis of the EGFR(+) NSCLC patient.
Owner:THE THIRD AFFILIATED HOSPITAL INST OF FIELD SURGERY OF PLA ARMY MEDICAL UNIV
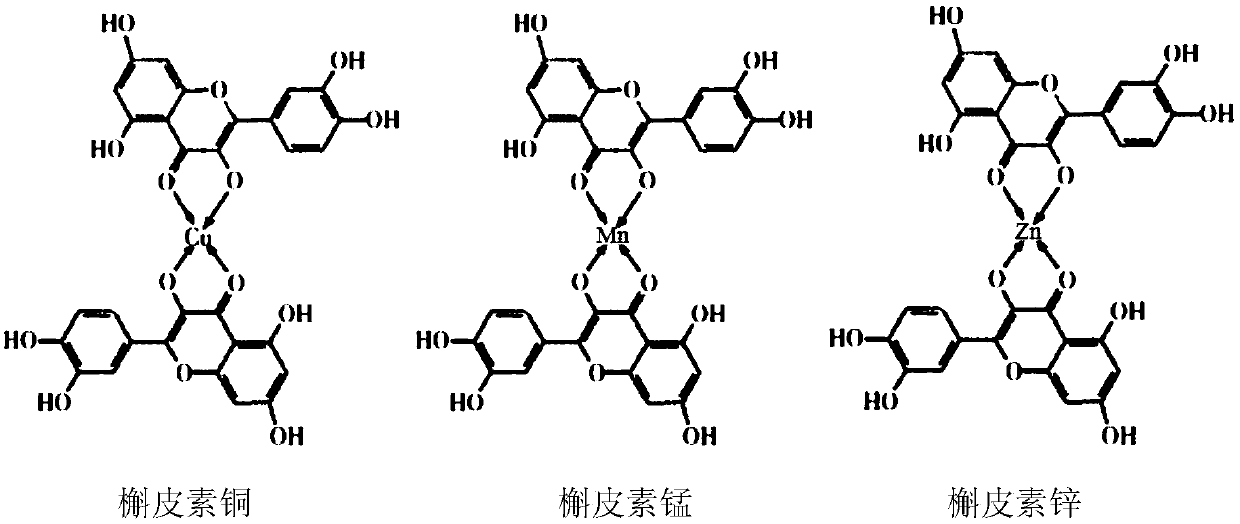
Pharmaceutical composition of osimertinib, and applications thereof
InactiveCN111053780AAddressing drug resistanceSolve the problems of high toxicity and side effectsOrganic active ingredientsAntineoplastic agentsSide effectTyrosine
A purpose of the invention is to solve the problems of drug resistance, high toxic and side effects and the like of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) in non-smallcell lung cancer (NSCLC) treatment and provide a pharmaceutical composition for targeted therapy of lung cancer, wherein the active ingredients of the pharmaceutical composition are quercetin metal coordination compound and osimertinib or a pharmaceutically acceptable salt thereof, and the quercetin metal coordination compound is selected from quercetin copper, quercetin manganese and quercetin zinc. The pharmaceutical composition provided by the invention has good water solubility, low drug resistance, high curative effect and few side effects.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Therapeutic agent for lung cancer that has acquired EGFR-TKI resistance
ActiveUS10813933B2Good treatment effectGood effectOrganic active ingredientsMicrobiological testing/measurementAntiendomysial antibodiesEGFR Antibody
A drug containing, as an active ingredient, a compound represented by ALK inhibitors such as brigatinib, AP26113-analog, and AZD3463 has been found to be effective against a non-small cell lung cancer having a point mutation at C797S in EGFR which has acquired a resistance to chemotherapy agents. Further, the drug used in combination with an anti-EGFR antibody demonstrates a notable suppression effect on the tumor growth. The drug has a potential to be a therapeutic agent effective against a non-small cell lung cancer which is resistant to gefitinib, a first generation therapeutic agent and osimertinib, a third generation therapeutic agent.
Owner:JAPANESE FOUND FOR CANCER RES
Novel method for catalytically synthesizing osimertinib intermediate with a graphene/Pd catalyst
The invention discloses a novel method for catalytically synthesizing an osimertinib intermediate, [2-[2-dimethylamineethylmethylamine]-4-methoxy-5-benzyloxyamide]phenylamine, with a graphene / Pd catalyst. In the method, an osimertinib intermediate, N-carbobenzoxy-2-methoxy-4-[2-dimethylamineethylmethylamine]-5-nitrophenylamine, is used as a raw material; the nitro group then is reduced into an amino group by hydrogen under light irradiation with the graphene / Pd catalyst, thereby producing the [2-[2-dimethylamineethylmethylamine]-4-methoxy-5-benzyloxyamide]phenylamine, yield being higher than 95% and purity being higher than 98%. Compared with other methods, the method has gentle reaction conditions and is more environment-friendly and has simple operations; the reaction is more high-effective and rapid, and the catalyst has high wear resistance and recycle rate. The intermediate and the final product have high quality; the method has advantages in industrial production.
Owner:UNIV OF JINAN
Crystal form of deuterated osimertinib pharmaceutical salt and preparation method thereof
InactiveCN111285852AImprove liquidityImprove solubilityOrganic chemistry methodsSulfonic acids salts preparationEGFR Tyrosine Kinase InhibitorsChemical compound
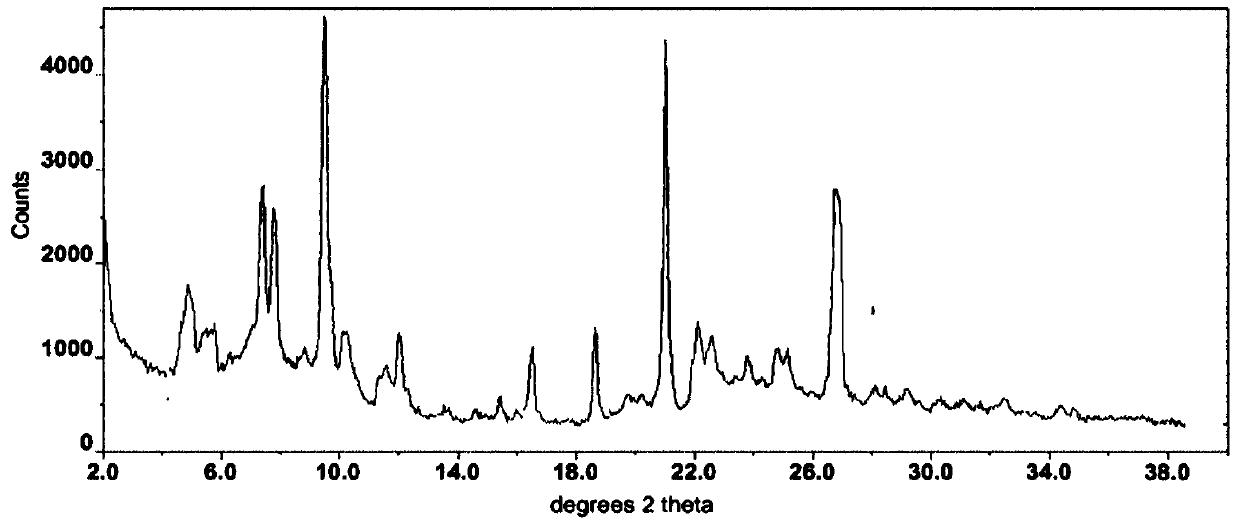
The invention relates to a compound crystal form and a preparation method thereof, in particular to a crystal form I of a deuterated osimertinib pharmaceutical salt as well as a preparation method andapplication thereof. An X-ray powder diffraction pattern of the crystal form represented by a 2theta angle has characteristic diffraction peaks at positions of 5.49 + / -0.2 degrees, 6.42 + / -0.2 degrees, 11.01 + / -0.2 degrees, 11.61 + / -0.2 degrees, 12.46 + / -0.2 degrees, 13.42 + / -0.2 degrees, 14.22 + / -0.2 degrees and 15.58 + / -0.2 degrees. The crystal form is not easy to absorb moisture, has higher solubility, better stability, better fluidity and preparation processability, and has important significance in preparation of EGFR tyrosine kinase inhibitor drugs. The invention also discloses the preparation method of the crystal form I of the deuterated osimertinib pharmaceutical salt, and the crystal form I obtained by the preparation method has higher purity and higher yield.
Owner:GUANGZHOU BOJI MEDICINE SERVICES
Anticancer compound osimertinib and synthesis method thereof
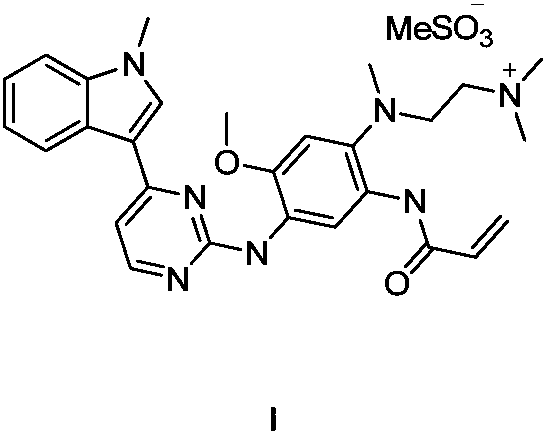
InactiveCN105646467AThe synthesis method is simpleReach costOrganic chemistryBenzeneSynthesis methods
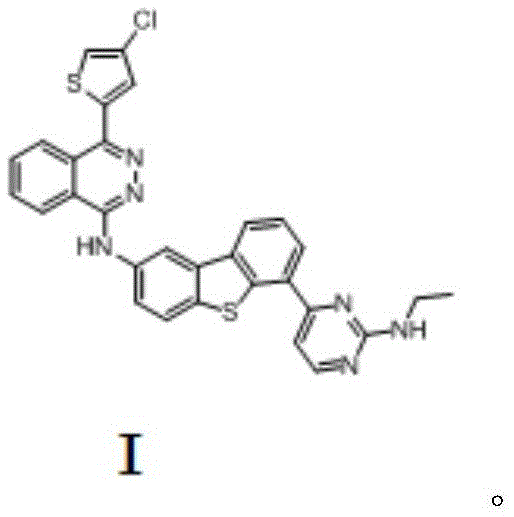
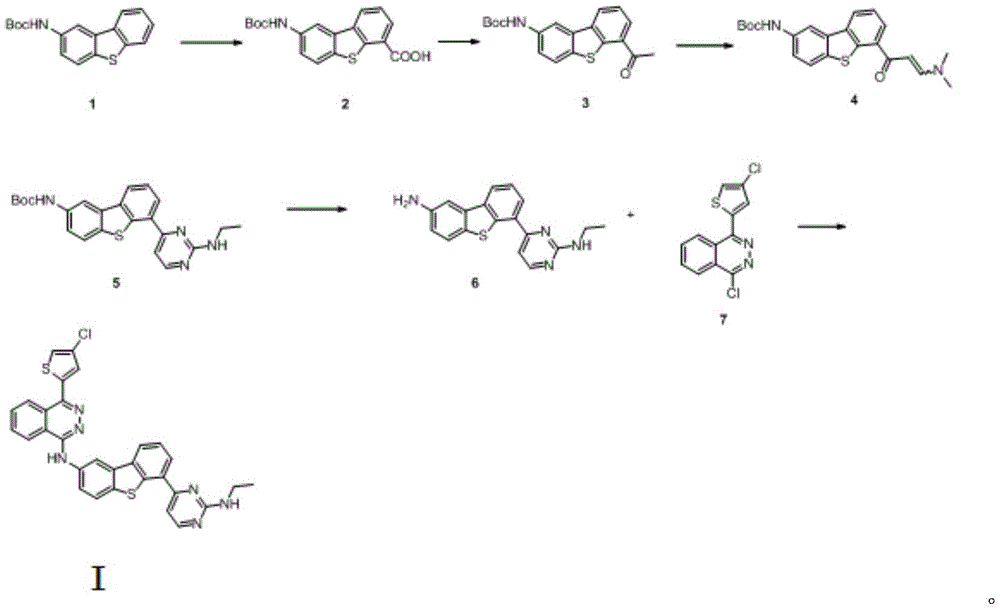
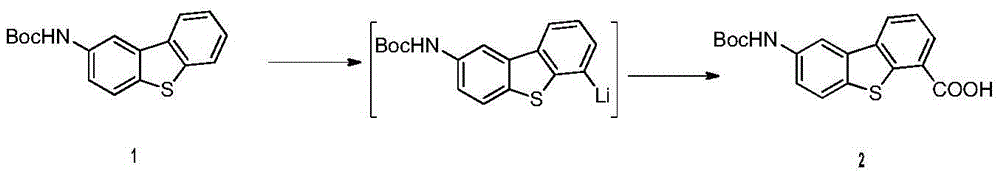
The invention provides an anticancer compound osimertinib and a synthesis method thereof. The chemical name of osimertinib is (4-(4-chlorothiophene-2-yl)-N-(6-(2-(ethylamino) pyridine-4-yl) diphenyl (b,d) thiophene-2-yl)-phthalazinyl-1-amine) with the structural formula shown in formula I. The synthesis process is shown in the specification. The compound is an effective and high-selectivity pan-Aurora kinase inhibitor and acts on Aurora kinase, so that growth of tumor cells is effectively inhibited, and the anticancer effect is realized. Besides, the synthesis method of the compound is simple and easy to implement, the cost is low, the yield is high, the product quality is good, and the synthesis method is suitable for large-scale industrial production.
Owner:南京迪缘医药科技有限公司
<99m>Tc-labeled complex and application thereof in diagnosis of non-small cell lung cancer
InactiveCN110183493AImprove stabilityEasy to makeOrganic chemistry methodsRadioactive preparation carriersDiseaseOncology
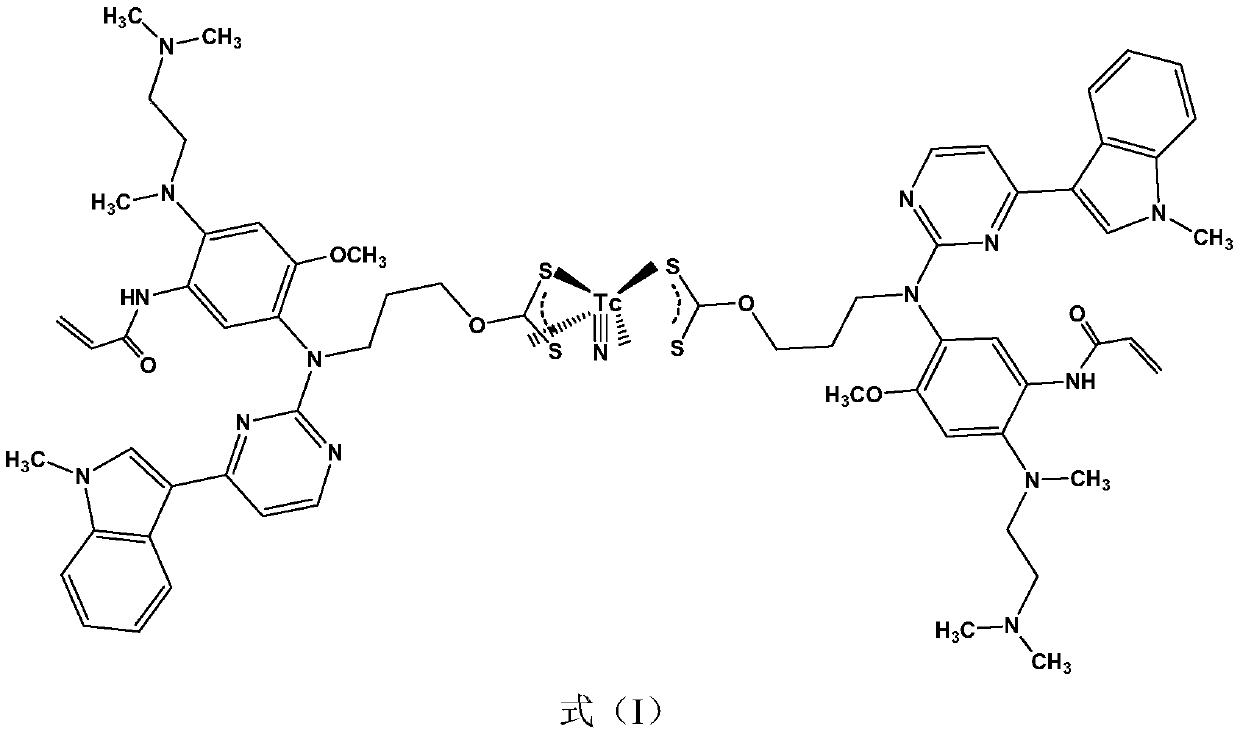
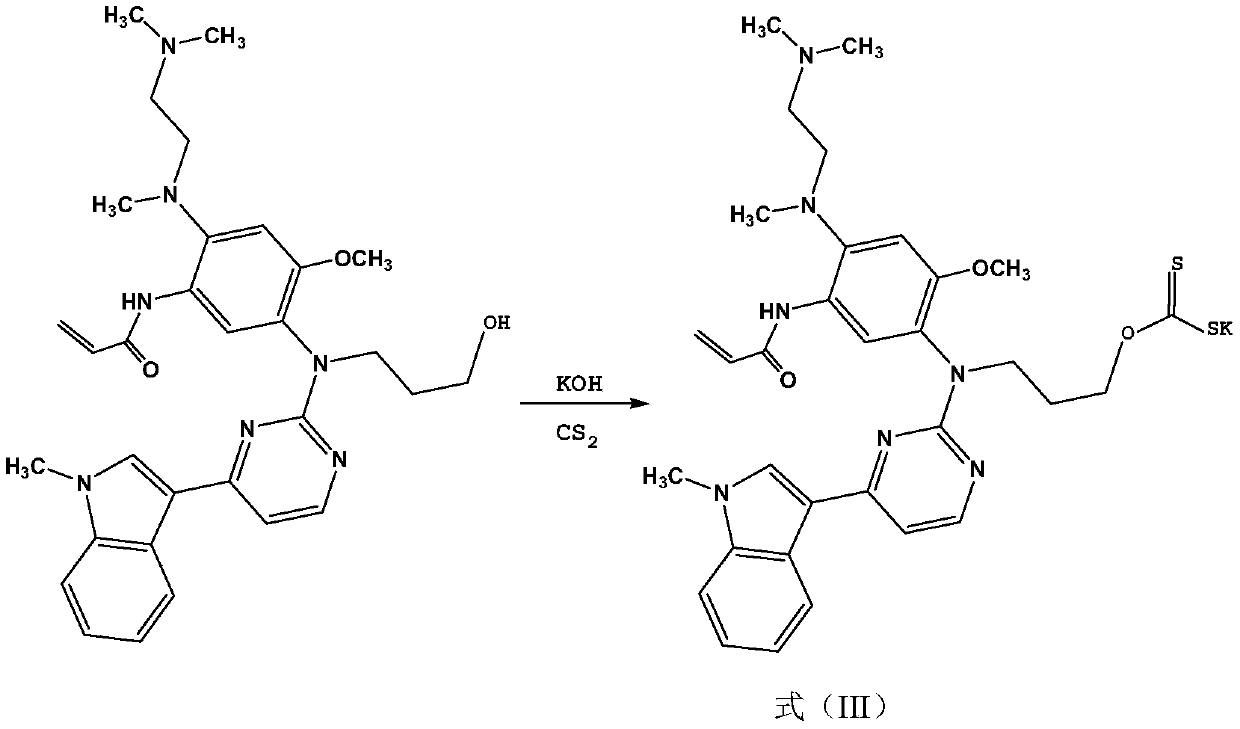
The invention discloses a <99m>Tc-labeled complex and an application thereof in the diagnosis of non-small cell lung cancer. Specifically, a ligand is formed between osimertinib and CS2, and then theligand and <99m>Tc are subjected to coordination to form a complex of a formula (I). The complex is a hydrophilic complex, the stability is good, the preparation is simple, and the complex has relatively high intake and good retention in lungs, has strong signal intensity, and can be applied in the diagnosis of lung cancer diseases, especially non-small cell lung cancer.
Owner:MUDANJIANG MEDICAL UNIV
Method for synthesizing osimertinib intermediate through micro-channel reactor
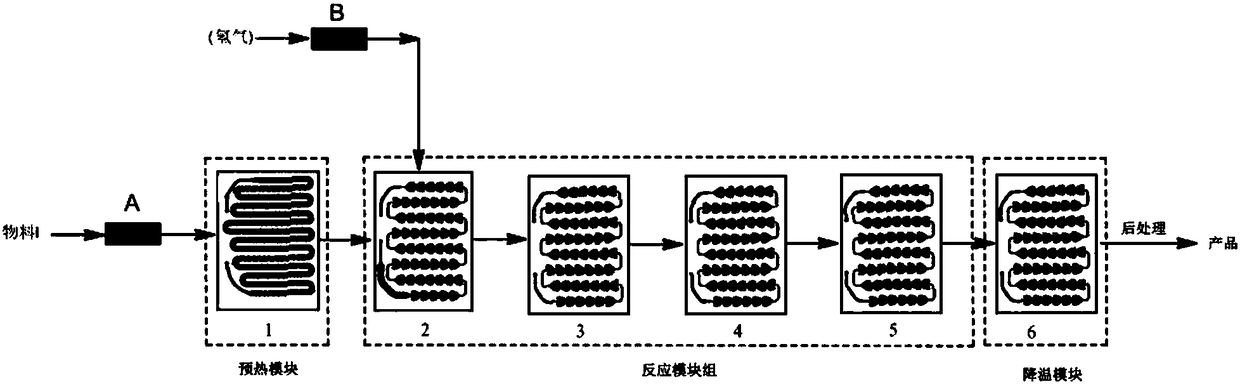
InactiveCN108484579AQuick responseSlow reaction speedOrganic chemistryOrganic synthesisMethyl-1H-indole
The invention discloses a method for synthesizing an osimertinib intermediate through a micro-channel reactor and belongs to the technical field of anticancer drug synthesis in organic synthesis. Aiming at the problems in the traditional high-pressure catalytic hydrogenation reactor synthesis process that the yield is low, the purity is low, violent explosion easily occurs to cause danger, and thecatalyst recycling and reusing frequency is low, the method for synthesizing the osimertinib intermediate through the micro-channel reactor is provided. The osimertinib intermediate is N-1-[2-(dimethylamino)ethyl]-5-methoxy-N1-methyl-N4-[4-(1-methyl-1H-indole-3-yl)-2-pyrimidyl]-1,2,4-triaminobenzene. The method comprises the following synthesis steps: adding N-(2-(dimethylamino-ethyl)-2-methoxy-N-(4-(1-methyl-1-indole-3-yl)-pyrimidyl-2-yl)-5-nitro-phenyl-1,4-diamine into a mixture of an organic solvent and concentrated hydrochloric acid in a microchannel reactor, adding an activated carbon supported noble metal catalyst, and preheating to form a material I; performing reaction on hydrogen and the preheated material I, thereby obtaining the osimertinib intermediate. The method is applicable to synthesis of anticancer drugs.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
2-(2,4,5-substituted phenylamino)pyrimidine derivative and crystal form B thereof
InactiveCN110003183AReduce generationImprove bioavailabilityOrganic chemistry methodsAntineoplastic agentsMedicineEffective treatment
The invention discloses a novel anti-lung cancer medicine, particularly relates to a 2-(2,4,5-substituted phenylamino)pyrimidine derivative I and a crystal form B thereof, and belongs to the field ofmedicinal chemistry. The 2-(2,4,5-substituted phenylamino)pyrimidine derivative adopts a structure as follows: FORMUAL. According to pharmacokinetic and pharmacodynamic studies, compared with osimertinib, dositinib significantly reduces production of an N-Me metabolite with relatively toxicity and maintains or improves the medicinal efficacy. The dositinib can be expected to be developed into a novel medicine which can treat the non-small cell lung cancer with EGFR mutation more safely and more effectively than the osimertinib.
Owner:HENAN GENUINE BIOTECH CO LTD
Osimertinib intermediate and preparation method thereof
InactiveCN108129342AReduce quality riskHigh reaction yieldOrganic compound preparationCarboxylic acid amides preparationArylStructural formula
The invention belongs to the field of medicine synthesis and particularly relates to an osimertinib intermediate and a preparation method thereof. The provided osimertinib intermediate is a compound in a formula C and has a structural formula in the description, wherein R is alkyl or aryl; R1 is alkyl, more preferably, methyl. After the compound in the formula C is hydrolyzed, the compound and a compound in a formula E are subjected to a condensation reaction, a compound in a formula F is prepared, wherein R is alkyl or aryl; R1 is alkyl, more preferably, methyl.
Owner:瑞博(杭州)医药科技有限公司
Refining method of osimertinib
The invention relates to a refining method of osimertinib. The refining method of osimertinib comprises the following steps of mixing an organic solvent with an osimertinib crude product to dissolve the osimertinib crude product, then cooling to separate out crystals, and collecting the crystals, wherein the organic solvent is selected from one or more of 2-butanone, ethyl acetate, methylbenzene and tetrahydrofuran. Different from a conventional purification method, the method disclosed by the invention has the advantages that the osimertinib crude product is recrystallized by virtue of the specific organic solvent, so that the purity of osimertinib is effectively improved, residues of reactants can be remarkably reduced, and process byproducts are remarkably reduced. The purity of osimertinib detected by HPLC can reach 98.5% or above, and the impurity limit is less than 0.1%.
Owner:ENANTIOTECH CORP
Preparation of biomass-derived palladium catalyst and its application in the synthesis of anticancer drug osimertinib
ActiveCN108187666BWide variety of sourcesLow priceOrganic chemistryCatalyst activation/preparationPtru catalystPalladium catalyst
The invention relates to a preparation method of a biomass derived palladium catalyst and application to synthesis of an anti-tumor drug osimertinib. The preparation method comprises the following process: enabling chitosan and palladium salt to react in the presence of a solvent to form a complex; then cooling to room temperature; drying a solid in vacuum to remove a solvent; under the protectionof argon gas, carbonizing at high temperature to obtain the catalyst; dissolving a raw material N-(2-dimethylamino-ethyl-2-methoxy-N-methyl-N-[4-(1-methyl-1H-indole-3-yl-pyrimidine-2-yl)-5-nitryl-benzene-1,4-diamine and adding the catalyst; stirring at temperature of 0 to 120 DEG C for 5 to 12h; dropwise adding hydrazine hydrate; after completely reacting, cooling to room temperature and filtering; spinning and drying the solvent to obtain a faint yellow solid. The preparation method provided by the invention has the advantages of wide raw material source, relatively low price, moderate reaction conditions and high yield; the catalyst has high activity, high stability, high dispersity of active components and long service life; the catalyst can be repeatedly used for a plurality of timesand the preparation method also has the advantages of short flow, easiness for controlling reaction and simple equipment requirements; complicated post-treatment is avoided and environment pollution is avoided.
Owner:HANGZHOU LUPU BIOTECH CO LTD
Novel crystal form of osimertinib monohydrate
ActiveCN113372331AEasy to prepareLow costOrganic chemistry methodsPhysical chemistryPharmaceutical drug
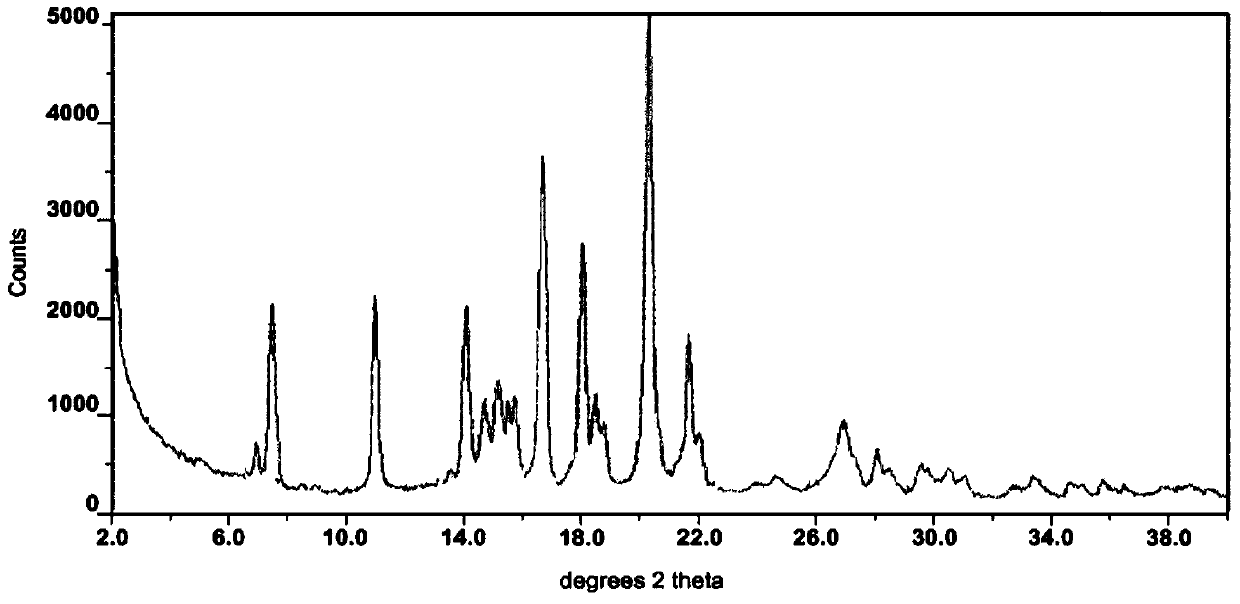
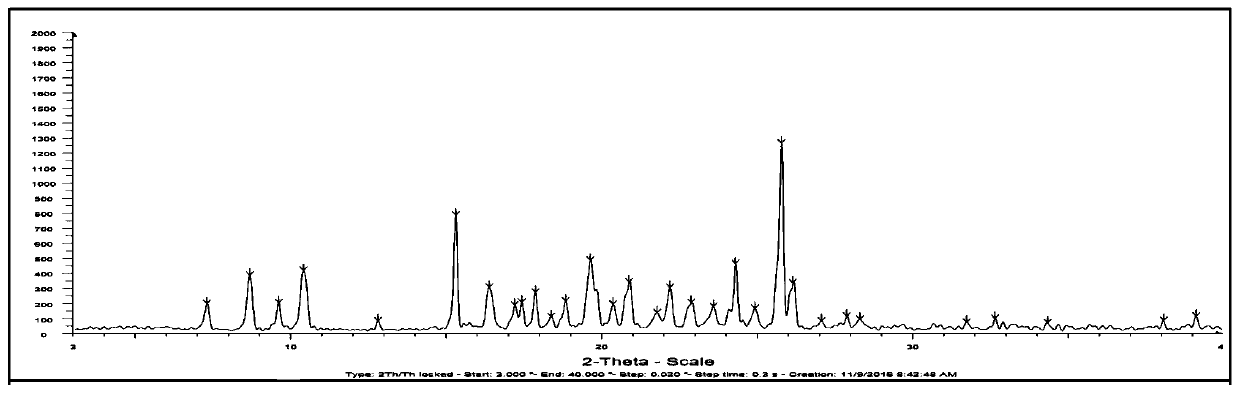
The invention belongs to the technical field of crystal forms of organic medicines, and discloses a novel crystal form of osimertinib monohydrate. According to the novel crystal form of the osimertinib monohydrate, Cu-K alpha radiation is used, and an X-ray diffraction spectrum represented by 2[theta] has characteristic diffraction peaks at the positions of 5.9 + / -0.2 degrees, 7.3 + / -0.2 degrees, 9.1 + / -0.2 degrees, 11.2 + / -0.2 degrees, 25.2 + / -0.2 degrees and 25.41 + / -0.2 degrees. Compared with the prior art, the crystal form provided by the invention is simple in preparation method and low in cost, and has important value for optimization and development of the medicine in the future. The novel crystal form prepared by the invention of the osimertinib monohydrate has the advantages of high yield, high purity, good stability and good dissolution rate.
Owner:LUNAN PHARMA GROUP CORPORATION
Osimertinib medicinal eutecticum and preparation method thereof
PendingCN114075169AHigh puritySimple and fast operationOrganic active ingredientsOrganic chemistry methodsPharmacologyHygroscopicities
The invention relates to the technical field of crystal form drug molecules and particularly provides an osimertinib eutecticum, a preparation method and application thereof. The medicinal eutecticumum disclosed by the invention is an osimertinib-malic acid eutecticumum, an osimertinib-malonic acid eutecticumum, an osimertinib-vanillic acid eutecticumum or an osimertinib-sorbic acid eutecticumum. According to the invention, the preparation method of the medicinal eutecticum is easy and convenient to operate, the prepared crystal is high in purity, and compared with an existing osimertinib crystal form, the osimertinib crystal form has better solubility and lower hygroscopicity and is more suitable for medicine development.
Owner:LUNAN PHARMA GROUP CORPORATION
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com