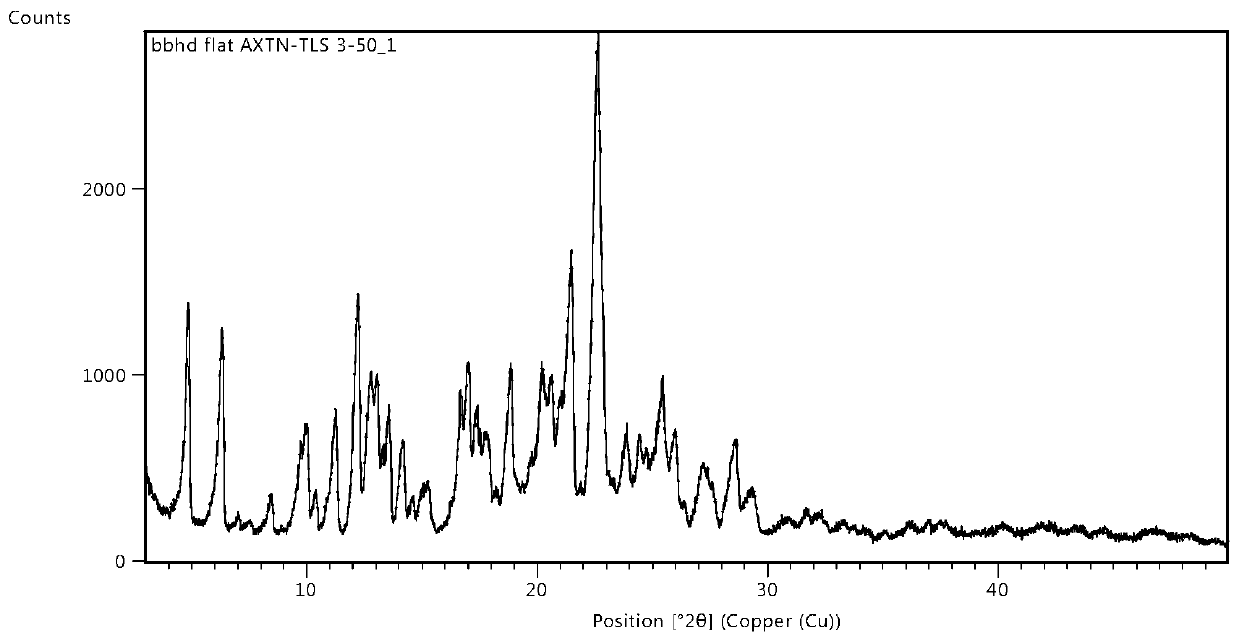
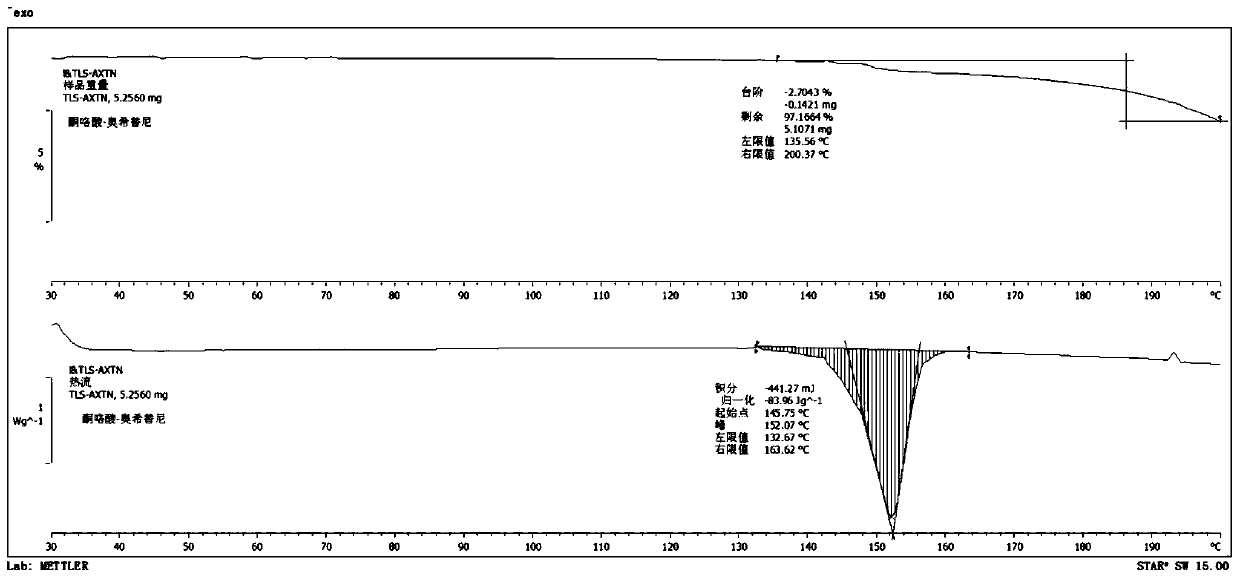
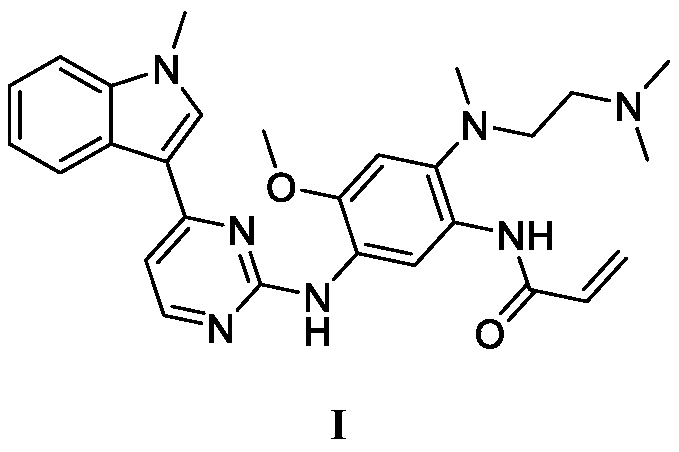
Osimertinib ketorolac salt crystal form and preparation method thereof
A technology of osimertinib ketorolate and tenidone ketone salt, which is applied in the field of medicine, can solve the problems of high humidity, easy deliquescence, and high hygroscopicity, and achieves simple and easy preparation methods, low hygroscopicity, and high toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] N-[2-[[2-(dimethylamino)ethyl](methyl)amino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3- Base) pyrimidin-2-yl] amino] phenyl] prop-2-enamide 5.01g and 5-benzoyl-2,3-dihydro-1H-pyrrolopyrrolidine-1-carboxylic acid 3.58g were added to 30mL In acetone, slowly heat until dissolved, stir for 10 hours, reduce the reaction to 10°C, filter with suction, and dry to obtain a solid with a yield of 94.7% and a purity of 99.9%.
Embodiment 2
[0052] N-[2-[[2-(dimethylamino)ethyl](methyl)amino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3- Base) pyrimidin-2-yl] amino] phenyl] prop-2-enamide 5.01g and 5-benzoyl-2,3-dihydro-1H-pyrrolopyrrolidine-1-carboxylic acid 4.34g were added to 35mL In dichloromethane, heat slowly until dissolved, stir for 9 hours, reduce the reaction to 12°C, filter with suction, and dry to obtain a solid with a yield of 94.1% and a purity of 99.9%.
Embodiment 3
[0054] N-[2-[[2-(dimethylamino)ethyl](methyl)amino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3- Base) pyrimidin-2-yl] amino] phenyl] prop-2-enamide 5.01g and 5-benzoyl-2,3-dihydro-1H-pyrrolopyrrolidine-1-carboxylic acid 5.10g were added to 40mL In methanol, slowly heat until dissolved, stir for 8 hours, reduce the reaction to 15°C, filter with suction, and dry to obtain a solid with a yield of 93.8% and a purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com