Method for synthesizing lenvatinib
A technology of lenvatinib and synthetic route, which is applied in the field of lenvatinib synthesis and can solve the problems of low yield and complicated synthetic route of lenvatinib.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
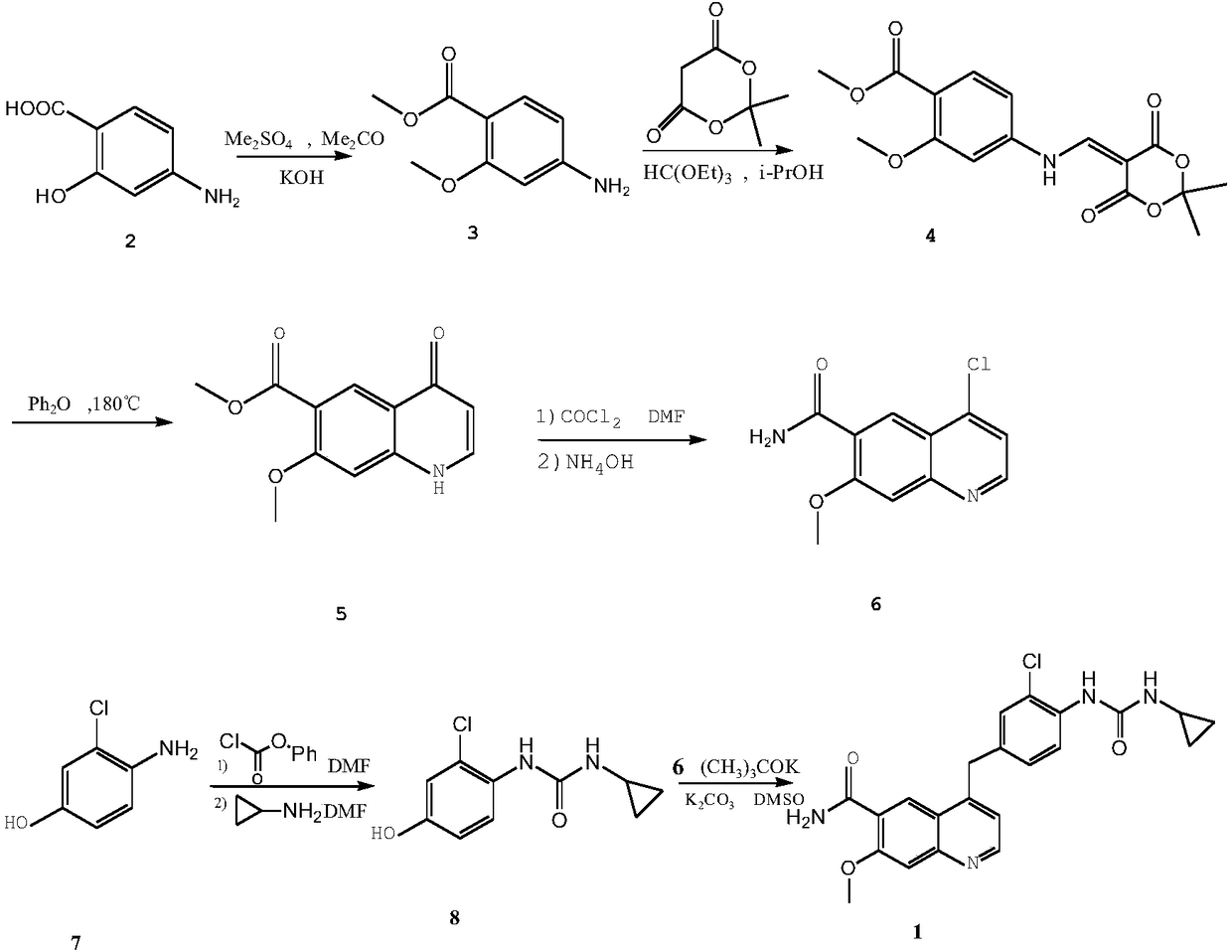
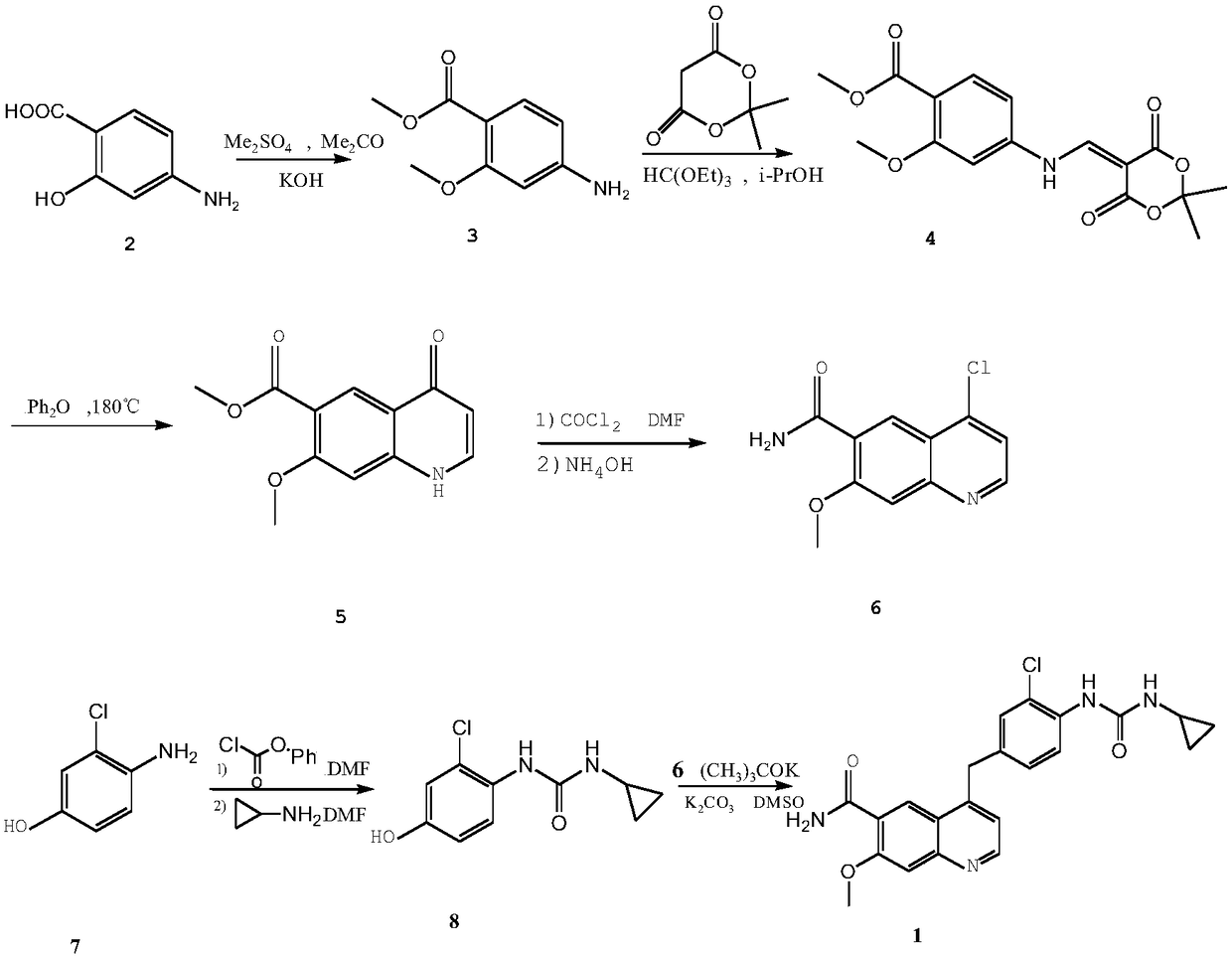
[0022] Embodiment 1: the synthesis of methyl 4-amino-2-methoxybenzoate (3)
[0023] Weigh 10g (65mmol) of p-aminosalicylic acid (2) into a 250mL three-neck flask, add 100mL of acetone, 10.45g (32.5mmol) of tetrabutylammonium bromide, stir until completely dissolved, then add 9.80g of potassium hydroxide (0.176mmol), control the reaction temperature at 20-30°C, stir until a large amount of precipitates are formed, add 15.40mL (16.3mmol) of dimethyl sulfate dropwise, control the temperature at 30-45°C, complete the dropwise addition within 20min, control the temperature , stirred for 2h. After the reaction was completed, the acetone was distilled off under reduced pressure, and 80 mL of ice water was added. A white solid precipitated out. Suction filtration, washing with water, and drying gave 10.51 g of white solid (3), yield 84%, mp 157-159°C.
Embodiment 2
[0024] Example 2: 5-[(3-methoxy-4-methoxycarbonylanilino)methylene]-2,2-dimethyl-1,3-dioxane-4,6-di Synthesis of Ketone (4)
[0025] Weigh 15g (104mmol) of McBurney's acid, add it into 50mL (300mmol) of triethyl orthoformate, and stir at 90°C for 3h. Then, 50 mL of isopropanol and 21.92 g (0.121 mmol) of methyl 4-amino-2-methoxybenzoate (3) were added, and the mixture was refluxed for 1 h. After the reaction was completed, a large amount of yellow precipitates were produced, cooled to room temperature, filtered, and the filter cake was fully washed with ether, and left to dry to obtain 29.76 g of light yellow solid (4), with a yield of 85%.
Embodiment 3
[0026] Example 3: Synthesis of 7-methoxy-4-oxo-1,4-dihydroquinoline-6-carboxylic acid methyl ester (5)
[0027] Weigh 45g (292mmol) of biphenyl and place it in a three-necked flask, add 150mL of diphenyl ether, heat the solvent to 180°C under nitrogen protection, and quickly add 18g (53.7mmol) of compound 4 under nitrogen atmosphere, a large amount of gas is released, Maintain the temperature at 170-185°C, react for 45 minutes, and stop heating. After cooling to room temperature, a large number of yellow solids precipitated, adding petroleum ether, filtering, and washing the filter cake with diethyl ether to obtain a crude product. The crude product was purified by slurrying with petroleum ether-ethyl acetate (volume ratio: 5:2), filtered by suction, and dried to obtain 10.11 g of yellow solid (5), with a yield of 80.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com