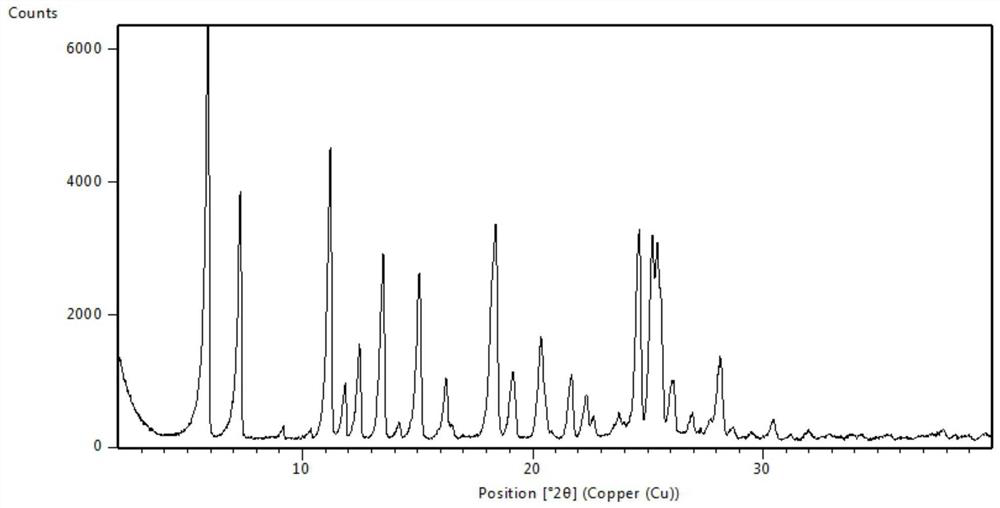
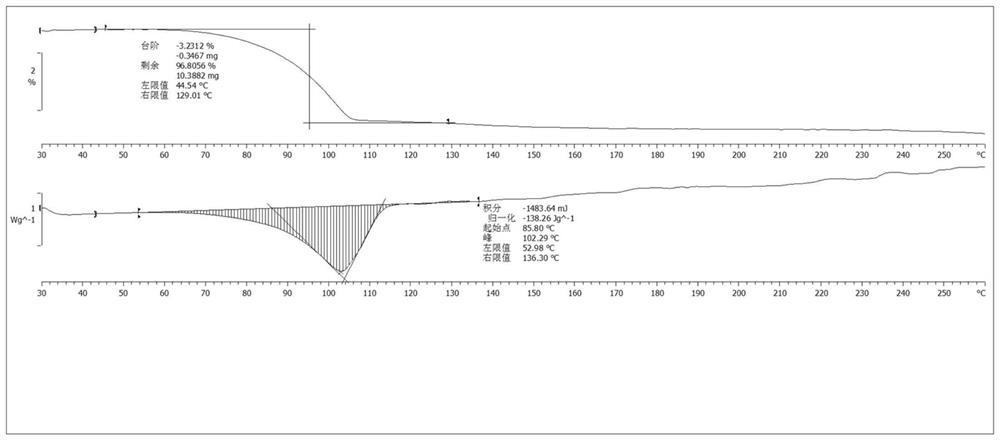
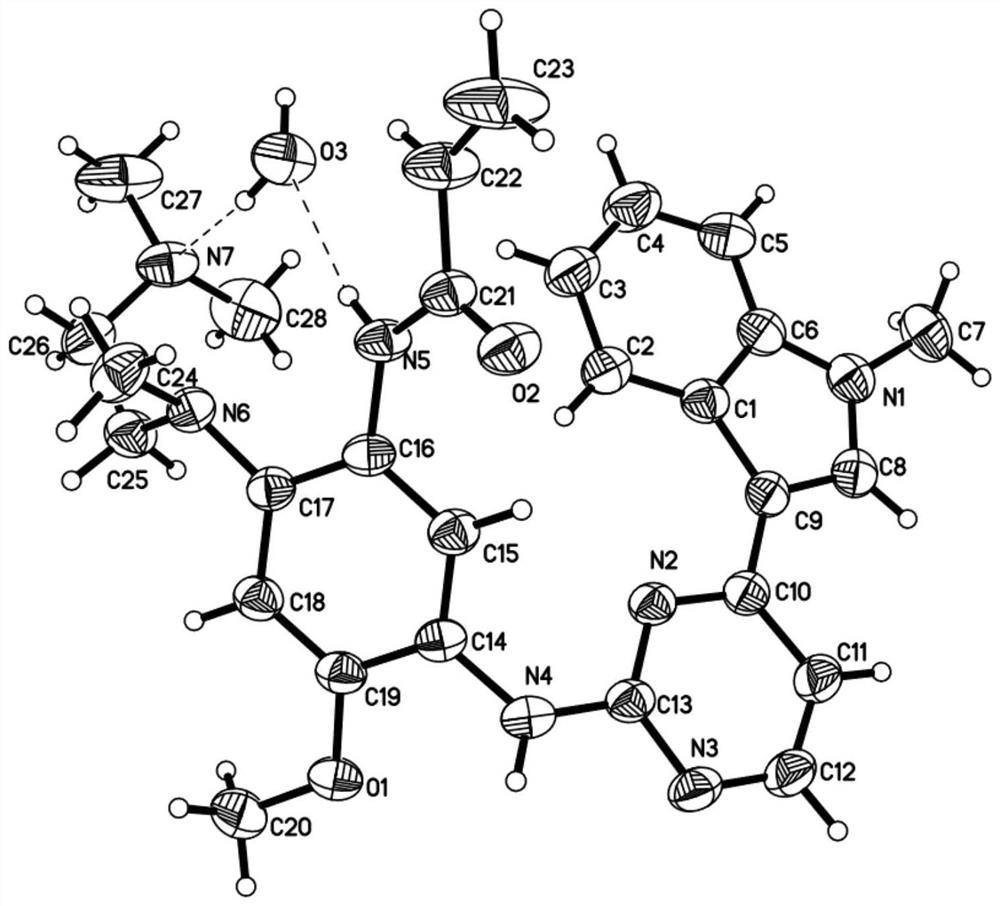
Novel crystal form of osimertinib monohydrate
A technology of monohydrate and crystal form, which is applied in the field of osimertinib monohydrate crystal form, which can solve the problems of high humidity, easy deliquescence, high toxicity, and unsuitability for drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) 10.0 g of N-[2-[[2-(dimethylamino)ethyl](methyl)amino]-4-methoxy-5-[[4-(1-methyl-1H -Indol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide was added to 70mL of acetonitrile, slowly heated to 50°C until the solution was dissolved;
[0056] (2) Add the dissolved clear solution dropwise to 14 mL of purified water, stirring while adding;
[0057] (3) Slowly lower the temperature to 25°C, and control the temperature for crystallization for 5 hours;
[0058] (4) Vacuum drying was carried out for 5 hours after the crystallization was completed, the drying temperature was 35°C, the yield was 97.23%, HPLC: 99.98%.
Embodiment 2
[0060] (1) 10.0 g of N-[2-[[2-(dimethylamino)ethyl](methyl)amino]-4-methoxy-5-[[4-(1-methyl-1H Add -indol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide into 80mL of ethanol, slowly heat to 55°C, and wait for the solution to dissolve;
[0061] (2) Add the dissolved clear solution dropwise to 12 mL of purified water, stirring while adding;
[0062] (3) Slowly lower the temperature to 30°C, and control the temperature for crystallization for 6 hours;
[0063] (4) Vacuum drying was carried out for 7 hours after the crystallization was completed, the drying temperature was 35°C, the yield was 96.57%, HPLC: 99.98%.
Embodiment 3
[0065] (1) 10.0 g of N-[2-[[2-(dimethylamino)ethyl](methyl)amino]-4-methoxy-5-[[4-(1-methyl-1H -Indol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide was added to 50mL methanol, slowly heated to 45°C until the solution was dissolved;
[0066] (2) Add the dissolved clear solution dropwise to 17mL of purified water, stirring while adding;
[0067] (3) Slowly lower the temperature to 15°C, and control the temperature for crystallization for 4 hours;
[0068] (4) Vacuum drying was carried out for 5 hours after the crystallization was completed, the drying temperature was 30°C, the yield was 95.84%, HPLC: 99.97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com