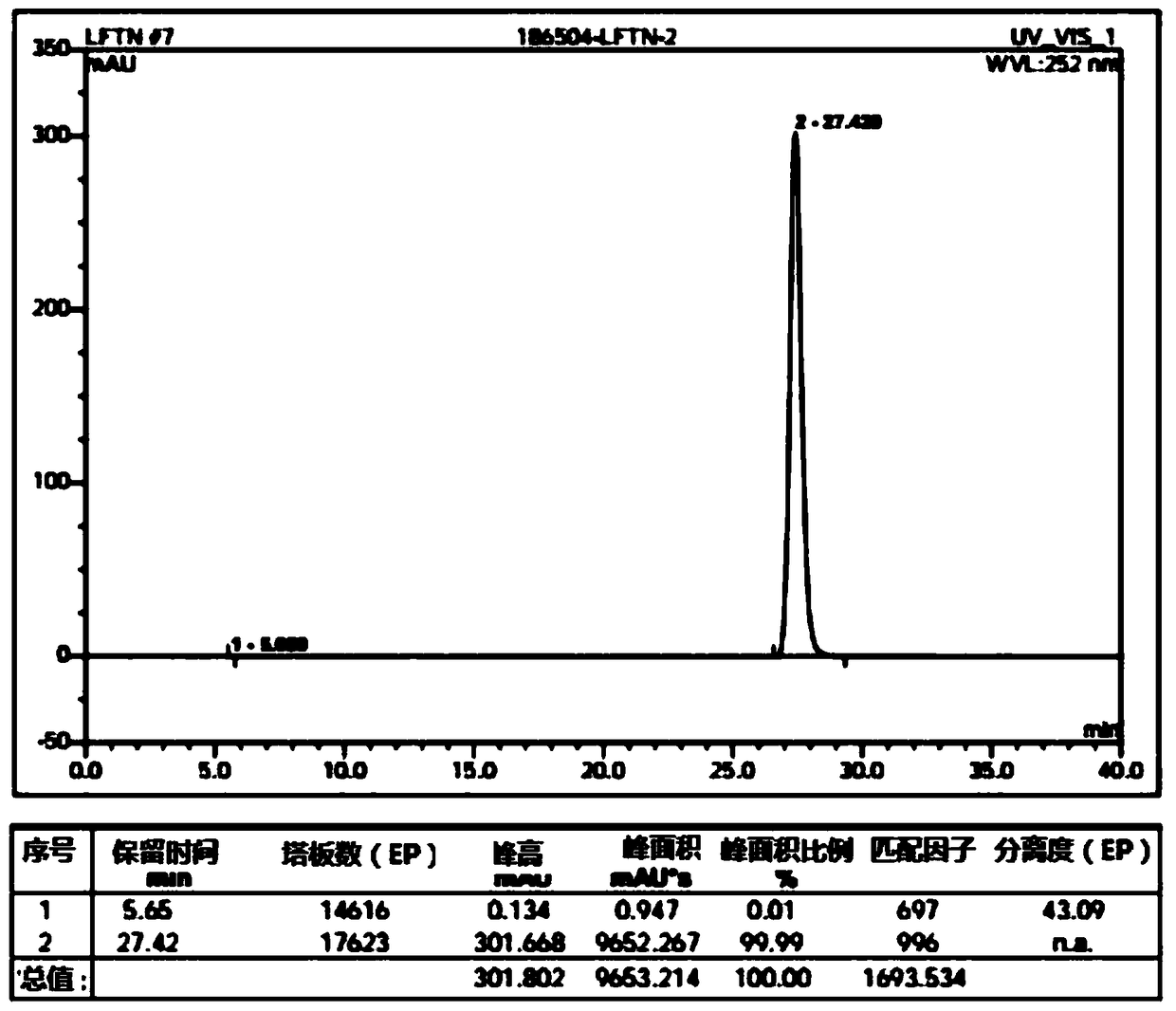
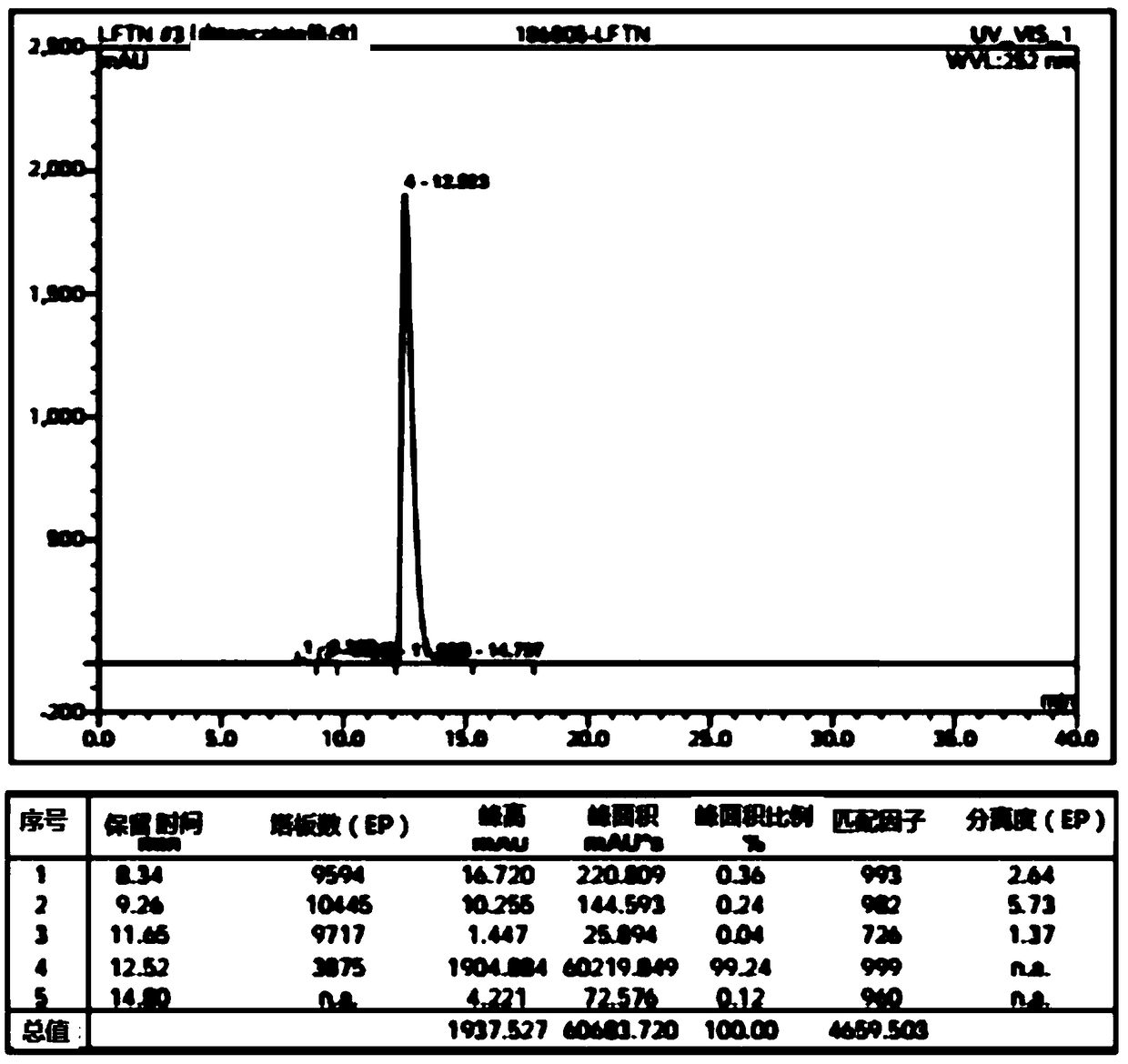
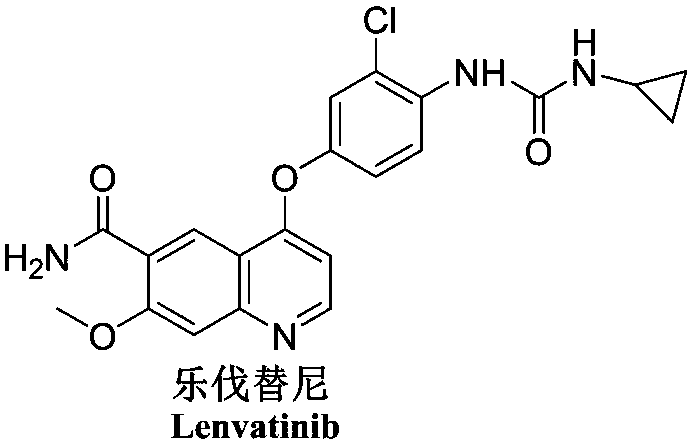
Lenvatinib intermediate, preparation thereof and preparation of lenvatinib
A technology of lenvatinib and intermediates, applied in the field of pharmaceutical chemical synthesis, to achieve the effects of cheap and easy-to-obtain reaction raw materials, avoid the use of organic solvents, and simplify the process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] (1) Compared with the reagent phenyl chloroformate used in the preparation of the existing lenvatinib intermediate 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (compound E), the present invention The reagent CDI (N,N'-carbonyldiimidazole) used is more environmentally friendly and cheap and easy to obtain. And it is also possible to directly use water as a solvent, and directly precipitate 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea with a purity greater than 98.5% from the water phase after the reaction, avoiding the reaction process and after-treatment The use of a large amount of organic solvents in the treatment process is more environmentally friendly.
[0066] (2) react with compound CDI earlier by cyclopropylamine and then react with 4-amino-3-chlorophenol to obtain key intermediate E, and then react with 4-chloro-7-methoxyquinoline-6-formamide (compound F) Condensation can greatly reduce the side reactions caused by the fact that 4-amino-3-chlorophenol has ...
Embodiment 1
[0074] Example 1: Synthesis of 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (compound E)
[0075] Step 1: Synthesis of N-cyclopropyl-1H-imidazole-1-carboxamide (Compound I)
[0076]
[0077] In a 250ml round-bottomed flask, add cyclopropylamine (10g, 0.175mol) and 50ml of water as a solvent, place it in an ice bath, add CDI (51.08g, 0.315mol) in three batches, after the addition is complete, continue at 0°C After reacting for 1 h, TLC monitored that the reaction was complete, and the reaction mixture was directly carried out to the next reaction without any post-treatment.
[0078] Step 2: Synthesis of 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (compound E)
[0079]
[0080] To the untreated N-cyclopropyl-1H-imidazole-1-carboxamide (Compound I) aqueous solution obtained in Step 1, directly add raw material 4-amino-3-chlorophenol (Compound A) (17.7g, 0.123mol) , the reaction mixture was heated in an oil bath at 78°C, and after 4 hours, TLC monitored that the react...
Embodiment 2
[0081]Example 2: Synthesis of 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (compound E)
[0082] Step 1: Synthesis of N-cyclopropyl-1H-imidazole-1-carboxamide (Compound I)
[0083]
[0084] In a 250ml round-bottomed flask, add cyclopropylamine (10g, 0.175mol) and 30ml of water as a solvent, place it in an ice bath, add CDI (51.08g, 0.315mol) in three batches, after the addition is complete, continue at 25°C After reacting for 1 h, TLC monitored that the reaction was complete, and the reaction mixture was directly carried out to the next reaction without any post-treatment.
[0085] Step 2: Synthesis of 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (compound E)
[0086]
[0087] To the untreated N-cyclopropyl-1H-imidazole-1-carboxamide (Compound I) aqueous solution obtained in Step 1, directly add raw material 4-amino-3-chlorophenol (Compound A) (17.7g, 0.123mol) , the reaction mixture was heated in an oil bath at 78°C, and after 4 hours, TLC monitored that the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com