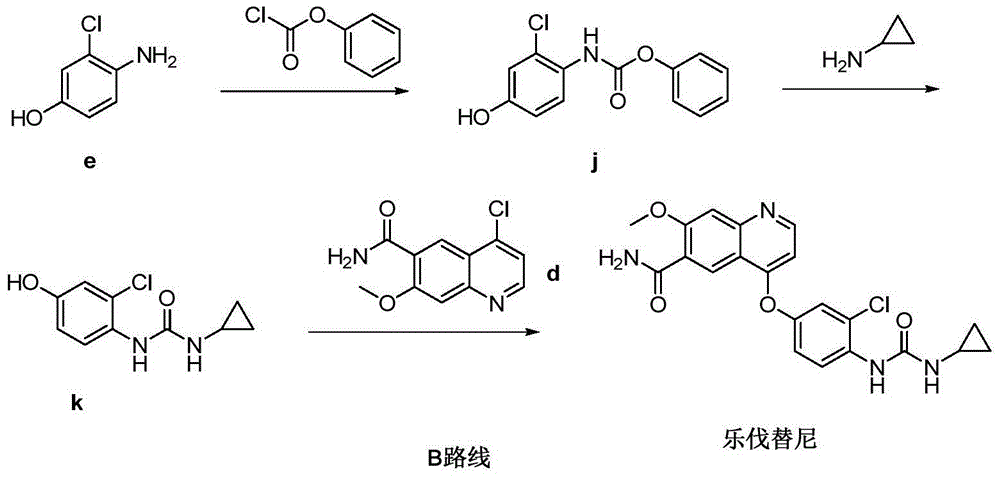
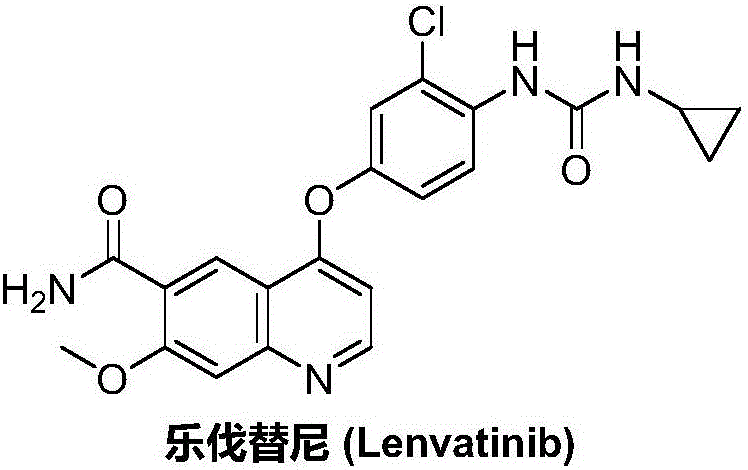
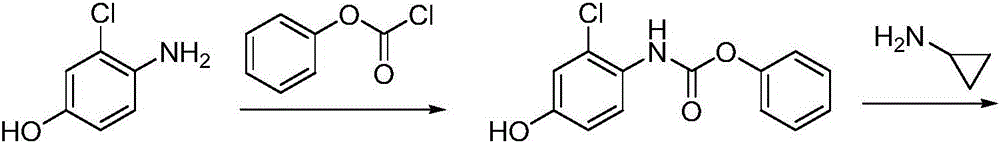
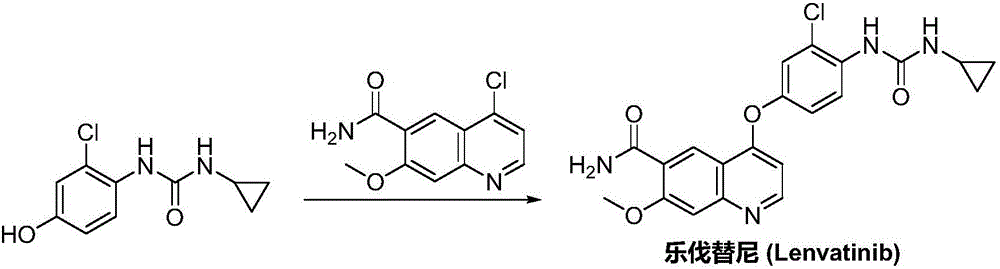
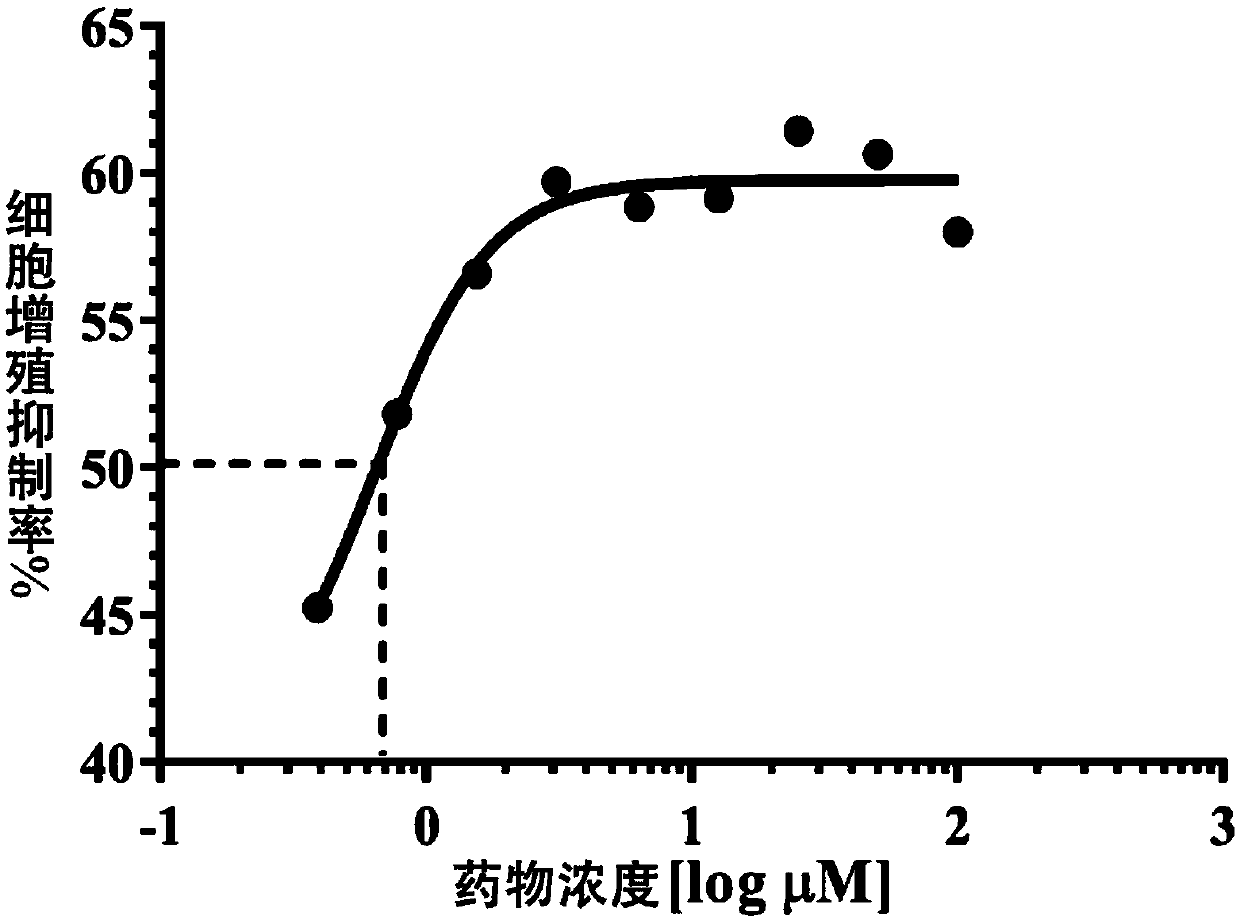
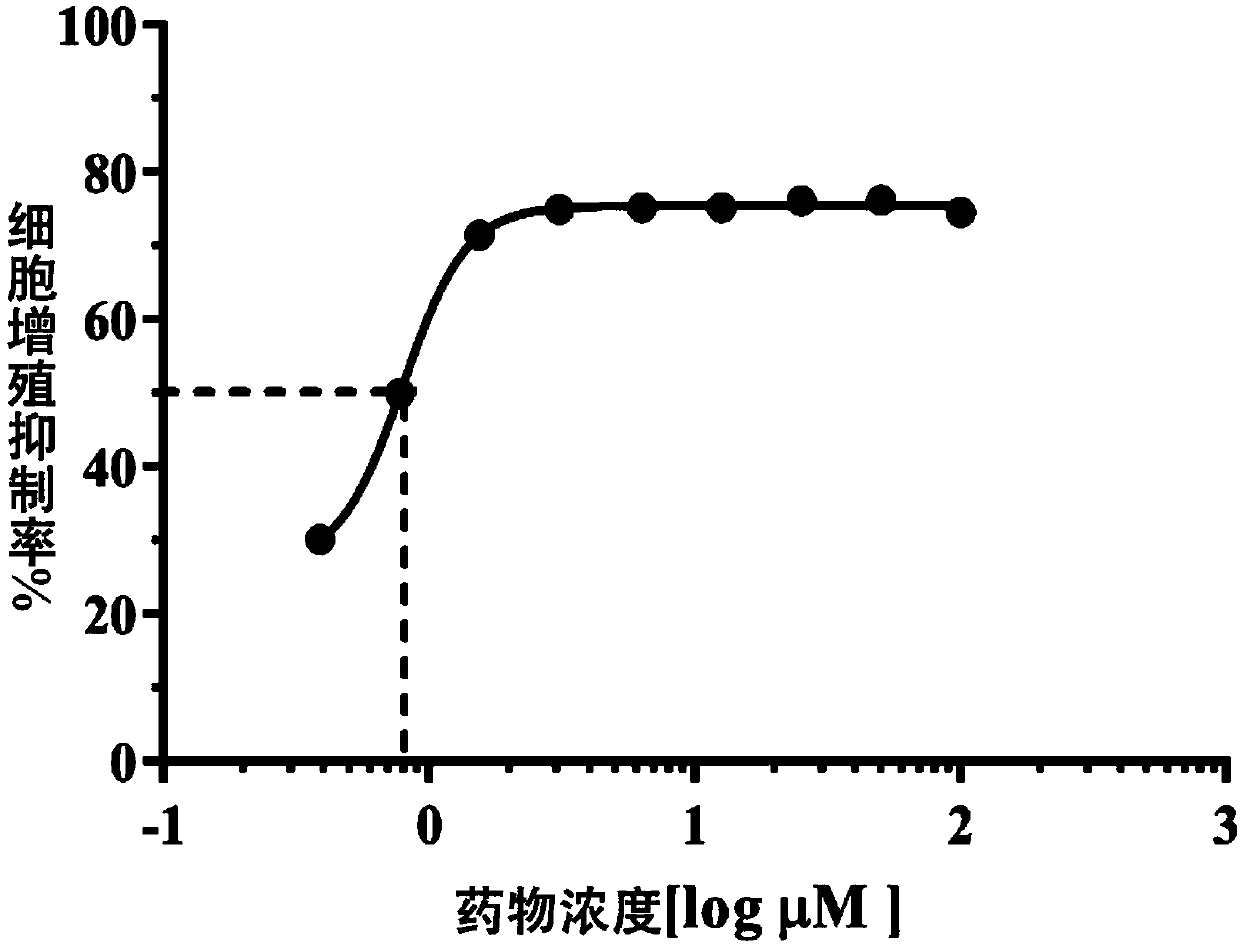
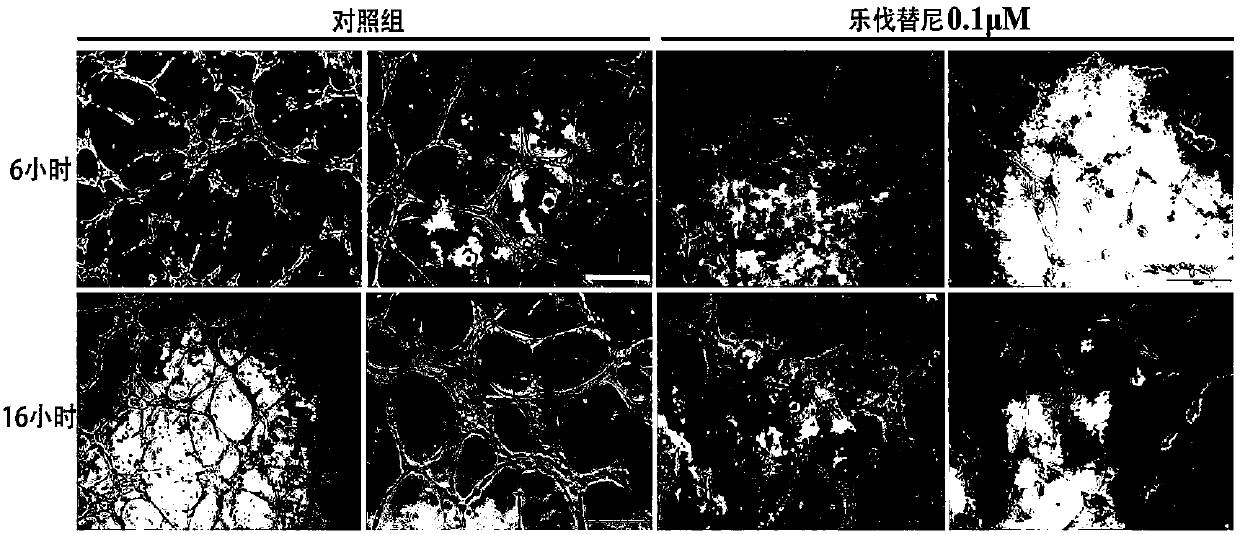
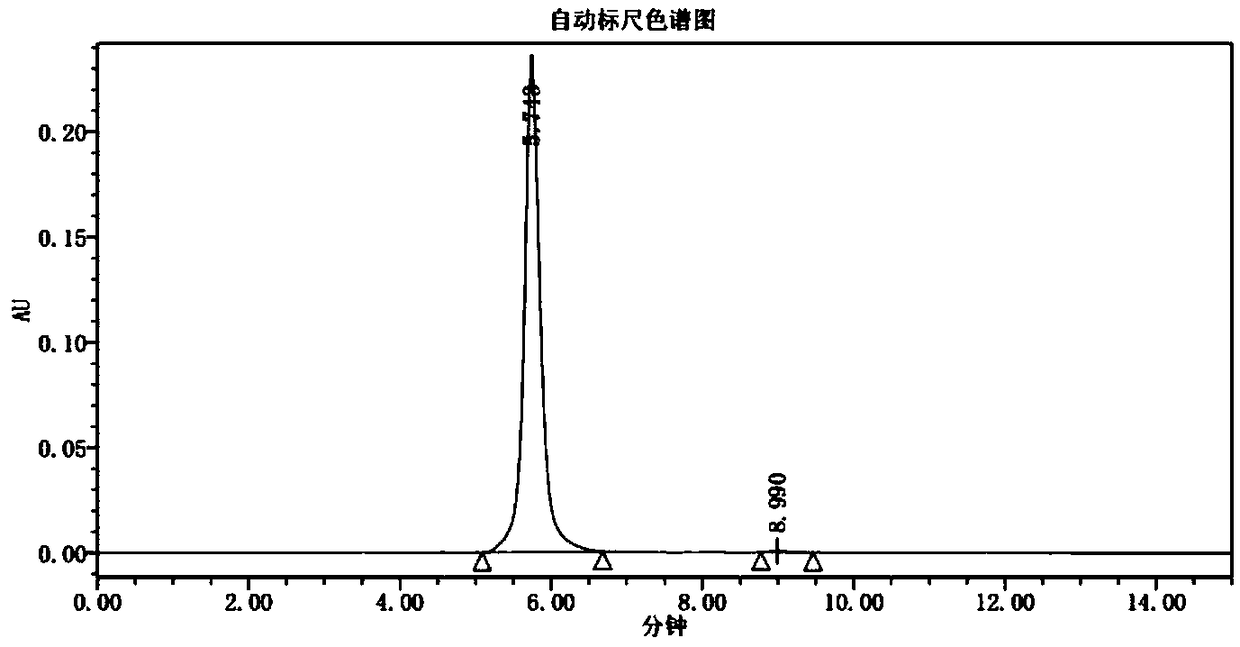
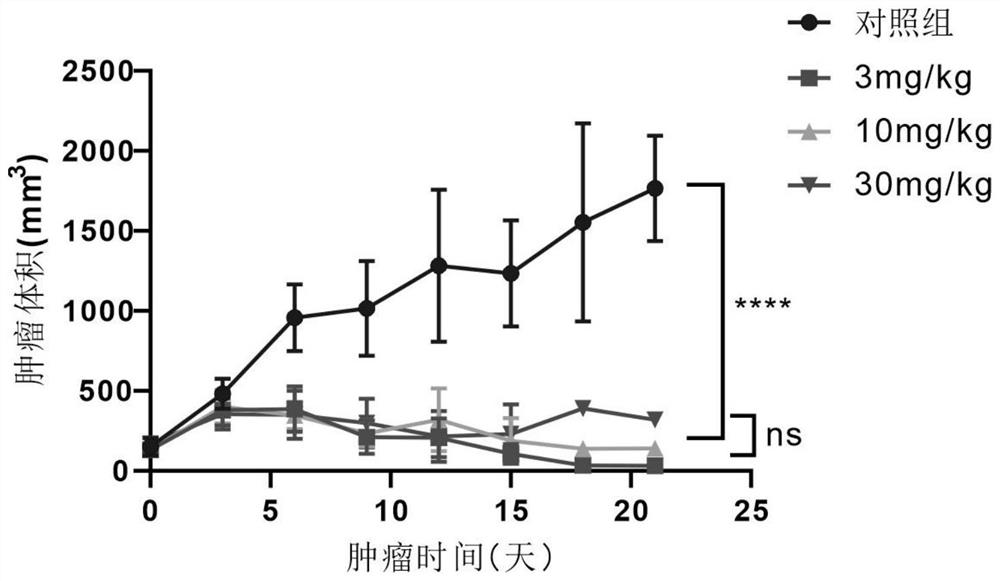
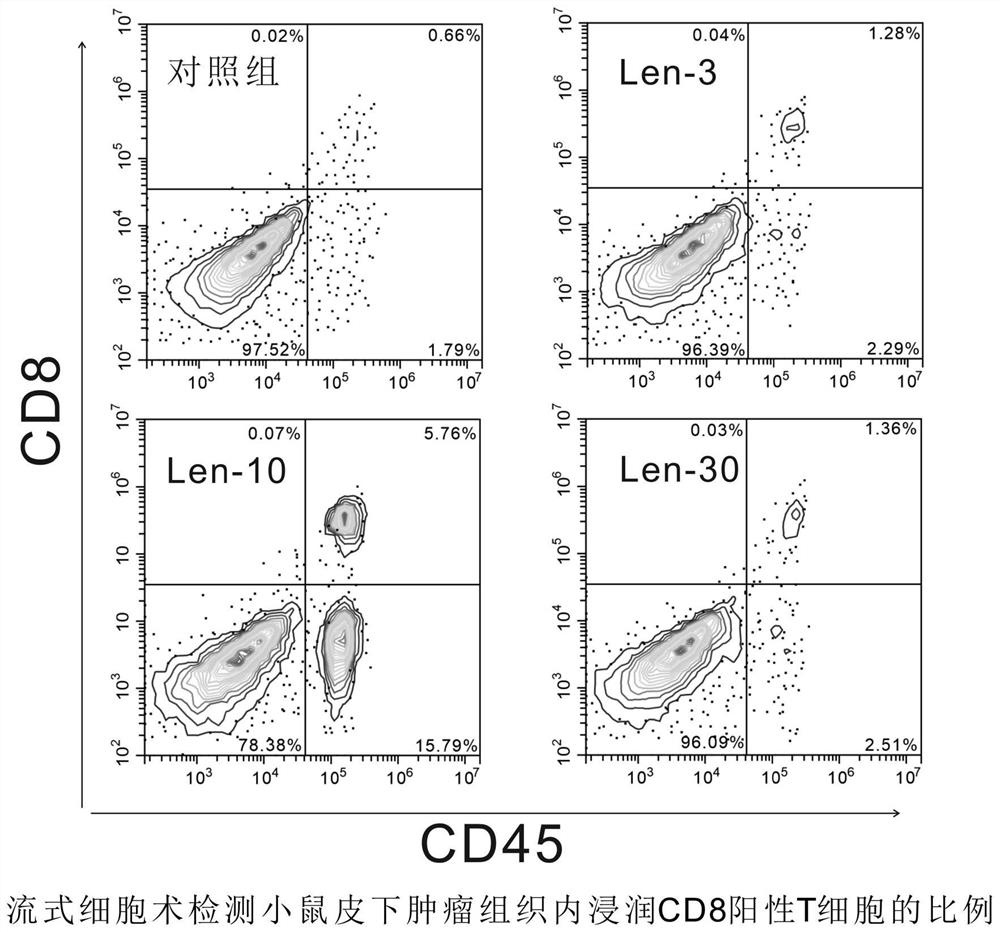
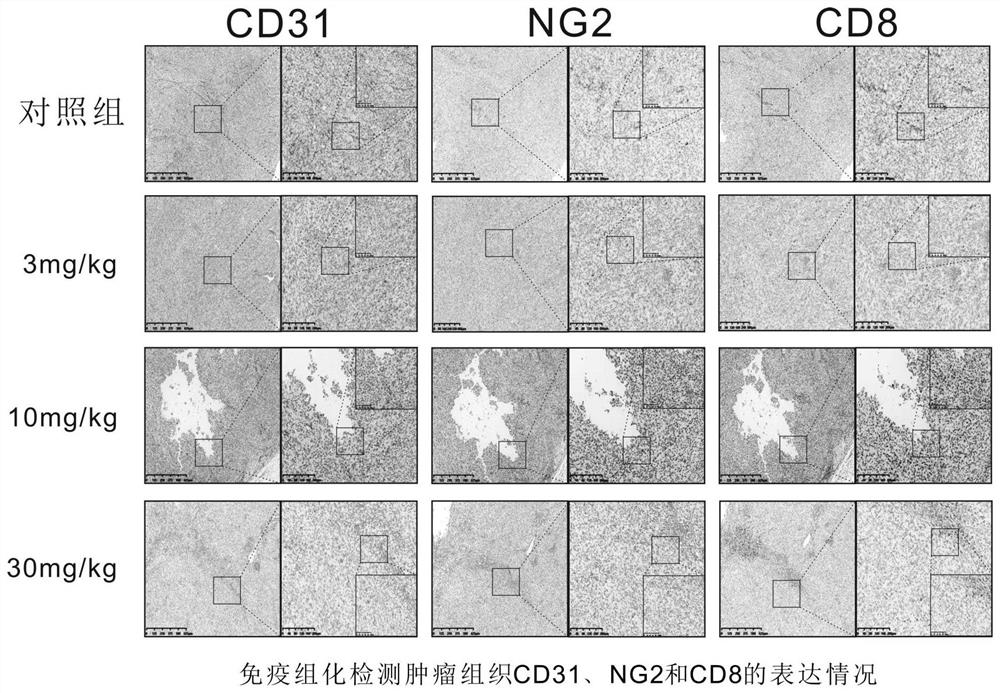
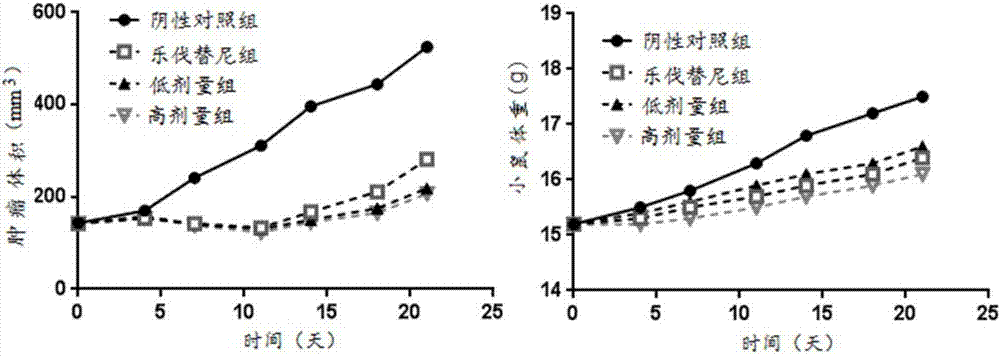
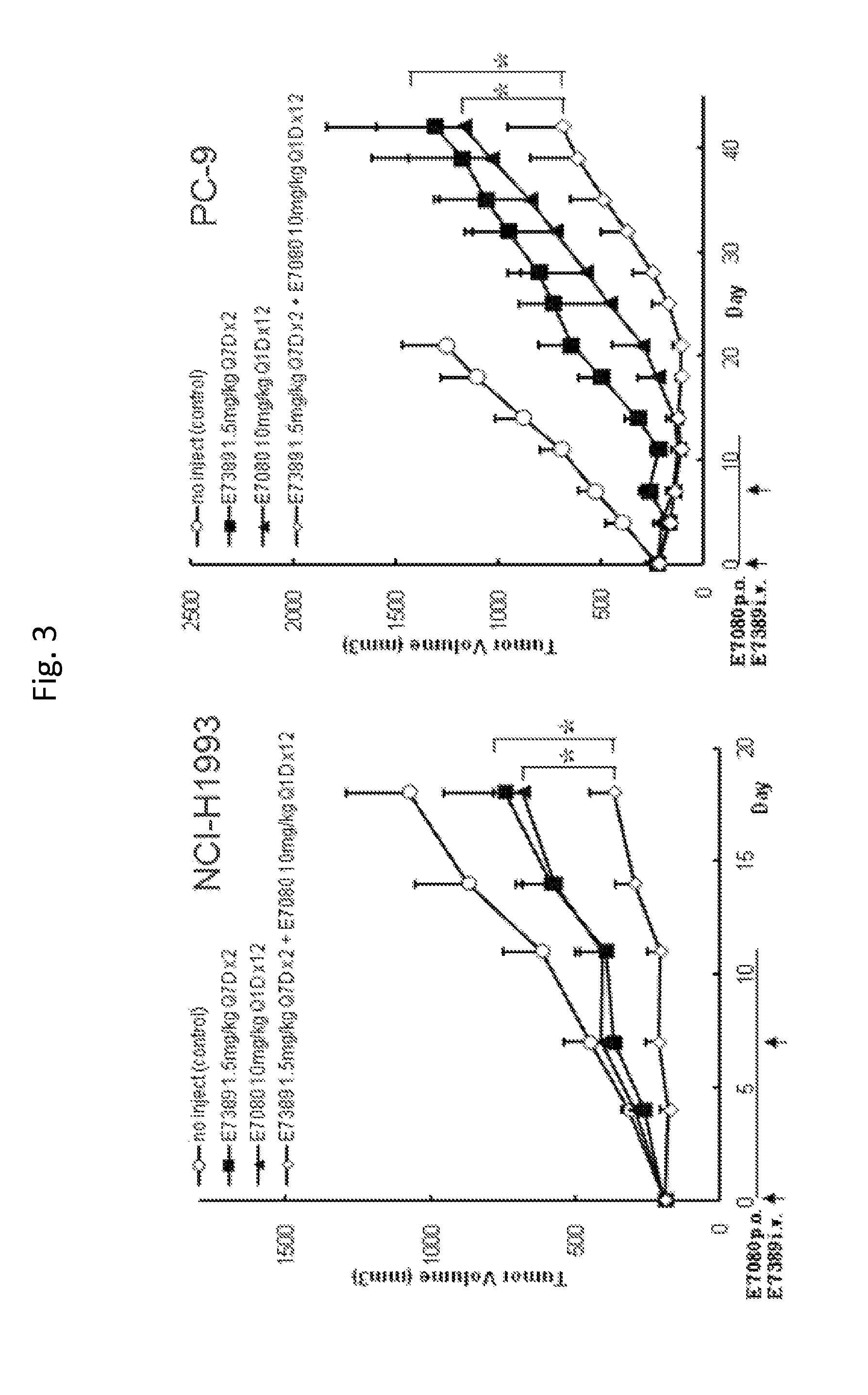
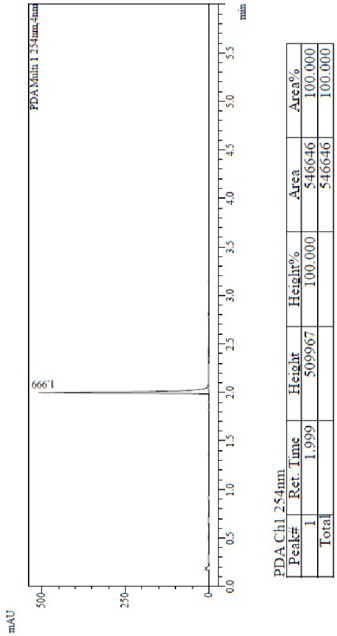
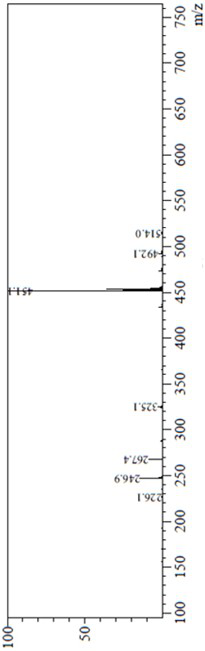
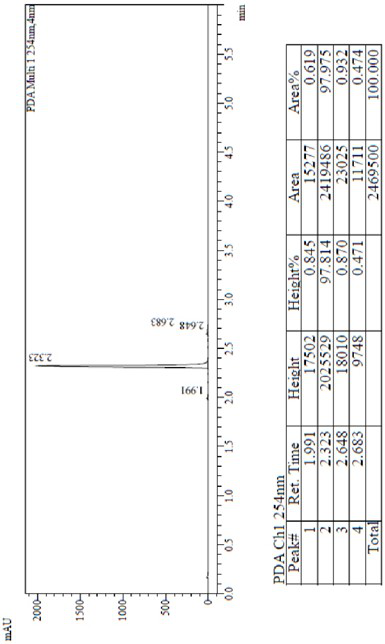
Patents
Literature
82 results about "Lenvatinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
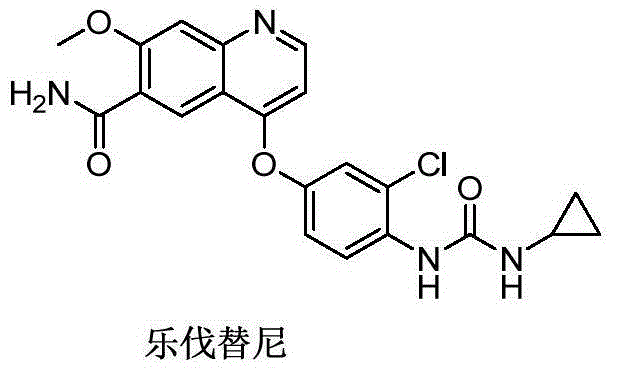
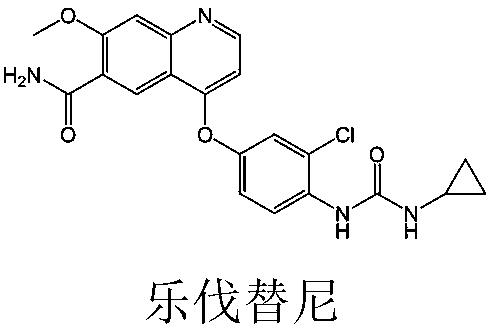
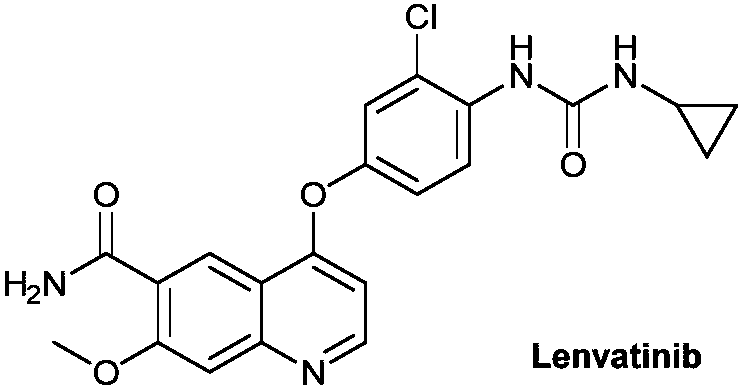
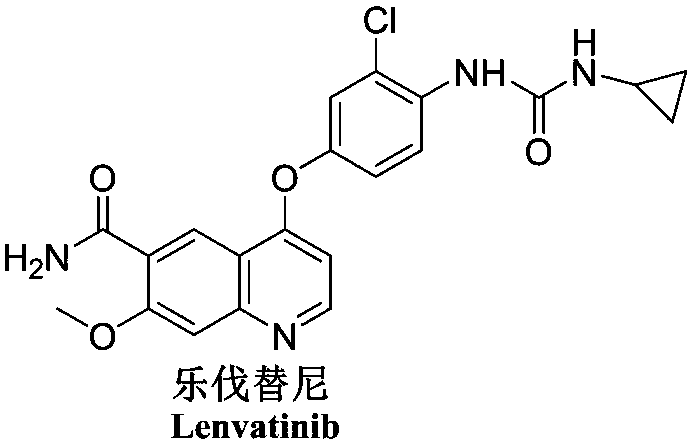
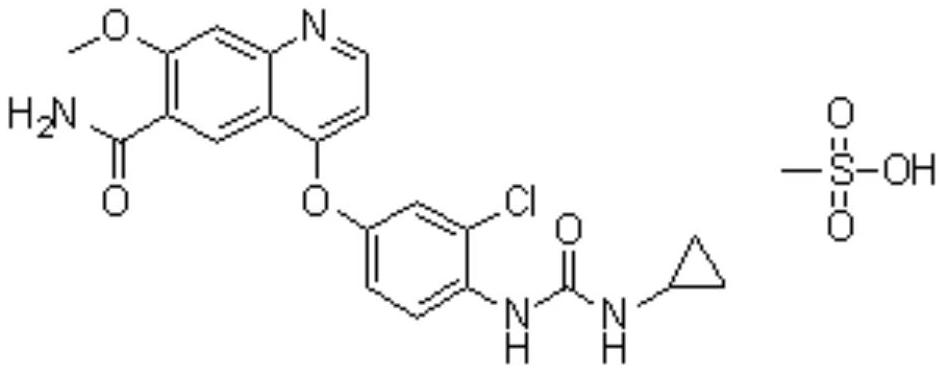
Lenvatinib (trade name Lenvima) is an anti-cancer drug for the treatment of certain kinds of thyroid cancer, and potentially for other cancers as well. It was developed by Eisai Co. and acts as a multiple kinase inhibitor against the VEGFR1, VEGFR2 and VEGFR3 kinases.
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
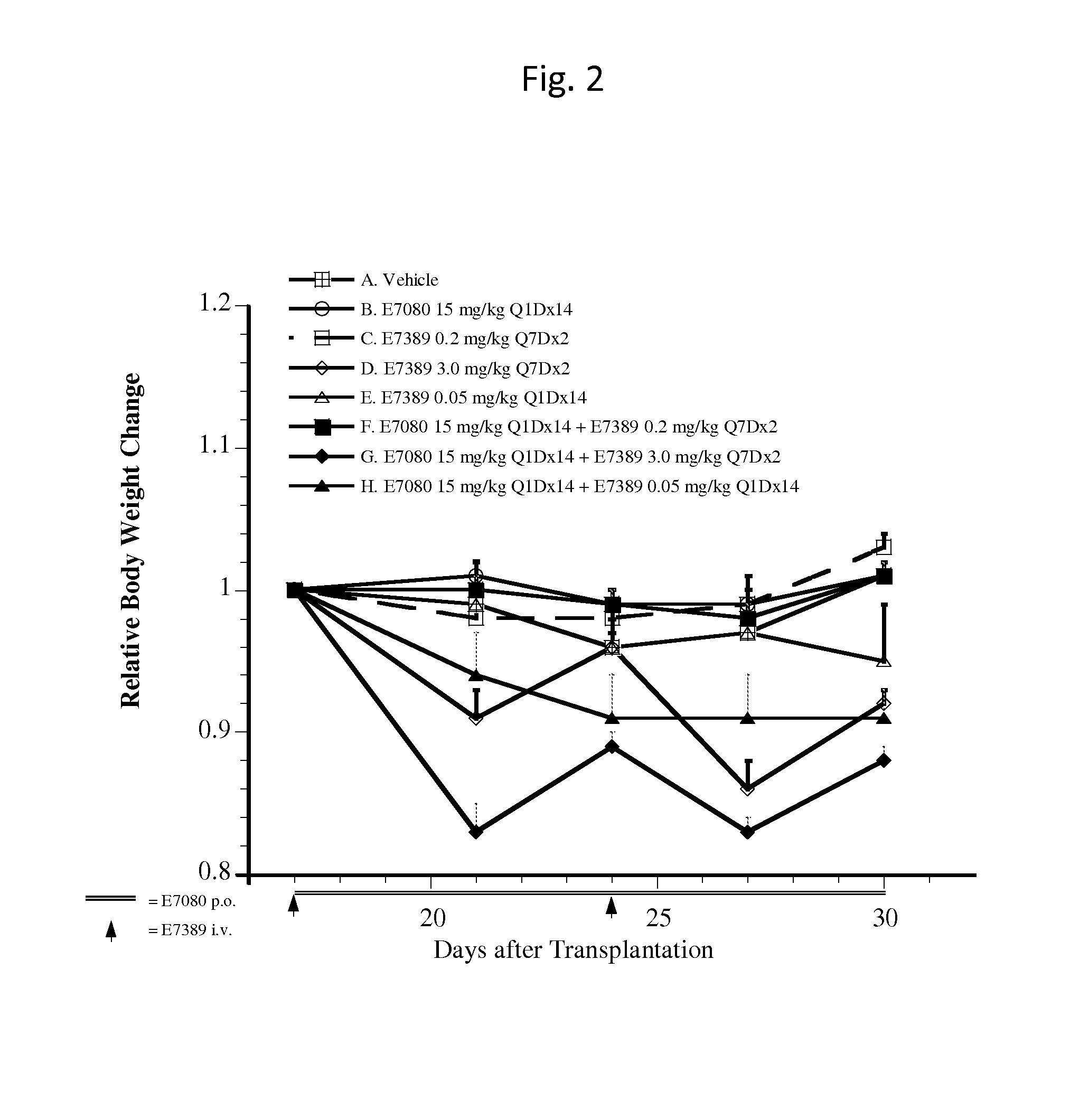
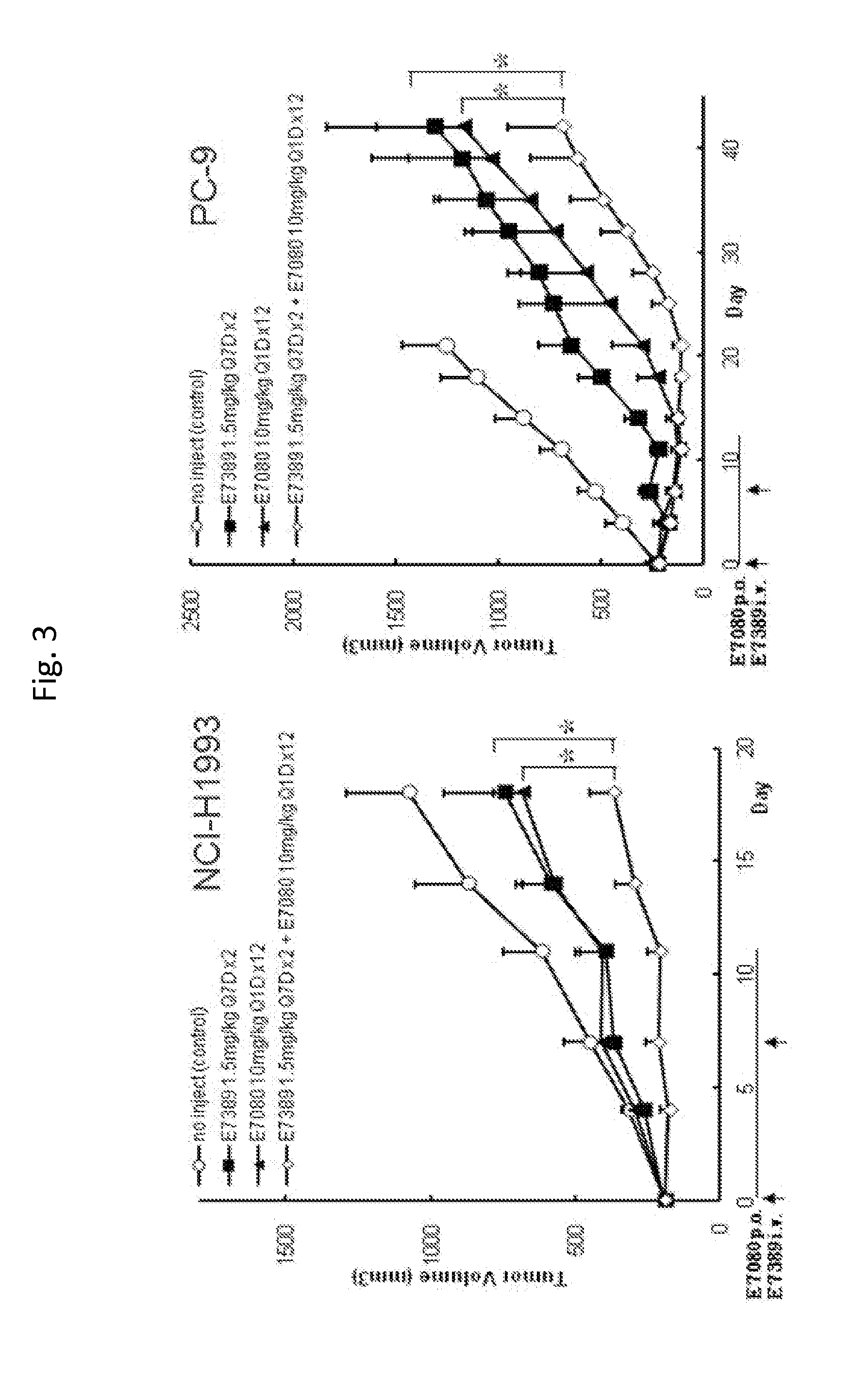
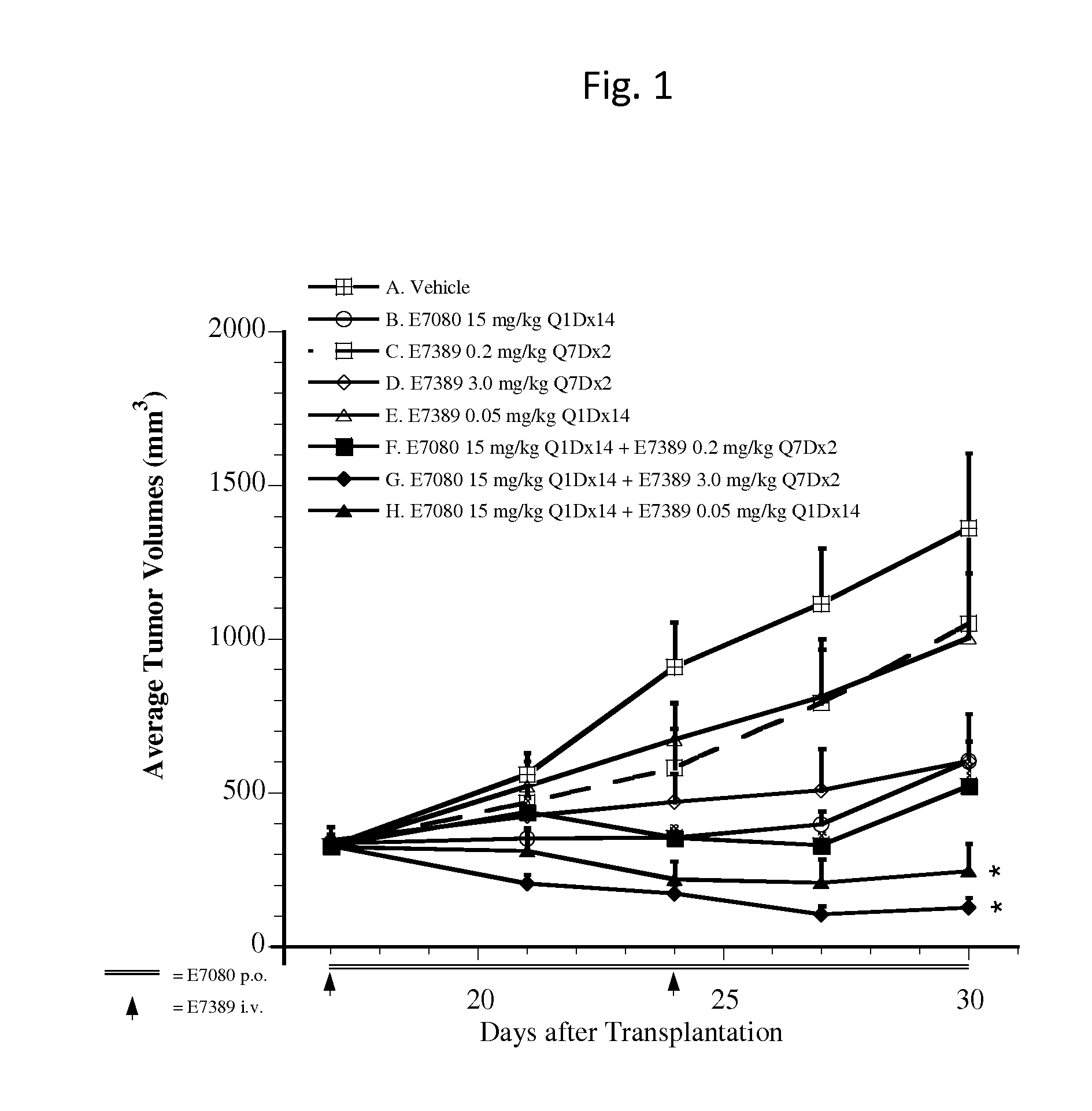
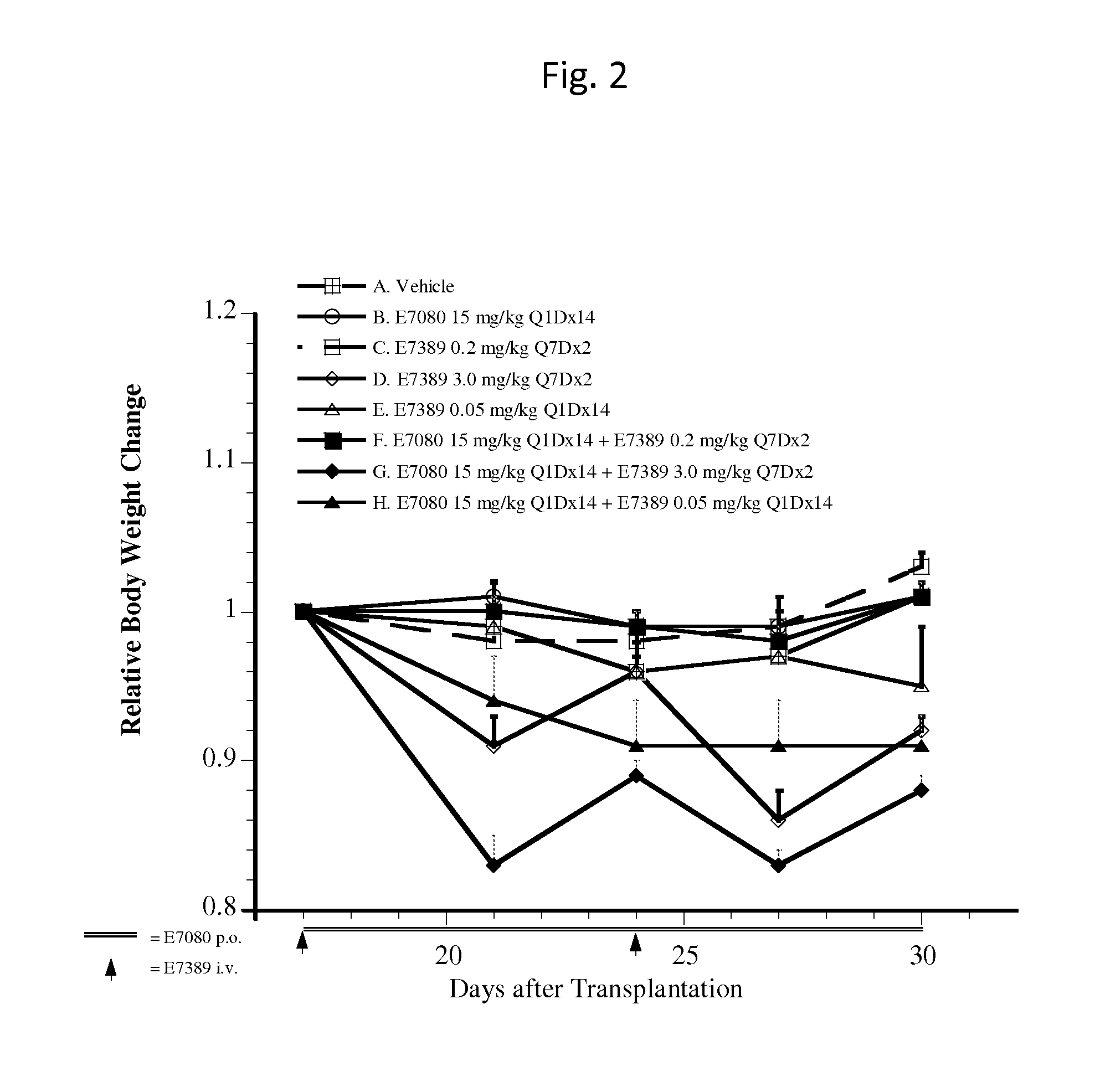
Use of eribulin and lenvatinib as combination therapy for treatment of cancer
ActiveUS20150005343A1Reduce in quantityPrevent and delay occurrenceBiocideAnimal repellantsLenvatinibEribulin
The invention provides methods and compositions for use in treating diseases associated with excessive cellular proliferation, such as cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
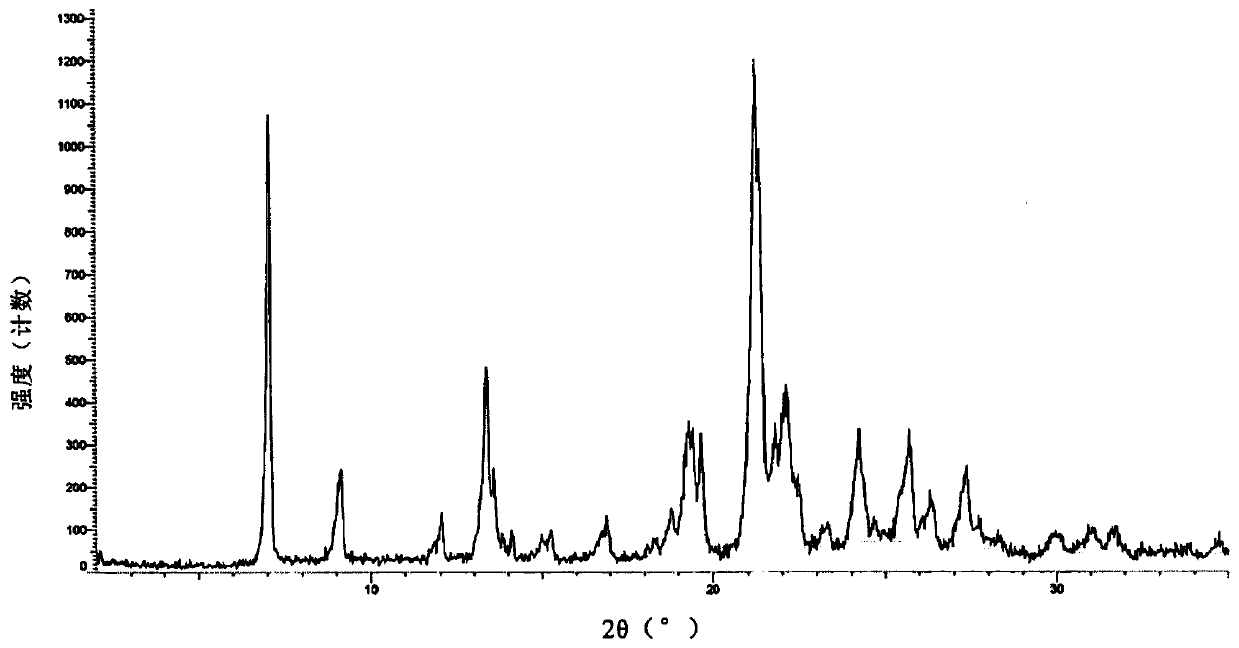
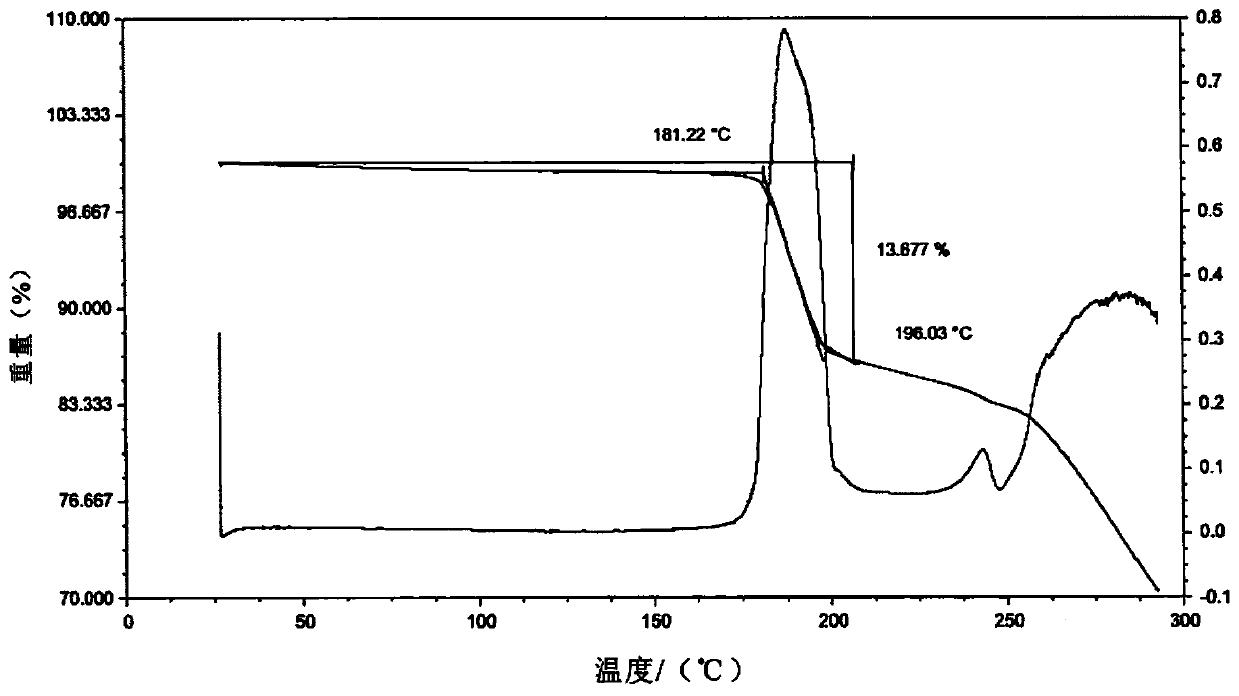
Lenvatinib mesilatee polymorph and preparation method thereof
InactiveCN109867626AGood crystal stabilityGood chemical stabilitySulfonic acids salts preparationAntineoplastic agentsLenvatinibChemical stability
The invention relates to a lenvatinib mesilatee polymorph and a preparation method thereof. In particular, the present invention provides a novel crystalline form of a lenvatinib mesilatee dimethyl sulfoxide solvate and a preparation method of the novel crystalline form. The novel crystalline form of the present invention has an excellent crystal form and chemical stability, and better flowability.
Owner:ANLITE SHANGHAI PHARMA TECH CO LTD +2
Stable lenvatinib pharmaceutical composition and preparation method thereof
InactiveCN106551911AExtended shelf lifeImprove stabilityPharmaceutical non-active ingredientsPill deliveryLenvatinibCoated tablets
The invention discloses a stable lenvatinib pharmaceutical composition and a preparation method thereof. The pharmaceutical composition consists of a cyclodextrin inclusion of an active ingredient lenvatinib and other pharmaceutically acceptable carriers; and the pharmaceutical composition is prepared by virtue of a freeze-drying dry granulation process by strictly controlling temperature and humidity in a preparation process. According to the lenvatinib pharmaceutical composition and the preparation method thereof, the lenvatinib is included in cyclodextrin, and meanwhile the preparation process of freeze-drying and dry granulation is adopted, so that the shortcomings of the lenvatinib, as a main drug, which is capable of absorbing moisture quite easily and quite poor in stability and cannot be prepared into an oral preparation, can be greatly overcome, and meanwhile, influence on disintegration and the dissolution of ingredients of the main drug can be avoided; and the finished lenvatinib pharmaceutical composition, which is not a coated tablet, can avoid the shortcoming of a conventional coated tablet which is relatively slow in dissolution.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method of lenvatinib
The invention relates to a preparation method of lenvatinib, in particular to the preparation of lenvatinib through a one-step urea forming reaction. The preparation method of lenvatinib according to the present invention produces less difficult-to-remove impurities, facilitates post-processing, facilitates the quality control of raw material drug production, and also provides convenience for subsequent research on preparations.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Lenvatinib synthesizing method
InactiveCN105801481AMeet the needs of useMild reaction conditionsOrganic chemistryLenvatinibBenzyl chloroformate
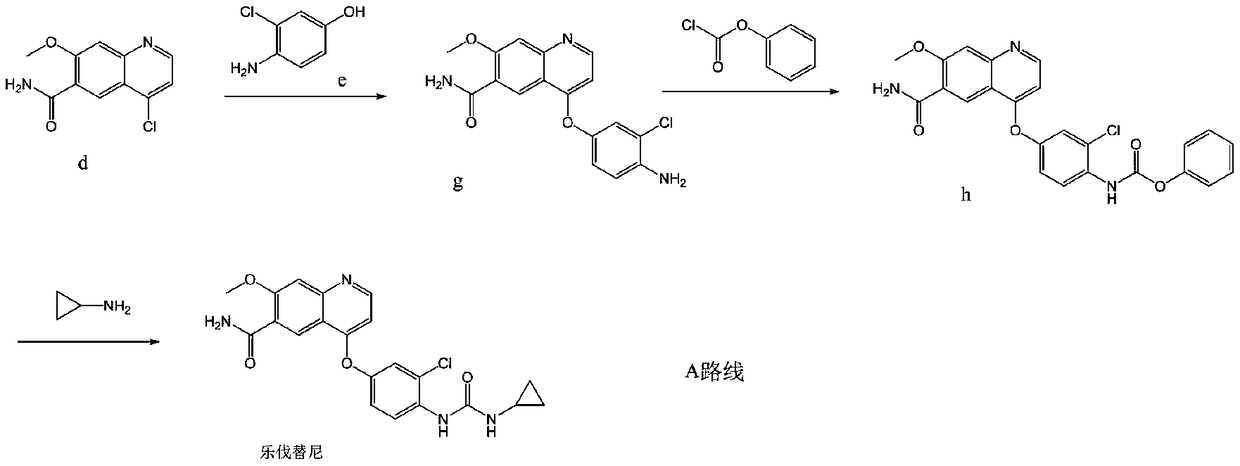
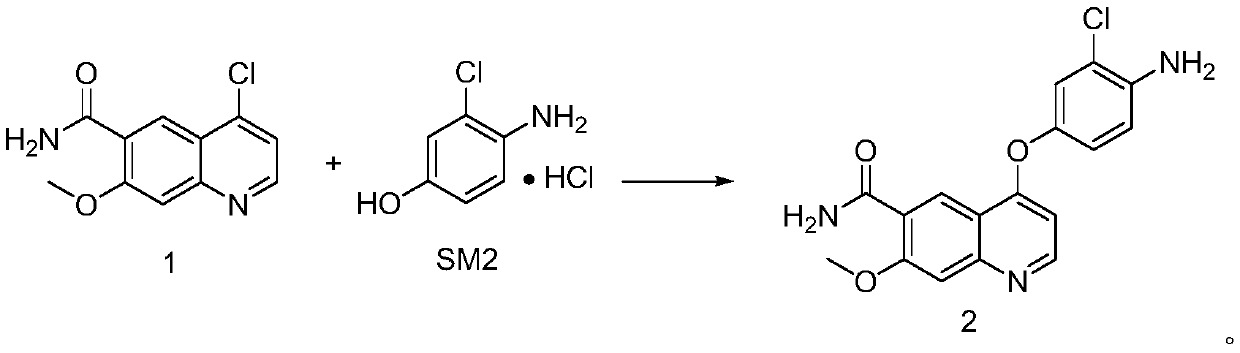
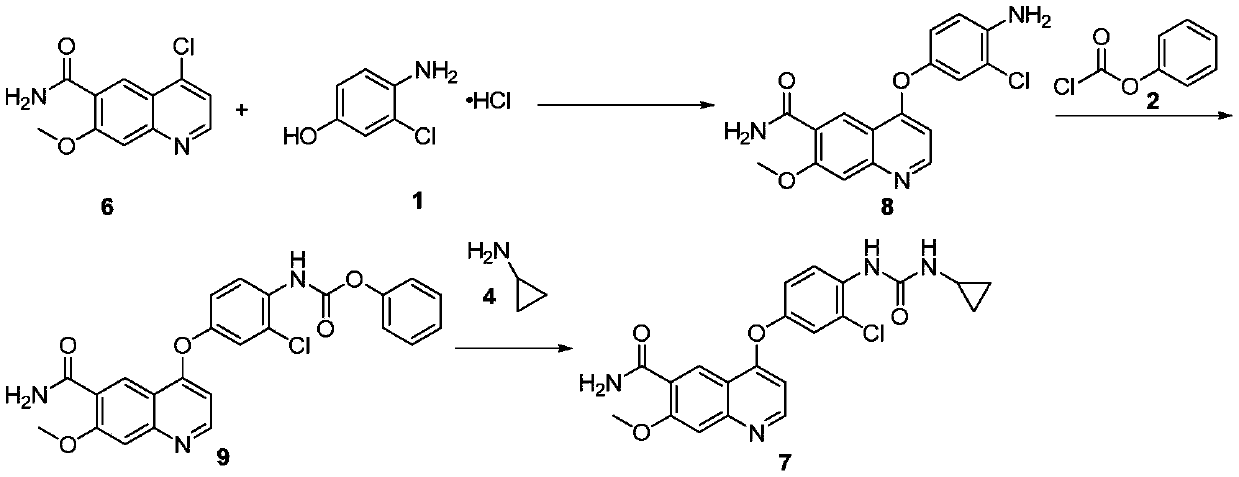
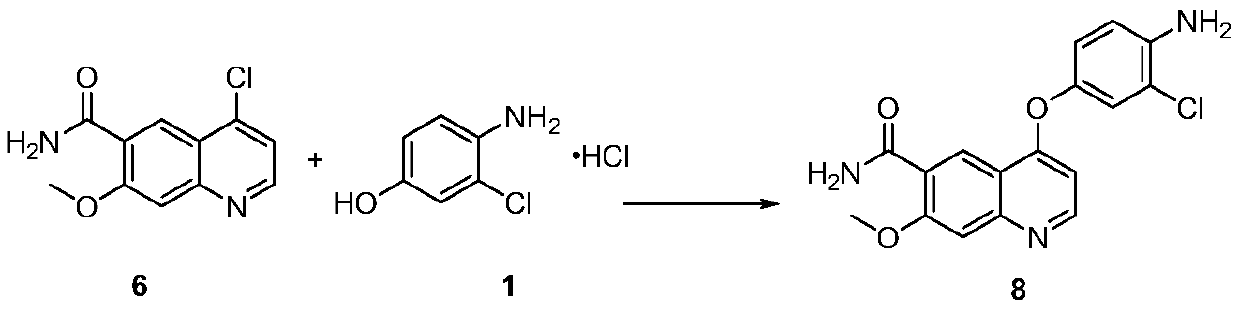
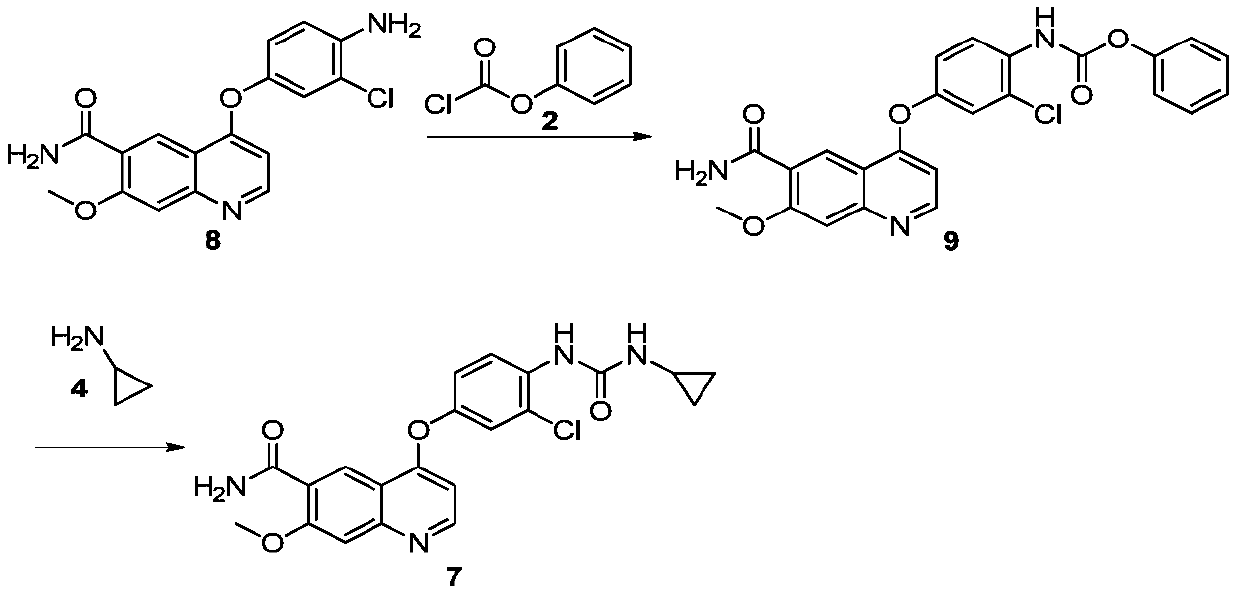
The invention discloses a lenvatinib synthesizing method.The method includes the steps that 4-amino-3-chlorophenol and benzyl chloroformate are subjected to amidation reaction, the obtained 4-(carbobenzoxy)amino-3-chlorophenol and 4-chlorine-7-methoxyquinoline-6-formamide are subjected to condensation reaction, the obtained 4-[3-chlorine-4-(carbobenzoxy)aminophenoxy]-7-methoxyquinoline-6-formamide and cyclopropylamine are subjected to amidation reaction, and the finished product lenvatinib is obtained.The method is short in process route step, operation is simplified, cost is low, and the method is environmentally friendly and suitable for industrial production.
Owner:湖南欧亚药业有限公司
Application of Lenvatinib to preparation of medicine for preventing and curing macular degeneration
The invention relates to application of Lenvatinib to preparation of medicine for preventing and curing macular degeneration, and belongs to the field of medicine. The problem that the conventional therapeutic medicine for macular degeneration is low in curative effect and low in patient compliance is solved, and the technical scheme is to provide application of Lenvatinib to preparation of medicine for preventing and curing macular degeneration. Lenvatinib is a multiple target point micromolecularly targeted inhibitor resist to VEGFR, PDGFR and bFGFR, and has been approved by FDA in the market for the treatment of tumors. The invention shows that Lenvatinib has potential for curing macular degeneration, and the application of Lenvatinib is verified by establishing an in vitro and vivo evaluation model, and the application has preferable clinic and social meanings.
Owner:GUANGDONG ZHONGSHENG PHARMA
Preparation method of lenvatinib
ActiveCN108623521ARaw materials are easy to getReduce energy consumptionOrganic chemistryLenvatinibPollution
The invention relates to a preparation method of lenvatinib, and belongs to the field of medicine chemistry. The preparation method of lenvatinib uses a brand-new preparation scheme; a target compoundmeeting the requirement can be obtained no matter from which intermediates. The method provided by the invention has the advantages that the steps are short; the reaction operation is simple; the safety and the reliability are realized; the yield is high; the cost is low; the purity is high; the pollution is low; the operation is simple, and the like. The total yield of lenvatinib can reach about84 percent; the purity can reach 99.7 percent.
Owner:YANCHENG TEACHERS UNIV +1
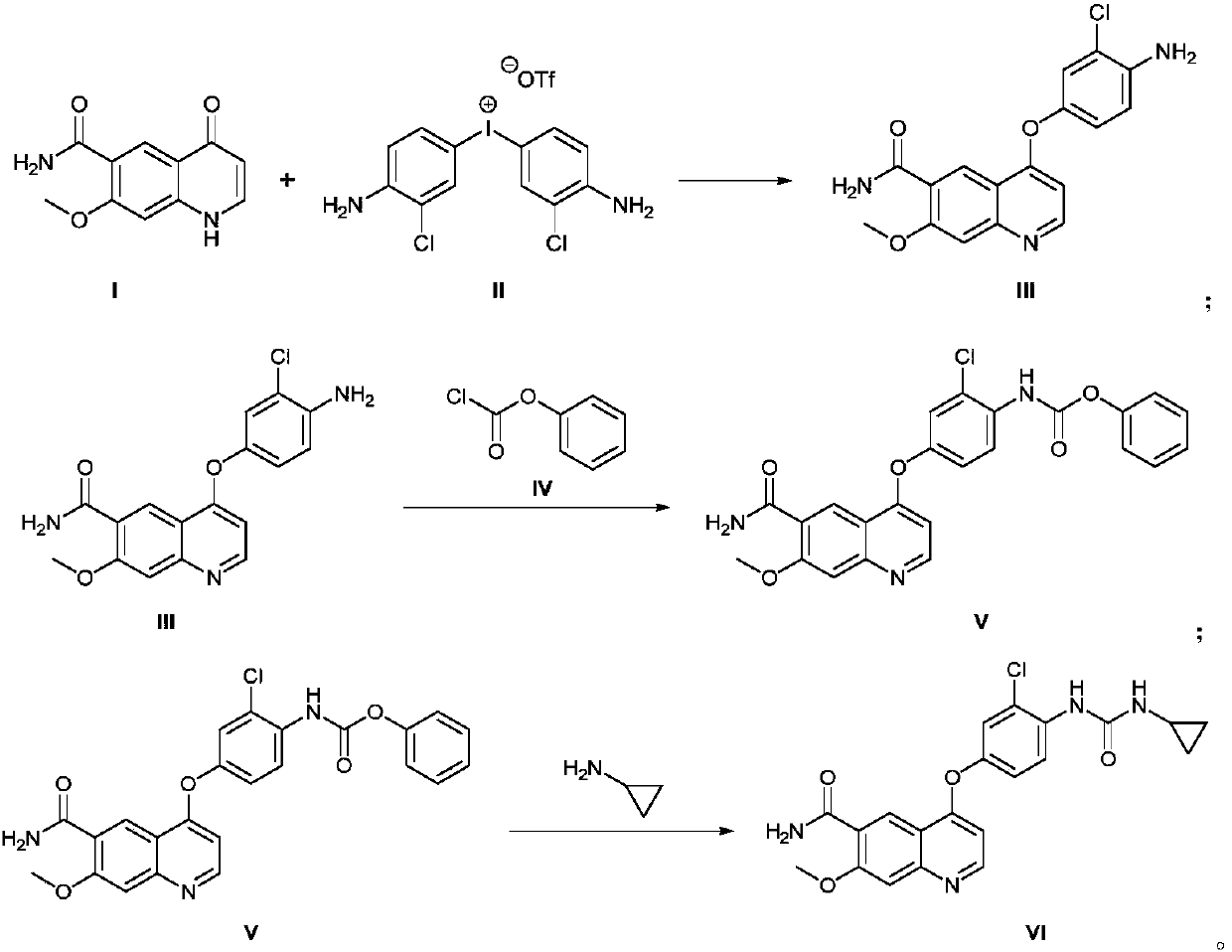
Method for synthesizing lenvatinib
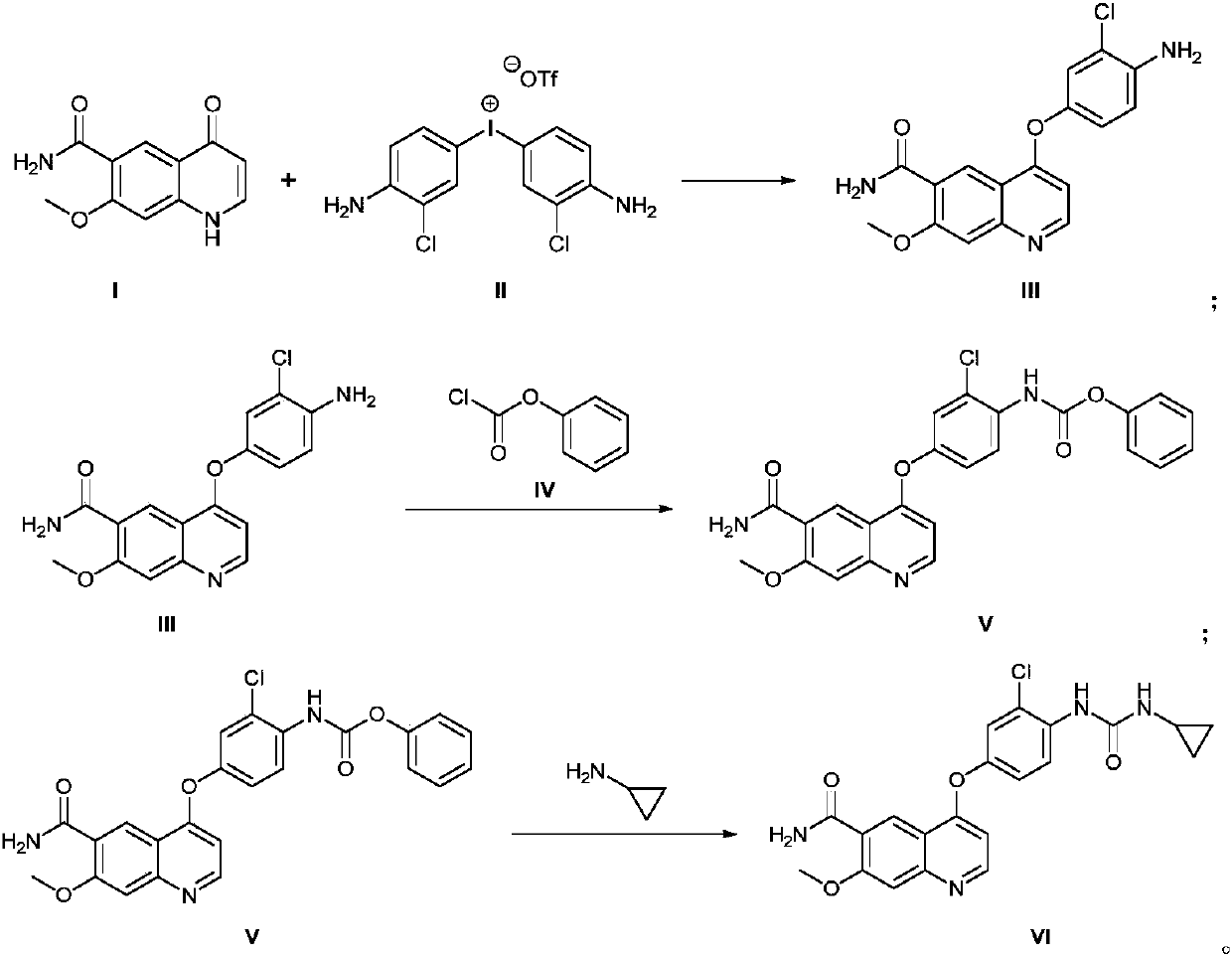
The invention discloses a method for synthesizing lenvatinib. The method comprises the following steps: with 7-methoxy-4-oxo-1,4-dihydro-quinoline-6-formamide as an initial raw material, carrying outan oxo-arylation reaction on the initial raw material and bis(3-chloro-4-amino)phenyl iodine trifluoro-methanesulfonate, carrying out an amidation reaction between 4-(4-amino-3-chloro-phenoxy)-7-methoxy-6-formamide, a product of oxo-arylation reaction, and phenyl chloroformate, and carrying out a urea reaction on a product of amidation reaction and cyromazine, thereby obtaining lenvatinib. The synthetic method has few process route steps and is simple in operation, 7-methoxy-4-oxo-1,4-dihydro-quinoline-6-formamide innovatively replaces 4-chloro-7-methoxyquinoline-6-formamide to serve as the initial raw material, and the method is environmental-friendly and is applicable to industrialized production.
Owner:NANJING CHICO PHARMA CO LTD
Preparation method of lenvatinib
PendingCN110981800ALow densityReduce the temperatureOrganic chemistryBulk chemical productionLenvatinibChlorobenzene
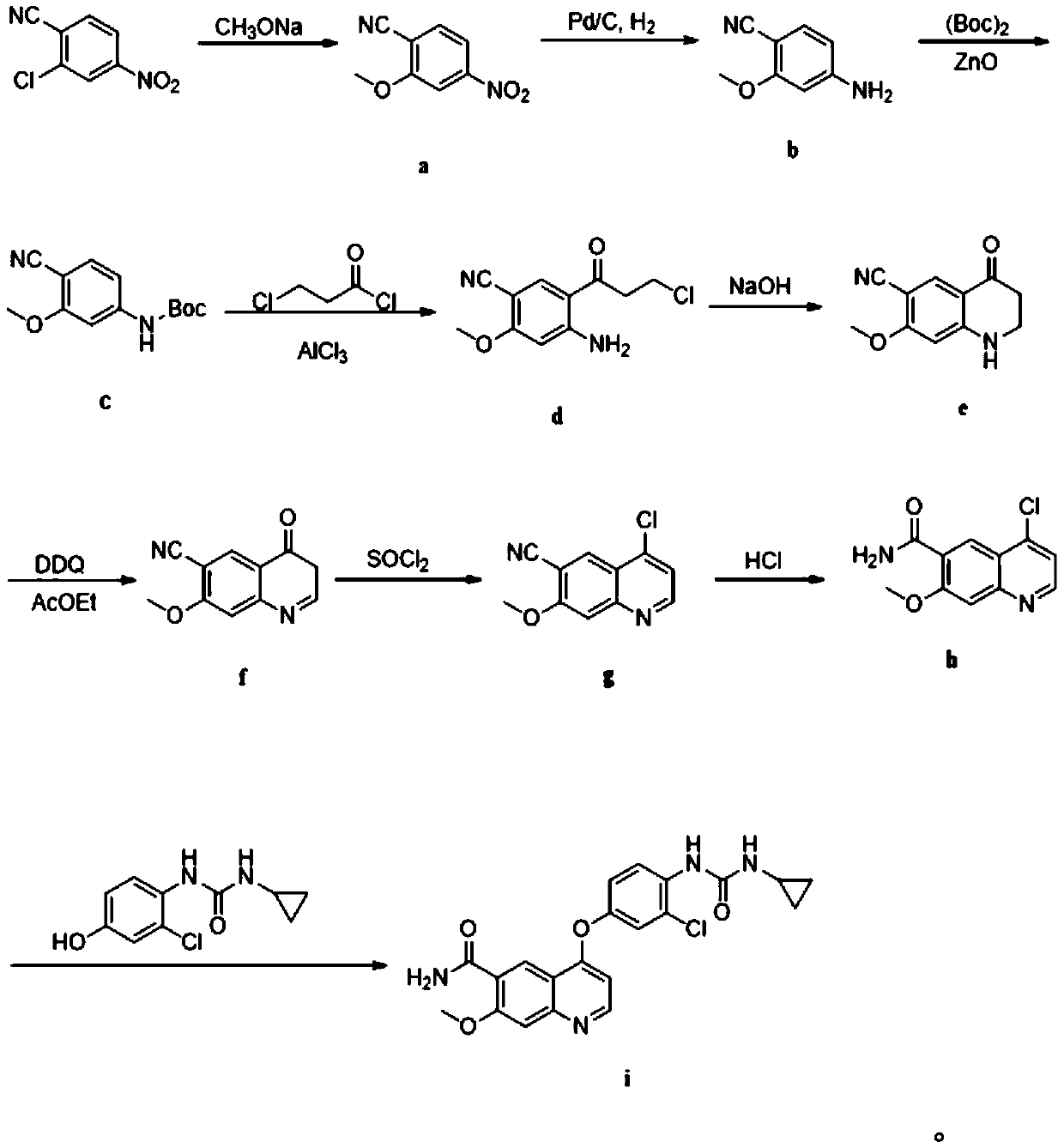
The invention discloses a preparation method of lenvatinib, which comprises the following steps: by using 4-nitro-2-chlorobenzonitrile as an initial raw material, introducing nitro into molecules forelectron withdrawing, thereby greatly lowering the electron cloud density of benzene rings, wherein it is beneficial to nucleophilic substitution reaction and can greatly lower the reaction temperature; carrying out amino protection: taking ZnO as Lewis acid, so that the reaction is green; carrying out Friedel-Crafts acylation, deprotection and intramolecular alkylation cyclization. Compared witha traditional high-temperature reaction, the method is novel in synthetic route, low in reaction temperature, mild in condition, green, free of dangerous steps and suitable for industrial production.
Owner:安徽省诚联医药科技有限公司
Application of combination of lenvatinib and PD-1 monoclonal antibody in preparation of anti-hepatoma drugs
PendingCN114191547APromote normalizationPromote infiltrationAntibody ingredientsImmunoglobulinsLenvatinibCo medication
The invention provides application of combination of lenvatinib and a PD-1 monoclonal antibody in preparation of an anti-liver cancer drug, and belongs to the technical field of medicines.The combination of low-dose lenvatinib and the PD-1 monoclonal antibody is adopted, infiltration of T lymphocytes in tumor tissue can be enhanced while normalizing of liver cancer blood vessels is promoted, and then the treatment effect on liver cancer is remarkably enhanced; the compound can be applied to liver cancer prevention and treatment. The invention provides a novel treatment combined medication scheme for the treatment of the liver cancer, and has a good application prospect in the aspect of preventing and treating the liver cancer.
Owner:SUN YAT SEN UNIV +1
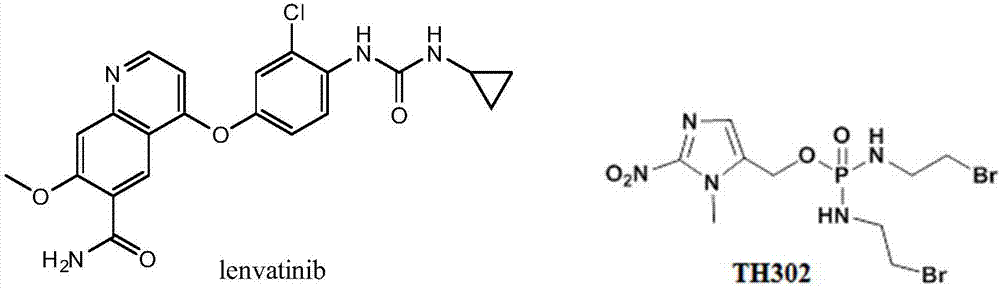
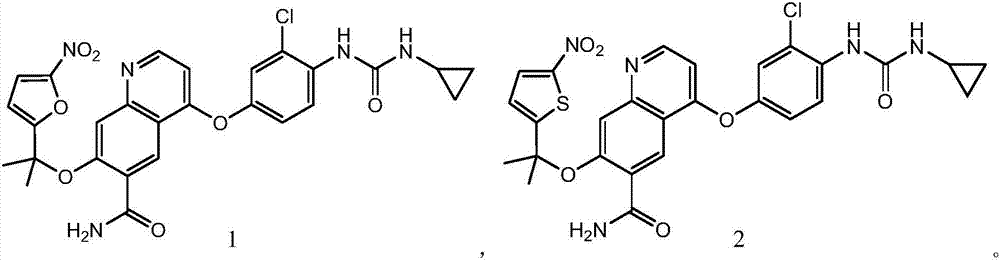
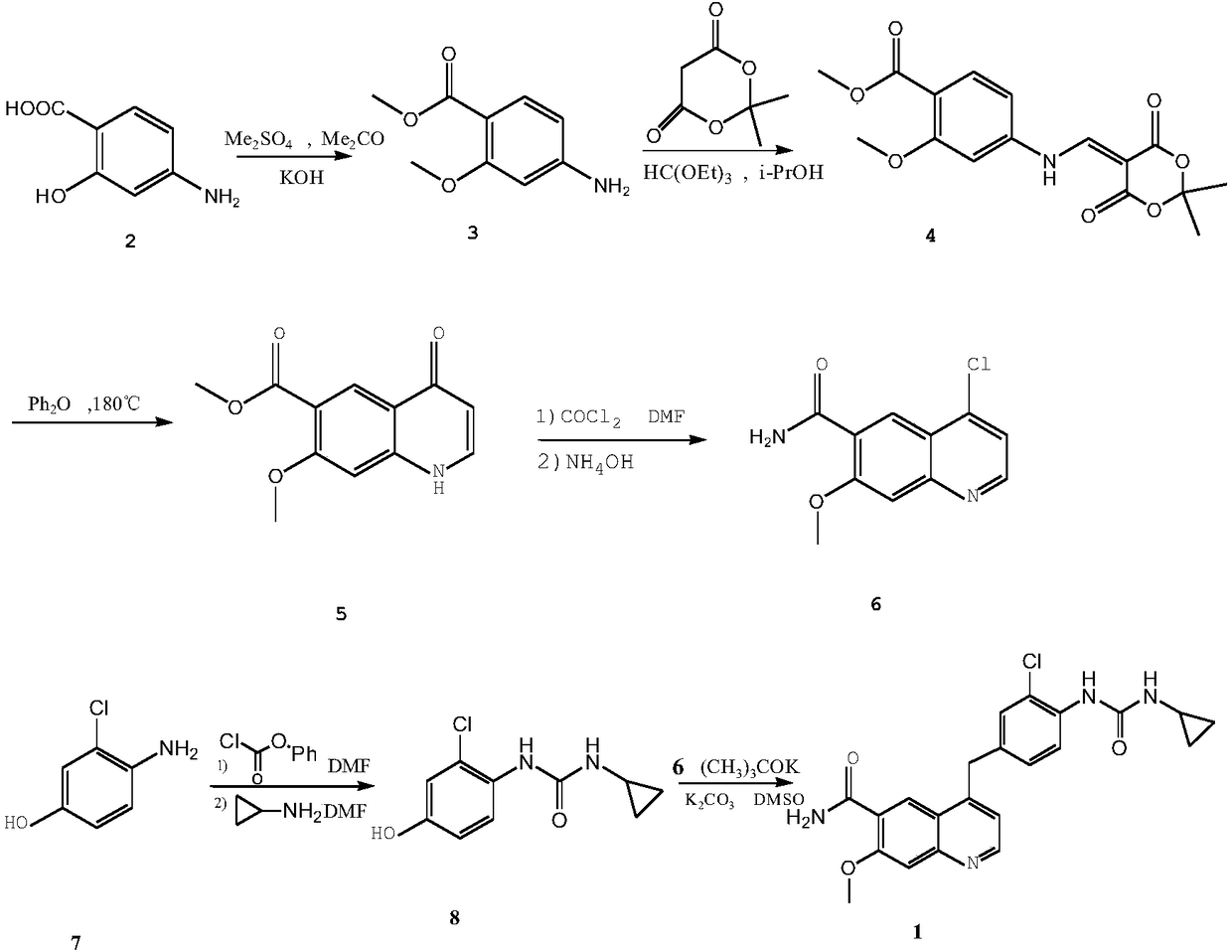
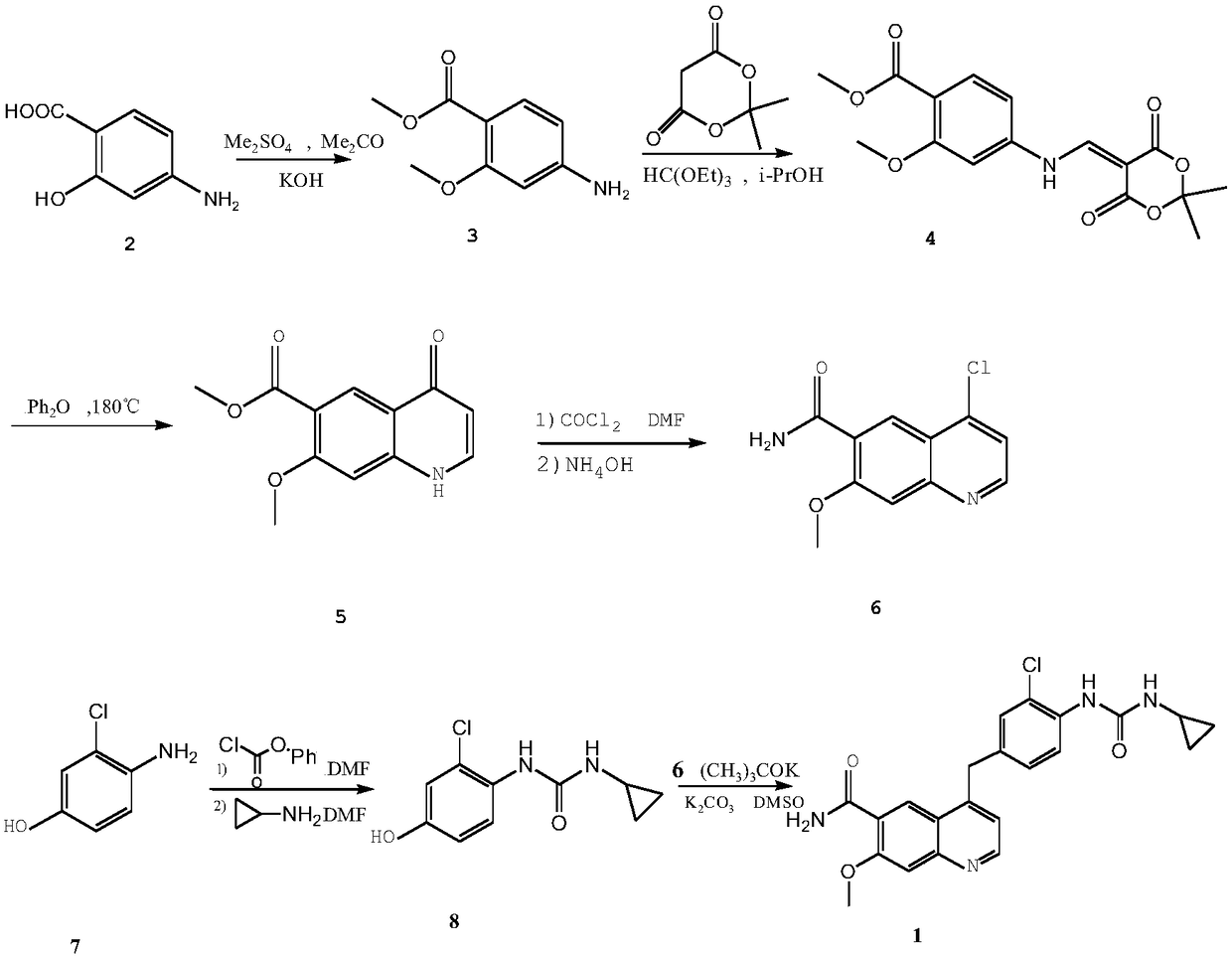
Hypoxia-activated prodrug of lenvatinib and application of hypoxia activated prodrug
InactiveCN107513057AReduced inhibitory activityStrong inhibitory activityOrganic active ingredientsOrganic chemistryLenvatinibHypoxia activated prodrug
The invention provides a hypoxia-activated prodrug of lenvatinib and application of the hypoxia activated prodrug. A structure of the hypoxia-activated prodrug accords with a general formula (I), wherein the formula (I) is shown in the description, wherein R is -O- and -S-. The drug provided by the invention is stable under normal conditions and has a relatively weak drug function. The hypoxia-activated prodrug is not stable under a hypoxia condition and can generate molecules with the stronger drug function. The drug provided by the invention has good drug function and safety and can be used for preparing drugs for treating tumors.
Owner:NANJING MEDICAL UNIV
Method for synthesizing lenvatinib
The invention belongs to the field of chemical pharmacy, and specifically relates to a method for synthesizing lenvatinib. The method comprises the following steps: step 1, taking 4-aminosalicylic acid as a raw material, and preparing 4-chloro-7-methoxyquinoline-6-formamide through methylation, condensation with meldrum's acid, high-temperature cyclization, chlorination and ammoniation; step 2, taking 3-chloro-4-aminophenol as a raw material, and reacting with phenyl chloroformate and cyclopropylamine to obtain 1-(2-chloro-4-hydroxy phenyl)-3-cyclopropyl urea; and step 3, enabling the 4-chloro-7-methoxyquinoline-6-formamide prepared in step 1 to react with the 1-(2-chloro-4-hydroxy phenyl)-3-cyclopropyl urea prepared in step 2 under action of potassium tert-butoxide to obtain the lenvatinib. The invention provides a brand-new route for synthesising the lenvatinib. The used reagent is cheap and is easily available, is simple in operation, has a yield higher than that of other methods, and is easy for industrial production.
Owner:南京天越星生物技术有限公司
Synthesis method for lenvatinib
InactiveCN108658859AReduce usageAvoid competing reactionsOrganic chemistryLenvatinibSynthesis methods
The invention provides a synthesis method for lenvatinib. The synthesis method takes m-chlorophenol as a raw material and a target product, i.e., the lenvatinib, is obtained through four-step reaction. According to the synthesis method provided by the invention, steps of an original synthesis route are reduced and relatively high yield is kept; and the cost of the raw material is reduced and the atom economy is improved.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Preparation method of lenvatinib and its salts
The invention discloses a preparation method of lenvatinib and its salts, wherein N-methylpyrrolidone is used as a reaction solvent in the step of condensation with phenyl chloroformate. The preparation method has the advantages that the danger is successfully avoided that N,N-dimethylformamide and phenyl chloroformate react to produce massive gas during large-scale production; the preparation method is stable, the reaction time is short so that better safety is ensured, posttreatment is facilitated, and quality control is facilitated for the production of active pharmaceutical ingredients.
Owner:YANGTZE RIVER PHARM GRP CO LTD
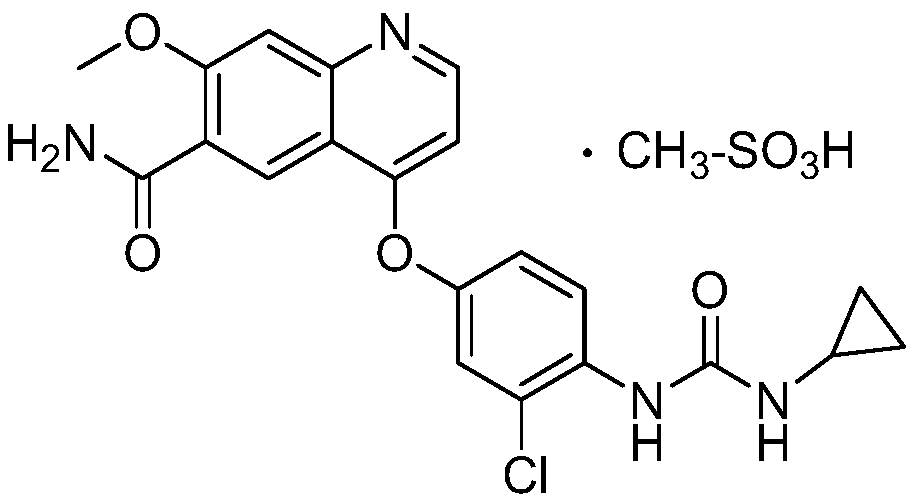
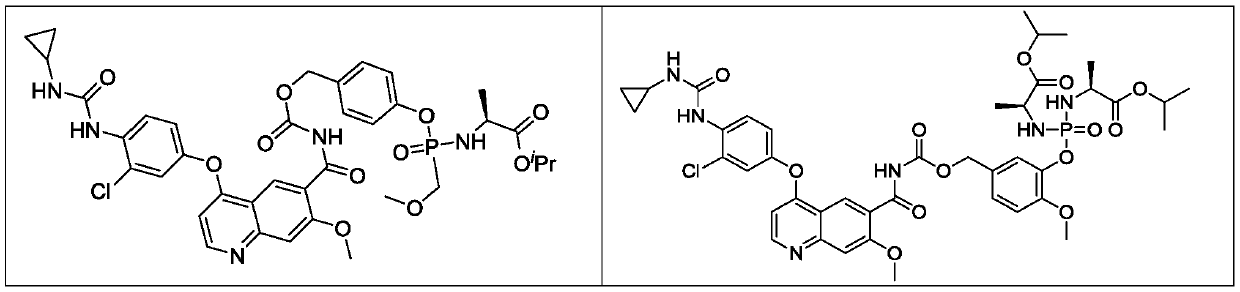
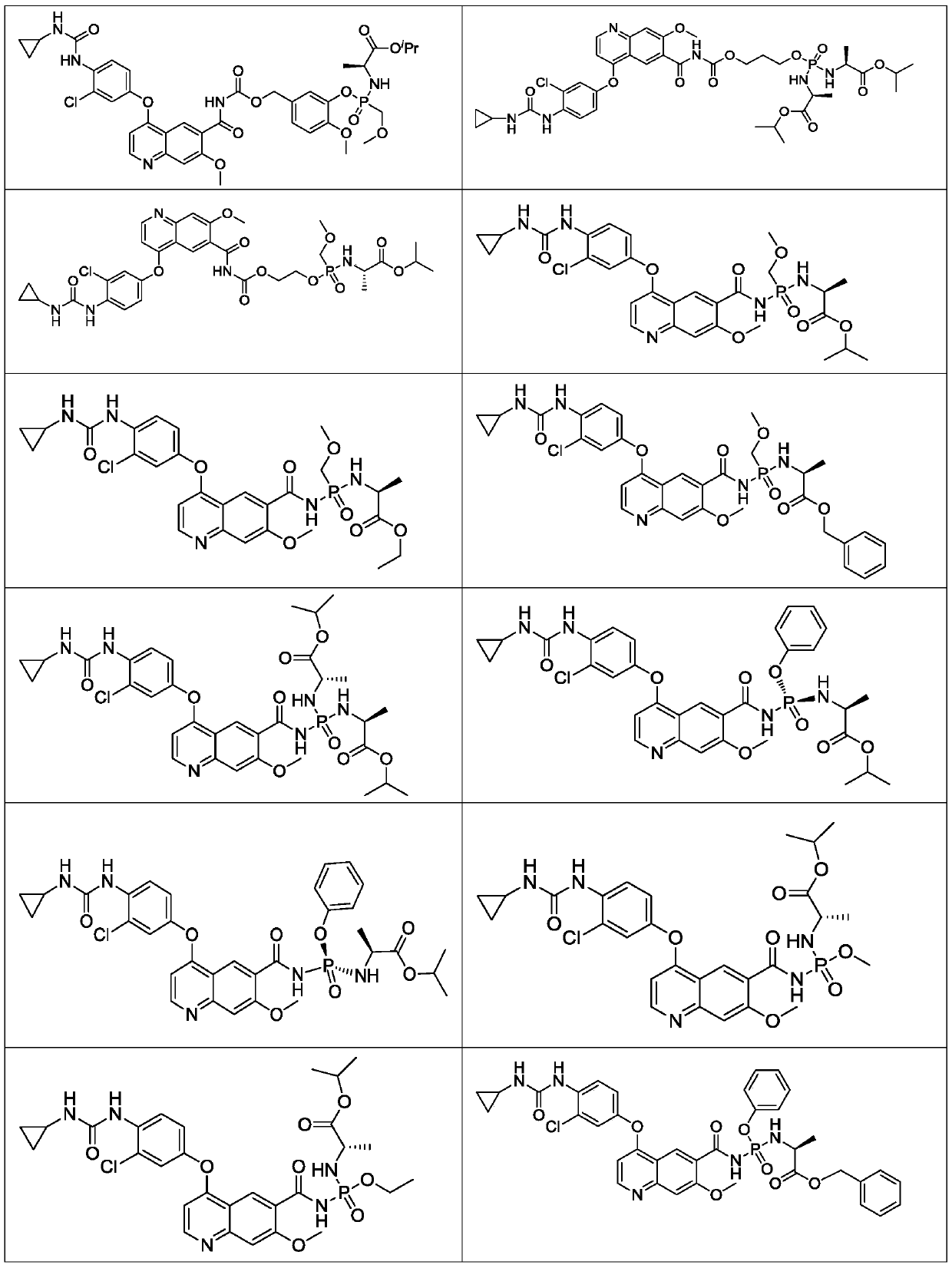
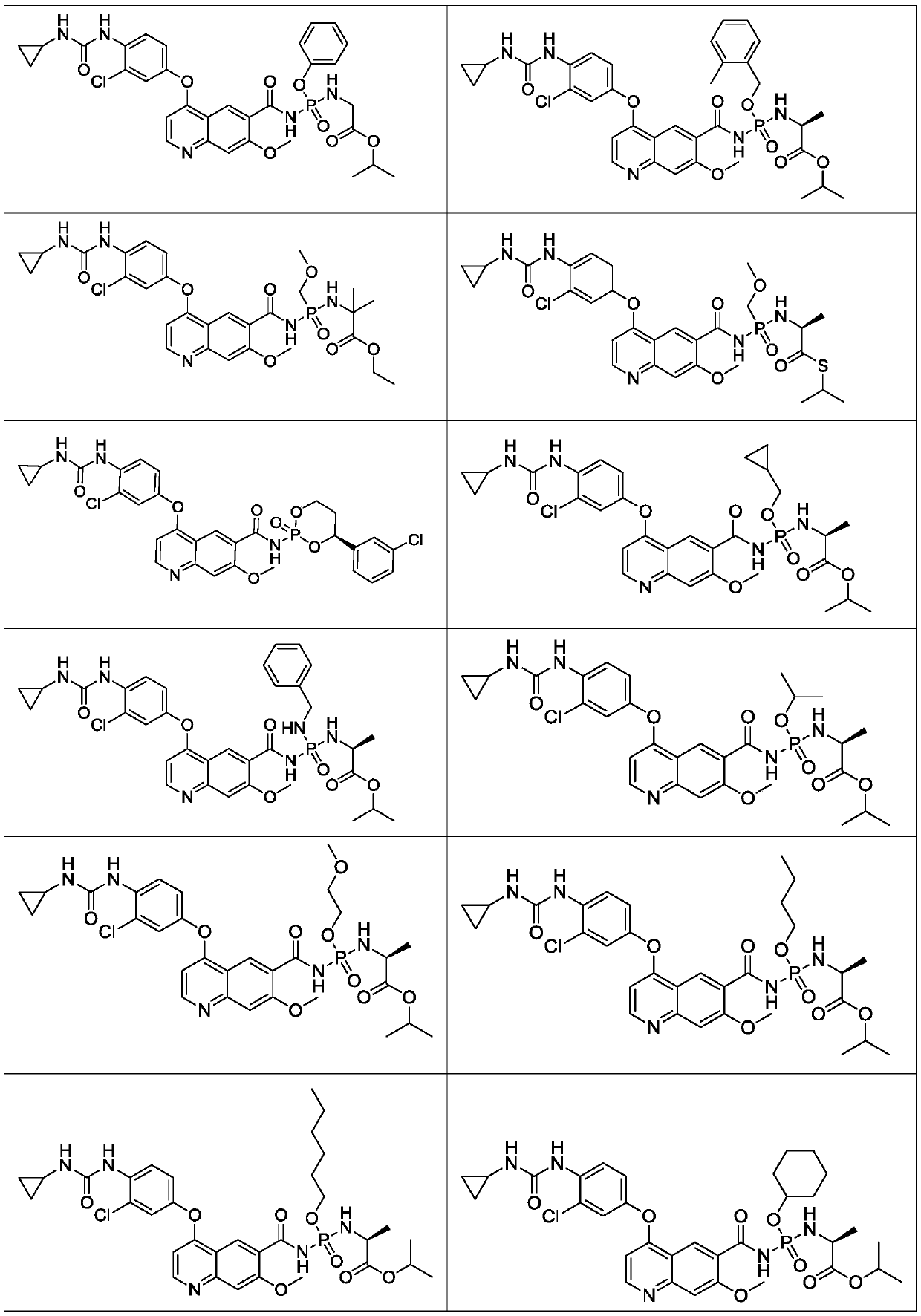
Lenvatinib derivatives, preparation method and application
ActiveCN110590839AGood effectStrong specificityOrganic active ingredientsPhosphorus organic compoundsStereoisomerismStereochemistry
Owner:SICHUAN HAISCO PHARMA CO LTD
Preparation method of high-purity lenvatinib and salt thereof
The invention relates to a preparation method of lenvatinib and salt thereof. The adopted method for preparing the lenvatinib has the advantages that the process is simple, the cost is low, the purity is high, the single-purity content meets the regulations, and the industrial production is easier.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Application of PERK inhibitor to preparation of synergist of liver cancer medicine
ActiveCN112807434AEnhance the effect of treating liver cancerHas clinical application valueAntibody ingredientsAntineoplastic agentsLenvatinibChemical compound
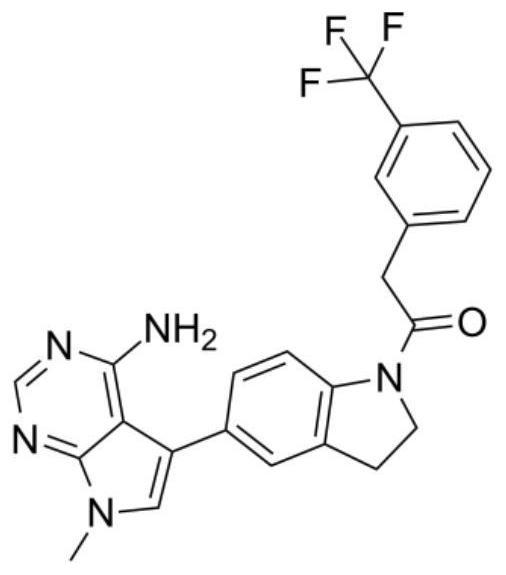
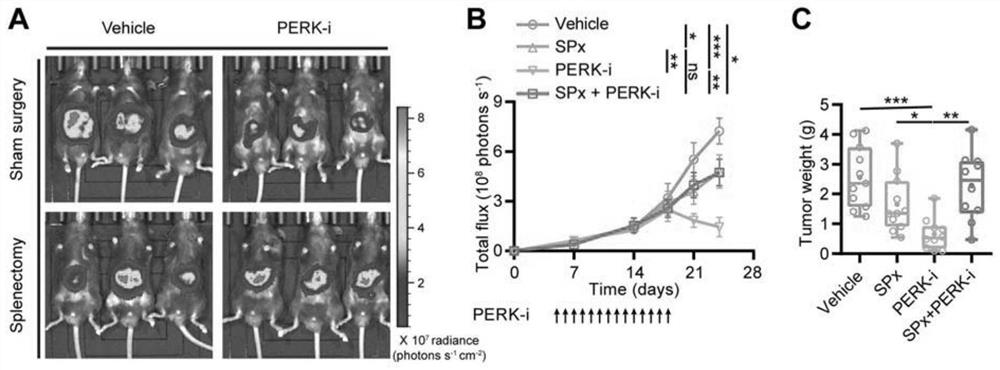
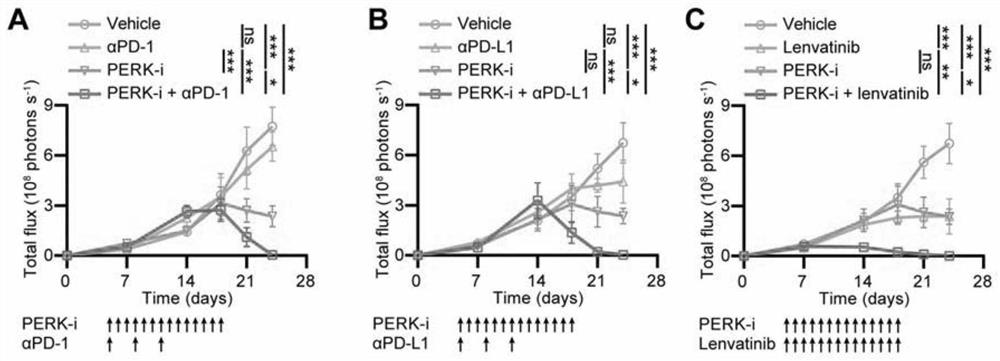
The invention discloses an application of a PERK inhibitor to preparation of a synergist of a liver cancer medicine. By researches, the following case is found that PERK is a target for liver cancer treatment, blockage of PERK activation through a small molecule compound is a new strategy of anti-liver cancer immunotherapy, which uses tumor-related spleen as a target spot, and the PERK has clinical application value. The invention shows that the PERK inhibitor GSK2606414 takes an anti-tumor effect by acting on an immune system in the spleen of a mouse suffering from liver cancer. Moreover, the PERK inhibitor GSK2606414 can significantly and synergistically enhance the liver cancer treatment effect of an anti-PD-1 / PD-L1 antibody and Lenvatinib, and can be used as a novel liver cancer treatment drug for treating the liver cancer or enhancing the curative effect.
Owner:SUN YAT SEN UNIV
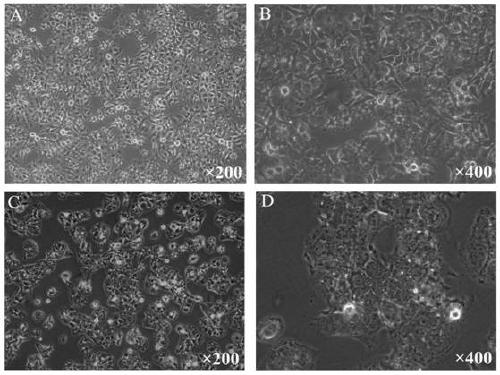
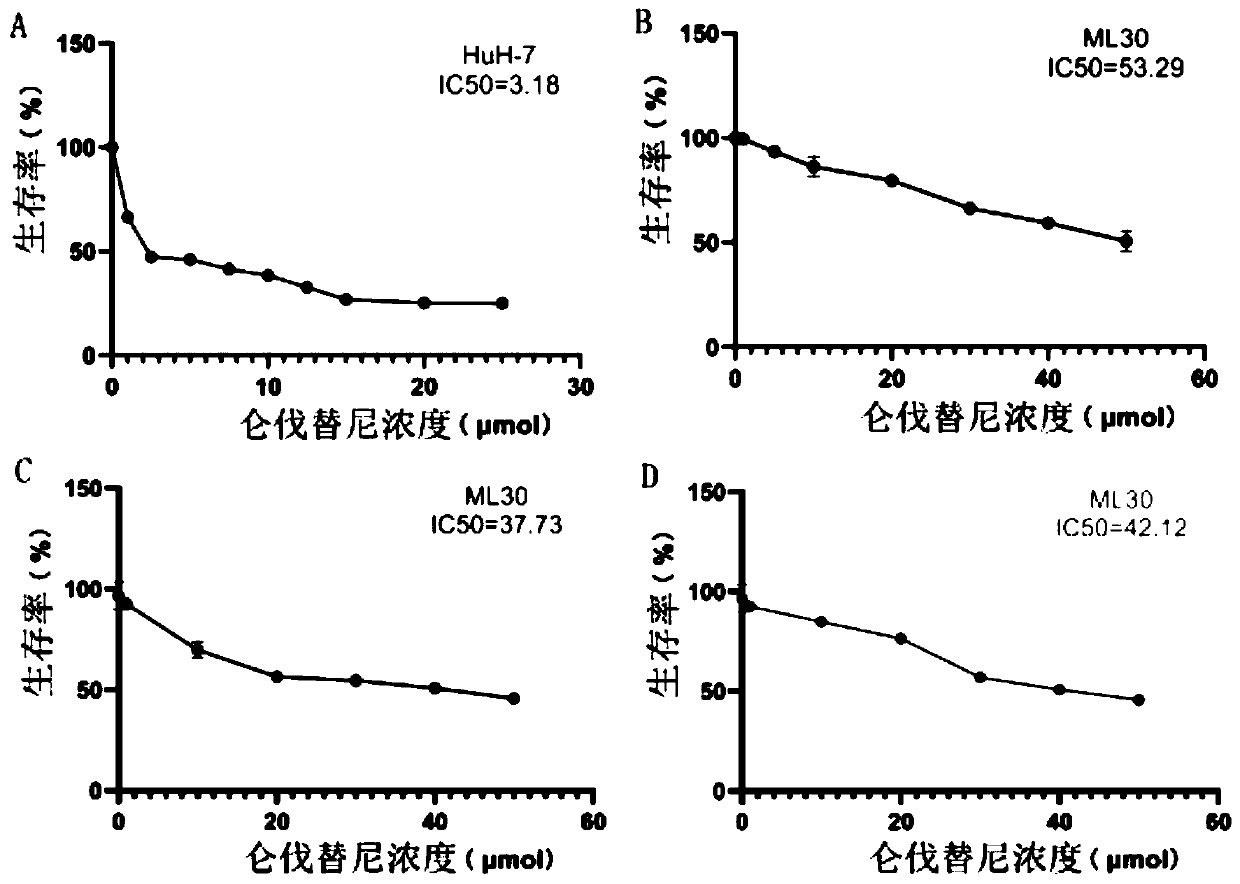
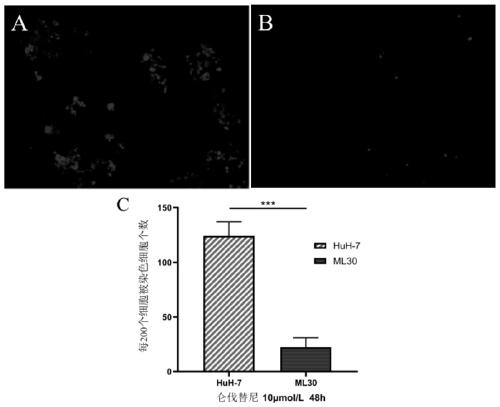
Hepatocellular carcinoma lenvatinib drug-resistance cell strain and preparation method and application thereof
ActiveCN110305845AMaintain drug resistanceHigh scientific researchCompound screeningApoptosis detectionLenvatinibCell strain
The invention discloses a hepatocellular carcinoma lenvatinib drug-resistance cell strain. The hepatocellular carcinoma lenvatinib drug-resistance cell strain is characterized in that the cell strainis named human hepatocellular carcinoma lenvatinib drug-resistance cell strain ML30, and the preservation number is (C2019127). A preparation method of the hepatocellular carcinoma lenvatinib drug-resistance cell strain comprises the following steps that 1, after resuscitation of human hepatocellular carcinoma cell strain HuH-7 cells, culturing is carried out in a 5% CO2 incubator at 37 DEG C by aDMEM medium containing fetal bovine serum of 10%; and 2, the human hepatocellular carcinoma cell strain HuH-7 cells in the logarithmic phase are taken, lenvatinib is added in a culture dish, the initial concentration is 3[mu]mol / L, and timely passage or liquid exchange is carried out; after each passage, the concentration of 0.5[mu]mol / L is increased, continuous culture is carried out for more than six months; until the human hepatocellular carcinoma cell strain HuH-7 cells which can grow rapidly in the range of being greater than 30[mu]mol / L are selected and used as the drug-resistance cellstrain, and the drug-resistance cell strain is named as the human hepatocellular carcinoma lenvatinib drug-resistance cell strain ML30. The hepatocellular carcinoma lenvatinib drug-resistance cell strain has important application prospects in studying the mechanism of liver cancer drug-resistance, reversing the drug-resistance of liver cancer cells, guiding the patient to use drugs and establishing a drug-resistance animal model.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Lenvatinib synthesis method
ActiveCN109734661ALower requirementMild reaction conditionsOrganic chemistryLenvatinibAlkyl transfer
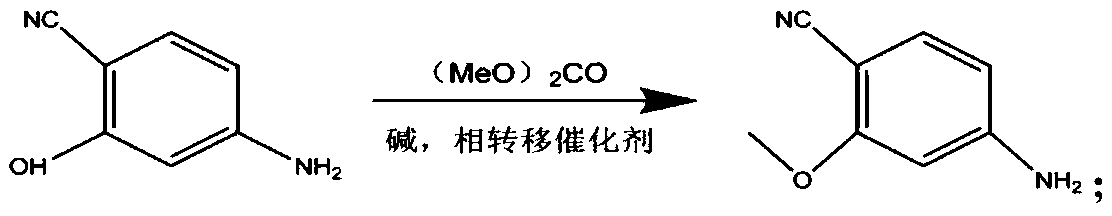
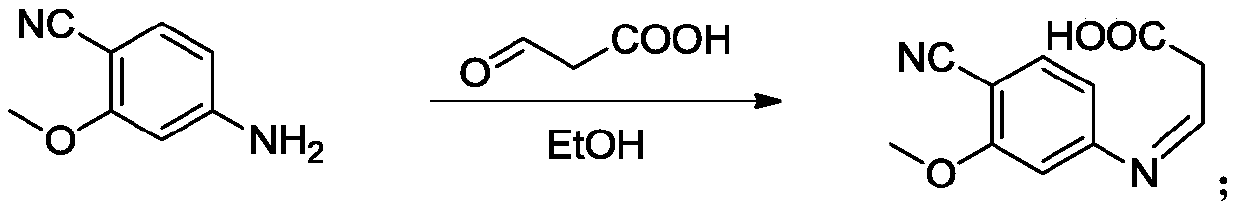
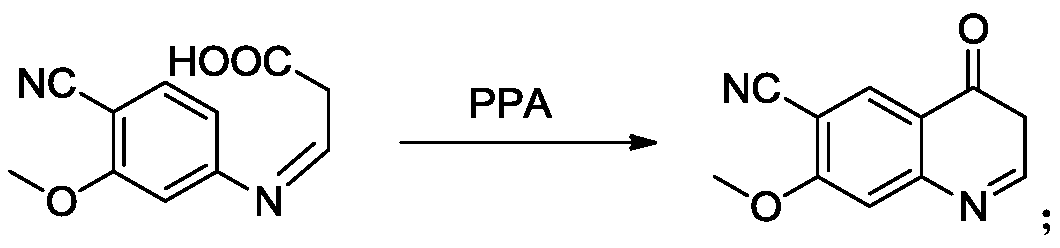
The invention provides a lenvatinib synthesis method. The method includes: taking 4-cyano-3-hydroxyaniline as a starting material, subjecting to dimethyl carbonate methylation and oximation through reaction with malonaldehydic acid at the room temperature, performing cyclization under the PPA condition to form 6-cyano-7-methoxy-4-quinolinone, forming 6-cyano-7-methoxy-4-chlorolinone under the action of thionyl chloride, performing cyano hydrolyzing synthesis of 6-formamido-7-methoxy-4-chloroquine which is an intermediate of lenvatinib under an acidic condition, subjecting 4-hydroxy-2-chloroaniline and cyanogen bromide to low-temperature reaction to form 4-hydroxy-2-chlorocyanogen amine, and subjecting 4-hydroxy-2-chlorocyanogen amine and bromopropane to ritter reaction to synthesize 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropyl urea which is another key intermediate of lenvatinib; finally, subjecting the two intermediates including 6-formamido-7-methoxy-4-chloroquine and 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropyl urea to alkylation reaction in an alkaline environment to obtain lenvatinib. By the scheme, mild reaction conditions, avoidance of highly toxic reagents, environmentally friendliness and the like are realized.
Owner:IANGSU COLLEGE OF ENG & TECH
Pharmaceutical composition for treatment of gastric cancer and application of pharmaceutical composition for treatment of gastric cancer
InactiveCN105832732AGrowth inhibitionImprove tumor inhibition rateAntineoplastic agentsHeterocyclic compound active ingredientsLenvatinibSide effect
The invention belongs to the technical field of medicines and relates to a pharmaceutical composition for treatment of gastric cancer and application of the pharmaceutical composition for treatment of gastric cancer. The pharmaceutical composition for treatment of gastric cancer is provided to overcome technical defects of strong side effects and poor efficacies of existing medicines for treating gastric cancer and is composed of lenvatinib and rhein. The two medicinal active components have a remarkable synergistic effect on inhibition of in-vivo proliferation of human gastric cancer MGC-803.
Owner:QINGDAO YUNTIAN BIOTECH
Nano-carrier medicine for liver cancer precise targeted treatment and preparation method thereof
InactiveCN108567735AAchieve targeted drug deliveryGood release effectInorganic non-active ingredientsPharmaceutical delivery mechanismLenvatinibTargeted therapy
The invention relates to the field of carrier medicine, and discloses nanometer carrier medicine for liver cancer precise targeted treatment and a preparation method thereof. The method comprises thefollowing preparation processes of (1) adding Lenvatinib raw medicine into methyl alcohol and urea to form a dispersion solution; (2) adding soluble bivalent metal salts, soluble trivalent metal saltand low-concentration hydrogen peroxide solution; performing stirring and heating for condensing backflow reaction; (3) using a sulfamic acid water solution for anionic intercalation modification; after the completion, performing washing and drying by distillation; preparing nanometer grade liver cancer precise targeted carrier medicine. The nanometer carrier medicine prepared by the method has the advantages that the Lenvatinib is used; the modified hydrotalcite has the functional performance of light, electricity, magnetism, biology and the like, the cell barrier targeted medication of the nano-carrier medicine can be effectively realized; the absorption and release control effect is good; the medicine can be widely used for liver cancer treatment.
Owner:CHENDU NEW KELI CHEM SCI CO LTD
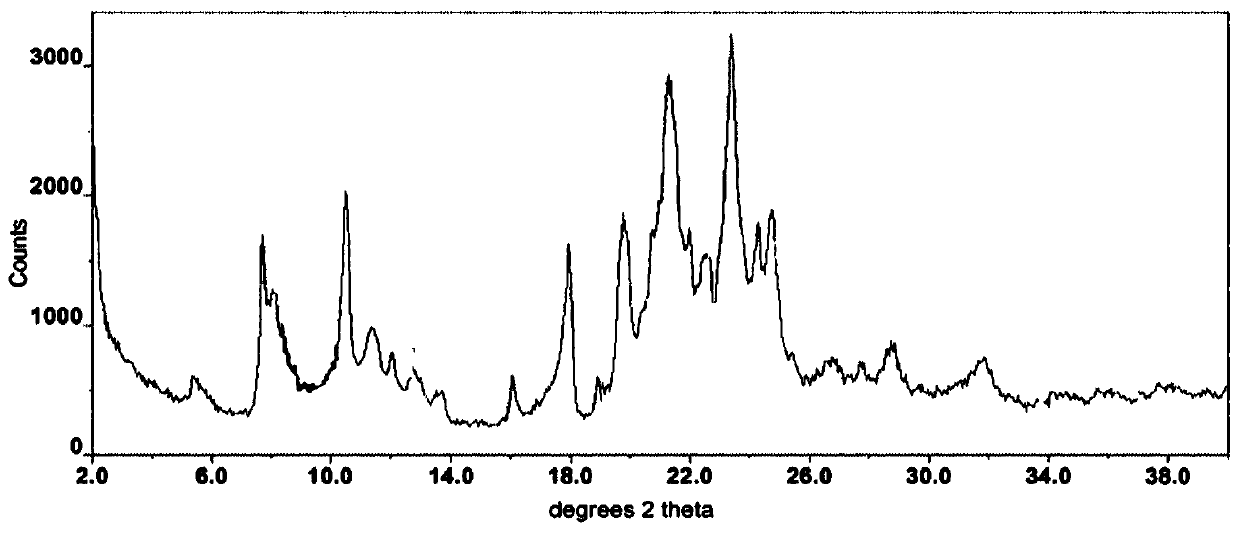
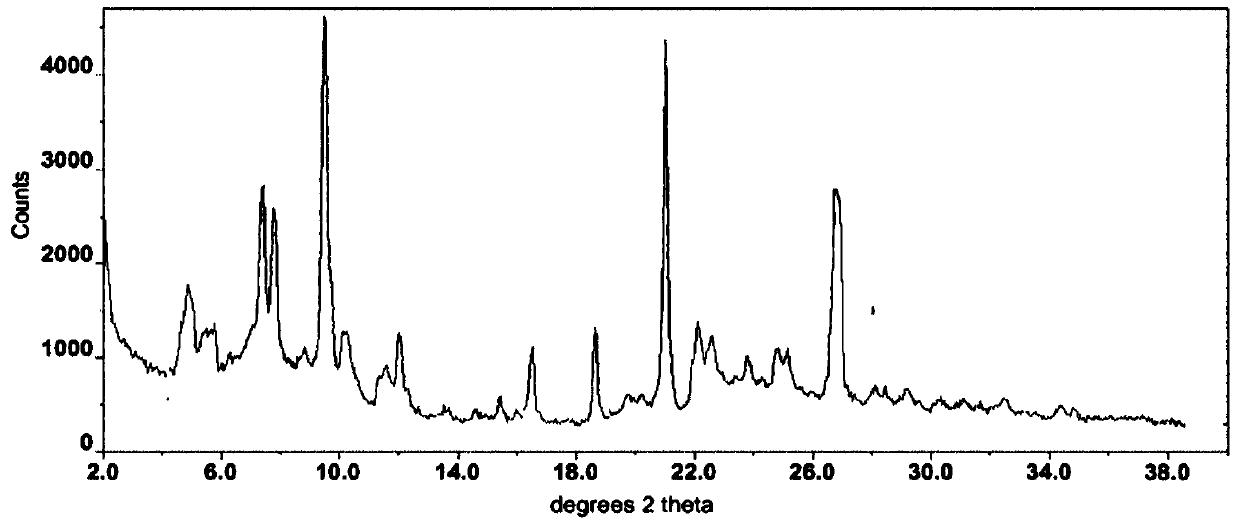
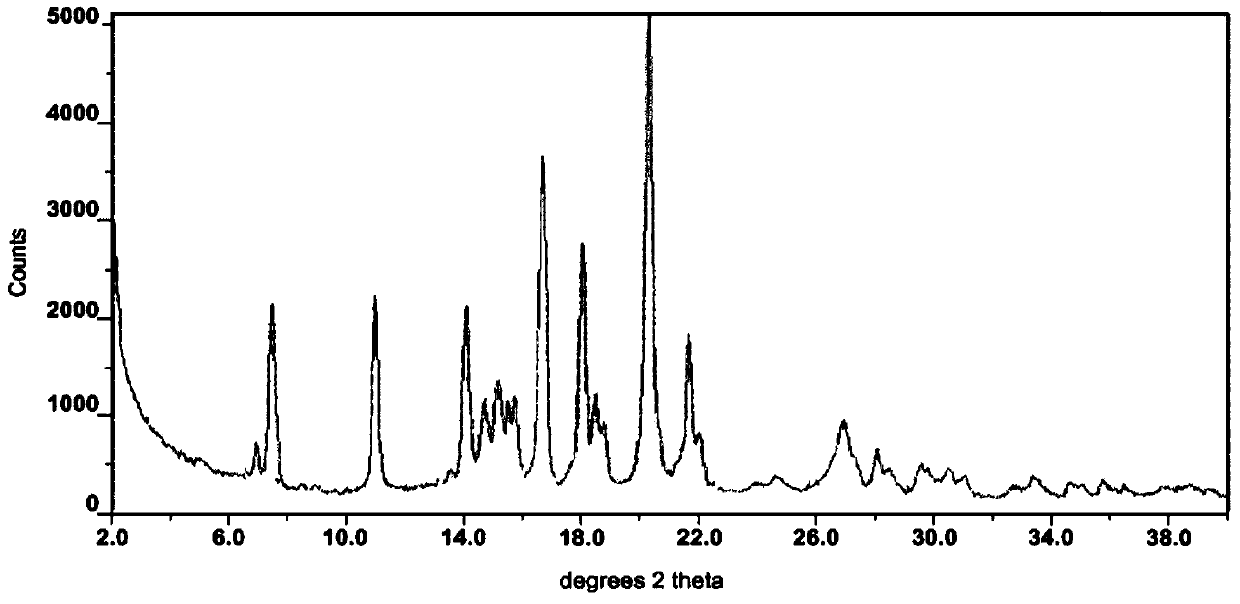
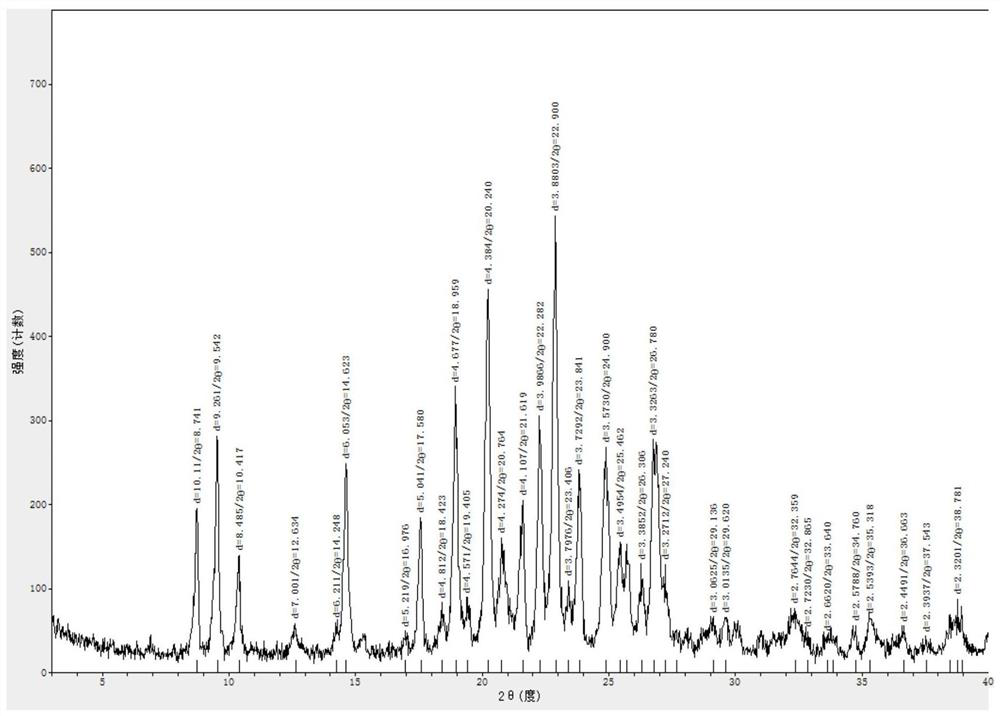
Lenvatinib and p-hydroxybenzoic acid eutectic crystal and preparation method thereof
ActiveCN111233762AFast dissolution rateImprove apparent solubilityAntipyreticOrganic chemistry methodsLenvatinibPhysical chemistry
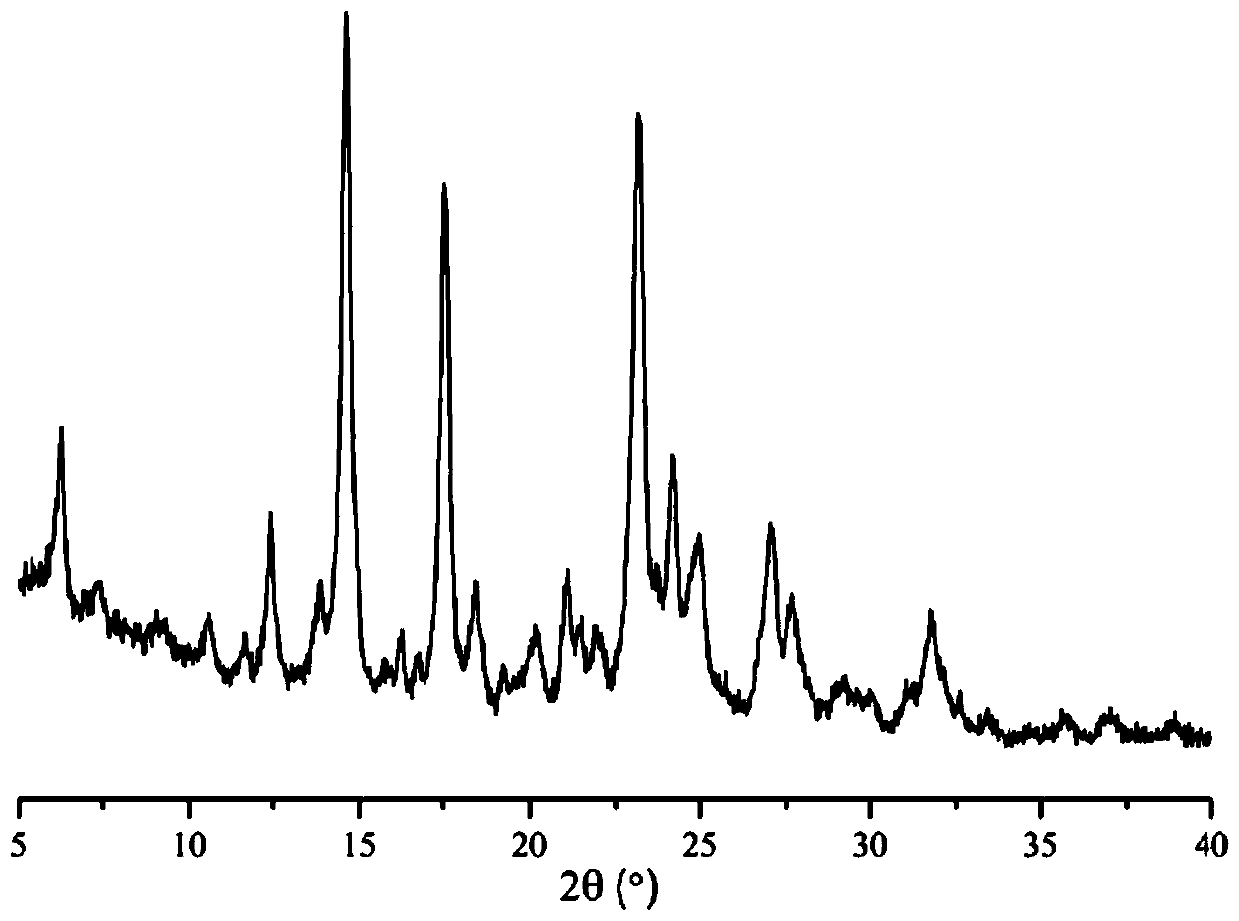
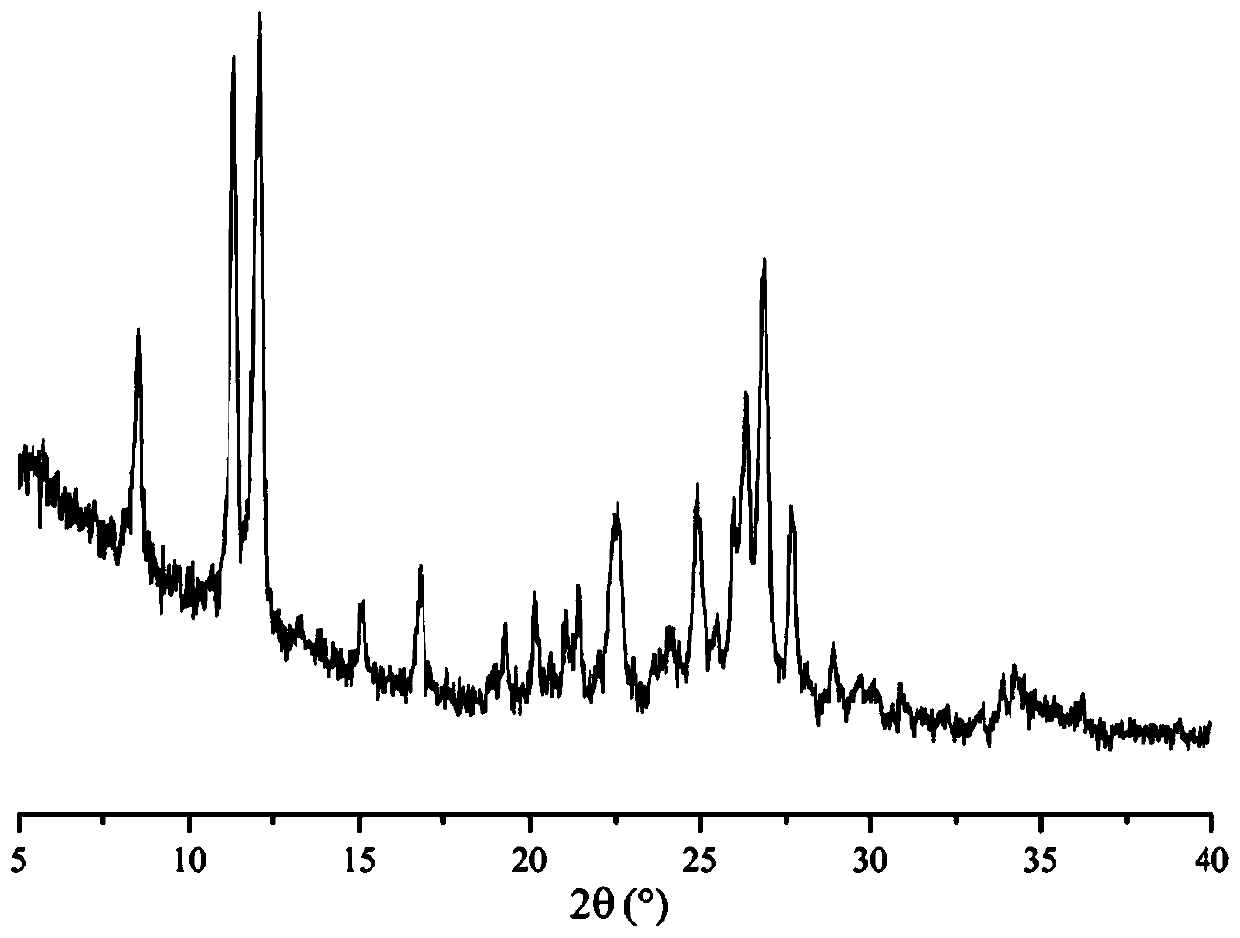
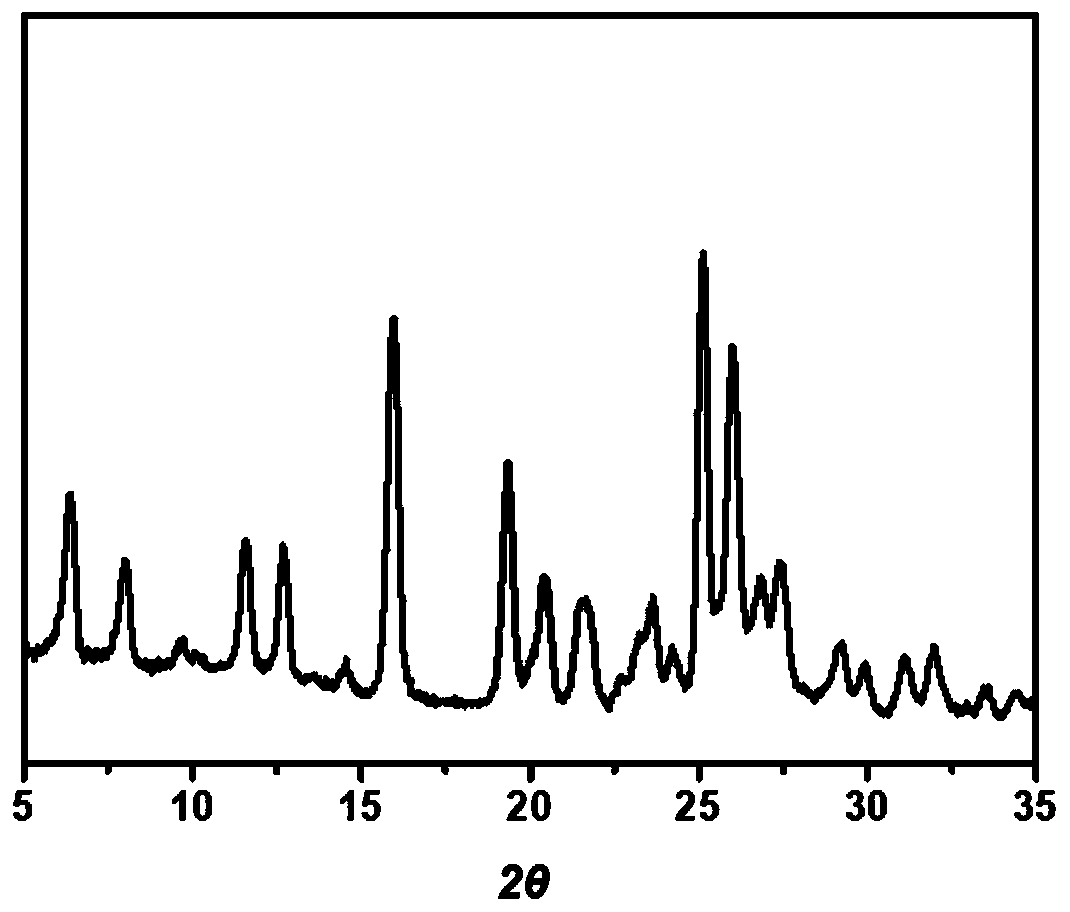
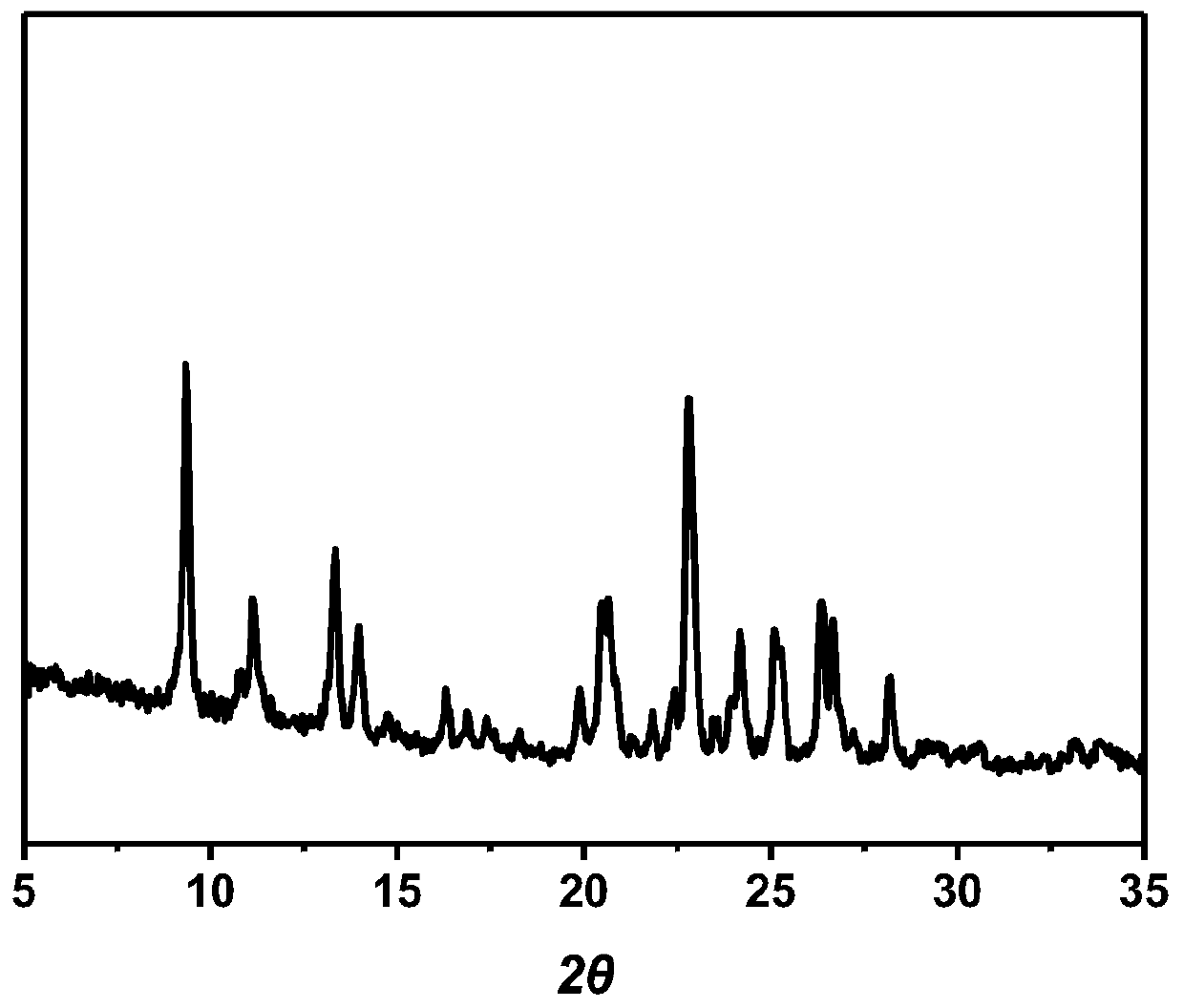
The invention discloses a lenvatinib and p-hydroxybenzoic acid eutectic crystal and a preparation method thereof. The eutectic crystal comprises lenvatinib and p-hydroxybenzoic acid in a molar ratio of 1: 1, an X-ray powder diffraction pattern of the eutectic crystal measured by Cu K alpha rays has characteristic peaks when diffraction angles 2theta are 6.3+ / -0.2 degrees, 10.6+ / -0.2 degrees, 12.4+ / -0.2 degrees, 14.6+ / -0.2 degrees, 17.5+ / -0.2 degrees and 18.4+ / -0.2 degrees. According to the preparation method, the lenvatinib is converted into a brand-new lenvatinib and p-hydroxybenzoic acid eutectic crystal for the first time, and the lenvatinib and p-hydroxybenzoic acid eutectic crystal has a relatively high dissolution rate and relatively high apparent solubility. The preparation method of the lenvatinib and p-hydroxybenzoic acid eutectic crystal is simple in process, easy to control the crystallization process, good in reproducibility, suitable for industrial production and wide in application prospect.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Lenvatinib intermediate, preparation thereof and preparation of lenvatinib
InactiveCN108997214AReduce usageSimple process routeUrea derivatives preparationOrganic compound preparationLenvatinibOrganic solvent
The invention relates to the field of drug synthesis and discloses a lenvatinib key intermediate 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea, preparation thereof and preparation of lenvatinib. Thelenvatinib intermediate has purity higher than 99% and is prepared by the following steps: (1) cyclopropylamine is subjected to reaction with a compound N,N'-carbonyldiimidazole in a solvent, and N-cyclopropyl-1H-imidazole-1-formamide is obtained; (2) N-cyclopropyl-1H-imidazole-1-formamide and 4-amino-3-chlorophenol are subjected to reaction, and 1-(2-chloro-hydroxyphenyl)-cyclopropylurea is obtained. The intermediate 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea and 4-chloro-7-methoxyquinoline-6-carboxamide are subjected to reaction, and a final product lenvatinib is obtained. The preparation methods are simple to operate, raw materials are cheap and easily available, few difficult-to-remove impurities are produced, water is directly used as a solvent, an organic solvent is not needed, the purity of the product can be higher than 99% with low environmental pollution and simple post-processing, and the methods are green, environmentally friendly, economic and suitable for large-scaleindustrial production.
Owner:CHENGDU DIAO PHARMA GROUP
Lervatinib-gallic acid eutectic crystal form and application thereof
PendingCN111574359AEfficiently obtainedEasy to operateOrganic chemistry methodsAntineoplastic agentsLenvatinibGallic acid ester
The invention discloses a lenvatinib-gallic acid eutectic crystal form and application thereof. The eutectic crystal is composed of a compound as shown in a formula (I) and a compound as shown in a formula (II). The eutectic crystal can be used for inhibiting vascular endothelial growth factor receptors, fibroblast growth factor receptors, platelet-derived growth factor receptors and proto-oncogenes, and can be used for preventing and / or treating thyroid cancer or liver cancer.
Owner:NOVAGENESIS THERAPEUTIX SUZHOU LTD
Lenvatinib medicine composition and preparation method thereof
ActiveCN112190583AImprove applicabilityImprove medication complianceDigestive systemInorganic non-active ingredientsLenvatinibBile Juice
The invention provides a new Lenvatinib medicine composition and a preparation method thereof. The medicine composition contains Lenvatinib or pharmaceutically acceptable salt of the Lenvatinib and ananti-acid anti-bile gastric mucosa protective agent, wherein the protective agent is hydrotalcite. The medicine composition can fully guarantee that a product is dissolved out, medicine degradation is avoided, and the quality of the product is more favorably guaranteed.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
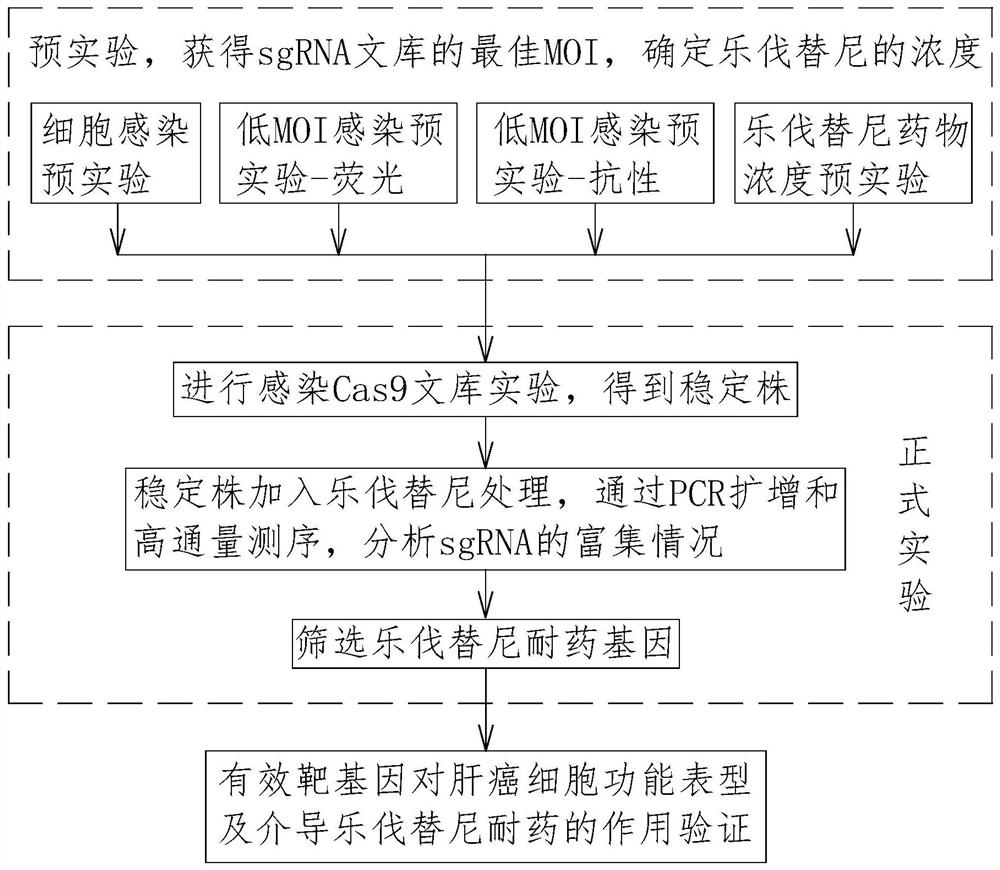
Lenvatinib drug-resistant gene NF1 as well as screening method and application thereof
PendingCN113005125ADelay drug resistanceMicrobiological testing/measurementNucleic acid vectorLenvatinibTarget gene
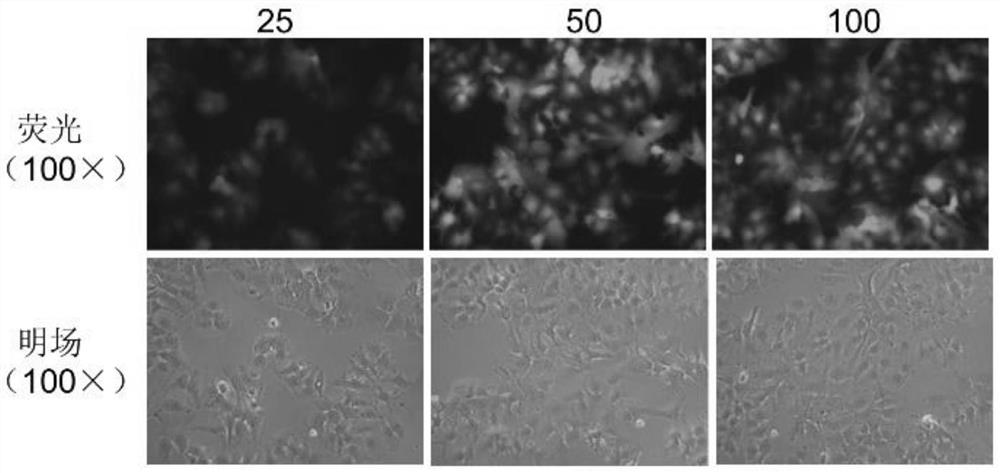
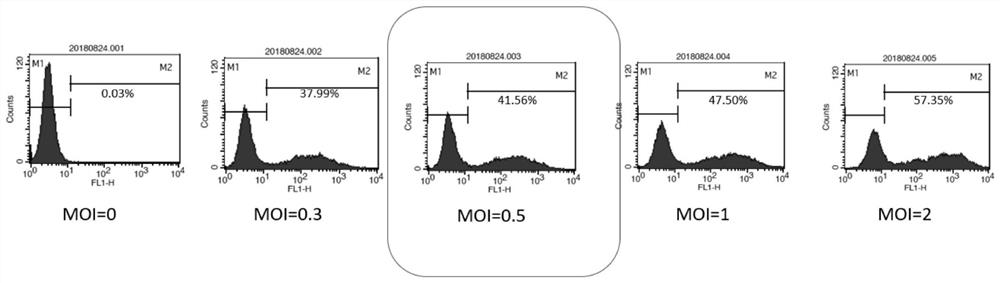
The invention relates to the technical field of tumor drug-resistant genes, in particular to a lenvatinib drug-resistant gene NF1 as well as a screening method and application thereof. The gene sequence of the NF1 is SEQ ID NO: 1. The screening method comprises the following steps: carrying out a pre-experiment to obtain the optimal MOI of the sgRNA library, and determining the concentration of lenvatinib; carrying out an infection Cas9 library experiment to obtain a stable strain; adding lenvatinib into the stable strain for treatment, and analyzing the enrichment condition of the sgRNA through PCR amplification and high-throughput sequencing; screening a drug-resistant gene of lenvatinib; and verifying the function of the effective target gene on liver cancer cell function phenotype and mediated lenvatinib drug resistance. The drug-resistant gene of the lenvatinib is obtained through CRISPR / Cas9 whole-genome library screening, a theoretical basis is provided for reducing the drug resistance of the lenvatinib in the future, a new drug-resistant target is provided for clinical application of the lenvatinib, and guidance is provided for clinical reasonable medication.
Owner:柳州市柳铁中心医院
Use of eribulin and lenvatinib as combination therapy for treatment of cancer
ActiveUS9549922B2Reduce in quantityPrevent and delay occurrenceAntineoplastic agentsHeterocyclic compound active ingredientsDiseaseLenvatinib
The invention provides methods and compositions for use in treating diseases associated with excessive cellular proliferation, such as cancer.
Owner:EISIA R&D MANAGEMENT CO LTD
Lenvatinib impurity and preparation method thereof
PendingCN112409255AQualitatively accurateQuantitatively accurateOrganic chemistryLenvatinibQuantitative determination
The present invention discloses a lenvatinib impurity and a preparation method thereof. The lenvatinib impurity has a structure represented by a formula VI, the impurity uses 2-fluoro-4-hydroxyanilineas a starting material, a lenvatinib impurity intermediate represented by a formula V is prepared through a two-step alkylation reaction, and then the lenvatinib impurity represented by the formula VI is prepared through a condensation reaction. The purity and yield of the prepared impurity are stable, the impurity can be used for quality control of lenvatinib intermediates, bulk drugs and related preparation products, and the quality VI of the bulk drugs and the related preparation products is improved by qualitative and quantitative determination of specific impurities.
Owner:南京方生和医药科技有限公司
Process for the preparation of lenvatinib
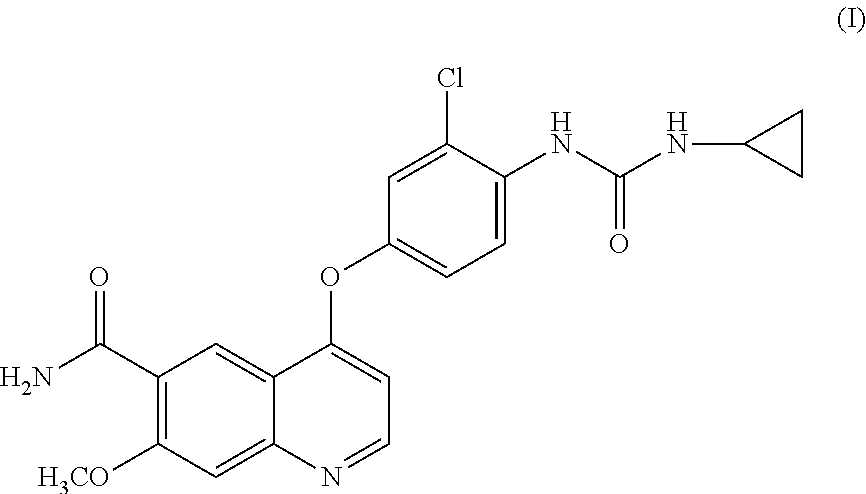
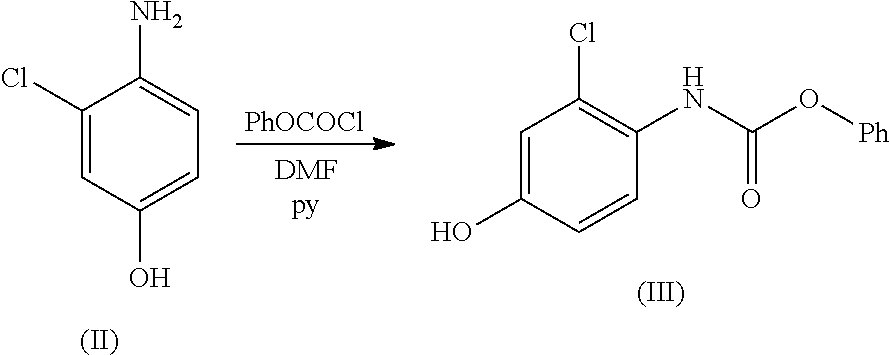
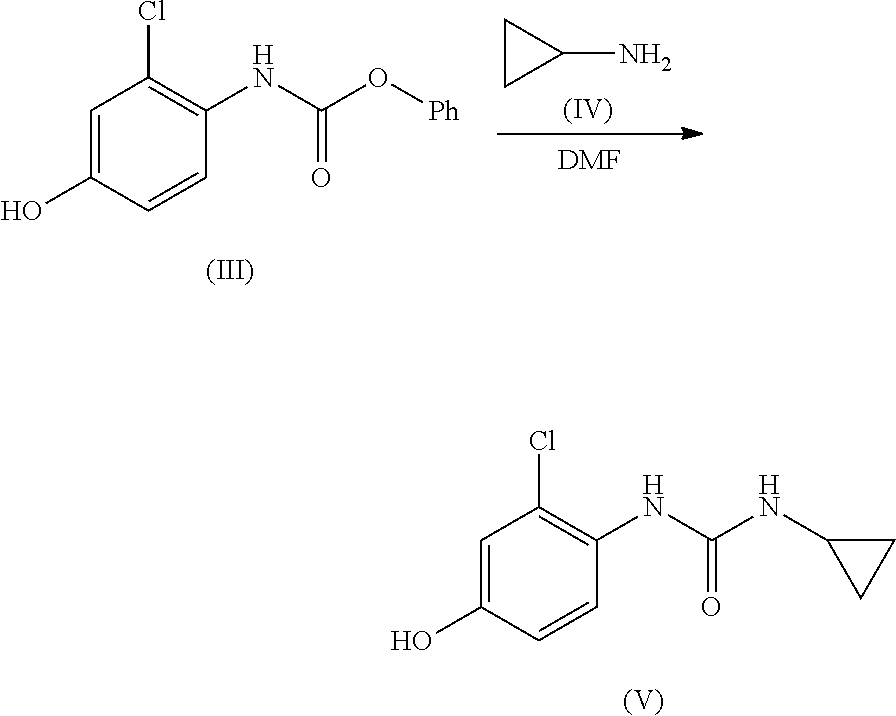
ActiveUS20210246107A1Organic chemistry methodsHeterocyclic compound active ingredientsLenvatinibBiochemical engineering
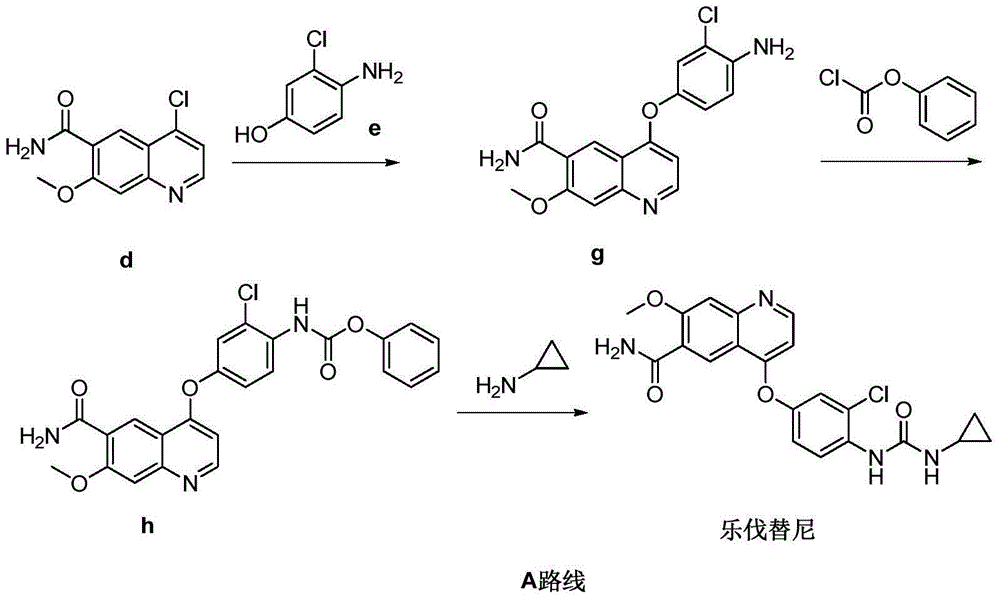
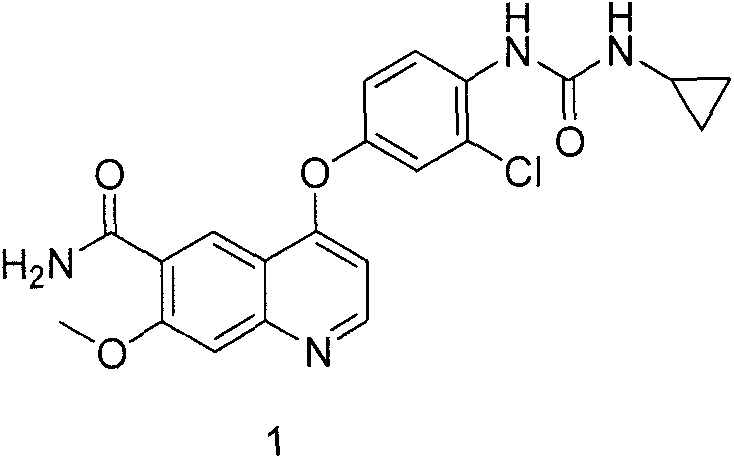
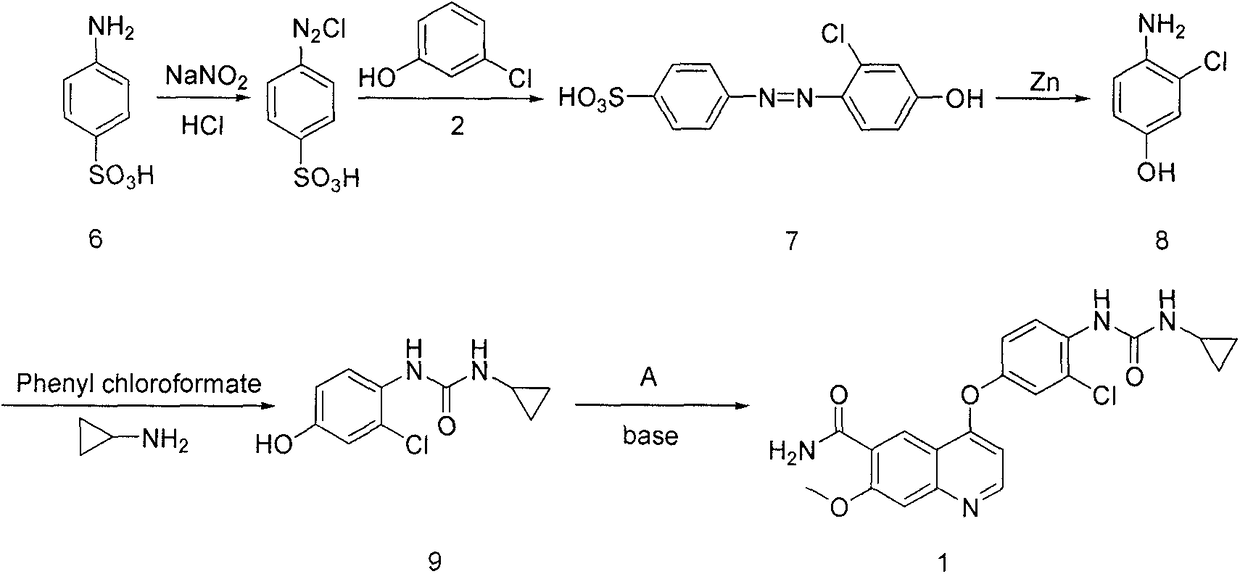
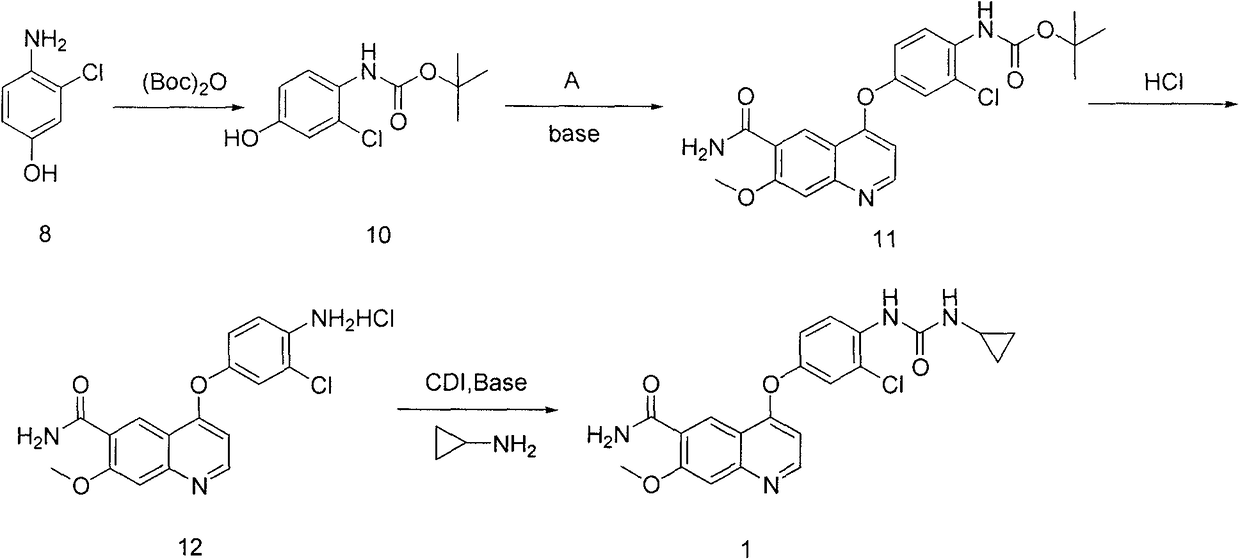
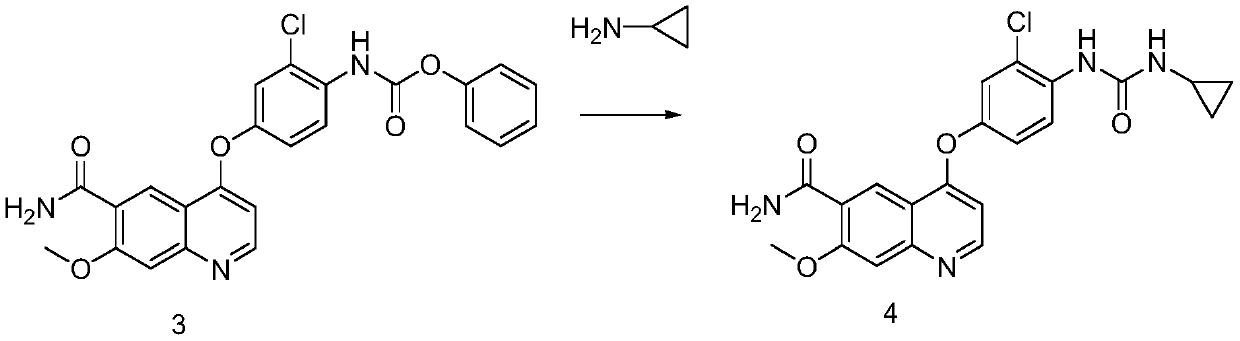
The present invention relates to a process for the preparation of Lenvatinib of formula (I) from 4-amino-3-chloro-phenol and 4-chloro-7-methoxyquinoline-6-carboxamide.
Owner:INDENA SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com