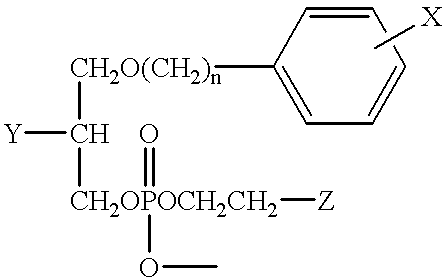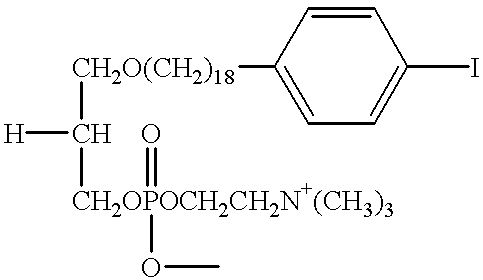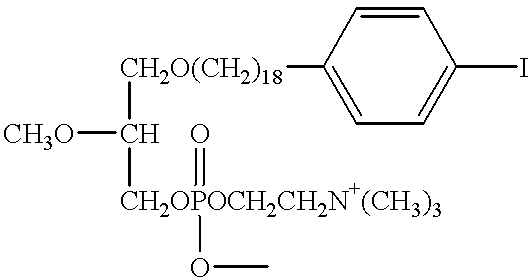Radioiodinated phospholipid ether analogs and methods of using the same
a technology of phospholipid ether and radioiodination, which is applied in the field of radiolabelled analogs of phospholipid ether, can solve the problems of tumor-specific radiopharmaceuticals which are tissue-destructive by more than on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] The synthesis of 18-(p-Iodophenyl)octadecanol (Compound VI), which is a preliminary compound for the illustrative preparatory schemes for the phospholipid ether analogs discussed in detail in Examples 2 through 4 below, was accomplished from commercially-available 15-(p-iodophenyl) pentadecanoic acid (Compound I) in accordance with the illustrative preparatory scheme shown in FIG. 1.
[0032] In general terms, aliphatic chain elongation from C-15 to C-18 was achieved by Li.sub.2CuCl.sub.4-catalyzed cross-coupling between Grignard reagent (Compound IV) prepared from benzyl 3-bromopropyl ether and 15-(p-iodophenyl) pentadecyl tosylate (Compound III). See, e.g., Fouquet and Schlosser. "Improved carbon-carbon linking by controlled copper catalysis," Angew. Chem. Int. Ed. 13:82-83 (1974); Schlosser and Bossert. "The two-fold reaction benchmark applied to the copper catalyzed assembling of 1, .omega.-difunctional hydrocarbon chains," Tetrahedron 47:6287-92 (1991). Cleavage of the benz...
example 2
[0039] Further conversion of 18-(p-iodophenyl) octadecanol into the desired phosphocholines was performed as described in detail for the C-12 analogs in Rampy et al., "Synthesis and biological evaluation of radio-iodinated phospholipid ether analogs," Nuci. Med. Biol. 22, 505-512 (1995). In one preferred embodiment, the improved phospholipid ether analog contemplated by the present invention is a simple straight chain alkyl phospholipid. 18-(p-iodophenyl)octadecyl phosphocholine (Compound XVI). The synthesis of Compound XVI was accomplished according to the illustrative preparatory scheme shown in FIG. 2.
[0040] As illustrated in FIG. 2, 2-chloro-2-oxo-1,3.2-dioxaphospholane (0.025 ml; 0.27 mmol) was added to the stirred solution of 18-(p-iodophenyl)octadecanol (Compound VI) (115 mg; 0.24 mmol) in dry benzene (3 ml) containing triethylamine (0.042 ml; 0.29 mmol). Stirring was continued overnight. The precipitated triethylamine hydrochloride was filtered off and the solvent was remove...
example 3
[0041] In another preferred embodiment, the improved phospholipid ether analogs contemplated by the present invention are constructed using a propanediol backbone. In this example, the synthesis of 1-O-[18-(p-Iodophenyl)octadecyl]-1,3-propanediol-3-phosphocholine (Compound XIV) was accomplished according to the illustrative preparatory scheme shown in FIG. 3.
[0042] To a solution of 18-(p-iodophenyl)octadecanol (Compound VI) (150 mg; 0.317 mmol) and triethylamine (0.07 ml; 0.475 mmol) in dry methylene chloride (2 ml) was added methane sulfonyl chloride (0.03 ml; 0.38 mmol) at 0.degree. C. Stirring was continued for 40 min and the reaction was quenched by addition of water. The reaction mixture was diluted with chloroform and washed several times with water. The chloroform layer was dried (Na.sub.2SO.sub.4) and evaporated. The residue was chromatographed in hexane-ethyl acetate (9:1). This afforded pure Compound VII, 18-(p-Iodophenyl)octadecyl methanesulfonate (142 mg; 82% yield).
[004...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com