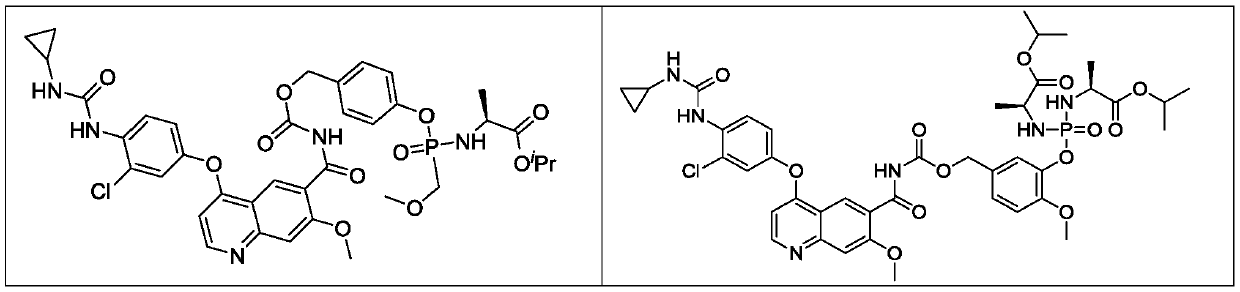
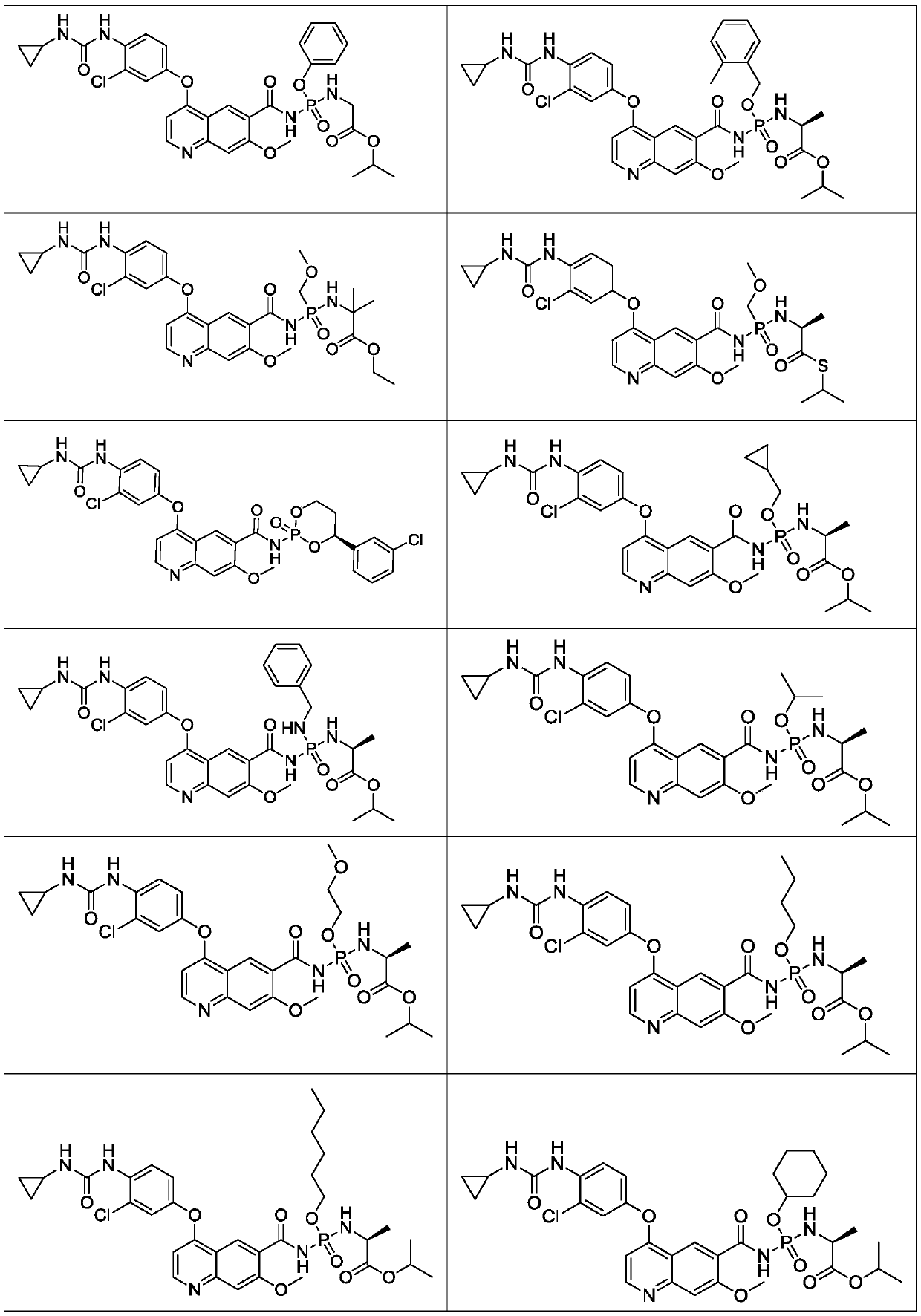
Lenvatinib derivatives, preparation method and application
A compound and selected technology, applied in the field of lenvatinib derivatives and their preparation, can solve the problems of limiting lenvatinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Isopropyl(2S)-2-[[[4-[[4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6- Carbonyl]carbamoyloxymethyl]phenoxy]-(methoxymethyl)phosphoryl]amino]propionate 2,2,2-trifluoroacetate (compound 1);
[0079] isopropyl
[0080] (2S)-2-[[[4-[[4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carbonyl]carbamoyloxymethyl]phenoxy]-(methoxymethyl)phosphoryl]amino] propanoate 2,2,2-trifluoroacetate.
[0081]
[0082] (1) The first step:
[0083] Isopropyl (2S)-2-[[[4-(hydroxymethyl)phenoxy]-(methoxymethyl)phosphoryl]amino]propionate;
[0084] isopropyl
[0085] (2S)-2-[[[4-(hydroxymethyl)phenoxy]-(methoxymethyl)phosphoryl]amino]propanoate.
[0086] Under nitrogen protection, dichloromethane (90 mL) was added into the reaction flask, methoxymethyl)phosphonic dichloride (8.96 g, 55 mmol) was added with stirring, and cooled to -30°C. Add L-alanine isopropyl ester hydrochloride (9.22g, 55mmol), slowly drop into a mixed solution of triet...
Embodiment 2
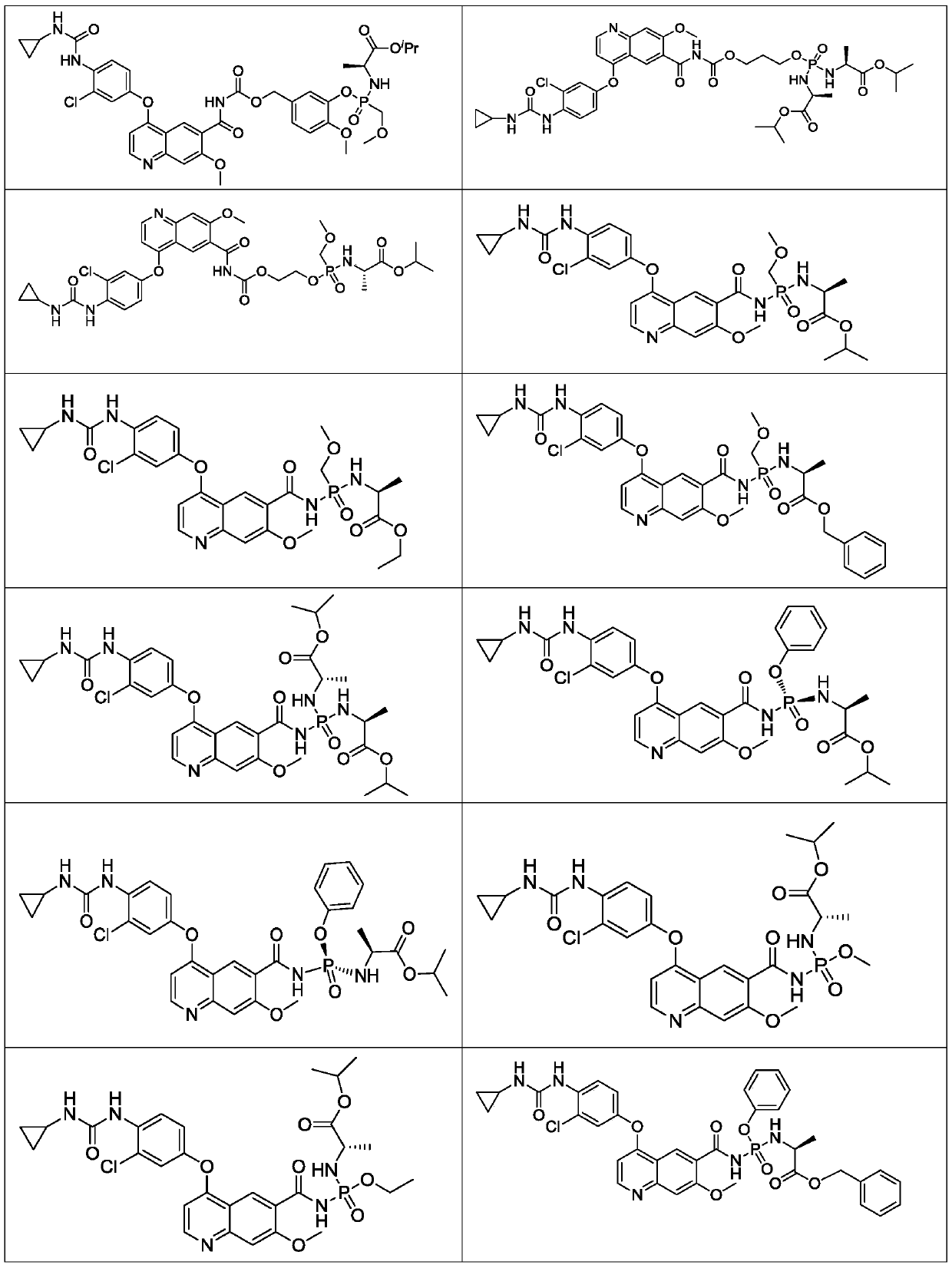
[0103] Isopropyl(2S)-2-[[[5-[[4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6- Carbonyl]carbamoyloxymethyl]-2-methoxy-phenoxy]-[[(1S)-2-isopropoxy-1-methyl-2-oxo-ethyl]amino]phosphine Acyl]amino]propionate (compound 2);
[0104] isopropyl
[0105] (2S)-2-[[[5-[[[4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carbonyl]carbamoyloxymethyl]-2-methoxy-phenoxy]-[[ (1S)-2-isopropoxy-1-methyl-2-oxo-ethyl]amino]phosphoryl]amino]propanoate;
[0106]
[0107] (1) The first step:
[0108] Isopropyl(2S)-2-[[(5-formyl-2-methoxy-phenoxy)-[[(1S)-2-isopropoxy-1-methyl-2-oxo- Ethyl]amino]phosphoryl]amino]propionate;
[0109] isopropyl
[0110] (2S)-2-[[(5-formyl-2-methoxy-phenoxy)-[[(1S)-2-isopropoxy-1-methyl-2-oxo-ethyl]amino]phosphoryl]amino]propanoate;
[0111] 2A (5g, 32.86mmol) was dissolved in 200mL of dichloromethane, phosphorus oxychloride (5g, 32.86mmol) was added at -78 degrees Celsius, and triethylamine (3.4g, 33.60mmol) was ...
Embodiment 3
[0136] Isopropyl(2S)-2[[[5-[[4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carbonyl ]carbamoyloxymethyl]-2-methoxy-phenoxy]-(methoxymethyl)phosphoryl]amino]propionate (compound 3)
[0137] isopropyl
[0138] (2S)-2-[[[5-[[[4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carbonyl]carbamoyloxymethyl]-2-methoxy-phenoxy]-(methoxymethyl ) phosphoryl] amino] propanoate.
[0139]
[0140] (1) The first step:
[0141] Under nitrogen protection, dichloromethane (90 mL) was added into the reaction flask, methoxymethyl)phosphonic dichloride (8.96 g, 55 mmol) was added with stirring, and cooled to -30°C. Add L-alanine isopropyl hydrochloride (9.22g, 55mmol), slowly drop into a mixed solution of triethylamine (11.11g, 110mmol) and dichloromethane (5mL), and react at -30°C for 30min after the addition is complete . Then 3A (7.50g, 50mmol) and triethylamine (5.05g, 50mmol) were added successively, and then raised to room temperature a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com