2-(2,4,5-substituted phenylamino)pyrimidine derivative and crystal form B thereof
A technology of pyrimidine derivatives and aniline, applied in the field of 2-pyrimidine derivatives and its crystal form B, new anti-lung cancer drugs, can solve problems such as T790M mutation, achieve good bioavailability and improve drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
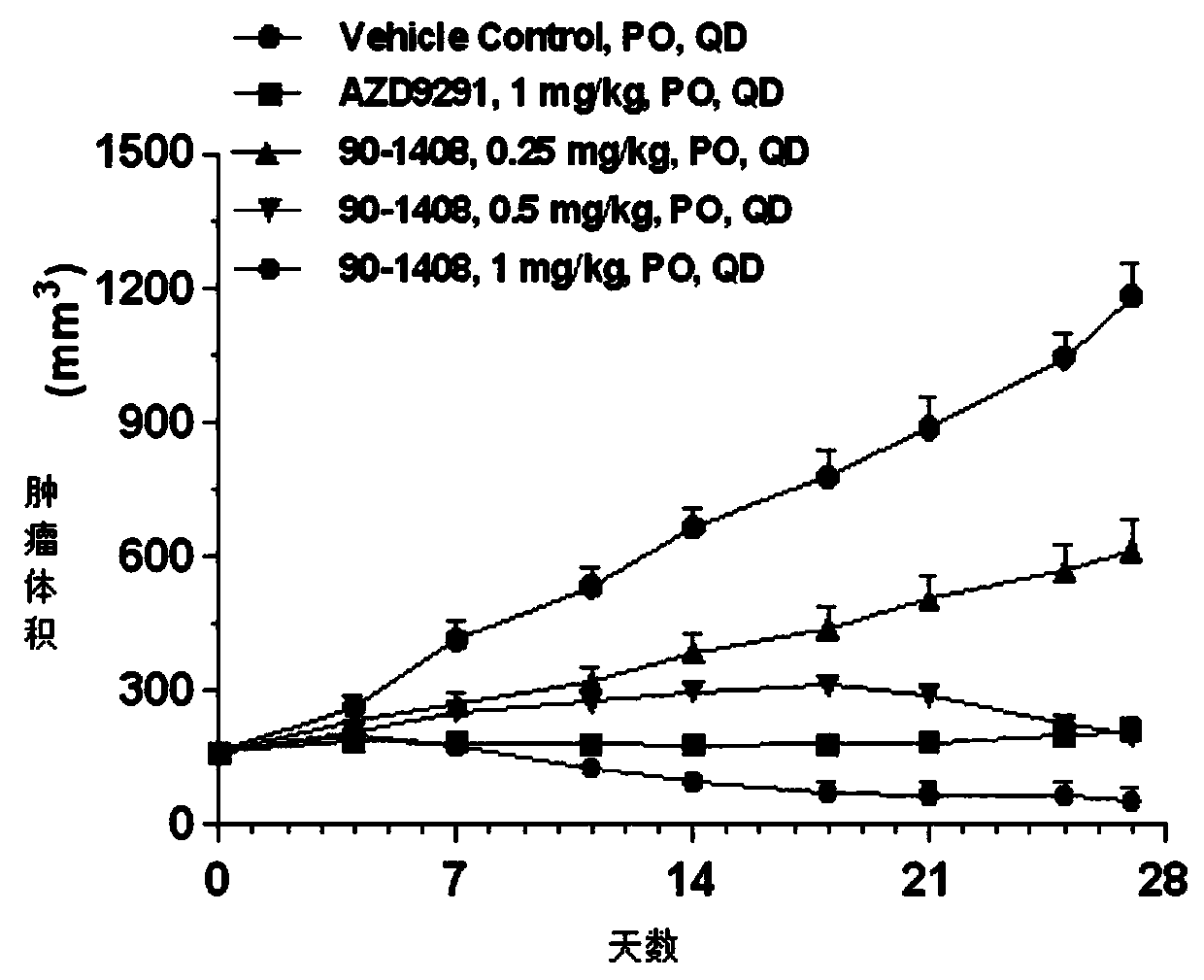
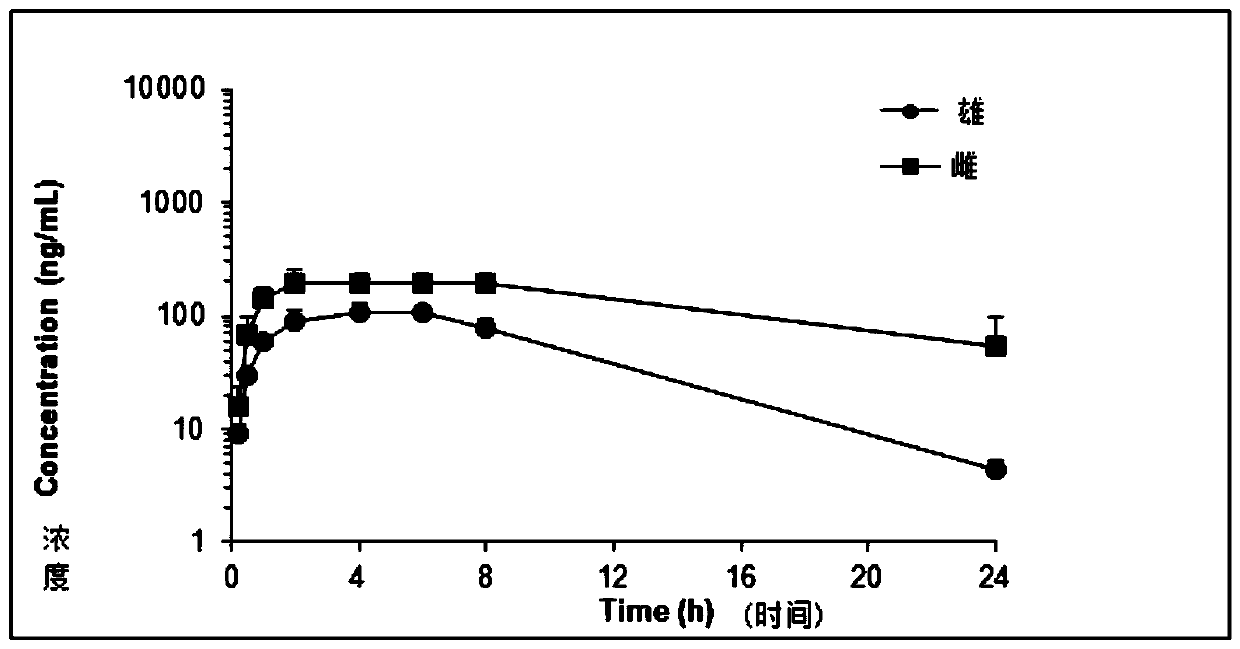
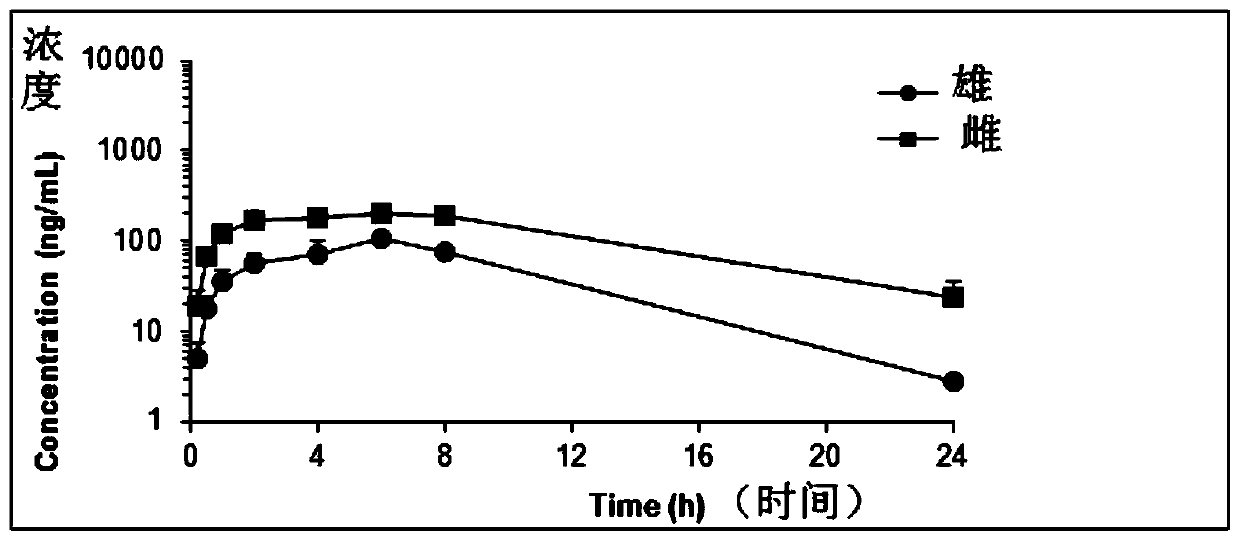
Image
Examples
Embodiment 1
[0025] Embodiment 1, preparation intermediate 9
[0026] first step:
[0027]
[0028] Compound 1 (2.00kg, 1.71mol) and KOH (1.44kg, 25.6mol) were added into DMF (6.0L) precooled at 0-15°C, and the mixture was stirred for 0.5h. Compound 2 (2.47kg, 17.1 mol) was dropped into the above mixture at 0-5°C within 4h, and the reaction mixture was stirred at 0-10°C for 2h, then at 5-15°C for 12h. After adding ice water (15.0L), extract with a mixture of petroleum ether (10L) and methyl tert-butyl ether (10.0L), wash the organic phase with brine, Na 2 SO 4 Drying, filtration, and evaporation of the solvent gave an oil (2.60 kg, 82% purity, containing solvent). 1 H NMR (400MHz, DMSO-d 6 )δppm 6.41-6.48(m,1H); 6.96-7.03(m,1H); 7.03-7.05(m,1H); 7.11-7.17 (m,1H); 7.24-7.26(m,1H); (m,1H).
[0029] second step
[0030]
[0031] Compound 4 (2.42kg, 16.24mol), DME (8.0L) and anhydrous FeCl 3 (2.64kg, 16.3mol) was added into the reactor, the reaction temperature was controlled at ...
Embodiment 2
[0038] Embodiment 2, preparation compound 12
[0039] first step
[0040]
[0041] Compound 9 (2.39g, 5mmol), MeOH (200mL), ammonium formate (2.39g) and palladium carbon (200 mg, 5%) were sequentially added to a one-necked flask, the reaction compound was stirred under a hydrogen balloon for 16h, filtered, and the filtrate was concentrated to dry, add water (100 mL), adjust pH to 9 with saturated sodium bicarbonate solution, extract the mixture with DCM (3 x 100 mL), combine organic phases, Na 2 SO 4 Drying, filtration, and evaporation of the solvent gave 10 as a white solid (2.05 g, 91%). 1 H-NMR (400MHz, DMSO-d 6 )δ2.18(s,6H),2.36(t,J=6.8Hz,2H), 2.64(s,3H),2.89(t,J=6.8Hz,2H),3.75(s,3H),4.58( br s,2H),6.77 (s,1H),7.23-7.27(m,3H),7.50-7.53(m,2H),7.79(s,1H),8.28(d, J=5.2Hz,1H), 8.30 (s, 1H), 8.43 (d, J=8.0Hz, 1H). LCMS[M+1] + :449.3.
[0042] second step
[0043]
[0044] Compound 10 (4.93g, 0.011mol), diethylphosphonoacetic acid (2.35g, 0.012mol) and N,N-diisopr...
Embodiment 3
[0048] The preparation of embodiment 3 compound I crystal B
[0049]
[0050] Compound 12 (5.04g, 10mmol) was added to acetone (55mL), then water (5 mL) was added at 50°C, methanesulfonic acid (0.94g, 9.8mmol) was added dropwise to the reaction solution, solids were precipitated, and stirred at 50°C 1h. Cool to room temperature, filter, wash the obtained solid with acetone (5 mL), and dry under vacuum at 25°C to obtain a solid product (5.5 g, 91%). 1 HNMR (400MHz, DMSO-d 6 ):δ2.72,2.75(ss,6H), 2.90(s,6H), 3.29(m,2H), 3.50(m,2H),4.03(s,3H),6.56(s,1H), 6.99( s,1H), 7.21(m,3H), 7.46(d,J=8.0Hz,1H),8.17(s,1H),8.26 (d,J=5.6Hz,1H),8.35(d,J=7.6 Hz, 1H), 8.67 (s, 1H). LCMS[M+1] + :505.3.
[0051] The characterization data of single crystal B of compound I are as follows:
[0052]
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com