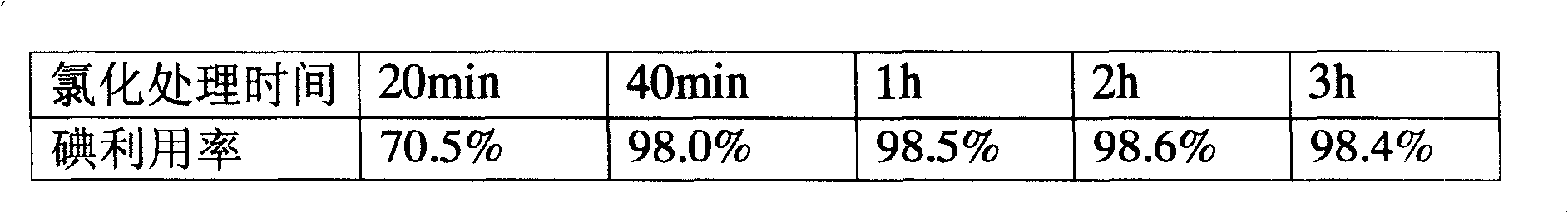
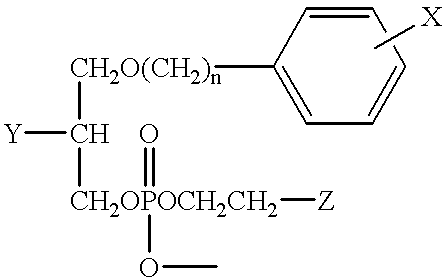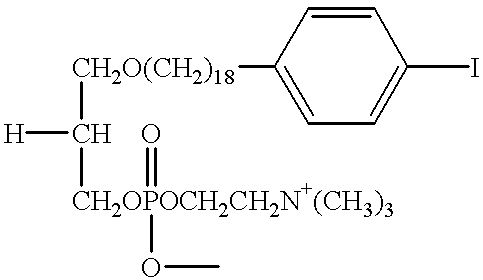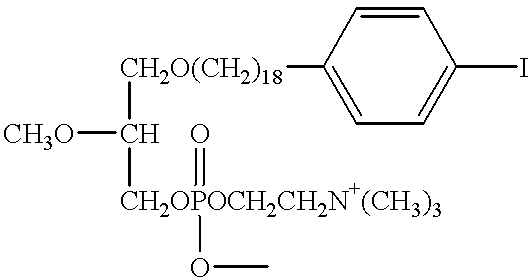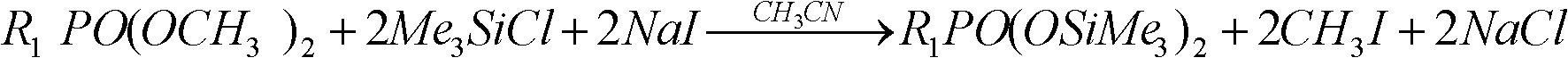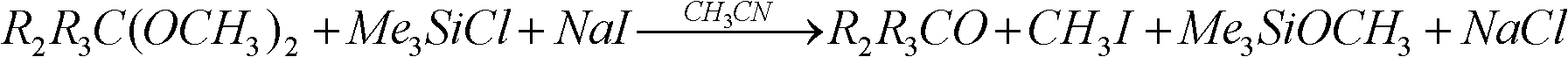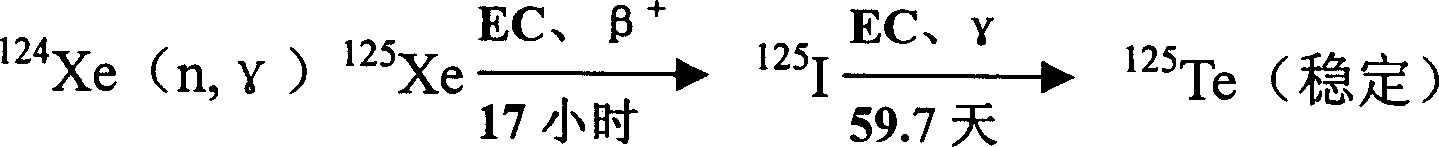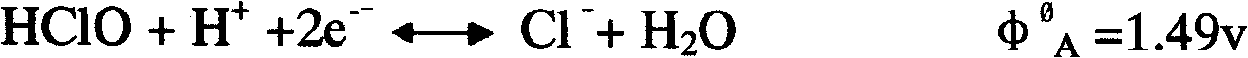Patents
Literature
240 results about "Radioactive iodine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Radioiodinated phospholipid ether analogs and methods of using the same
InactiveUS20020065429A1Radioactive preparation carriersIsotope introduction to organic compoundsHalf-lifeCancer therapy
The present invention provides improved radioiodinated phospholipid ether analogs which demonstrate significant tumor avidity and longer plasma half-life than shorter-chain analogs. The radioiodinated phospholipid ether analogs of the present invention provide superior imaging and visualization of neoplastic lesions and tumor-specific cytotoxic cancer therapy.
Owner:RGT UNIV OF MICHIGAN
Methods for treating thyroid cancer
The invention provides methods for enhancing iodine absorption in a thyroid in a subject and treating thyroid cancer by administering to the subject a composition which includes a multi-kinase inhibitor. Furthermore, the invention provides methods for improving a medical diagnostic procedure based on radioactive iodine in a subject by administering to the subject a composition comprising a multi-kinase inhibitor.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
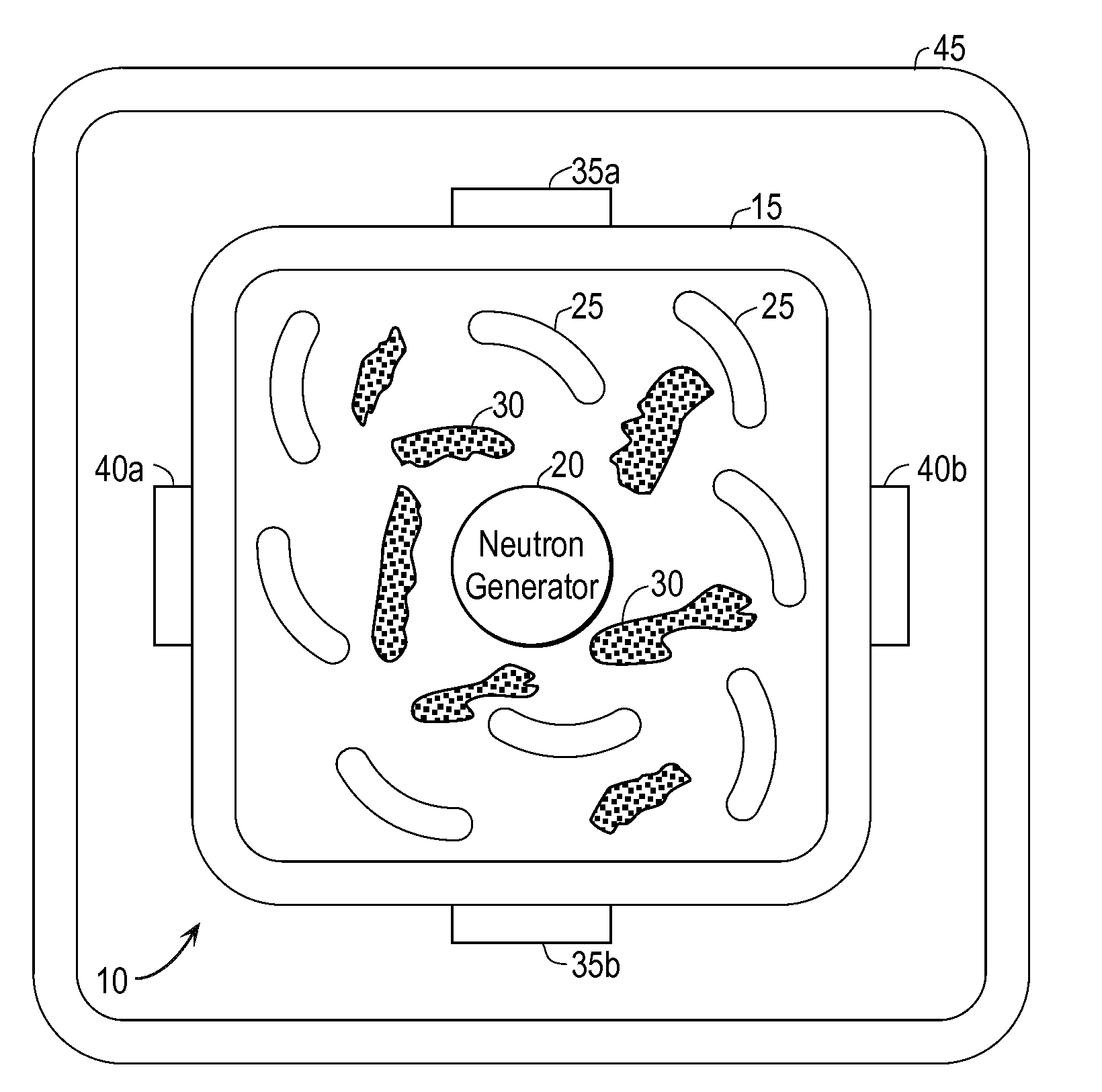
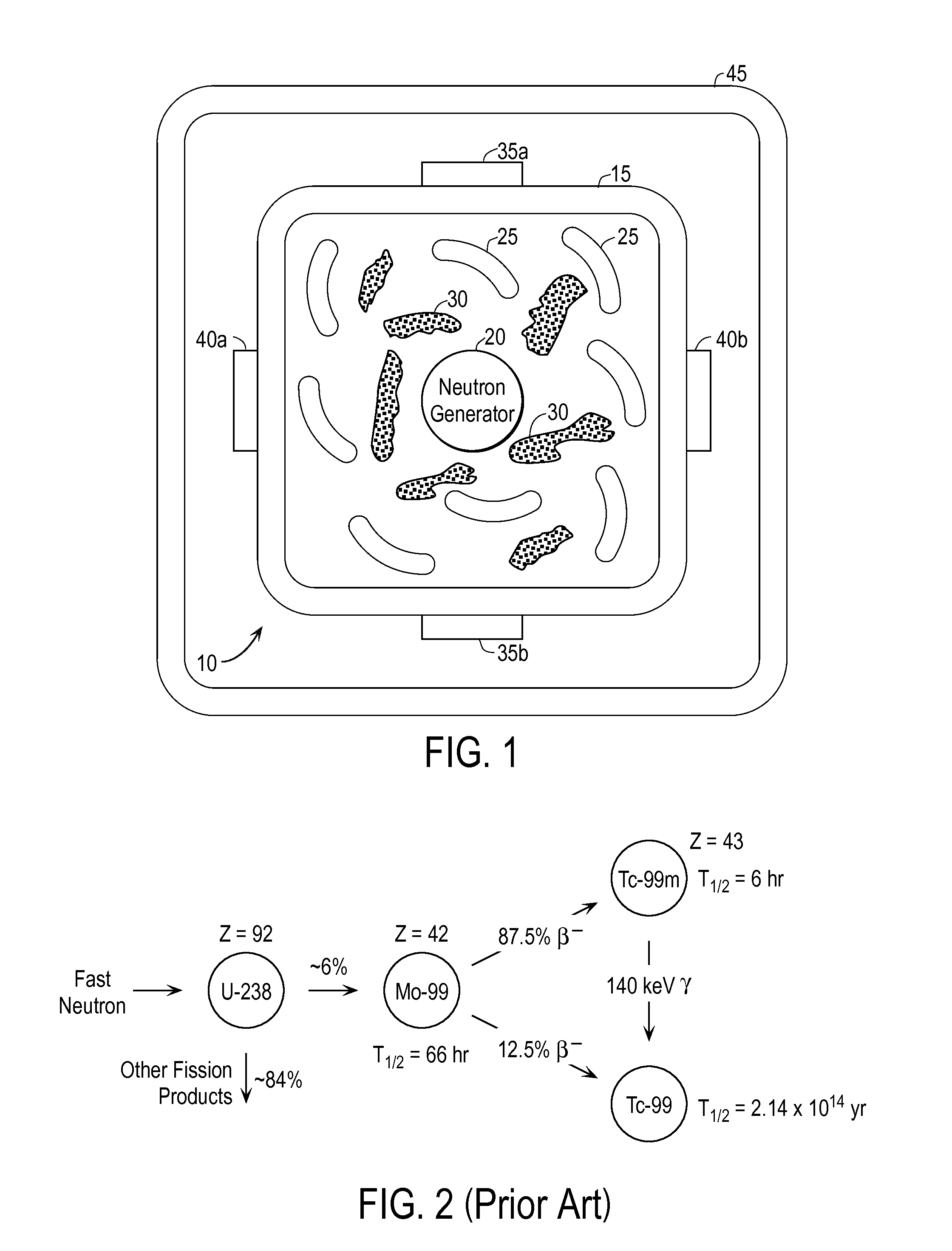
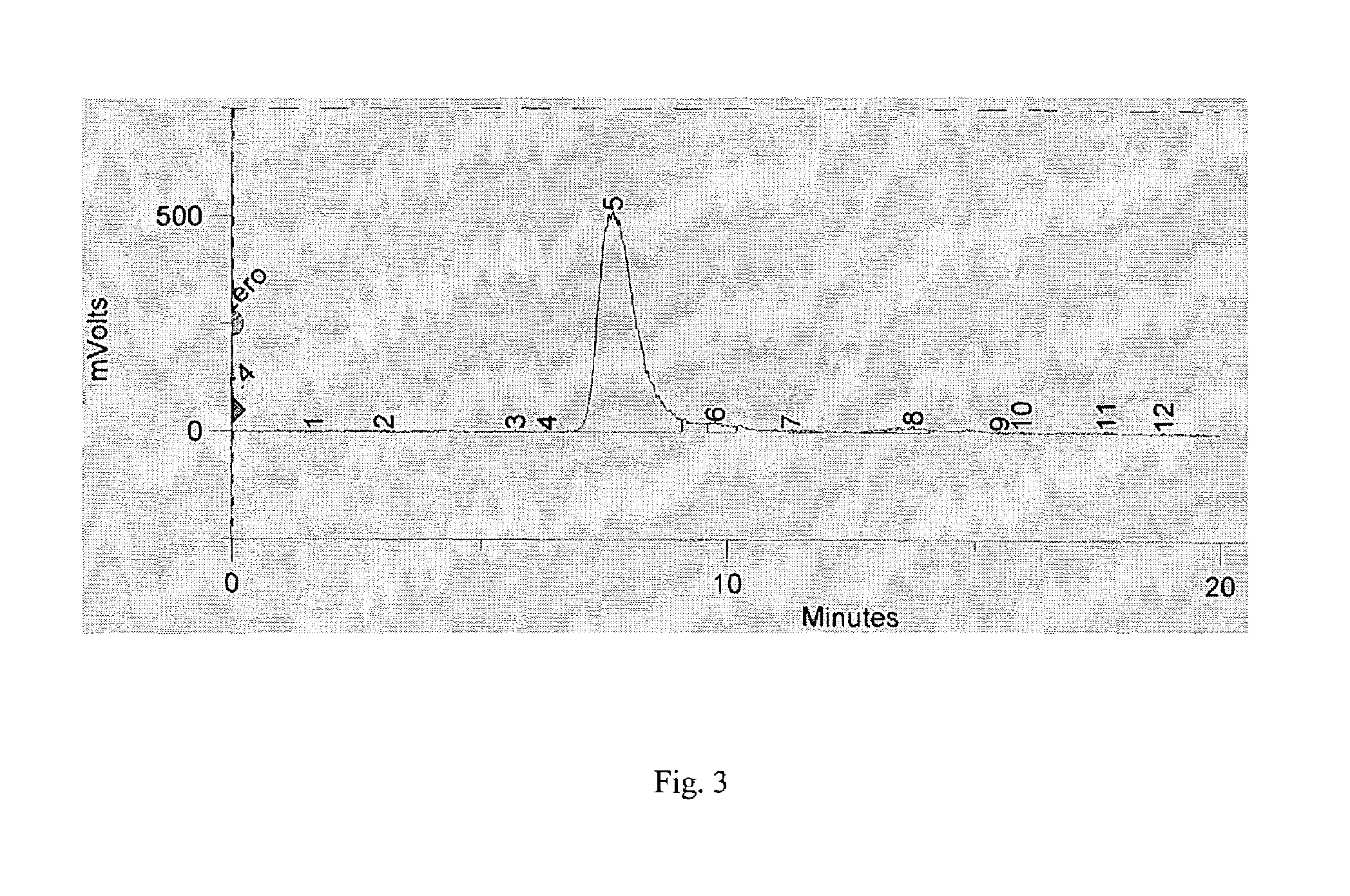
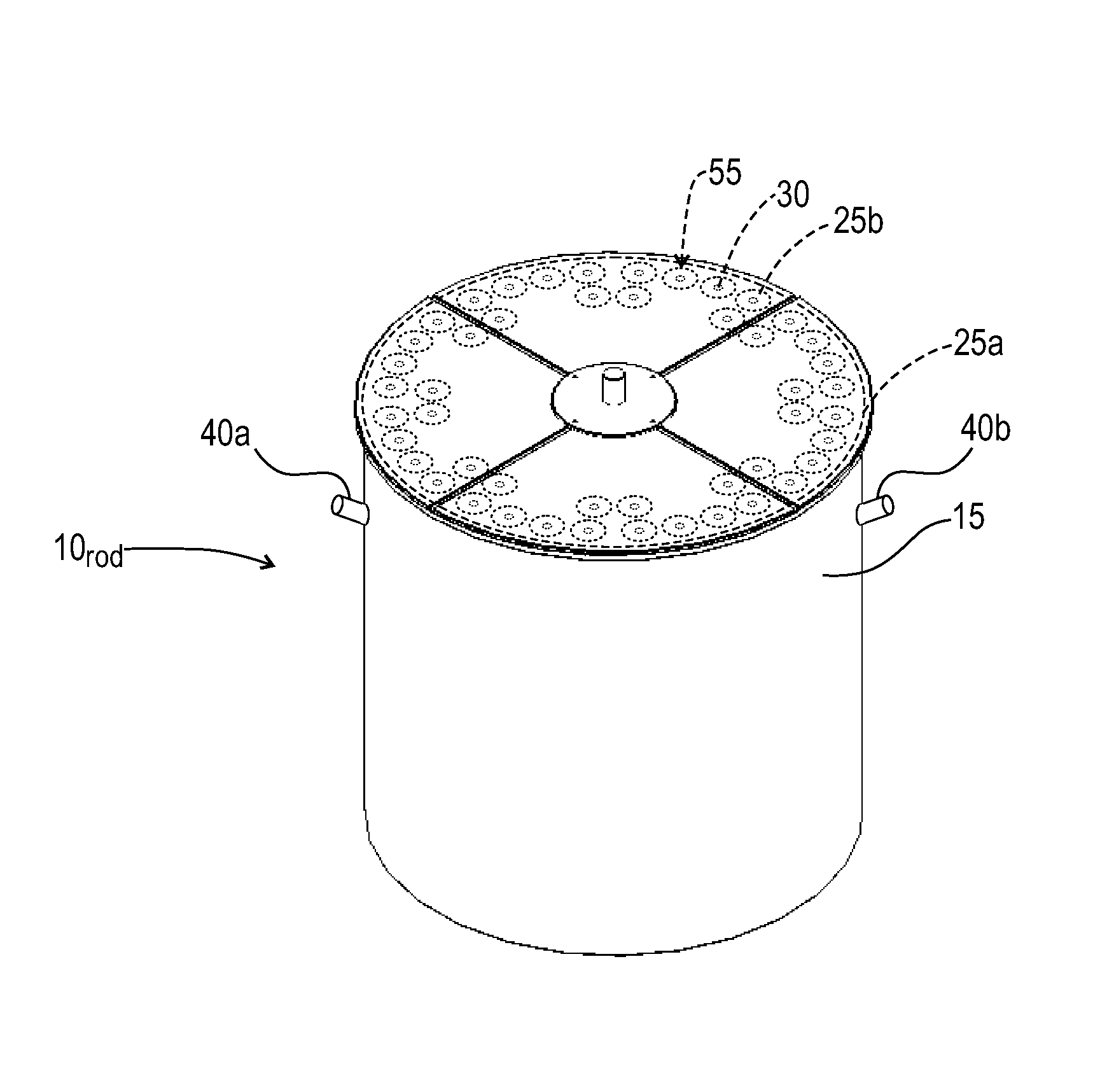
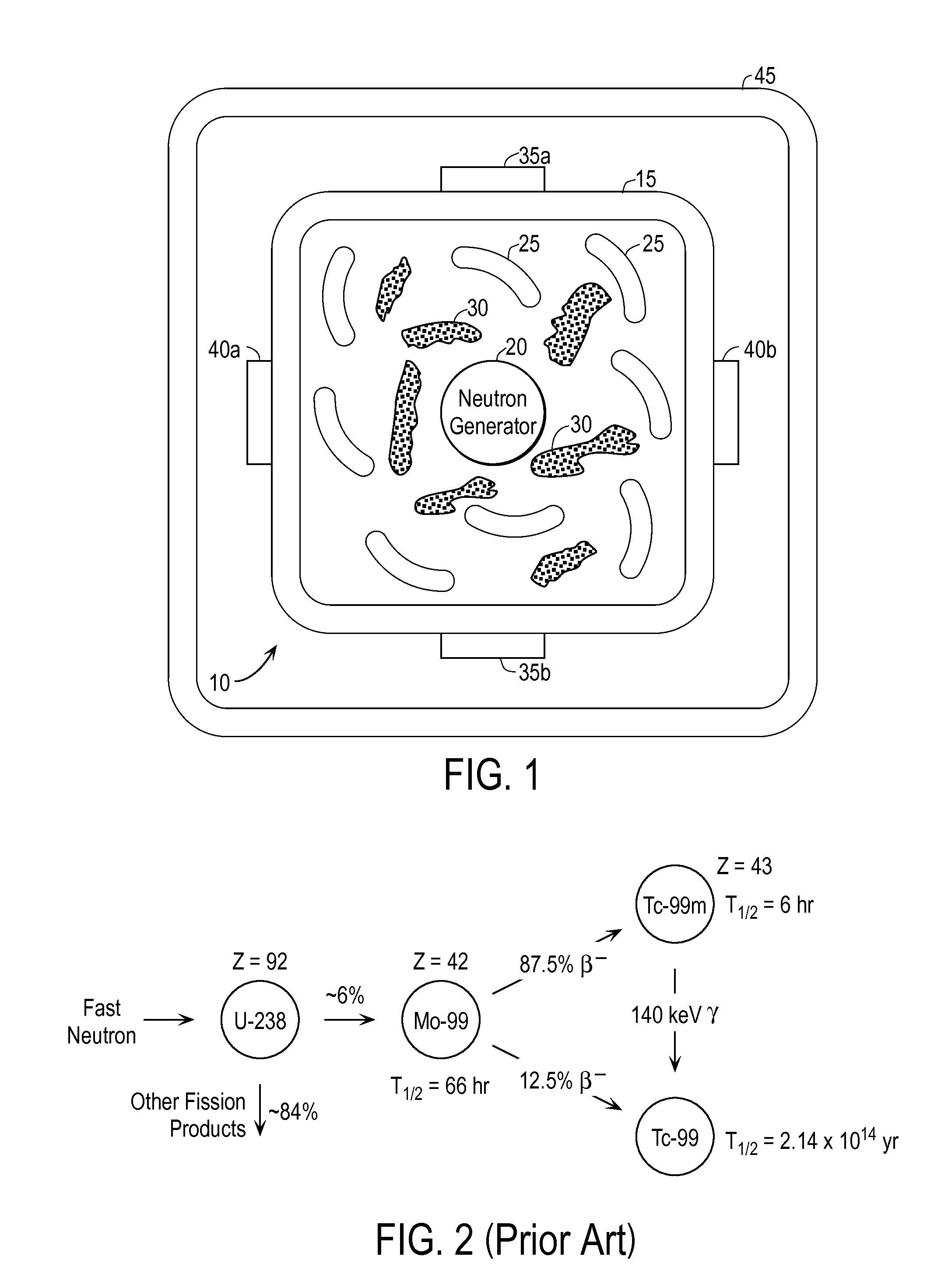
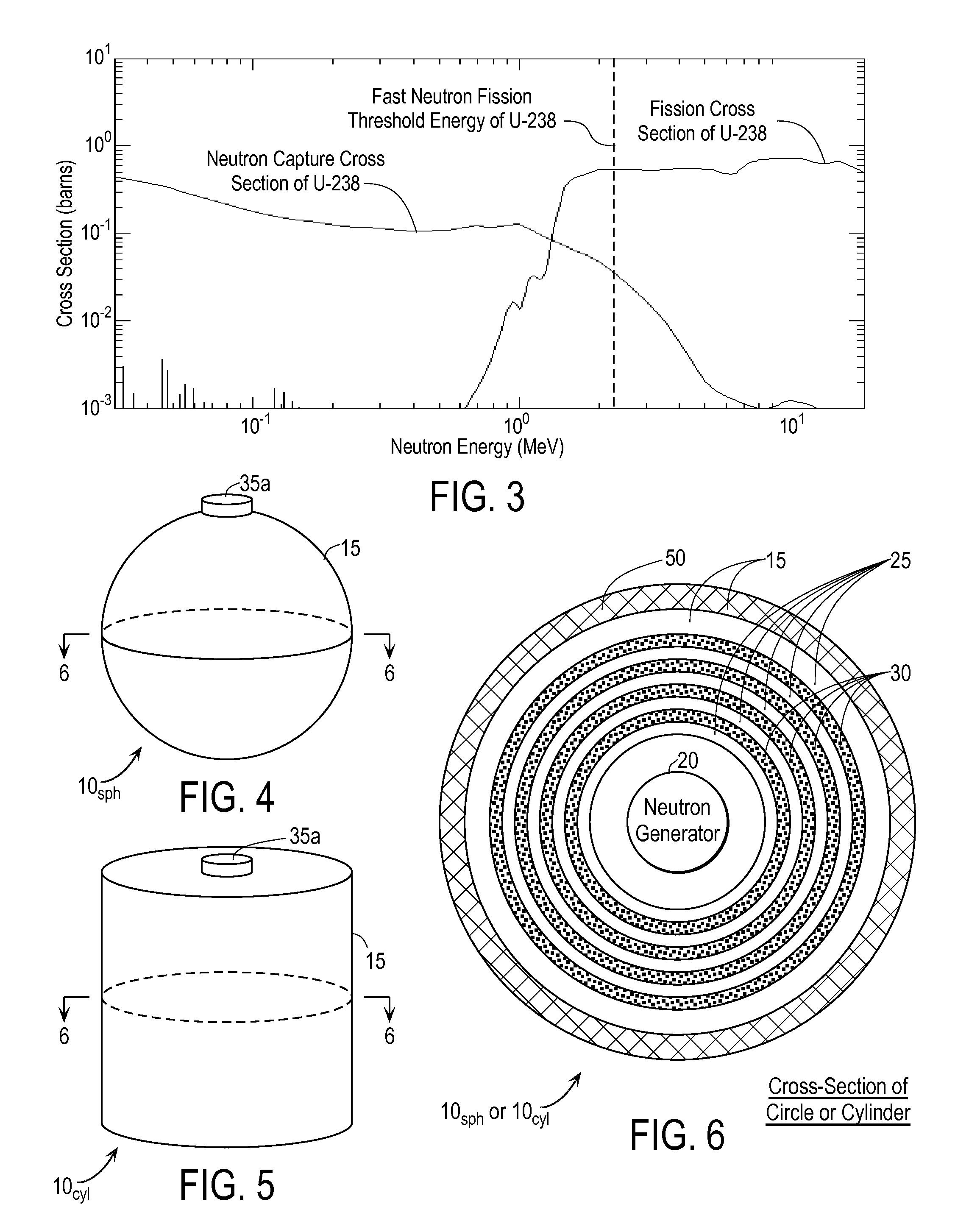
Techniques for On-Demand Production of Medical Isotopes Such as Mo-99/Tc-99m and Radioactive Iodine Isotopes Including I-131
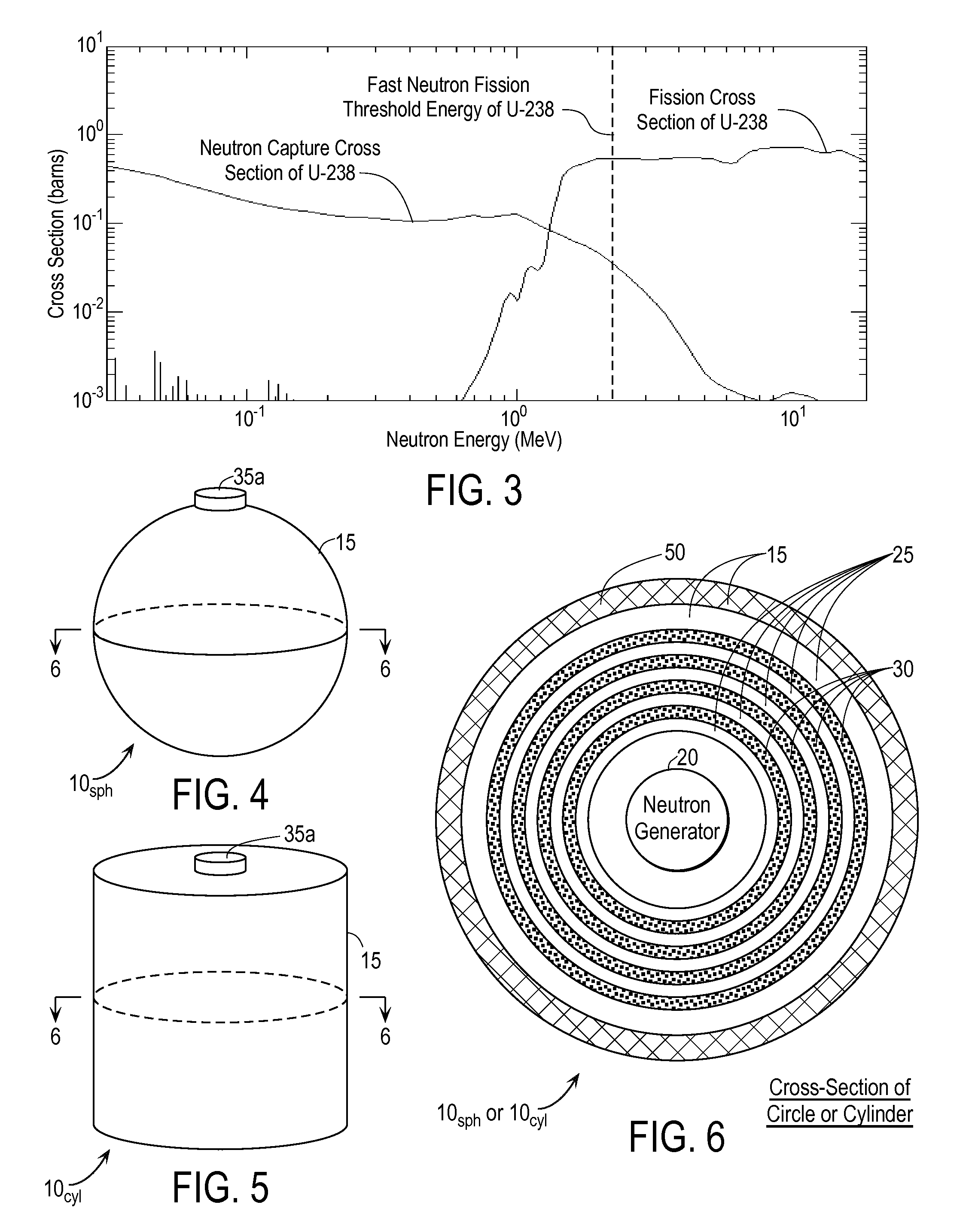
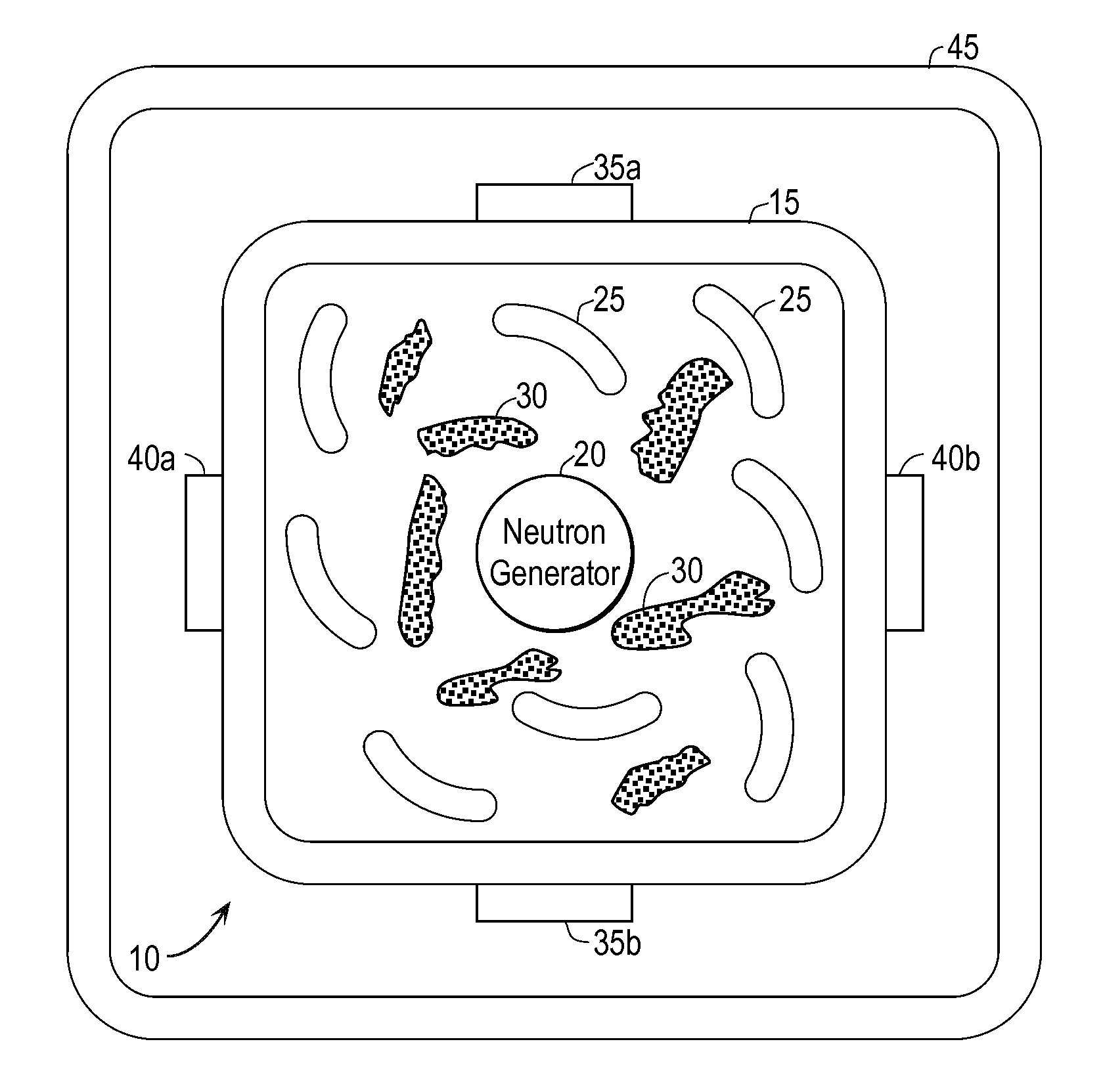
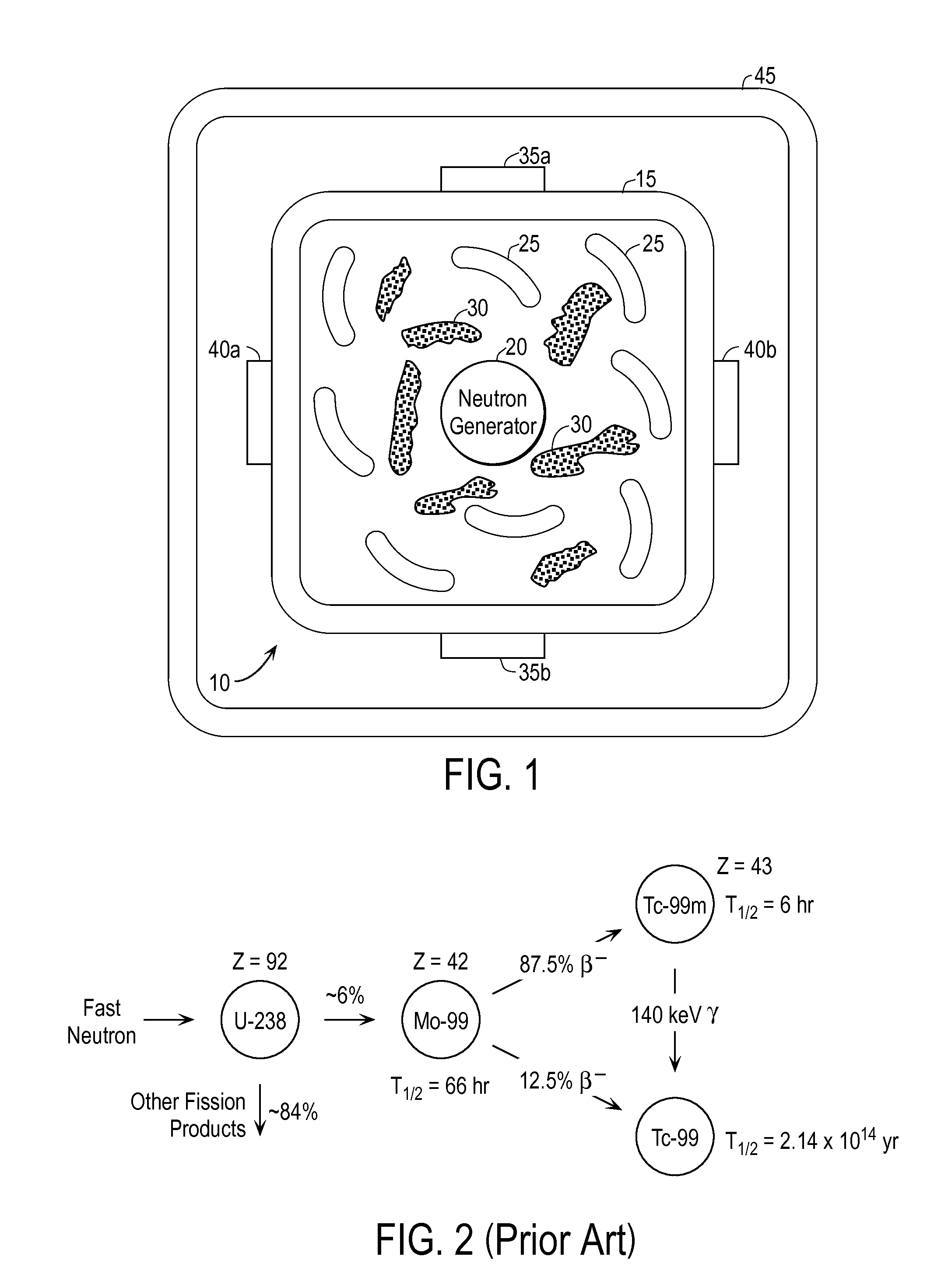
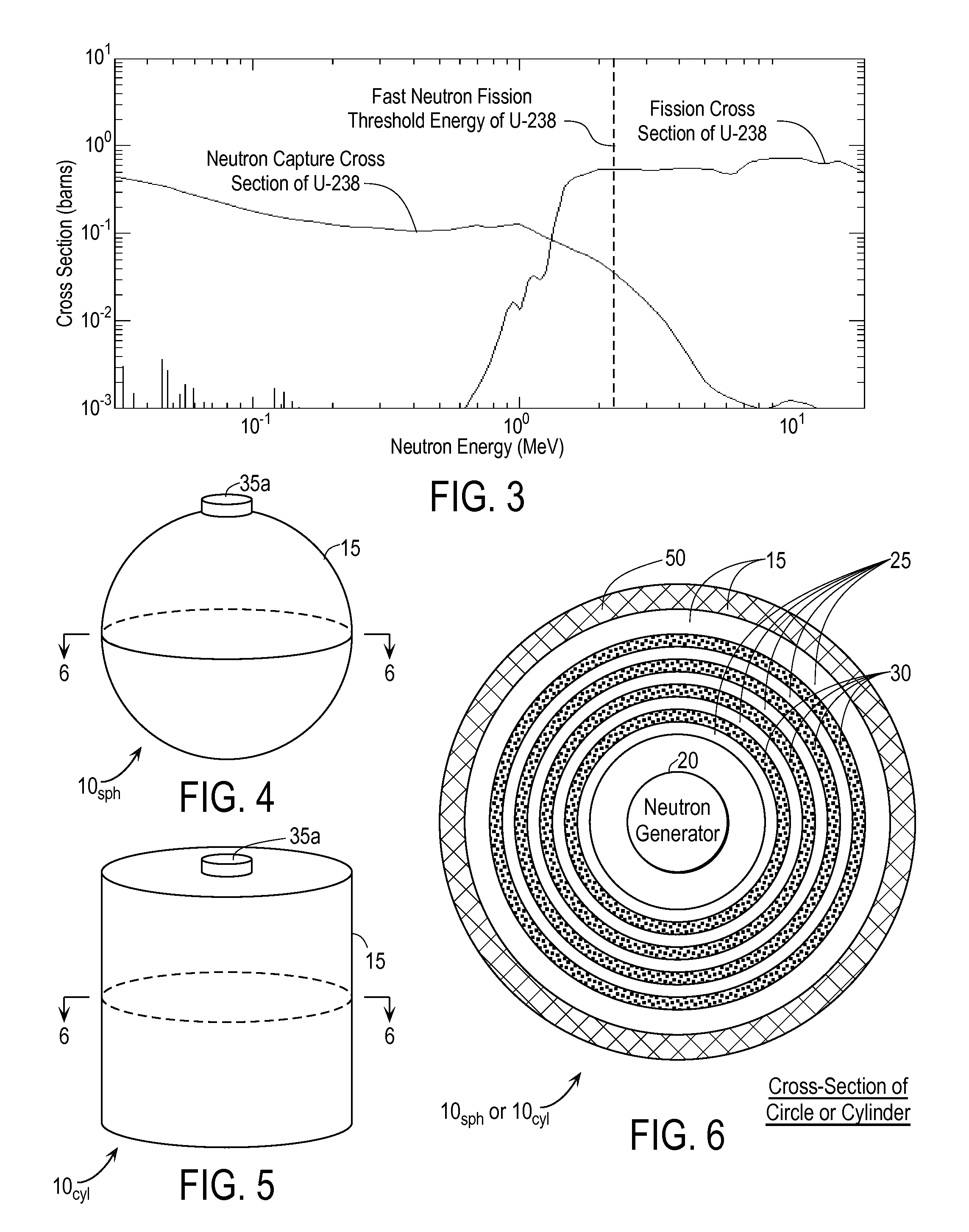
InactiveUS20110280356A1Minimize neutron absorptionReduce productionSpecific isotope recoveryConversion outside reactor/acceleratorsRadio isotopesNuclear engineering
A system for radioisotope production uses fast-neutron-caused fission of depleted or naturally occurring uranium targets in an irradiation chamber. Fast fission can be enhanced by having neutrons encountering the target undergo scattering or reflection to increase each neutron's probability of causing fission (m, f) reactions in U-238. The U-238 can be deployed as layers sandwiched between layers of neutron-reflecting material, or as rods surrounded by neutron-reflecting material.
Owner:GLOBAL MEDICAL ISOTOPE SYTEMS LLC +1
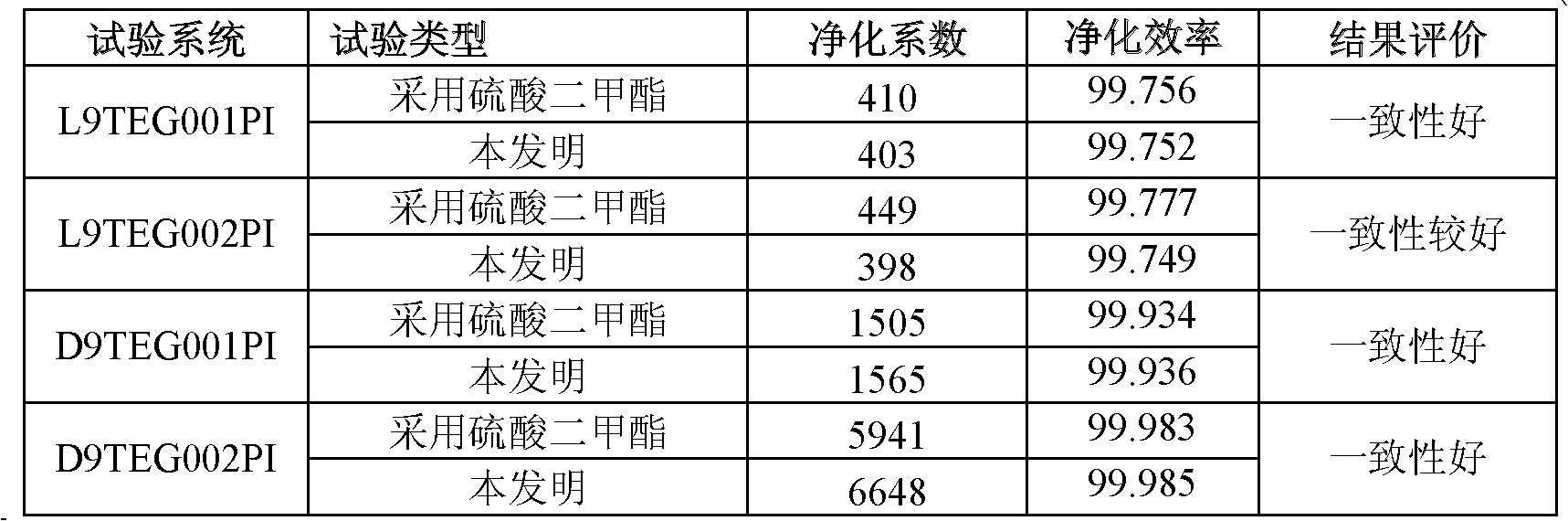
Preparation method of preparation for radioactive gas purifying capability test in nuclear power plant
The invention discloses a preparation method of a preparation for a radioactive gas purifying capability test in a nuclear power plant, which comprises the following steps of: mixing by using a methyl phosphate compound or a dimethylacetal compound, acetonitrile, trimethylchlorosilane and a radioactive iodine source as raw materials, and then reacting for 10-40 minutes at the temperature of 20-50DEG C to obtain the preparation for the radioactive gas purifying capability test in the nuclear power plant. The invention provides the preparation method of the preparation for the radioactive gas purifying capability test in the nuclear power plant, wherein reactants are not virulent, the reaction can be carried out at high speed and high efficiency without high temperature, and other productsexcept methyl iodide have no harm or small toxicity to nuclear-grade impregnated active carbon in an iodine filter.
Owner:CHINA GENERAL NUCLEAR POWER CORP +2
Techniques for On-Demand Production of Medical Radioactive Iodine Isotopes Including I-131
InactiveUS20110286565A1Avoid short travelEnhance fast fissionSpecific isotope recoveryConversion outside reactor/acceleratorsIodineIsotope
A system for radioisotope production uses fast-neutron-caused fission of depleted or naturally occurring uranium targets in an irradiation chamber. Fast fission can be enhanced by having neutrons encountering the target undergo scattering or reflection to increase each neutron's probability of causing fission (n, f) reactions in U-238. The U-238 can be deployed as one or more layers sandwiched between layers of neutron-reflecting material, or as rods surrounded by neutron-reflecting material. The gaseous fission products can be withdrawn from the irradiation chamber on a continuous basis, and the radioactive iodine isotopes (including I-131) extracted.
Owner:MIPOD NUCLEAR +1
estimation method for a critical accident environment release source item of MOX fuel
The invention provides an estimation method for a critical accident environment release source item of MOX fuel. The method comprises the following steps: (1) determining the total number of fissures;(2) determining the release of aerosol; (3) determining the release of inert gas and radioactive iodine; (4) determining the influence of ventilation and exhaust equipment and purification measures;And (5) calculating the critical accident radiation quantity. The method provided by the invention can estimate the critical accident release amount of the MOX fuel.
Owner:CHINA INST FOR RADIATION PROTECTION
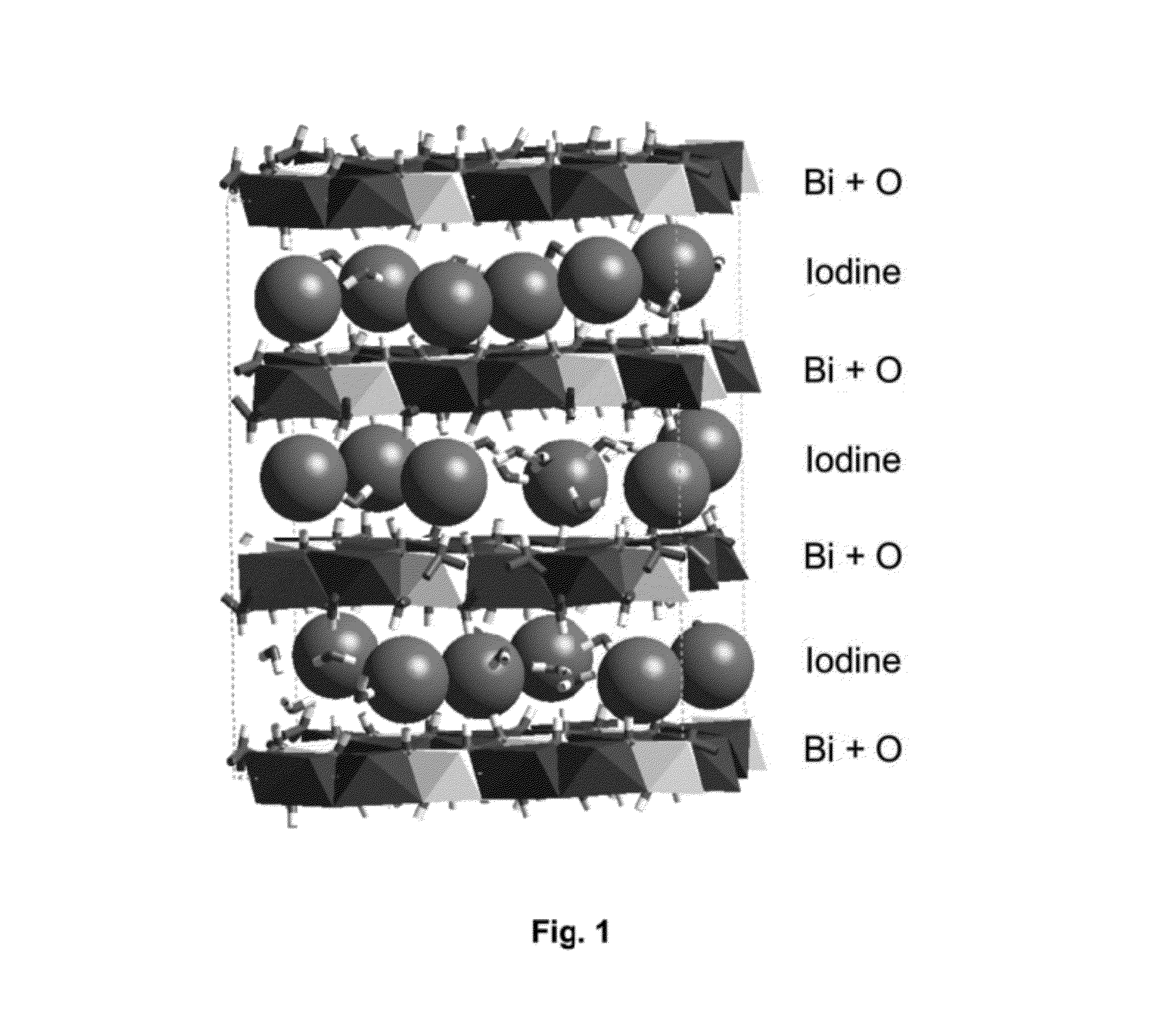
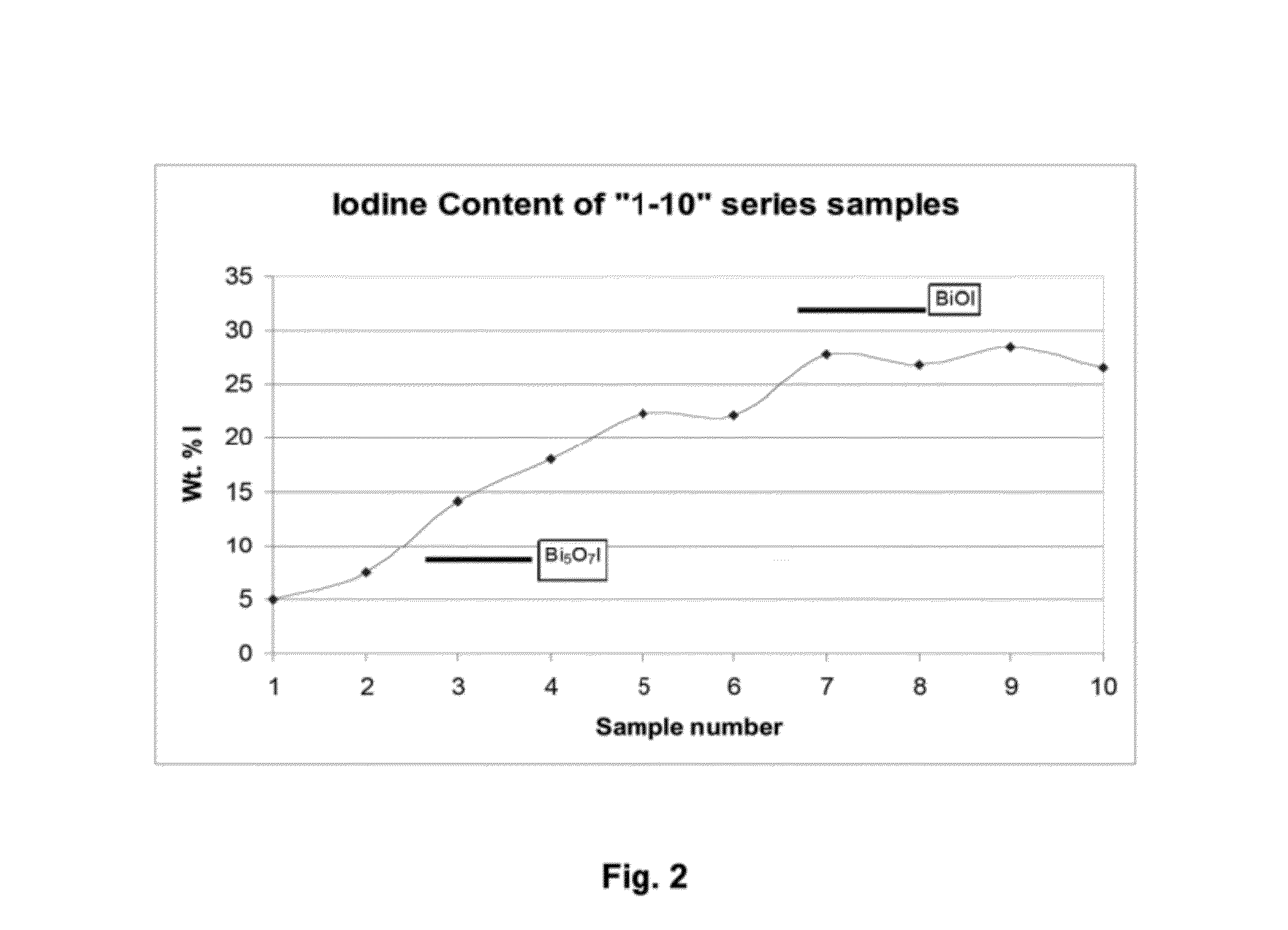
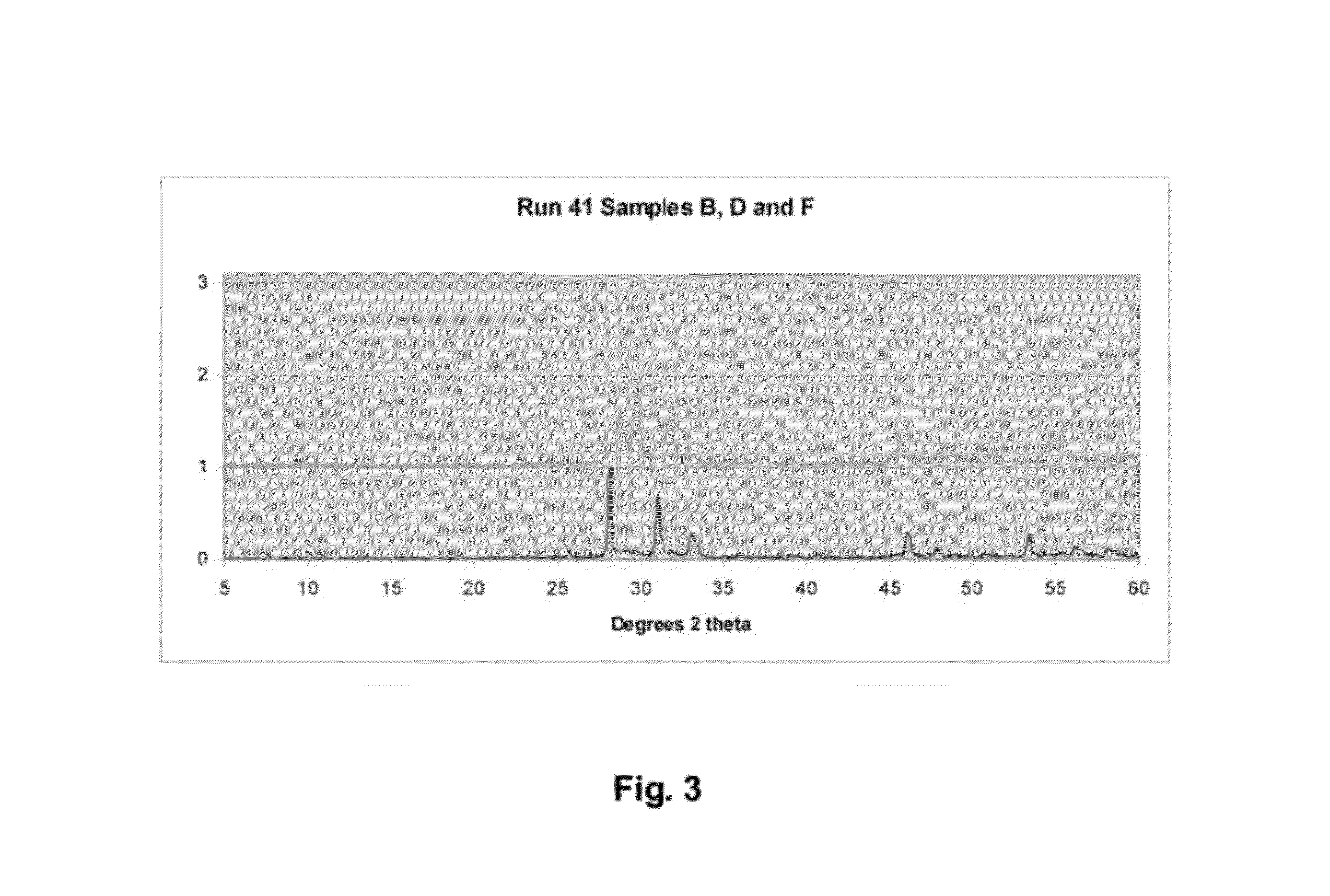
Mixed-layered bismuth-oxygen-iodine materials for capture and waste disposal of radioactive iodine
ActiveUS8383021B1Transuranic element compoundsSolid waste disposalSolubilityWaste Isolation Pilot Plant
Materials and methods of synthesizing mixed-layered bismuth oxy-iodine materials, which can be synthesized in the presence of aqueous radioactive iodine species found in caustic solutions (e.g. NaOH or KOH). This technology provides a one-step process for both iodine sequestration and storage from nuclear fuel cycles. It results in materials that will be durable for repository conditions much like those found in Waste Isolation Pilot Plant (WIPP) and estimated for Yucca Mountain (YMP). By controlled reactant concentrations, optimized compositions of these mixed-layered bismuth oxy-iodine inorganic materials are produced that have both a high iodine weight percentage and a low solubility in groundwater environments.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
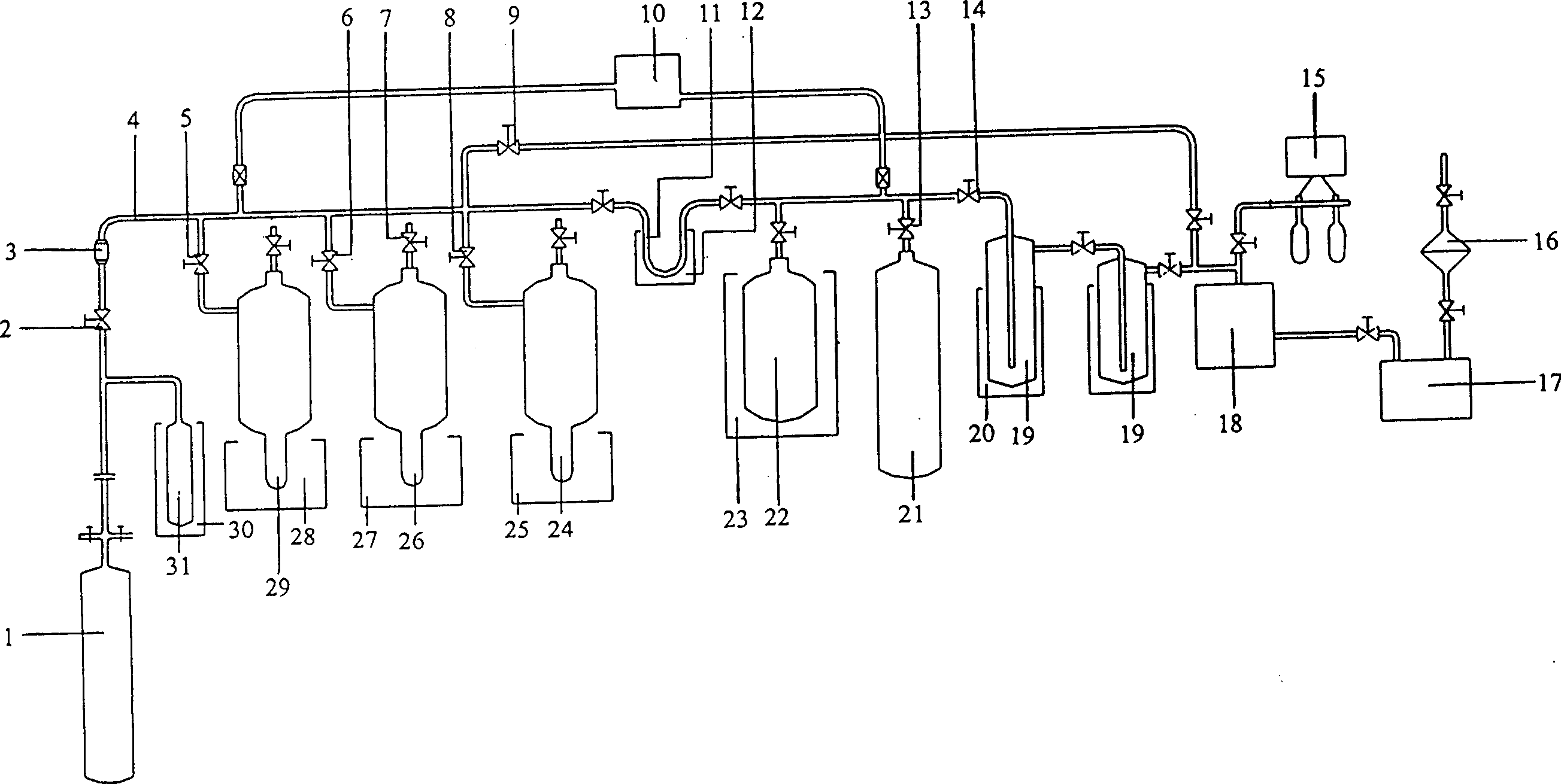
Method for preparing radioactive 125I and intermittent circulation loop device
InactiveCN1571073ALower requirementGuaranteed decay periodConversion outside reactor/acceleratorsIodineEngineering
The present invention belongs to the field of preparation of radioactive nuclide. It discloses a kind of preparation method of radioactive iodine and the intermittent closed circuit apparatus that used in this method. The characteristic of this method is irradiated the Xe gas in the irradiation bottle that placed in the reactor active area and transferred it out of the reactor to disintegrate, separate, recycle, purify, split charging, etc. At the same time, the circulating irradiated Xe gas would be inflated to the irradiation bottle of reactor active area, and the process of irradiation and disintegration would be carried on. The characteristic of this apparatus is that except irradiation bottle is in the active area of reactor, all the other equipments are placed out of the noumenon of reactor and connected to the irradiation bottle through main pipe. And there are 2 to 7 used disintegration bottle. The needle-shape tube with the cooling trap is connected to the main pipe between the irradiation bottle and aspirator. íí
Owner:HTA CO LTD +1
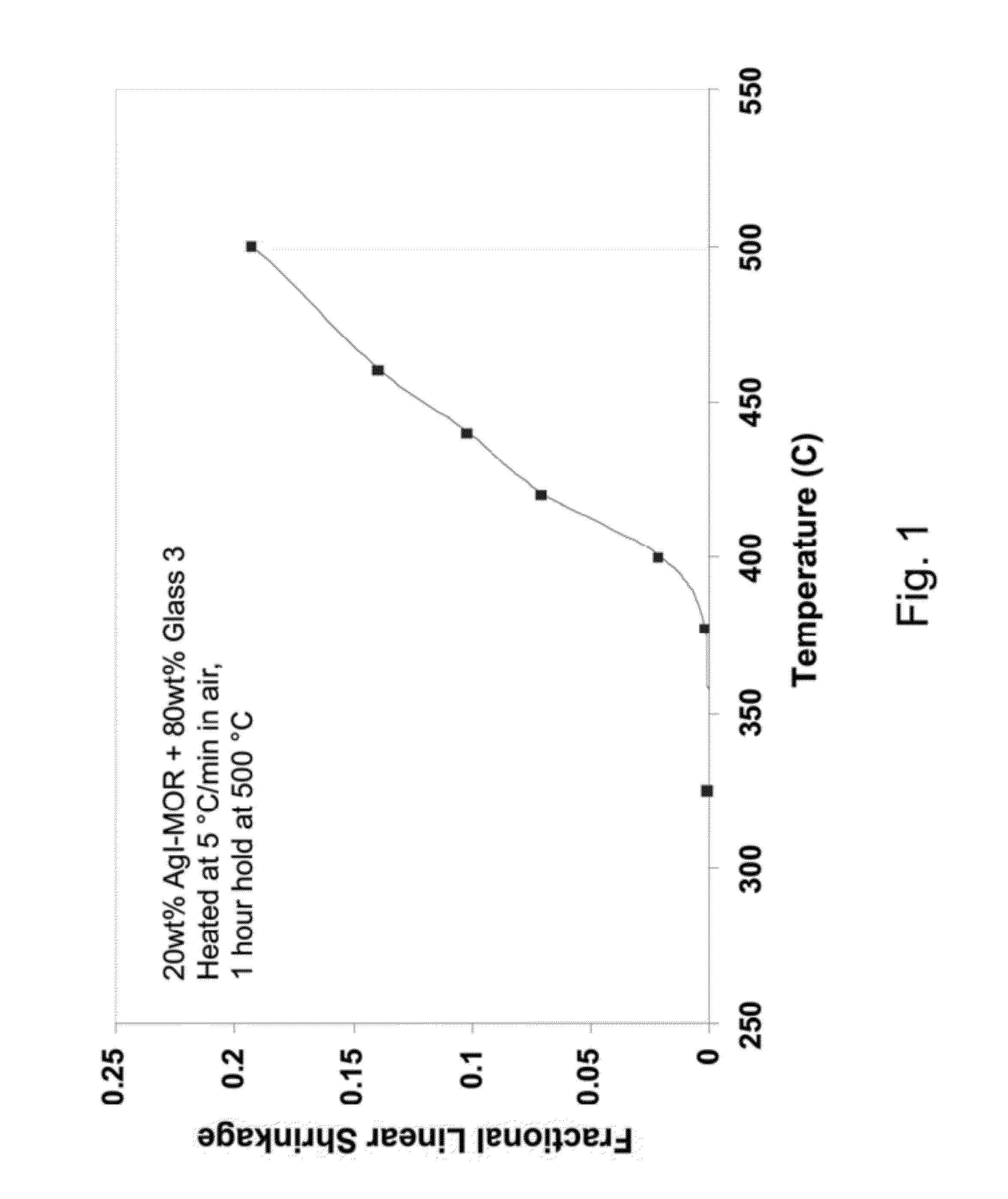
Low sintering temperature glass waste forms for sequestering radioactive iodine
ActiveUS8262950B1Stable structureLow leaching rateOther chemical processesTransuranic element compoundsSilver iodideSorbent
Materials and methods of making low-sintering-temperature glass waste forms that sequester radioactive iodine in a strong and durable structure. First, the iodine is captured by an adsorbant, which forms an iodine-loaded material, e.g., AgI, AgI-zeolite, AgI-mordenite, Ag-silica aerogel, ZnI2, CuI, or Bi5O7I. Next, particles of the iodine-loaded material are mixed with powdered frits of low-sintering-temperature glasses (comprising various oxides of Si, B, Bi, Pb, and Zn), and then sintered at a relatively low temperature, ranging from 425° C. to 550° C. The sintering converts the mixed powders into a solid block of a glassy waste form, having low iodine leaching rates. The vitrified glassy waste form can contain as much as 60 wt % AgI. A preferred glass, having a sintering temperature of 500° C. (below the silver iodide sublimation temperature of 500° C.) was identified that contains oxides of boron, bismuth, and zinc, while containing essentially no lead or silicon.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
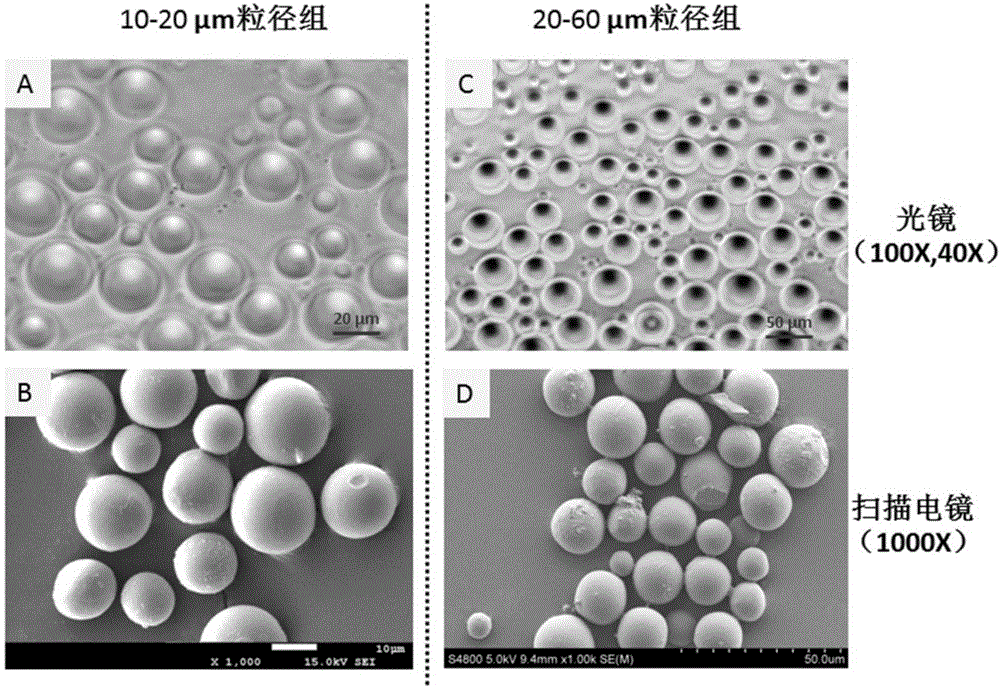
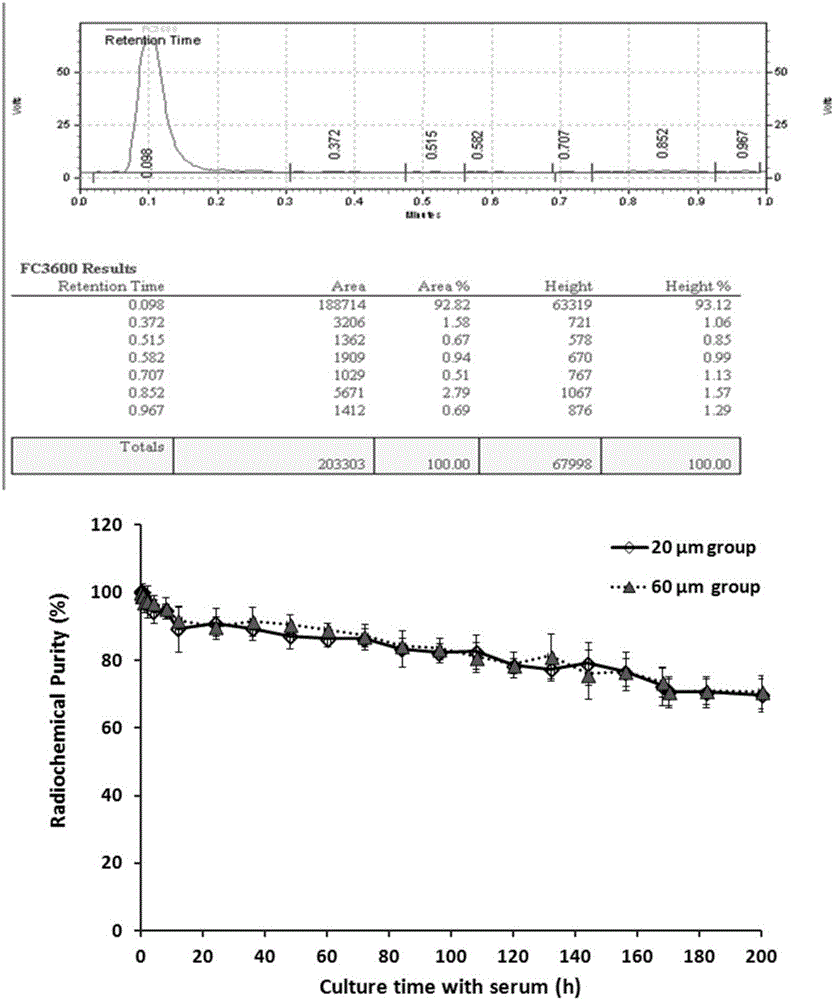
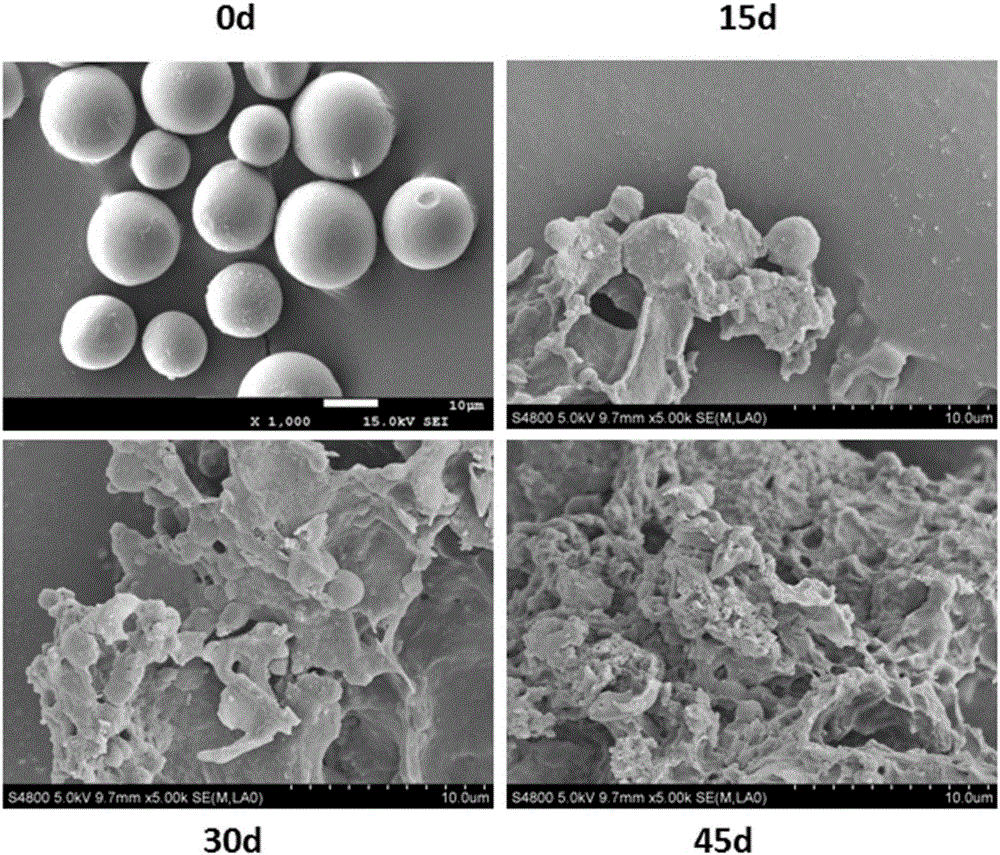
Preparation method and application of radioiodinated biodegradable chitosan-collagen composite microsphere medicine
InactiveCN106344939AGood biocompatibilityStable degradationPowder deliveryRadioactive preparation carriersMicrosphereBiocompatibility Testing
The invention discloses a composite microsphere. The composite microsphere is formed in a way that chitosan and collagen are combined. The invention further discloses a radioactive microsphere for radioactive therapy, and radionuclides are marked on the microsphere. The invention further discloses a preparation method and application of the microsphere. According to the composite microsphere, the preparation method and the application, a biodegradable chitosan-collagen composite microsphere is used as nuclide and a drug carrier, both the chitosan and the collagen have good biocompatibility, are biodegradable and can be stably combined with a plurality of radionuclides and drugs (such as chemotherapy drugs, bioactive macromolecules and the like), and clinical application prospects are good.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
SSTR1-selective analogs
InactiveUS20060155107A1Improve bindingHigh selectivityPeptide/protein ingredientsSomatostatinsNervous systemScreening method
Analogs of SRIF which are selective for SSTR1 in contrast to the other cloned SRIF receptors. These analogs are useful in determining the tissue and cellular expression of the receptor SSTR1 and its biological role in the endocrine, exocrine and nervous system, as well as in regulating tumor growth. SRIF analog peptides, such as des-AA1,2,5[D-Trp8, IAmp9, Tyr11]-SRIF and counterparts incorporating Cbm at the N-terminus, as well as radioiodinated versions thereof, inhibit the binding of a universal SRIF radioligand to the cloned human receptor SSTR1, but they do not bind with significant affinity to human SSTR2, SSTR3, SSTR4 or SSTR5. By incorporating an iodinated tyrosine in position-2 or in position-11 in these SSTR1-selective SRIF analogs, a labeled compound useful in drug-screening methods is provided. The N-terminus accommodates bulky moieties without loss of selectivity, and a carbamoyl moiety or a conjugating agent that will accept a radioactive nuclide or will link to a cytotoxin may be present at the N-terminus.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Treatment method of radioactive iodine waste
The invention belongs to the technical field of radioactive waste treatment, and relates to a treatment method of radioactive iodine waste. The treating method sequentially comprises the following steps: (1) the radioactive iodine waste reacts with sodium hydroxide, and radioactive iodine waste conversion product powder is obtained; (2) 4A zeolite and the radioactive iodine waste conversion product powder are mixed and then ground, and after drying and weighing, deionized water is added for further grinding, refining and homogenizing treatment, a uniformly wetted mixture is obtained; and (3) the uniformly wetted mixture is hydrolyzed, evaporated to be dry and then subjected to heating sintering, and an iodine sodalite solidified body is obtained. Through the treatment method of the radioactive iodine waste, the radioactive iodine waste can be treated in the modes of simple technological process, energy conservation, environment protection, safety and reliability.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Stabilized radiolabeled Anti-cd45 immunoglobulin compositions
ActiveUS20170326259A1Provide stabilityPharmaceutical delivery mechanismMammal material medical ingredientsAntigenDiagnostic agent
Compositions and methods are described for stabilizing a radio-iodinated monoclonal IgG antibody for up to 17 days against radiolytic decomposition. The stabilized radiolabeled murine antibody binding the CD45 antigen expressed on various forms of lymphomas is useful as a radio-therapeutic and diagnostic agent in the treatment of human malignancies of hematopoietic origin, including lymphomas.
Owner:ACTINIUM PHARMA
Radioactive iodine marked protein binding ligand and application thereof
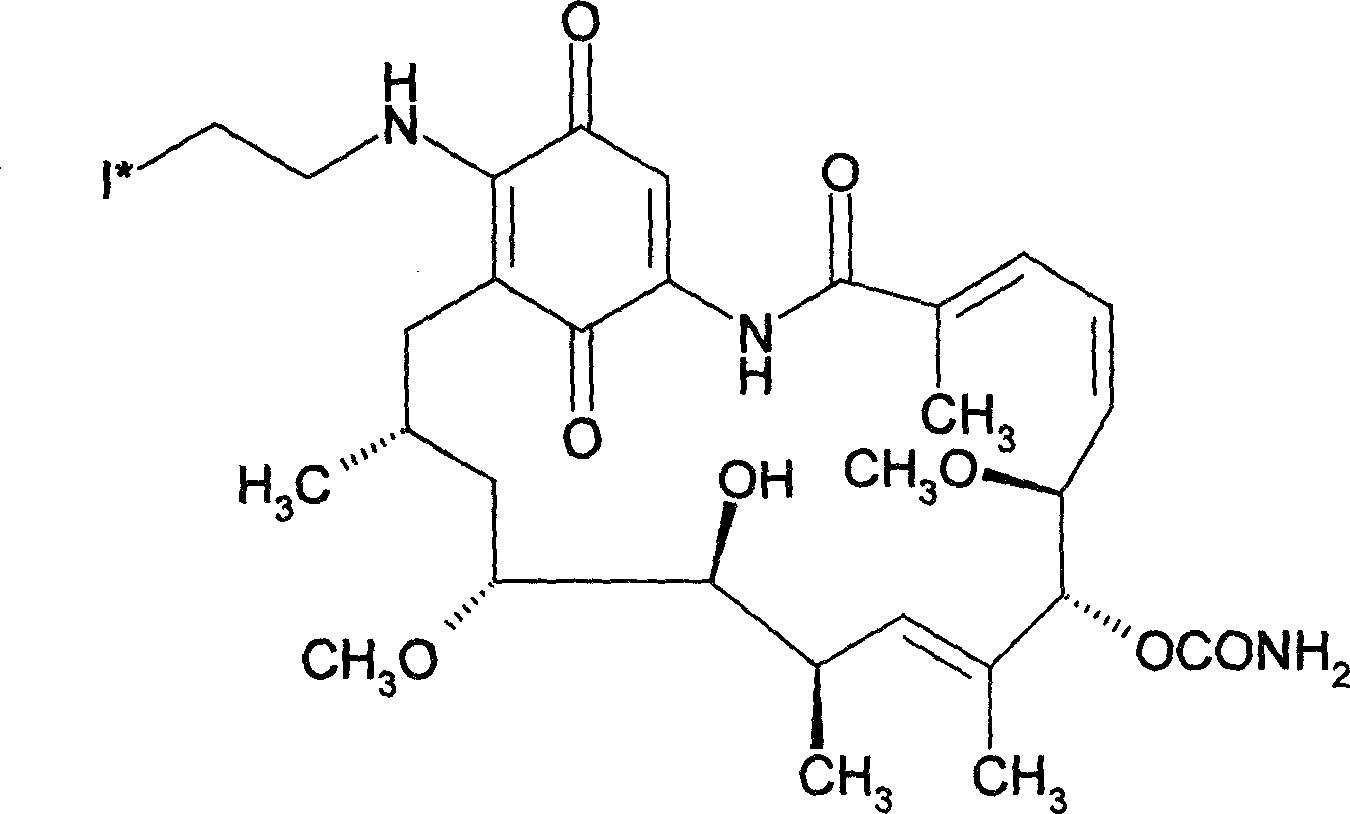
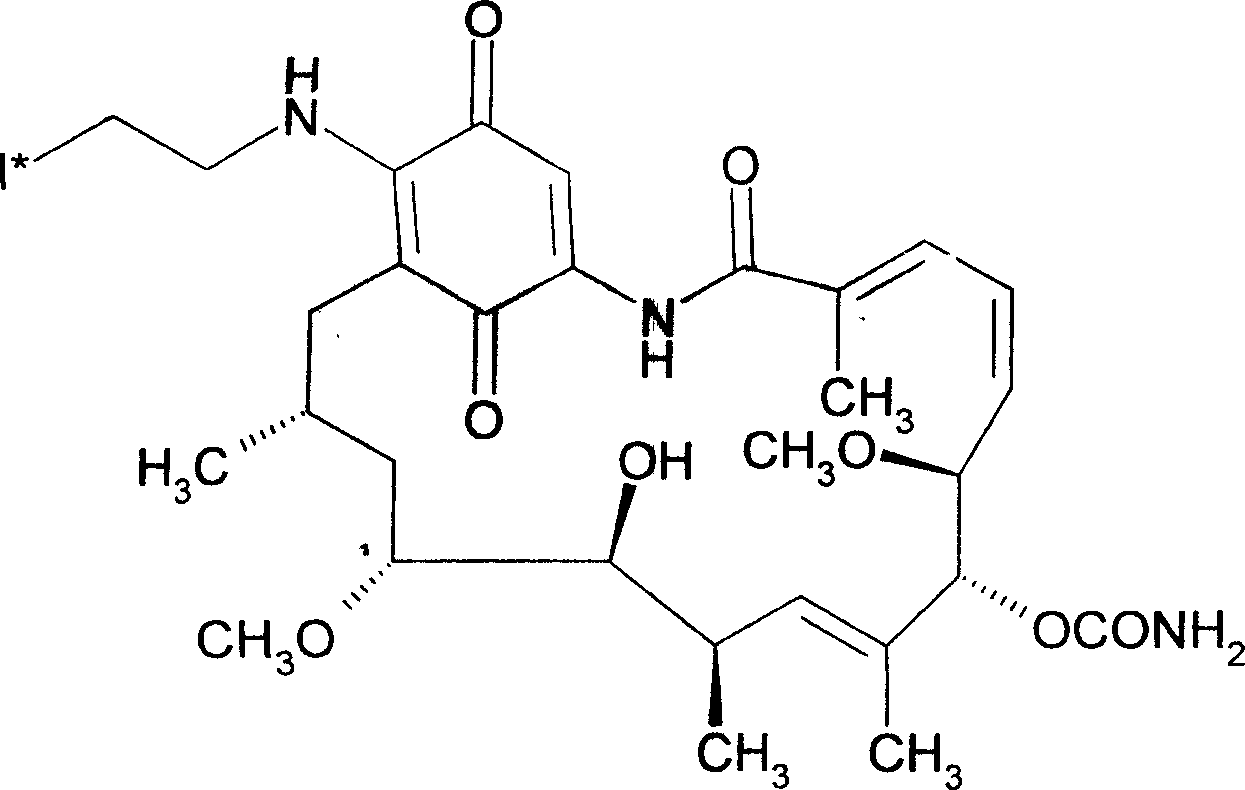
ActiveCN108434468AImprove stabilityIncrease intakeOrganic compound preparationRadioactive preparation carriersStructural formulaRadioactive Label
The invention discloses a radioactive iodine marked protein binding ligand and application thereof. A structural formula of the radioactive iodine marked protein binding ligand is shown in a followingimage, wherein the I is radioactive iodine nuclide, the R is OH or derived from PEG, folic acid, RGD, octreotide, epidermal growth factor, protein, nucleic acid or polysaccharide. The radioactive iodine marked protein binding ligand disclosed by the invention has the advantages of simple marking method, low cost and good stability. Animal live test results show that the albumin binding ligand hashigher uptake and longer-time retention in blood and further has a higher target / non-target specific value. When the radioactive iodine marked protein binding ligand is modified to a target group orother functional groups, pharmacokinetic characters of compound can be obviously improved, a blood half-life period of the compound is prolonged, and the compound is suitable for being utilized as a blood pool, lymph and tumor diagnosing and treating reagent.
Owner:XIAMEN UNIV
Filter device for gas flow loaded with aerosoles and/or gaseous iodine
InactiveUS20140007550A1Promote absorptionMore thermal energyCombination devicesGas treatmentFilter mediaIodine
The invention relates to a filter device for filtering a gas flow that is loaded with aerosols and / or gaseous radioactive iodine including a housing that is closed fluid tight, including at least one raw gas inlet, a clean gas outlet, at least one filter element including a filter medium, the filter element is arranged in the housing so that a gas flow to be filtered moves from the at least one raw gas inlet to the clean gas outlet only through the filter element, at least one tubular element penetrating the housing from a first pass-through cross-section to a second pass through cross-section which is arranged in vertical direction above the first pass through cross-section, so that an entire inner cavity of the tubular element is exclusively in contact with an ambient fluid surrounding the filter device.
Owner:YIT GERMANY
Micro-nano structure bismuth oxide material and preparation method thereof
InactiveCN105060341AImprove adsorption capacityEasy to prepareMaterial nanotechnologyBismuth compoundsMicro nanoTremella
The invention discloses a micro-nano structure bismuth oxide material and a preparation method thereof. The component of the material is non-stoichiometric bismuth oxide with a molecular formula of Bi2O2.33, and the material is in a tremella shape formed by vertical cross-linking of non-stoichiometric bismuth oxide nanosheets. The specific surface area of the tremella shaped non-stoichiometric bismuth oxide is larger than or equal to 26.6m<2> / g, and the nanosheets composing the tremella shaped non-stoichiometric bismuth oxide have a thickness of 5-10nm and a height of 2-5 micrometers. The preparation method includes: firstly using ethanol and ethylene glycol to prepare an organic solvent, then dissolving a soluble bismuth salt in the organic solvent to obtain a soluble bismuth salt solution, then subjecting the soluble bismuth salt solution to sealed reaction to obtain a reaction solution, and then carrying out solid-liquid separation, washing and drying treatment on the reaction solution in order, thus obtaining the target product. The micro-nano structure bismuth oxide material has adsorption capacity to I<-> and IO<3-> ions, and can be widely applied to high performance adsorption of radioactive iodine in water environment.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Low-temperature curing method for glass ceramic silver-coating silica gel
ActiveCN109775994ALow curing temperatureLow iodine leaching rateRadioactive decontaminationLow temperature curingSilica gel
The invention discloses a low-temperature curing method for glass ceramic silver-coating silica gel. The method comprises the following steps that silver-coating silica gel particles containing radioactive iodine and glass ceramic powder are mixed, deionized water is added for grinding, and a humid moisture is obtained, wherein the glass ceramic is composed of B2O3, Bi2O3, ZnO and SiO2; the humidmixture is dried and processed to obtain a dried mixture; the dried mixture is placed into a sintering device for sintering, after sintering is completed, cooling is performed to obtain a glass ceramic sintering body. The obtained glass ceramic sintering body has the advantages of being low in curing temperature, low in iodine leaching efficiency, low in heat release rate, and dispersion of radioactive iodine in the environment can be well processed. The method has the advantages of being simple in technological process, saving energy, being environmentally friendly, safe and reliable and thelike, and has the good industrial application prospect.
Owner:SOUTHWEAT UNIV OF SCI & TECH
Preparation of 131I-thyroid stimulating hormone (TSH) and application thereof
InactiveCN101787077ASimple preparation processStable markerRadioactive preparation carriersDepsipeptidesTyrosineUndifferentiated Thyroid Tumor
The invention relates to the preparation of 131I-thyroid stimulating hormone (TSH) and an application thereof, belonging to the field of radionuclide therapeutic drugs. The compound 131I-thyroid stimulating hormone is a radioiodine marker for thyroid stimulating hormone with pathoclisis in vivo acting on thyrocyte. A preparation method of the 131I-thyroid stimulating hormone comprises the following steps of: carrying out iodine 131 marking on the thyroid stimulating hormone by utilizing a chloramine-T method, a peroxide oxidation method or an iodogen method, and introducing radionuclide iodine 131 for treatment into tyrosine residues in thyroid stimulating hormone molecules. The marking rate of the prepared 131I-TSH is 85.9 percent, the radiochemical purity after purification achieves more than 90 percent, and the prepared 131I-TSH can be stored stably for more than one week at room temperature; the 131I-TSH is mainly metabolized through the liver and excreted through the kidneys; the percentage of ID / g of the 131I-TSH in the thyroid gland is not obviously reduced in four hours; and the 131I-TSH can be concentrated in thyroid gland issues sealed by an iodine solution. Concerning low / undifferentiated thyroid tumours incapable of iodine uptake, the 131I-TSH can increase the radioiodine uptake of thyroid gland cancer cells, and the radioiodine 131 can be chosen for treatment.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Method of preparing iodine-125 sealing seed source core
ActiveCN101401944AIncrease profitImprove uniformityRadioactive preparation carriersAntineoplastic agentsRadioactive iodineChemistry
The invention discloses a method for preparing an iodine-125 brachytherapy source core for treating tumor, which comprises the following steps: firstly, putting a standby filamentary silver in a 20-80 percent sodium hypochlorite solution, and performing oscillatory reaction at room temperature for one hour so that the surface of the filamentary silver is covered with a layer of compact AgCl deposition; and secondly, putting the filamentary silver into a mixture solution of an acetic acid buffer solution and a Na<125>I solution to react for one hour so that a layer of even and compact Ag<125>I deposition which is firm and hard to fall off is formed on the surface of the filamentary silver. The reagents used in the method are all conventional ones which are convenient to purchase and have low prices; irritant chlorine is not generated in the process of preparation; the surface of the iodine-125 brachytherapy source core is even and compact; the utilization rate of the radioiodine is high; and the process time is short, thus mass production is easy to realize.
Owner:CHENGDU YUNKE PHARMA
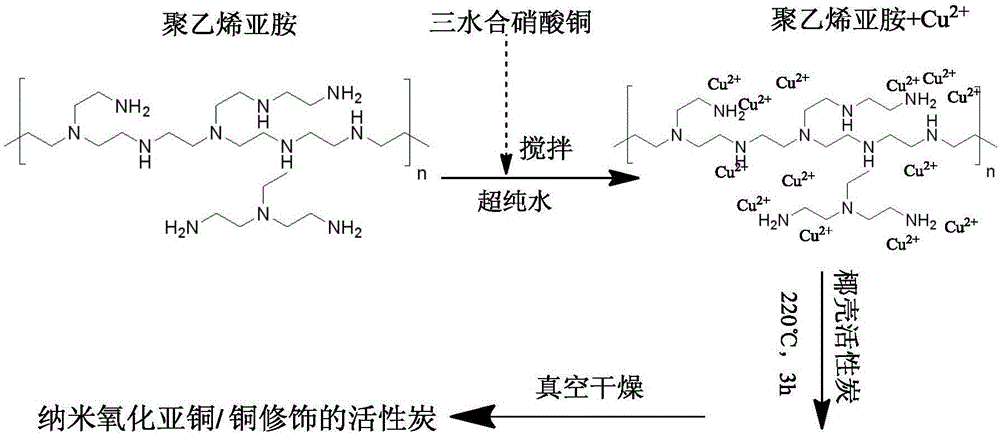
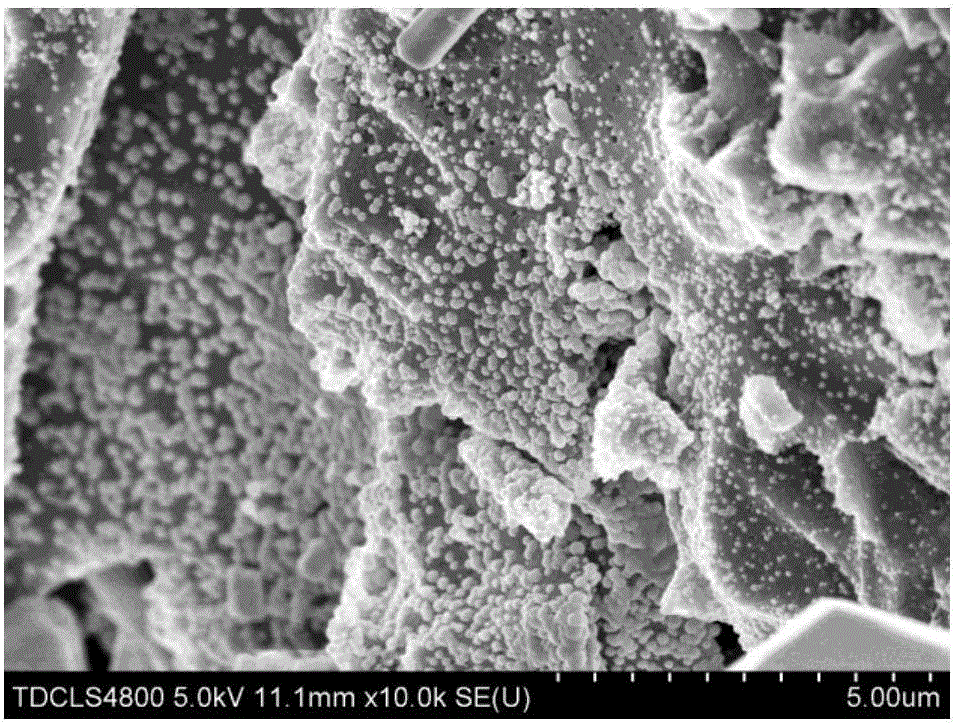
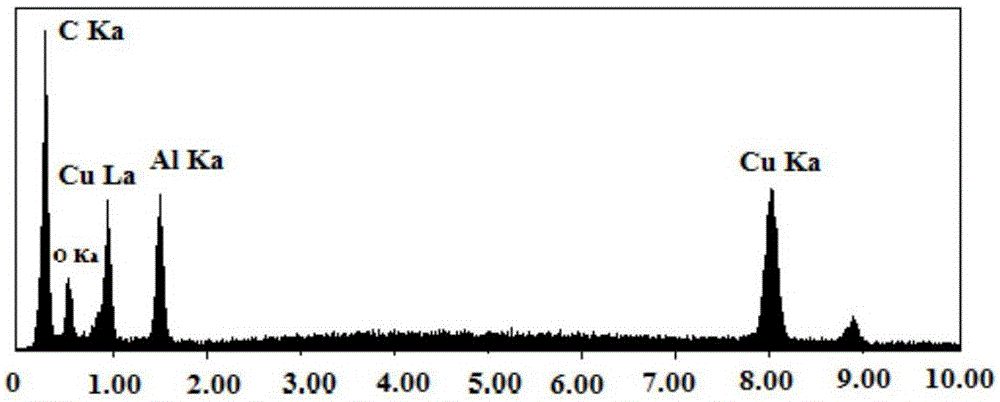
Preparation method of carbonaceous adsorbent modified by nanometer cuprous oxide/copper and application in iodine removal
InactiveCN106824084ASimple processReduce energy consumptionOther chemical processesRadioactive decontaminationSorbentCopper nitrate
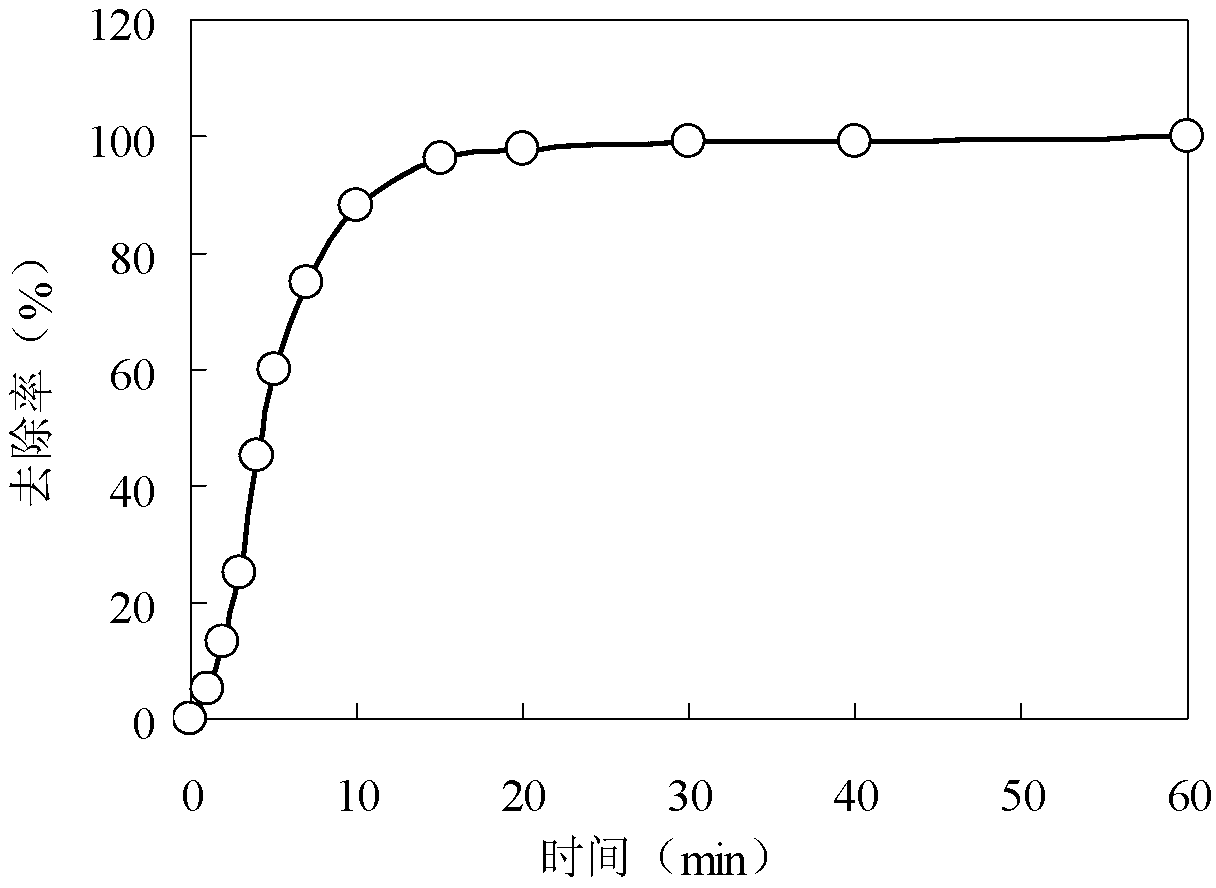
The invention relates to a preparation method of carbonaceous adsorbent modified by nanometer cuprous oxide / copper and an application in iodine removal. The preparation method includes steps of adding polyethyleneimine in ultra-pure water, and then mixing and stirring with copper nitrate; adding active carbon and placing it in a high-pressure kettle; sealing the reaction kettle, performing the hydrothermal reaction for 3-12 hours at 80-220 DEG C; naturally cooling the high-pressure kettle to room temperature, washing a solid sample by de-ionized water, and vacuum-drying at 50-80 DEG C; acquiring the active carbon modified by nanometer cuprous oxide / copper. The active carbon modified by nanometer cuprous oxide / copper is feed into radioactive water containing iodine ion, and has good absorbing performance to the iodine ion in the water body; under the condition that the initial concentration is 1-30 mg / L and the absorbent feeding amount is 1g / L, the removal effect is 90% above; the method can be applied to the emergency treatment of water body polluted by radioactive iodine ion; therefore, the preparation method of carbonaceous adsorbent modified by nanometer cuprous oxide / copper is an economic and practicable method for removing the radioactive iodide ion.
Owner:TIANJIN UNIV
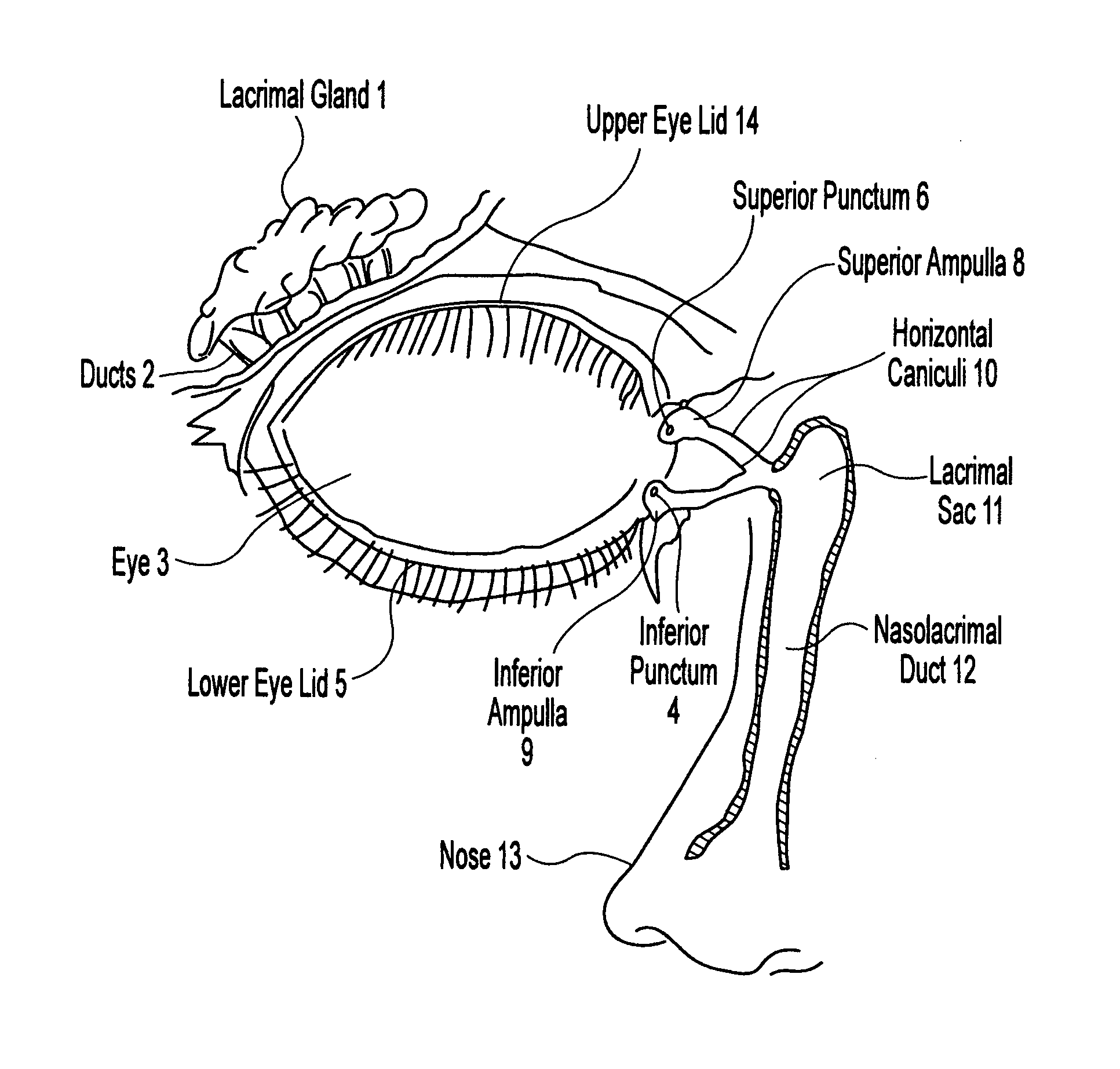
Method for preventing nasolacrimal duct obstruction
The invention is directed to a method for preventing nasolacrimal duct obstruction (NLDO) in a patient receiving high dose radioactive iodine for treatment of cancer. The method includes administering an effective amount of perchlorate anion to the eyes of the patient.
Owner:KEM HLDG
Low-temperature curing method for silver coated silica gel
ActiveCN109920574AHigh bulk densityLow nuclide leaching rateRadioactive decontaminationPrillLow temperature curing
The application of the invention discloses a low-temperature curing method for silver coated silica gel. The low-temperature curing method comprises the following steps: mixing a silver coated silicagel particle containing radioactive iodine with a raw material powder of borate, and adding deionized water to grind, to obtain a damp mixture, wherein the raw material powder of borate comprises boron oxide, bismuth oxide and zinc oxide; drying the damp mixture to obtain a dried mixture; sintering the dried mixture in a sintering device, and cooling after finishing sintering, to obtain a glass ceramic sintering body. The glass ceramic sintering body obtained according to the application has the advantages of relatively high volume density, relatively low nuclide leaching rate and the like, and the migration of the radioactive iodine in the natural world is excellently inhibited. In addition, according to the low-temperature curing method provided by the application, the silver coated silica gel particle containing radioactive iodine is directly mixed with the raw material powder of borate, the operation of sintering at the high temperature is eliminated, and the low-temperature curingmethod has the characteristics of simple craft process, energy saving, environment protection, safety, reliability and the like, and has an excellent industrial application prospect.
Owner:SOUTHWEAT UNIV OF SCI & TECH
Collagen-polyvinyl alcohol embolism microspheres loaded with iodine 131 and preparation method thereof
ActiveCN111229139ARadioactive shortImprove distributionSurgeryMicroballoon preparationPolyvinyl alcoholMicrosphere
The invention discloses collagen-polyvinyl alcohol embolism microspheres loaded with iodine 131 and a preparation method thereof. The preparation method comprises the following steps: firstly, carrying out a crosslinking reaction on polyvinyl alcohol and type I collagen to prepare collagen-polyvinyl alcohol embolism microspheres, and then carrying out radioactive iodine 131 labeling on the collagen-polyvinyl alcohol embolism microspheres to obtain iodine 131-collagen-polyvinyl alcohol embolism microspheres. The embolism microspheres prepared by the invention not only can slow down the growth speed of tumors and prolong the longest lifetime of patients, but also have the advantages of small specific gravity, easiness in perfusion, long half-life period and capability of releasing gamma raysfor imaging monitoring, do not need expensive activation equipment, and are beneficial to reducing the cost.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Borate glass ceramic low-temperature solidification method of silver-coated silica gel
ActiveCN109748509AHigh bulk densityLow nuclide leaching rateGlass furnace apparatusBorate glassIodine
The invention discloses a borate glass ceramic low-temperature solidification method of silver-coated silica gel. The borate glass ceramic low-temperature solidification method of silver-coated silicagel comprises the following steps: mixing silver-coated silica gel granules containing radioactive iodine and a borate glass ceramic powder, adding deionized water and grinding to obtain a humid mixture, wherein the borate glass ceramic consists of boron oxide, bismuth oxide and zinc oxide; dryin the humid mixture to obtain a dried mixture; and putting the dried mixture into a sintering device, sintering and cooling after sintering to obtain a glass ceramic sintering body. The glass ceramic sintering body provided by the invention contains silver-coated silica gel containing radioactive iodine; and the glass ceramic sintering body has the advantages of high volume density, low nuclide leaching rate and the like and can inhibit migration of the radioactive iodine in the natural world well.In addition, the low-temperature solidification method has the characteristics of simple technological process, energy conservation, environmental friendliness, safety, reliability and the like, andhas a good industrial application prospect.
Owner:SOUTHWEAT UNIV OF SCI & TECH
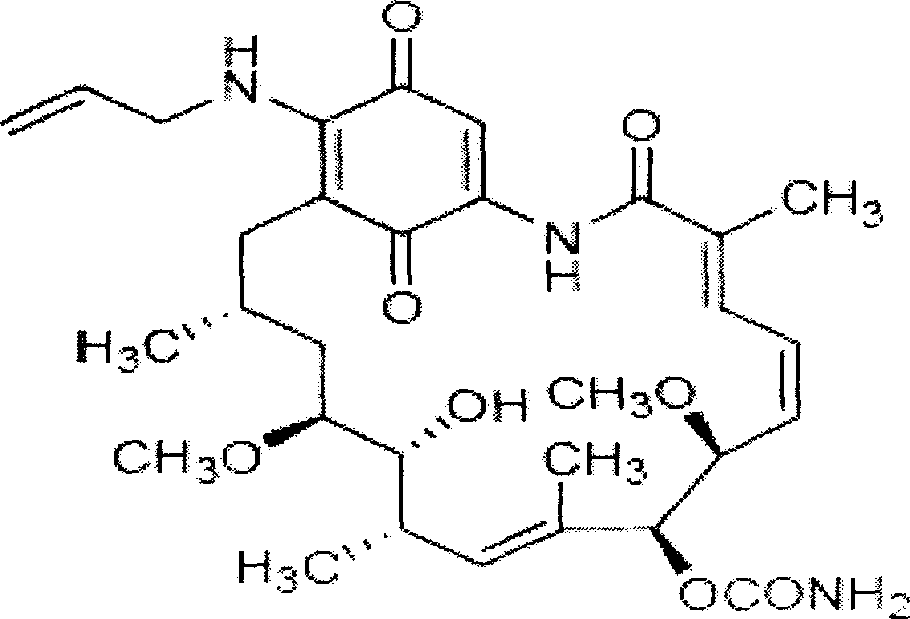
Radioactive iodine-17- allylamino-17-demethoxygeldanamycin and its preparation method
InactiveCN1995020AGood for cancer treatmentGood for diagnosisRadioactive preparation carriersIsotope introduction to organic compoundsTreatment effectGeldanamycin
The invention discloses a making method of radioactive iodine-17-allylamine-17-normethoxy geldanamycin in the nuclear medical, tumour and medical agent technical domain, which is characterized by the following: marking the product of 17-allylamine-17-normethoxy geldanamycin through iodine for HSP90; modifying the structure of 17-allylamine-17-normethoxy geldanamycin; guiding radioactive nuclear iodine on the end of double-bond; providing the medical acceptable drug or drug composition to treat tumor or cancer.
Owner:SOUTHEAST UNIV
Water treatment method of removing radioactive iodine pollutants by using permanganate and activated carbon together
ActiveCN103345954AEfficient removalStrong oxidation abilityRadioactive decontaminationActivated carbonIodine
Owner:HARBIN INST OF TECH
Adsorption material enriching radioactive iodine in seawater and preparation method of adsorption material
InactiveCN109569535AEasy to manufactureLow costOther chemical processesSeawater treatmentSorbentIodine
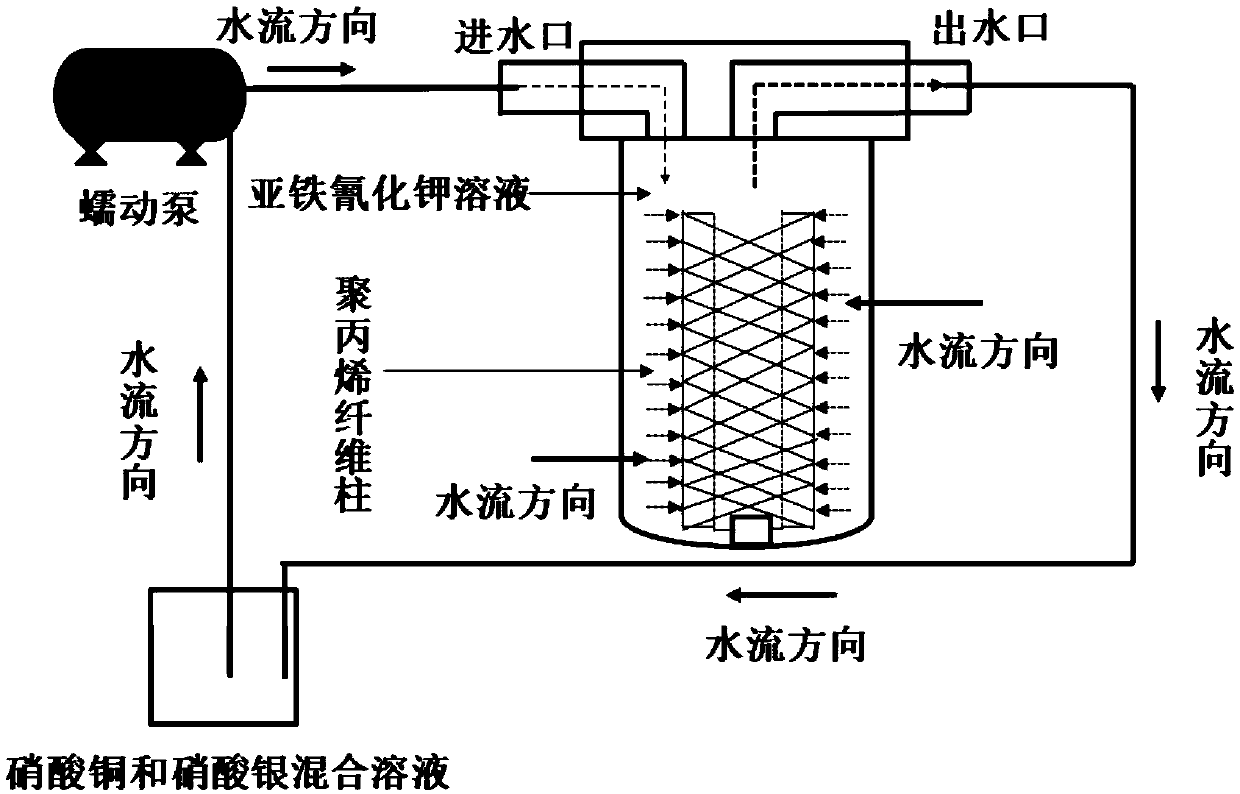
The invention discloses an adsorption material enriching radioactive iodine in seawater and a preparation method of the adsorption material. An adsorbent of the adsorption material is CuFC / AgFC, and aloading body is a polypropylene fiber filter element. The adsorption material is simple and convenient to make and low in cost, can rapidly enrich the radioactive iodine in the seawater within a short time and can be applied to large-sized surface and small-sized middle (bottom) seawater samples. The efficiency of a single-time method is 55%, and the efficiency of an eight-time cyclic adsorptionmethod is 100%. The analysis time of the adsorption material applied to enrichment of the radioactive iodine in the seawater samples is shortened to 25-30 hours, and detection limit is reduced to be 0.4-1.2Bq*m<-3>. Besides, the adsorption material can jointly enrich 134 and 137Cs in the seawater, can be applied to joint emergency monitoring of radioactive sites in the seawater, is beneficial to improvement of existing marine environment radioactivity monitoring capacity and provides technical support for marine radioactivity supervision.
Owner:EAST CHINA NORMAL UNIV
Preparation method of radioactive methyl iodide
InactiveCN104591955AReduce corrosionReduce operational instabilityPreparation by halogen replacementIsotope introduction to organic compoundsSodium iodideAir cleaning
The invention belongs to the field of a nuclear air cleaning technology and relates to a novel preparation method of radioactive methyl iodide for detecting active carbon performances and iodine absorber efficiency of a nuclear air cleaning system. The preparation method utilizes an alcohol-water mixed solution of nonradioactive iodomethane as a reagent and comprises the following steps of mixing a prepared nonradioactive iodomethane reactive liquid and a radioactive sodium iodide solution to obtain a radioactive methyl iodide generation liquid, standing the generation liquid for some time so that iodine isotopic exchange is finished and radioactive iodomethane is obtained, putting the radioactive iodomethane generation liquid into a methyl iodide generator, carrying out radioactive iodomethane generation, and injecting the product into a test loop for detecting iodine absorber efficiency. The preparation method prevents a risk caused by use and operation of a large amount of dimethyl sulphate as a highly toxic product, reduces radioactive methyl iodide generator pipe corrosion caused by a test reagent and instability of generator operation caused by corrosion, reduces maintenance frequency of the methyl iodide generator and prolongs a service life of the methyl iodide generator.
Owner:CHINA INST FOR RADIATION PROTECTION
Methods for the purification of stable radioiodine conjugates
InactiveUS7521531B2Improve efficiencyReduce contentNervous disorderElectrotherapyComputational chemistryRadioactive iodine
The present invention is directed toward a method for preparing and purifying a conjugate of a radioiodinated aminopolycarboxylate-appended peptide and a targeting agent. The method involves (A) providing a solution comp rising (i) unbound radioiodine (ii) a radioiodinated aminopolycarboxylate-appended peptide that is not conjugated to a targeting agent (iii) and a radioiodinated aminopolycarboxylate-appended peptide that is conjugated to the targeting agent; (B) contacting the solution with an anion-exchange resin; and (C) passing the anion-exchange resin and solution together through a filter capable of trapping anion-exchange resin particles.
Owner:IMMUNOMEDICS INC
Techniques for on-demand production of medical radioactive iodine isotopes including I-131
InactiveUS8989335B2Avoid short travelEnhance fast fissionSpecific isotope recoveryConversion outside reactor/acceleratorsIodineIsotope
A system for radioisotope production uses fast-neutron-caused fission of depleted or naturally occurring uranium targets in an irradiation chamber. Fast fission can be enhanced by having neutrons encountering the target undergo scattering or reflection to increase each neutron's probability of causing fission (n, f) reactions in U-238. The U-238 can be deployed as one or more layers sandwiched between layers of neutron-reflecting material, or as rods surrounded by neutron-reflecting material. The gaseous fission products can be withdrawn from the irradiation chamber on a continuous basis, and the radioactive iodine isotopes (including I-131) extracted.
Owner:MIPOD NUCLEAR +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com